Abstract
Intermetallic compounds (IMCs) have attracted significant attention in recent years due to their unique properties and potential applications in various fields, particularly in catalysis. This review aims to provide an in-depth understanding of IMCs, including their synthesis methods, structural characteristics, and diverse catalytic applications. The review begins with an introduction to IMCs, highlighting their distinct features and advantages over traditional catalyst materials. It then delves into the synthesis techniques employed to prepare IMCs and explores their structural properties. Subsequently, catalytic applications of the IMCs are introduced, focusing on the key reactions and highlighting their superior catalytic performance compared to conventional catalysts. Future perspectives for, and challenges to, the catalysis of IMCs are then proposed.
1. Introduction
Recently, significant progress has been made in the fields of various metal nanocatalyst developments and their catalytic research. However, single-metal catalysts still face several issues, such as having only one type of active site, being prone to sintering at high temperatures, and susceptibility to competitive adsorption by poisons [1,2]. The development of bimetallic catalysts is a particularly effective strategy to solve the above problems. The introduction of a second metal leads to interactions not only between the metal and the support but also between the two metals, which can alter the structure of the active sites and stabilize the active centers [3,4]. Among the various multi-metallic catalysts, due to the unique electronic effects and surface structures, IMCs exhibit excellent activities and selectivities for many reactions [5,6,7].
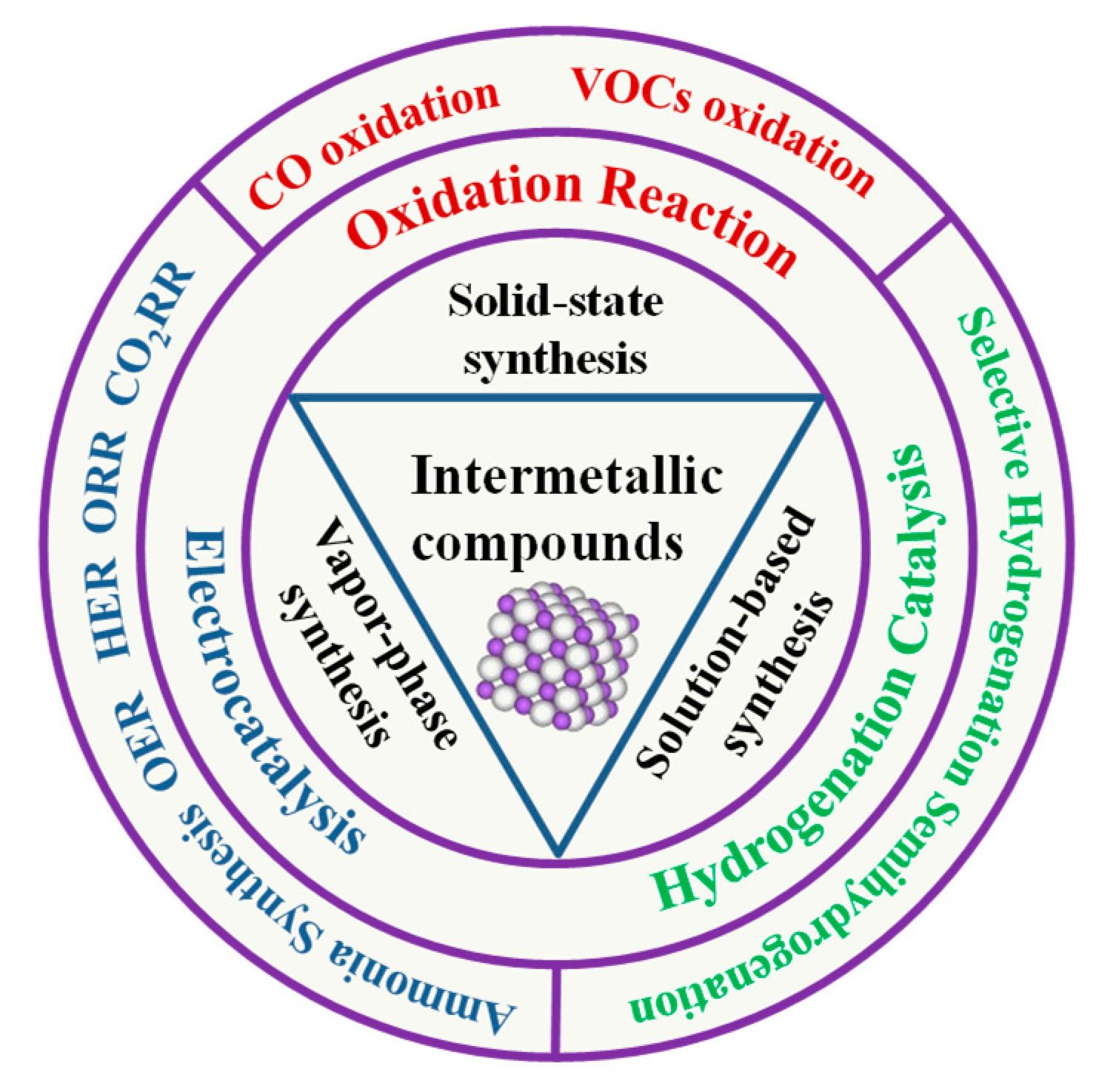
IMCs show special properties that distinguish them from traditional multi-metallic catalysts, such as diverse electronic and crystal structures, unique chemical bonding, ordered crystal structures, and diverse chemical potentials. These properties often possess high melting points, excellent thermal stability, and good mechanical properties [8,9]. Furthermore, their ordered structures provide well-defined active sites for catalytic reactions. The combination of these characteristics makes IMCs highly attractive for catalytic applications. In this review article, we shall give an overview of the current commonly used strategies for the preparation of IMCs and their applications in catalysis, including catalytic oxidation, hydrogen production, and electrocatalysis (Figure 1).
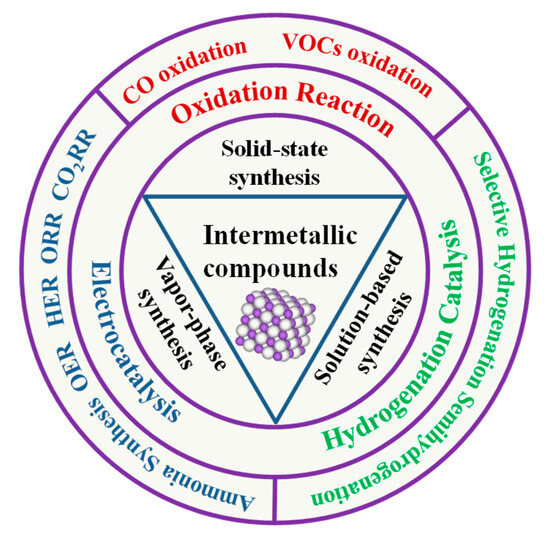
Figure 1.
Preparation strategies and applications of IMCs in catalysis.
2. Advantages of IMCs in Catalysis
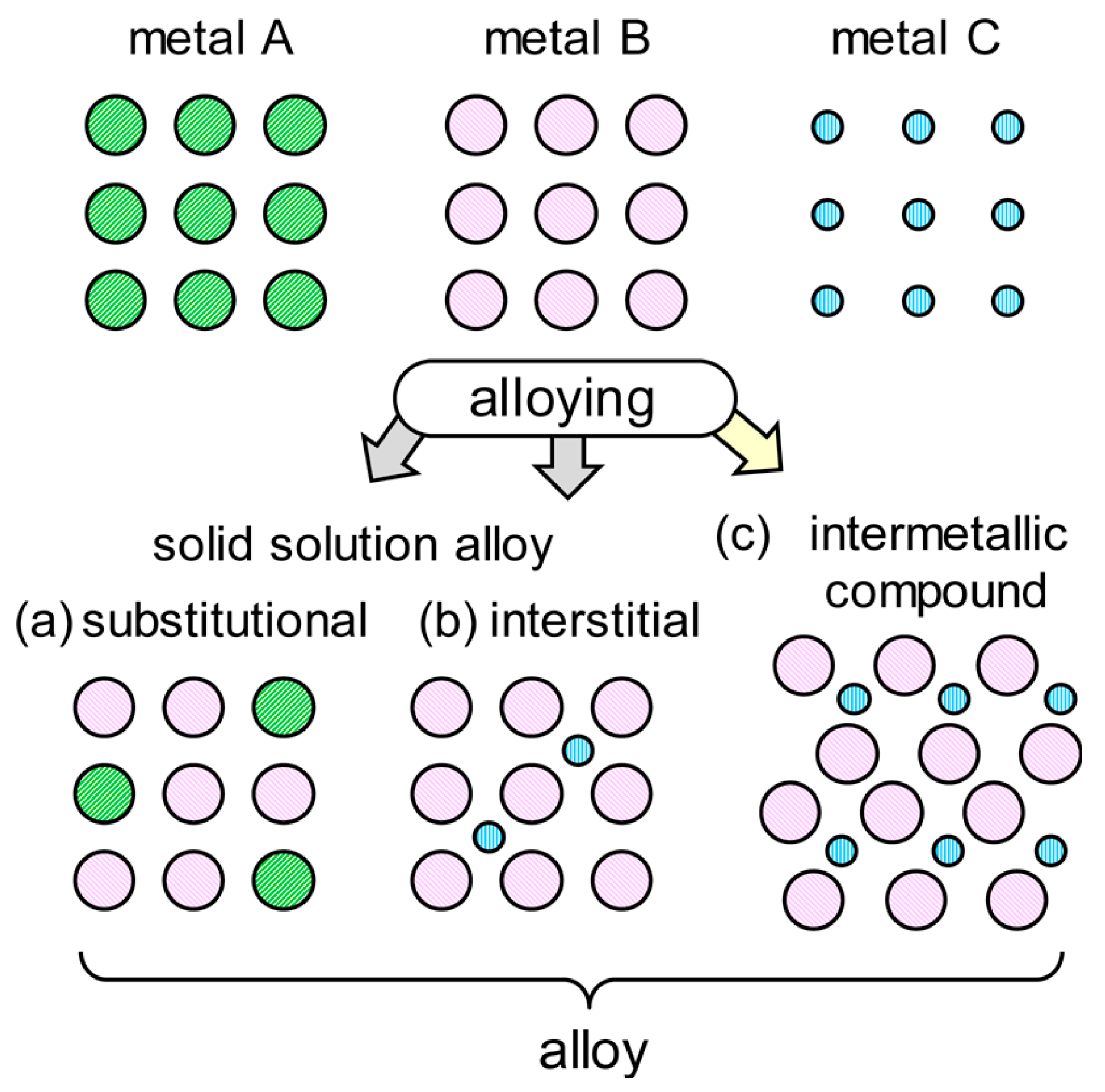
IMCs are a class of solid-state materials composed of two or more metallic elements [5,8]. Unlike solid solution alloys (Figure 2), which typically have random atomic arrangements, IMCs exhibit ordered atomic structures [10,11,12]. It should be noted that the formation of interstitial solid solution alloy requires a smaller atomic radium of the element. Usually, non-metallic elements (e.g., C, N, and H) are more suitable as interstitial elements [13,14]. It is also possible for smaller metal atoms to be inserted into the interstitial positions of solid solutions. For instance, an interstitial solid solution of Hf5GaxSn3 (x = 0–1) was formed based on the Hf5Sn3 binary compound, in which the Ga atoms occupied the Wyckoff position 2b at the centers of the Hf6 octahedral interstices [15]. Similar findings [16,17] also confirmed that metal C (Figure 2) was feasible to be present in the interstitial positions. These compounds can be categorized in binary, ternary, and higher-order compounds, based on the number of the metal elements present [18]. Additionally, quasicrystals, a special type of IMC, possess long-range orders but lack translational symmetry [4].
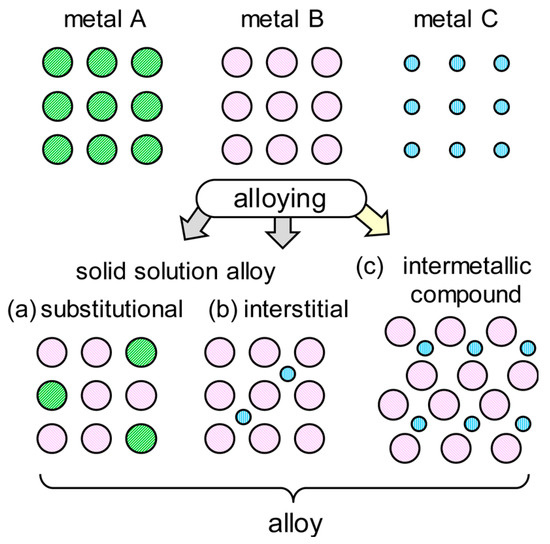
Figure 2.
Schematic illustration of bimetallic alloys structure [11]. Reprinted with permission from Ref. [11]. Copyright 2017, copyright American Chemical Society.
IMCs offer several advantages over conventional catalyst materials, making them valuable for catalytic applications.
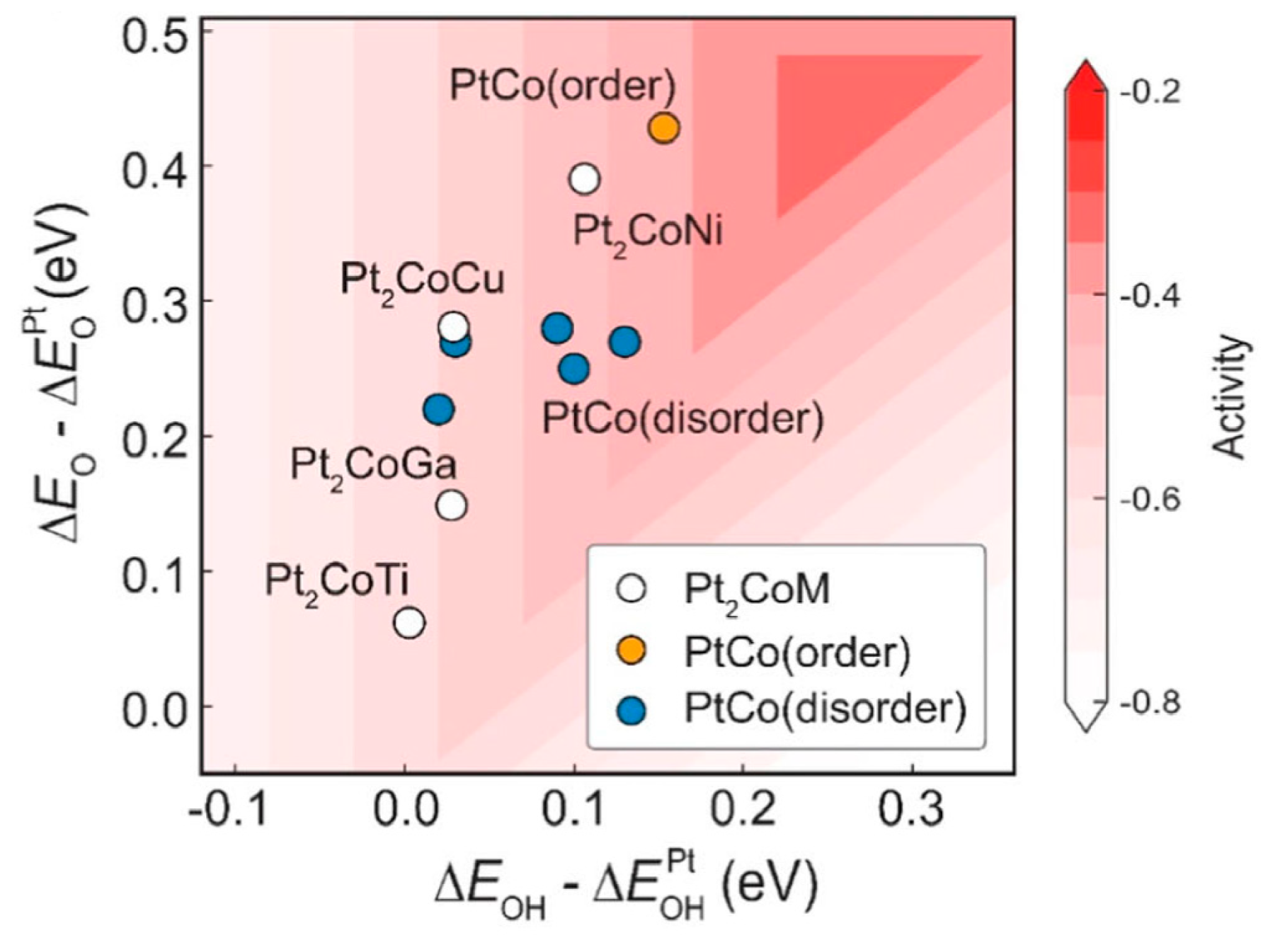
(i) Selectivity and efficiency [19,20,21,22,23,24]: IMCs exhibit high selectivity in specific reactions due to their ordered atomic structures (Figure 3), which enable the precise control of reactant adsorption and reaction pathways. For example, the structural motif observed in the IMC Al13Fe4 stands in marked contrast to that of elemental iron [19]. It should be pointed out that the “structural motif” refers to the characteristic arrangement of atoms or the recurring pattern of elements within the crystal structure of a material. Unlike iron, where a high density of adjacent iron atoms fosters robust adsorption of the reactants and thus catalyzes C–C coupling reactions with high efficiency [20], such as in Fischer–Tropsch catalysis and carbon nanotube synthesis, the Al13Fe4 compound shows a distinct atomic arrangement. This compound’s structure mimics that of the intermetallic GaPd, wherein gallium atoms co-ordinate solely with palladium, a configuration that exhibits a remarkable efficacy as a semi-hydrogenation catalyst [21]. It indicates that the IMCs are not a unique case in their catalytic potential. This discovery underscores the promise of IMCs as catalysts with tailored selectivity and activity, offering a viable alternative to the conventional metal catalysts. Additionally, unique electronic properties and synergistic effects between different metal elements of IMCs enhance catalytic activity and efficiency [25,26,27,28]. For example, Nørskov and coworkers [25] uncovered a novel Ni–Ga catalyst which was proficient in transforming carbon dioxide into methanol at normal atmospheric pressure. Such a discovery was facilitated by a descriptor-based analysis, complemented by computational techniques, which led to the identification of Ni–Ga IMCs as potentially stable and highly active candidates for the reduction process. It was experimentally verified that Ni5Ga3 was a standout performer in terms of both activity and selectivity.
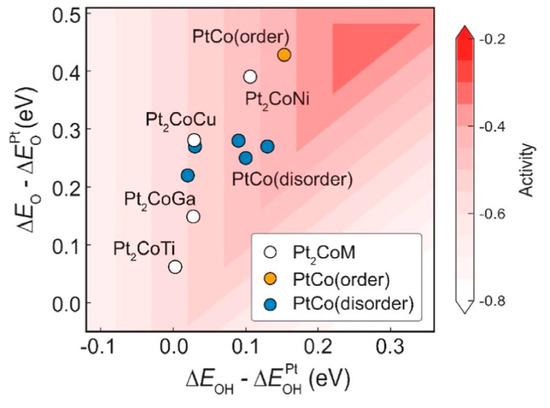
Figure 3.
The ORR activity volcano plot of four ordered Pt2CoM, ordered PtCo, and five disordered PtCo. [24] Reprinted with permission from Ref. [24]. Copyright 2024, copyright Springer Nature.
(ii) Stability: Traditional noble metal catalysts are prone to agglomeration and sintering of the active centers at high temperatures, leading to a decrease in activity. However, the introduction of base metals to the noble metal particles (Pd, Pt, etc.) to form IMCs results in a strong mutual interaction that enhances the binding strength to the support and slows down the particle migration rate, thereby greatly improving stability of the catalyst [29,30,31,32,33,34]. For instance, under the demanding environment of a high-purity propane stream at 580 °C, the 1Pd1Zn/MZ catalyst demonstrated remarkable endurance, maintaining its propane conversion rate at an impressive level for more than 20 days before its efficiency declined to half of its initial rate. This remarkable durability stood in stark contrast to the conventional PtZn catalysts, which typically experienced a rapid loss in activity within just a few days [29]. In addition, the synthesis of the catalysts with special structures, based on the evolution of intermetallic precursors, could likewise achieve improved stability [30,31,32,33,34]. Previous studies by our group revealed that the catalysts prepared by intermetallic precursors exhibited a novel metal composite oxide structure and extremely high thermal stability in methane oxidation. No significant decrease in activity was observed after hydrothermal aging at 750 °C. On the other hand, the traditional commercial Pdnp/Al2O3 catalyst was significantly deactivated and the T50 (the temperature for achieving a methane conversion of 50%) increased by 50 °C. Characterization results revealed that this was due to the formation of metal composite oxide (PdGaOx) after calcination of the IMC (Pd5Ga3), which not only ensured a high dispersion of the noble metal (Pd), but also effectively prevented the agglomeration of the noble metal by the strong interaction with the alumina support [30]. Similarly, in the utilized multicomponent nanoparticles with surface oxygenation to fabricate the catalysts, it needed to confirm featuring an oxygen-enriched metal surface layer coupled with a disordered core of a ternary alloy. The result of this study [31] demonstrated that the catalyst exhibited a complex kinetic behavior characterized by fluctuating dynamics due to the ongoing processes of lattice dilation and contraction, and phase transitions between order and disorder, as well as the creation of the oxygenated surface sites and reaction intermediates. Such a synergistic effect on catalysis successfully lowered the oxidation temperature by nearly 100 °C and demonstrated remarkable durability under the hydrothermal aging conditions at 800 °C.
(iii) Tailorable properties: due to their compositions and structures, IMCs can be tailored, and this enables the optimization of catalytic performance for specific reactions. By adjusting the types and ratios of metal elements, as well as the crystal structure, researchers can finely tune catalytic properties of the IMCs to achieve the desired selectivity, activity, and stability [35,36,37,38]. As is well known, the deposition of chlorine species is the main cause of catalytic deactivation for the oxidation of chlorinated volatile organic compounds (CVOCs). A site-isolation strategy could enhance the resistance against chlorine formed during the oxidation of CVOCs. As reported by our research team, we developed a bifunctional catalyst that featured an atomic-scale interface between metal and oxide, and meticulously engineered from nanocrystals of an IMC [35]. The introduction of trichloroethylene (TCE) exerted a minimal negative impact on the activity of the bimetallic catalyst, and the partial deactivation caused by TCE introduction was reversible. Over the site-isolated Pt–Sn catalyst, the abundance of oxygen species adsorbed on the electronegative surface of platinum proved to be exceptionally potent for the cleavage of C–Cl bonds at lower temperatures. The proximal presence of the promoter (Sn–O) served the dual role of the acidic sites, furnishing ample protons necessary for HCl generation on the catalyst surface. This mechanism is pivotal in sustaining the catalytic activity of Pt by eliminating chlorine and curbing the proliferation of polychlorinated byproducts. Furthermore, by introducing a third metal (M = Mn, W, Nb) [1] to construct ternary metal catalysts, the dual enhancement of chlorine and water resistance was achieved. Such an improvement was primarily due to the presence of the Brønsted acid sites on the catalyst surface, which were capable of reacting with the adsorbed chlorine atoms to form hydrochloric acid, thereby accelerating the detachment of chlorine species from the catalyst surface. Moreover, the addition of water vapor served as a dual function in the reaction process. On the one hand, it could provide protons to react with chlorine species to form HCl, on the other hand, it participated in the oxidation of VOCs by reacting with the adsorbed oxygen species to form new oxygen species, accelerating the removal of the chlorine species from the catalyst surface and reducing the more toxic polychlorinated byproducts. In electrocatalytic applications, Peter et al. [36] successfully modulated the electronic environment of the Pd active site by replacing the Pd site with a Pt atom, achieving a significant increase in ORR activity and selectivity while maintaining excellent stability.
In addition, the rational use of IMCs represents a potent approach to reduce the cost of catalysts. IMCs frequently incorporate lower-priced metals (Fe, Cu, Al, etc.), and their incorporation can significantly reduce the cost of catalysts. For instance, the integration of a diverse array of metals into intermetallic catalysts based on noble metals can substantially decrease the requisite amount of these costly elements. Moreover, the synergistic effects between multiple metal components can confer high activities over some lower-priced metals, enabling them to reach or even exceed catalytic activities of the precious metal catalysts, thereby further economizing the catalyst costs. Additionally, as previously discussed, the enhanced stability of IMCs can prolong the operational lifespan of catalysts. This durability serves as a cost-reduction mechanism by diminishing the frequency of replacement and maintenance, consequently curtailing operational expenditure.
3. Synthesis Methods of IMCs
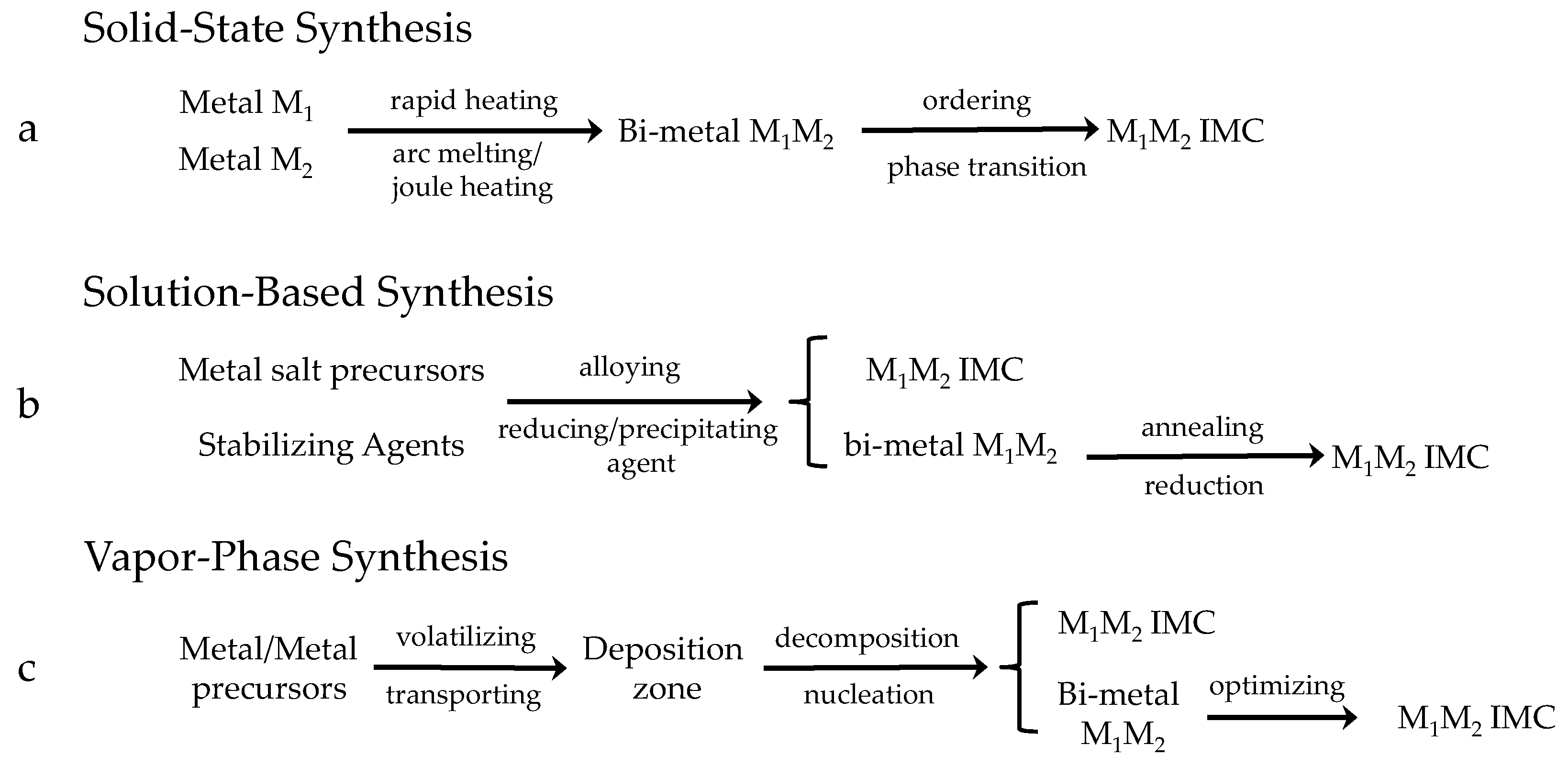
3.1. Solid-State Synthesis
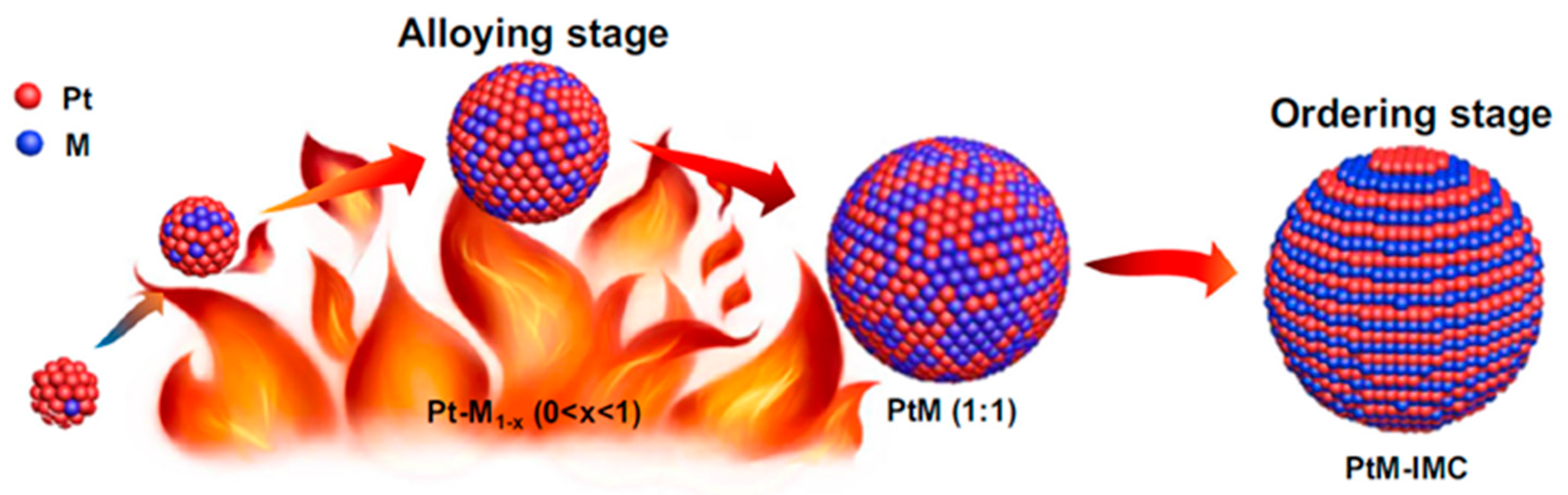
Solid-state synthesis generally refers to the use of solid-phase precursors to re-arrange metal atoms at high temperatures to form IMCs, as shown in Figure 4 and Figure 5a. Currently, the commonly used method is the arc melting method, which uses the principle of arc discharge by applying a high voltage over a short distance to cause an electrical discharge and generate high temperatures to melt the metals. This process mainly relies on an arc furnace. The arc melting method exhibits the following characteristics: (i) the melting temperature can reach 1980 °C, at which most refractory metals are melt; (ii) the melting time is 30–60 s, within which elemental volatilization is minimized; (iii) the intense arc-striking ensures complete stirring of the melt, resulting in excellent homogeneity; and (iv) high-purity inert gases (e.g., argon) can be used to protect the melt. To date, there has been considerable research on the preparation of IMCs using the arc-melting method [39,40,41,42,43,44,45,46]. It should be noted that the IMCs prepared by the arc-melting method are difficult to reduce in particle size and control due to being obtained under the high-temperature melting conditions. They usually require a physical crushing process to ensure the certain particle sizes, and the energy consumption is relatively high, owing to the requirement of high-temperature melting conditions.
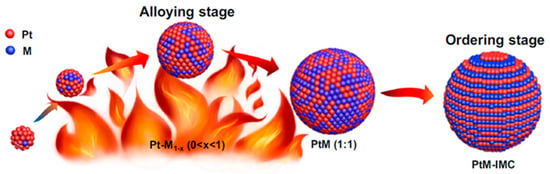
Figure 4.
Schematic illustration of the arc-melting synthesis of the structurally ordered Pt–M (M = Fe, Co, and Ni) IMCs (IMCs) [46]. Reprinted with permission from Ref. [46]. Copyright 2022, copyright Springer Nature.
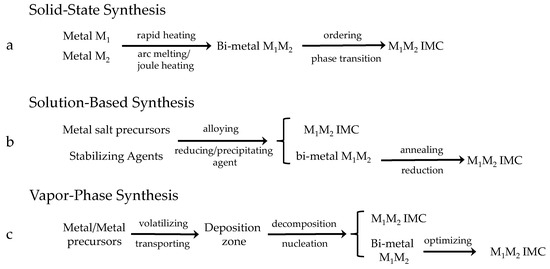
Figure 5.
Schematic illustration of different syntheses of intermetallic compounds.
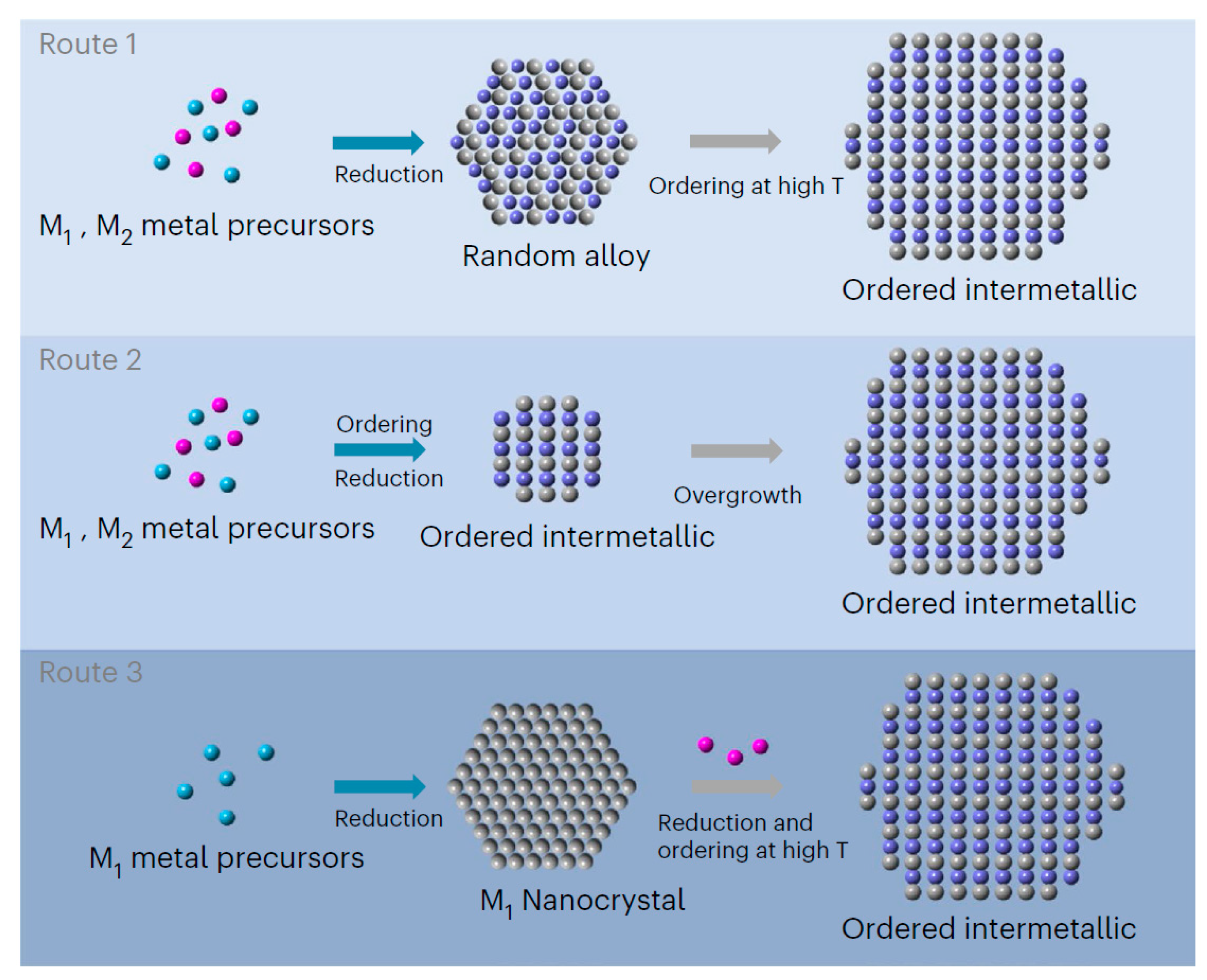
As mentioned above, it is difficult to reduce the particle size and control since the IMCs are obtained under the high-temperature melting conditions. In this regard, some studies have also reported the synthesis of IMCs first, using the ball-milling method that mixes the metal precursors and fuses them at elevated temperatures [47,48]. As described by Gao and Chen [47], the mixture of the commercial Al–Mg alloy and Ti powders was ball-milled in an argon environment using a planetary ball-milling machine. The mixed powders were heated to 660 °C for different durations, ranging from 7.5 min to 24 h in an argon atmosphere, thus preparing the Al18Ti2Mg3 and Al3Ti IMCs. In order to reduce the synthesis temperatures, however, some researchers [49] chose different metal precursors (palladium acetate and ferrous acetate to synthesize the ordered PdFe IMCs at lower temperatures). In addition, Kato and coworkers [50] used the precursor alloys, meticulously crafted with Mo, Co, Fe, or Cr, as the pore-forming agents and Ni, alongside Mg, as the sacrificial and molten components, respectively, to carry out a rigorous thermal treatment within a magnesium melt at temperatures ranging from 700 to 900 °C. This rapid dealloying procedure, propelled by the swift dissolution of Ni into the melt, engendered a bicontinuous nanoporous framework at the alloy/melt boundary. The subsequent cooling process solidified the entrapped Mg, which was later meticulously removed via the acid-etching process, hence unveiling the intricate architecture of the IMC. The high-temperature LMD surpassed the kinetic limitations, fostered the direct emergence of the chemically ordered intermetallic phases, and culminated in the formation of nanoporous IMCs with remarkably diminutive characteristic dimensions, which were attributed to the intermetallic effect that mitigated the surface diffusivity and curtailed the thermal coarsening during the dealloying process. Furthermore, with the rapid development of synthesis techniques, the goal of rapid temperature rise and fall has been achievable. By regulating the temperature ramp to the extremely rapid level, it can orchestrate the ordering of an extensive array of metallic elements. This innovative approach is particularly applicable to the realm of ultra-small nanoparticles, unlocking new possibilities in materials science. Hu and coworkers [51] presented a novel approach of disorder-to-order phase transition for synthesizing the ultra-small and stable multi-principal elemental intermetallic (MPEI) nanoparticles containing up to eight different elements. Joule heating was applied for only 5 min to facilitate the phase transition into the L10 intermetallic structure, which was then preserved by rapid cooling.
3.2. Solution-Based Synthesis
3.2.1. Chemical Reduction Method
The chemical reduction method (Figure 5b) involves the mixing of the metal precursors in an appropriate solvent, which are then reduced by a reducing agent (e.g., sodium borohydride, borane-tetrahydrofuran complex, or hydrogen) in the presence of the stabilizing agents or surfactant ligands (e.g., citrate, alkyl thiols, or thiol ethers). Additionally, polymer ligands (e.g., polyvinylpyrrolidone (PVP) and polyvinyl alcohol (PVA)) can be added to prevent the aggregation and growth of the resulting nanoparticles, and hence achieve the controllable particle sizes [52,53,54,55]. There has been considerable work on the preparation of IMCs via the chemical reduction route. For example, Hu and coworkers [54] utilized a solvothermal synthesis strategy to prepare a series of hollow-structured Pd–Sn alloy nanocrystals. This method entailed the adjustment of the stoichiometric ratios of palladium and tin precursors, yielding a variety of nanocrystalline structures (e.g., the face-centered cubic solid solution of Pd(Sn) and IMCs Pd2Sn and Pd3Sn2). The structural nuances of these nanocrystals were scrutinized through an integrated suite of characterization techniques, encompassing electron microscopy, spectroscopic analysis, elemental mapping, and X-ray diffraction. The solvothermal synthesis conducted under the precise thermal and pressure conditions culminated in the formation of nanocrystals with hollow architectures. Prominently, the Pd3Sn2 intermetallic phase exhibited a superior catalytic activity in methanol oxidation, which had potential as a high-performance electrocatalyst. Chou and Schaak et al. [56] synthesized the Sn-based IMCs (M–Sn, M = Fe, Co, Ni, and Pd) using PVP and 2-ethyl-2-oxazoline as the stabilizers, and sodium borohydride as the reducing agent, in a tetraethylene glycol solvent. Hou et al. [30] fabricated the Pd5Ga3 intermetallic nanocrystals by co-reducing palladium acetylacetonate and gallium chloride with tert-butylamine borane (TBAB) as a reducing agent in an oleylamine solvent.
Although the chemical reduction method appears simple in principle and operation, controlling the simultaneous nucleation of two metals is challenging due to the significant differences in the chemical reduction potentials and atomic radii of the metals. If a strong reducing agent is used, the reduction process can be very rapid, thus making it difficult to control the formation of IMCs [7]. For example, Sra and Schaak [57] studied the reduction process using sodium borohydride as a reducing agent, and found that gold and copper were reduced very quickly, resulting in separate nucleation and no alloy formation. Similarly, Li et al. [58] used hydrazine as a reducing agent to prepare the Ni–Co and Ni–Cu alloys. Although the Ni–Co and Ni–Cu alloys were formed, the nucleation and growth during the reduction process were uncontrollable, and no IMCs were generated. In addition, the control of particle size is also one of the difficulties. To address this issue, the main solution methods are the use of milder reducing agents and other stabilizing agents (e.g., oleylamine and oleic acid). Michael Ruck et al. [59] synthesized Bi2Pd5 and BiPd3 using Bi(NO3)3·5H2O and Pd(CH3COO)2 as the precursors, and ethylene glycol (EG) as the reducing agent. The Bi2Pd, BiPd, and Bi12Pd31 were also fabricated using similar methods. Wu et al. [60] also used EG as the reducing agent, and poly(diallyldimethylammonium) chloride (PDDA) as the stabilizer, to obtain the AgAu.
3.2.2. Annealing Reduction Method
The annealing reduction method primarily involves the deposition of the metal precursors onto a support via the simple impregnation or precipitation route, thus forming a composite. The catalyst is then exposed to a hydrogen atmosphere at high temperatures, where the composite on the catalyst surface is reduced, and the bimetallic species are re-arranged in hydrogen to generate the stable bimetallic or IMCs [61,62,63,64], as shown in Figure 6. For example, Du et al. [61] used a co-impregnation method to load Co(NO3)2 and H2PtCl6 onto Ketjen Black (KB), and sonicated it for 3 to 6 h. After being dried, the resultant powders were then subjected to a calcination process in a hydrogen atmosphere and a tube furnace. The temperature was ramped up to 900 °C and maintained for 2 h in a flow of hydrogen. The powders, after undergoing the high-temperature treatment, formed the PtCo IMC catalyst. Such a catalyst with a high intrinsic activity was prepared for the use of a cathode catalyst, so that its performance was compared with that of the commercial Pt/C catalyst. Similarly, Scelza et al. [64] used the impregnation method to load the PtFe or PtSn chloride precursors onto different carbon supports (CV, CN, and CN-P), followed by drying and reduction in a hydrogen atmosphere at 350 °C for 3 h to obtain the PtFe or PtSn IMC catalyst. Komatsu et al. [65] adopted a co-impregnation method to load the Pt and Co precursors onto various supports, followed by reduction in a hydrogen atmosphere at 600 or 800 °C (depending on the support used) for 2 h to generate the Pt3Co/MOx (M = La, Ca, Mg, Al, and Si) IMCs catalysts. Muramatsu et al. [66] also used a volumetric impregnation method to prepare the PtCu/Al2O3 IMCs catalysts after reduction at 800 °C in a hydrogen atmosphere.
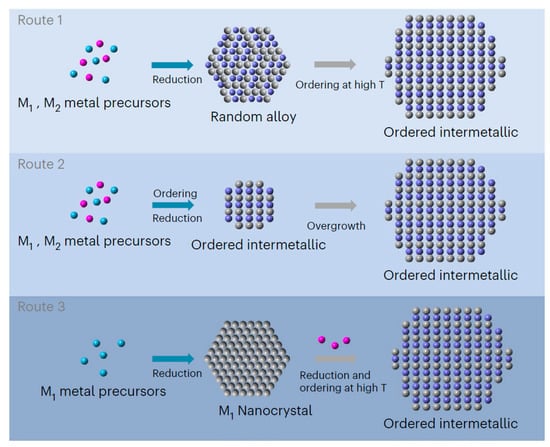
Figure 6.
Schematic illustrations of traditional synthetic strategies for ordered intermetallic nanocrystals [27]. Reprinted with permission from Ref. [27]. Copyright 2023, copyright Springer Nature.
It is worth noting that, during the impregnation process, one can first impregnate the precious metals that have a hydrogen activation effect and then deposit another metal, thus obtaining the IMCs. This is because precious metals can exert a hydrogen activation effect, and the activated hydrogen species will overflow to the nearby sites for reduction, allowing the two metals to make full contact and form IMCs. For example, Komatsu et al. [67] first used the volumetric impregnation method to prepare Pd/SiO2, then continued to impregnate Pd/SiO2 with the corresponding nitrates of Ge, In, Sn, and Zn, and finally obtain PdmMn/SiO2 (M = Ge, In, Sn, Zn) intermetallic catalysts after reduction at 800 °C for 2 h.
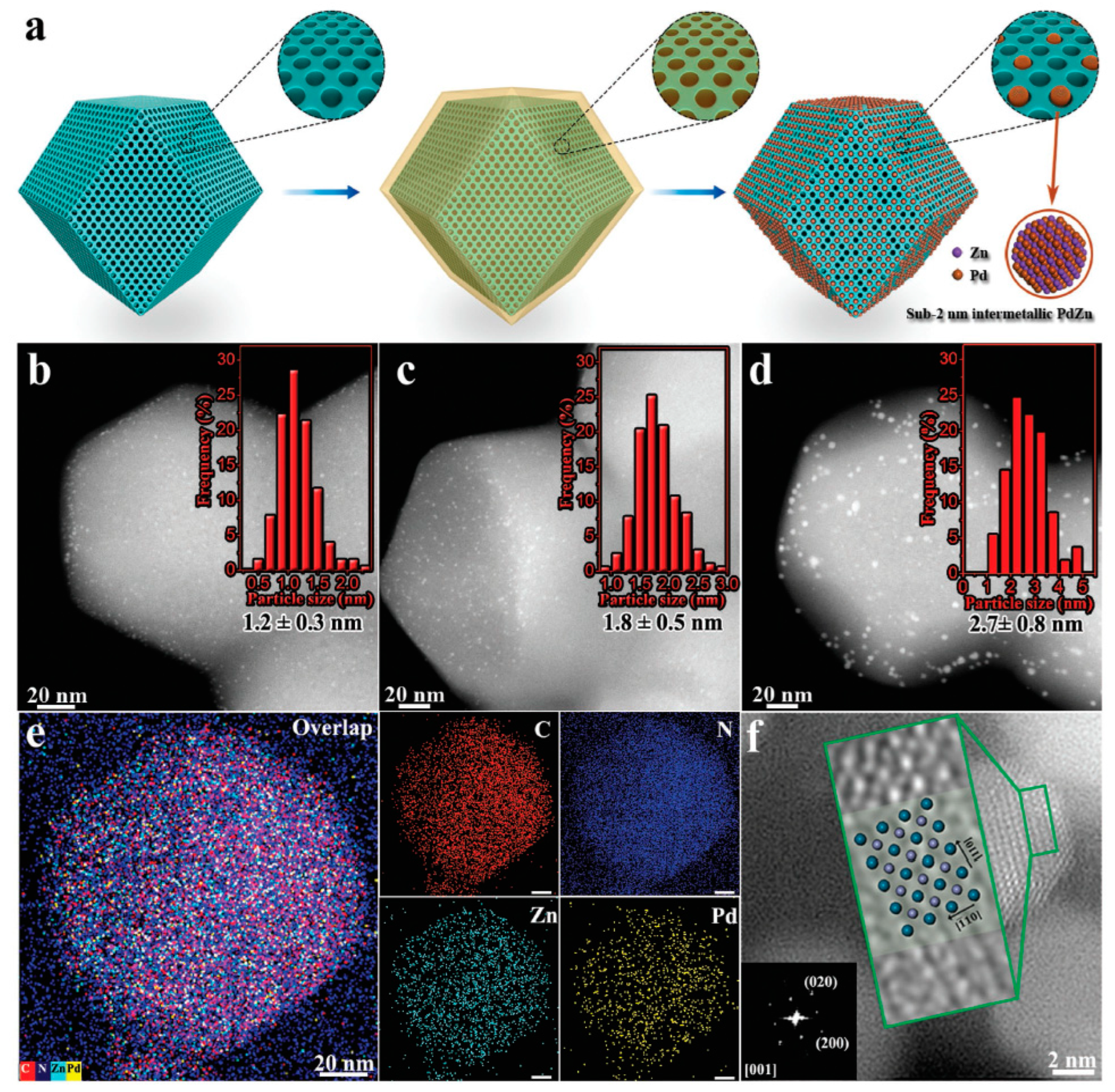
In order to better control particle sizes of the IMCs and inhibit their agglomeration during the annealing process, Liang and coworkers [68] prepared the porous sulfur-doped, carbon-supported, platinum-based IMCs by the sulfur-anchored method. There were IMC libraries containing 46 combinations of platinum with 16 other metal elements, and their effects on the electrocatalytic activity of the oxygen reduction reaction were investigated. These IMCs exhibited efficient mass activity in the proton exchange membrane fuel cells, capable of achieving high activities of 1.3–1.8 A/mgPt at 0.9 V. Zhou and coworkers [69] adeptly fabricated sub-2 nm intermetallic palladium–zinc (PdZn) nanoparticles encapsulated within the porous ZIF-8C (PdZn–sub-2@ZIF-8C) matrix, as shown in Figure 7. This innovative approach capitalized on the regular porous architecture of ZIF-8C, facilitating the synchronized in situ reduction of the palladium and zinc precursors. Acting as a molecular “cage”, the ZIF-8C framework circumvented the nanoparticle agglomeration, bestowing the resultant sub-2 nm intermetallic PdZn nanoparticles with superior size uniformity and thermal stability. Remarkably, the nanoparticles maintained their size distribution intact, even after subjection to a rigorous calcination at 600 °C.
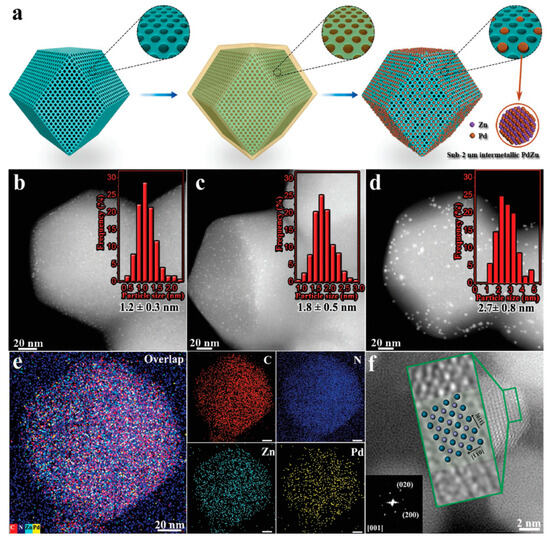
Figure 7.
(a) Schematic preparation process of the PdZn–sub-2@ZIF-8C using a MOF-confined co-reduction strategy, (b) HAADF–STEM image of PdZn–1.2@ZIF-8C, (c) HAADF–STEM image of PdZn–1.8@ZIF-8C, (d) HAADF–STEM image of PdZn–2.7/ZIF-8C, and the insets of panels (b–d) are the corresponding histograms of particle-size distributions of the PdZn–1.2@ZIF-8C, PdZn–1.8@ZIF-8C, and PdZn–2.7/ZIF-8C, respectively, (e) EDS elementary mapping images of the PdZn–1.2@ZIF-8C, scale bar = 20 nm, and (f) high-resolution HAADF–STEM image of PdZn–10/ZIF-8C [69]. Reprinted with permission from Ref. [69]. Copyright 2018, copyright John Wiley and Sons.
In addition to the impregnation method, many researchers have also used the precipitation approach to precipitate the precursors from the solution. For instance, Behrens et al. [70] first adjusted the pH value of the mixed precursor solution to precipitate the metals, and then calcined and reduced the obtained metal precipitation mixtures to obtain the binary or ternary IMCs. This method is relatively simple to operate, but the reduction with high-temperature hydrogen is dangerous, and the presence of hydrogen can also damage the structure of some oxide supports. Therefore, some researchers have utilized hydrogen substitutes (e.g., argon and other inert atmospheres) to treat the bimetallic compounds at high temperatures to generate the IMCs. For example, Matsumoto et al. [71] used chloroplatinic acid and lead nitrate as the metal precursors, reduced these precursors with sodium borohydride, and then calcined the mixture in an argon atmosphere at 600 °C to obtain the Pt3Pb IMC.
3.3. Vapor-Phase Synthesis
The chemical vapor deposition method (CVD) for synthesizing IMCs typically involves introducing a second metal precursor in the form of vapor directly into the matrix metal, followed by reduction, deposition, and alloying (Figure 5c). At the optimal temperature threshold, the reduction and deposition processes predominantly take place on the active metal surface. The selective targeting of the metal surface facilitates a more directed and efficient deposition (Figure 8) [72]. To enable the second metal to be introduced into the reactor in the form of vapor, the precursors (e.g., silane [73] and tetraalkyl compounds [74]), with sufficiently high vapor pressures, are required. It can be seen that the amount of the metal loaded in the CVD process largely depends on the temperature and time. Therefore, the appropriate and precise conditions should be required to control the loading of the second metal.
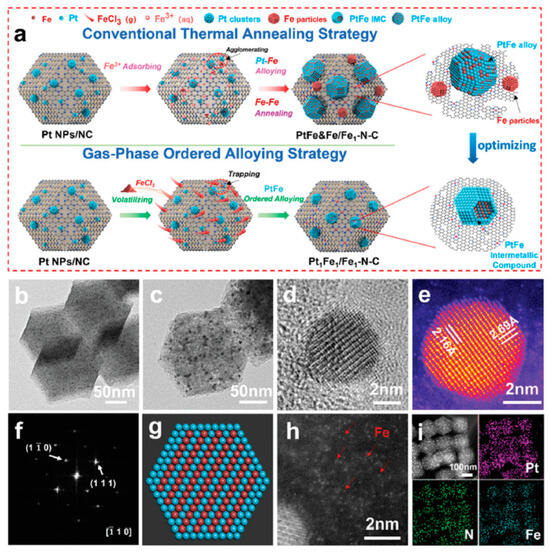
Figure 8.
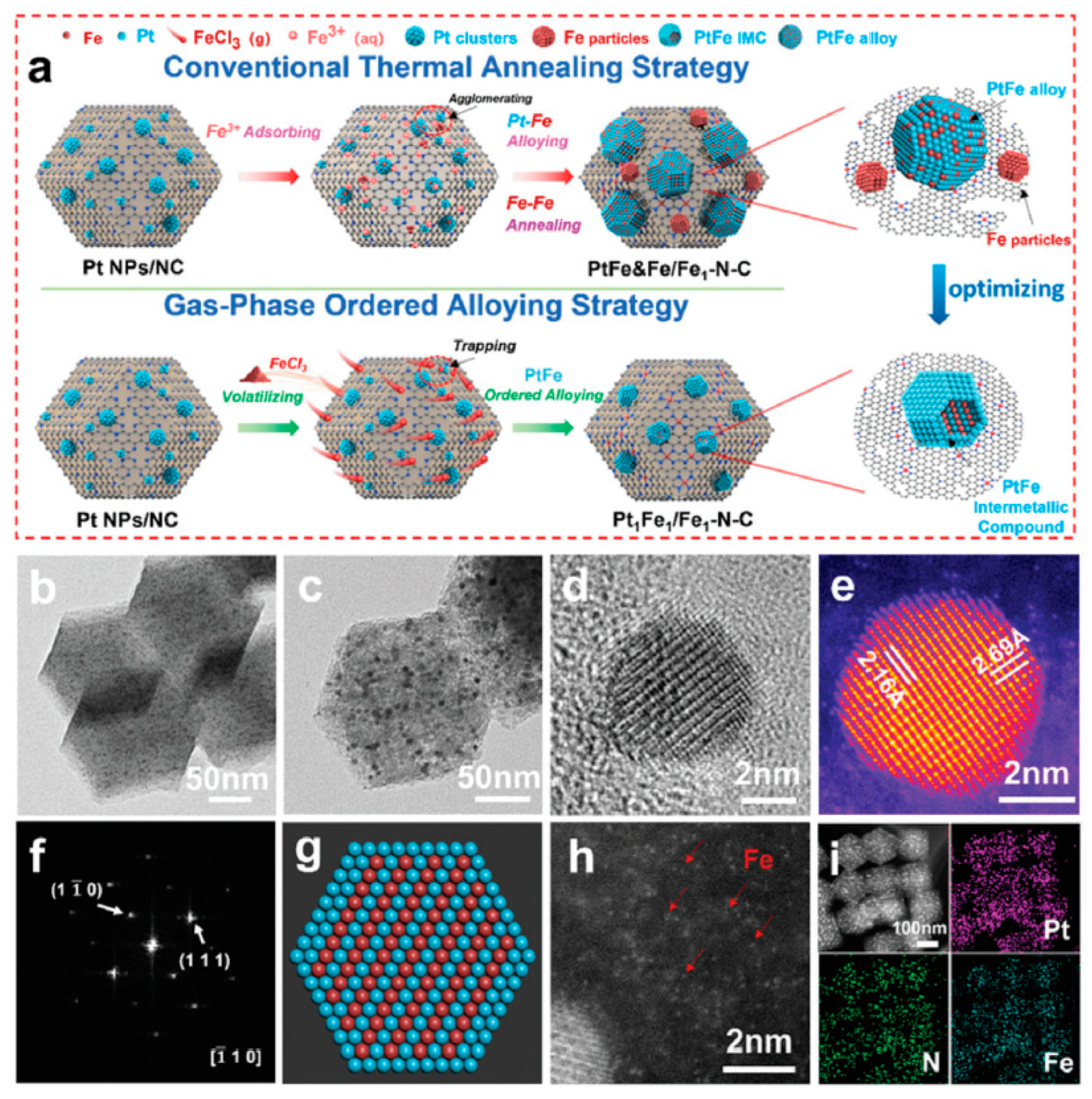
(a) Schematic illustration of gas-phase ordered alloying strategy for the preparation of Pt1Fe1/Fe1–N–C and conventional thermal annealing strategy for the preparation of contrast sample PtFe&Fe/Fe1–N–C, (b) TEM image of pre-synthesized Pt nanoparticles/NC, (c) TEM, (d) HRTEM images of Pt1Fe1/Fe1–N–C, (e,f) atomic-resolution HAADF–STEM image and corresponding FFT pattern of Pt1Fe1 IMC, (g) schematic diagram of the Pt1Fe1 IMC structure, (h) aberration-corrected HAADF–STEM image, and (i) EDS elemental mappings of Pt1Fe1/Fe1–N–C [72]. Reprinted with permission from Ref. [72]. Copyright 2023, copyright John Wiley and Sons.
A typical synthesis example is from Komatsua et al. [75], who first introduced Pt to MCM-41 through a ion-exchange process, reduced the Pt precursor at 200 °C for 1 h to obtain Pt/MCM-41, and then introduced Ge(CH3)4 in the form of vapor to Pt/MCM-41 at 150 °C with hydrogen as the carrier gas, and finally reduced the mixture at 600 °C in hydrogen for 1 h to generate the Pt–Ge IMC. Liang et al. [73] used Ni as the matrix metal and reacted it with silane in the vapor state in a hydrogen atmosphere to synthesize the Ni2Si, NiSi, and NiSi2 IMCs with different Ni/Si ratios. The IMCs synthesized by the CVD method usually have high phase-purity and can be controlled. In addition to silane and tetraalkyl compounds, Wu and coworkers [72] reported the synthesis of Pt1Fe1/Fe1–N–C by using FeCl3 as the precursor. Specifically, as shown in Figure 7, Pt nanoparticles/NC and FeCl3 were placed separately in a tube furnace and heated in a nitrogen flow first at 600 °C for 2 h and then at 900 °C for 1 h. After cooling to room temperature, the Pt1Fe1 IMCs and Fe single atoms on NC were obtained. The characterization findings revealed that PtFe was uniformly dispersed, generating the IMCs within the samples obtained by the gas-phase ordered alloying approach, as opposed to those produced by the traditional impregnation methods. This gas-phase ordered alloying strategy is extolled for its efficacy in preventing sintering and phase segregation, thereby yielding a homogeneous distribution of the active sites. Such a uniform distribution is instrumental in enhancing catalytic performance and robustness of the material in question.
In order to control the growth of IMCs, Nakamura and coworkers [76] carried out the synthesis of FeGeγ (γ = ca. 1.52) on the Si substrate using the seed-assisted epitaxy (SAE) strategy. For example, the SAE process was initiated with the meticulous formation of epitaxial nanoseeds of FeGeγ on a Si(001) substrate. This entailed the introduction of undoped Si(001) substrate into a high-vacuum molecular beam epitaxy (MBE) chamber, followed by the creation of the pristine Si surface through the epitaxial deposition of Si buffer layers at 500 °C for 6 h. A precise deposition of three monolayers (MLs) of Ge at the ambient temperature, succeeded by 2 MLs of Fe, unfolded on the pristine Si(001) surface. The subsequent annealing of the Fe/Ge layers within the MBE chamber at 400 °C for 30 min catalyzed the epitaxial emergence of FeGeγ nanoseeds. Thereafter, a co-deposition procedure of Fe (250 MLs) and Ge (375 or 450 MLs) was conducted on the pre-established epitaxial FeGeγ nanoseeds at room temperature, thus yielding the as-grown NS-FeGeγ specimens. These specimens were subjected to a solid-phase epitaxy (SPE) annealing step in a nitrogenous atmosphere at 350 °C for 10 min. The critical annealing phase fostered the epitaxial proliferation of FeGeγ at the nanoseed interface, hence effectively precluding emergence of the non-desired stable phases through interdiffusion.
Table 1 provides an overview of the existing methodologies for IMC synthesis. However, the key challenge in current research is to devise a synthesis approach that is efficient and broadly applicable under benign conditions. Concurrently, scaling up the production of nanoscale IMCs is another significant hurdle in their industrial applications.

Table 1.
A summary of synthesis methods and catalytic performance of IMCs.
4. Catalytic Applications of IMCs
4.1. Oxidative Reaction
The oxidation of CO (Equation (1)) is a commonly used probe reaction. Although the process is simple, it plays a significant role in fundamental chemical research, automotive exhaust removal, and fuel cell investigations. A large number of researchers in the fields of catalysis chemistry and surface science have made many efforts to thoroughly understand the oxidation of CO [77,78,79,80]. In this field, noble metals (e.g., Pt, Pd, and Rh) have been the focus of catalytic material research, especially their (110) crystal facets [77]. Additionally, there has been considerable work on IMC catalysts for CO oxidation. For instance, Saravanan and Abe et al. [81] reported the preparation of Pt3Ti/SiO2 and its application for CO oxidation, and found that, compared to Pt/SiO2, Pt3Ti/SiO2 exhibited superior catalytic activity: the CO conversion rate was up to three times higher than that of the single Pt-loaded catalyst, and the ignition temperature (125 °C over Pt3Ti/SiO2, but 200 °C over Pt/SiO2) was decreased by 75 °C. The authors attributed this result to the weaker adsorption of CO on Pt3Ti, compared to that on Pt, thereby reducing the poisoning effect of CO on the catalyst, which was confirmed by the thermal desorption characterization results. After investigating the various Pt-based IMC catalysts (PtmMn/SiO2; M = Co, Cu, Fe, Ge, Sn, Tl) for CO oxidation, Komatsu et al. [74] pointed out that Pt3Co and PtCu showed the best catalytic activities. Through IR-TPD combined characterization, these authors discovered: (i) during the oxidation process, Cu was enriched on the surface of PtCu, and (ii) the adsorption of CO on the surface of copper was significantly weaker than that on the surface of platinum [66]. Moreover, kinetic studies indicated that the introduction of copper promoted the adsorption of O2 and competitive adsorption with CO. The as-obtained results suggested that the introduction of copper not only promoted the adsorption of O2 but also inhibited the poisoning effect of CO, hence enhancing the activity of the catalyst.
2CO(g) + O2(g) → 2CO2(g)
Furthermore, it is well known that Au exhibits the best catalytic activity for the oxidation of CO. Researchers have also conducted extensive investigations on the gold-based IMC catalysts. For example, Zhang et al. [82] prepared the IMC Au3Cu and loaded it on SBA-15, and found that a complete conversion of CO to CO2 was achieved over the Au3Cu/SBA-15 catalyst at room temperature. In contrast, the supported single-metal Au or Cu catalyst showed lower catalytic activity. It was confirmed that the surface of the catalyst was a mixture of Au and CuOx. There might be a synergistic interaction between Au and Cu, with the CuOx serving as an effective oxygen activation center and Au as a dual active site, thus achieving the low-temperature CO oxidation. In addition to the AuCu IMC, Huang et al. [83] fabricated the very rare IMC Au2Na using an alkali metal as the second metal, over which a near-room-temperature conversion of CO (with an apparent activation energy for CO oxidation of 31.6 kJ/mol) was achieved. Through DFT calculations, it was found that: (i) after the catalyst was exposed to oxygen, the second layer of Na migrated to the surface as a binding site for oxygen, and (ii) this assumption allowed CO to directly react with O2 to form an OOCO intermediate, which was kinetically favorable, as shown in Figure 9.
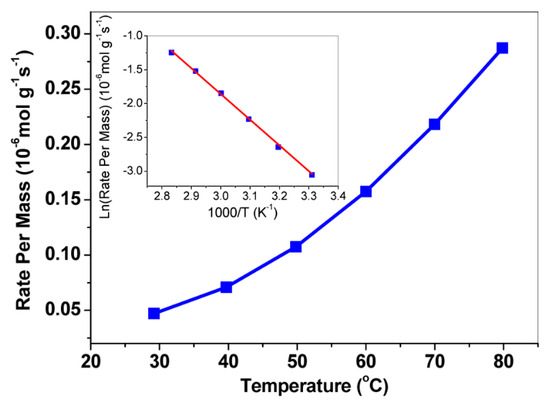
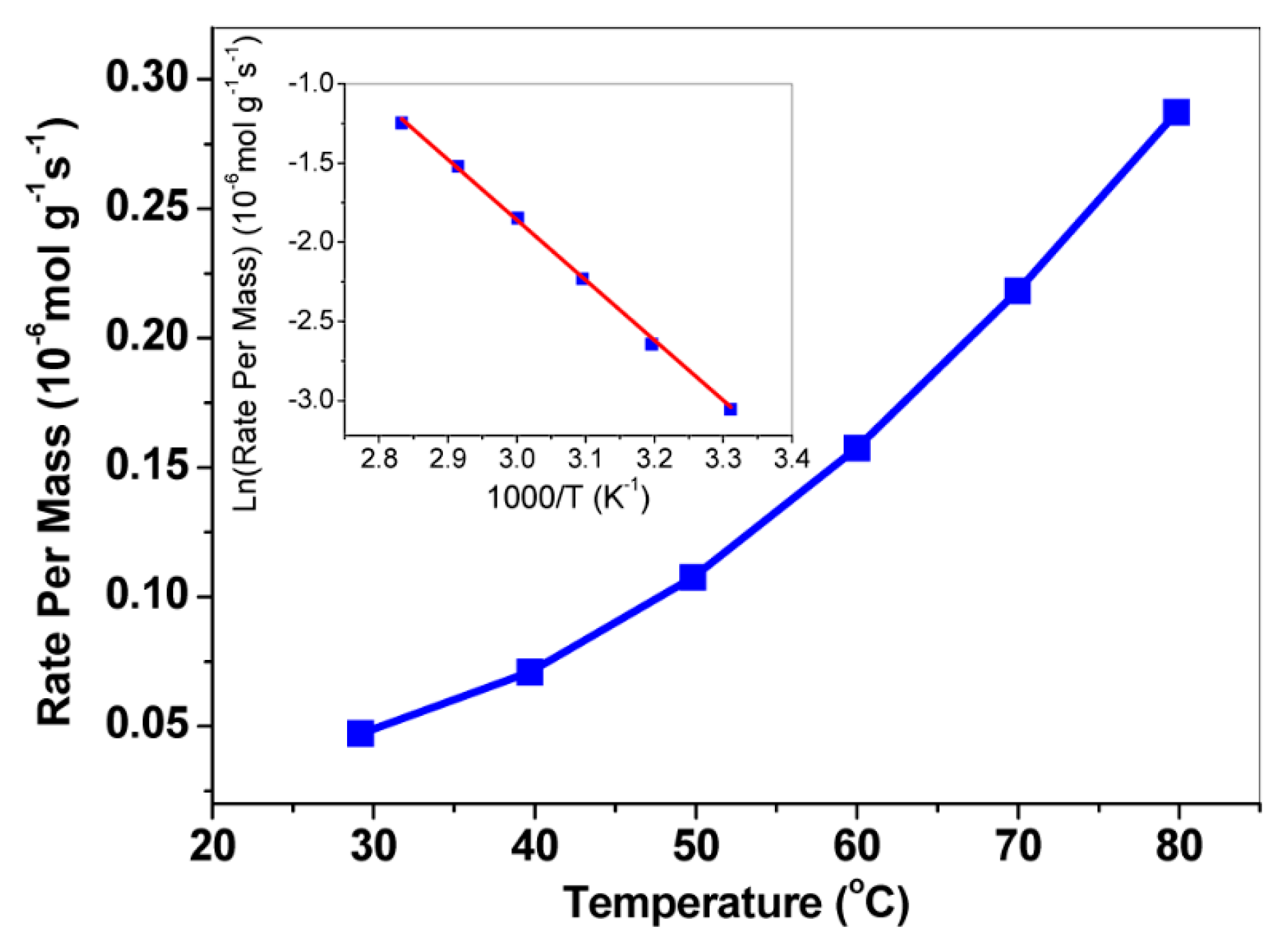
Figure 9.
Catalytic activity of NaAu2 for CO oxidation. Reaction rates were measured in a plug-flow reactor with 150 mg of NaAu2 powders. The reaction gases were composed of CO (14 mL/min), O2 (35 mL/min), and He (31 mL/min) at 1 atm, and the Arrhenius plot showed that the activation energy of NaAu2 for CO oxidation was 31.6 ± 0.7 kJ/mol (inset) [83]. Reprinted with permission from Ref. [83]. Copyright 2013, copyright American Chemical Society.
In addition, the application of IMCs in the oxidation of volatile organic compounds (VOCs, Equation (2)) is particularly noteworthy. Our group probed the use of RuMn IMCs supported on mesoporous carriers (meso-TiO2 and meso-Fe2O3) for the oxidation of methyl ethyl ketone (MEK) and typical aromatics [84,85]. It was observed that these catalysts exhibited excellent performance and stability, which was essential for the industrial applications aiming to reduce VOC emissions. IMCs have demonstrated superior catalytic performance in various oxidative reactions. Similarly, Li and coworkers [86] reported that the PdCu intermetallic nanoparticles showed enhanced activity and durability for the oxidation of formic acid compared to their disordered and commercial counterparts. The improved performance was attributed to the strong electronic interaction and homogeneous catalytic active sites in the ordered intermetallic structure.
CxHy(g) + O2(g) → CO2(g) + H2O(g)
As described in the above synthesis methods, the synthesis process of IMCs generally requires maintaining a reduced or inert atmosphere. During the oxidation processes, however, it was necessary to maintain an oxidative environment. Consequently, IMCs tend to undergo a phase separation during the oxidation process, leading to the formation of a mixture of two metal oxides. Due to the different properties of the metals, the composite metal oxides formed from the separation of IMCs may exhibit various structures, such as core-shell, heterojunctions, and solid solutions. These unique structures can catalyze the specific reactions. For instance, Shan and coworkers [31] emphasized that the surface oxygenation of multicomponent nanoparticles was needed for the active and stable oxidation catalysts. The surface oxygenation of multicomponent nanoparticles (e.g., Pt-alloyed with Ni and Co) was shown to create the active sites that were crucial for the total oxidation of hydrocarbons (e.g., propane). This approach could give rise to a catalyst with a decreased oxidation temperature and a high catalytic stability under harsh reaction conditions.
In conclusion, IMCs hold great promise in the field of catalysis, especially for the oxidative reactions. Their unique properties, combined with the advanced synthetic methods and theoretical insights, enable the development of the highly efficient and stable catalysts for the catalytic applications from environmental remediation to energy production. The ongoing research work is aimed at further optimizing these materials and exploring their potential in the novel and existing catalytic processes.
4.2. Hydrogenation Catalysis
4.2.1. Hydrogenation of Multiple Bonds
IMCs, typically composed of base metal elements, are sensitive to air, and thus are suitable for chemical reactions conducted in inert or reduced atmospheres. Hydrogenation of multiple bonds, such as C=C, C≡C, C=O, and N=O compounds, represents a broad class of probe reactions involving IMC catalysis. This section discusses the catalytic performance of the IMCs using the hydrogenation of carbon oxides (CO or CO2, Equation (3)) as an example.
CO or CO2(g) + H2(g) → CH4(g) + H2O(g)
The methanation of CO or CO2 is widely studied in the research of coal-to-natural gas. Generally speaking, the fourth-period transition metal-based IMCs (MxNy; M = Cu, Ni, Co, and Fe; N = Th, U, Si, and Ti) [87,88,89] are considered excellent hydrogen storage materials and are extensively used for the hydrogenation of carbon oxides. Liang et al. [90] prepared the Ni–Si IMCs supported on silica, and found that the catalyst containing an intermetallic Ni–Si phase (Ni2Si, NiSi or NiSi2) exhibited higher CO conversion and CH4 selectivity, and better stability in CO hydrogenation, compared to the single nickel-loaded counterpart. The authors attributed the increased activity to the strong interaction between nickel and silicon, which prevented nanoparticle sintering and carbon deposition. In addition, Nørskov and collaborators [25] reported that the IMC Ni5Ga3 catalyst showed a high catalytic activity for the hydrogenation of CO2 to methanol, with the CO production being significantly lower than that of the traditional copper/zinc oxide/alumina. Apart from nickel-based catalysts, palladium-based catalysts for the hydrogenation of CO2 to methanol have also been reported. For instance, Ota et al. [70] fabricated nanoscale IMC Pd2Ga and PdZn catalysts for the hydrogenation of CO2, and observed that both catalysts also exhibited higher CO2 conversions and methanol selectivities compared to the single metal catalysts.
4.2.2. Semi-Hydrogenation
The selective semi-hydrogenation of alkynols to alkenols (Equation (4)) is a critical reaction in the production of fine chemicals and pharmaceuticals. IMCs, such as PdZn stabilized on ZnO/N-decorated carbon hollow spheres, have demonstrated high activities and selectivities for the above transformation reactions. The electronic modulation provided by the intermetallic structure enhanced the catalytic performance, as shown in the study by Ye and colleagues [91]. Meanwhile, Armbrüster and coworkers [23] used Al13Fe4 as a potential low-cost alternative to palladium for heterogeneous hydrogenation. Such an IMC exhibited high stability and promising catalytic performance in the semi-hydrogenation of acetylene. Almisbaa et al. [92] used the DFT-based modeling approach to study the selective hydrogenation of acetylene over the Ni, Ni3In, NiIn, and Ni2In3 intermetallic surface. The authors underscored the significance of taking into account the oligomerization reaction, the impact of surface coverage, and the constituents of the feedstock when assessing the selectivity of a catalyst. In addition, PdGa/Al2O3 [93], Pd2Ga/Al2O3 [93], Pd2Ga/C [94], and PdZn/ZnO [95] used for the semi-hydrogenation of acetylene were found to exhibit good catalytic activities. The introduction of Ga or Zn weakened the adsorption of the target product (i.e., enhanced its desorption) and improved the dissociation capability of H2, thereby enhancing the selectivity and yield of the target product.
R–C≡C–CH2–OH + H2 → R–C=C–CH2–OH
4.2.3. Selective Hydrogenation
The conversion of acetylene to ethylene (Equation (5)) via hydrogenation represents a reaction of significant industrial value. Hu and coworkers [96] reported the use of MOF-confined sub-2 nm intermetallic PdZn nanoparticles for this reaction. These nanoparticles displayed excellent size uniformity, thermal stability, and catalytic performance, which were attributed to the synergistic geometric and electronic effects of the intermetallic phase. The PdZn/ZnO [97], Pd3Pb/SiO2, and Pd3Bi/SiO2 [98] catalysts used for the hydrogenation of fatty alkynes were observed to show higher selectivities toward fatty alkenes. Wang and colleagues [99] synthesized the twinned intermetallic Pt2Mo nanocrystals that exhibited high selectivities and resistance to CO poisoning during the hydrogenation of nitroarenes. The twin boundaries and local regions of the Pt2Mo catalyst weakened CO adsorption, hence enhancing the catalytic performance.
H–C≡C–H + H2 → H–C=C–H
4.2.4. Hydroformylation
Traditionally, hydroformylation (i.e., the process of converting alkenes to aldehydes, Equation (6)) relies on homogeneous catalysts. However, Chen and coworkers [69] prepared the RhZn intermetallic nanoparticles that showed a superior turnover frequency and chemoselectivity compared to the homogeneous catalyst. DFT calculation results demonstrated that the intermetallic surface weakened the binding strength of the reaction intermediates, thus leading to lower activation energy barriers.
R–CH=CH2 + H2 + CO → R–CH2–CH2–CHO
4.2.5. Selective Hydrogenation of Unsaturated Aldehydes
Yu and coauthors [100] reported the synthesis of Ni-based IMCs (IMCs) derived from the layered double hydroxide (LDHs) precursors. These IMCs, particularly NiBi, showed a high selectivity in the hydrogenation of C=O (Equation (7)) in various unsaturated aldehydes. The unique surface atomic structure of NiBi IMCs induced a vertical adsorption configuration of the substrate, hence favoring selective hydrogenation.
In summary, IMCs have shown great potential in various hydrogenation reactions due to their unique properties. They offer a means to enhance catalytic activity and selectivity, develop the CO-resistant catalysts, and provide low-cost alternatives to noble metals.
R–CH=CH–CHO + H2 → R–CH=CH–CH2OH
4.3. Electrocatalysis
IMCs have emerged as a promising class of materials for electrocatalysis due to their unique electronic structures, tunable compositions, and enhanced stability. These materials offer a rich platform for the development of highly efficient catalysts for various electrochemical reactions, including hydrogen evolution reaction (HER, Equation (8)) [101,102,103], oxygen reduction reaction (ORR, Equation (9)), oxygen evolution reaction (OER, Equation (10)), fuel cells [68,72], carbon dioxide reduction reaction (CO2RR) [38], ammonia synthesis [104], and so on. Table 1 lists the catalytic activities of some of the electrocatalysts.
4.3.1. HER Electrocatalysis
Pu and coworkers [101] discussed the overarching methods for preparing carbon-group intermetallic catalysts based on transition metals, which were designed to catalyze hydrogen evolution effectively across a diverse pH range. They emphasized the application of specific IMCs and their synthesis via a molten-salt-assisted route. These compounds exhibited excellent catalytic activities and durability, with the PtSi catalyst showing particularly impressive performance in the HER with low overpotentials and high faradaic efficiency. Shi and coworkers [102] presented an investigation on the multicomponent intermetallic nanoparticles (i.e., Mo(NiFeCo)4), which were integrated on a hierarchical nickel network (i.e., Mo(NiFeCo)4/Ni). These intermetallic nanoparticles served as the robust hydrogen-evolution electrocatalysts with improved activities and durability. The intermetallic MoNi4 matrix, enriched with a high-entropy NiFeCo sublattice, furnished the dual-functional electroactive sites that facilitated the dissociation of water and the adsorption/combination of hydrogen, thus enhancing the thermodynamic stability. The self-supported nanoporous Mo(NiFeCo)4/Ni electrode demonstrated the exceptional HER electrocatalytic performance with a low Tafel slope, a high current density, and a long-term stability in a KOH aqueous solution (1 mol/L). Ordered mesoporous intermetallic trimetals also show similar catalytic performance. Liu’s research group [23] investigated the ordered mesoporous intermetallic trimetal MI-PtZnCo catalyst, which exhibited a high mass activity and a high specific activity for the HER in alkaline media. This trimetallic catalyst showed a remarkable turnover frequency and cycling stability. The authors pointed out that the introduction of Co into the PtZn intermetallic structure enriched the surface with electrons, accelerated the Tafel kinetics, and enhanced the HER activity and stability of the electrocatalyst.
2H+(aq) + 2e− → H2(g)
4.3.2. Oxygen Reduction Reaction (ORR)
The rational design and synthesis of intermetallic nanocatalysts with well-defined structural and compositional features can lead to significant advancements in the field of electrocatalysis, particularly in energy conversion and storage. Based on IMCs, Bu and coworkers [105] synthesized a novel class of intermetallic Pt–Pb–Ni octahedra using a simple chemical approach. These nanostructures featured a unique intermetallic core, active surface composition, and exposed facets that significantly enhanced the ORR performance. The optimized PtPb1.12Ni0.14 octahedra catalyst demonstrated superior specific and mass activities for the ORR, which were approximately 20 and 11 times higher than those of the commercial Pt/C catalysts, respectively. Furthermore, the PtPb1.12Ni0.14 octahedra catalyst exhibited excellent stability, with negligible activity decay after over 15,000 potential sweeps. The distinctive electronic configuration of an IMC exerts a beneficial influence on the ORR, thus enhancing its overall efficiency and performance. Mondal et al. [36] explored the use of the chemically modulated, ordered, intermetallic catalysts (i.e., Pd2Ge with the site-selective Pt substitution) for the ORR in alkaline fuel cells, among which the Pt0.2Pd1.8Ge catalyst exhibited an improved half-wave potential, mass activity, and stability compared to the state-of-the-art Pt/C catalyst. In situ spectroscopic characterization results revealed a complete 4e− transfer pathway, which minimized the formation of hydrogen peroxide and demonstrated the potential of these IMCs in enhancing the ORR performance. The atomic ordered structures of IMCs are considered to be the primary reason for enhancing catalytic activity and stability of the ORR due to their thermodynamic stability and strong orbital interactions [62,63]. These characteristics contribute to the improved catalytic performance, as the ordered arrangement of atoms in intermetals leads to a more stable and effective catalytic site for the ORR process. Liang et al. [62] prepared a series of intermetallic PtFe catalysts with varying ordering degrees (10–70%) using a wet-impregnation method and a two-step annealing process. A positive correlation was found between the ordering degree and the ORR performance. The highly ordered PtFe/Pt catalyst showed superior mass activity and durability. The improved performance of such a catalyst was attributed to the compressive strain effect induced by the intermetallic PtFe core and the more thermodynamically stable intermetallic structure compared to the disordered alloys.
O2(g)+4H+(aq)+4e− → 2H2O(l)
4.3.3. Oxygen Evolution Reaction (OER)
IMCs have emerged as the promising candidates for the OER due to their unique electronic structures, high conductivity, and rich active sites [106,107]. IMCs often exhibit synergistic effects between different metal atoms, which can enhance their catalytic activities for the OER by providing an optimal balance of adsorption and desorption energies for the reaction intermediates. Meanwhile, the ordered arrangement of atoms in intermetallic structures often leads to a high density of active sites, which is beneficial for accelerating OER kinetics [108,109]. Chen et al. [110] revealed that the enhanced OER activity of the catalyst was attributed to the increased degeneracy of the Ir 5d electron of the surface IrOx sites induced by the intermetallic IrGa core. This modification increased the adsorption capacity of the IrOx layer for O and OH binding, which lowered the energy barrier of the rate-determining step in the OER. Zhang et al. [111] reported the synthesis of Laves phase Ir2Sm intermetallic nanoparticles (which were used as the highly active electrocatalysts for the acidic OER). The alloying of Sm with Ir in the intermetallic nanoparticles modulated the electronic property of Ir, hence enhancing the OER activity. Such research provided insights into the design and applications of the high-performance rare earth alloy catalysts for the energy-related electrochemical processes.
2H2O(l) → O2(g) + 4H+(aq) + 4e−
In summary, the preparation and catalytic applications in HER, ORR, and OER of IMCs with well-defined atomic arrangements and tunable electronic properties have made significant advancements. They offer a pathway to design the catalysts with high activities, stability, and selectivities for a range of electrochemical reactions. The development of synthesis methods, such as molten-salt-assisted and alloying/dealloying approaches, allows for the creation of nanostructures with optimized performance. These materials hold a great potential for practical applications in energy conversion and storage, particularly in the drive towards the production of sustainable hydrogen and clean energies.
4.4. Carbon–Carbon Coupling Reactions
Carbon–carbon coupling is essential in organic chemistry for building complex molecules and synthesizing bioactive compounds. This process is vital for creating carbon skeletons, making it significant in the fields of medicinal chemistry and materials science. In the pursuit of efficient Suzuki cross-coupling reactions, there has been a focus on developing heterogeneous catalysts. Most of these efforts are concentrated on developing palladium catalysts with electron-rich supports, or utilizing photogenerated or hot electrons in heterojunctions to lower activation barriers [112].
The conventional approach in homogeneous palladium catalysis involves electron-donating ligands creating electron-rich active sites, facilitating substrate activation. Some researchers [112,113] have proposed that combining rare earth metals with active transition metals could induce charge transfer, creating negatively charged active sites. This charge transfer could enable electron movement from the catalyst to the lowest unoccupied molecular orbitals (LUMOs) of adsorbed molecules, weakening their chemical bonds and reducing the energy required for molecular dissociation. The research work highlighted the development and use of IMCs (e.g., Y3Pd2 and Pd-ZrC) as efficient and stable catalysts for the Suzuki cross-coupling reactions. The intermetallic catalysts demonstrated the significantly higher catalytic activity compared to the traditional palladium-based catalysts. For instance, Y3Pd2 showed a ten-fold increase in activity and a reduction in activation energy by nearly 35%. Meanwhile, the catalysts exhibited excellent stability, with Y3Pd2 maintaining its activity even after 20 cycles of use [102]. This result indicates a high potential for practical applications in chemical industries. This research provides insights into the catalytic mechanism, suggesting that the electron-rich nature of the palladium sites in these IMCs facilitates the activation of aryl halides (a rate-determining step in the Suzuki reaction). In addition, the addition of some transition metal elements can also achieve the modulation of the electronic structure of Pd. It was reported that the Pd3Cu IMC nanoplates could effectively catalyze the Suzuki coupling reaction without the leaching of Pd ions [114], which was a common issue involved in the monometallic and short-range-ordered Pd bimetallic nanocatalysts. The authors found that the presence of Cu in the Pd3Cu IMC altered the electronic structure of Pd, leading to a lower energy barrier for the activation and cleavage of the C–Br bond, which was the rate-determining step in the coupling reaction. This discovery suggests that the IMCs offer a robust and efficient catalytic pathway for the C–C coupling reactions, providing a promising strategy for designing the nanocatalysts used for the synthesis of value-added chemicals.
4.5. Other Catalytic Applications
4.5.1. Steam Reforming
The process of steam reforming that is applied to fuels (including methane, methanol, and various hydrocarbons) is a prevalent method for hydrogen generation. This is attributed to their high hydrogen yields and the ease with which they can be stored and transported. Consequently, these feedstocks have garnered a broad acceptance as potent precursors for catalytic hydrogen production through cracking. Armbrüster et al. [115] have recently reported the catalytic effect of bimetallic systems on methanol steam reforming, and found that a crystalline phase of IMCs, such as PdIn or Pd2In3, would be generated on PdO/In2O3 after hydrogen treatment at 300 or 390 °C. These catalysts containing IMC phases showed a good selectivity toward carbon dioxide in methanol steam reforming and maintained good activity stability even after 100 h of on-stream reaction. The performance of IMCs in the steam-reforming reactions is often attributed to the synergistic effects between the metal and its oxide, or between different metals within the intermetallic structure. These effects can lead to improved CO2 selectivity and enhanced catalytic activity [116,117]. For instance, Ota et al. [70] found that the formation of PdZn and Pd2Ga IMCs resulted in improved activities and selectivities for methanol synthesis and methanol steam reforming (MSR). Rameshan and coworkers [118] pointed out that the Cu(Zn)0/Zn(ox) interface was crucial for the bifunctional catalyst operation, with the Cu(Zn)0 regions favoring the selective methanol dehydrogenation and the Cu(Zn)0Zn(ox) sites assisting the water activation.
4.5.2. Reverse Water Gas Shift Reaction
Wang and coworkers [119] conducted an exhaustive investigation on the In–Ni IMCs, which were derived from the layered double hydroxides (LDHs), for their application as catalysts in the reverse water gas shift (RWGS) reaction. The results indicated that by increasing the In/Ni ratio in the IMCs, the selectivity toward CO production was enhanced. Such an enhancement in CO selectivity was achieved by inhibiting the adsorption of CO* through a mechanism called as the “active site isolation”, where Ni, the active site for CO2 hydrogenation, was isolated by In. Density functional theory (DFT) calculations uncovered that the hydrogenation of CO2 predominantly resulted in the formation of CO instead of CH4 and/or CH3OH. Moreover, the redox mechanism was found to be advantageous for the RWGS reaction when this reaction was catalyzed by the In–Ni IMCs. This catalyst demonstrated high performance with 99.8% CO selectivity at a CO2 conversion of 50.7% and did not show significant deactivation after over 250 h of on-stream reaction, demonstrating its potential for industrial applications. Zhao and coworkers [120] prepared a Ru-Sn/La2O2CO3 catalytic system that showed a CO selectivity of over 99% for CO2 hydrogenation at 400 °C. Characterization studies have revealed that the electron transfer from Sn to Ru alters the adsorption and activation modes of CO2 on Ru, inhibiting methane formation and promoting the RWGS process. An ultra-low, Ru-loaded (0.01 wt%) Ru-Sn/La2O2CO3 catalyst has exhibited a CO2 hydrogenation rate of 103 times higher than the best data reported in the literature, and it was successfully applied to the hydrogenation of blast furnace gas (BFG) to syngas, showing a high stability at 850 °C for 1000 h.
4.5.3. Propane Dehydrogenation
Ye and coworkers [121] prepared the highly ordered hexagonal Pt1Sn1 IMCs. It was found that the Pt–Sn co-ordination number significantly affected catalytic performance, with turnover rates (TORs) increasing and propylene selectivity remaining high. The Pt–Sn bimetallic catalysts exhibited improved performance for propane dehydrogenation due to the geometric and electronic effects of Sn on Pt. To reduce catalyst costs, Laursen’s research group [122] developed a non-precious IMC catalyst (i.e., Ni3Ga) for the purpose of directly converting propane into propylene via dehydrogenation. This catalyst exhibited a high selectivity (ca. 94%), a comparable activity (turnover frequency (TOF) = 4.7 × 10−2 s−1), and good stability (the selectivity decreased from 94 to 81% in an 82-h test at 600 °C). The synthesis method used to stabilize the Ni3Ga phase allowed the surface composition of the catalyst nanoparticles to be tuned by adjusting Ni and Ga loadings, which could improve the selectivity toward propylene. The Ni component on the active surface of the catalyst played an essential role in propelling the dehydrogenation reaction and bolstering the conversion rate. Concurrently, the incorporation of Ga was crucial for moderating the surface reactivity and ensuring a balanced catalytic performance, hence improving the desirable product selectivity and catalyst stability. Traditional catalytic PDH requires temperatures above 600 °C, leading to side reactions and catalyst deactivation. Furukawa’s team [123] presented a new concept involving the double decoration of PtGa intermetals with Pb and Ca to enhance the catalyst stability. This design leveraged the geometric and electronic promotion effects, respectively, leading to a high catalytic stability at 600 °C with minimal deactivation over one month. To lower the reaction temperature, Furukawa and colleagues [124] introduced a novel catalytic system for the low-temperature PDH by integrating the surface protonics with the intermetallic active sites. The application of an electric current to the intermetallic Pt–In/TiO2 catalyst achieved a propylene yield of 10.2% with high selectivity at 250 °C, surpassing the thermodynamic equilibrium yield of 0.15%. The electro-assisted proton collisions with propane facilitated an unconventional reaction pathway for the low-temperature PDH. Alloying Pt with In significantly enhanced the activity and selectivity due to the increased electron density of Pt.
5. Conclusions and Perspectives
In summary, the foray into the realm of IMCs for catalytic applications has unveiled a trove of opportunities to revolutionize industrial and environmental processes. As we reflect on the journey from fundamental principles to state-of-the-art applications, it is evident that IMCs are more than mere curiosities of materials science—they are potent agents of change in the catalysis domain. This review offers a survey of the synthesis methods, structural attributes, and catalytic roles of IMCs. The prevalent synthesis methods, categorized by their media into solid-phase, liquid-phase, and gas-phase processes, employ physical or chemical strategies to orchestrate the precise arrangement of disparate metal atoms. These methods facilitate the creation of IMCs with tailored compositions and architectures, predicated on their distinctive, ordered crystal lattices, electronic configurations, and phase stabilities, which in turn endow them with exceptional performance in hydrogenation, oxidation, electrocatalysis, and carbon–carbon coupling reactions. This review may help researchers in the field of catalysis to understand the properties of IMCs and their applications in different catalytic reactions.
The study of IMCs in catalytic processes is of great scientific and technological importance. While extensive research has already been conducted on these compounds, there remains a constellation of aspects that promote their application in catalysis. Looking ahead, there is ample scope for further exploration within these domains, with the potential to significantly accelerate advancements and foster the evolution of catalytic science.
(i) While significant strides have been made in the synthesis of IMCs, challenges such as the scalability, long-term stability, and component leaching persist. Addressing these issues requires a concerted effort across disciplines, from materials chemistry to surface science and engineering;
(ii) The advent of computational chemistry has empowered researchers with the ability to simulate and predict the behavior of intermetallic catalysts. This predictive power is a double-edged sword, offering both profound insights and the necessity for experimental validation;
(iii) Improved understanding of catalytic mechanisms: investigating catalytic behaviors of the IMCs provides insights into the fundamental mechanisms underlying the catalytic reactions. By elucidating the interactions between reactants and the catalyst’s surface, as well as the role of different metal species, one can gain a deeper understanding of the reaction kinetics and selectivity, thus enabling the rational design of more efficient catalysts.
(iv) Development of novel catalysts: by exploring different metal combinations and crystal structures, one can identify the IMCs with unique catalytic properties, uncover new catalytic reactions, or improve existing reactions. This knowledge can give rise to the development of more efficient and sustainable catalytic processes.
Author Contributions
The manuscript was written with the contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFB3504101 and 2022YFB3506200), the National Natural Science Foundation of China (22306008, 21876006, 21976009, and 21961160743), the Foundation on the Creative Research Team Construction Promotion Project of Beijing Municipal Institutions (IDHT20190503), the Natural Science Foundation of Beijing Municipal Commission of Education (KM201710005004), and the Development Program for the Youth Outstanding—Notch Talent of Beijing Municipal Commission of Education (CIT&TCD201904019).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, R.Y.; Tian, X.R.; Ding, X.L.; Hou, Z.Q.; Li, Z.Y.; Yu, X.H.; Wang, J.; Wu, L.K.; Jing, L.; Deng, J.G.; et al. Regulating catalytic stability of PtSnM/CeO2 (M = Mn, W, Nb) catalysts via the closely coupled multi-active sites to promote multicomponent VOCs oxidation. Chem. Eng. J. 2023, 471, 144456. [Google Scholar] [CrossRef]
- Lin, F.W.; Zhang, Z.M.; Li, N.; Yan, B.B.; He, C.; Hao, Z.P.; Chen, G.Y. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem. Eng. J. 2021, 404, 126534. [Google Scholar] [CrossRef]
- Xing, F.L.; Ma, J.M.; Shimizu, K.; Furukawa, S. High-entropy intermetallics on ceria as efficient catalysts for the oxidative dehydrogenation of propane using CO2. Nat. Commun. 2022, 13, 5065. [Google Scholar] [CrossRef]
- Sharma, H.R.; Shimoda, M.; Tsai, A.P. Quasicrystal surfaces: Structure and growth of atomic overlayers. Adv. Phys. 2007, 56, 403–464. [Google Scholar] [CrossRef]
- Feng, S.M.; Geng, Y.Y.; Liu, H.Y.; Li, H. Targeted intermetallic nanocatalysts for sustainable biomass and CO2 valorization. ACS Catal. 2022, 12, 14999–15020. [Google Scholar] [CrossRef]
- Wang, Y.F.; Sun, D.; Chowdhury, T.; Wagner, J.S.; Kempa, T.J.; Hall, A.S. Rapid room-temperature synthesis of a metastable ordered intermetallic electrocatalyst. J. Am. Chem. Soc. 2019, 141, 2342–2347. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Gschneidner Jr, K.; Russell, A.; Pecharsky, A.; Morris, J.; Zhang, Z.H.; Lograsso, T.; Hsu, D.; Chester Lo, C.H.; Ye, Y.Y.; Slager, A.; et al. A family of ductile intermetallic compounds. Nat. Mater. 2003, 2, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Wei, M. intermetallic compound catalysts: Synthetic scheme, structure characterization and catalytic application. J. Mater. Chem. A. 2020, 8, 2207–2221. [Google Scholar] [CrossRef]
- Furukawa, S.; Komatsu, T. intermetallic compounds: Promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 2017, 7, 735–765. [Google Scholar] [CrossRef]
- Zhang, J.M.; Shen, L.F.; Jiang, Y.X.; Sun, S.G. Random alloy and intermetallic nanocatalysts in fuel cell reactions. Nanoscale 2020, 12, 19557–19581. [Google Scholar] [CrossRef]
- Liu, C.; Lu, W.J.; Xia, W.Z.; Du, C.W.; Rao, Z.Y.; Best, J.P.; Brinckmann, S.; Lu, J.; Gault, B.; Dehm, G.; et al. Massive interstitial solid solution alloys achieve near-theoretical strength. Nat. Commun. 2022, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xie, Z.; Yao, B.D.; Si, J.X.; Wu, L.; Wu, X.Y.; Wang, Y.X. Theoretical investigation on solid solution effect in dilute Zr alloys: Insight into mechanical and thermal properties. J. Mater. Res. Technol. 2024, 29, 738–750. [Google Scholar] [CrossRef]
- Voznyaka, I.; Tokaychuka, Y.; Hlukhyyb, V.; Fässlerb, T.F.; Gladyshevskiia, R. Interstitial solid solution Hf5GaxSn3 (x = 0–1). J. Alloys Compd. 2012, 512, 246–251. [Google Scholar] [CrossRef]
- Xin, J.Z.; Zhang, Y.; Wu, H.J.; Zhu, T.J.; Fu, T.Z.; Shen, J.J.; Pennycook, S.J.; Zhao, X.B. Multiscale defects as strong phonon scatters to enhance thermoelectric performance in Mg2Sn1–xSbx solid solutions. Small Methods 2019, 3, 1900412. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.G.; Bai, H.Y.; Ning, Z.L.; Sun, J.F.; Huang, Y.J. Rationality of two-phase coexistence with no element segregation in a CuZr-based amorphous alloy composite. J. Alloys Compd. 2023, 962, 171185. [Google Scholar] [CrossRef]
- Williams, B.P.; Qi, Z.Y.; Huang, W.Y.; Tsung, C.K. The impact of synthetic method on the catalytic application of intermetallic nanoparticles. Nanoscale 2020, 12, 18545–18562. [Google Scholar] [CrossRef] [PubMed]
- Armbrüster, M.; Kovnir, K.; Friedrich, M.; Teschner, D.; Wowsnick, G.; Hahne, M.; Gille, P.; Szentmiklósi, L.; Feuerbacher, M.; Heggen, M.; et al. Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation. Nat. Mater. 2012, 11, 690–693. [Google Scholar] [CrossRef]
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer―Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Armbrüster, M.; Kovnir, K.; Behrens, M.; Teschner, D.; Grin, Y.; Schlögl, R. Pd―Ga intermetallic compounds as highly selective semi-hydrogenation catalysts. J. Am. Chem. Soc. 2010, 132, 14745–14747. [Google Scholar] [CrossRef] [PubMed]
- Han, A.J.; Zhang, J.; Sun, W.M.; Chen, W.X.; Zhang, S.L.; Han, Y.H.; Feng, Q.C.; Zheng, L.R.; Gu, L.; Chen, C.; et al. Isolating contiguous Pt atoms and forming Pt-Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation. Nat. Commun. 2019, 10, 3787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Lv, H.; Sun, L.Z.; Jia, F.R.; Liu, B. Ordered mesoporous intermetallic trimetals for efficient and pH-universal hydrogen evolution electrocatalysis. Adv. Energy Mater. 2022, 12, 2201478. [Google Scholar] [CrossRef]
- Yin, P.; Niu, X.F.; Li, S.B.; Chen, K.; Zhang, X.; Zuo, M.; Zhang, L.; Liang, H.W. Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts. Nat. Commun. 2024, 15, 415. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni–Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef]
- Ji, Y.L.; Chen, Z.; Wei, R.L.; Yang, C.; Wang, Y.H.; Xu, J.; Zhang, H.; Guan, A.X.; Chen, J.T.; Sham, T.K.; et al. Selective CO-to-acetate electroproduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 2022, 5, 251–258. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Y.Z.; Sun, L.Z.; Yamauchi, Y.; Liu, B. A general protocol for precise syntheses of ordered mesoporous intermetallic nanoparticles. Nat. Protoc. 2023, 18, 3126–3154. [Google Scholar] [CrossRef]
- Feng, G.; An, L.; Li, B.; Zuo, Y.X.; Song, J.; Ning, F.H.; Jiang, N.; Cheng, X.P.; Zhang, Y.F.; Xia, D.G. Atomically ordered non-precious Co3Ta intermetallic nanoparticles as high-performance catalysts for hydrazine electrooxidation. Nat. Commun. 2019, 10, 4514. [Google Scholar] [CrossRef]
- Han, S.W.; Park, H.; Han, J.; Kim, J.C.; Lee, J.; Jo, C.; Ryoo, R. PtZn intermetallic compound nanoparticles in mesoporous zeolite exhibiting high catalyst durability for propane dehydrogenation. ACS Catal. 2021, 11, 9233–9241. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Liu, Y.X.; Deng, J.G.; Lu, Y.; Xie, S.H.; Fang, X.Z.; Dai, H.X. Highly active and stable Pd–GaOx/Al2O3 catalysts derived from intermetallic Pd5Ga3 nanocrystals for methane combustion. ChemCatChem 2018, 10, 5637–5648. [Google Scholar] [CrossRef]
- Shan, S.Y.; Li, J.; Maswadeh, Y.; O’Brien, C.; Kareem, H.; Tran, D.T.; Lee, I.C.; Wu, Z.P.; Wang, S.; Yan, S.; et al. Surface oxygenation of multicomponent nanoparticles toward active and stable oxidation catalysts. Nat. Commun. 2020, 11, 4201. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yao, B.Q.; Pillai, H.S.; Zang, W.J.; Han, X.; Liu, Y.Q.; Yu, S.W.; Yan, Z.H.; Min, B.; Zhang, S.; et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat. Synth. 2023, 2, 624–634. [Google Scholar] [CrossRef]
- Guo, M.; Ma, P.J.; Wei, L.; Wang, J.Y.; Wang, Z.W.; Zheng, K.; Cheng, D.J.; Liu, Y.X.; Dai, H.X.; Guo, G.S.; et al. Highly selective activation of C–H bond and inhibition of C–C bond cleavage by tuning strong oxidative Pd sites. J. Am. Chem. Soc. 2023, 145, 11110–11120. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ma, P.J.; Wang, J.Y.; Xu, H.X.; Zheng, K.; Cheng, D.J.; Liu, Y.X.; Guo, G.S.; Dai, H.X.; Duan, E.H.; et al. Synergy in Au-CuO janus structure for catalytic isopropanol oxidative dehydrogenation to acetone. Angew. Chem. Int. Ed. 2022, 61, e202203827. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.Y.; Zhang, M.C.; Liu, Y.X.; Xie, S.H.; Deng, J.G.; Ke, X.X.; Jing, L.; Hou, Z.Q.; Zhang, X.; Liu, F.D.; et al. Engineering platinum catalysts via a site-isolation strategy with enhanced chlorine resistance for the elimination of multicomponent VOCs. Environ. Sci. Technol. 2022, 56, 9672–9682. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bagchi, D.; Riyaz, M.; Sarkar, S.; Singh, A.K.; Vinod, C.P.; Peter, S.C. In situ mechanistic insights for the oxygen reduction reaction in chemically modulated ordered intermetallic catalyst promoting complete electron transfer. J. Am. Chem. Soc. 2022, 144, 11859–11869. [Google Scholar] [CrossRef] [PubMed]
- Cesar, L.G.; Yang, C.; Lu, Z.; Ren, Y.; Zhang, G.H.; Miller, J.T. Identification of a Pt3Co surface intermetallic alloy in Pt–Co propane dehydrogenation catalysts. ACS Catal. 2019, 9, 5231–5244. [Google Scholar] [CrossRef]
- Ratschmeier, B.; Paulsen, C.; Stallberg, K.; Roß, G.; Daum, W.; Pöttgen, R.; Braunschweig, B. Cu/Au(111) surfaces and AuCu intermetallics for electrocatalytic reduction of CO2 in ionic liquid electrolytes. ACS Catal. 2024, 14, 1773–1784. [Google Scholar] [CrossRef]
- Onda, A.; Komatsu, T.; Yashima, T. Characterization and catalytic properties of Ni–Sn intermetallic compounds in acetylene hydrogenation. Phys. Chem. Chem. Phys. 2000, 2, 2999–3005. [Google Scholar] [CrossRef]
- Endo, N.; Ito, S.; Tomishige, K.; Kameoka, S.; Tsai, A.P.; Hirata, T.; Nishimura, C. CO hydrogenation over a hydrogen-induced amorphization of intermetallic compound CeNi2. Catal. Today 2011, 164, 293–296. [Google Scholar] [CrossRef]
- Endo, N.; Kameoka, S.; Tsai, A.P.; Lingling, Z.; Hirata, T.; Nishimura, C. Hydrogen absorption properties of intermetallic compounds in the Au–Zr binary system. J. Alloys Compd. 2009, 485, 588–592. [Google Scholar] [CrossRef]
- Zou, P.F.; Zheng, C.H.; Hu, L.; Wang, H.P. Rapid growth of TiNi intermetallic compound within undercooled Ti50Ni50 alloy under electrostatic levitation condition. J. Mater. Sci. Technol. 2021, 77, 82–89. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wu, C.S.; Shi, L.; Su, H. Evolution of microstructures and intermetallic compounds at bonding interface in friction stir welding of dissimilar Al/Mg alloys with/without ultrasonic assistance. J. Mater. Sci. Technol. 2023, 139, 31–46. [Google Scholar] [CrossRef]
- Ma, H.; Qin, G.L.; Geng, P.H.; Ao, Z.Y.; Chen, Y. Effect of intermetallic compounds on the mechanical property and corrosion behavior of aluminum alloy/steel hybrid fusion-brazed welded structure. J. Manuf. Process 2022, 75, 170–180. [Google Scholar] [CrossRef]
- Zhao, B.K.; Zhang, Q.H.; Fu, X.Q.; Qiao, D.X.; Zhang, L.; Chen, X.; Gu, L.; Lu, Y.P.; Yu, Q. Brittle-to-ductile transition in Ti–Pt intermetallic compounds Beikai. Sci. Bull. 2021, 66, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.J.; Wang, C.; Yan, Q.Q.; Yin, P.; Tong, L.; Liang, H.W. Phase diagrams guide synthesis of highly ordered intermetallic electrocatalysts: Separating alloying and ordering stages. Nat. Commun. 2022, 13, 7654. [Google Scholar] [CrossRef]
- Gao, M.; Chen, T.J. Formation of intermetallic compounds during reaction between Ti and Al–Mg alloys with various Mg contents. J. Mater. Sci. Technol. 2023, 159, 225–243. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.I.; Choi, S.; Choi, Y.; Park, S.; Ryu, J. Nonporous oxide-terminated multicomponent bulk anode enabling energy-dense sodium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 26576–26584. [Google Scholar] [CrossRef]
- Gong, M.X.; Shen, T.; Deng, Z.P.; Yang, H.Y.; Li, Z.R.; Zhang, J.J.; Zhang, R.; Hu, Y.Z.; Zhao, X.; Xin, H.L.; et al. Surface engineering of PdFe ordered intermetallics for efficient oxygen reduction electrocatalysis. Chem. Eng. J. 2021, 408, 127297. [Google Scholar] [CrossRef]
- Song, R.R.; Han, J.H.; Okugawa, M.; Belosludov, R.; Wada, T.; Jiang, J.; Wei, D.X.; Kudo, A.; Tian, Y.; Chen, M.W.; et al. Ultrafine nanoporous intermetallic catalysts by high-temperature liquid metal dealloying for electrochemical hydrogen production. Nat. Commun. 2022, 13, 5157. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.J.; Yang, C.P.; Hwang, S.; Yang, M.H.; Overa, S.; Dong, Q.; Yao, Y.G.; Brozena, A.H.; Cullen, D.A.; Chi, M.F.; et al. Multi-principal elemental intermetallic nanoparticles synthesized via a disorder-to-order transition. Sci. Adv. 2022, 8, eabm4322. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.B.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- Xue, J.N.; Hu, Z.; Li, H.; Zhang, Y.; Liu, C.; Li, M.; Yang, Q.H.; Hu, S. Pd-Sn alloy nanoparticles for electrocatalytic methanol oxidation: Phase evolution from solid solution to intermetallic compounds. Nano Res. 2022, 15, 8819–8825. [Google Scholar] [CrossRef]
- Li, F.; Zong, Y.; Ma, Y.L.; Wang, M.X.; Shang, W.; Tao, P.; Song, C.Y.; Deng, T.; Zhu, H.; Wu, J.B. Atomistic imaging of competition between surface diffusion and phase transition during the intermetallic formation of faceted particles. ACS Nano 2021, 15, 5284–5293. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.H.; Schaak, R. Shape-controlled conversion of β-Sn nanocrystals into intermetallic M–Sn (M = Fe, Co, Ni, Pd) nanocrystals. J. Am. Chem. Soc. 2007, 129, 7339–7345. [Google Scholar] [CrossRef] [PubMed]
- Sra, A.K.; Schaak, R.E. Synthesis of atomically ordered AuCu and AuCu3 nanocrystals from bimetallic nanoparticle precursors. J. Am. Chem. Soc. 2004, 126, 6667–6672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Li, L.Q.; Liao, H.W.; Wang, H.R. Preparation of pure nickel, cobalt, nickel–cobalt and nickel–copper alloys by hydrothermal reduction. J. Mater. Chem. 1999, 9, 2675–2677. [Google Scholar] [CrossRef]
- Heise, M.; Chang, J.H.; Schönemann, R.; Herrmannsdörfer, T.; Wosnitza, J.; Ruck, M. Full access to nanoscale bismuth–palladium intermetallics by low-temperature syntheses. Chem. Mater. 2014, 26, 5640–5646. [Google Scholar] [CrossRef]
- Li, C.C.; Sun, L.; Sun, Y.Q.; Teranishi, T. One-pot controllable synthesis of Au@Ag heterogeneous nanorods with highly tunable plasmonic absorption. Chem. Mater. 2013, 25, 2580–2590. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lin, H.; Huo, J.L.; Ma, T.M.; Du, L. Multi-scale exploring the output performance mechanism in MEA with high intrinsic activity PtCo intermetallic compounds. J. Clean. Prod. 2024, 434, 140072. [Google Scholar] [CrossRef]
- Song, T.W.; Chen, M.X.; Yin, P.; Tong, L.; Zuo, M.; Chu, S.Q.; Chen, P.; Liang, H.W. Intermetallic PtFe electrocatalysts for the oxygen reduction reaction: Ordering degree-dependent performance. Small 2022, 18, 2202916. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.T.; Chen, K.; He, Y.H.; Karakalos, S.; Zhang, H.G.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co intermetallic nanoparticles derived from metal–organic frameworks for oxygen reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Stassi, J.P.; Zgolicz, P.D.; Miguel, S.R.; Scelza, S.O. Formation of different promoted metallic phases in PtFe and PtSn catalysts supported on carbonaceous materials used for selective hydrogenation. J. Catal. 2013, 306, 11–29. [Google Scholar] [CrossRef]
- Furukawa, S.; Ehara, K.; Komatsu, T. Unique reaction mechanism of preferential oxidation of CO over intermetallic Pt3Co catalysts: Surface-OH-mediated formation of a bicarbonate intermediate. Catal. Sci. Technol. 2016, 6, 1642–1650. [Google Scholar] [CrossRef]
- Komatsu, T.; Takasaki, M.; Ozawa, K.; Furukawa, S.; Muramatsu, A. PtCu intermetallic compound supported on alumina active for preferential oxidation of CO in hydrogen. J. Phys. Chem. C 2013, 117, 10483–10491. [Google Scholar] [CrossRef]
- Furukawa, S.; Endo, M.; Komatsu, T. Bifunctional catalytic system effective for oxidative dehydrogenation of 1-butene and n-butane using Pd-based intermetallic compounds. ACS Catal. 2014, 4, 3533–3542. [Google Scholar] [CrossRef]
- Yang, C.L.; Wang, L.N.; Yin, P.; Liu, J.Y.; Chen, M.X.; Yan, Q.Q.; Wang, Z.S.; Xu, S.L.; Chu, S.Q.; Cui, C.H.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Hu, M.Z.; Zhao, S.; Liu, S.J.; Chen, C.; Chen, W.X.; Zhu, W.; Liang, C.; Cheong, W.C.; Wang, Y.; Yu, Y.; et al. MOF-confined sub-2 nm atomically ordered intermetallic PdZn nanoparticles as high-performance catalysts for selective hydrogenation of acetylene. Adv. Mater. 2018, 30, 1801878. [Google Scholar] [CrossRef]
- Ota, A.; Kunkes, E.L.; Kasatkin, I.; Groppo, E.; Ferri, D.; Poceiro, B.; Yerga, R.M.N.; Behrens, M. Comparative study of hydrotalcite-derived supported Pd2Ga and PdZn intermetallic nanoparticles as methanol synthesis and methanol steam reforming catalysts. J. Catal. 2012, 293, 27–38. [Google Scholar] [CrossRef]
- Gunji, T.; Tanabe, T.; Jeevagan, A.J.; Usui, S.; Tsuda, T.; Kaneko, S.; Saravanan, G.; Abe, H.; Matsumoto, F. Facile route for the preparation of ordered intermetallic Pt3P–PtPb core-shell nanoparticles and its enhanced activity for alkaline methanol and ethanol oxidation. J. Power Sources 2015, 273, 990–998. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Ruan, Y.E.; Zhu, M.Z.; Gao, X.P.; Guo, W.X.; Liu, X.K.; Wang, W.Y.; Chen, M.; Wu, G.; Yao, T.; et al. Coupling single-atom sites and ordered intermetallic PtM nanoparticles for efficient catalysis in fuel cells. Small 2023, 19, 2302328. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Guan, J.C.; Wang, X.K.; Williams, C.T.; Liang, C.H. Nickel-silicon intermetallics with enhanced selectivity in hydrogenation reactions of cinnamaldehyde and phenylacetylene. Ind. Eng. Chem. Res. 2012, 51, 3604–3611. [Google Scholar] [CrossRef]
- Komatsu, T.; Tamura, A. Pt3Co and PtCu intermetallic compounds: Promising catalysts for preferential oxidation of CO in excess hydrogen. J. Catal. 2008, 258, 306–314. [Google Scholar] [CrossRef]
- Komatsu, T.; Sou, K.; Ozawa, K. Preparation and catalytic properties of fine particles of Pt–Ge intermetallic compound formed inside the mesopores of MCM-41. J. Mol. Catal. A 2010, 319, 71–77. [Google Scholar] [CrossRef]
- Terada, T.; Kitaura, R.; Ishigaki, S.; Ishibe, T.; Naruse, N.; Mera, Y.; Asahi, R.; Nakamura, Y. Seed-assisted epitaxy of intermetallic compounds with interface-determined orientation: Incommensurate Nowotny chimney-ladder FeGeγ epitaxial film. Acta Mater. 2022, 236, 118130. [Google Scholar] [CrossRef]
- Santra, A.K.; Goodman, D.W. Catalytic oxidation of CO by platinum group metals: From ultrahigh vacuum to elevated pressures. Electrochim. Acta 2002, 47, 3595–3609. [Google Scholar] [CrossRef]
- Over, H.; Muhler, M. Catalytic CO oxidation over ruthenium-bridging the pressure gap. Prog. Surf. Sci. 2003, 72, 3–17. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous gold-based catalysis for green chemistry: Low-temperature CO oxidation and propene oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Freund, H.J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Saravanan, G.; Abe, H.; Xu, Y.; Sekido, N.; Hirata, H.; Matsumoto, S.I.; Yoshikawa, H.; Yamabe-Mitarai, Y. Pt3Ti nanoparticles: Fine dispersion on SiO2 supports, enhanced catalytic CO oxidation, and chemical stability at elevated temperatures. Langmuir 2010, 26, 11446–11451. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.Q.; Li, L.; Zhang, T.; Mou, C.Y.; Lee, J.F. Structural changes of Au-Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 2011, 278, 288–296. [Google Scholar] [CrossRef]
- Xiao, C.X.; Wang, L.L.; Maligal-Ganesh, R.V.; Smetana, V.; Walen, H.; Thiel, P.A.; Miller, G.J.; Johnson, D.D.; Huang, W.Y. Intermetallic NaAu2 as a heterogeneous catalyst for low-temperature CO oxidation. J. Am. Chem. Soc. 2013, 135, 9592–9595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, L.Y.; Deng, J.G.; Liu, Y.X.; Jing, L.; Pei, W.B.; Hou, Z.Q.; Zhang, X.; Yu, X.H.; Dai, H.X. Experimental and density functional theory investigations on the oxidation of typical aromatics over the intermetallic compounds-derived AuMn/meso-Fe2O3 catalysts. J. Catal. 2022, 405, 273–287. [Google Scholar] [CrossRef]
- Wang, J.; Dai, L.Y.; Deng, J.G.; Liu, Y.X.; Jing, L.; Hao, X.Q.; Pei, W.B.; Yu, X.H.; Rastegarpanah, A.; Dai, H.X. An investigation on catalytic performance and reaction mechanism of RuMn/meso-TiO2 derived from RuMn intermetallic compounds for methyl ethyl ketone oxidation. Appl. Catal. B 2021, 296, 120361. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Zhang, J.J.; Yan, B.; Jin, C.Q.; Dou, J.J.; Li, M.Y.; Feng, X.H.; Liu, G. No annealing synthesis of ordered intermetallic PdCu nanocatalysts for boosting formic acid oxidation. Chem. Mater. 2022, 34, 1385–1391. [Google Scholar] [CrossRef]
- Baglin, E.G.; Atkinson, G.B.; Nicks, L.J. Methanol synthesis catalysts from thorium-copper intermetallics. Preparation and evaluation. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 87–90. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Goncalves, A.P.; Gasche, T.A.; Ferraria, A.M.; Rego, A.M.B.D.; Correia, M.R.; Bola, A.M.; Branco, J.B. Partial oxidation of methane over bimetallic copper-and nickel-actinide oxides (Th, U). J. Alloys Compd. 2010, 497, 249–258. [Google Scholar] [CrossRef]
- Sasikala, R.; Gupta, N.M.; Kulshreshtha, S.K.; Iyer, R.M. Carbon monoxide methanation over FeTi1+ x intermetallics. J. Catal. 1987, 107, 510–521. [Google Scholar] [CrossRef]
- Chen, X.; Jin, J.H.; Sha, G.Y.; Li, C.; Zhang, B.S.; Su, D.S.; Williams, C.T.; Liang, C.H. Silicon-nickel intermetallic compounds supported on silica as a highly efficient catalyst for CO methanation. Catal. Sci. Technol. 2014, 4, 53–61. [Google Scholar] [CrossRef]
- Ye, C.L.; Chen, X.J.; Li, S.S.; Feng, B.B.; Fu, Y.H.; Zhang, F.M.; Chen, D.L.; Zhu, W.D. PdZn intermetallic compound stabilized on ZnO/nitrogen-decorated carbon hollow spheres for catalytic semihydrogenation of alkynols. Nano Res. 2022, 15, 3090–3098. [Google Scholar] [CrossRef]
- Almisbaa, Z.; Aljama, H.A.; Almajnouni, K.; Cavallo, L.; Sautet, P. Acetylene semi-hydrogenation on intermetallic Ni–In catalysts: Ni ensemble and acetylene coverage effects from a theoretical analysis. ACS Catal. 2023, 13, 7358–7370. [Google Scholar] [CrossRef]
- Armbrüster, M.; Wowsnick, G.; Friedrich, M.; Heggen, M.; Cardoso-Gil, R. Synthesis and catalytic properties of nanoparticulate intermetallic Ga-Pd compounds. J. Am. Chem. Soc. 2011, 133, 9112. [Google Scholar] [CrossRef]
- Shao, L.D.; Zhang, W.; Armbrüster, M.; Teschner, D.; Girgsdies, F.; Zhang, B.; Timpe, O.; Friedrich, M.; Schlögl, R.; Su, D.S. Nanosizing intermetallic compounds onto carbon nanotubes: Active and selective hydrogenation catalysts. Angew. Chem. Int. Ed. 2011, 50, 10231–10235. [Google Scholar] [CrossRef]
- Zhou, H.R.; Yang, X.F.; Li, L.; Liu, X.Y.; Huang, Y.Q.; Pan, X.L.; Wang, A.Q.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd–Zn–Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Chen, M.; Gupta, G.; Ordonez, C.W.; Lamkins, A.R.; Ward, C.J.; Abolafia, C.A.; Zhang, B.Y.; Roling, L.T.; Huang, W.Y. Intermetallic nanocatalyst for highly active heterogeneous hydroformylation. J. Am. Chem. Soc. 2021, 143, 20907–20915. [Google Scholar] [CrossRef] [PubMed]
- Tew, M.W.; Emerich, H.; Bokhoven, J.A. Formation and characterization of PdZn alloy: A very selective catalyst for alkyne semihydrogenation. J. Phys. Chem. C 2011, 115, 8457–8465. [Google Scholar] [CrossRef]
- Furukawa, S.; Komatsu, T. Selective hydrogenation of functionalized alkynes to (E)-alkenes, using ordered alloys as catalysts. ACS Catal. 2016, 6, 2121–2125. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Yao, Z.; Zhang, L.; Zhang, L.Y.; Yang, X.S.; Li, Y.B.; Wang, Y.G.; Li, Y.; Yang, F. Kinetic diffusion–controlled synthesis of twinned intermetallic nanocrystals for CO-resistant catalysis. Sci. Adv. 2022, 8, eabo4599. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.S.; Chen, L.F.; Li, Z.H.; Liu, W.; Xu, E.Z.; Zhang, Y.J.; Hong, S.; Zhang, X.; Wei, M. NiBi intermetallic compounds catalyst toward selective hydrogenation of unsaturated aldehydes. Appl. Catal. B 2020, 277, 119273. [Google Scholar] [CrossRef]
- Pu, Z.H.; Liu, T.T.; Zhang, G.X.; Chen, Z.S.; Li, D.S.; Chen, N.; Chen, W.F.; Chen, Z.X.; Sun, S.H. Intermetallic catalysts for efficient electrocatalytic hydrogen evolution in wide pH range. Adv. Energy Mater. 2022, 12, 2200293. [Google Scholar] [CrossRef]
- Shi, H.; Sun, X.Y.; Liu, Y.; Zeng, S.P.; Zhang, Q.H.; Gu, L.; Wang, T.H.; Han, G.F.; Wen, Z.; Fang, Q.R.; et al. Multicomponent intermetallic nanoparticles on hierarchical metal network as versatile electrocatalysts for highly efficient water splitting. Adv. Funct. Mater. 2023, 33, 2214412. [Google Scholar] [CrossRef]
- Shen, S.J.; Zhang, H.H.; Song, K.; Wang, Z.P.; Shang, T.T.; Gao, A.; Zhang, Q.H.; Gu, L.; Zhong, W.W. Multi-d electron synergy in LaNi1–xCoxRu intermetallics boosts electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2024, 63, e202315340. [Google Scholar] [CrossRef]
- Gong, Y.T.; Li, H.C.; Wu, J.Z.; Song, X.Y.; Yang, X.Q.; Bao, X.B.; Han, X.; Kitano, M.; Wang, J.J.; Hosono, H. Unique catalytic mechanism for Ru-loaded ternary intermetallic electrides for ammonia synthesis. J. Am. Chem. Soc. 2022, 144, 8683–8692. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.Z.; Shao, Q.; E, B.; Guo, J.; Yao, J.L.; Huang, X.Q. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2017, 139, 9576–9582. [Google Scholar] [CrossRef]
- Masa, J.; Piontek, S.; Wilde, P.; Antoni, H.; Eckhard, T.; Chen, Y.T.; Muhler, M.; Apfel, U.P.; Schuhmann, W. Ni-metalloid (B, Si, P, As, and Te) alloys as water oxidation electrocatalysts. Adv. Energy Mater. 2019, 16, 1900796. [Google Scholar] [CrossRef]
- Menezes, P.W.; Panda, C.; Garai, S.; Walter, C.; Guiet, A.; Driess, M. Structurally ordered intermetallic cobalt stannide nanocrystals for high-performance electrocatalytic overall water-splitting. Angew. Chem. In. Ed. 2018, 57, 15237–15242. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Wang, P.T.; Shao, Q.; Huang, X.Q. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Menezes, P.W.; Driess, M. Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 2021, 12, 8603–8631. [Google Scholar] [CrossRef]
- Chen, L.W.; He, F.X.; Shao, R.Y.; Yan, Q.Q.; Yin, P.; Zeng, W.J.; Zuo, M.; He, L.X.; Liang, H.W. Intermetallic IrGa-IrOx core–shell electrocatalysts for oxygen evolution. Nano Res. 2022, 15, 1853–1860. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, L.L.; Li, Q.Q.; Wang, S.Y.; Wang, W.H.; Du, Y.P. Laves phase Ir2Sm intermetallic nanoparticles as a highly active electrocatalyst for acidic oxygen evolution reaction. Chem. Sci. 2023, 14, 5887–5893. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.N.; Lu, Y.F.; Xiao, Z.W.; Li, J.; Nakao, T.; Abe, H.; Niwa, Y.; Kitano, M.; Tada, T.; Hosono, H. Palladium-bearing intermetallic electride as an efficient and stable catalyst for Suzuki cross-coupling reactions. Nat. Commun. 2019, 10, 5653. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Ye, T.N.; Park, S.W.; Li, J.; Sasase, M.; Abe, H.; Niwa, Y.; Kitano, M.; Hosono, H. Intermetallic ZrPd3-embedded nanoporous ZrC as an efficient and stable catalyst of the Suzuki cross-coupling reaction. ACS Catal. 2020, 10, 14366–14374. [Google Scholar] [CrossRef]
- Liu, R.; Xu, C.H.; Chen, Y.N.; Zhao, H.C.; Sun, J.F.; He, Z.L.; Shan, W.Y.; Li, G.; Shi, Q.T.; Fang, L.P.; et al. Existence of a heterogeneous pathway in palladium-catalyzed carbon–carbon coupling reaction: Evidence from Ag@Pd3Cu intermetallic nanoplates. CCS Chem. 2022, 4, 671–682. [Google Scholar] [CrossRef]
- Neumann, M.; Teschner, D.; Knop-Gericke, A.; Reschetilowski, W.; Armbrüster, M. Controlled synthesis and catalytic properties of supported In-Pd intermetallic compounds. J. Catal. 2016, 340, 49–59. [Google Scholar] [CrossRef]
- Penner, S.; Nezhad, P.D.K. Steering the catalytic properties of intermetallic compounds and alloys in reforming reactions by controlled in situ decomposition and self-activation. ACS Catal. 2021, 11, 5271–5286. [Google Scholar] [CrossRef] [PubMed]
- Armbrüster, M.; Behrens, M.; Föttinger, K.; Friedrich, M.; Gaudry, É.; Matam, S.K.; Sharma, H.R. The intermetallic compound ZnPd and its role in methanol steam reforming. Catal. Rev. 2013, 55, 289–367. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Penner, S.; Lorenz, H.; Memmel, N.; Hävecker, M.; Blume, R.; Teschner, D.; Rocha, T.; Zemlyanov, D.; et al. Hydrogen production by methanol steam reforming on copper boosted by zinc-assisted water activation. Angew. Chem. In. Ed. 2012, 51, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Wang, Z.Y.; Li, J.L.; Wang, Z. In–Ni intermetallic compounds derived from layered double hydroxides as efficient catalysts toward the reverse water gas shift reaction. ACS Catal. 2022, 12, 4026–4036. [Google Scholar] [CrossRef]
- Li, H.C.; Ma, B.; Tian, J.Q.; Zhao, C. Manipulation of CO2 hydrogenation selectivity over RuSn/La2O2CO3 catalysts with intermetallic electron transfer. Chem. Eng. J. 2023, 464, 142572. [Google Scholar] [CrossRef]
- Ye, C.L.; Mao, P.; Wang, Y.H.; Zhang, N.Q.; Wang, D.S.; Jiao, M.L.; Miller, J.T. Surface hexagonal Pt1Sn1 intermetallic on Pt nanoparticles for selective propane dehydrogenation. ACS Appl. Mater. Interfaces 2020, 12, 25903–25909. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Song, Y.J.; Cullen, D.A.; Laursen, S. Selective and stable non-noble-metal intermetallic compound catalyst for the direct dehydrogenation of propane to propylene. J. Am. Chem. Soc. 2018, 140, 14010–14014. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Xing, F.L.; Ham, H.; Shimizu, K.; Furukawa, S. Doubly decorated platinum–gallium intermetallics as stable catalysts for propane dehydrogenation. Angew. Chem. Int. Ed. 2021, 60, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.S.; Ma, R.Y.; Ham, H.; Shimizu, K.; Furukawa, S. Electroassisted propane dehydrogenation at low temperatures: Far beyond the equilibrium limitation. JACS Au 2021, 1, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).