Facile Synthesis of Ni(OH)2 through Low-Temperature N-Doping for Efficient Hydrogen Evolution
Abstract
:1. Introduction
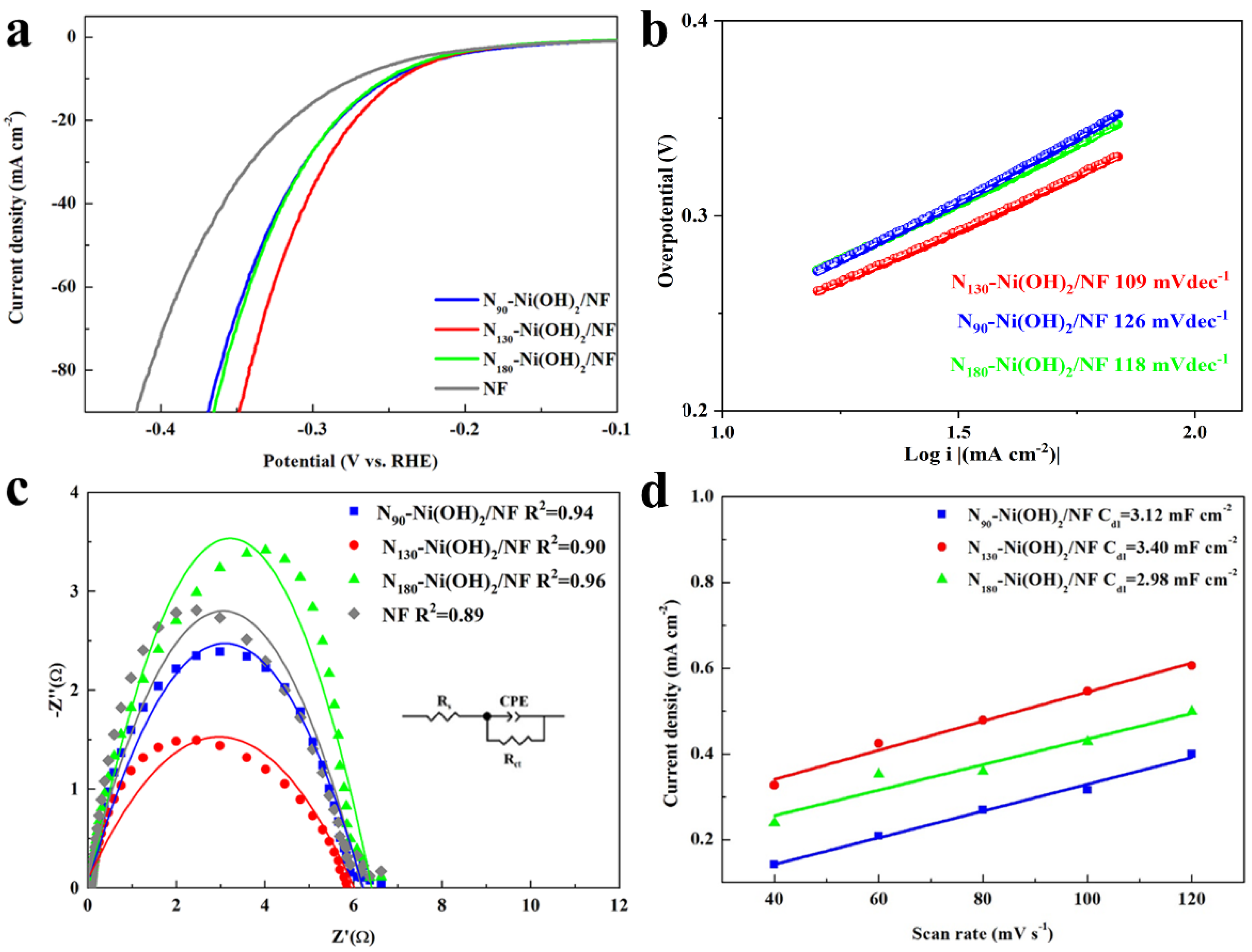
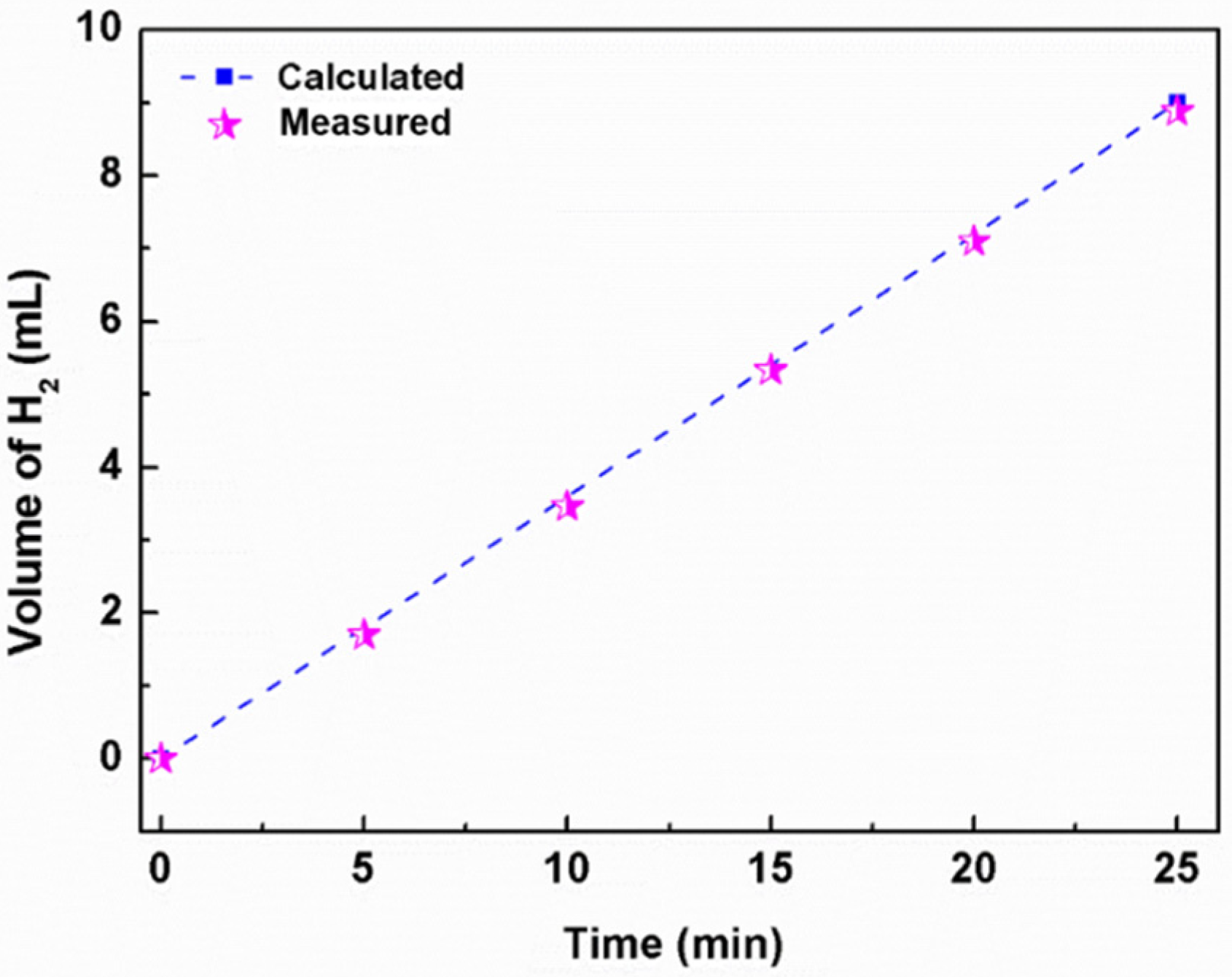
2. Results and Discussion
3. Experimental Section
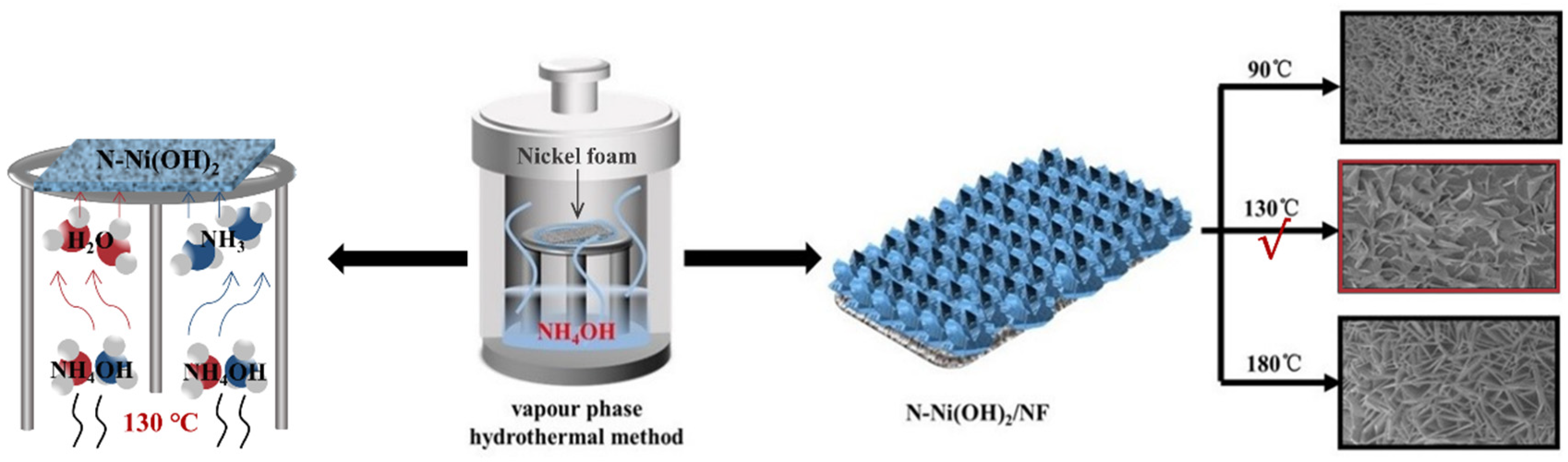
3.1. Synthesis of N-Ni(OH)2/NF
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Ren, X.; Sun, S.; Liu, Z.; Xi, S.; Xu, Z.J. Engineering high-spin state cobalt cations in spinel zinc cobalt oxide for spin channel propagation and active site enhancement in water oxidation. Angew. Chem. Int. Ed. 2021, 60, 14536–14544. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Shamskhou, K.; Awada, H.; Yari, F.; Aljabour, A.; Schofberger, W. A molecular binuclear nickel (II) schiff base complex for efficient HER electrocatalysis. Catalysts 2023, 13, 1348. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, Z.J.; Hu, X.F.; Li, Y.H.; Bao, J.C.; Fang, M.; Wu, Y. CoxMoNyOzHw microrods grown on Ni foam for large-current-density alkaline hydrogen evolution with ultralow overpotential. J. Solid State Chem. 2023, 321, 123870. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, W.L.; Zhao, J.; Fu, J.Y.; Dong, B.; Wang, F.L.; Yu, J.F.; Liu, C.G.; Chai, Y.M. Self-supported FexNi1-xMoO4 with synergistic morphology and composition for efficient overall water splitting at large current density. Chin. Chem. Lett. 2023, 34, 107422. [Google Scholar] [CrossRef]
- An, C.H.; Wang, Y.C.; Jiao, P.G.; Wu, S.; Gao, L.X.; Zhu, C.Y.; Li, J.S.; Hu, N. Se-Doped Ni5P4 nanocatalysts for high-efficiency hydrogen evolution reaction. Catalysts 2022, 12, 1055. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.; Hou, J.; Ma, W.; Jian, T.; Yan, S.; Liu, H. Duplex interpenetrating-phase FeNiZn and FeNi3 heterostructure with low-Gibbs free energy interface coupling for highly efficient overall water splitting. Nano-Micro Lett. 2023, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Exner, K.S.; Vines, F.; Illas, F. Theoretical study of the mechanism of the hydrogen evolution reaction on the V2C MXene: Thermodynamic and kinetic aspects. J. Catal. 2023, 421, 252–263. [Google Scholar] [CrossRef]
- Fu, G.; Kang, X.; Zhang, Y.; Yang, X.; Wang, L.; Fu, X.Z.; Zhang, J.; Luo, J.L.; Liu, J. Coordination effect-promoted durable Ni(OH)2 for energy-saving Hydrogen evolution from water/methanol Co-electrocatalysis. Nano-Micro Lett. 2022, 14, 200. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Liu, H.C.; Zhu, H.Y.; Jiang, X.Y.; Zhu, L.Q.; Li, W.P.; Chen, H.N. Amorphous/amorphous Ni–P/Ni(OH)2 heterostructure nanotubes for an efficient alkaline hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 10169–10179. [Google Scholar] [CrossRef]
- Wu, K.L.; Sun, K.; Liu, S.J.; Cheong, W.C.; Chen, Z.; Zhang, C.; Pan, Y.; Cheng, Y.S.; Zhuang, Z.W.; Wei, X.W.; et al. Atomically dispersed Ni-Ru-P interface sites for high-efficiency pH-universal electrocatalysis of hydrogen evolution. Nano Energy 2021, 80, 105467. [Google Scholar] [CrossRef]
- Qian, L.H.; Dong, W.W.; Xu, M.D.; Cao, Y.B.; Wu, X.; Song, X.J.; Ding, Y.; Wang, X. ZIF-67 derived hybrid CuCo2S4@MoS2 catalyst for efficient electrocatalytic hydrogen evolution reaction. J. Solid State Chem. 2022, 319, 123809. [Google Scholar] [CrossRef]
- Zhong, B.; Wen, S.J.; Kuang, P.Y.; Cheng, B.; Yu, L.; Yu, J.G. Crystalline/amorphous Ni/NixSy supported on hierarchical porous nickel foam for high-current-density hydrogen evolution. Appl. Catal. B Environ. 2024, 340, 123195. [Google Scholar] [CrossRef]
- Chen, M.P.; Liu, D.; Chen, Y.Y.; Liu, D.; Du, X.Y.; Feng, J.X.; Zhou, P.F.; Zi, B.Y.; Liu, Q.J.; Lo, K.H.; et al. Insightful view on the active sites of Ni/NixP for hydrogen evolution reaction. Appl. Mater. Today 2022, 26, 101343. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Wang, H.Y.; Gao, H.T.; Liu, X.E.; Wang, L.; She, X.L.; Zhan, T.R. Controllable synthesis and phase-dependent catalytic performance of dual-phase nickel selenides on Ni foam for overall water splitting. Appl. Catal. B Environ. 2022, 303, 120915. [Google Scholar] [CrossRef]
- Shen, J.J.; Zheng, X.Q.; Peng, L.S.; Waterhouse, G.I.N.; Tan, L.Q.; Yang, J.; Li, L.; Wei, Z.D. Heteroatom modification of nanoporous nickel surfaces for electrocatalytic water splitting. ACS Appl. Nano Mater. 2020, 3, 11298–11306. [Google Scholar] [CrossRef]
- Liu, H.H.; Zeng, S.; He, P.; Dong, F.Q.; He, M.Q.; Zhang, Y.; Wang, S.; Li, C.X.; Liu, M.Z.; Jia, L.P. Samarium oxide modified Ni-Co nanosheets based three-dimensional honeycomb film on nickel foam: A highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 299, 405–414. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Xu, Z.F.; Pan, H.L.; Lin, Y.; Yang, Z.; Wang, J.L. A 3D well-matched electrode pair of Ni-Co-S//Ni-Co-P nanoarrays grown on nickel foam as a high-performance electrocatalyst for water splitting. J. Mater. Chem. A 2018, 6, 12506–12514. [Google Scholar] [CrossRef]
- Battiato, S.; Bruno, L.; Terrasi, A.; Mirabella, S. Superior performances of electroless-deposited Ni-P films decorated with an ultralow content of Pt for water-splitting reactions. ACS Appl. Energy Mater. 2022, 5, 2391–2399. [Google Scholar] [CrossRef]
- Yang, J.H.; Xu, X.H.; Chen, M.M.; Yang, D.; Lu, H.Y.; Sun, Y.Z.; Shao, C.; Song, Q.Q.; Zhang, J.; Gao, L.; et al. Morphology-controllable nanocrystal β-Ni(OH)2/NF designed by hydrothermal etching method as high-efficiency electrocatalyst for overall water splitting. J. Electroanal. Chem. 2021, 882, 115035. [Google Scholar] [CrossRef]
- Liu, J.N.; Cui, J.S.; Sun, J.H.; Liu, H.; Li, W.; Han, C.; Chen, Y.N.; Yang, G.C.; Shan, Y.P. Hierarchical nickel-vanadium nanohybrid with strong electron transfer for accelerated hydrogen evolution reaction. Appl. Surf. Sci. 2020, 528, 146982. [Google Scholar] [CrossRef]
- Ye, F.; Yang, Y.K.; Liu, P.; Feng, Y.C.; Cao, Y.P.; Cao, D.H.; Ta, L.; Ma, X.F.; Xu, C. In-situ porous flake heterostructured NiCoP/Ni foam as electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2022, 423, 140578. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.W.; Liu, Y.X.; Zhang, Y.; Shi, J.W.; Xiong, J.; Zhou, S.F.; Li, J.; Fan, L.Y.; Cai, W.W. Soft-template derived Ni/Mo2C hetero-sheet arrays for large current density hydrogen evolution reaction. J. Colloid Interface Sci. 2023, 635, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Sun, Z.; Zhang, F.; Wang, X.N.; Xu, J.C.; Shi, X.Q.; Wang, S.P.; Pan, H. N and V coincorporated Ni nanosheets for enhanced hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 16525–16531. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Fan, R.Y.; Zhao, Z.Y.; Zhang, X.Y.; Dong, B.; Lv, Q.X.; Liu, X.; Liu, B.; Chai, Y.M. Precipitation/dissolution equilibrium to achieve trace iron doping on the surface of β-Ni(OH)2 for electrocatalytic oxygen evolution. Fuel 2023, 332, 125780. [Google Scholar] [CrossRef]
- Qiao, X.S.; Kang, H.J.; Li, Y.; Cui, K.; Jia, X.; Liu, H.H.; Qin, W.; Pupucevski, M.; Wu, G. Porous Fe-doped β-Ni(OH)2 nanopyramid array electrodes for water splitting. ACS Appl. Mater. Interfaces 2020, 12, 36208–36219. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Zhao, B.; Chen, M.X.; Wang, L.; Fu, X.Z.; Luo, J.L. Electrodeposited porous spherical Ni(OH)2@Ni on carbon paper for high-efficiency hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 1540–1547. [Google Scholar] [CrossRef]
- Shao, D.; Wang, Q.; Yao, X.Z.; Zhou, Y.T.; Yu, X.Y. Phase-engineering of nickel hydroxide in the Ni/Ni(OH)2 interface for efficient hydrogen evolution and hydrazine-assisted water splitting in seawater. J. Mater. Chem. A 2022, 10, 21848–21855. [Google Scholar] [CrossRef]
- Chen, D.C.; Chen, Z.W.; Zhang, X.X.; Lu, Z.L.; Xiao, S.; Xiao, B.B.; Singh, C.V. Exploring single atom catalysts of transition-metal doped phosphorus carbide monolayer for HER: A first-principles study. J. Energy Chem. 2021, 52, 155–162. [Google Scholar] [CrossRef]
- Xu, L.N.; Li, W.; Luo, J.Q.; Chen, L.Y.; He, K.C.; Ma, D.M.; Lv, S.H.; Xing, D.F. Carbon-based materials as highly efficient catalysts for the hydrogen evolution reaction in microbial electrolysis cells: Mechanisms, methods, and perspectives. Chem. Eng. J. 2023, 471, 144670. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Tang, J.; Tanaka, S.; Kaneti, Y.V.; Na, J.; Jiang, B.; Yamauchi, Y.; Bando, Y.; Sugahara, Y. Multiscale structural optimization: Highly efficient hollow iron-doped metal sulfide heterostructures as bifunctional electrocatalysts for water splitting. Nano Energy 2020, 75, 104913. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Kumar, J.S.; Murmu, N.C.; Ganesh, R.S.; Inokawa, H.; Kuila, T. Optimization of active surface area of flower like MoS2 using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium. Appl. Catal. B Environ. 2019, 254, 432–442. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Y.; Liu, S.; Zhang, M.; Guo, Q.; Shen, W.; He, R.; Su, W.; Li, M. Regulating the electron density of dual transition metal sulfide heterostructures for highly efficient hydrogen evolution in alkaline electrolytes. Nanoscale 2019, 11, 14016–14023. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, J.; Zhang, H.; Lu, M.; Peng, Y.; Huang, B.; Xi, P.; Yan, C. Atomic Arrangement in Metal-Doped NiS2 Boosts the Hydrogen Evolution Reaction in Alkaline Media. Angew. Chem. Int. Ed. 2019, 58, 18676–18682. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, A.; Jiao, Y.; Zheng, H.; Wang, X.; Xie, Y.; Wang, L.; Tian, C.; Fu, H. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew. Chem. Int. Ed. 2021, 133, 6747–6755. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Xie, Y.; Sun, B.; Jiang, J.; Jiang, W.-J.; Xi, S.; Yang, H.Y.; Yan, K.; Wang, S.; et al. Constructing Atomic Heterometallic Sites in Ultrathin Nickel-Incorporated Cobalt Phosphide Nanosheets via a Boron-Assisted Strategy for Highly Efficient Water Splitting. Nano Lett. 2021, 21, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.-L.; Shang, X.; Li, Z.; Dong, B.; Li, X.; Gao, W.-K.; Chi, J.-Q.; Chai, Y.-M.; Liu, C.-G. Ternary mixed metal Fe-doped NiCo2O4 nanowires as efficient electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 2017, 416, 371–378. [Google Scholar] [CrossRef]
- Cheng, N.; Liu, Q.; Asiri, A.M.; Xing, W.; Sun, X. A Fe-doped Ni3S2 particle film as a high-efficiency robust oxygen evolution electrode with very high current density. J. Mater. Chem. A 2015, 3, 23207–23212. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhang, K.; Lin, H.L.; Li, X.; Chan, H.C.; Yang, L.C.; Gao, Q.S. MoS2-Ni3S2 heteronanorods as efficient and stable bifunctional electrocatalysts for overall water splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: An efficient 3D electrode for overall water splitting. Energy Environ. Sci. 2015, 9, 478–483. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-Z.; Fan, R.-Y.; Yu, N.; Zhou, Y.-N.; Zhang, X.-Y.; Dong, B.; Yan, Z.-F. Facile Synthesis of Ni(OH)2 through Low-Temperature N-Doping for Efficient Hydrogen Evolution. Catalysts 2024, 14, 534. https://doi.org/10.3390/catal14080534
Liu Z-Z, Fan R-Y, Yu N, Zhou Y-N, Zhang X-Y, Dong B, Yan Z-F. Facile Synthesis of Ni(OH)2 through Low-Temperature N-Doping for Efficient Hydrogen Evolution. Catalysts. 2024; 14(8):534. https://doi.org/10.3390/catal14080534
Chicago/Turabian StyleLiu, Zi-Zhang, Ruo-Yao Fan, Ning Yu, Ya-Nan Zhou, Xin-Yu Zhang, Bin Dong, and Zi-Feng Yan. 2024. "Facile Synthesis of Ni(OH)2 through Low-Temperature N-Doping for Efficient Hydrogen Evolution" Catalysts 14, no. 8: 534. https://doi.org/10.3390/catal14080534




