Application of Different Waveforms of Pulsed Current in the Classical Electro-Cocatalytic Process for Effective Removal of Sulfamethoxazole: Oxidation Mechanisms
Abstract
1. Introduction
2. Results and Discussion
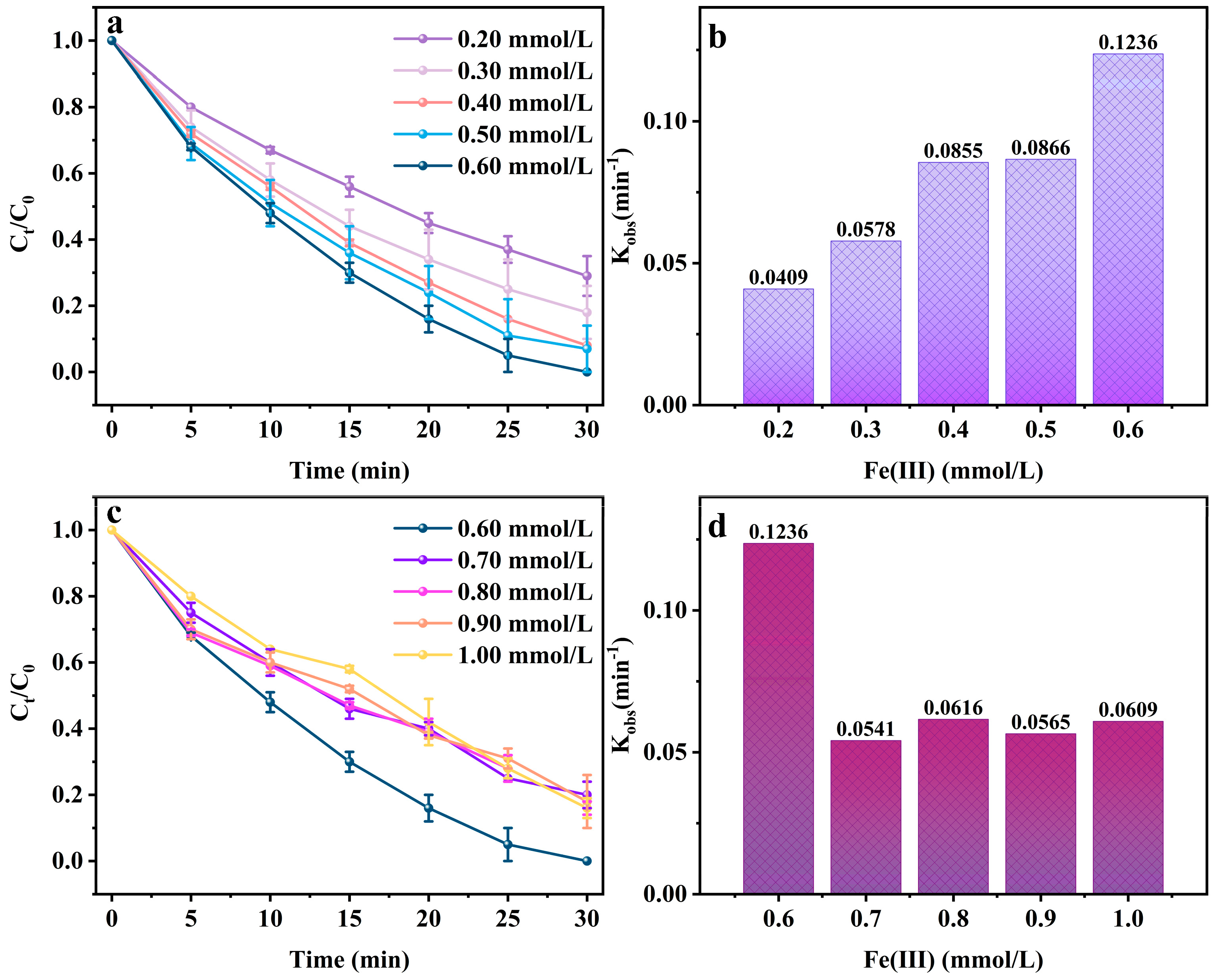
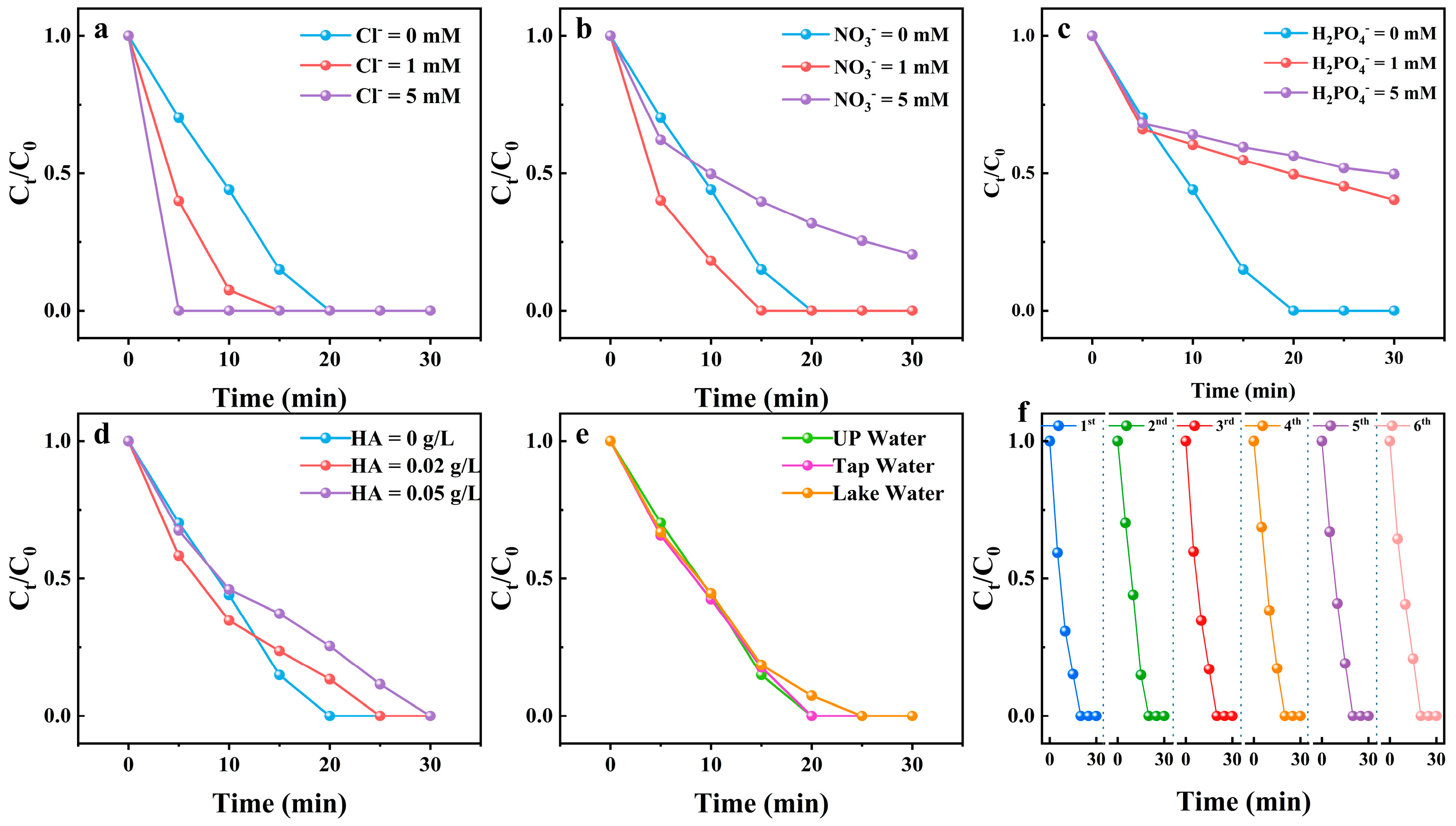
2.1. Impact of System Parameters on Degradation Performance
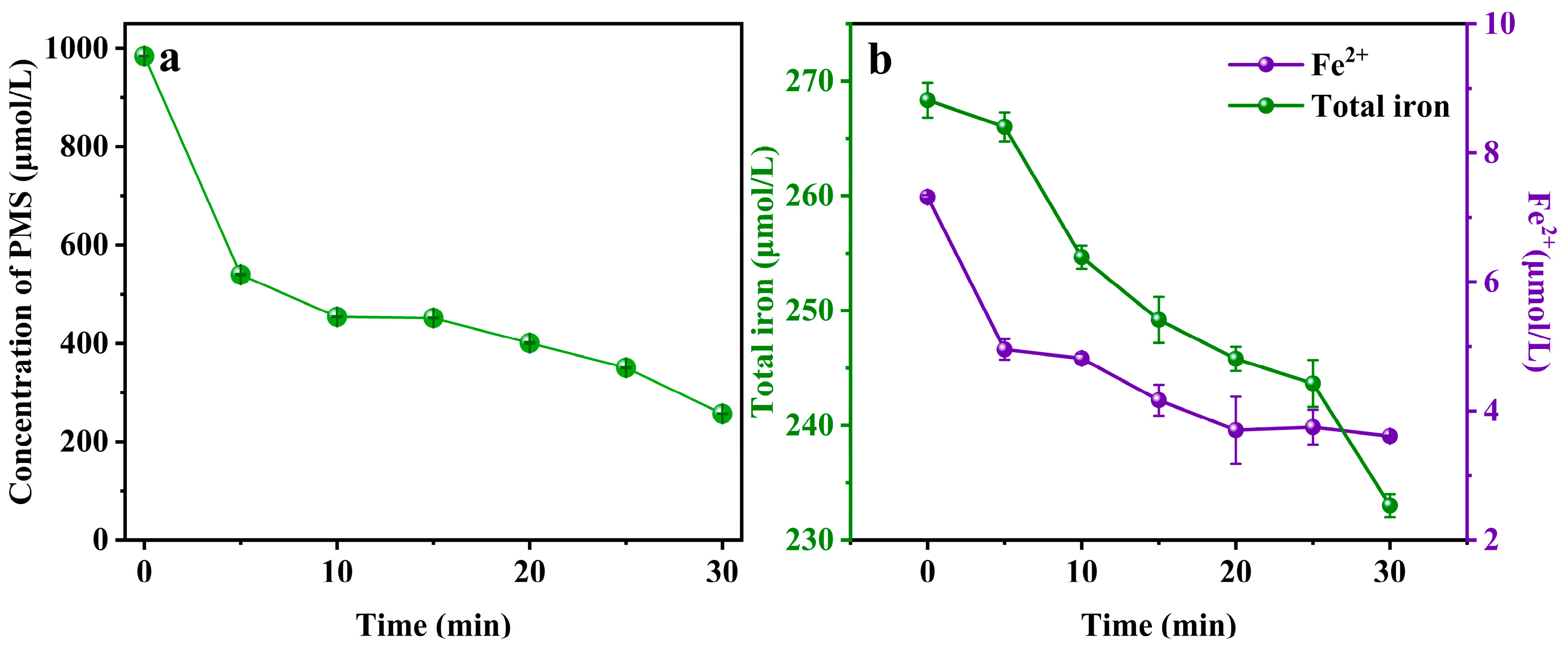
2.2. Effectiveness of Iron Species and PMS in SIN/PMS/Fe(III) Process
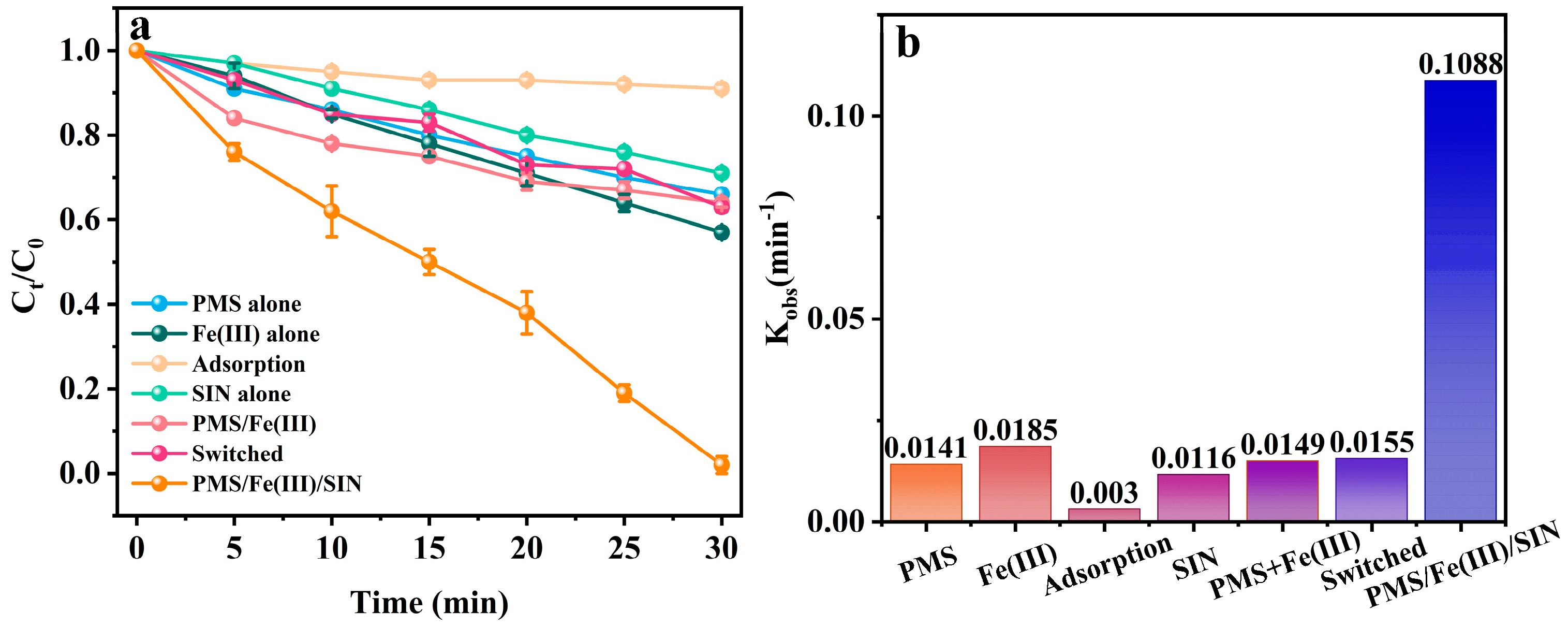
2.3. Removal Efficiency of SMX in Various Processes
2.4. Identification of Reactive Species and Mechanisms of SIN/PMS/Fe(III) Process
2.4.1. EPR Test
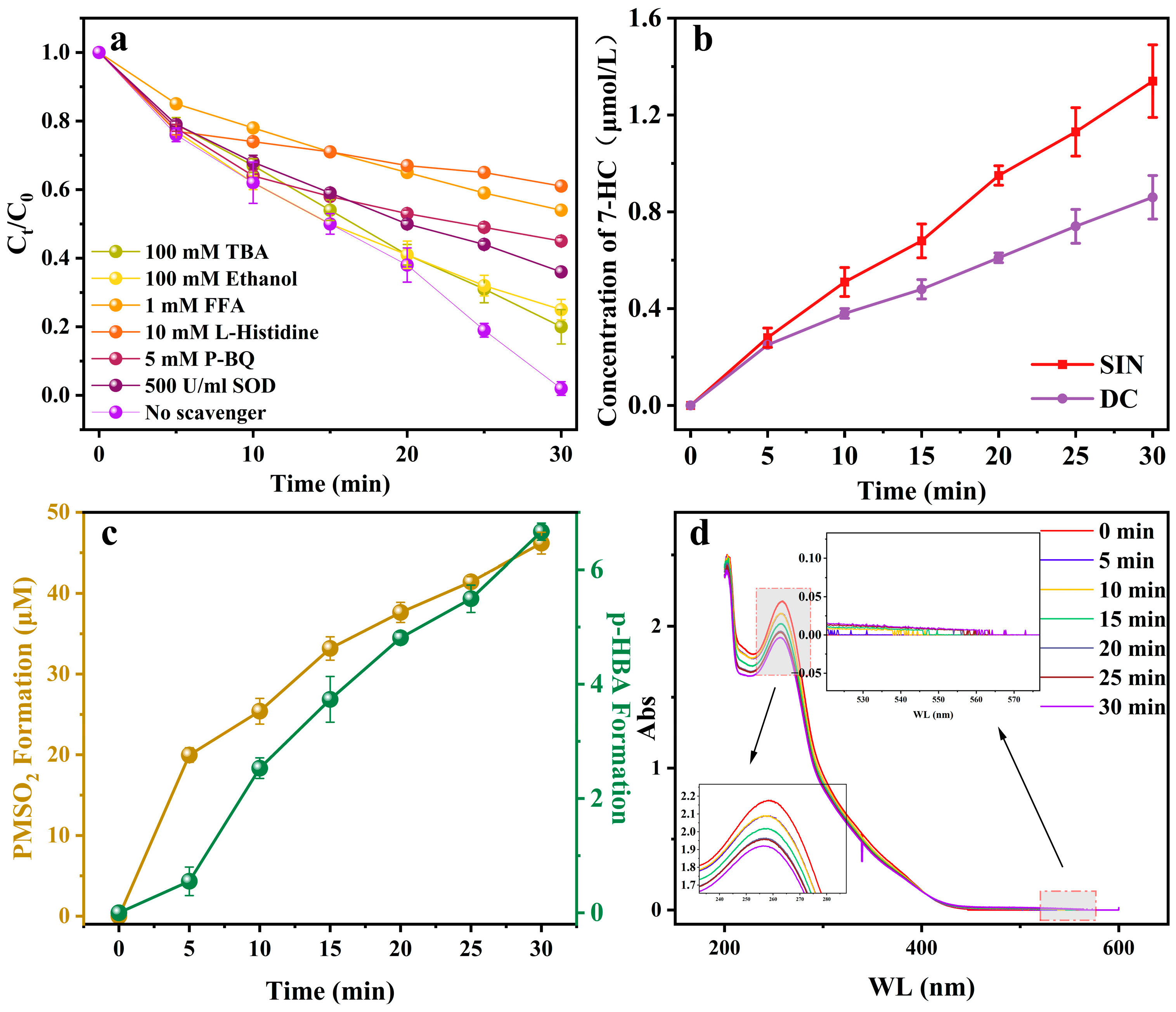
2.4.2. Quenching Experiment
2.4.3. Chemical Probe Experiments
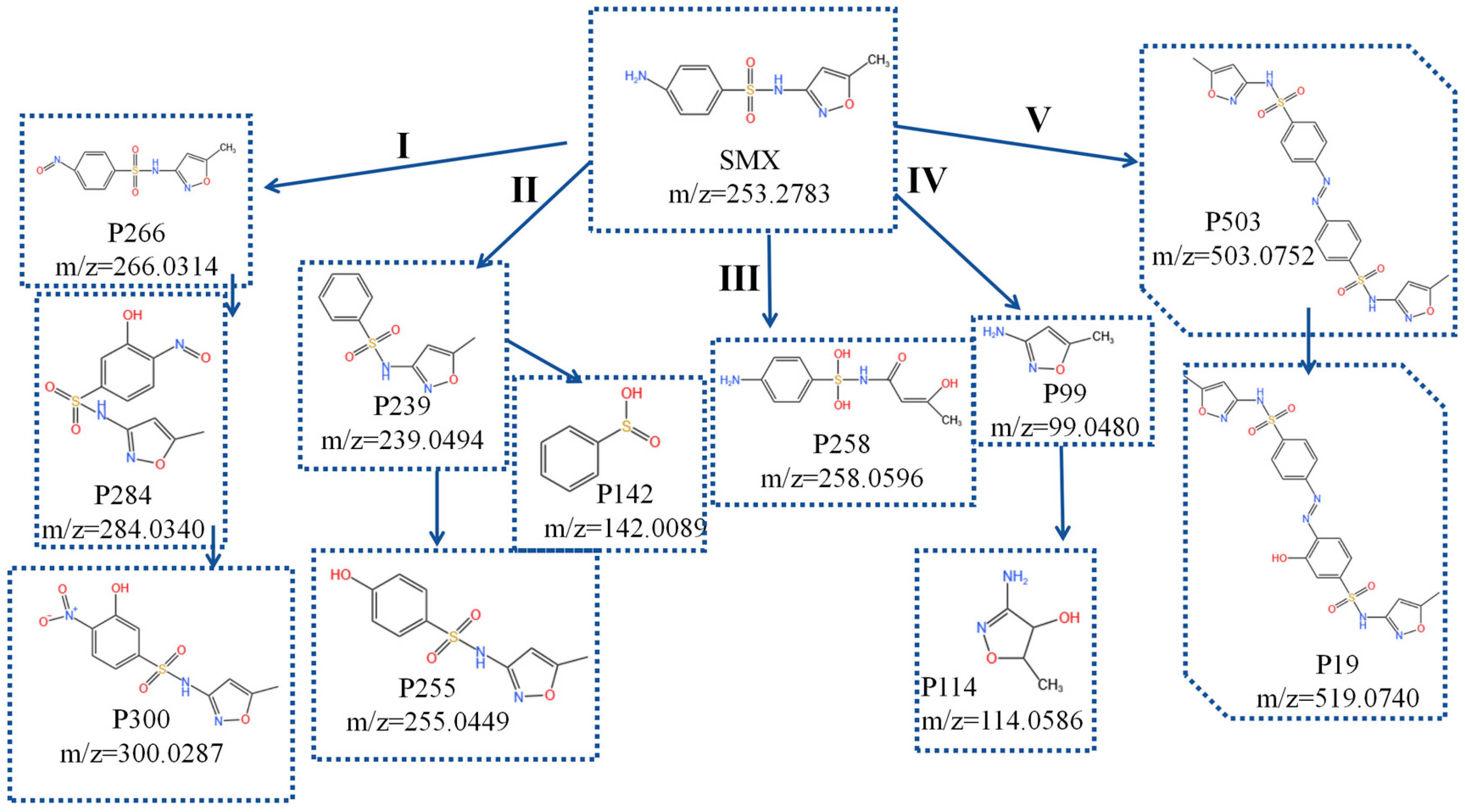
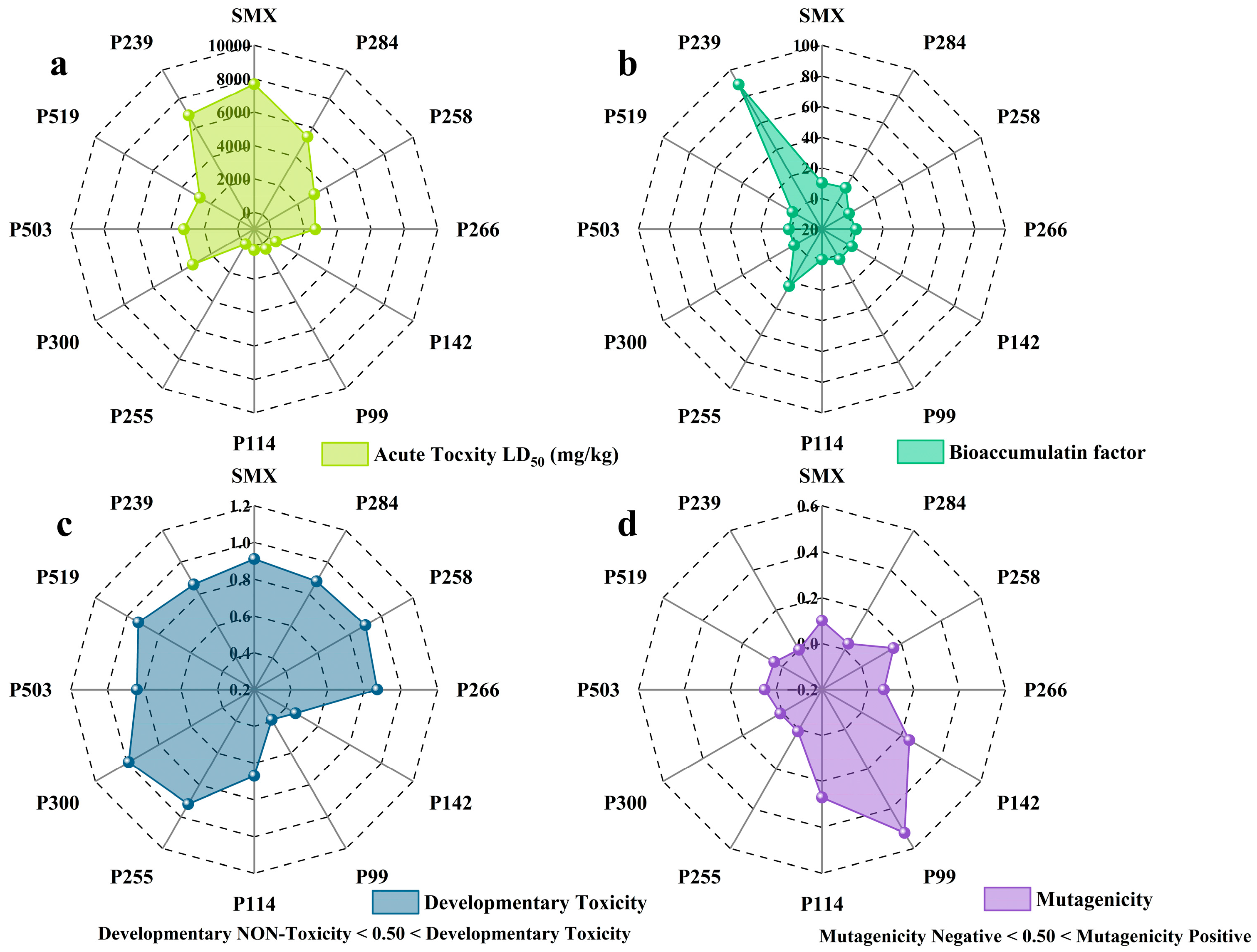
2.5. Degradation Pathways of SMX and Toxicity Evaluation
2.6. Practical Application of the SIN/PMS/Fe(III) System
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Degradation Experiment
3.3. Analytical Methods
3.4. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Khanal, S.K.; Zhang, H.; Chen, G.-H.; Lu, H. Sulfamethoxazole degradation in anaerobic sulfate-reducing bacteria sludge system. Water Res. 2017, 119, 12–20. [Google Scholar] [CrossRef]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Thi Thuy, D.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic oxidation of organic pollutants: Anode fabrication, process hybrid and environmental applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of nitrophenols and their derivatives by chemical redox: A review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci.-Wat. Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. Protect. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, M.; Tang, D.; Xu, Y.; Ran, H.; He, J.; Chen, K.; Sun, J. High H2O2 selectivity and enhanced Fe2+ regeneration toward an effective electro-Fenton process based on a self-doped porous biochar cathode. Appl. Catal. B-Environ. 2022, 315, 121523. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Abdalrhman, A.S.; El-Din, M.G. Degradation of organics in real oil sands process water by electro-oxidation using graphite and dimensionally stable anodes. Chem. Eng. J. 2020, 389, 124406. [Google Scholar] [CrossRef]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Hoyos-Arbelaez, J.; Vazquez, M.; Contreras-Calderon, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, D.; Feng, J.; Tang, T.; Cheng, H. Rapid quantitative detection of luteolin using an electrochemical sensor based on electrospinning of carbon nanofibers doped with single-walled carbon nanoangles. Anal. Methods 2023, 15, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M. An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, C.; Zhang, W.; Yang, X.; Ding, J.; Gao, G. Electrical Pulse-Driven Periodic Self-Repair of Cu-Ni Tandem Catalyst for Efficient Ammonia Synthesis from Nitrate. Angew. Chem.-Int. Edit. 2023, 62, 17337. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Wang, L.; Xue, Q.; Hu, Z.; Pan, B. Multivariate optimization of the pulse electrochemical oxidation for treating recalcitrant dye wastewater. Sep. Purif. Technol. 2020, 230, 115851. [Google Scholar] [CrossRef]
- Lei, J.; Xu, Z.; Xu, H.; Qiao, D.; Liao, Z.; Yan, W.; Wang, Y. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, H.; Lei, Z.; Yi, X.; Feng, C.; Dang, Z. A binder-free electrode for efficient H2O2 formation and Fe2+ regeneration and its application to an electro-Fenton process for removing organics in iron-laden acid wastewater. Chin. Chem. Lett. 2022, 33, 920–925. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Pazos, M.; Angeles Sanroman, M.; Gonzalez-Romero, E. Double benefit of electrochemical techniques: Treatment and electroanalysis for remediation of water polluted with organic compounds. Electrochim. Acta 2019, 320, 134628. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, X.; Ni, J. Electrochemical oxidation of phenol at boron-doped diamond electrode in pulse current mode. Electrochim. Acta 2011, 56, 5310–5315. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Zhu, K.; Zhang, H. Degradation of Acid Orange 7 using peroxymonosulfate catalyzed by granulated activated carbon and enhanced by electrolysis. Chemosphere 2017, 188, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Wang, X.; Zhou, M. Electro-enhanced activation of peroxymonosulfate by a novel perovskite-Ti4O7 composite anode with ultra-high efficiency and low energy consumption: The generation and dominant role of singlet oxygen. Water Res. 2023, 232, 119682. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Zhao, Y.; Wang, L.; Lu, D.; Ma, J. Electrified ceramic membrane actuates non-radical mediated peroxymonosulfate activation for highly efficient water decontamination. Water Res. 2022, 225, 119140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, T.; Tong, L.; Gao, Y.; Zhang, H.; Zhang, Y.; Wang, Z.; Zhu, S. Non-free Fe dominated PMS activation for enhancing electro-Fenton efficiency in neutral wastewater. J. Electroanal. Chem. 2023, 928, 117062. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Wang, J.; Long, X.; Zhang, I.Y.; Huang, R. Pulsed versus direct current electrochemical co-catalytic peroxymonosulfate-based system: Elevated degradation and energy efficiency with enhanced oxidation mechanisms. J. Hazard. Mater. 2023, 458, 132004. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Ganiyu, S.O.; Martinez-Huitle, C.A.; Mousset, E.; Olvera-Vargas, H.; Trellu, C.; Zhou, M.; Oturan, M.A. Recent advances in electro-Fenton process and its emerging applications. Crit. Rev. Environ. Sci. Technol. 2023, 53, 887–913. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Gao, P.; Li, H. New insight into the mechanism of electro-assisted pyrite minerals activation of peroxymonosulfate: Synergistic effects, activation sites and electron transfer. Sep. Purif. Technol. 2021, 274, 118817. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, N.; Hu, X. A novel strategy of pulsed electro-assisted pyrite activation of peroxymonosulfate for the degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2022, 280, 119781. [Google Scholar] [CrossRef]
- Zhou, P.; Ren, W.; Nie, G.; Li, X.; Duan, X.; Zhang, Y.; Wang, S. Fast and Long-Lasting Iron(III) Reduction by Boron Toward Green and Accelerated Fenton Chemistry. Angew. Chem.-Int. Edit. 2020, 59, 16517–16526. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, P.; Sun, Y.; Zhang, P.; Zhou, C.; Xiong, Z.; Zhang, H.; Liang, J.; Lai, B. Reducing agents enhanced Fenton-like oxidation (Fe(III)/Peroxydisulfate): Substrate specific reactivity of reactive oxygen species. Water Res. 2022, 218, 118412. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Li, J.; Yang, C.; Meng, X.; Gao, J. Insights into the effects of pulsed parameters on H2O2 synthesis by two-electron oxygen reduction under pulsed electrocatalysis. Electrochem. Commun. 2023, 146, 107414. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, J.; Fang, J.; Huang, Y.; Wang, P.; Ma, J. Production of Hydroxyl Radical via the Activation of Hydrogen Peroxide by Hydroxylamine. Environ. Sci. Technol. 2015, 49, 10373–10379. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.-T.; Yao, R.-Q.; Wan, W.-B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.-Y.; Zheng, W.-T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Lv, F.; Sun, M.; Hu, Y.; Xu, J.; Huang, W.; Han, N.; Huang, B.; Li, Y. Near-unity electrochemical conversion of nitrate to ammonia on crystalline nickel porphyrin-based covalent organic frameworks. Energy Environ. Sci. 2023, 16, 201–209. [Google Scholar] [CrossRef]
- Ma, X.; He, C.; Yan, Y.; Chen, J.; Feng, H.; Hu, J.; Zhu, H.; Xia, Y. Energy-efficient electrochemical degradation of ciprofloxacin by a Ti-foam/PbO2-GN composite electrode: Electrode characteristics, parameter optimization, and reaction mechanism. Chemosphere 2023, 315, 137739. [Google Scholar] [CrossRef]
- Long, X.; Huang, R.; Li, Y.; Wang, J.; Zhang, M.; Zhang, I.Y. Understanding the electro-cocatalytic peroxymonosulfate-based systems with BDD versus DSA anodes: Radical versus nonradical dominated degradation mechanisms. Sep. Purif. Technol. 2023, 309, 123120. [Google Scholar] [CrossRef]
- Kashani, M.R.K.; Wang, Q.; Khatebasreh, M.; Li, X.; Asadi, A.M.S.; Boczkaj, G.; Ghanbari, F. Sequential treatment of landfill leachate by electrocoagulation/aeration, PMS/ZVI/UV and electro-Fenton: Performance, biodegradability and toxicity studies. J. Environ. Manag. 2023, 338, 117781. [Google Scholar] [CrossRef]
- Eary, L.E.; Rai, D. Chromate Reduction by Subsurface Soils under Acidic Conditions. Soil Sci. Soc. Am. J. 1991, 55, 676–683. [Google Scholar] [CrossRef]
- Tanaka, E.; Hatanaka, N.; Muguruma, M.; Ishikawa, T.; Nakayama, T. Influence of anions on the formation of artificial steel rust particles prepared from acidic aqueous Fe(III) solution. Corros. Sci. 2013, 66, 136–141. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, H.; Xiong, Z.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2020, 391, 123604. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Q.; Xu, P.; Zhu, P.; Yang, Z.; Crittenden, J.C. Rapid determination of monopersulfate with bromide ion-catalyzed oxidation of 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS). Chem. Eng. J. 2022, 433, 133551. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Zhu, L.; Cui, Z.; Yuan, B. Multi-wavelength spectrophotometric measurement of persulfates using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) as indicator. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2019, 216, 214–220. [Google Scholar] [CrossRef]
- Xue, Q.; Yu, B.; Dagnew, M.; Li, W.; Ding, H.; Zhang, J.; Sun, Z.; Wang, P.; Zhao, C. Rapid in situ regeneration of phenol-saturated activated carbon fiber by an electro-permonosulfate-ozone process: Performance, operators and mechanism. J. Environ. Chem. Eng. 2024, 12, 111932. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Li, J.; Feng, Y.; Feng, L.; Han, S.; Pan, T.; Zhang, T.; Wu, S.; Ke, Z.; et al. Study on the performance efficiency, mechanism, power consumption and biochemical properties of E/Ce(IV)/PMS on the enhanced removal of RB19. Environ. Res. 2023, 232, 116271. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Z.; Kang, X.; Cheng, C.; Mao, X.; Li, R.; Lin, H.; Zhang, H. Electro-enhanced heterogeneous activation of peroxymonosulfate via acceleration of Fe(III)/Fe(II) redox cycle on Fe-B catalyst. Electrochim. Acta 2021, 377, 138073. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B-Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Xiong, Z.; Yao, G.; Lai, B. The electrochemical advanced oxidation processes coupling of oxidants for organic pollutants degradation: A mini-review. Chin. Chem. Lett. 2019, 30, 2139–2146. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B-Environ. Energy 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, H.; Zhao, C.; Wang, T.; Wang, P.; Dionysiou, D.D. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential. Water Res. 2019, 159, 111–121. [Google Scholar] [CrossRef]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, Y.; Lei, X.; Liang, X.; Cheng, S.; Ouyang, G.; Yang, X. Assessing the Use of Probes and Quenchers for Understanding the Reactive Species in Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 5433–5444. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Z.; Yu, Y.; Wang, X.; Zhou, H.; Huang, B.; Wu, Z.; Yu, C.; Chen, T.; Pan, Z.; et al. Efficient degradation of carbamazepine by electro-Fenton system without any extra oxidant in the presence of molybdate: The role of slow release of iron ions. Appl. Catal. B-Environ. 2021, 298, 120506. [Google Scholar] [CrossRef]
- Long, X.; Xiong, Z.; Huang, R.; Yu, Y.; Zhou, P.; Zhang, H.; Yao, G.; Lai, B. Sustainable Fe(III)/Fe(II) cycles triggered by co-catalyst of weak electrical current in Fe(III)/peroxymonosulfate system: Collaboration of radical and non-radical mechanisms. Appl. Catal. B-Environ. 2022, 317, 121716. [Google Scholar] [CrossRef]
- Long, X.; Shi, H.; Huang, R.; Gu, L.; Liu, Y.; He, C.-s.; Du, Y.; Xiong, Z.; Liu, W.; Lai, B. Identifying the evolution of primary oxidation mechanisms and pollutant degradation routes in the electro-cocatalytic Fenton-like systems. J. Hazard. Mater. 2023, 445, 130577. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C-TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef]
- Lai, W.; Chen, Z.; Ye, S.; Xu, Y.; Xie, G.; Kuang, C.; Li, Y.; Zheng, L.; Wei, L. BiVO4 prepared by the sol-gel doped on graphite felt cathode for ciprofloxacin degradation and mechanism in solar-photo-electro-Fenton. J. Hazard. Mater. 2021, 408, 124621. [Google Scholar] [CrossRef]
- Tokumura, M.; Morito, R.; Hatayama, R.; Kawase, Y. Iron redox cycling in hydroxyl radical generation during the photo-Fenton oxidative degradation: Dynamic change of hydroxyl radical concentration. Appl. Catal. B-Environ. 2011, 106, 565–576. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Gao, Y.; Liu, X.; Wei, Y.; Liang, Z. Enhanced atrazine degradation in the Fe(III)/peroxymonosulfate system via accelerating Fe(II) regeneration by benzoquinone. Chem. Eng. J. 2022, 427, 131995. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Dzakpasu, M.; Xu, S.; Ding, D.; Wang, G.; Chen, R.; Jin, P.; Wang, X.C. Galvanic corrosion of zero-valent iron to intensify Fe2+ generation for peroxymonosulfate activation. Chem. Eng. J. 2021, 417, 128023. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Yu, X.; Jin, X.; Li, M.; Yu, Y.; Liu, H.; Zhou, R.; Yin, A.; Shi, J.; Sun, J.; Zhu, L. Mechanism and security of UV driven sodium percarbonate for sulfamethoxazole degradation using DFT and metabolomic analysis. Environ. Pollut. 2023, 323, 121352. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Y.; Metz, J.; He, S.; Alvarez, P.J.J.; Long, M. Persistent free radicals in biochar enhance superoxide-mediated Fe(III)/Fe (II) cycling and the efficacy of CaO2 Fenton-like treatment. J. Hazard. Mater. 2022, 421, 126805. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yan, Y.; Yan, J.; Pan, Y.; Zhang, Y.; Lai, B. Enhanced sulfamethoxazole degradation by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron (S-mFe0): Performance, mechanisms, and the role of sulfur species. Chem. Eng. J. 2019, 376, 03.178. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, X.; Ma, J.; Tan, H.; Ye, W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity. Chem. Eng. J. 2014, 249, 6–14. [Google Scholar] [CrossRef]
- Wang, F.; Fu, H.; Wang, F.-X.; Zhang, X.-W.; Wang, P.; Zhao, C.; Wang, C.-C. Enhanced catalytic sulfamethoxazole degradation via peroxymonosulfate activation over amorphous CoSx@SiO2 nanocages derived from ZIF-67. J. Hazard. Mater. 2022, 423, 126998. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, L.; Wang, M.; Shu, J.; Shao, P.; Yang, L.; Meng, X.; Fan, Y.; Li, M. Highly effective recovery of palladium from a spent catalyst by an acid- and oxidation-resistant electrospun fibers as a sorbent. Chem. Eng. J. 2023, 466, 143171. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, J.; Jiang, Y.; Yang, S.; Yang, Y.; Wang, Z. FeCo bimetallic metal organic framework nanosheets as peroxymonosulfate activator for selective oxidation of organic pollutants. Chem. Eng. J. 2022, 443, 136483. [Google Scholar] [CrossRef]
- Wang, W.-L.; Wu, Q.-Y.; Huang, N.; Wang, T.; Hu, H.-Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Q.; Lian, L.; Li, Y.; Wang, S.; Li, C.; Guan, X. Degradation of Organic Contaminants in the Fe(II)/Peroxymonosulfate Process under Acidic Conditions: The Overlooked Rapid Oxidation Stage. Environ. Sci. Technol. 2021, 55, 15390–15399. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, L.; Huang, W.; Zhou, H.; Li, J.; Liu, C.; Lai, B.; Li, N. Effective peroxymonosulfate activation by natural molybdenite for enhanced atrazine degradation: Role of sulfur vacancy, degradation pathways and mechanism. J. Hazard. Mater. 2022, 435, 128899. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Yu, Y.; Cui, X.; Yan, B.; Song, Y.; Li, N.; Chen, G.; Wang, S. Effect of phosphates on oxidative species generation and sulfamethoxazole degradation in a pig manure derived biochar activated peroxymonosulfate system. Sep. Purif. Technol. 2022, 295, 121255. [Google Scholar] [CrossRef]
- Duan, P.; Liu, X.; Liu, B.; Akram, M.; Li, Y.; Pan, J.; Yue, Q.; Gao, B.; Xu, X. Effect of phosphate on peroxymonosulfate activation: Accelerating generation of sulfate radical and underlying mechanism. Appl. Catal. B-Environ. 2021, 298, 120532. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Wang, Y.; Wang, J.; Zhang, I.Y.; Huang, R. Application of Different Waveforms of Pulsed Current in the Classical Electro-Cocatalytic Process for Effective Removal of Sulfamethoxazole: Oxidation Mechanisms. Catalysts 2024, 14, 532. https://doi.org/10.3390/catal14080532
Fang J, Wang Y, Wang J, Zhang IY, Huang R. Application of Different Waveforms of Pulsed Current in the Classical Electro-Cocatalytic Process for Effective Removal of Sulfamethoxazole: Oxidation Mechanisms. Catalysts. 2024; 14(8):532. https://doi.org/10.3390/catal14080532
Chicago/Turabian StyleFang, Jingkai, Yongjian Wang, Jiahao Wang, Igor Ying Zhang, and Rongfu Huang. 2024. "Application of Different Waveforms of Pulsed Current in the Classical Electro-Cocatalytic Process for Effective Removal of Sulfamethoxazole: Oxidation Mechanisms" Catalysts 14, no. 8: 532. https://doi.org/10.3390/catal14080532
APA StyleFang, J., Wang, Y., Wang, J., Zhang, I. Y., & Huang, R. (2024). Application of Different Waveforms of Pulsed Current in the Classical Electro-Cocatalytic Process for Effective Removal of Sulfamethoxazole: Oxidation Mechanisms. Catalysts, 14(8), 532. https://doi.org/10.3390/catal14080532





