Amorphous Carbon and Cyano-Group Self-Modified P-Doped g-C3N4 for Boosting Photocatalytic H2 Evolution
Abstract
1. Introduction
2. Results and Discussions
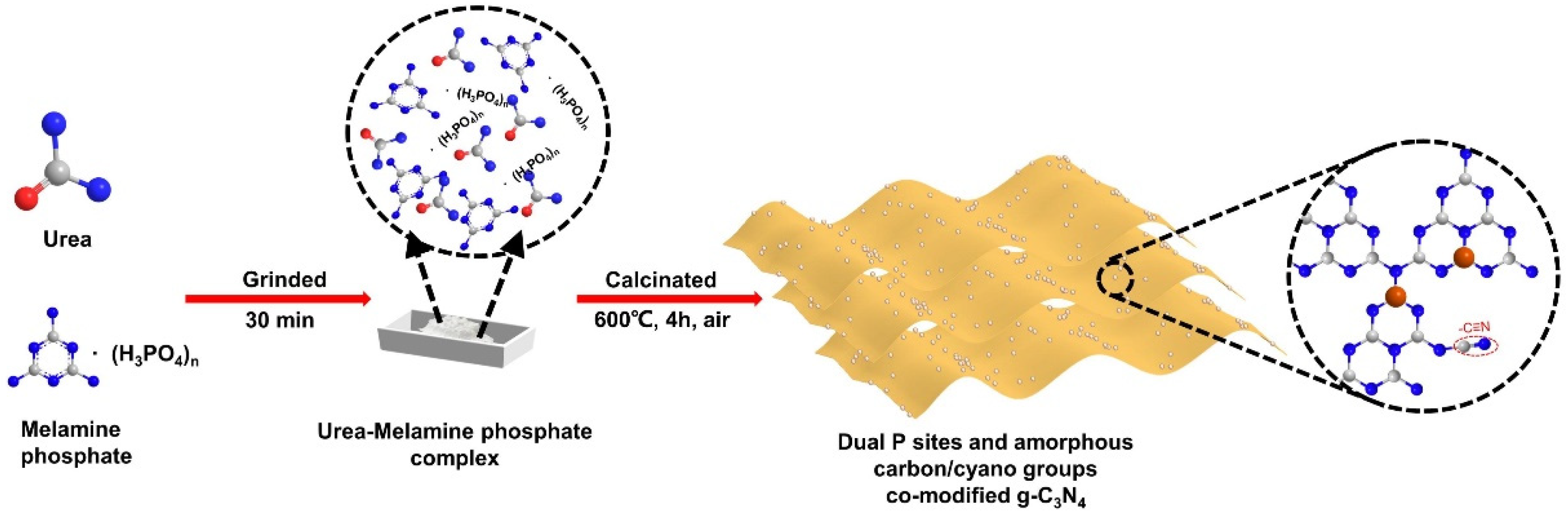
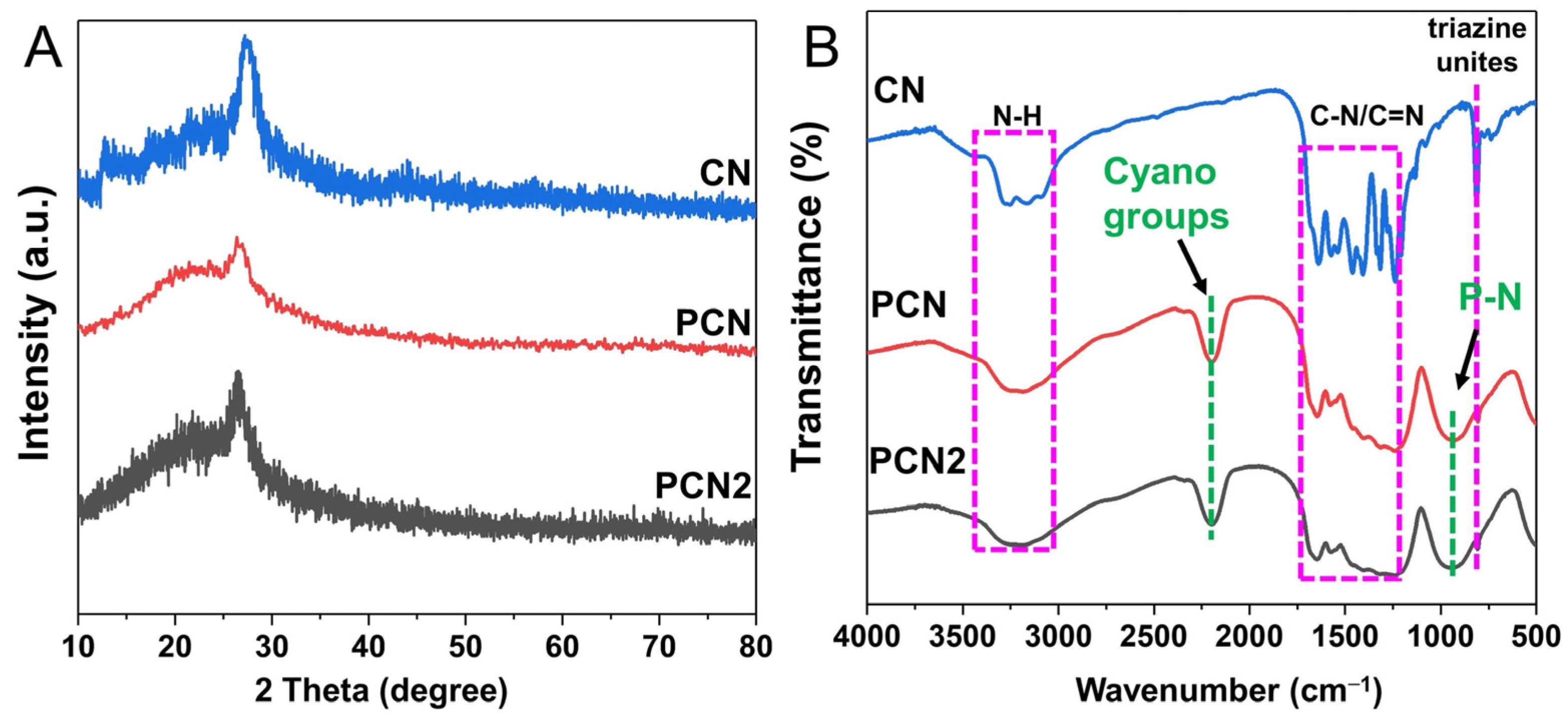
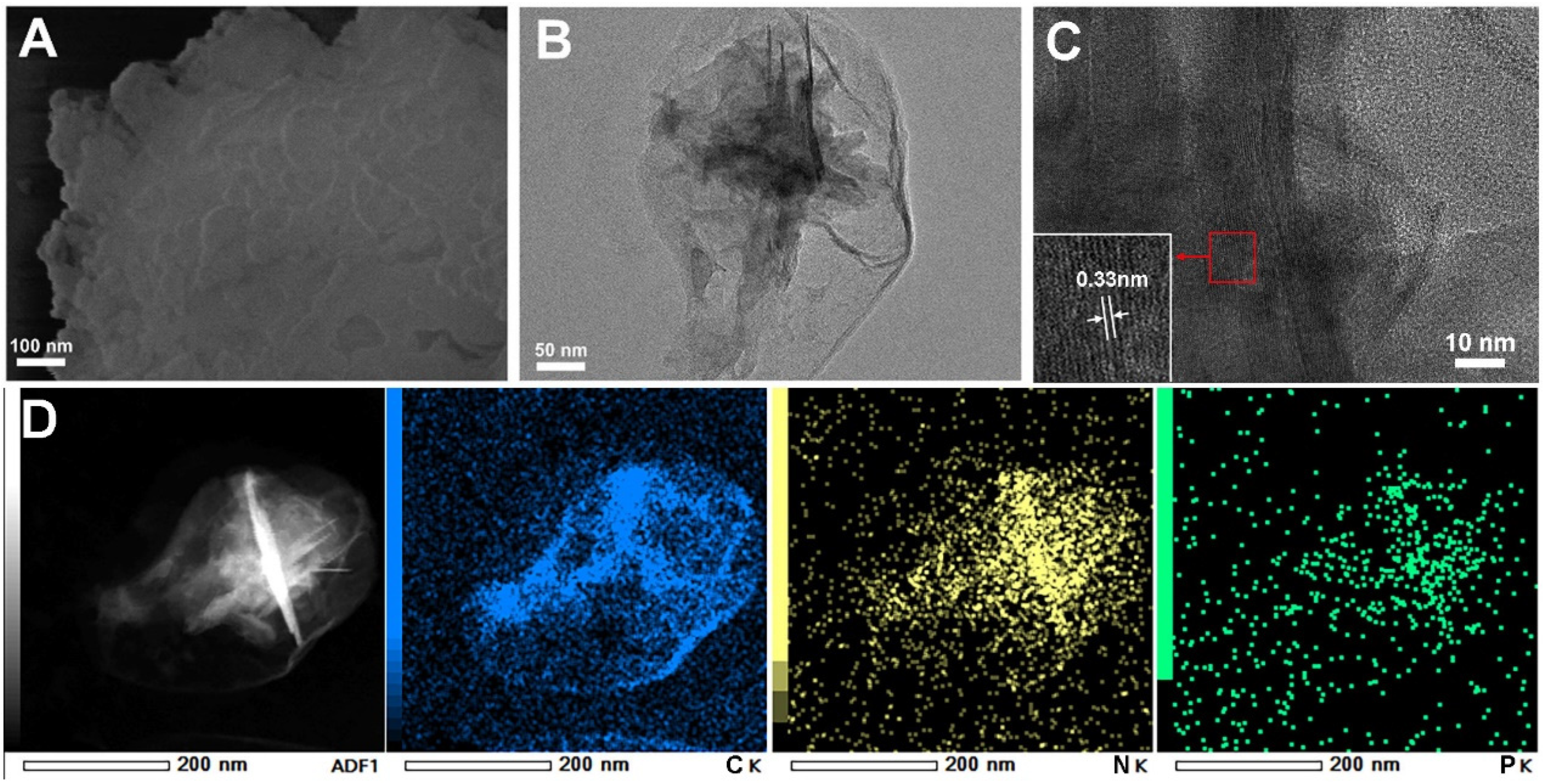
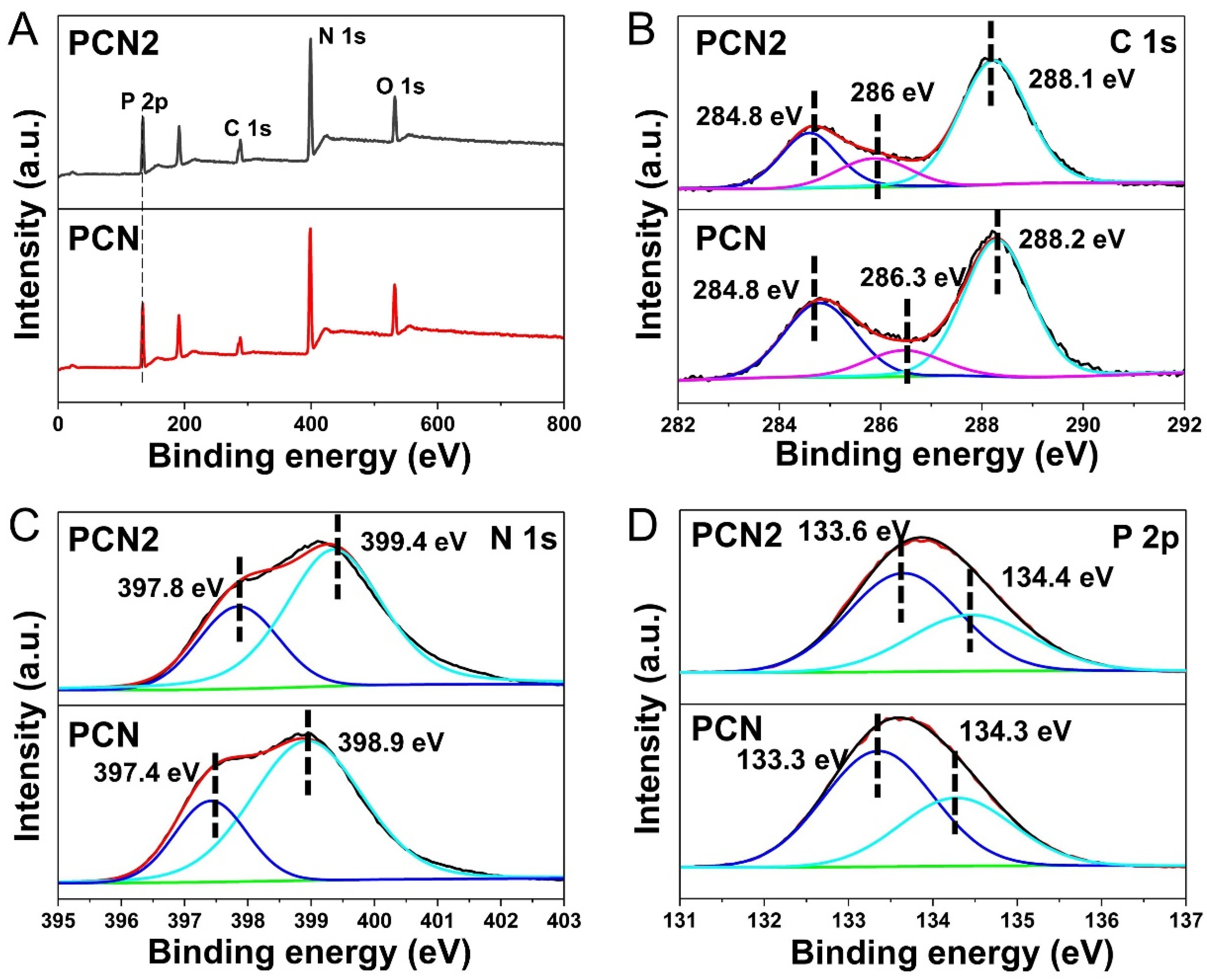
2.1. Photocatalyst Characterization
2.2. Photocatalytic Performance of Catalysts
2.3. Mechanism of Photocatalytic H2 Production
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Photocatalysts
3.3. Characterizations of Photocatalysts
3.4. Photocatalytic Hydrogen Production Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, Y.; Cassinese, A.; Li, G. Engineered 2D Metal Oxides for Photocatalysis as Environmental Remediation: A Theoretical Perspective. Catalysts 2022, 12, 1613. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Shawky, A. Promoted visible-light-driven H2 production over hydrothermally synthesized YVO4 nanorods coupled with Pt/AgInS2 nanospheres. Ceram. Int. 2023, 49, 15015–15023. [Google Scholar] [CrossRef]
- Benlembarek, M.; Salhi, N.; Benrabaa, R.; Djaballah, A.M.; Boulahouache, A.; Trari, M. Synthesis, physical and electrochemical properties of the spinel CoFe2O4: Application to the photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 9239–9247. [Google Scholar] [CrossRef]
- Alhaddad, M.; El-Hout, S.I. Hydrothermal fabrication of visible-light photoactive Ag2O/BaSnO3 nanocomposite and its application in hydrogen production. Opt. Mater. 2023, 146, 114517. [Google Scholar] [CrossRef]
- Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, S.; Zhang, Y.; Li, G.; Zhang, B.; Ma, W.; Rao, B.; Song, R.; Zhang, L.; Zhang, Y.; et al. ortho-Terphenylene Viologens with Through-Space Conjugation for Enhanced Photocatalytic Oxidative Coupling and Hydrogen Evolution. J. Am. Chem. Soc. 2022, 144, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-K.; Jia, Y.J.; Huang, Y.Y.; Xu, D.B.; Wu, X.J.; Chen, M.; Shi, W.D. Near-Infrared Light-Driven Photocatalytic Reforming Lignocellulose into H2 and Chemicals over Heterogeneous Carbon Nitride. ACS Catal. 2023, 13, 13768–13776. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, M.; Cheng, Z.; Liu, B.; Ma, Z.; Ma, J.; Zhang, J.; Ma, X.; Ma, P.; Lin, J. Defect-Repaired g-C3N4 Nanosheets: Elevating the Efficacy of Sonodynamic Cancer Therapy Through Enhanced Charge Carrier Migration. Angew. Chem. Int. Ed. 2024, 63, e202401758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Zhao, E.; Yang, W.; Lin, J.; Zhong, Q.; Qi, H.; Deng, A.; Yang, S.; Zhang, H.; et al. Atomically Dispersed Silver-Cobalt Dual-Metal Sites Synergistically Promoting Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2301840. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, F.; Liu, J.; Zhou, G.; Chen, D.; Liu, Z.; Fang, J. Design and Architecture of P-O Co-Doped Porous g-C3N4 by Supramolecular Self-Assembly for Enhanced Hydrogen Evolution. Catalysts 2022, 12, 1583. [Google Scholar] [CrossRef]
- Santiago-Aliste, A.; Sánchez-Hernández, E.; Andrés-Juan, C.; Chamorro-Posada, P.; Antorrena, G.; Martín-Gil, J.; Martín-Ramos, P. F,O,S-Codoped Graphitic Carbon Nitride as an Efficient Photocatalyst for the Synthesis of Benzoxazoles and Benzimidazoles. Catalysts 2023, 13, 385. [Google Scholar] [CrossRef]
- Tian, C.; Li, C.; Zhao, C.; Liu, D.; He, X. A Novel Synthetic 3D Interconnected Porous Carbon-Rich Graphitic Carbon Nitride for Boosting Visible Light Photocatalytic Hydrogen Production and Dye Contaminant Degradation. Catalysts 2023, 13, 1345. [Google Scholar] [CrossRef]
- Hammoud, L.; Marchal, C.; Caps, V.; Toufaily, J.; Hamieh, T.; Keller, V. Influence of low level of non-metal doping on g-C3N4 performance for H2 production from water under solar light irradiation. Int. J. Hydrogen Energy 2024, 51, 285–300. [Google Scholar] [CrossRef]
- Yu, D.; Jia, T.; Deng, Z.; Wei, Q.; Wang, K.; Chen, L.; Wang, P.; Cui, J. One-Dimensional P-Doped Graphitic Carbon Nitride Tube: Facile Synthesis, Effect of Doping Concentration, and Enhanced Mechanism for Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1759. [Google Scholar] [CrossRef]
- Sun, S.C.; Peng, B.; Song, Y.; Liu, B.; Song, H.T.; Lin, W. Boosting photoelectron transfer by Fermi and doping levels regulation in carbon nitride towards efficient solar-driven hydrogen production. Chem. Eng. J. 2024, 495, 153547. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, H.; Yang, J.; Luan, X.; Fang, D.; Yang, L.; Zi, J.; Lian, Z. Modulation of electronic density in ultrathin g-C3N4 for enhanced photocatalytic hydrogen evolution through an efficient hydrogen spillover pathway. Appl. Catal. B 2024, 341, 123334. [Google Scholar] [CrossRef]
- Yu, G.; Gong, K.; Xing, C.; Hu, L.; Huang, H.; Gao, L.; Wang, D.; Li, X. Dual P-doped-site modified porous g-C3N4 achieves high dissociation and mobility efficiency for photocatalytic H2O2 production. Chem. Eng. J. 2023, 461, 142140. [Google Scholar] [CrossRef]
- Tian, X.; Xue, M.; Yang, X.; Jiang, D.; Yuan, Y. Up-cycling of waste paper for increased photo-catalytic hydrogen generation of graphitic carbon nitride under visible light exposure. J. Taiwan Inst. Chem. Eng. 2021, 127, 259–264. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Zhang, X.; Jin, Z. Based on amorphous carbon C@ZnxCd1-xS/Co3O4 composite for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 8405–8417. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Zhu, S.; Lei, L.; Wu, X.; Feng, C.; Wang, W.; Ma, L. Engineering doping and defect in graphitic carbon nitride by one-pot method for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2023, 49, 6729–6738. [Google Scholar] [CrossRef]
- Li, K.Q.; Jiang, Y.Q.; Li, Y.D.; Wang, Z.; Liu, X.; Wang, P.; Xia, D.B.; Fan, R.Q.; Lin, K.F.; Yang, Y.L. In situ preparation of graphitic carbon nitride bonded with cyano groups for enhanced photocatalytic activity. Int. J. Hydrogen Energy 2020, 45, 9683–9694. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, B.; Yu, J.; Liu, G. Making co-condensed amorphous carbon/g-C3N4 composites with improved visible-light photocatalytic H2-production performance using Pt as cocatalyst. Carbon 2017, 118, 241–249. [Google Scholar] [CrossRef]
- Huang, T.; Wang, R.; Zhang, J.; Wang, J.; Ge, H.; Ren, J.; Zheng, Z. Cyano group modified graphitic carbon nitride supported Ru nanoparticles for enhanced CO2 methanation. Chem. Eng. J. 2023, 467, 143469. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Sun, R.; Luo, Y.; Xin, L.; Wang, D. Interfacial hot electron injection in Cu2O/MXene-g-C3N4 p-n heterojunction for efficient photocatalytic CO2 reduction. Colloids Surf. A Physicochem. Eng. Aspects 2024, 684, 133236. [Google Scholar] [CrossRef]
- Hoang, T.V.A.; Nguyen, P.A.; Shin, E.W. Effect of Morphological Modification over g-C3N4 on Photocatalytic Hydrogen Evolution Performance of g-C3N4-Pt Photocatalysts. Catalysts 2023, 13, 92. [Google Scholar] [CrossRef]
- Saman, F.; Bahruji, H.; Mahadi, A.H.; Ling, C.H.S. Pd/g-C3N4 photocatalyst for hydrogen production: Role of experimental condition for Schottky barrier. Fuel 2023, 349, 128725. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Lei, L.; Wu, X.; Wang, W.; Ma, L. Generation Mechanism of the Defects in g-C3N4 Synthesized in N2 Atmosphere and the Method for Improving Photocatalysis Activity. Catalysts 2023, 13, 269. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Yang, F.; Zhang, Y.; Yan, L.; Li, K.; Guo, H.; Yan, J.; Lin, J. Construction of Au/g-C3N4/ZnIn2S4 plasma photocatalyst heterojunction composite with 3D hierarchical microarchitecture for visible-light-driven hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2900–2913. [Google Scholar] [CrossRef]
- Yuan, J.; Tian, N.; Zhu, Z.; Yu, W.; Li, M.; Zhang, Y.; Huang, H. P, K doped crystalline g-C3N4 grafted with cyano groups for efficient visible-light-driven H2O2 evolution. Chem. Eng. J. 2023, 467, 143379. [Google Scholar] [CrossRef]
- Yu, G.; Gong, K.; Hu, L.; Xing, C.; Hu, Y.; Wang, D.; Li, X. Controllably solar-driven C–C coupling organic synthesis integrated with H2 production over P-doped g-C3N4 with NiS nanoparticles modification. Appl. Mater. Today 2023, 32, 101794. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Shi, W.; Guo, F. High-crystalline/amorphous g-C3N4 S-scheme homojunction for boosted photocatalytic H2 production in water/simulated seawater: Interfacial charge transfer and mechanism insight. Appl. Surf. Sci. 2022, 593, 153281. [Google Scholar] [CrossRef]
- Katsumata, H.; Islam Molla, M.A.; Islam, J.B.; Tateishi, I.; Furukawa, M.; Kaneco, S. Dual Z-scheme heterojunction g-C3N4/Ag3PO4/AgBr photocatalyst with enhanced visible-light photocatalytic activity. Ceram. Int. 2022, 48, 21898–21905. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Mohamed, R.M.; Alhaddad, M.; Basaleh, A.; Al-Hajji, L.A.; Ismail, A.A. Green synthesis of porous Cu2ZnSnS4/g-C3N4 heterostructured for promoted photocatalytic degradation of trichloroethylene. Ceram. Int. 2022, 48, 11736–11746. [Google Scholar] [CrossRef]
- Roškarič, M.; Zavašnik, J.; Zámbó, D.; Kotnik, T.; Kovačič, S.; Žerjav, G.; Pintar, A. Optimization Method Based on Simplex for Surface Area Improved Photocatalytic Performance of g-C3N4. ACS Catal. 2023, 13, 13282–13300. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ho, P.N.T.; Huang, C.P.; Doong, R.-a.; Chen, L.; Chen, C.-W.; Dong, C.-D. Peroxymonosulfate-assisted visible light sensitive 0D/3D Z-scheme NiCo2O4@g-C3N4 photocatalyst for effective degradation of ibuprofen in water. Chem. Eng. J. 2023, 478, 147332. [Google Scholar] [CrossRef]
- Kwon, N.H.; Park, J.; Jin, X.; Kim, S.-J.; Kim, H.; Hwang, S.-J. Defect-Regulated Two-Dimensional Superlattice of Holey g-C3N4–TiO2 Nanohybrids: Contrasting Influence of Vacancy Content on Hybridization Impact and Photocatalyst Performance. ACS Nano 2023, 17, 23732–23745. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, M.; Liu, X.; Li, H.; Yu, G.; Wang, D. Ni7S6 decorated g-C3N4/graphene oxide composites for enhanced visible light photocatalytic hydrogen evolution. Diamond Relat. Mater. 2024, 143, 110869. [Google Scholar] [CrossRef]
- Lv, S.; Ng, Y.H.; Zhu, R.; Li, S.; Wu, C.; Liu, Y.; Zhang, Y.; Jing, L.; Deng, J.; Dai, H. Phosphorus vapor assisted preparation of P-doped ultrathin hollow g-C3N4 sphere for efficient solar-to-hydrogen conversion. Appl. Catal. B 2021, 297, 120438. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Baburao Mane, S.K.; Faheem, M.B.; Ali, A.; Zhao, Y.; Han, S.; Cai, C.; Li, W.; et al. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B 2020, 270, 118867. [Google Scholar] [CrossRef]
- Xu, C.; Wu, S.; Xiong, G.; Guo, X.; Yang, H.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. Nanoconfined fusion of g-C3N4 within edge-rich vertically oriented graphene hierarchical networks for high-performance photocatalytic hydrogen evolution utilizing superhydrophillic and superaerophobic responses in seawater. Appl. Catal. B 2021, 280, 119461. [Google Scholar] [CrossRef]
- Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Aleman, M.; Cervantes-Uribe, A.; Torres-Torres, J.G.; Kesarla, M.K.; Collins-Martínez, V.; Godavarthi, S.; Martínez-Gómez, L. Facile Synthesis of Zn Doped g-C3N4 for Enhanced Visible Light Driven Photocatalytic Hydrogen Production. Top. Catal. 2020, 64, 65–72. [Google Scholar] [CrossRef]
- Ye, P.; Liu, X.; Iocozzia, J.; Yuan, Y.; Gu, L.; Xu, G.; Lin, Z. A highly stable non-noble metal Ni2P co-catalyst for increased H2generation by g-C3N4 under visible light irradiation. J. Mater. Chem. A 2017, 5, 8493–8498. [Google Scholar] [CrossRef]
- Xu, K.; Xu, H.; Feng, G.; Feng, J. Photocatalytic hydrogen evolution performance of NiS cocatalyst modified LaFeO3/g-C3N4 heterojunctions. New J. Chem. 2017, 41, 14602–14609. [Google Scholar] [CrossRef]
- Li, Y.; Lai, C.; Zhong, J.; Li, J. Largely elevated photocatalytic hydrogen generation over Eu doped g-C3N4 photocatalyst. Int. J. Hydrogen Energy 2023, 48, 24356–24368. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhong, J.; Li, J. Facile construction of CuO/g-C3N4 heterojunctions with promoted photocatalytic hydrogen generation behaviors. Fuel 2023, 353, 129224. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Yang, Z.; Shen, R.; Fang, Y.; Ma, S.; Chen, X.; Li, X. Earth-abundant WC nanoparticles as an active noble-metal-free co-catalyst for the highly boosted photocatalytic H2 production over g-C3N4 nanosheets under visible light. Catal. Sci. Technol. 2017, 7, 1193–1202. [Google Scholar] [CrossRef]
- Cao, L.; Qiao, S.; Li, X.; Li, Q. Synthesis and photocatalytic performance of g-C3N4/MeTMC-COP composite photocatalyst. Front. Chem. 2023, 11, 1138789. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Haidry, A.A.; Amin, T.; Hussain, A.A.; Shah, S.A.M.H.; Ahsan, M. Boosting the water splitting and hydrogen production of S-scheme fabricated porous g-C3N4 modified with CuO. Diamond Relat. Mater. 2024, 141, 110703. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Wang, X.; Chu, X.-S.; Wang, F.; Wang, X.-C.; Wang, C.-Y. Enhanced photoresponse and fast charge transfer: Three-dimensional macroporous g-C3N4/GO-TiO2 nanostructure for hydrogen evolution. J. Mater. Chem. A 2020, 8, 19533–19543. [Google Scholar] [CrossRef]
- He, R.; Liang, H.; Li, C.; Bai, J. Enhanced photocatalytic hydrogen production over Co3O4@g-C3N4 p-n junction adhering on one-dimensional carbon fiber. Colloids Surf. A 2020, 586, 124200. [Google Scholar] [CrossRef]
- Liu, T.; Hao, L.; Bai, L.; Liu, J.; Zhang, Y.; Tian, N.; Huang, H. Z-scheme junction Bi2O2(NO3)(OH)/g-C3N4 for promoting CO2 photoreduction. Chem. Eng. J. 2022, 429, 132268. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Li, X.; Li, B.; et al. Molecular engineering of polymeric carbon nitride for highly efficient photocatalytic oxytetracycline degradation and H2O2 production. Appl. Catal. B 2020, 272, 118970. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.H.; Kang, F.; Yang, Q.H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon Quantum Dot Implanted Graphite Carbon Nitride Nanotubes: Excellent Charge Separation and Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Liang, H.; Liu, B.J.; Tang, B.; Zhu, S.C.; Li, S.; Ge, X.Z.; Li, J.L.; Zhu, J.R.; Xiao, F.X. Atomically Precise Metal Nanocluster-Mediated Photocatalysis. ACS Catal. 2022, 12, 4216–4226. [Google Scholar] [CrossRef]
- Liu, X.W.; Lin, J.H.; Jong, W.J.; Shih, H.C. The effect of pressure control on a thermally stable a-C:N thin film with low dielectric constant by electron cyclotron resonance-plasma. Thin Solid Films 2002, 409, 178–184. [Google Scholar] [CrossRef]
- Chandra, M.; Guharoy, U.; Pradhan, D. Boosting the Photocatalytic H2 Evolution and Benzylamine Oxidation using 2D/1D g-C3N4/TiO2 Nanoheterojunction. ACS Appl. Mater. Interfaces 2022, 14, 22122–22137. [Google Scholar] [CrossRef]
- Su, C.; Zhou, Y.; Zhang, L.; Yu, X.; Gao, S.; Sun, X.; Cheng, C.; Liu, Q.; Yang, J. Enhanced n→π* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance. Ceram. Int. 2020, 46, 8444–8451. [Google Scholar] [CrossRef]




| Catalysts | Light Source | Scavengers | Dosage of Photocatalyst | H2 Evolution Rate (µmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|
| PCN2 | 300 W Xe lamp | TEOA | 100 mg | 157.86 | This work |
| g-C3N4-Zn−1@Pt | 250 W Xe lamp | TEOA | 75 mg | 78.7 | [45] |
| g-C3N4/Ni2P | 300 W Xe lamp | TEOA | 20 mg | 82.5 | [46] |
| LaFeO3/g-C3N4/NiS | 300 W Xe lamp | TEOA | 100 mg | 121 | [47] |
| Eu/CN | 300 W Xe lamp | TEOA | 50 mg | 128.8 | [48] |
| CuO/CN | 300 W LED lamp | TEOA | 50 mg | 130.1 | [49] |
| WC/g-C3N4 | 300 W Xe lamp | TEOA | 50 mg | 146.1 | [50] |
| g-C3N4/MeTMC-COP | 300 W Xe lamp | TEOA | 10 mg | 11.8 | [51] |
| CuO/pCN | 400 W Xe lamp | TEOA | 50 mg | 30 | [52] |
| p-CNGT | 300 W Xe lamp | TEOA | 30 mg | 33.1 | [53] |
| Co3O4@g-C3N4/CNFs | 300 W Xe lamp | TEOA | 5 mg | 67.17 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhang, M.; Li, H.; Zhang, Y.; Song, C.; Wang, D. Amorphous Carbon and Cyano-Group Self-Modified P-Doped g-C3N4 for Boosting Photocatalytic H2 Evolution. Catalysts 2024, 14, 523. https://doi.org/10.3390/catal14080523
Gao H, Zhang M, Li H, Zhang Y, Song C, Wang D. Amorphous Carbon and Cyano-Group Self-Modified P-Doped g-C3N4 for Boosting Photocatalytic H2 Evolution. Catalysts. 2024; 14(8):523. https://doi.org/10.3390/catal14080523
Chicago/Turabian StyleGao, Hang, Minghao Zhang, Huixin Li, Yiran Zhang, Caixia Song, and Debao Wang. 2024. "Amorphous Carbon and Cyano-Group Self-Modified P-Doped g-C3N4 for Boosting Photocatalytic H2 Evolution" Catalysts 14, no. 8: 523. https://doi.org/10.3390/catal14080523
APA StyleGao, H., Zhang, M., Li, H., Zhang, Y., Song, C., & Wang, D. (2024). Amorphous Carbon and Cyano-Group Self-Modified P-Doped g-C3N4 for Boosting Photocatalytic H2 Evolution. Catalysts, 14(8), 523. https://doi.org/10.3390/catal14080523










