Selective Oligomerization of Isobutylene in Mixed C4 with Co/BETA-Loaded Molecular Sieve Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst
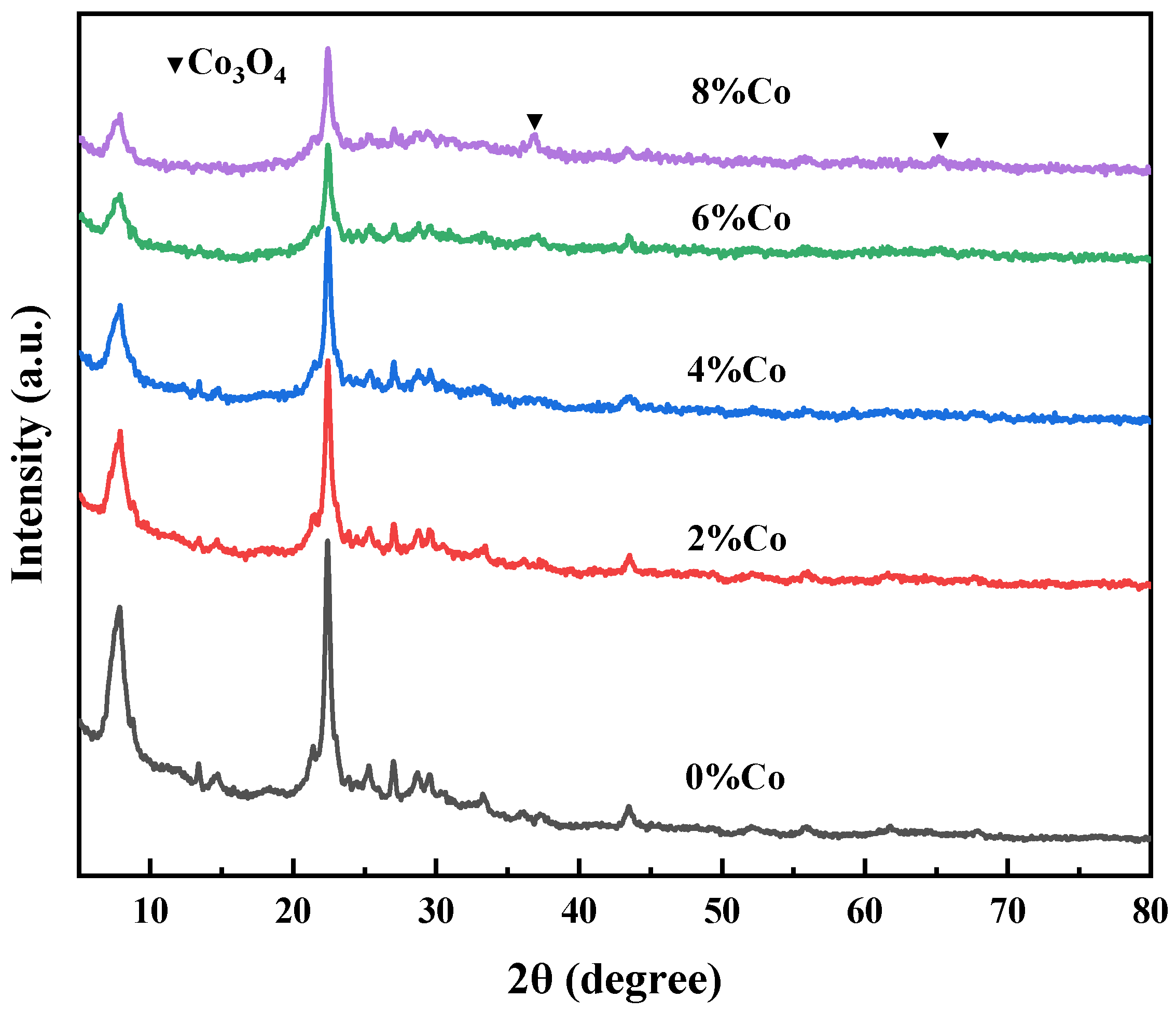
2.1.1. XRD Analysis
2.1.2. BET Analysis
2.1.3. NH3-TPD Analysis
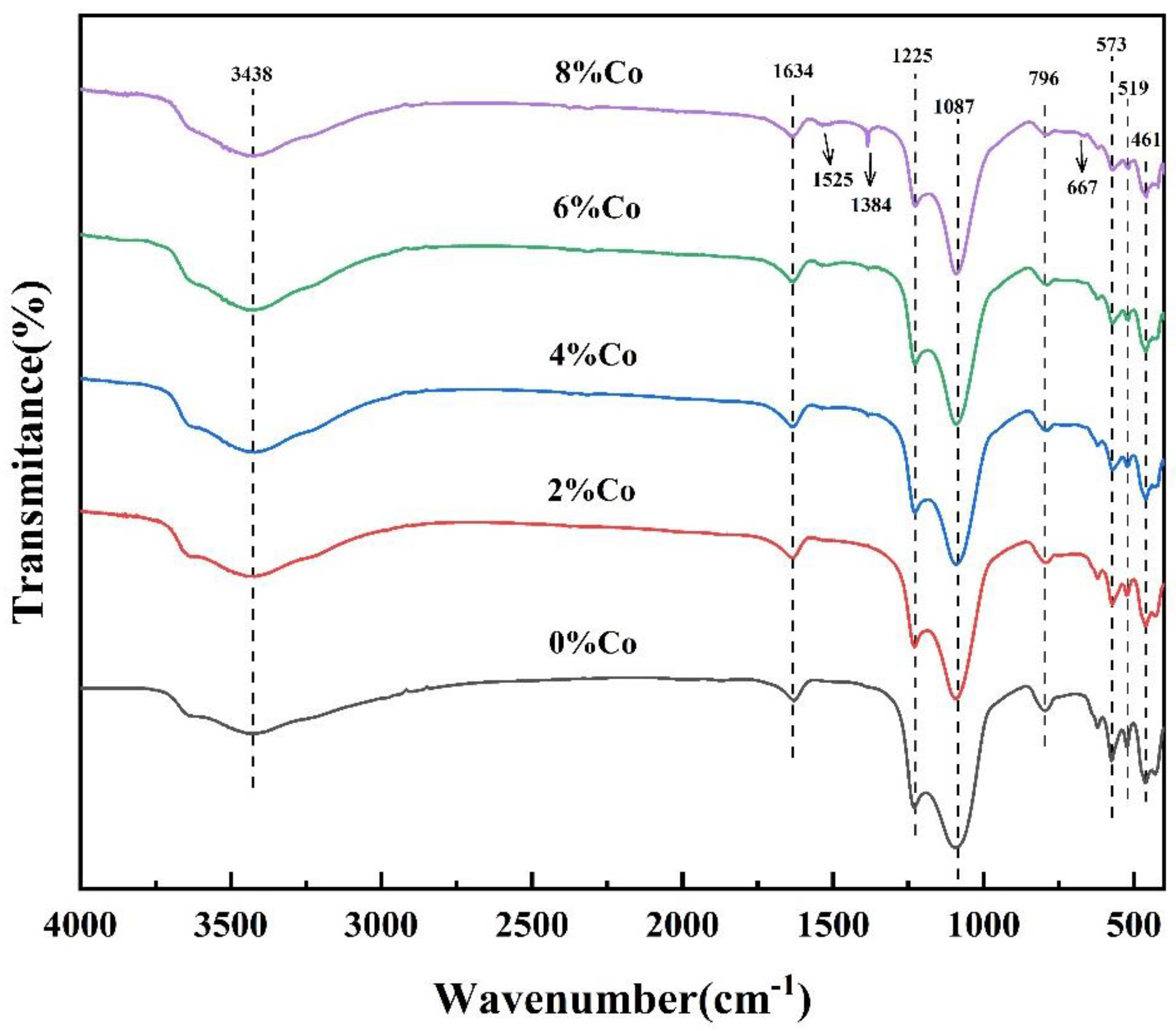
2.1.4. FI-IR Analysis
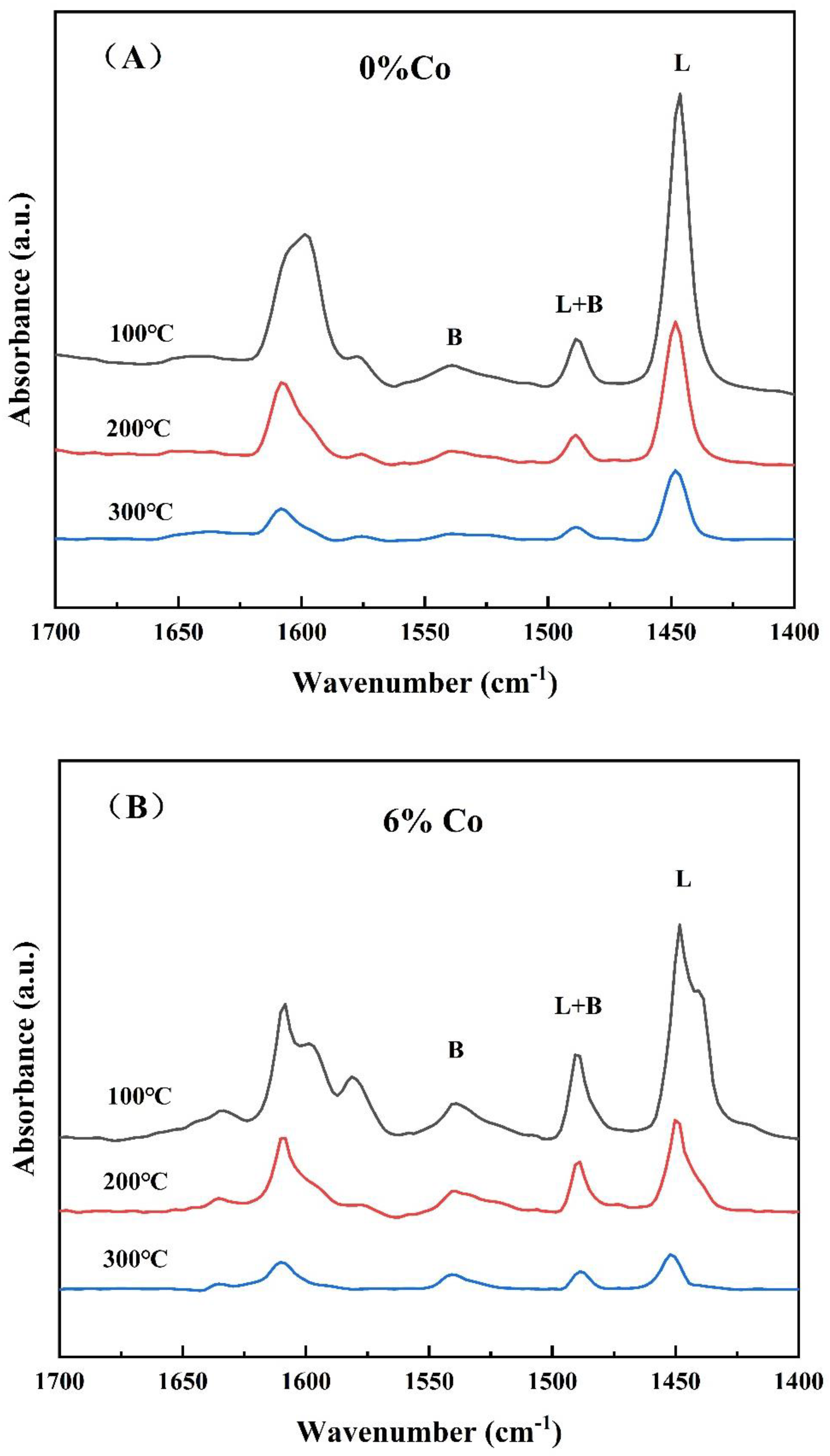
2.1.5. Py-FTIR Analysis
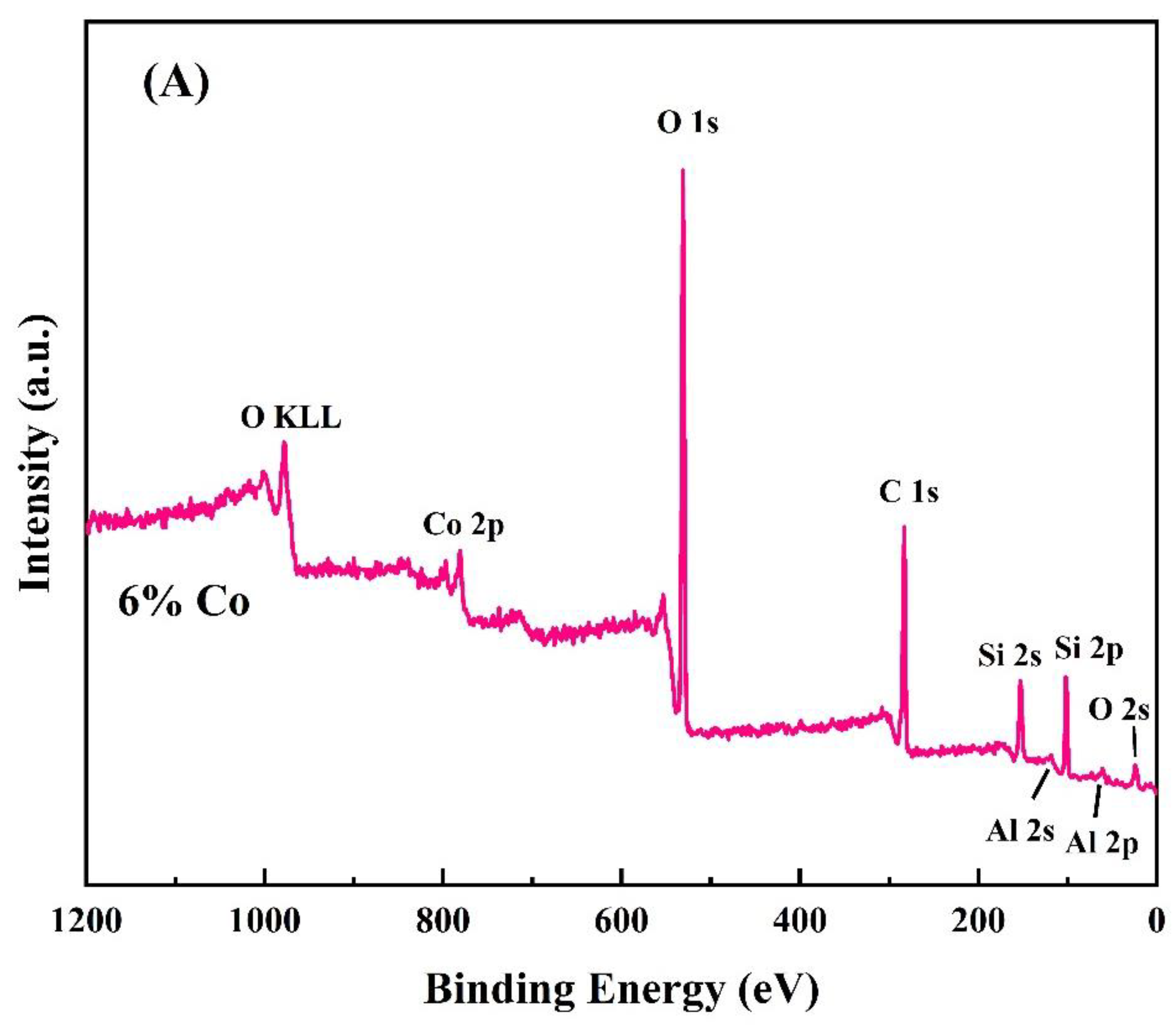
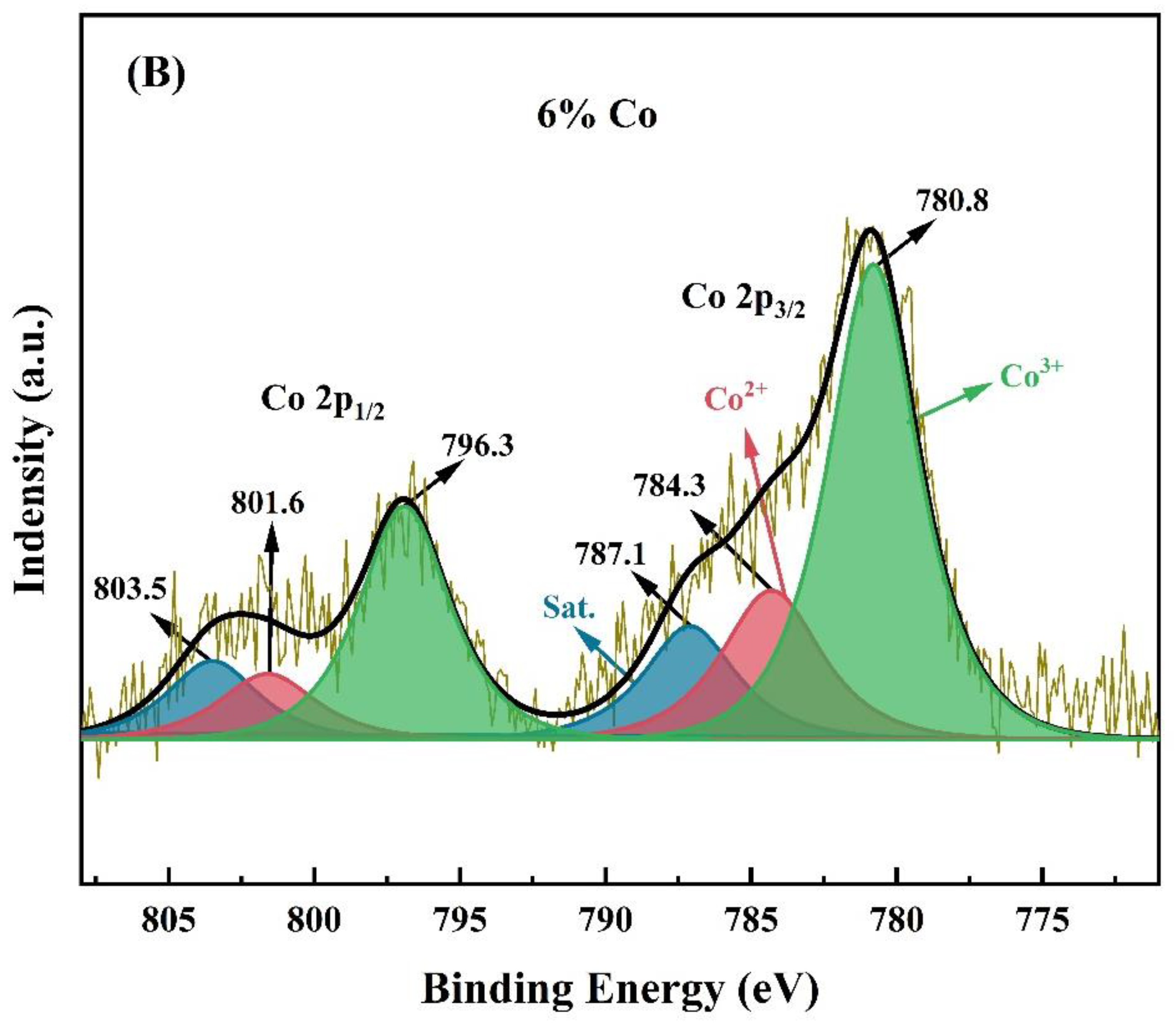
2.1.6. XPS Analysis
2.2. Catalyst Evaluation Results and Analysis
2.2.1. Evaluation Results of Different Co Loadings
2.2.2. Evaluation Results of Different Reaction Temperatures
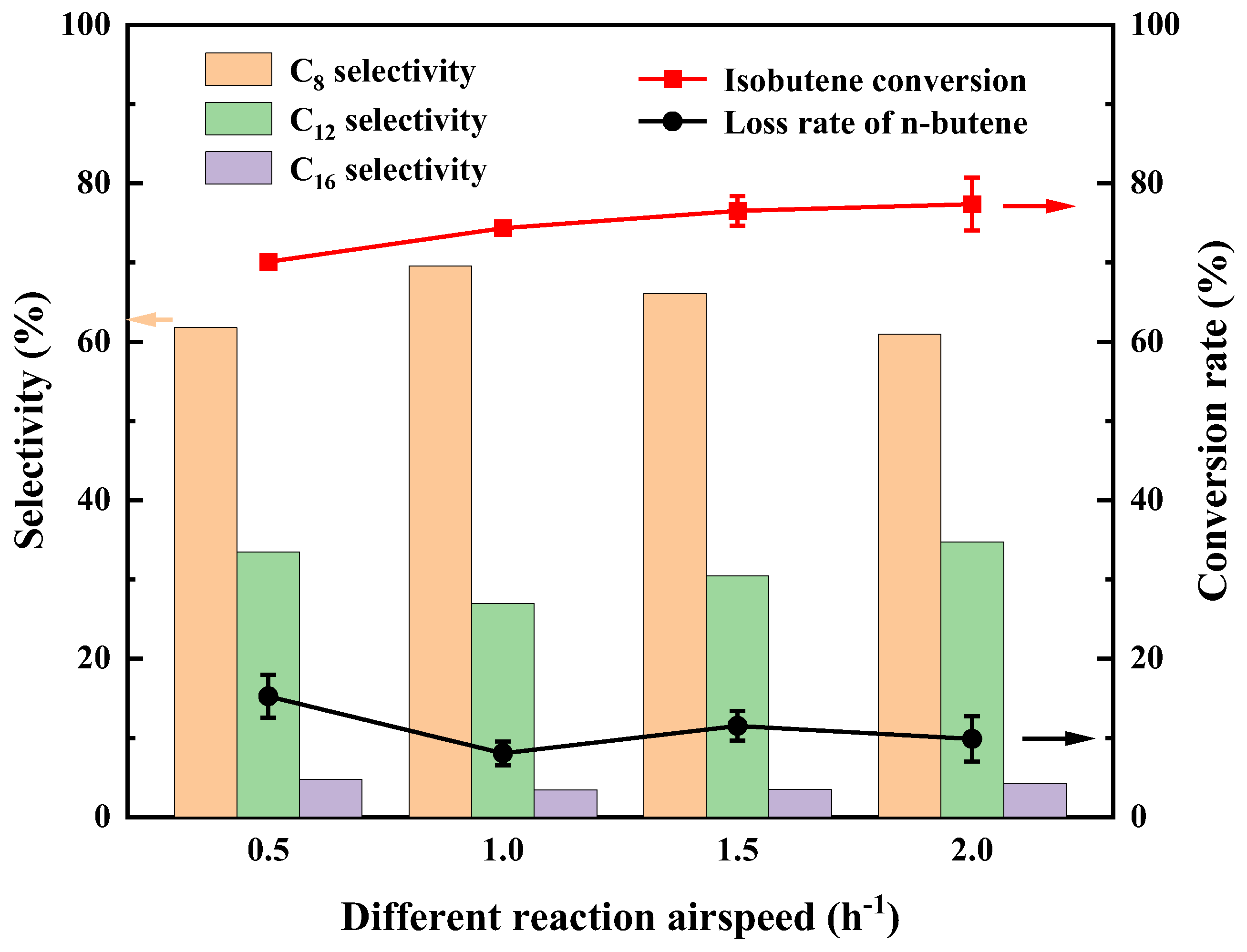
2.2.3. Evaluation Results of Different Reaction Air Velocities
3. Materials and Methods
3.1. Experimental Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.3.1. XRD Analysis
3.3.2. BET Analysis
3.3.3. NH3-TPD Analysis
3.3.4. XPS Analysis
3.3.5. FI-IR Analysis
3.3.6. Py-IR Analysis
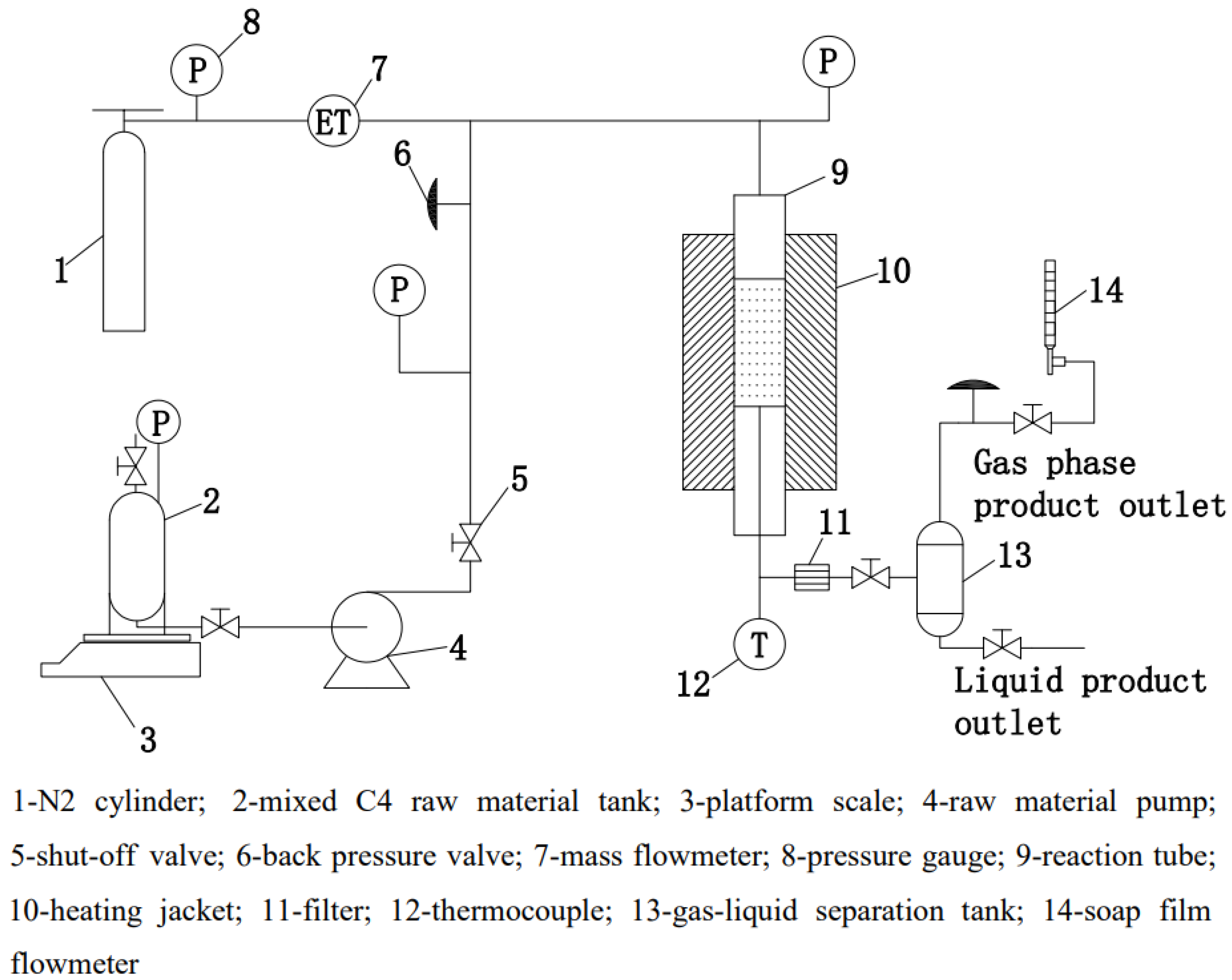
3.4. Experimental Setup
3.5. Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Shang, J.; Zhang, S.; Yang, B.; Deng, Y. Highly efficient trimerization of isobutylene over silica-supported chloroaluminate ionic liquid using C4 feed. Catal. Today 2013, 200, 41–48. [Google Scholar] [CrossRef]
- Díaz, M.; Epelde, E.; Valecillos, J.; Izaddoust, S.; Aguayo, A.T.; Bilbao, J. Coke deactivation and regeneration of HZSM-5 zeolite catalysts in the oligomerization of 1-butene. Appl. Catal. B Environ. 2021, 291, 120016. [Google Scholar] [CrossRef]
- Díaz, M.; Epelde, E.; Aguayo, A.T.; Bilbao, J. Low-pressure oligomerization of 1-butene to liquid fuels on HZSM-5 zeolite catalysts: Effect of operating conditions. J. Ind. Eng. Chem. 2020, 87, 234–241. [Google Scholar] [CrossRef]
- Fernandez-Morales, J.M.; Castillejos, E.; Asedegbega-Nieto, E.; Dongil, A.B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Comparative Study of Different Acidic Surface Structures in Solid Catalysts Applied for the Isobutylene Dimerization Reaction. Nanomaterials 2020, 10, 1235. [Google Scholar] [CrossRef]
- Cao, P.; Zheng, L.; Sun, W.; Zhao, L. Multiscale Modeling of Isobutane Alkylation with Mixed C4 Olefins Using Sulfuric Acid as Catalyst. Ind. Eng. Chem. Res. 2019, 58, 6340–6349. [Google Scholar] [CrossRef]
- Malaika, A.; Rechnia-Gorący, P.; Kot, M.; Kozłowski, M. Selective and efficient dimerization of isobutylene over H3PO4/activated carbon catalysts. Catal. Today 2018, 301, 266–273. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Liu, S.; Tian, H. Preparation of supported Fe2O3-Cl/β-zeolite catalyst and its application in the selective oligomerization reaction of isobutylene in mixed C4. J. Chem. Technol. Biotechnol. 2022, 97, 1893–1899. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, S.-S.; Pinnavaia, T.J.; Tzompantzi, F.; Prince, J.; Valente, J.S. Selective Isobutylene Oligomerization by Mesoporous MSU-SBEA Catalysts. J. Phys. Chem. C 2011, 115, 5809–5816. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Zhang, Z.; Chen, X.; Liu, Q.; Fei, Z.; Tang, J.; Qiao, X. Promoting di-isobutylene selectivity over ZnO/ZrO2-SO4 in isobutylene oligomerization. Chin. J. Chem. Eng. 2021, 38, 165–171. [Google Scholar] [CrossRef]
- Tang, S.; Scurto, A.M.; Subramaniam, B. Improved 1-butene/isobutane alkylation with acidic ionic liquids and tunable acid/ionic liquid mixtures. J. Catal. 2009, 268, 243–250. [Google Scholar] [CrossRef]
- Feller, A.; Zuazo, I.; Guzman, A.; Barth, J.O.; Lercher, J.A. Common mechanistic aspects of liquid and solid acid-catalyzed alkylation of isobutane with n -butene. J. Catal. 2003, 216, 313–323. [Google Scholar] [CrossRef]
- Klerk, A.D.; Leckel, D.O.; Prinsloo, N.M. Butene Oligomerization by Phosphoric Acid Catalysis: Separating the Effects of Temperature and Catalyst Hydration on Product Selectivity. Ind. Eng. Chem. Res. 2006, 45, 6127–6136. [Google Scholar] [CrossRef]
- Antunes, B.M.; Rodrigues, A.E.; Lin, Z.; Portugal, I.; Silva, C.M. Alkenes oligomerization with resin catalysts. Fuel Process. Technol. 2015, 138, 86–99. [Google Scholar] [CrossRef]
- Kriván, E.; Hancsók, J. Oligomerization of Light FCC Naphtha with Ion Exchange Resin Catalyst. Top. Catal. 2015, 58, 939–947. [Google Scholar] [CrossRef]
- Liu, J.; Ding, N.; Ge, Y.; Zhou, X.; Wang, J.A.; Li, C. Dimerization of Isobutylene in C4 Mixtures in the Presence of Ethanol Over Acid Ion-Exchange Resin DH-2. Catal. Lett. 2019, 149, 1277–1285. [Google Scholar] [CrossRef]
- Sun, D.; Liu, S.; Yu, F.; Tian, H.; Zhao, Q. Selective oligomerization of isobutylene in mixed C4 catalyzed by supported Fe(NO3)3/β catalyst. J. Chem. Technol. Biotechnol. 2021, 96, 2588–2595. [Google Scholar] [CrossRef]
- Yang, J.-B.; Hui, Y.; Qin, Y.-C.; Zhang, X.-T.; Wang, H.; Song, L.-J. Effect of Lewis acid sites of FER zeolite on the catalytic transformation of isobutylene. J. Fuel Chem. Technol. 2021, 49, 1326–1335. [Google Scholar] [CrossRef]
- Yi, F.; Chen, H.; Huang, L.; Hu, C.; Wang, J.; Li, T.; Wang, H.; Tao, Z.; Yang, Y.; Li, Y. Effects of the acidity and shape selectivity of dealuminated zeolite beta on butene transformations. Fuel 2021, 300, 120694. [Google Scholar] [CrossRef]
- Al-Kinany, M.C.; Al-Drees, S.A.; Al-Megren, H.A.; Alshihri, S.M.; Alghilan, E.A.; Al-Shehri, F.A.; Al-Hamdan, A.S.; Alghamdi, A.J.; Al-Dress, S.D. High-quality fuel distillates produced from oligomerization of light olefin over supported phosphoric acid on H-Zeolite-Y. Appl. Petrochem. Res. 2019, 9, 35–45. [Google Scholar] [CrossRef]
- Huo, W.; Liu, W.; Yu, Q.; An, J.; Zhu, X.; Qin, Y.; Li, X. Exploration of Isobutene Oligomerization Reaction on MWW Molecular Sieve. Chem. Prog. 2023, 42, 5205–5212. [Google Scholar]
- Yu, Y.; Zhang, J.; Lv, Z.; Ji, M. The effect of Ni modified HZSM-5 catalyst on isobutene oligomerization in mixed C4. Ind. Catal. 2023, 31, 63–68. [Google Scholar]
- Lv, L.; Wang, H.; Chen, J.; Cao, Y.; Wang, H.; Ren, B.; Zhang, S. Fabrication of Ionic Liquid-Based Pickering Emulsion and Its Enhancement for Tri-isobutylene Formation in Isobutylene Oligomerization. Ind. Eng. Chem. Res. 2020, 59, 10436–10446. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Deng, L.; Wang, H.; Li, Z. Synthesis, characterization, and application of metal-free acidic ionic liquids as catalysts for oligomerization of isobutylene. Fuel 2021, 299, 120876. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Zhu, Y.; Wang, H.; Wang, H. Dual-site eutectic ionic liquids based microemulsion for boosting selective dimerization of isobutylene. Chem. Eng. Sci. 2023, 266, 118263. [Google Scholar] [CrossRef]
- Fütyű, J.; Ispán, D.; Fehér, C.; Szegedi, Á.; Juzsakova, T.; Hancsók, J.; Skoda-Földes, R. Recyclable supported Brønsted acidic ionic liquid catalysts with non-aromatic cations for the oligomerization of isobutylene under mild conditions. Mol. Catal. 2022, 518, 112075. [Google Scholar] [CrossRef]
- Zhang, J.; Ohnishi, R.; Okuhara, T.; Kamiya, Y. Preferential oligomerization of isobutylene in mixtures of isobutylene and 1-butene over 12-tungstosilicic acid supported on silica. Appl. Catal. A General. 2009, 353, 68–73. [Google Scholar] [CrossRef]
- Kocaman, E.; Akarçay, Ö.; Bağlar, N.; Çelebi, S.; Uzun, A. Isobutylene oligomerization on MCM-41-supported tungstophosphoric acid. Mol. Catal. 2018, 457, 41–50. [Google Scholar] [CrossRef]
- Liu, P.; Redekop, E.; Gao, X.; Liu, W.C.; Olsbye, U.; Somorjai, G.A. Oligomerization of Light Olefins Catalyzed by Bronsted-Acidic Metal-Organic Framework-808. J. Am. Chem. Soc. 2019, 141, 11557–11564. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Morales, J.M.; Lozano, L.A.; Castillejos-López, E.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Zamora, J.M. Direct sulfation of a Zr-based metal-organic framework to attain strong acid catalysts. Microporous Mesoporous Mater. 2019, 290, 109686. [Google Scholar] [CrossRef]
- Joanna, D.; Marzena, B.; Patrycja, P.; Adriana, Z.M. Dipicolinate Oxovanadium (IV) Complexes-Well-Defined, Universal Precatalysts for Ethylene Polymerization and Polar Monomers Oligomerization. Chem. Sel. 2024, 9, e202303255. [Google Scholar]
- Yu, P.H.; Luan, C.H.; Cao, M.; Sun, D.H.; Tian, H. Effect of ethanol on selective oligomerization of isobutene and the simulation of reactive distillation process. Asia Pac. J. Chem. Eng. 2024, 18, e2933. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, Z.; Zhong, H.; Bai, W.; Ji, M. Supported Metal Sulfate Catalysts FexZny/SiO2 for Selective Dimerization of Isobutylene in Mixed C4 to Isooctenes. Eur. J. Inorg. Chem. 2022, 26, e202200631. [Google Scholar] [CrossRef]
- Xu, Z.; Chada, J.P.; Zhao, D.; Carrero, C.A.; Kim, Y.T.; Rosenfeld, D.C.; Rogers, J.L.; Rozeveld, S.J.; Hermans, I.; Huber, G.W. Production of Linear Octenes from Oligomerization of 1-Butene over Carbon-Supported Cobalt Catalysts. ACS Catal. 2016, 6, 3815–3825. [Google Scholar] [CrossRef]
- Jonathan, A.; Dastidar, R.G.; Wang, C.; Dumesic, J.A.; Huber, G.W. Effect of catalyst support on cobalt catalysts for ethylene oligomerization into linear olefins. Catal. Sci. Technol. 2022, 12, 3639–3649. [Google Scholar] [CrossRef]
- Jian, J.; Kuang, D.; Wang, X.; Zhou, H.; Gao, H.; Sun, W.; Yuan, Z.; Zeng, J.; You, K.; Luo, H.A. Highly dispersed Co/SBA-15 mesoporous materials as an efficient and stable catalyst for partial oxidation of cyclohexane with molecular oxygen. Mater. Chem. Phys. 2020, 246, 122814. [Google Scholar] [CrossRef]
- Selvaraj, M.; Lee, T.G. Direct Synthesis of Well-Ordered and Unusually Reactive MnSBA-15 Mesoporous Molecular Sieves with High Manganese Content. J. Phys. Chem. B 2006, 110, 21793–21802. [Google Scholar] [CrossRef]
- Wang, C.; Lim, S.; Du, G.; Loebicki, C.Z.; Li, N.; Derrouiche, S.; Haller, G.L. Synthesis, Characterization, and Catalytic Performance of Highly Dispersed Co-SBA-15. J. Phys. Chem. C 2009, 113, 14863–14871. [Google Scholar] [CrossRef]
- Deng, R.; You, K.; Yi, L.; Zhao, F.; Jian, J.; Chen, Z.; Liu, P.; Ai, Q.; Luo, H.A. Solvent-Free, Low-Temperature, Highly Efficient Catalytic Nitration of Toluene with NO2 Promoted by Molecular Oxygen over Immobilized AlCl3–SiO2. Ind. Eng. Chem. Res. 2018, 57, 12993–13000. [Google Scholar] [CrossRef]
- Bin, F.; Song, C.; Lv, G.; Song, J.; Cao, X.; Pang, H.; Wang, K. Structural Characterization and Selective Catalytic Reduction of Nitrogen Oxides with Ammonia: A Comparison between Co/ZSM-5 and Co/SBA-15. J. Phys. Chem. C 2012, 116, 26262–26274. [Google Scholar] [CrossRef]
- Alegre, C.; Busacca, C.; Di Blasi, A.; Di Blasi, O.; Aricò, A.S.; Antonucci, V.; Modica, E.; Baglio, V. Electrospun carbon nanofibers loaded with spinel-type cobalt oxide as bifunctional catalysts for enhanced oxygen electrocatalysis. J. Energy Storage 2019, 23, 269–277. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, Z.; Huang, L.-a.; Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Pan, J. Phosphorus-modified cobalt single-atom catalysts loaded on crosslinked carbon nanosheets for efficient alkaline hydrogen evolution reaction. Nanoscale 2023, 15, 3550–3559. [Google Scholar] [CrossRef] [PubMed]





| Catalyst | Specific Surface Area (m2 g−1) | Pore Size (nm) | Pore Volume (mL g−1) | ||
|---|---|---|---|---|---|
| Vtotal | Vmicro | Vmeso | |||
| 0%Co | 423 | 3.26 | 0.33 | 0.15 | 0.18 |
| 2%Co | 422 | 3.36 | 0.35 | 0.16 | 0.19 |
| 4%Co | 391 | 3.43 | 0.32 | 0.14 | 0.18 |
| 6%Co | 353 | 3.46 | 0.29 | 0.13 | 0.16 |
| 8%Co | 341 | 3.5 | 0.29 | 0.12 | 0.17 |
| Catalyst | 0% Co | 6% Co | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Brønsted Acid | Lewis Acid | Total Acid Content | B/L | Brønsted Acid | Lewis Acid | Total Acid Content | B/L |
| 100 | 21.48 | 129.17 | 150.65 | 0.17 | 30.76 | 116.50 | 147.26 | 0.26 |
| 200 | 12.56 | 58.77 | 71.33 | 0.21 | 24.07 | 37.16 | 61.23 | 0.65 |
| 300 | 7.70 | 29.17 | 36.87 | 0.26 | 10.37 | 12.89 | 23.26 | 0.81 |
| Temperature/℃ | Conversion/% | Selectivity/% | Yield of C8=/% | |||
|---|---|---|---|---|---|---|
| Isobutylene | n-Butene | C8= | C12= | C16= | ||
| 50 | 57.87 | 8.23 | 66.58 | 28.50 | 4.92 | 38.53 |
| 55 | 60.40 | 10.71 | 73.55 | 23.19 | 3.26 | 44.42 |
| 60 | 74.34 | 8.11 | 69.54 | 27.01 | 3.45 | 51.69 |
| 65 | 80.48 | 12.53 | 57.01 | 35.65 | 7.34 | 45.88 |
| 70 | 80.19 | 9.50 | 59.87 | 30.35 | 9.78 | 48.01 |
| Raw Material Composition | Propane | Propylene | Isobutane | n-Butane | trans-2-Butene | n-Butene | Isobutylene | cis-2-Butene |
|---|---|---|---|---|---|---|---|---|
| Mass fraction (%) | 0.96 | 0.42 | 27.11 | 10.89 | 14.18 | 10.19 | 27.22 | 9.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yu, P.; Tian, H.; Xiang, S. Selective Oligomerization of Isobutylene in Mixed C4 with Co/BETA-Loaded Molecular Sieve Catalysts. Catalysts 2024, 14, 533. https://doi.org/10.3390/catal14080533
Chen X, Yu P, Tian H, Xiang S. Selective Oligomerization of Isobutylene in Mixed C4 with Co/BETA-Loaded Molecular Sieve Catalysts. Catalysts. 2024; 14(8):533. https://doi.org/10.3390/catal14080533
Chicago/Turabian StyleChen, Xiaoping, Panhu Yu, Hui Tian, and Shuguang Xiang. 2024. "Selective Oligomerization of Isobutylene in Mixed C4 with Co/BETA-Loaded Molecular Sieve Catalysts" Catalysts 14, no. 8: 533. https://doi.org/10.3390/catal14080533





