Catalytic Effect of Alkali Metal Ions on the Generation of CO and CO2 during Lignin Pyrolysis: A Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Binding Sites between Na+/K+ and Lignin Monomer Model Compounds
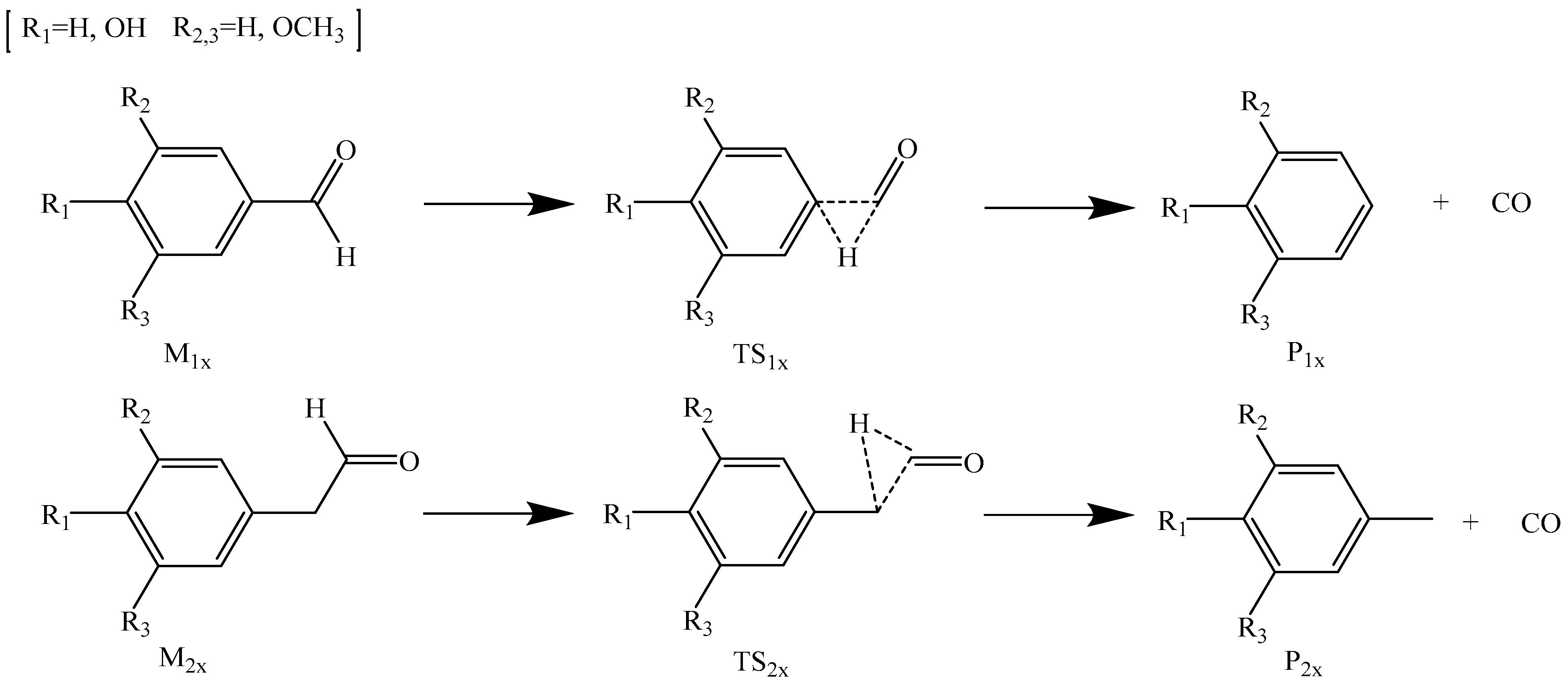
2.2. Catalytic Influence of Na+ and K+ on the Formation of CO during Lignin Pyrolysis
2.2.1. Catalytic Influence of Na+ on the Formation of CO
2.2.2. Catalytic Influence of K+ on the Formation of CO
2.2.3. Summary
2.3. Catalytic Influence of Na+ and K+ on the Formation of CO2 during Lignin Pyrolysis
2.3.1. Catalytic Influence of Na+ on the Formation of CO2
2.3.2. Catalytic Influence of K+ on the Formation of CO2
2.3.3. Discussion
3. Calculation Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.-H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T.; Saidina Amin, N.A. Current status of biohydrogen production from lignocellulosic biomass, technical challenges and commercial potential through pyrolysis process. Energy 2021, 226, 120433. [Google Scholar] [CrossRef]
- Kulik, T.; Nastasiienko, N.; Palianytsia, B.; Ilchenko, M.; Larsson, M. Catalytic pyrolysis of lignin model compound (ferulic acid) over alumina: Surface complexes, kinetics, and mechanisms. Catalysts 2021, 11, 1508. [Google Scholar] [CrossRef]
- Yang, H.M.; Appari, S.; Kudo, S.; Hayashi, J.; Norinaga, K. Detailed chemical kinetic modeling of vapor-phase reactions of volatiles derived from fast pyrolysis of lignin. Ind. Eng. Chem. Res. 2015, 54, 6855–6864. [Google Scholar] [CrossRef]
- Zhang, L.; Choi, C.; Machida, H.; Norinaga, K. Production of light hydrocarbons from organosolv lignin through catalytic hydrogenation and subsequent fast pyrolysis. J. Anal. Appl. Pyrol. 2021, 156, 105096. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C. Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis. Energ. Convers. Manag. 2016, 120, 370–377. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, W.; Hu, B.; Zhang, B.; Li, K. Formation mechanism of CH4 during lignin pyrolysis: A theoretical study. J. Energy Inst. 2022, 100, 237–244. [Google Scholar] [CrossRef]
- Xie, W.L.; Hu, B.; Liu, Y.; Fu, H.; Liu, J.; Zhang, B.; Lu, Q. Unraveling the radical chain mechanism in the pyrolysis of β-O-4 linked lignin: The role of aliphatic substituents. Proc. Combust. Inst. 2023, 39, 3303–3311. [Google Scholar] [CrossRef]
- Liu, C.; Lei, M.; Fan, Y.; Kong, X.; Zhang, H.; Xiao, R. Mechanism insights into enol ether intermediate formation during β-O-4-type lignin pyrolysis. J. Anal. Appl. Pyrol. 2023, 176, 106262. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Jiang, J.; Zhang, Y.; Song, M.; Zhang, R.; Dong, Z.; Yang, H.; Yu, H. Revealing G-lignin model compounds pyrolysis behavior: β-O-4 and 5-5′ dimer and trimer. Fuel 2022, 317, 123531. [Google Scholar] [CrossRef]
- Li, L.; Ouyang, X.; Qian, Y. Exploring factors affecting the dissociation energies of C–O and C–C bonds in lignin oligomers. Chem. Eng. Sci. 2024, 297, 120296. [Google Scholar] [CrossRef]
- Eskay, T.P.; Britt, P.F.; Buchanan, A.C. Does decarboxylation lead to cross-linking in low-rank coals? Energy Fuels 1996, 10, 1257–1261. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Tong, H.; Li, W.; Wu, D. A density functional theory study on formation mechanism of CO, CO2 and CH4 in pyrolysis of lignin. Comput. Theor. Chem. 2014, 1045, 1–9. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M.; Brydson, R.M.D.; Ross, A.B. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Lane, D.J.; van Eyk, P.J.; Ashman, P.J.; Kwong, C.W.; de Nys, R.; Roberts, D.A.; Cole, A.J.; Lewis, D.M. Release of Cl, S, P, K, and Na during thermal conversion of algal biomass. Energy Fuels 2015, 29, 2542–2554. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, B.; Xie, W.; Jiang, X.; Liu, J.; Lu, Q. Recent progress in quantum chemistry modeling on the pyrolysis mechanisms of lignocellulosic biomass. Energy Fuels 2020, 34, 10384–10440. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Q.; Hu, B.; Chen, D.; Liu, J.; Dong, C. Influence of inherent alkali metal chlorides on pyrolysis mechanism of a lignin model dimer based on DFT study. J. Therm. Anal. Calorim. 2018, 137, 151–160. [Google Scholar] [CrossRef]
- Cen, K.; Cao, X.; Chen, D.; Zhou, J.; Chen, F.; Li, M. Leaching of alkali and alkaline earth metallic species (AAEMs) with phenolic substances in bio-oil and its effect on pyrolysis characteristics of moso bamboo. Fuel Process. Technol. 2020, 200, 106332. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Fang, Y.; Yin, L.; Yang, H.; Gong, X.; Chen, Y.; Chen, H. Catalytic mechanisms of potassium salts on pyrolysis of β-O-4 type lignin model polymer based on DFT study. Proc. Combust. Inst. 2021, 38, 3969–3976. [Google Scholar] [CrossRef]
- Jeong, K.; Jeong, H.J.; Lee, G.; Kim, S.H.; Kim, K.H.; Yoo, C.G. Catalytic effect of alkali and alkaline earth metals in lignin pyrolysis: A density function theory study. Energy Fuels 2020, 34, 9734–9740. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, K.; Kim, S.-S.; Brown, R.C. Kinetic understanding of the effect of Na and Mg on pyrolytic behavior of lignin using a distributed activation energy model and density functional theory modeling. Green Chem. 2019, 21, 1099–1107. [Google Scholar] [CrossRef]
- Tran, Q.K.; Ly, H.V.; Hwang, H.T.; Kim, J.; Kim, S.S. Study on pyrolysis of Organosolv lignin impregnated with alkali and alkaline earth metals: Kinetics, thermodynamics, and product characterization. Fuel 2022, 329, 125472. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of alkali and alkaline earth metals on in-situ catalytic fast pyrolysis of lignocellulosic biomass: A microreactor study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Li, J.; Burra, K.G.; Wang, Z.; Liu, X.; Gupta, A.K. Effect of alkali and alkaline metals on gas formation behavior and kinetics during pyrolysis of pine wood. Fuel 2021, 290, 120081. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Karton, A.; O’Reilly, R.J.; Random, L. Assessment of theoretical procedures for calculating barrier heights for a diverse set of water-catalyzed proton-transfer reactions. J. Phys. Chem. A. 2012, 116, 4211–4221. [Google Scholar] [CrossRef]
- Yu, H.; Wang, S.; Sun, Y.; Zhang, W.; Li, R.; Kang, X. Pyrolysis mechanism law of β-O-4 lignin dimer model compounds: A density functional theory study. Ind. Crop. Prod. 2022, 180, 114746. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
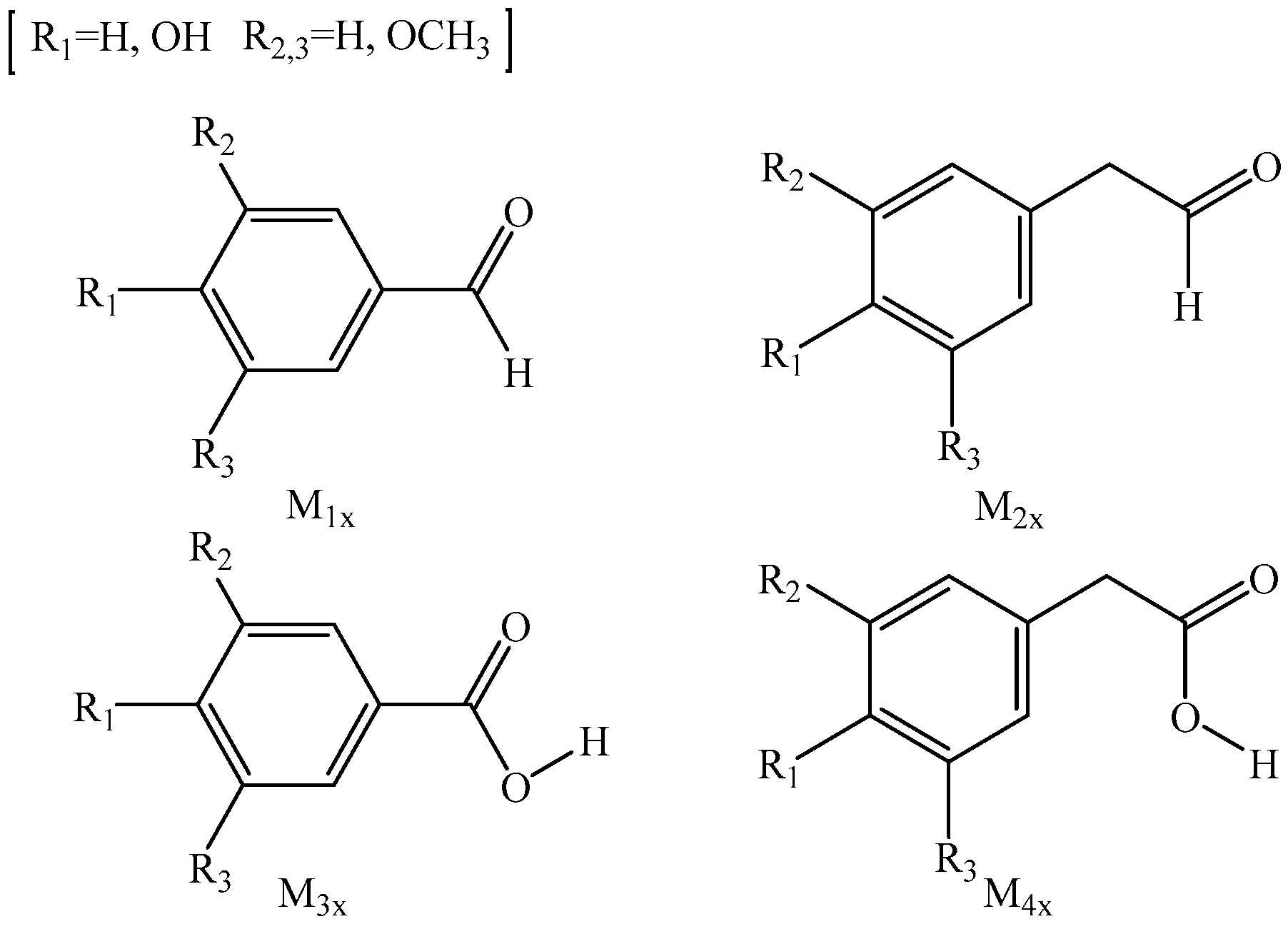
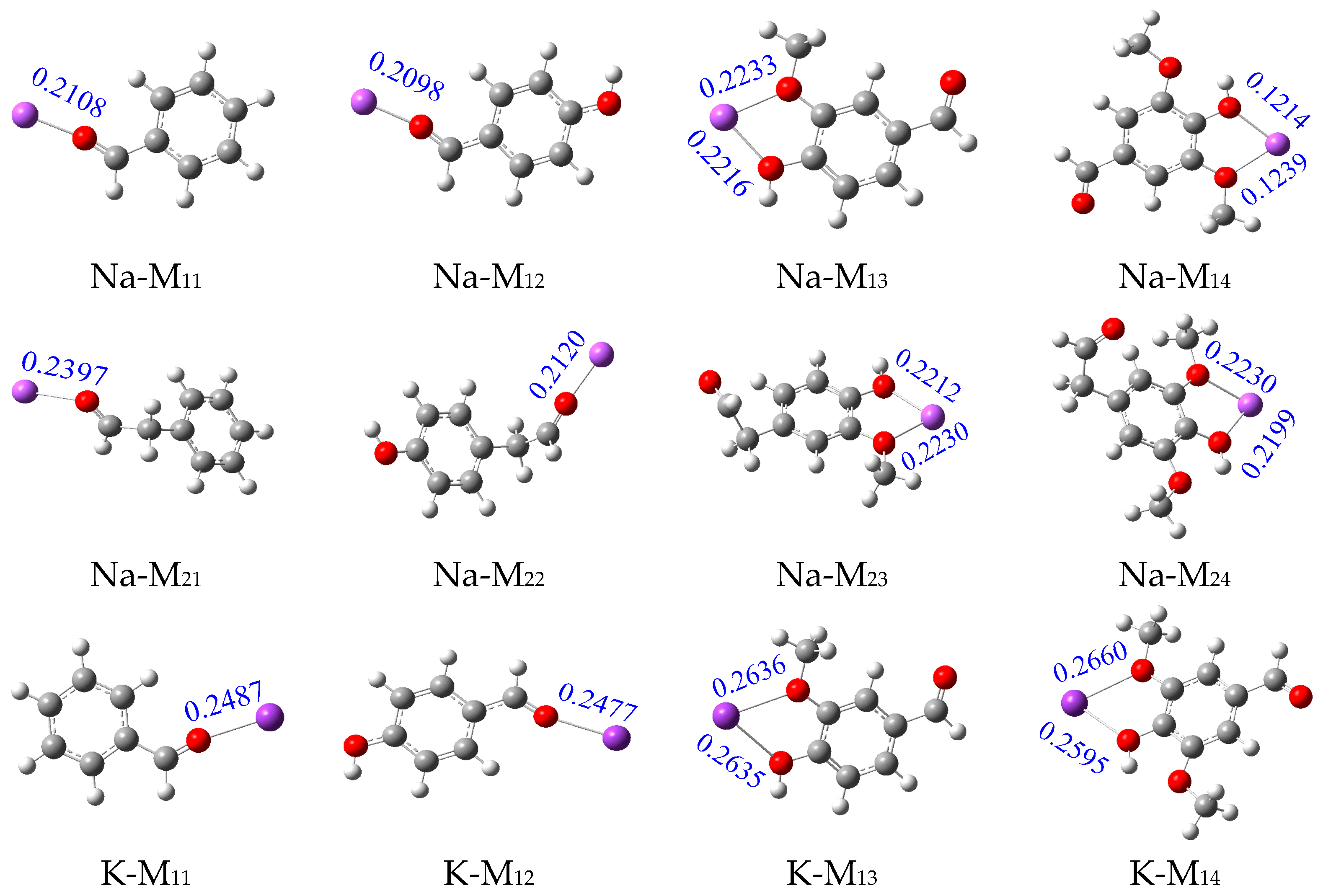
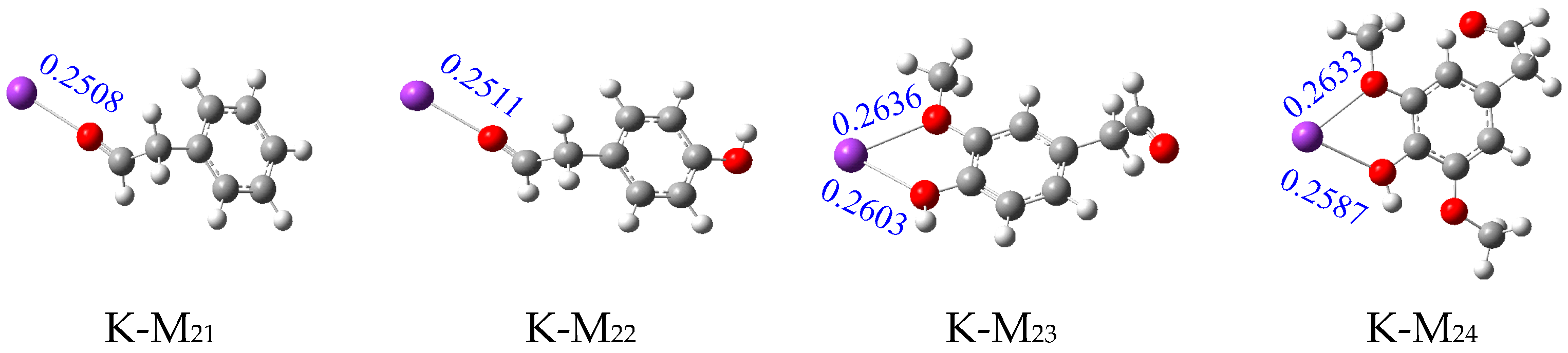
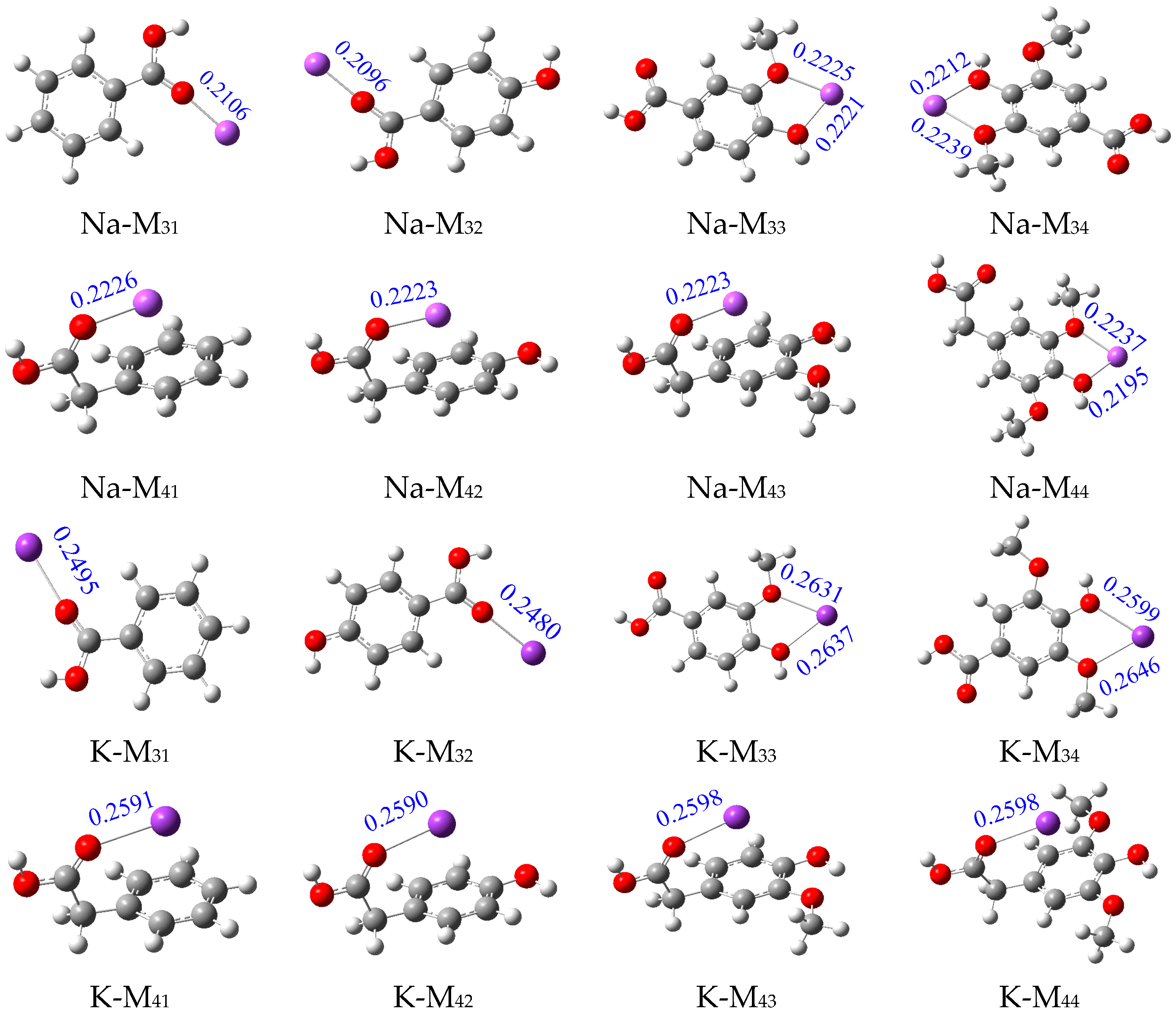

| Types | Substituent | Uncatalyzed (kJ/mol) | Catalyzed (kJ/mol) | Numerical Difference (kJ/mol) | |||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Na+ | K+ | Na+ | K+ | |||
| Benzaldehyde-based lignin monomers | M11 | H | H | H | 383.8 | 417.3 | 412.9 | 33.5 | 29.1 |
| M12 | OH | H | H | 388.7 | 396.1 | 403.2 | 7.4 | 14.5 | |
| M13 | OH | OCH3 | H | 389.2 | 361.2 | 364.3 | −28.0 | −24.9 | |
| M14 | OH | OCH3 | OCH3 | 387.9 | 361.9 | 364.3 | −26.0 | −23.6 | |
| phenylacetaldehyde-based lignin monomer | M21 | H | H | H | 329.9 | 305.0 | 316.4 | −24.9 | −13.5 |
| M22 | OH | H | H | 332.2 | 308.0 | 318.0 | −24.2 | −14.2 | |
| M23 | OH | OCH3 | H | 336.4 | 306.4 | 309.2 | −30.0 | −27.2 | |
| M24 | OH | OCH3 | OCH3 | 332.7 | 316.6 | 318.8 | −16.1 | −13.9 | |
| Types | Substituent | Uncatalyzed (kJ/mol) | Catalyzed (kJ/mol) | Numerical Difference (kJ/mol) | |||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Na+ | K+ | Na+ | K+ | |||
| Benzoic acid-based lignin monomers | M31 | H | H | H | 299.9 | 321.8 | 323.7 | 21.9 | 23.8 |
| M32 | OH | H | H | 299.1 | 320.2 | 320.2 | 21.1 | 21.1 | |
| M33 | OH | OCH3 | H | 288.6 | 316.3 | 314.4 | 27.7 | 25.8 | |
| M34 | OH | OCH3 | OCH3 | 287.2 | 314.4 | 313.2 | 27.2 | 26.0 | |
| Phenylacetic acid-based lignin monomers | M41 | H | H | H | 294.4 | 332.2 | 326.1 | 37.8 | 31.7 |
| M42 | OH | H | H | 296.4 | 332.9 | 326.6 | 36.5 | 30.2 | |
| M43 | OH | OCH3 | H | 297.8 | 330.3 | 326.4 | 32.5 | 28.6 | |
| M44 | OH | OCH3 | OCH3 | 295.9 | 330.5 | 324.2 | 34.6 | 28.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Han, Y.; Li, B.; Liu, J.; Zhou, G.; Du, X.; Wei, S.; Meng, H.; Hu, B. Catalytic Effect of Alkali Metal Ions on the Generation of CO and CO2 during Lignin Pyrolysis: A Theoretical Study. Catalysts 2024, 14, 537. https://doi.org/10.3390/catal14080537
Jiang X, Han Y, Li B, Liu J, Zhou G, Du X, Wei S, Meng H, Hu B. Catalytic Effect of Alkali Metal Ions on the Generation of CO and CO2 during Lignin Pyrolysis: A Theoretical Study. Catalysts. 2024; 14(8):537. https://doi.org/10.3390/catal14080537
Chicago/Turabian StyleJiang, Xiaoyan, Yiming Han, Baojiang Li, Ji Liu, Guanzheng Zhou, Xiaojiao Du, Shougang Wei, Hanxian Meng, and Bin Hu. 2024. "Catalytic Effect of Alkali Metal Ions on the Generation of CO and CO2 during Lignin Pyrolysis: A Theoretical Study" Catalysts 14, no. 8: 537. https://doi.org/10.3390/catal14080537
APA StyleJiang, X., Han, Y., Li, B., Liu, J., Zhou, G., Du, X., Wei, S., Meng, H., & Hu, B. (2024). Catalytic Effect of Alkali Metal Ions on the Generation of CO and CO2 during Lignin Pyrolysis: A Theoretical Study. Catalysts, 14(8), 537. https://doi.org/10.3390/catal14080537








