α-L-Arabinofuranosidases of Glycoside Hydrolase Families 43, 51 and 62: Differences in Enzyme Substrate and Positional Specificity between and within the GH Families
Abstract
:1. Introduction
2. Results
2.1. Arabinofuranosidase Activity on the Chromogenic Substrate
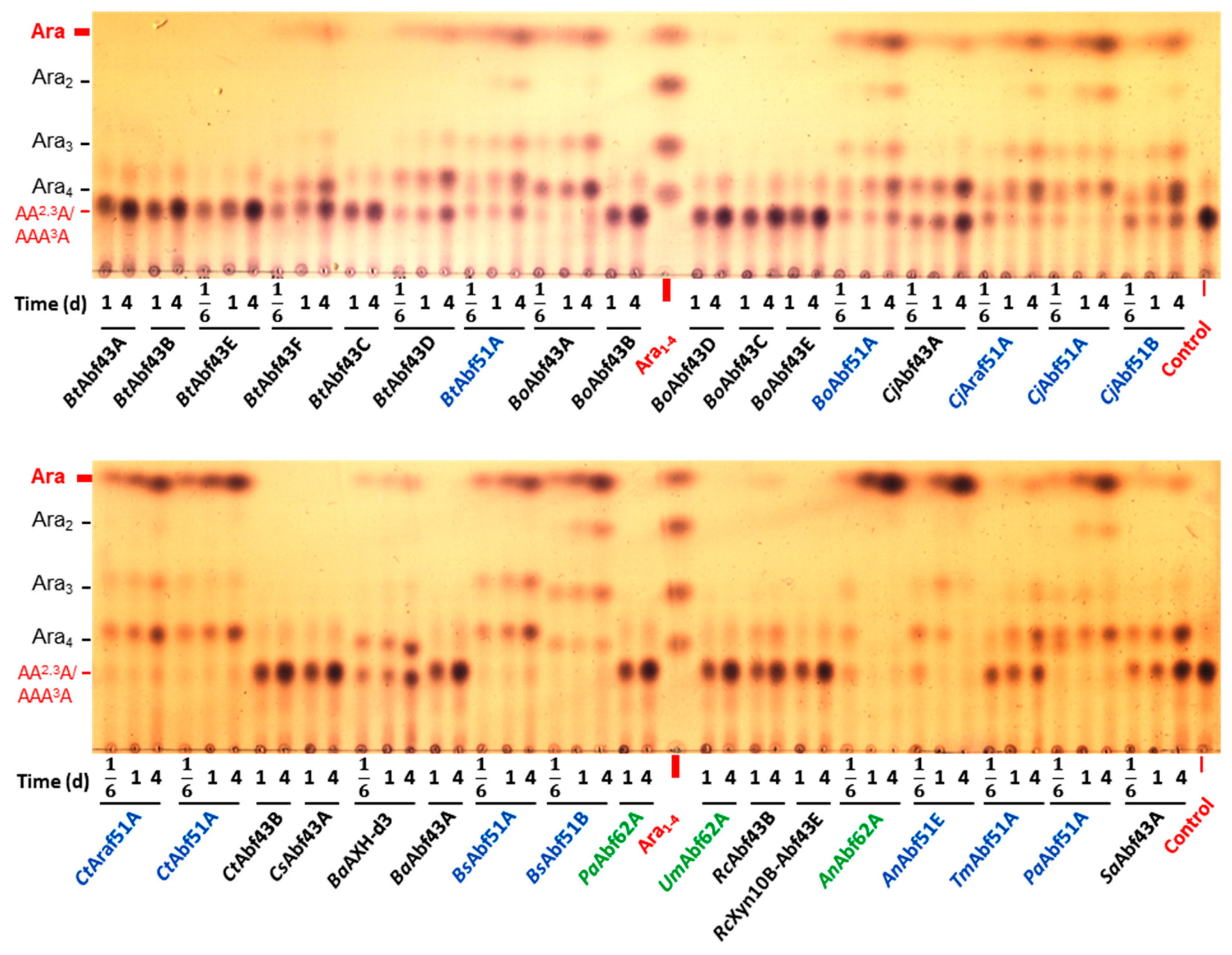
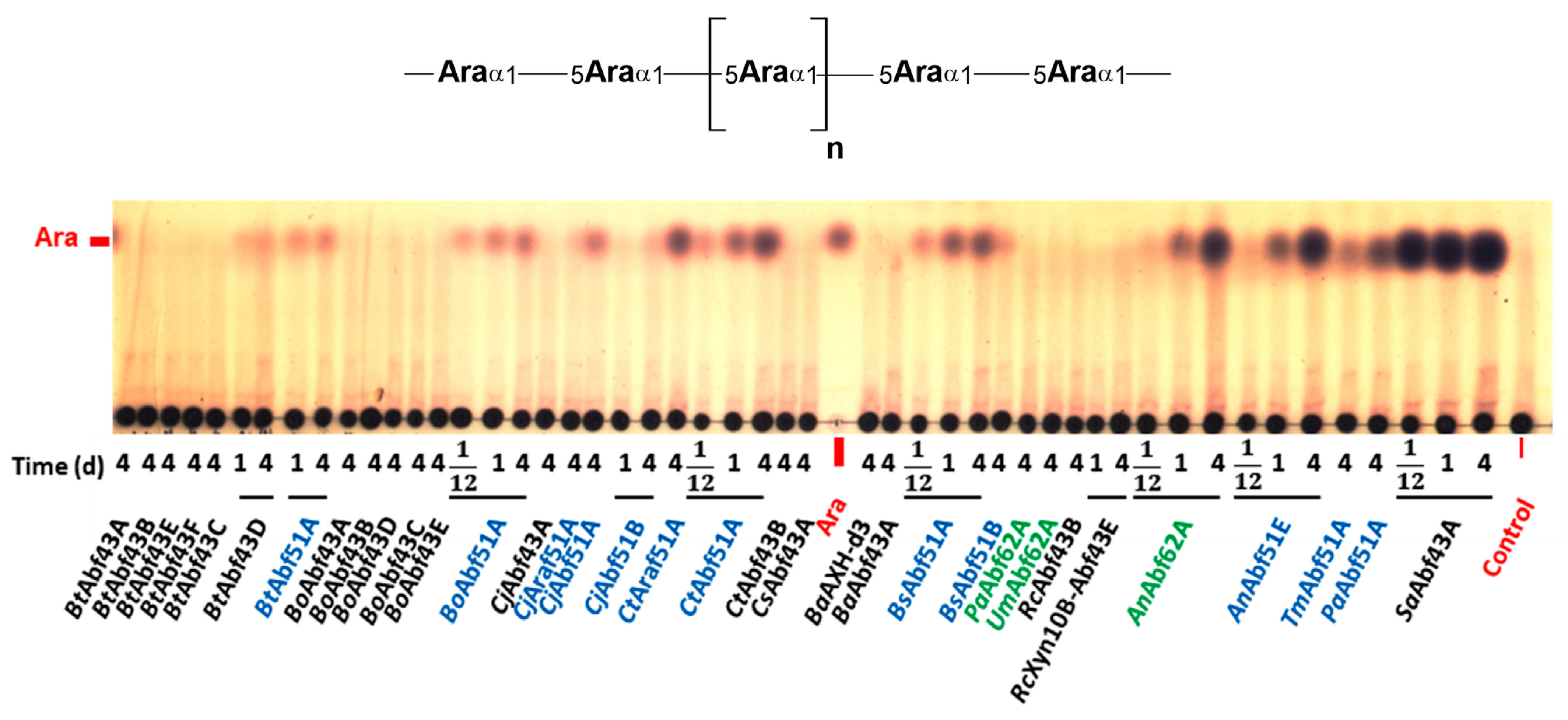
2.2. Specificity of GH43 α-L-Arabinofuranosidases
2.3. Specificity of GH51 α-L-Arabinofuranosidases
2.4. Specificity of GH62 α-L-Arabinofuranosidases
3. Discussion
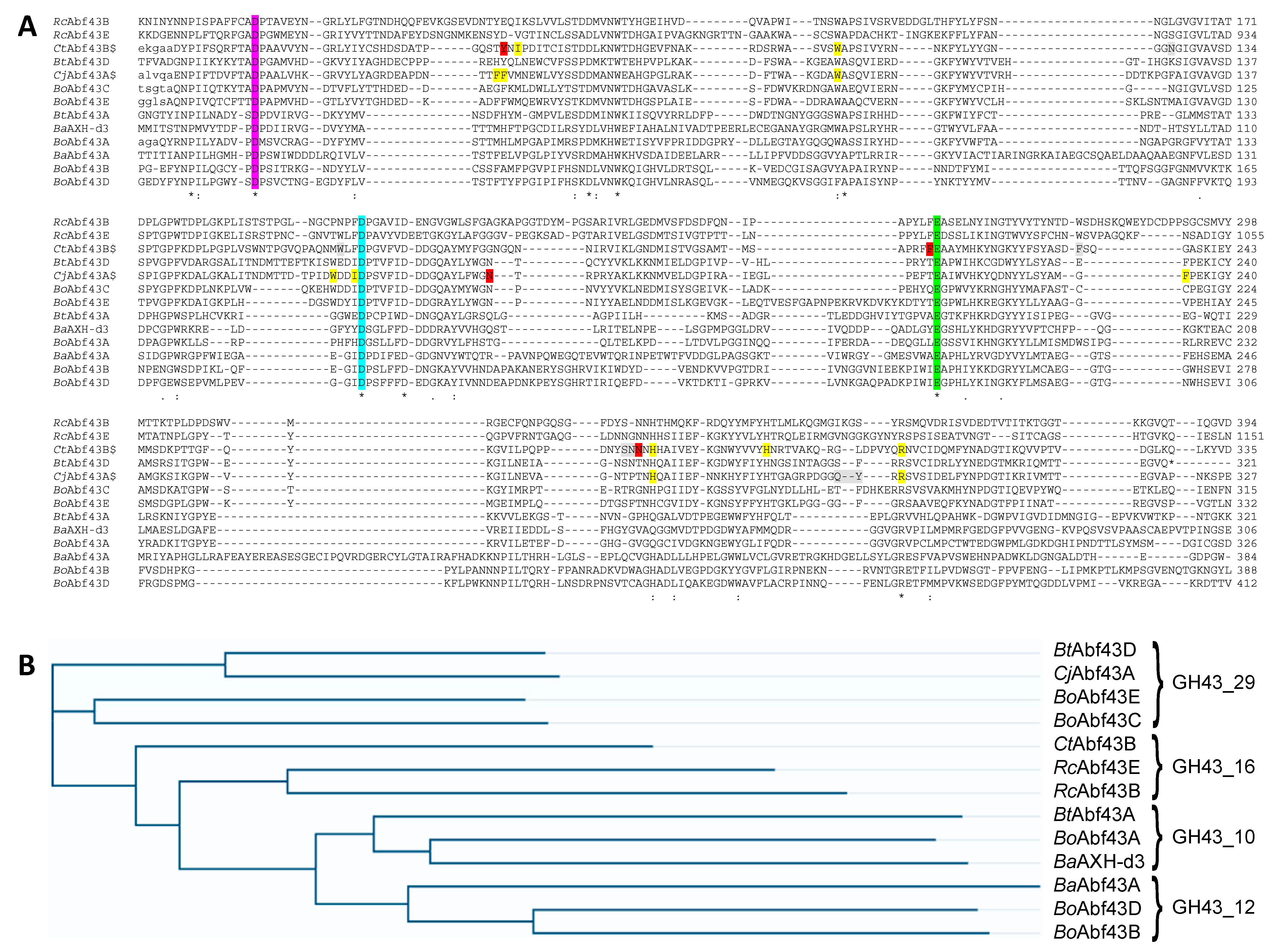
3.1. Specificity of GH43 α-L-Arabinofuranosidases
3.2. Specificity of GH51 α-L-Arabinofuranosidases
3.3. Specificity of GH62 α-L-Arabinofuranosidases
4. Materials and Methods
4.1. Enzymes and Substrates
4.2. Enzyme Assays
4.3. Hydrolysis of Natural Substrates
4.4. Analysis of the Hydrolysates
4.5. Comparison of Amino Acid Sequences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Taylor, E.J.; Smith, N.L.; Turkenburg, J.P.; D’Souza, S.; Gilbert, H.J.; Davies, G.J. Structural insight into the ligand specificity of a thermostable family 51 arabinofuranosidase, Araf51, from Clostridium thermocellum. Biochem. J. 2006, 395, 31–37. [Google Scholar] [CrossRef]
- Beylot, M.-H.; Mckie, V.A.; Voragen, A.G.J.; Doeswijk-Voragen, C.H.L.; Gilbert, H.J. The Pseudomonas cellulosa glycoside hydrolase family 51 arabinofuranosidase exhibits wide substrate specificity. Biochem. J. 2001, 358, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, A.; Briggs, J.A.; Mortimer, J.C.; Tryfona, T.; Terrapon, N.; Lowe, E.C.; Baslé, A.; Morland, C.; Day, A.M.; Zheng, H.; et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun. 2015, 6, 7481. [Google Scholar] [CrossRef]
- Kormelink, F.J.M.; Searl-Van Leeuwen, M.J.F.; Wood, T.M.; Voragen, A.G.J. Purification and characterization of a (1,4)-β-D-arabinoxylan arabinofuranohydrolase from Aspergillus awamori. Appl. Microbiol. Biotechnol. 1991, 35, 753–758. [Google Scholar] [CrossRef]
- Lagaert, S.; Pollet, A.; Courtin, C.M.; Volckaert, G. β-Xylosidases and α-L-arabinofuranosidases: Accessory enzymes for arabinoxylan degradation. Biotechnol. Adv. 2014, 32, 316–332. [Google Scholar] [CrossRef]
- Jones, D.R.; Thomas, D.; Alger, N.; Ghavidel, A.; Inglis, G.D.; Abbott, D.W. SACCHARIS: An automated pipeline to streamline discovery of carbohydrate-active enzyme activities within polyspecific families and de novo sequence datasets. Biotechnol. Biofuels 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Borsenberger, V.; Dornez, E.; Desrousseaux, M.-L.; Massou, S.; Tenkanen, M.; Courtin, C.M.; Dumon, C.; O’Donohue, M.J.; Fauré, R. A 1H NMR study of the specificity of α-L-arabinofuranosidases on natural and unnatural substrates. Biochim. Biophys. Acta 2014, 1840, 3106–3114. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ogura, A.; Inui, M.; Tokuda, S.; Hosokawa, S.; Ihara, H.; Kasai, N. Identification of a GH62 α-L-arabinofuranosidase specific for arabinoxylan produced by Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2011, 90, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, A.; McKee, L.S.; Peña, M.J.; Larsbrink, J.; Brumer, H.; Kaneko, S.; Ichinose, H.; Lewis, R.J.; Viksø-Nielsen, A.; Gilbert, H.J.; et al. The structure and function of an arabinan-specific α-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases. J. Biol. Chem. 2011, 286, 15483–15495. [Google Scholar] [CrossRef]
- Goyal, A.; Ahmed, S.; Sharma, K.; Gupta, V.; Bule, P.; Alves, V.D.; Fontes, C.M.G.A.; Najmudin, S. Molecular determinants of substrate specificity revealed by the structure of Clostridium thermocellum arabinofuranosidase 43A from glycosyl hydrolase family 43 subfamily 16. Acta Cryst. 2016, D72, 1281–1289. [Google Scholar] [CrossRef]
- Lagaert, S.; Pollet, A.; Delcour, J.A.; Lavigne, R.; Courtin, C.M.; Volckaert, G. Substrate specificity of three recombinant α-L-arabinofuranosidases from Bifidobacterium adolescentis and their divergent action on arabinoxylan and arabinoxylan oligosaccharides. Biochem. Biophys. Res. Commun. 2010, 402, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Till, M.; Goldstone, D.; Card, G.; Attwood, G.T.; Moon, C.D.; Arcus, V.L. Structural analysis of the GH43 enzyme Xsa43E from Butyrivibrio proteoclasticus. Acta Cryst. 2014, F70, 1193–1198. [Google Scholar] [CrossRef]
- Leschonski, K.P.; Kaasgaard, S.G.; Spodsberg, N.; Krogh, K.B.R.M.; Kabel, M.A. Two subgroups within the GH43_36 α-L-arabinofuranosidase subfamily hydrolyze arabinosyl from either mono- or disubstituted xylosyl units in wheat arabinoxylan. Int. J. Mol. Sci. 2022, 23, 13790. [Google Scholar] [CrossRef]
- Sørensen, H.; Jørgensen, C.T.; Hansen, C.H.; Jørgensen, C.I.; Pedersen, S.; Meyer, A.S. A novel GH43 α-L-arabinofuranosidase from Humicola insolens: Mode of action and synergy with GH51 α-L-arabinofuranosidases on wheat arabinoxylan. Appl. Microbiol. Biotechnol. 2022, 73, 850–861. [Google Scholar] [CrossRef]
- Van Laere, K.; Beldman, G.; Voragen, A.G.J. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl. Microbiol. Biotechnol. 1997, 47, 231–235. [Google Scholar] [CrossRef]
- Mewis, K.; Lenfant, N.; Lombard, V.; Henrissat, B. Dividing the large glycoside hydrolase family 43 into subfamilies: A motivation for detailed enzyme characterization. Appl. Environ. Microbiol. 2016, 82, 1686–1692. [Google Scholar] [CrossRef]
- Moraïs, S.; David, Y.B.; Bensoussan, L.; Duncan, S.H.; Koropatkin, N.M.; Martens, E.C.; Flint, H.J.; Bayer, E.A. Enzymatic profiling of cellulosomal enzymes from the human gut bacterium, Ruminococcus champanellensis, reveals a fine-tuned system for cohesin-dockerin recognition. Environ. Microbiol. 2016, 18, 542–556. [Google Scholar] [CrossRef]
- Sakka, K.; Yoshikawa, K.; Kojima, Y.; Karita, S.; Ohmiya, K.; Shimada, K. Nucleotide sequence of the Clostridium stercorarium xylA gene encoding a bifunctional protein with β-D-xylosidase and α-L-arabinofuranosidase activities, and properties of the translated product. Biosci. Biotechnol. Biochem. 1993, 57, 268–272. [Google Scholar] [CrossRef]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Luis, A.S.; Baslé, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70. [Google Scholar] [CrossRef]
- Ichinose, H.; Yoshida, M.; Fujimoto, Z.; Kaneko, S. Characterization of a modular enzyme of exo-1,5-α-L-arabinofuranosidase and arabinan binding module from Streptomyces avermitilis NBRC14893. Appl. Microbiol. Biotechnol. 2008, 80, 399–408. [Google Scholar] [CrossRef]
- Fujimoto, Z.; Ichinose, H.; Maehara, T.; Honda, M.; Kitaoka, M.; Kaneko, S. Crystal structure of an exo-1,5-α-L-arabinofuranosidase from Streptomyces avermitilis provides insights into the mechanism of substrate discrimination between exo- and endo-type enzymes in glycoside hydrolase family 43. J. Biol. Chem. 2010, 285, 34134–34143. [Google Scholar] [CrossRef] [PubMed]
- McKee, L.S.; Peña, M.J.; Rogowski, A.; Jackson, A.; Lewis, R.J.; York, W.S.; Krogh, K.B.R.M.; Viksø-Nielsen, A.; Skjøt, M.; Gilbert, H.J.; et al. Introducing endo-xylanase activity into an exo-acting arabinofuranosidase that targets side chains. Proc. Natl. Acad. Sci. USA 2012, 109, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Hoffmam, Z.B.; Oliveira, L.C.; Cota, J.; Alvarez, T.M.; Diogo, J.A.; Neto, M.O.; Citadini, A.P.S.; Leite, V.B.P.; Squina, F.M.; Murakami, M.T.; et al. Characterization of a hexameric exo-acting GH51 α-L-arabinofuranosidase from the mesophilic Bacillus subtilis. Mol. Biotechnol. 2013, 55, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Sano, M.; Kusakabe, I. Purification and some properties of α-L-arabinofuranosidase from Bacillus subtilis 3-6. Appl. Environ. Microbiol. 1994, 60, 3425–3428. [Google Scholar] [CrossRef] [PubMed]
- Inácio, J.M.; Correia, I.L.; de Sá-Nogueira, I. Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in Bacillus subtilis. Microbiology 2008, 154, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K. Hyperthermophilic α-L-arabinofuranosidase from Thermotoga maritima MSB8: Molecular cloning, gene expression, and characterization of the recombinant protein. Extremophiles 2005, 9, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stott, K.; et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 2018, 3, 210–219. [Google Scholar] [CrossRef]
- Couturier, M.; Haon, M.; Coutinho, P.M.; Henrissat, B.; Lesage-Meessen, L.; Berrin, J.-G. Podospora anserina hemicellulases potentiate the Trichoderma reesei secretome for saccharification of lignocellulosic biomass. Appl. Environ. Microbiol. 2011, 77, 237–246. [Google Scholar] [CrossRef]
- Siguier, B.; Haon, M.; Nahoum, V.; Marcellin, M.; Burlet-Schiltz, O.; Coutinho, P.M.; Henrissat, B.; Mourey, L.; O’Donohue, M.J.; Berrin, J.-G.; et al. First structural insights into α-L-arabinofuranosidases from the two GH62 glycoside hydrolase subfamilies. J. Biol. Chem. 2014, 289, 5261–5273. [Google Scholar] [CrossRef]
- McCleary, B.V.; McKie, V.A.; Draga, A.; Rooney, E.; Mangan, D.; Larkin, J. Hydrolysis of wheat flour arabinoxylan, acid-debranched wheat flour arabinoxylan and arabino-xylo-oligosaccharides by β-xylanase, α-L-arabinofuranosidase and β-xylosidase. Carbohydr. Res. 2015, 407, 79–96. [Google Scholar] [CrossRef]
- Wilkens, C.; Andersen, S.; Petersen, B.O.; Li, A.; Busse-Wicher, M.; Birch, J.; Cockburn, D.; Nakai, H.; Christensen, H.E.M.; Kragelund, B.B.; et al. An efficient arabinoxylan-debranching α-L-arabinofuranosidase of family GH62 from Aspergillus nidulans contains a secondary carbohydrate binding site. Appl. Microbiol. Biotechnol. 2016, 100, 6265–6277. [Google Scholar] [CrossRef]
- Contesini, F.J.; Liberato, M.V.; Rubio, M.V.; Calzado, F.; Zubieta, M.P.; Riaño-Pachón, D.M.; Squina, F.M.; Bracht, F.; Skaf, M.S.; Damasio, A.R. Structural and functional characterization of a highly secreted α-L-arabinofuranosidase (GH62) from Aspergillus nidulans grown on sugarcane bagasse. Biochim. Biophys. Acta 2017, 1865, 1758–1769. [Google Scholar] [CrossRef]
- Aspeborg, H.; Coutinho, P.M.; Wang, Y.; Brumer, H., III; Henrissat, B. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Ecol. Evol. 2012, 12, 186. [Google Scholar] [CrossRef]
- Orlando, M.; Buchholz, P.C.F.; Lotti, M.; Pleiss, J. The GH19 Engineering Database: Sequence diversity, substrate scope, and evolution in glycoside hydrolase family 19. PLoS ONE 2021, 16, e0256817. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Orlando, M.; Bombardi, L.; Fusco, S.; Mangiagalli, M.; Lotti, M. Evolutionary history and activity towards oligosaccharides and polysaccharides of GH3 glycosidases from an Antarctic marine bacterium. Int. J. Biol. Macromol. 2024, 275, 133449. [Google Scholar] [CrossRef]
- Lebreton, A.; Vuillemin, M.; Pilgaard, B.; Hornung, B.V.H.; Drula, E.; Lombard, V.; Garron, M.-L.; Helbert, W.; Henrissat, B.; Terrapon, N. Subdivision of Family GH2 for improved functional prediction. In Proceedings of the 15th Carbohydrate Bioengineering Meeting, Ghent, Belgium, 5–8 May 2024; Ghent University: Ghent, Belgium, 2024; p. 230. [Google Scholar]
- Pilgaard, B.; Vuillemin, M.; Gippert, G.P.; Fjermedal, S.; Vincentelli, R.; Henrissat, B. Large scale functional exploration of underexplored subfamilies in the GH5 family. In Proceedings of the 15th Carbohydrate Bioengineering Meeting, Ghent, Belgium, 5–8 May 2024; Ghent University: Ghent, Belgium, 2024; p. 23. [Google Scholar]
- De Doncker, M.; De Graeve, C.; Franceus, J.; Beerens, K.; Křen, V.; Pelantová, H.; Vercauteren, R.; Desmet, T. Exploration of GH94 sequence space for enzyme discovery reveals a novel glucosylgalactose phosphorylase specificity. ChemBioChem 2021, 22, 3319–3325. [Google Scholar] [CrossRef]
- Meitil, I.K.S.; Gippert, G.P.; Barrett, K.; Hunt, C.J.; Henrissat, B. Diversity of sugar-diphospholipid-utilizing glycosyltransferase families. Commun. Biol. 2024, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Dilokpimol, A.; Mäkelä, M.R.; Aguilar-Pontes, M.V.; Benoit-Gelber, I.; Hildén, K.S.; de Vries, R.P. Diversity of fungal feruloyl esterases: Updated phylogenetic classification, properties, and industrial applications. Biotechnol. Biofuels 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
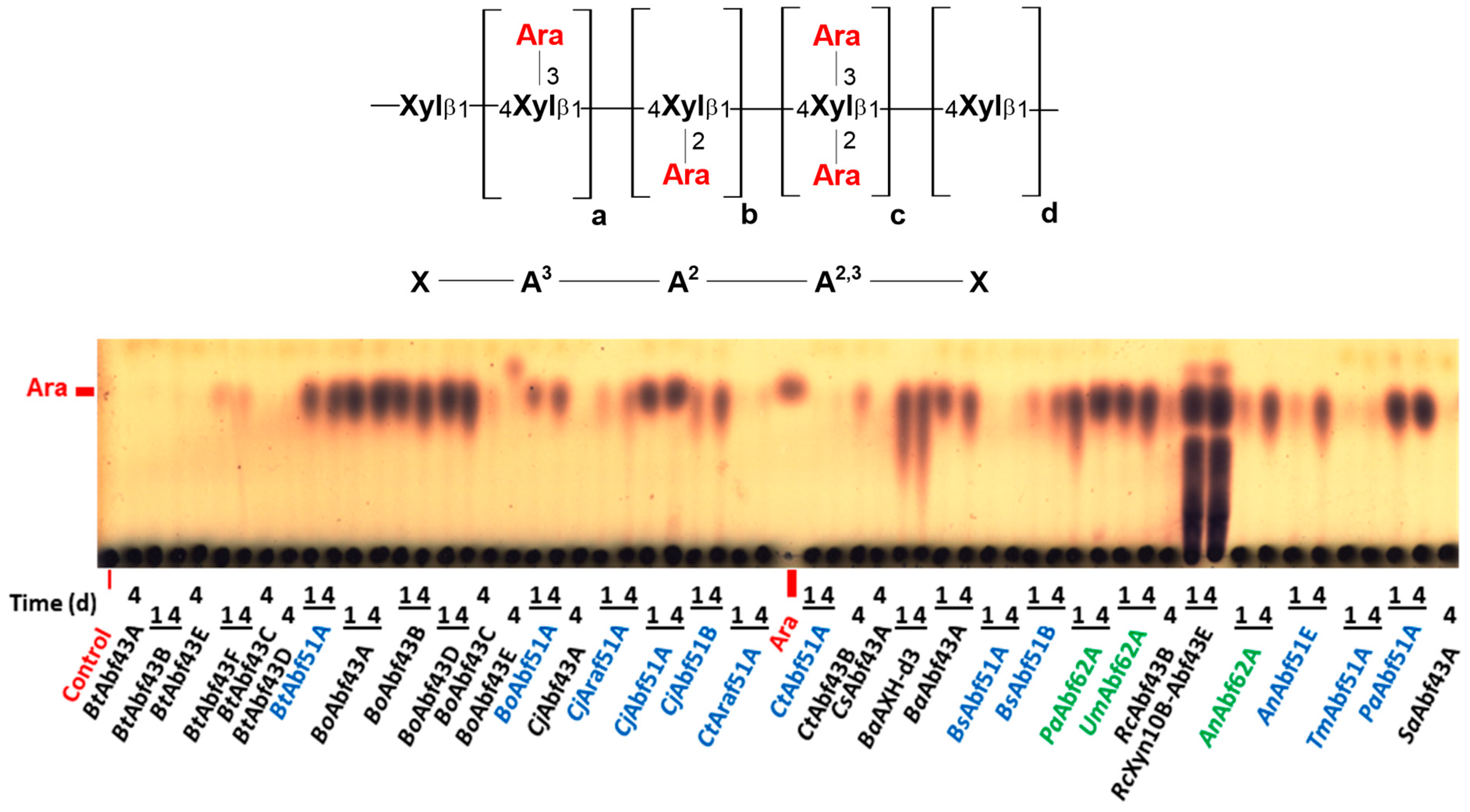
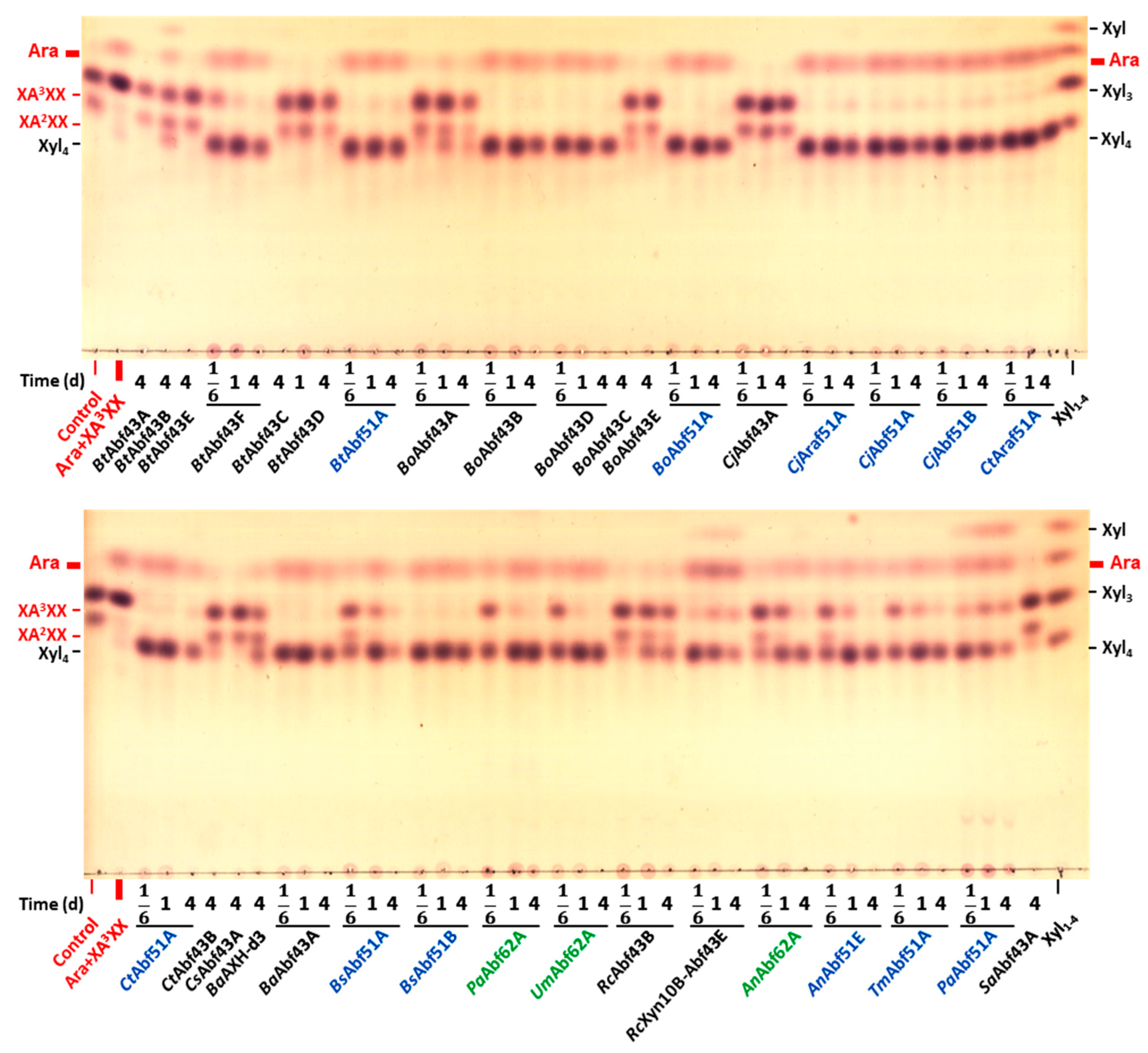
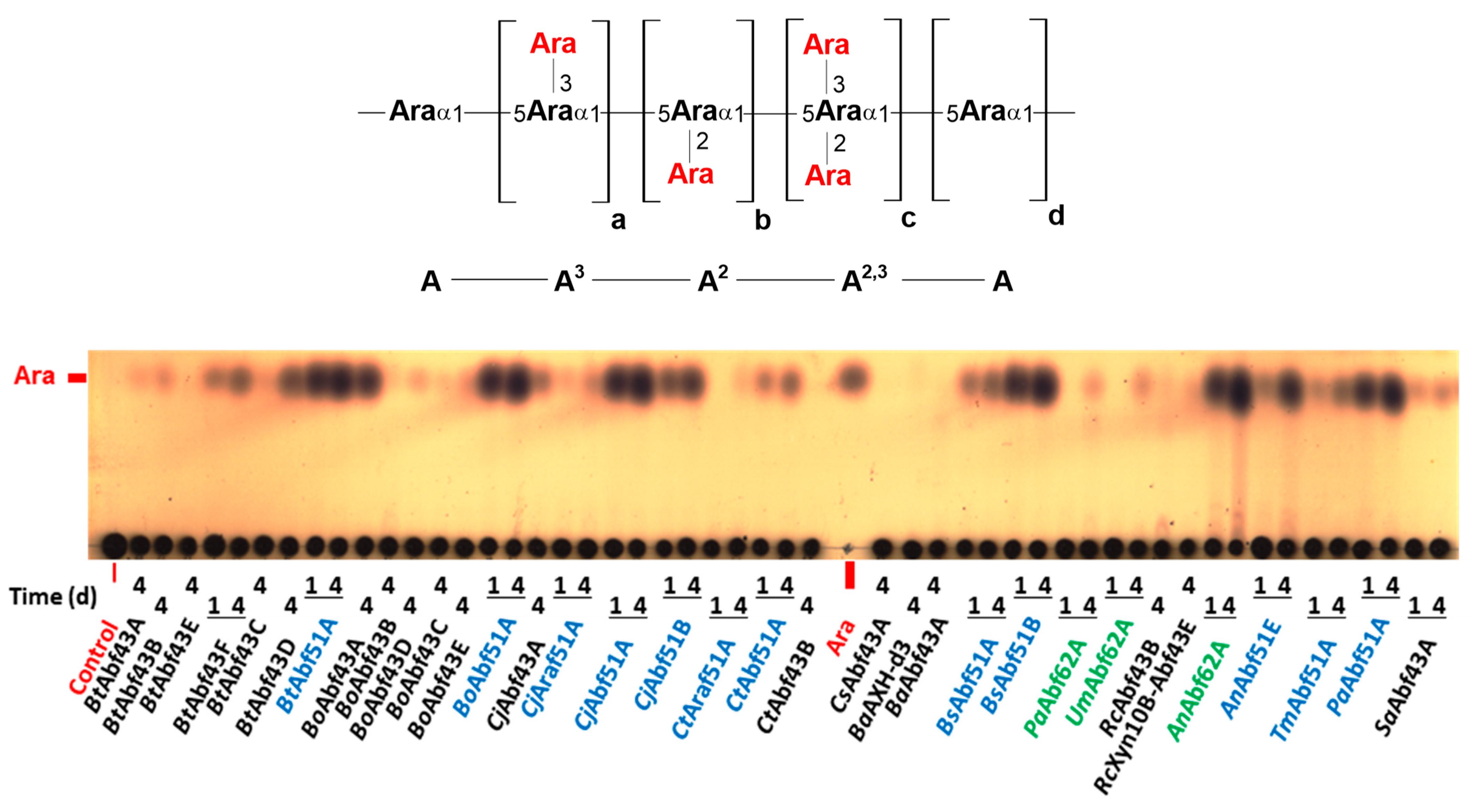
| Catalogue Number (a) | Abbreviated Enzyme Name | Organism | GH (Sub)Family Classification | Gene | GenBank Accession | Uniprot Accession | Specific Activity (mU/mg) |
|---|---|---|---|---|---|---|---|
| E-AFASE | AnAbf51E | Aspergillus niger CBS 513.88 | 51_2 | abfE | ACE00420.1 | B3GQR2 | 3620 |
| E-ABFAN | AnAbf62A | Aspergillus nidulans FGSC A4 | 62 | AN7908.2 | EAA59562.1 | Q5AUX2 | 1880 |
| CZ0951 | BaAbf43A | Bifidobacterium adolescentis ATCC 15703 | 43_12 | BAD_0423 | BAF39204.1 | A1A0H1 | 319 |
| E-AFAM2 | BaAXH-d3 | Bifidobacterium adolescentis ATCC 15703 | 43_10 | BAD_0301 | AAO67499.1 | Q5JB56 | 15.9 |
| CZ0485 | BoAbf43A | Bacteroides ovatus ATCC 8483 | 43_10 | BACOVA_03417 | ALJ48320.1 | A7LZZ1 | 4630 |
| CZ0487 | BoAbf43B | Bacteroides ovatus ATCC 8483 | 43_12 | BACOVA_03421 | ALJ48322.1 | A7LZZ4 | 421 |
| CZ0489 | BoAbf43C | Bacteroides ovatus ATCC 8483 | 43_29 | BACOVA_03424 | ALJ48325.1 | A7LZZ7 | 47.0 |
| CZ0490 | BoAbf43D | Bacteroides ovatus ATCC 8483 | 43_12 | BACOVA_03425 | ALJ48326.1 | A7LZZ8 | 86.5 |
| CZ0492 | BoAbf43E | Bacteroides ovatus ATCC 8483 | 43_29 | BACOVA_03436 | ALJ48337.1 | A7M009 | 347 |
| CZ0778 | BoAbf51A | Bacteroides ovatus V975 (ATCC 8483) | 51_1 | asdII (BACOVA_01708) | AAA50393.1 (EDO12566.1) | Q59219 (A7LV58) | 74,200 |
| CZ0807 | BsAbf51A | Bacillus subtilis subsp. subtilis str. 168 | 51_1 | abfA BSU_28720 | CAA99595.1 | P94531 | 87,700 |
| CZ0771 | BsAbf51B | Bacillus subtilis subsp. subtilis str. 168 | 51_1 | abf2 BSU_28510 | CAA99576.1 | P94552 | 118,000 |
| CZ0136 | BtAbf43A | Bacteroides thetaiotaomicron VPI-5482 | 43_10 | Bt2852 | AAO77958.1 | Q8A3V2 | 3.60 |
| CZ0137 | BtAbf43B | Bacteroides thetaiotaomicron VPI-5482 | 43_19 | Bt3655 | AAO78760.1 | Q8A1K6 | 110 |
| CZ0138 | BtAbf43C | Bacteroides thetaiotaomicron VPI-5482 | 43_NC | BT3094 | AAO78200.1 | Q8A361 | 72.0 |
| CZ0201 | BtAbf43D | Bacteroides thetaiotaomicron VPI-5482 | 43_29 | BT0369 | AAO75476.1 | Q8AAU5 | 10,400 |
| CZ0826 | BtAbf43E | Bacteroides thetaiotaomicron VPI-5482 | 43_18 | BT1021 | AAO76128.1 | Q8A8Z6 | 6.20 |
| CZ0882 | BtAbf43F | Bacteroides thetaiotaomicron VPI-5482 | 43_34 | BT3662 | AAO78767.1 | Q8A1J9 | 5320 |
| CZ0841 | BtAbf51A | Bacteroides thetaiotaomicron VPI-5482 | 51_1 | BT0348 | AAO75455.1 | Q8AAW4 | 95,300 |
| CZ0198 | CjAbf43A | Cellvibrio japonicus Ueda107 | 43_29 | CJA_3018 | ACE83886.1 | B3PD60 | 39,100 |
| CZ0554 | CjAbf51A | Cellvibrio japonicus | 51_1 | abfA | AAK84947.1 | Q93LE0 | 52,900 |
| CZ0707 | CjAbf51B | Cellvibrio japonicus Ueda 107 | 51_1 | abf51A CJA_2769 | ACE86344.1 | B3PBK2 | 67,900 |
| E-ABFCJ | CjAraf51A | Cellvibrio japonicus Ueda107 | 51_1 | CJA_2769 | ACE86344.1 | B3PBK2 | 7070 |
| CZ0329 | CsAbf43A | Thermoclostridium stercorarium F-9 | 43_35 | xylA | BAA02527.1 | P48790 | 8.99 |
| CZ0209 | CtAbf43B | Acetivibrio thermocellus ATCC 27405 | 43_16 | Cthe_1271 | ABN52503.1 | A3DEX4 | 8.04 |
| CZ0024 | CtAbf51A | Acetivibrio thermocellus ATCC 27405 | 51_1 | Cthe_2548 | ABN53749.1 | A3DIH0 | 45,900 |
| E-ABFCT | CtAraf51A | Acetivibrio thermocellus ATCC 27405 | 51_1 | Cthe_2548 | ABN53749.1 | A3DIH0 | 17,800 |
| CZ0291 | PaAbf51A | Podospora anserina S mat+ | 51_2 | abf51A | CAP62201.1 | B2AFI2 | 27,000 |
| CZ0292 | PaAbf62A | Podospora anserina S mat+ | 62 | Pa_0_1370 | CAP62336.1 | B2AFW5 | 10.5 |
| CZ0903 | RcAbf43B | Ruminococcus champanellensis 18P13 | 43_16 | RUM_14020 | CBL17514.1 | D4LD19 | 367 |
| CZ0995 | RcXyn10B-Abf43E (b) | Ruminococcus champanellensis 18P13 | 43_16 | RUM_15940 | CBL17682.1 | D4LDI7 | 907 |
| CZ0313 | SaAbf43A | Streptomyces avermitilis MA-4680 | 43_26 | SAV1043 | BAC68753.1 | Q82P90 | 10,500 |
| CZ0805 | TmAbf51A | Thermotoga maritima MSB8 | 51_1 | TM0281 | AAD35369.1 | Q4R1J9 | 5950 |
| E-ABFUM | UmAbf62A | Ustilago maydis 521 | 62 | UM04309 | KIS67202.1 (c) | A0A0D1BZQ4 | 27.9 |
| Abbreviated Enzyme Name | Subfamily | WAX | RAX | AXOS | DA | Linear AOS | SBA | Branched AOS | AG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A3X | A2XX | XA3XX | XA2XX | A2,3XX | XA2,3XX | Ara2 | Ara3 | Ara4 | AA3A | AAA3A | AA2,3A | |||||||
| BaAXH-d3 | GH43_10 | 229 | 172 | n.d. | n.d. | n.d. | n.d. | 245 | 179 | n.d. | n.d. | n.d. | n.d. | 10.3 | n.d. | n.d. | 202 | n.d. |
| BoAbf43A | 344 | 115 | n.d. | n.d. | n.d. | n.d. | 306 | 256 | n.d. | n.d. | n.d. | n.d. | 71.6 | n.d. | n.d. | 331 | n.d. | |
| BtAbf43A | n.d. | n.d. | n.d. | n.d. | <6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <6 | n.d. | n.d. | n.d. | n.d. | |
| BaAbf43A | GH43_12 | 40.1 | 103 | 958 | 328 | 199 | 178 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BoAbf43B | 504 | 458 | 1010 | 686 | 429 | 368 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| BoAbf43D | 286 | 258 | 494 | 343 | 214 | 184 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CtAbf43B | GH43_16 | 138 | 206 | n.d. | n.d. | 10.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| RcAbf43B | n.d. | n.d. | 151 | 9.53 | 20.4 | 19.9 | 7.66 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 11.1 | 6.13 | n.d. | <6 | |
| RcXyn10B-Abf43E (a) | 1150 | 1030 | 504 | 229 | 355 | 331 | <6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <6 | 20.4 | n.d. | n.d. | |
| BtAbf43E | GH43_18 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BtAbf43B | GH43_19 | n.d. | <6 | 353 | n.d. | 10.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 11.5 | 7.94 | <6 | n.d. | 57.3 |
| SaAbf43A | GH43_26 | n.d. | n.d. | <6 | n.d. | n.d. | n.d. | n.d. | n.d. | 9630 | 1480 | 1010 | 763 | 57.3 | n.d. | 98.0 | n.d. | n.d. |
| BoAbf43C | GH43_29 | 40.1 (b) | 28.6 (b) | 25.2 | n.d. | <6 | <6 | <6 | 17.1 | n.d. | n.d. | n.d. | n.d. | n.d. | <6 | 6.13 | 9.19 | 17.2 |
| BoAbf43E | 114 (b) | 45.8 (b) | 6.30 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 8.59 | <6 | <6 | n.d. | <6 | |
| BtAbf43D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 34.4 | n.d. | n.d. | n.d. | 28.6 | 12.7 | 24.5 | 221 | n.d. | |
| CjAbf43A | n.d. | n.d. | n.d. | 12.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 68.8 | n.d. | n.d. | 588 | n.d. | |
| BtAbf43F | GH43_34 | 68.8 | 6.55 | 1310 | 458 | 245 | 349 | 30.6 | 154 | n.d. | n.d. | n.d. | n.d. | 160 | 610 | 147 | n.d. | 34.4 |
| CsAbf43A | GH43_35 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BtAbf43C | GH43_NC | n.d. | n.d. | <6 | n.d. | <6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 19.1 | 16.3 | n.d. | 22.9 |
| Abbreviated Enzyme Name | GH Family | WAX | RAX | AXOS | DA | Linear AOS | SBA | Branched AOS | AG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A3X | A2XX | XA3XX | XA2XX | A2,3XX | XA2,3XX | Ara2 | Ara3 | Ara4 | AA3A | AAA3A | AA2,3A | |||||||
| BoAbf51A | GH51_1 | 92.8 | 67.6 | 967 | 313 | 208 | 181 | 40.8 | n.d. | 550 | 210 | 336 | 381 | 539 | 763 | 172 | n.d. | 11.5 |
| BsAbf51A | n.d. | n.d. | 4030 | 763 | 723 | 441 | 28.3 | n.d. | 3300 | 5330 | 4030 | 3050 | 321 | 3040 | 980 | 1470 | 19.5 | |
| BsAbf51B | 115 | 22.9 | 2020 | 381 | 466 | 346 | 51 | n.d. | 28.6 | 247 | 151 | 153 | 516 | 1530 | 490 | 735 | n.d. | |
| BtAbf51A | 172 | 97.4 | 927 | 343 | 225 | 175 | 123 | 17.1 | 57.3 | 185 | 504 | 419 | 344 | 763 | 32.7 | n.d. | 28.6 | |
| CjAraf51A | 51.6 | 24.1 | 995 | 240 | 199 | 177 | 245 | n.d. | 12.0 | 37.0 | 42.0 | 31.8 | 34.4 | 381 | 123 | 148 | n.d. | |
| CjAbf51A | 286 | 109 | 1010 | 267 | 208 | 184 | 276 | n.d. | 20.1 | 173 | 353 | 343 | 441 | 763 | 221 | 331 | <6 | |
| CjAbf51B | 321 | 65.5 | 4030 | 1220 | 735 | 712 | 980 | n.d. | 34.4 | 123 | 202 | 254 | 458 | 2750 | 196 | 294 | n.d. | |
| CtAbf51A | n.d. | n.d. | 974 | 343 | 165 | 179 | 30.6 | n.d. | 100 | 1410 | 1010 | 763 | 68.8 | 763 | 245 | 368 | n.d. | |
| CtAraf51A | <6 | n.d. | 933 | 329 | 159 | 167 | 27.5 | n.d. | 85.9 | 1330 | 961 | 746 | 10.6 | 757 | 239 | 348 | n.d. | |
| TmAbf51A | 45.8 | 13.1 | 3630 | 610 | 613 | 740 | 598 | n.d. | 114 | 493 | 1210 | 915 | 91.7 | 610 | 196 | n.d. | n.d. | |
| AnAbf51E | GH51_2 | 45.8 | 59.0 | 958 | 114 | 123 | 110 | 30.6 | n.d. | 229 | 221 | 555 | 378 | 71.6 | 458 | 208 | 312 | <6 |
| PaAbf51A | 1030 | 413 | 2020 | 458 | 404 | 368 | 245 | n.d. | 172 | 271 | 633 | 534 | 401 | 1520 | 490 | 705 | <6 | |
| AnAbf62A | GH62 | 57.3 | 32.7 | 876 | 76.3 | 20.4 | 92.9 | 20.4 | n.d. | 110 | 185 | 504 | 455 | 229 | 648 | 180 | 294 | 17.2 |
| PaAbf62A | 201 | 235 | 212 | 95.3 | 135 | 165 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 14.3 | n.d. | n.d. | n.d. | <6 | |
| UmAbf62A | 258 | 228 | 252 | 153 | 195 | 171 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 15.8 | n.d. | n.d. | n.d. | n.d. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathallah, W.; Puchart, V. α-L-Arabinofuranosidases of Glycoside Hydrolase Families 43, 51 and 62: Differences in Enzyme Substrate and Positional Specificity between and within the GH Families. Catalysts 2024, 14, 536. https://doi.org/10.3390/catal14080536
Fathallah W, Puchart V. α-L-Arabinofuranosidases of Glycoside Hydrolase Families 43, 51 and 62: Differences in Enzyme Substrate and Positional Specificity between and within the GH Families. Catalysts. 2024; 14(8):536. https://doi.org/10.3390/catal14080536
Chicago/Turabian StyleFathallah, Walid, and Vladimír Puchart. 2024. "α-L-Arabinofuranosidases of Glycoside Hydrolase Families 43, 51 and 62: Differences in Enzyme Substrate and Positional Specificity between and within the GH Families" Catalysts 14, no. 8: 536. https://doi.org/10.3390/catal14080536






