Biogenic Synthesis Based on Cuprous Oxide Nanoparticles Using Eucalyptus globulus Extracts and Its Effectiveness for Removal of Recalcitrant Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Nanoparticles
2.1.1. Synthesis of Nanoparticles Supported
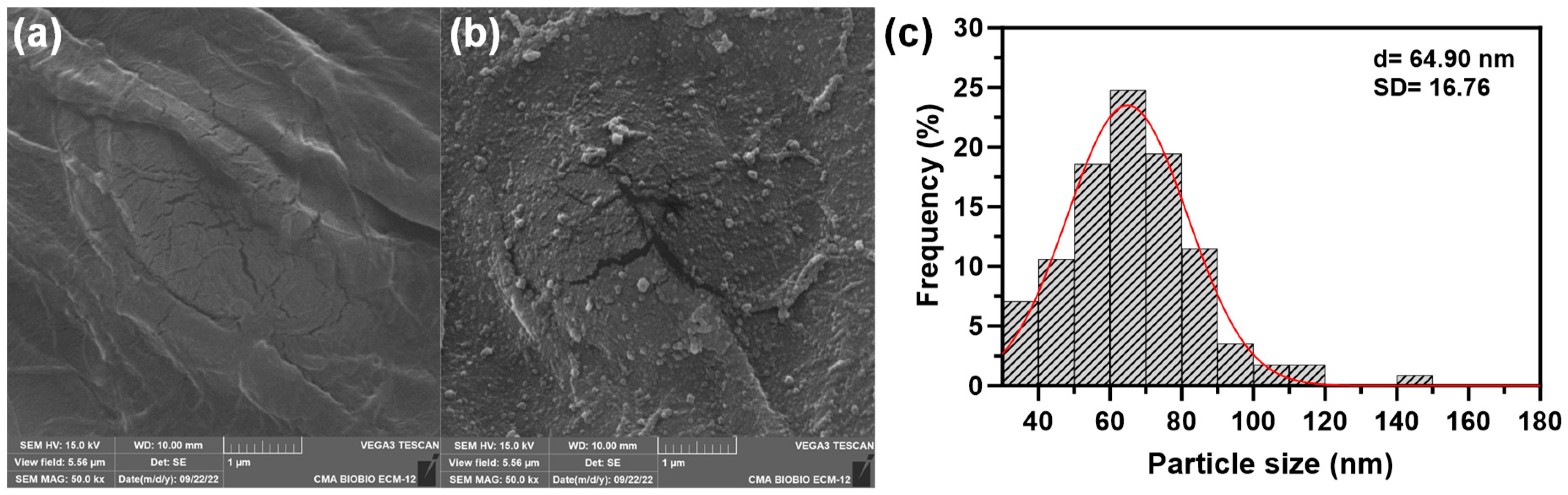
2.1.2. SEM Analysis
2.1.3. Raman Analysis
2.1.4. XRD Analysis
2.1.5. FTIR Analysis
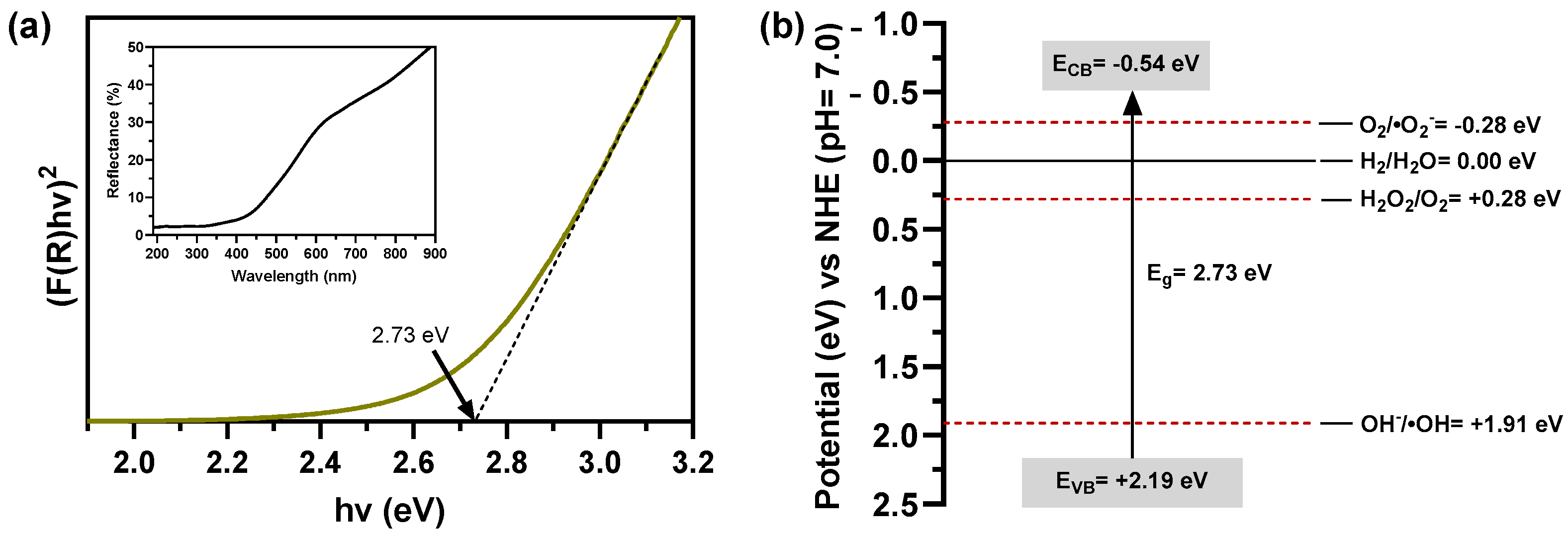
2.1.6. Optical Analysis
2.2. Identification of Biomolecules Involved in the Synthesis of Cu2O Nanoparticles
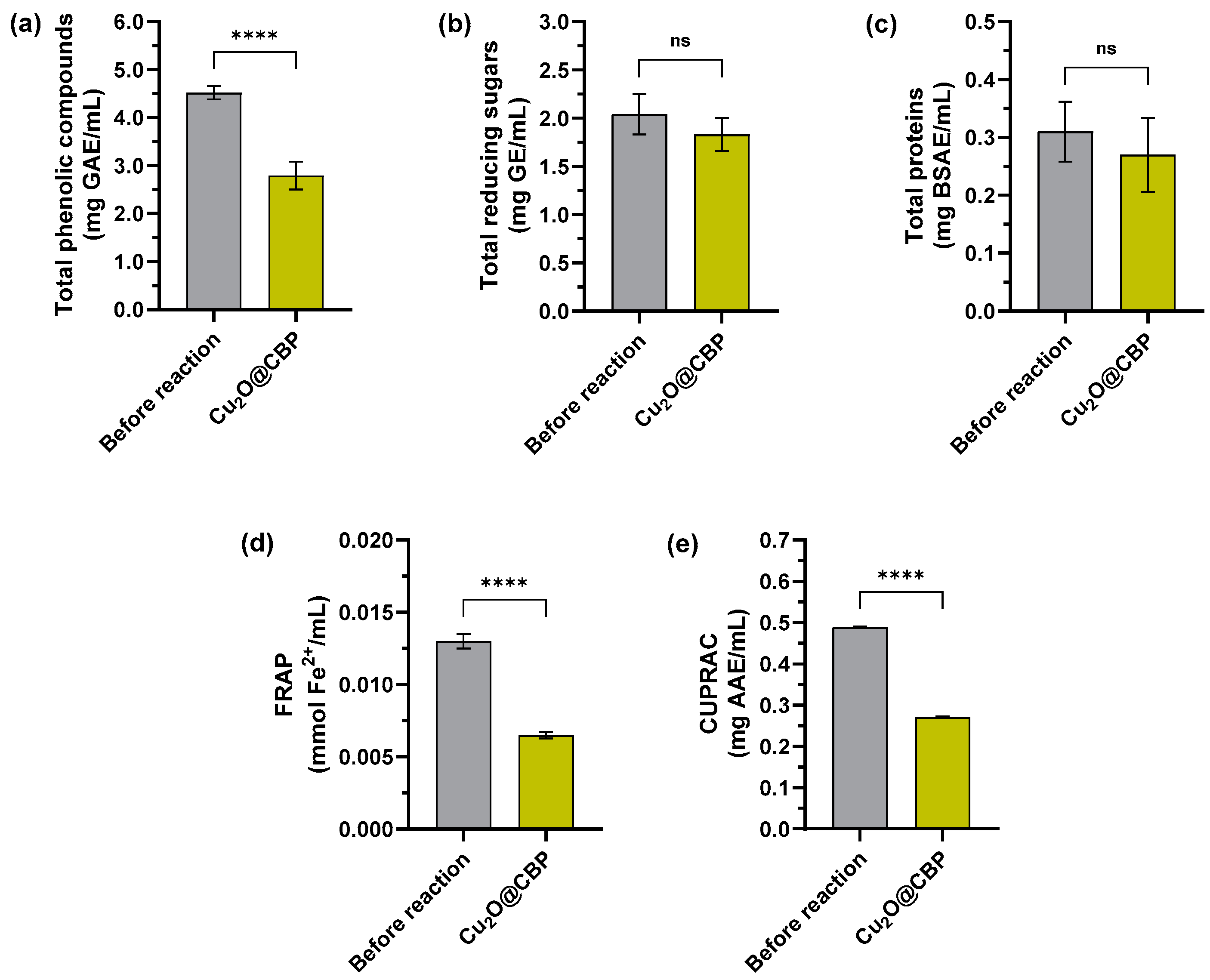
2.2.1. Spectrophotometric Analysis of Extracts before and after Formation Cu2O Nanoparticles
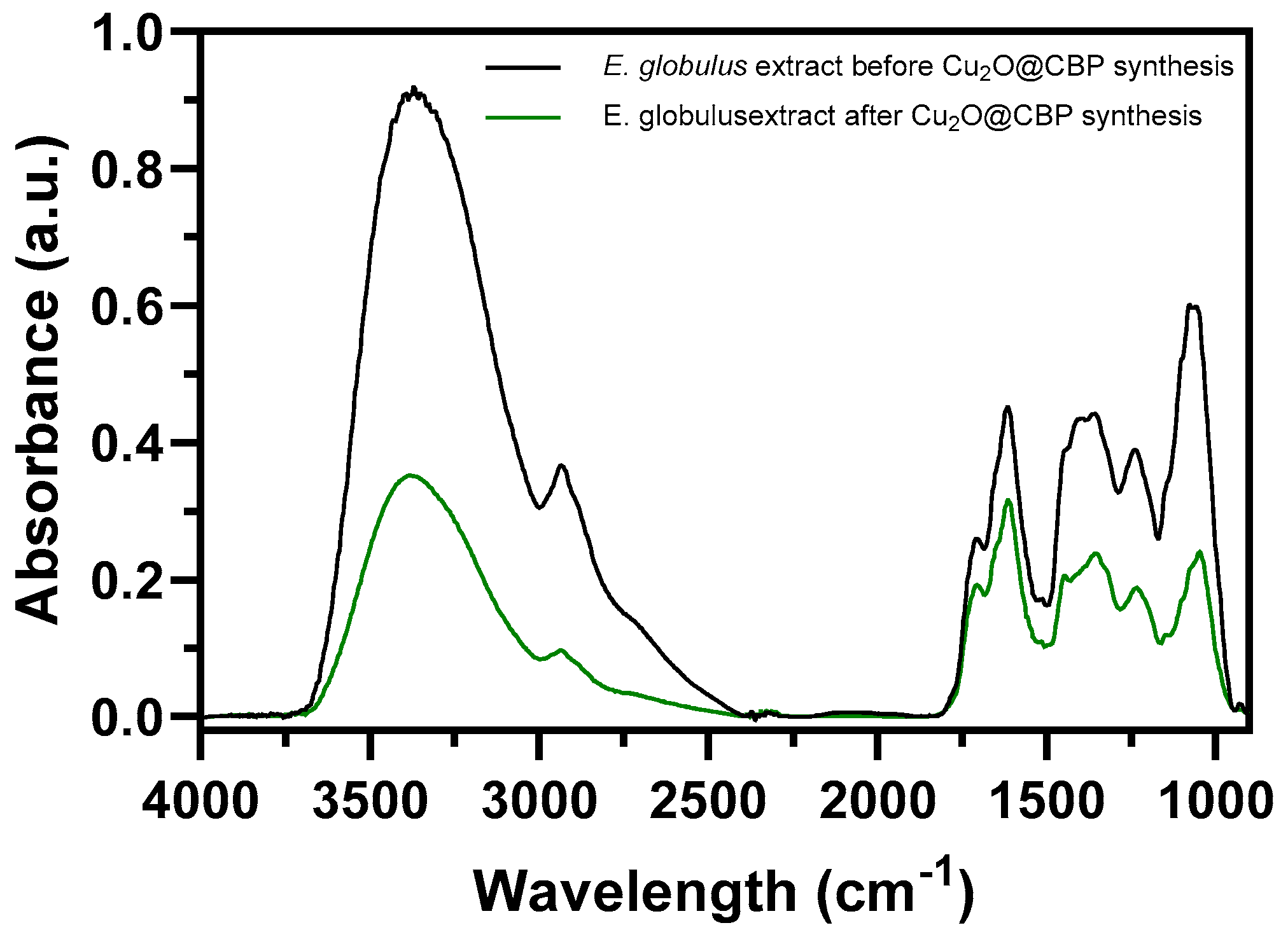
2.2.2. FTIR Analysis of Extracts before and after Formation of Cu2O Nanoparticles
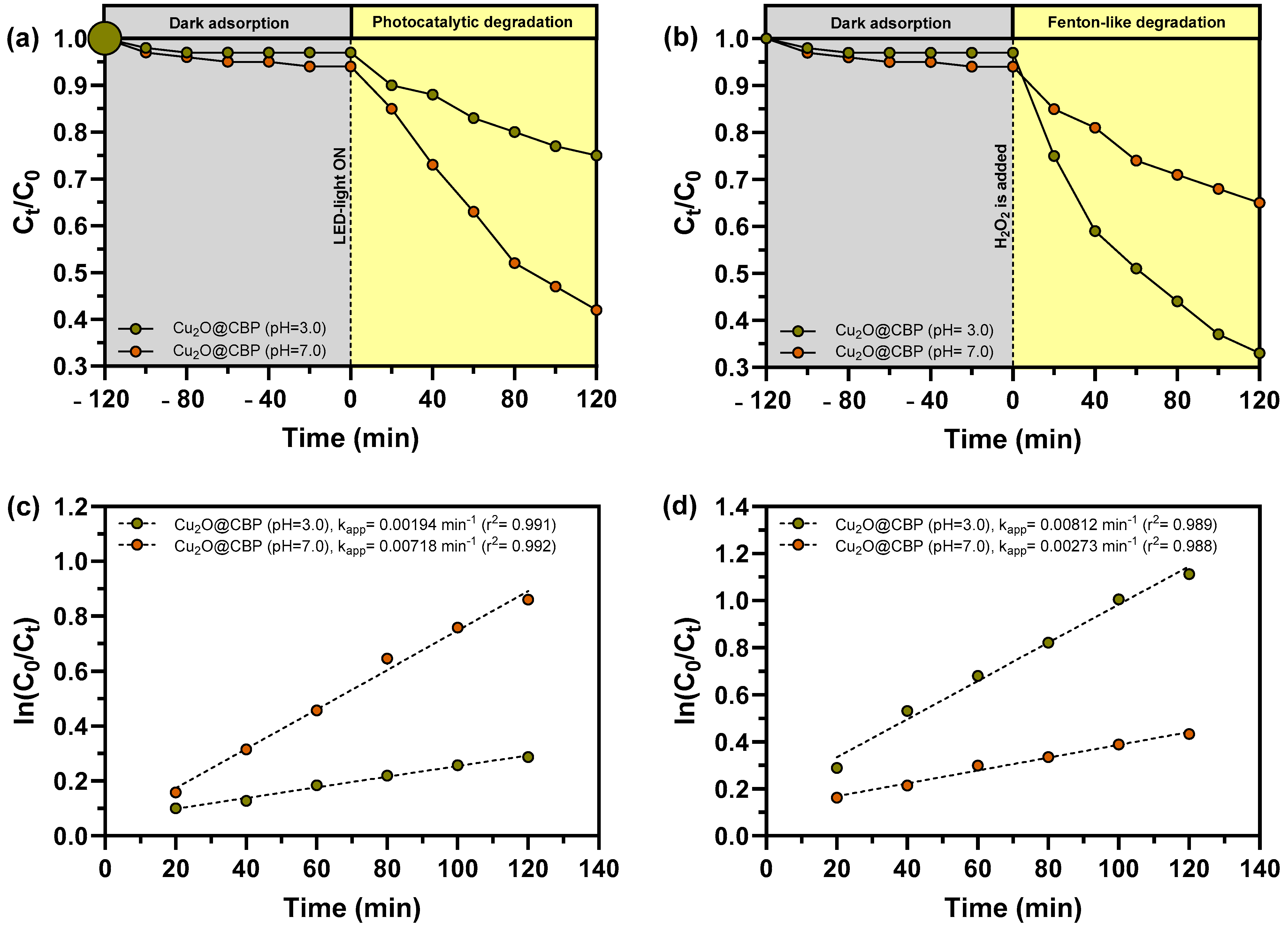
2.3. Photocatalytic and Fenton-Like Degradation of MB
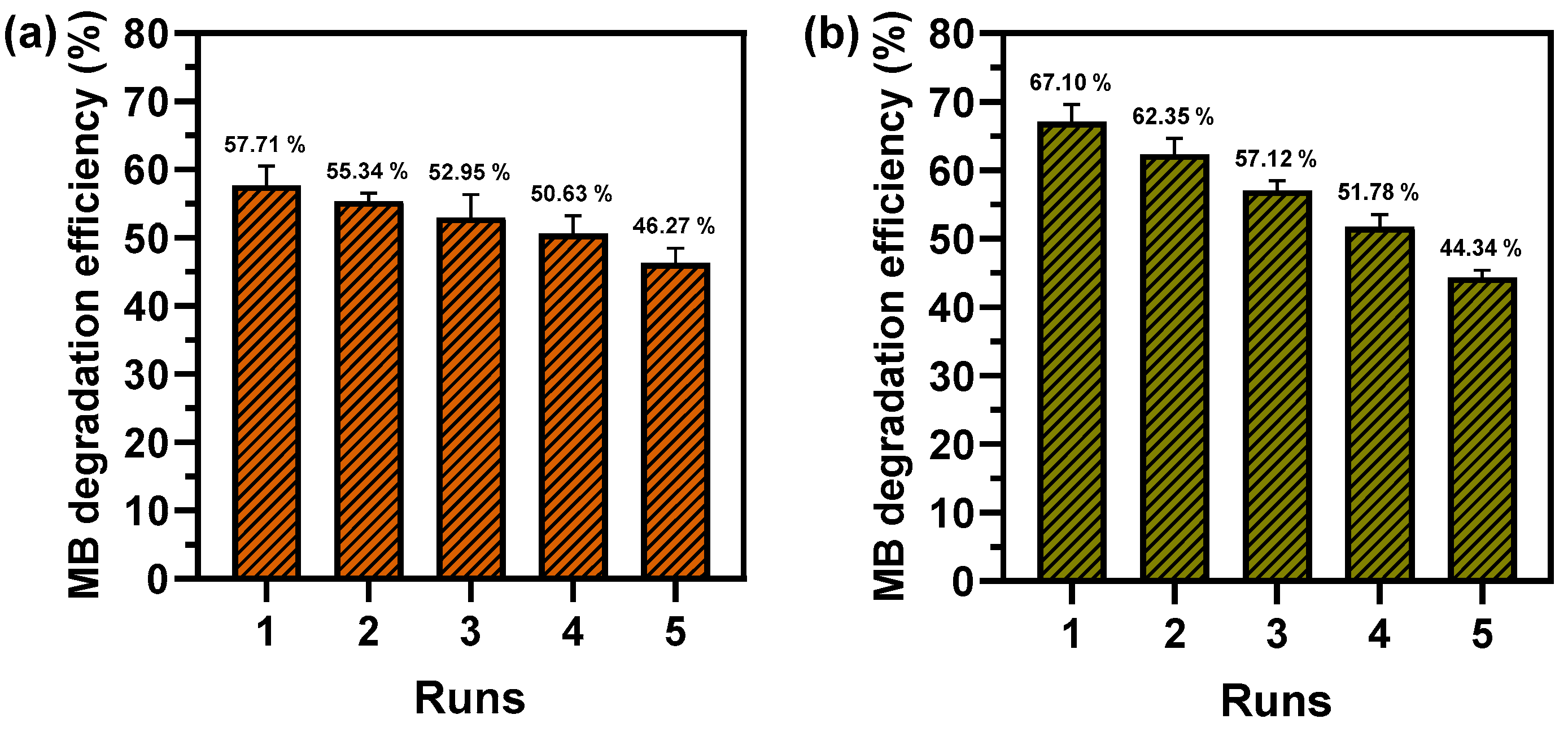
2.4. Reusability of Photocatalytic and Fenton-Like Degradation of MB
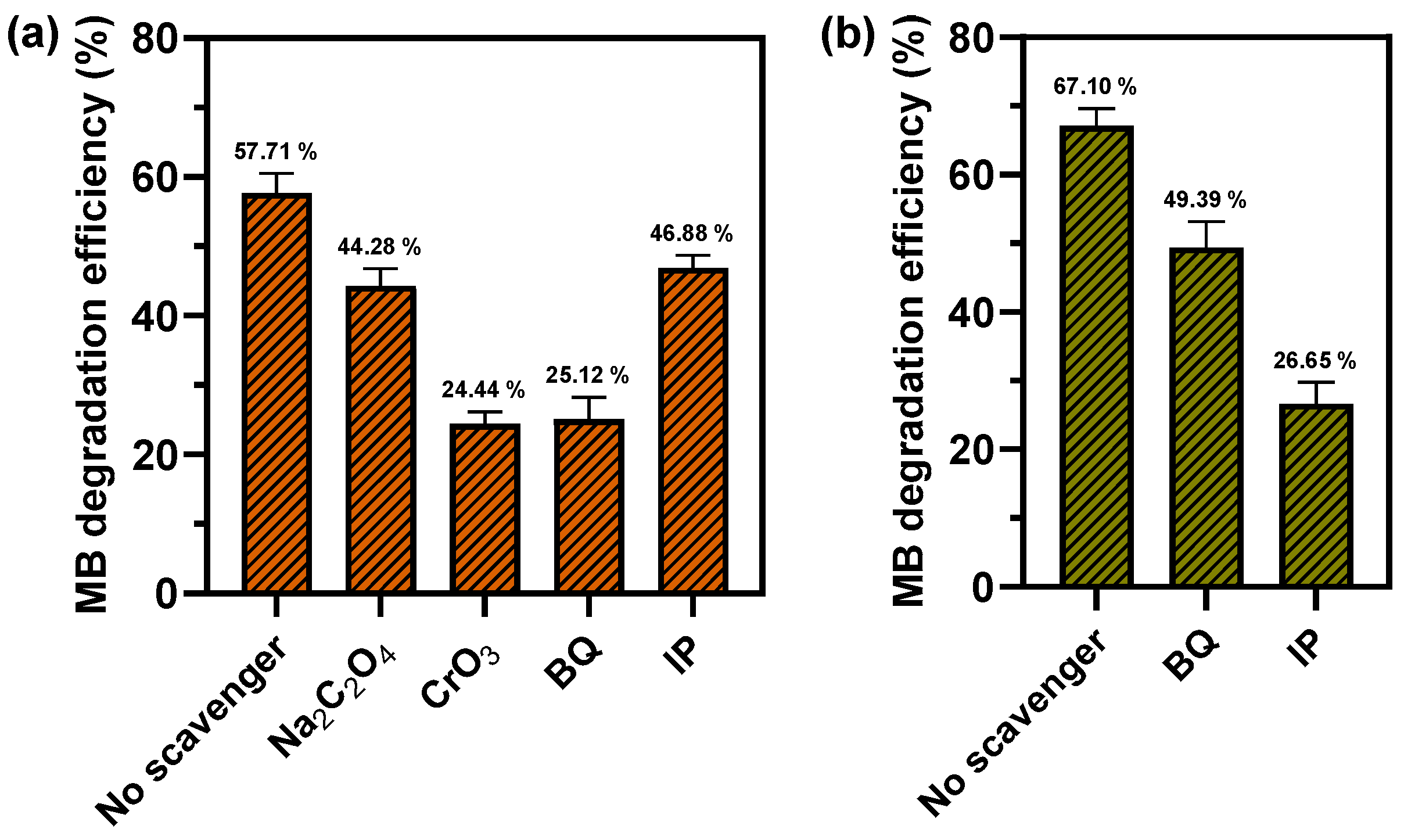
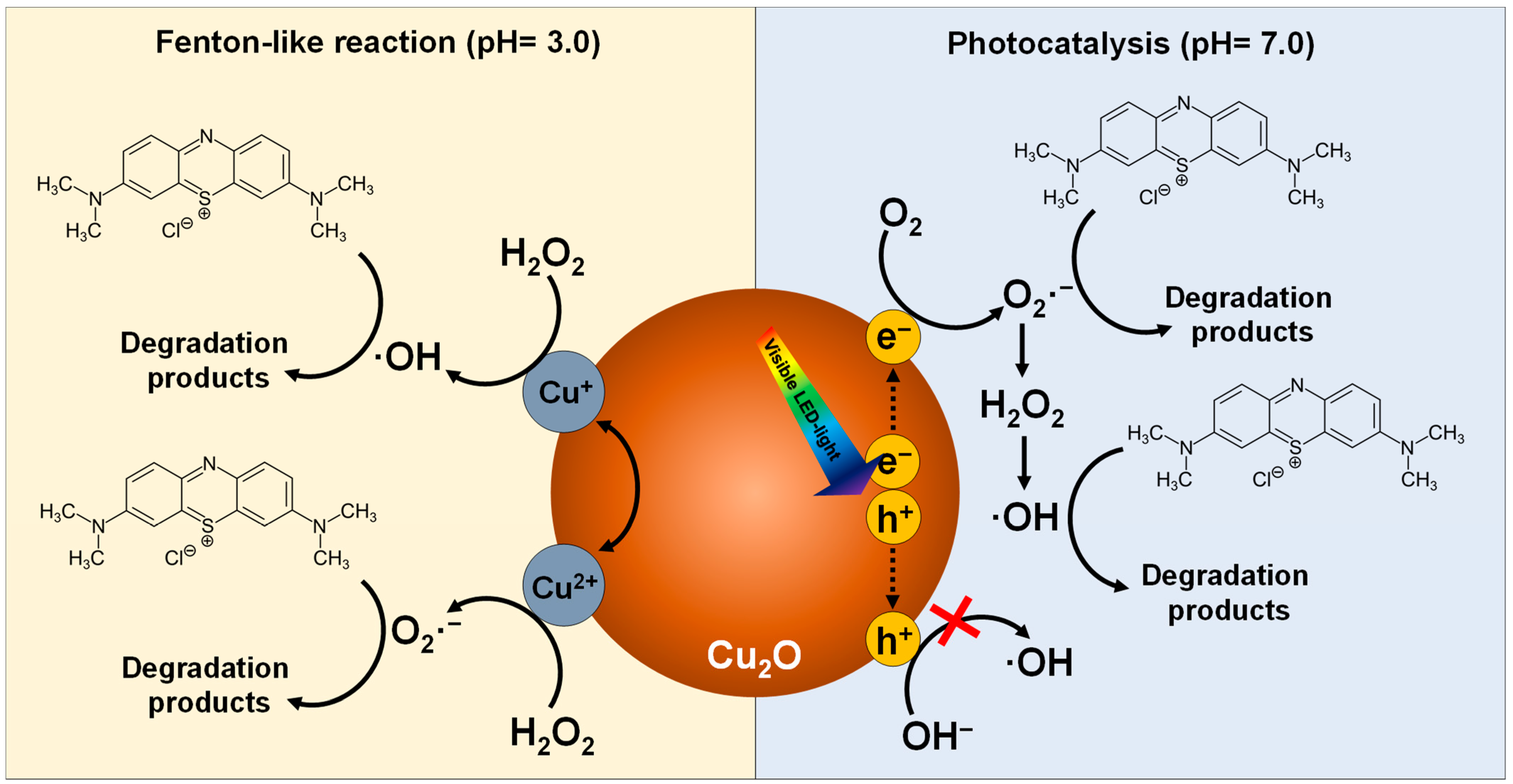
2.5. Reactive Species in Photocatalytic and Fenton-Like Degradation of MB
3. Materials and Methods
3.1. Preparation of E. Globulus Extracts
3.2. Synthesis of Cu2O Nanoparticles on Cellulose Paper
3.3. Characterization Techniques
3.4. Photocatalytic and Fenton-Like Degradation of MB
3.5. Reusability Studies
3.6. Reactive-Species-Trapping Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.; Huang, L.; Li, Y.; Zhu, M.; Liu, S.; Shu, S.; Huang, L.; Wu, X. Enhanced photo-degradation of pollutants in three dimensions by rGO/Bi12O15Cl6/InVO4: LED-light photocatalytic performance and mechanism investigation. Ceram. Int. 2023, 49, 19422–19433. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X.S. Degradation of Organic Dyes over Fenton-Like Cu2O–Cu/C Catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Frontela, J.L.; Vidal, G. Optimization of Fenton Technology for Recalcitrant Compounds and Bacteria Inactivation. Catalysts 2020, 10, 1483. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Durán, Y.; Mansilla, H.; Contreras, D. The Reactivity and Reaction Pathway of Fenton Reactions Driven by Substituted 1,2-Dihydroxybenzenes. Environ. Sci. Technol. 2017, 51, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, M.; You, L.; Wei, Y.; Cai, C.; Wei, Q.; Zhang, H.; Zhou, K. 3D printed hierarchically porous zero-valent copper for efficient pollutant degradation through peroxymonosulfate activation. Sep. Purif. Technol. 2023, 305, 122437. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Albornoz, M.; Mansilla, H.; Vidal, G.; Contreras, D. Effects of pH and substituted 1,2-dihydroxybenzenes on the reaction pathway of Fenton-like systems. Appl. Catal. B Environ. 2018, 226, 93–102. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton reaction driven by iron ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, C.; Ji, F.; Chen, M.; Zhong, Z.; Xing, W. Micro-Octahedron Cu2O-Based Photocatalysis-Fenton for Organic Pollutant Degradation: Proposed Coupling Mechanism in a Membrane Reactor. Ind. Eng. Chem. Res. 2022, 61, 7255–7265. [Google Scholar] [CrossRef]
- Tony, M.A.; Mansour, S.A. Microwave-assisted catalytic oxidation of methomyl pesticide by Cu/Cu2O/CuO hybrid nanoparticles as a Fenton-like source. Int. J. Environ. Sci. Technol. 2020, 17, 161–174. [Google Scholar] [CrossRef]
- Lopis, A.D.; Choudhari, K.S.; Sai, R.; Kanakikodi, K.S.; Maradur, S.P.; Shivashankar, S.A.; Kulkarni, S.D. Laddered type-1 heterojunction: Harvesting full-solar-spectrum in scavenger free photocatalysis. Sol. Energy 2022, 240, 57–68. [Google Scholar] [CrossRef]
- Dong, J.; Xu, H.; Zhang, F.; Chen, C.; Liu, L.; Wu, G. Synergistic effect over photocatalytic active Cu2O thin films and their morphological and orientational transformation under visible light irradiation. Appl. Catal. A Gen. 2014, 470, 294–302. [Google Scholar] [CrossRef]
- Akbal, F. Photocatalytic degradation of organic dyes in the presence of titanium dioxide under UV and solar light: Effect of operational parameters. Environ. Prog. 2005, 24, 317–322. [Google Scholar] [CrossRef]
- Su, Y.; Nathan, A.; Ma, H.; Wang, H. Precise control of Cu2O nanostructures and LED-assisted photocatalysis. RSC Adv. 2016, 6, 78181–78186. [Google Scholar] [CrossRef]
- Salgado, P.; Mártire, D.O.; Vidal, G. Eucalyptus extracts-mediated synthesis of metallic and metal oxide nanoparticles: Current status and perspectives. Mater. Res. Express 2019, 6, 082006. [Google Scholar] [CrossRef]
- Salgado, P.; Márquez, K.; Rubilar, O.; Contreras, D.; Vidal, G. The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity. Appl. Nanosci. 2019, 9, 371–385. [Google Scholar] [CrossRef]
- Kerour, A.; Boudjadar, S.; Bourzami, R.; Allouche, B. Eco-friendly synthesis of cuprous oxide (Cu2O) nanoparticles and improvement of their solar photocatalytic activities. J. Solid State Chem. 2018, 263, 79–83. [Google Scholar] [CrossRef]
- Amrani, M.A.; Srikanth, V.V.S.S.; Labhsetwar, N.K.; Al-Fatesh, A.S.; Shaikh, H. Phoenix dactylifera mediated green synthesis of Cu2O particles for arsenite uptake from water. Sci. Technol. Adv. Mater. 2016, 17, 760–768. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Niranjani, S.; Barnabas, K.S.; Narayanan, V.; Raju, T.; Venkatachalam, K. Green Route Synthesis and Characterization of Cuprous Oxide (Cu2O): Visible light Irradiation photocatalytic activity of MB Dye. Mater. Today Proc. 2019, 14, 563–568. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Wang, Q.; Lu, A.; Zhang, L. Portable Visible-Light Photocatalysts Constructed from Cu2O Nanoparticles and Graphene Oxide in Cellulose Matrix. J. Phys. Chem. C 2014, 118, 7202–7210. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, L.; Zhou, M.; Zhong, Z.; Xing, W. Membrane enhanced COD degradation of pulp wastewater using Cu2O/H2O2 heterogeneous Fenton process. Chin. J. Chem. Eng. 2018, 26, 1896–1903. [Google Scholar] [CrossRef]
- Su, X.; Chen, W.; Han, Y.; Wang, D.; Yao, J. In-situ synthesis of Cu2O on cotton fibers with antibacterial properties and reusable photocatalytic degradation of dyes. Appl. Surf. Sci. 2021, 536, 147945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Chen, Z.; Tang, Y.; Yu, Y. Preparation of Fenton reagent with H2O2 generated by solar light-illuminated nano-Cu2O/MWNTs composites. Appl. Catal. A Gen. 2006, 299, 292–297. [Google Scholar] [CrossRef]
- Sabbaghan, M.; Argyropoulos, D.S. Synthesis and characterization of nano fibrillated cellulose/Cu2O films; micro and nano particle nucleation effects. Carbohydr. Polym. 2018, 197, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kalubowila, K.D.R.N.; Gunewardene, M.S.; Jayasingha, J.L.K.; Dissanayake, D.; Jayathilaka, C.; Jayasundara, J.M.D.; Gao, Y.; Jayanetti, J.K.D.S. Reduction-Induced Synthesis of Reduced Graphene Oxide-Wrapped Cu2O/Cu Nanoparticles for Photodegradation of Methylene Blue. ACS Appl. Nano Mater. 2021, 4, 2673–2681. [Google Scholar] [CrossRef]
- Kumar, M.; Das, R.R.; Samal, M.; Yun, K. Highly stable functionalized cuprous oxide nanoparticles for photocatalytic degradation of methylene blue. Mater. Chem. Phys. 2018, 218, 272–278. [Google Scholar] [CrossRef]
- Pariona, N.; Basurto Cereceda, S.; Mondaca, F.; Carrión, G.; Mtz-Enriquez, A.I. Antifungal activity and degradation of methylene blue by ZnO, Cu, and Cu2O/Cu nanoparticles, a comparative study. Mater. Lett. 2021, 301, 130182. [Google Scholar] [CrossRef]
- Zhang, Z.; Che, H.; Wang, Y.; Gao, J.; Zhao, L.; She, X.; Sun, J.; Gunawan, P.; Zhong, Z.; Su, F. Facile Synthesis of Mesoporous Cu2O Microspheres with Improved Catalytic Property for Dimethyldichlorosilane Synthesis. Ind. Eng. Chem. Res. 2012, 51, 1264–1274. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, S.; Kim, Y.; Ryu, Y.B. Antimicrobial Activity of Morphology-Controlled Cu2O Nanoparticles: Oxidation Stability under Humid and Thermal Conditions. Materials 2024, 17, 261. [Google Scholar] [CrossRef]
- Prabhakaran, G.; Murugan, R. Synthesis of Cu2O Nanospheres and Cubes: Their Structural, Optical and Magnetic Properties. Adv. Mater. Res. 2014, 938, 114–117. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N.; Kaushik, S.D.; Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 2016, 390, 974–983. [Google Scholar] [CrossRef]
- Yu, P.Y.; Shen, Y.R.; Petroff, Y. Resonance Raman scattering in Cu2O at the blue and indigo excitons. Solid State Commun. 1973, 12, 973–975. [Google Scholar] [CrossRef]
- Solache-Carranco, H.; Juarez-Diaz, G.; Galvan-Arellano, M.; Martinez-Juarez, J.; Romero-Paredes, R.G.; Pena-Sierra, R. Raman scattering and photoluminescence studies on Cu2O. In Proceedings of the 2008 5th International Conference on Electrical Engineering, Computing Science and Automatic Control, Mexico City, Mexico, 12–14 November 2008; pp. 421–424. [Google Scholar]
- Ansari, A.; Jha, A.K.; Akhtar, M.J. Preparation and characterization of oxidized electrolytic copper powder and its dielectric properties at microwave frequency. Powder Technol. 2015, 286, 459–467. [Google Scholar] [CrossRef]
- Sander, T.; Reindl, C.T.; Klar, P.J. Breaking of Raman selection rules in Cu2O by intrinsic point defects. MRS Online Proc. Libr. 2013, 1633, 81–86. [Google Scholar] [CrossRef]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, structural and phase transition properties of Cu2O, CuO and Cu2O/CuO: Their photoelectrochemical sensor applications. Int. J. Hydrogen Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational Study (Raman, SERS, and IR) of Plant Gallnut Polyphenols Related to the Fabrication of Iron Gall Inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farwell, D.W.; Williams, A.C. FT-Raman spectrum of cotton: A polymeric biomolecular analysis. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 807–811. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S. Detection and quantitation of cellulose II by Raman spectroscopy. Cellulose 2021, 28, 9069–9079. [Google Scholar] [CrossRef]
- Gupta, D.; Meher, S.R.; Illyaskutty, N.; Alex, Z.C. Facile synthesis of Cu2O and CuO nanoparticles and study of their structural, optical and electronic properties. J. Alloys Compd. 2018, 743, 737–745. [Google Scholar] [CrossRef]
- Gupta, A.; Maruthapandi, M.; Das, P.; Saravanan, A.; Jacobi, G.; Natan, M.; Banin, E.; Luong, J.H.T.; Gedanken, A. Cuprous Oxide Nanoparticles Decorated Fabric Materials with Anti-biofilm Properties. ACS Appl. Bio Mater. 2022, 5, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Montazer, M.; Samadi, N. Synthesis of nano Cu2O on cotton: Morphological, physical, biological and optical sensing characterizations. Carbohydr. Polym. 2014, 110, 489–498. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-L.; Li, S.-P.; Wang, Y.-J.; Wang, L.-L.; Bao, X.-C.; Yang, R.-Q. Fabrication of Cu2O@Cu2O core–shell nanoparticles and conversion to Cu2O@Cu core–shell nanoparticles in solution. Trans. Nonferrous Met. Soc. China 2015, 25, 3643–3650. [Google Scholar] [CrossRef]
- Grasel, F.d.S.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Chinnaiah, K.; Maik, V.; Kannan, K.; Potemkin, V.; Grishina, M.; Gohulkumar, M.; Tiwari, R.; Gurushankar, K. Experimental and Theoretical Studies of Green Synthesized Cu2O Nanoparticles Using Datura metel L. J. Fluoresc. 2022, 32, 559–568. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Varada Rajalu, A.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef] [PubMed]
- Musino, D.; Rivard, C.; Landrot, G.; Novales, B.; Rabilloud, T.; Capron, I. Hydroxyl groups on cellulose nanocrystal surfaces form nucleation points for silver nanoparticles of varying shapes and sizes. J. Colloid Interface Sci. 2021, 584, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol–cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Mannarmannan, M.; Biswas, K. Phytochemical-Assisted Synthesis of Cuprous Oxide Nanoparticles and Their Antimicrobial Studies. ChemistrySelect 2021, 6, 3534–3539. [Google Scholar] [CrossRef]
- Salgado, P.; Contreras, D.; Mansilla, H.D.; Márquez, K.; Vidal, G.; Cobos, C.J.; Mártire, D.O. Experimental and computational investigation of the substituent effects on the reduction of Fe3+ by 1,2-dihydroxybenzenes. New J. Chem. 2017, 41, 12685–12693. [Google Scholar] [CrossRef]
- Taherzadeh Soureshjani, P.; Shadi, A.; Mohammadsaleh, F. Algae-mediated route to biogenic cuprous oxide nanoparticles and spindle-like CaCO3: A comparative study, facile synthesis, and biological properties. RSC Adv. 2021, 11, 10599–10609. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Montazer, M.; Mianehro, A.; Eslahi, N.; Mahmoudi Rad, M. Preparation of Antibacterial Cellulose Fabric via Copper (II) Oxide and Corn Silk (Stigma maydis) Nanocomposite. Starch Stärke 2023, 75, 2200156. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Alp, E. The Facile Synthesis of Cu2O-Cu hybrid cubes as efficient visible-light-driven photocatalysts for water remediation processes. Powder Technol. 2021, 394, 1111–1120. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Das, S.; Deka, T.; Ningthoukhangjam, P.; Chowdhury, A.; Nair, R.G. A critical review on prospects and challenges of metal-oxide embedded g-C3N4-based direct Z-scheme photocatalysts for water splitting and environmental remediation. Appl. Surf. Sci. Adv. 2022, 11, 100273. [Google Scholar] [CrossRef]
- da Costa, W.V.; Pereira, B.d.S.; Montanha, M.C.; Kimura, E.; Hechenleitner, A.A.W.; de Oliveira, D.M.F.; Pineda, E.A.G. Hybrid materials based on cotton fabric-Cu2O nanoparticles with antibacterial properties against S. aureus. Mater. Chem. Phys. 2017, 201, 339–343. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Madasu, M.; Huang, M.H. Modified Semiconductor Band Diagrams Constructed from Optical Characterization of Size-Tunable Cu2O Cubes, Octahedra, and Rhombic Dodecahedra. J. Phys. Chem. C 2018, 122, 13027–13033. [Google Scholar] [CrossRef]
- Sreeju, N.; Rufus, A.; Philip, D. Nanostructured copper (II) oxide and its novel reduction to stable copper nanoparticles. J. Phys. Chem. Solids 2019, 124, 250–260. [Google Scholar] [CrossRef]
- Chen, T.-N.; Kao, J.-C.; Zhong, X.-Y.; Chan, S.-J.; Patra, A.S.; Lo, Y.-C.; Huang, M.H. Facet-Specific Photocatalytic Activity Enhancement of Cu2O Polyhedra Functionalized with 4-Ethynylanaline Resulting from Band Structure Tuning. ACS Cent. Sci. 2020, 6, 984–994. [Google Scholar] [CrossRef]
- Ke, W.-H.; Hsia, C.-F.; Chen, Y.-J.; Huang, M.H. Synthesis of Ultrasmall Cu2O Nanocubes and Octahedra with Tunable Sizes for Facet-Dependent Optical Property Examination. Small 2016, 12, 3530–3534. [Google Scholar] [CrossRef]
- Higashimoto, S.; Nishi, T.; Yasukawa, M.; Azuma, M.; Sakata, Y.; Kobayashi, H. Photocatalysis of titanium dioxide modified by catechol-type interfacial surface complexes (ISC) with different substituted groups. J. Catal. 2015, 329, 286–290. [Google Scholar] [CrossRef]
- Savić, T.D.; Čomor, M.I.; Nedeljković, J.M.; Veljković, D.Ž.; Zarić, S.D.; Rakić, V.M.; Janković, I.A. The effect of substituents on the surface modification of anatase nanoparticles with catecholate-type ligands: A combined DFT and experimental study. Phys. Chem. Chem. Phys. 2014, 16, 20796–20805. [Google Scholar] [CrossRef]
- Wang, Y.; Miska, P.; Pilloud, D.; Horwat, D.; Mücklich, F.; Pierson, J.F. Transmittance enhancement and optical band gap widening of Cu2O thin films after air annealing. J. Appl. Phys. 2014, 115, 073505. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Verma, N.; Anupama, A.V.; Choudhary, H.K.; Philip, R.; Sahoo, B. Effect of the band gap and the defect states present within band gap on the non-linear optical absorption behaviour of yttrium aluminium iron garnets. Opt. Mater. 2020, 108, 110163. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Mabhouti, K.; Golzan, M.M.; Naderali, R. Investigation of structural, morphological and optical characteristics of Mn substituted Al-doped ZnO NPs: A Urbach energy and Kramers-Kronig study. Optik 2020, 204, 164227. [Google Scholar] [CrossRef]
- Yadav, S.; Jain, A.; Malhotra, P. A review on the sustainable routes for the synthesis and applications of cuprous oxide nanoparticles and their nanocomposites. Green Chem. 2019, 21, 937–955. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Pinto, R.J.B.; Rocha, S.M.; Marques, P.A.A.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Unveiling the Chemistry behind the Green Synthesis of Metal Nanoparticles. ChemSusChem 2014, 7, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Ullah Khan, A. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Djamila, B.; Eddine, L.S.; Abderrhmane, B.; Nassiba, A.; Barhoum, A. In vitro antioxidant activities of copper mixed oxide (CuO/Cu2O) nanoparticles produced from the leaves of Phoenix dactylifera L. Biomass Convers. Biorefinery 2024, 14, 6567–6580. [Google Scholar] [CrossRef]
- Dou, L.; Zhang, X.; Zangeneh, M.M.; Zhang, Y. Efficient biogenesis of Cu2O nanoparticles using extract of Camellia sinensis leaf: Evaluation of catalytic, cytotoxicity, antioxidant, and anti-human ovarian cancer properties. Bioorg. Chem. 2021, 106, 104468. [Google Scholar] [CrossRef]
- Atri, A.; Echabaane, M.; Bouzidi, A.; Harabi, I.; Soucase, B.M.; Ben Chaâbane, R. Green synthesis of copper oxide nanoparticles using Ephedra Alata plant extract and a study of their antifungal, antibacterial activity and photocatalytic performance under sunlight. Heliyon 2023, 9, e13484. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Dhara, M.; Kisku, K.; Naik, U.C. Biofunctionalized cuprous oxide nanoparticles synthesized using root extract of Withania somnifera for antibacterial activity. Appl. Nanosci. 2022, 12, 3555–3571. [Google Scholar] [CrossRef]
- Akter, J.; Sapkota, K.P.; Hanif, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically controlled selective synthesis of Cu2O and CuO nanoparticles toward enhanced degradation of methylene blue using ultraviolet and sun light. Mater. Sci. Semicond. Process. 2021, 123, 105570. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Messaadia, L.; Kiamouche, S.; Lahmar, H.; Masmoudi, R.; Boulahbel, H.; Trari, M.; Benamira, M. Solar photodegradation of Rhodamine B dye by Cu2O/TiO2 heterostructure: Experimental and computational studies of degradation and toxicity. J. Mol. Model. 2023, 29, 38. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, Z.; Yang, S.; Jiang, W.; Gu, Y.; Chen, J.; Yang, G. High efficiency degradation of 2,4-dichlorophenol using Cu2S/Cu2O decorated nanoscale zerovalent iron via adsorption and photocatalytic performances. J. Clean. Prod. 2022, 378, 134587. [Google Scholar] [CrossRef]
- Ai, Z.; Xiao, H.; Mei, T.; Liu, J.; Zhang, L.; Deng, K.; Qiu, J. Electro-Fenton Degradation of Rhodamine B Based on a Composite Cathode of Cu2O Nanocubes and Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 11929–11935. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Zhou, P.; Li, C.; Yang, D. Oxidative degradation of organic pollutants using cuprous oxide in acidic solution: Hydroxyl radical generation. Desalin. Water Treat. 2019, 171, 262–269. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; Anis, B. Gallic acid-assisted growth of cuprous oxide within polyvinyl alcohol; a separable catalyst for oxidative and reductive degradation of water pollutants. J. Clean. Prod. 2021, 279, 123826. [Google Scholar] [CrossRef]
- Wu, M.; He, S.; Ha, E.; Hu, J.; Ruan, S. A facile synthesis of PEGylated Cu2O@SiO2/MnO2 nanocomposite as efficient photo−Fenton−like catalysts for methylene blue treatment. Front. Bioeng. Biotechnol. 2022, 10, 1023090. [Google Scholar] [CrossRef]
- Salgado, P.; Rubilar, O.; Salazar, C.; Márquez, K.; Vidal, G. In Situ Synthesis of Cu2O Nanoparticles Using Eucalyptus globulus Extract to Remove a Dye via Advanced Oxidation. Nanomaterials 2024, 14, 1087. [Google Scholar] [CrossRef]
- Aguilar, M.S.; Rosas, G. A new synthesis of Cu2O spherical particles for the degradation of methylene blue dye. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100195. [Google Scholar] [CrossRef]
- Xu, L.; Xu, H.; Wu, S.; Zhang, X. Synergy effect over electrodeposited submicron Cu2O films in photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2012, 258, 4934–4938. [Google Scholar] [CrossRef]
- Sorekine, G.; Anduwan, G.; Waimbo, M.N.; Osora, H.; Velusamy, S.; Kim, S.; Kim, Y.S.; Charles, J. Photocatalytic studies of copper oxide nanostructures for the degradation of methylene blue under visible light. J. Mol. Struct. 2022, 1248, 131487. [Google Scholar] [CrossRef]
- Dustgeer, M.R.; Asma, S.T.; Jilani, A.; Raza, K.; Hussain, S.Z.; Shakoor, M.B.; Iqbal, J.; Abdel-wahab, M.S.; Darwesh, R. Synthesis and characterization of a novel single-phase sputtered Cu2O thin films: Structural, antibacterial activity and photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 128, 108606. [Google Scholar] [CrossRef]
- Athariq, M.; Rauf, M.R.; Khairany, I.W.; Adani, I.F.; Sutrisno, M.G. Synthesis and characterization of nanocube Cu2O thin film at room temperature for methylene blue photodegradation application. Chem. Mater. 2023, 2, 67–71. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Debut, A.; Cumbal, L. Andean Sacha Inchi (Plukenetia volubilis L.) Leaf-Mediated Synthesis of Cu2O Nanoparticles: A Low-Cost Approach. Bioengineering 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Rajendran, A. Photocatalytic dye degradation activities of green synthesis of cuprous oxide nanoparticles from Sargassum wightii extract. Chem. Phys. Impact 2023, 6, 100208. [Google Scholar] [CrossRef]
- Sun, H.W.; Chu, D.Q.; Liu, L.; Wang, L.M. Preparation of Nanocrystalline Cu2O and Its Catalytic Degradation of Methylene Blue. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–3. [Google Scholar]
- Cheng, Y.; Lin, Y.; Xu, J.; He, J.; Wang, T.; Yu, G.; Shao, D.; Wang, W.-H.; Lu, F.; Li, L.; et al. Surface plasmon resonance enhanced visible-light-driven photocatalytic activity in Cu nanoparticles covered Cu2O microspheres for degrading organic pollutants. Appl. Surf. Sci. 2016, 366, 120–128. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, C.; Wang, B.; Xing, S. Enhanced Photocatalytic and Fenton-like Performance of CuOx-Decorated ZnFe2O4. ACS Appl. Mater. Interfaces 2017, 9, 41927–41936. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Huang, M.H. Facet-dependent photocatalytic properties of Cu2O crystals probed by using electron, hole and radical scavengers. J. Mater. Chem. A 2017, 5, 15116–15123. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2023, 442, 130014. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, N.; Guo, J.; Wang, J. Catalytic activation of O2 by Al0-CNTs-Cu2O composite for Fenton-like degradation of sulfamerazine antibiotic at wide pH range. J. Hazard. Mater. 2020, 396, 122751. [Google Scholar] [CrossRef]
- Salgado, P.; Bustamante, L.; Carmona, D.J.; Meléndrez, M.F.; Rubilar, O.; Salazar, C.; Pérez, A.J.; Vidal, G. Green synthesis of Ag/Ag2O nanoparticles on cellulose paper and cotton fabric using Eucalyptus globulus leaf extracts: Toward the clarification of formation mechanism. Surf. Interfaces 2023, 40, 102928. [Google Scholar] [CrossRef]

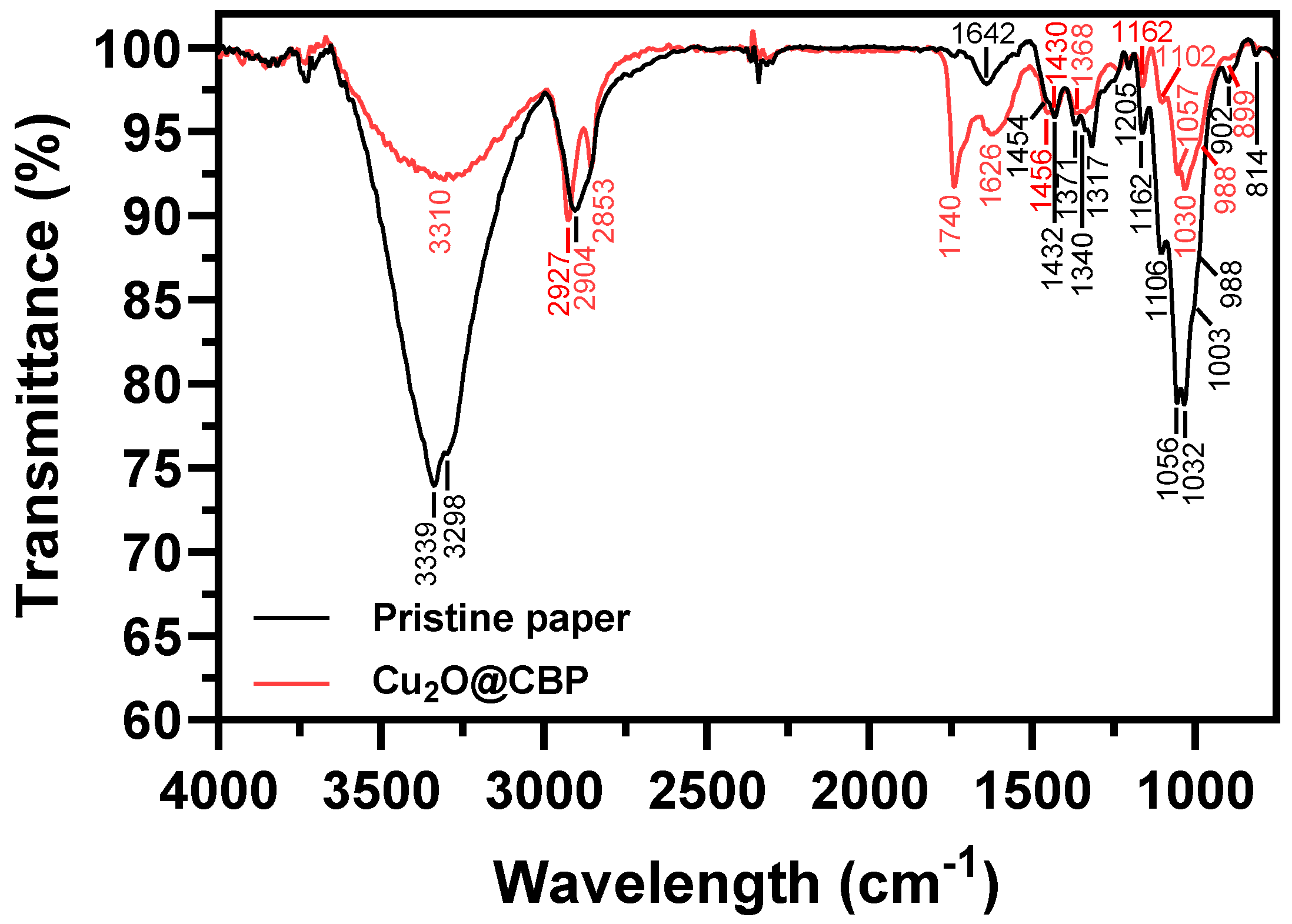

| Wavenumber (cm−1) | Vibration | Reference | |
|---|---|---|---|
| Pristine Paper | Cu2O@CBP | ||
| 3339 | - | Intra-molecular hydrogen bonding C(3)OH⋯O(5) or C(6)O⋯(O)H | [46] |
| 3298 | 3310 | Inter-molecular hydrogen bonding C(3)OH⋯C(6)O | [46] |
| 2904 | 2927 | Asymmetric stretching vibration of –CH2 | [47] |
| 2853 | 2853 | Symmetric stretching vibration of –CH2 | [47] |
| - | 1740 | C=O stretching | [48] |
| 1642 | - | O–H bending of adsorbed water | [46] |
| - | 1626 | C=C of aromatic ring | [49] |
| 1454 | 1456 | O–H in-plane deformation | [46] |
| 1432 | 1430 | CH2 scissoring | [50] |
| 1371 | 1368 | C–OH bending | [48] |
| 1340 | - | C–O stretching | [48] |
| 1317 | - | C–N stretch of aromatic amines | [51] |
| 1205 | - | C–O stretching | [46] |
| 1162 | 1162 | Anti-symmetrical bridge C–O–C stretching | [50] |
| 1106 | 1102 | C–OH bending | [49] |
| 1056 | 1057 | Stretching vibration of C–O–C in the pyranose skeletal ring | [18] |
| 1032 | 1030 | C–OH groups of cellulose | [46] |
| 1003 | - | C–O–H stretching vibration | [52] |
| 988 | 988 | C–O and ring stretching modes | [46] |
| 902 | 899 | Glycosidic deformation –C1–O–C4 characteristic of the β-glycosidic bond of cellulose | [50] |
| 814 | − | Plane bending of =C–H | [53] |
| Catalyst | Nanoparticles Synthesis | Time (min) | MB (mg/L) | Removal (%) | Ref. |
|---|---|---|---|---|---|
| Cu2O@CBP (~50 mg/L of Cu2O) | Green synthesis | 120 | 50 | 57.71 | This study |
| Cu2O (5 mg/L) | NaBH4 reduction | 120 | 1000 | 70 | [82] |
| Cu2O (3150 mg/L) | Green synthesis | 80 | 5 | 60 | [92] |
| Cu/Cu2O (250 mg/L) | Green synthesis | 150 | 10 | <10 | [29] |
| Cu2O (400 mg/L) | Ascorbic acid | 120 | 10 | ~10 | [27] |
| Cu2O on film | Electrodeposition | 150 | 6.25 | <10 | [93] |
| Cu/Cu2O/CuO (1000 mg/L) | Chemical synthesis | 120 | 10 | 46 | [94] |
| Cu2O thin films | Magnetron sputtering | 105 | 50 | 69.6 | [95] |
| Cu2O thin film | Electrodeposition | 120 | 5 | 62 | [96] |
| Cu2O (600 mg/L) | Ascorbic acid | 105 | 10 | 83 | [28] |
| Cu2O | Green synthesis | 150 | 10 | 78.9 | [97] |
| Cu2O-octaedral (60 mg/L) | Membrane-assisted precipitation | 120 | 50 | 68 | [10] |
| Cu2O-spherical (60 mg/L) | Membrane-assisted precipitation | 120 | 50 | 49 | [10] |
| Cu2O-micro-octahedrons (60 mg/L) | Membrane-assisted precipitation | 120 | 50 | 33 | [10] |
| Cu2O-microspheres (60 mg/L) | Membrane-assisted precipitation | 120 | 50 | 6.5 | [10] |
| Cu2O (50 mg/L) | Green synthesis | 180 | 1000 | ~60 | [98] |
| Catalyst | Nanoparticles Synthesis | Time (min) | MB (mg/L) | H2O2 Concentration | Removal (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu2O@CBP (~50 mg/L of Cu2O) | Green synthesis | 120 | 50 | 0.05 M | 67.10 | This study |
| Cu/Cu2O (250 mg/L) | Green synthesis | 150 | 10 | 0.55 M | ~70 | [29] |
| Cu2O (60 mg/L) | Membrane- assisted precipitation | 120 | 50 | 0.1 M | 74 | [10] |
| Cu2O (200 mg/L) | Sol-gel | 130 | 10 | 12.63% | ~75 | [99] |
| Cu2O/Cu (300 mg/L) | D-glucose | 60 | 10 | 9.8 × 10−3 M | 28 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, P.; Márquez, K.; Vidal, G. Biogenic Synthesis Based on Cuprous Oxide Nanoparticles Using Eucalyptus globulus Extracts and Its Effectiveness for Removal of Recalcitrant Compounds. Catalysts 2024, 14, 525. https://doi.org/10.3390/catal14080525
Salgado P, Márquez K, Vidal G. Biogenic Synthesis Based on Cuprous Oxide Nanoparticles Using Eucalyptus globulus Extracts and Its Effectiveness for Removal of Recalcitrant Compounds. Catalysts. 2024; 14(8):525. https://doi.org/10.3390/catal14080525
Chicago/Turabian StyleSalgado, Pablo, Katherine Márquez, and Gladys Vidal. 2024. "Biogenic Synthesis Based on Cuprous Oxide Nanoparticles Using Eucalyptus globulus Extracts and Its Effectiveness for Removal of Recalcitrant Compounds" Catalysts 14, no. 8: 525. https://doi.org/10.3390/catal14080525
APA StyleSalgado, P., Márquez, K., & Vidal, G. (2024). Biogenic Synthesis Based on Cuprous Oxide Nanoparticles Using Eucalyptus globulus Extracts and Its Effectiveness for Removal of Recalcitrant Compounds. Catalysts, 14(8), 525. https://doi.org/10.3390/catal14080525





