Strengthened Removal of Tetracycline by a Bi/Ni Co-Doped SrTiO3/TiO2 Composite under Visible Light
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Photocatalysts
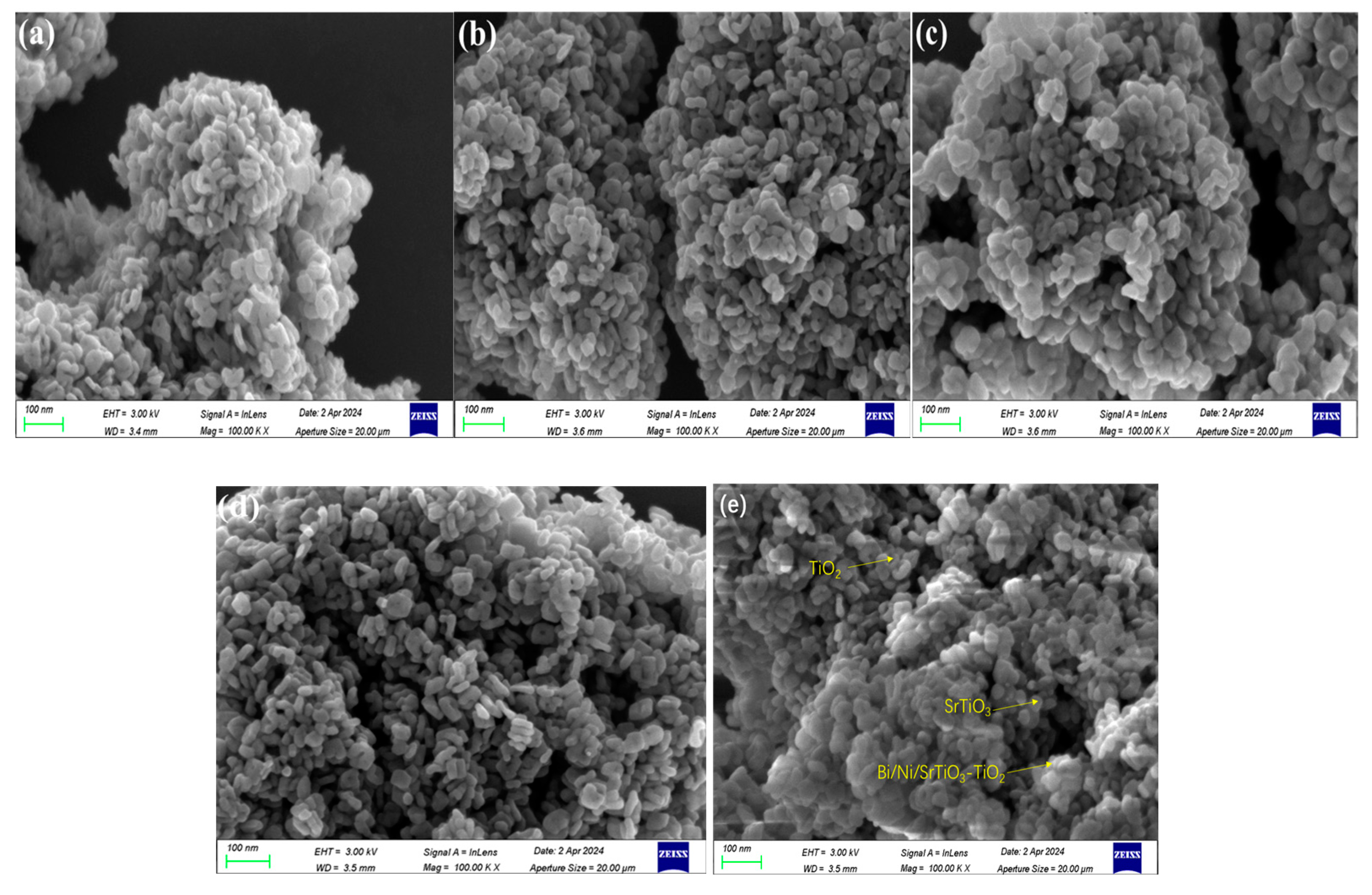
2.1.1. Morphology
2.1.2. Crystal Structures
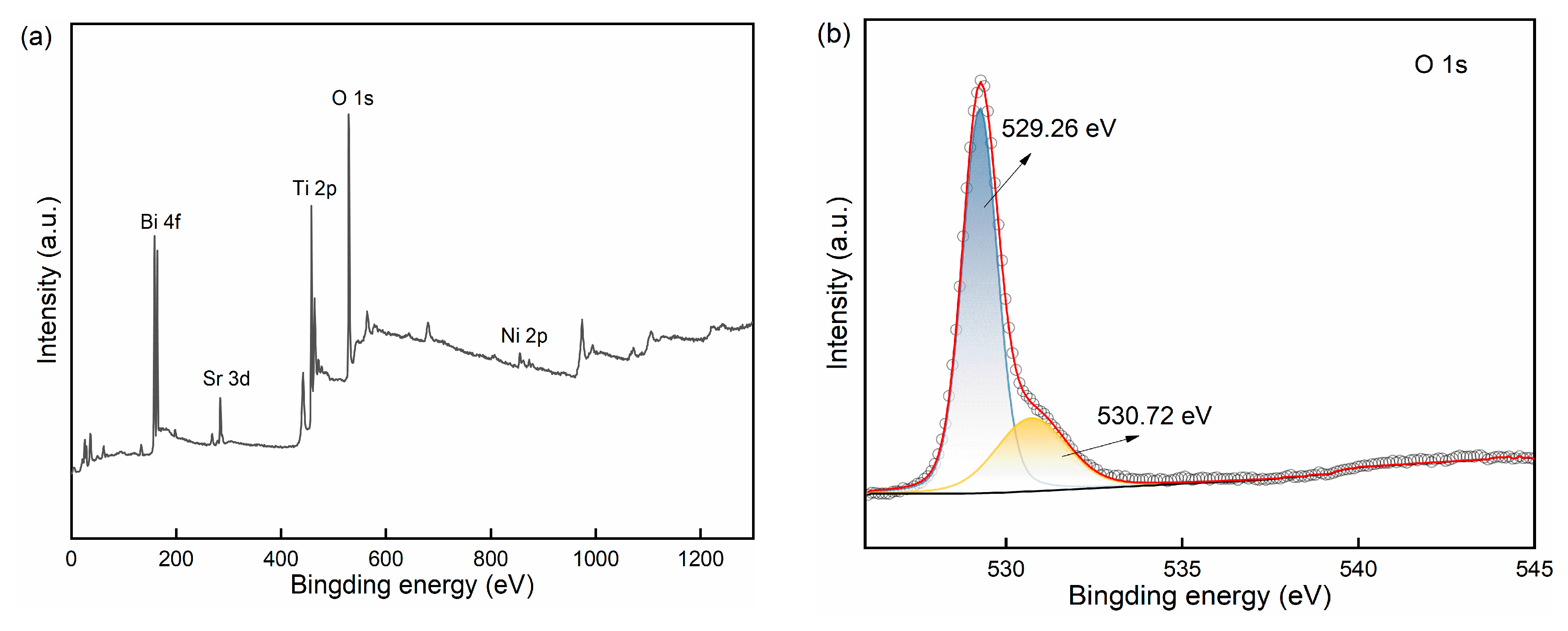
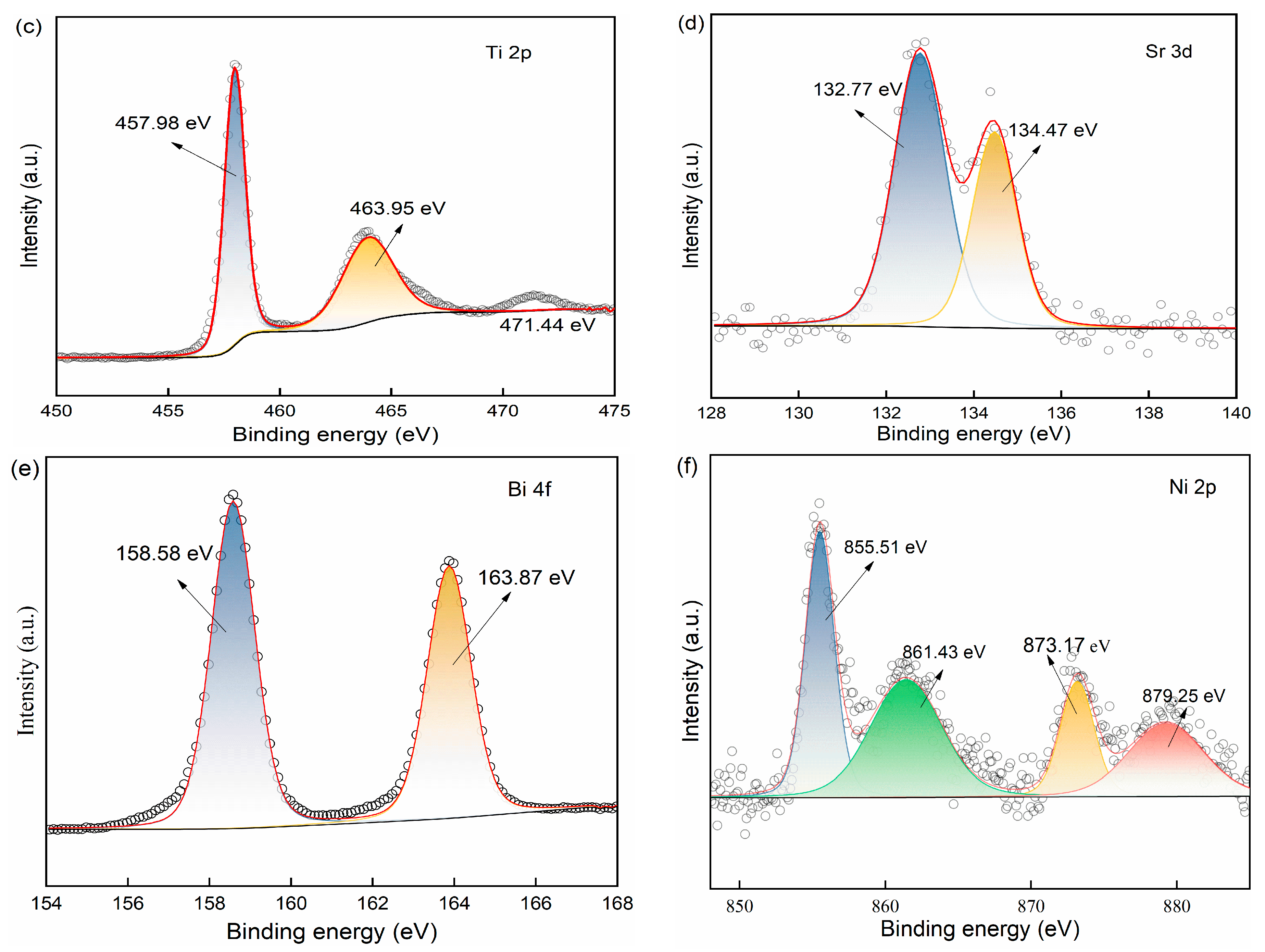
2.1.3. Elemental Composition and Chemical State Analysis
2.1.4. Optical Properties
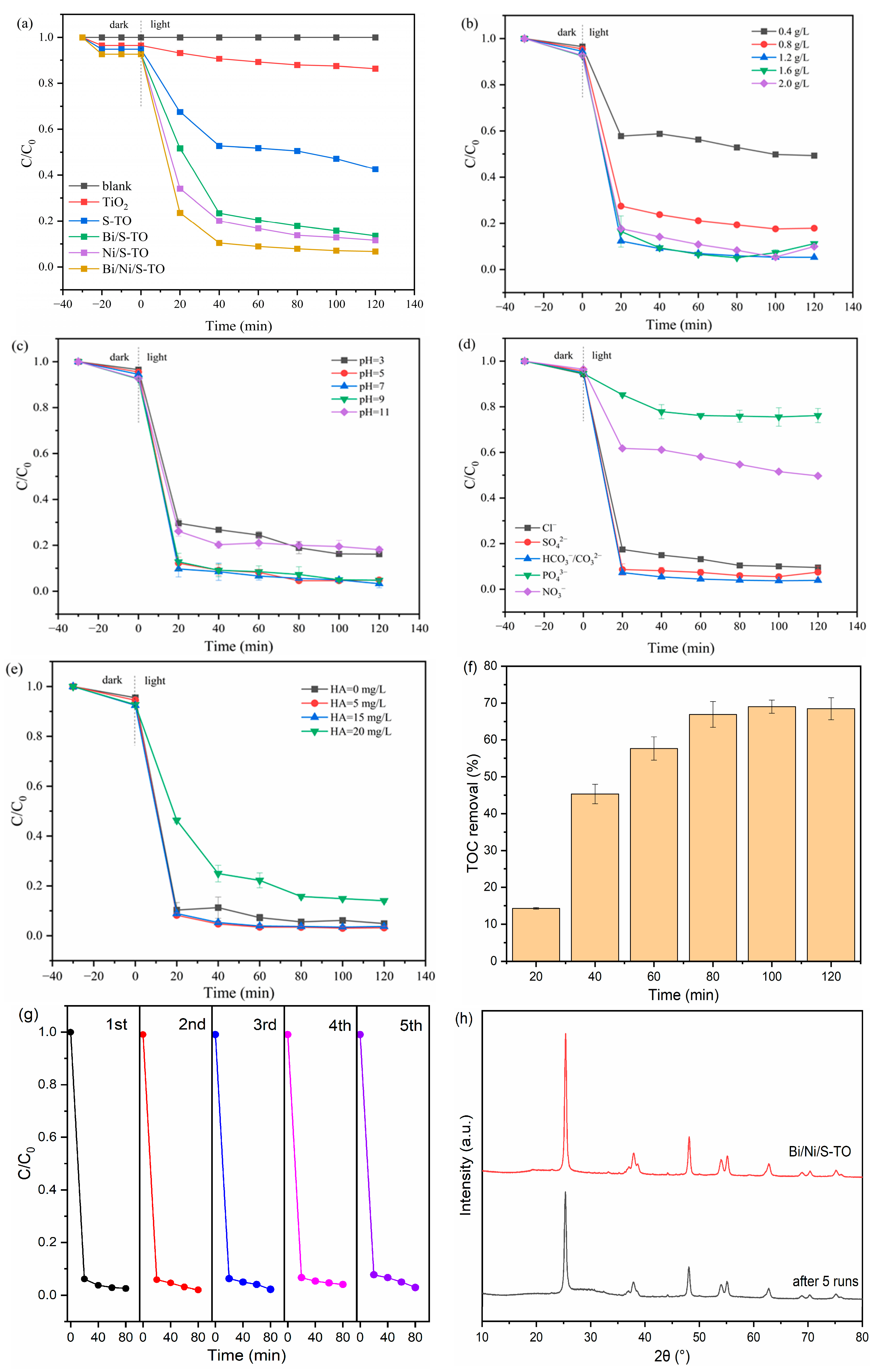
2.2. Photocatalytic Tetracycline Removal
2.3. Mechanism of Tetracycline Removal
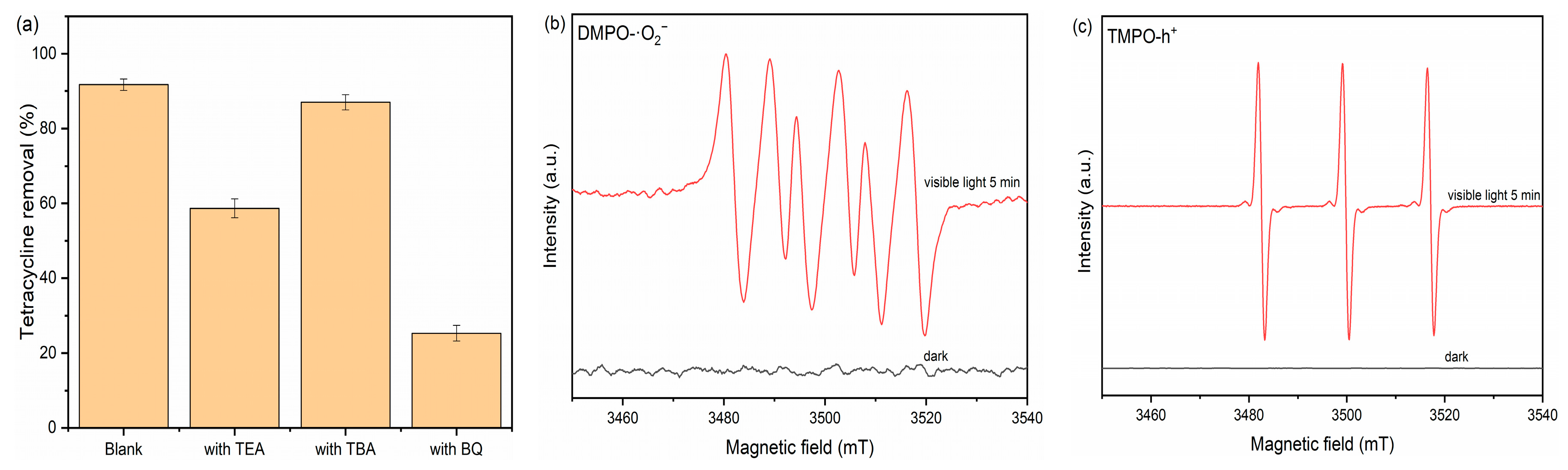
2.3.1. Free Radical Trapping and EPR Analysis
2.3.2. Mechanism of Tetracycline Removal by Bi/Ni/S-TO
3. Materials and Methods
3.1. Materials
3.2. Material Preparation
3.2.1. Preparation of SrTiO3/TiO2
3.2.2. Preparation of Bi/SrTiO3/TiO2 and Ni/SrTiO3/TiO2
3.2.3. Preparation of Bi/Ni/SrTiO3/TiO2
3.3. Characterizations
3.4. Evaluation of Photocatalytic Performance
3.5. Investigation on Factors Affecting Photocatalytic Performance
3.6. Free Radical Trapping and Electron Paramagnetic Resonance (EPR) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Bao, Y.; Xu, B. Seasonal Variation of Antibiotics in Surface Water of Pudong New Area of Shanghai, China and the Occurrence in Typical Wastewater Sources. Chemosphere 2020, 239, 124816. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Niu, Y.; Shen, T.; Dong, K.; Wang, D. Magnetic Biochar Prepared by a Dry Process for the Removal of Sulfonamides Antibiotics from Aqueous Solution. J. Mol. Liq. 2024, 400, 124576. [Google Scholar] [CrossRef]
- Nassri, I.; khattabi rifi, S.; Sayerh, F.; Souabi, S. Occurrence, Pollution Sources, and Mitigation Prospects of Antibiotics, Anti-Inflammatories, and Endocrine Disruptors in the Aquatic Environment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100878. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, Y.; Yin, L.; Chen, Y.; Xue, Y.; Wang, X.; Mao, D.; Luo, Y. Unraveling the Influence of Human Fecal Pollution on Antibiotic Resistance Gene Levels in Different Receiving Water Bodies Using crAssphage Indicator Gene. J. Hazard. Mater. 2023, 442, 130005. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Cheng, J.; Han, Y.; Ma, H.; Jiang, X.; Liu, Y. Molecular Mechanism Underlying the Degradation of Tetracycline by Apiotrichum loubieri MFZ-16. Environ. Technol. Innov. 2024, 33, 103489. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, Y.; Mustafa, G.; Wang, C.; Bai, Z.; Wei, L.; Ma, W. A Novel ZIF67 Self-Assembled Wrapped Fe3O4 Piezoelectric Material for Ultrasonic Degradation of Tetracycline. Sep. Purif. Technol. 2024, 344, 127319. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, C.; Tang, Y.; Wang, C.; Lin, S. Tetracycline Degradation by Dual-Frequency Ultrasound Combined with Peroxymonosulfate. Ultrason. Sonochem. 2024, 106, 106886. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, W.; Cui, M.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Li, L.; Du, Q.; et al. Filtration and Adsorption of Tetracycline in Aqueous Solution by Copper Alginate-Carbon Nanotubes Membrane Which Has the Muscle-Skeleton Structure. Chem. Eng. Res. Des. 2022, 183, 424–438. [Google Scholar] [CrossRef]
- Song, X.; Jo, C.; Zhou, M. Enhanced Tetracycline Removal Using Membrane-like Air-Cathode with High Flux and Anti-Fouling Performance in Flow-through Electro-Filtration System. Water Res. 2022, 224, 119057. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A Comprehensive Review on Biodegradation of Tetracyclines: Current Research Progress and Prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, Y.; Zhu, Q. Enhanced Photocatalysis of MgAl Layered Double Oxide Nanosheets with B,N-Codoped Carbon Coating for Tetracycline Removal. Appl. Clay Sci. 2020, 193, 105694. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ofomaja, A.E. Facile Microwave Synthesis of Pine Cone Derived C-Doped TiO2 for the Photodegradation of Tetracycline Hydrochloride under Visible-LED Light. J. Environ. Manag. 2018, 223, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Emzhina, V.; Kuzin, E.; Babusenko, E.; Krutchinina, N. Photodegradation of Tetracycline in Presence of H2O2 and Metal Oxide Based Catalysts. J. Water Process Eng. 2021, 39, 101696. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z.; Yin, Y.; Huang, B.; He, G.; Chen, H. A Facile Solvothermal Syntheses of NiFe Layered Double Hydroxide-Bi2MoO6 Heterostructure/Reduced Graphene Oxide with Efficient Photodegradation for Tetracycline. Environ. Res. 2022, 204, 112037. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhong, S.; Gu, B.; Chen, L.; Wu, J.; Fan, G. Synergistic Promotion Effects of MoS2 Integrated Transition Metal Sulfides Nanoparticles towards Efficient Tetracycline Destruction: Performance, Mechanism and Toxicity Assessment. J. Environ. Chem. Eng. 2024, 12, 112756. [Google Scholar] [CrossRef]
- Zhu, B.; Zhou, J.; Ni, L.; Diao, G. Rapid Removal of Tetracycline by G-C3N4/High Elastic Graphene Sponge via Synergy Effect of Adsorption and Photocatalysis under Visible Light. Solid State Sci. 2023, 139, 107158. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. One-Pot in-Situ Hydrothermal Synthesis of Ternary In2S3/Nb2O5/Nb2C Schottky/S-Scheme Integrated Heterojunction for Efficient Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2022, 628, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Xiao, X.; Xu, Q. Coordination Chemistry in Modulating Electronic Structures of Perovskite-Type Oxide Nanocrystals for Oxygen Evolution Catalysis. Coord. Chem. Rev. 2023, 485, 215109. [Google Scholar] [CrossRef]
- Singh, N.K.; Sharma, P.; Yadav, M.K.; Parihar, R. Oxygen Evolution Electrocatalytic Properties of Perovskite-Type Oxides Obtained by PVP Sol-Gel Route: Part II. The Effect of Partial Substitution of Sm for Sr in La0.4Sr0.6CoO3. Int. J. Electrochem. Sci. 2020, 15, 7001–7012. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.; Luo, J.; Ma, F.; Wang, C. Improved Photocatalytic NO Removal Activity of SrTiO3 by Using SrCO3 as a New Co-Catalyst. Appl. Catal. B Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Ni, W.; Ji, Y.; Liang, L.; Ding, B.; Li, X.; Lu, H. Constructing N-Type Doped Perovskite from Ti-Bearing Solid Waste to Boost the NO-to-NO2 Oxidation. J. Clean. Prod. 2022, 372, 133553. [Google Scholar] [CrossRef]
- Mizera, A.; Drożdż, E. Studies on Structural, Redox and Electrical Properties of Ni-Doped Strontium Titanate Materials. Ceram. Int. 2020, 46, 24635–24641. [Google Scholar] [CrossRef]
- Hu, M.; Chen, W.; Wang, J. Photocatalytic Degradation of Tetracycline by La-Fe Co-Doped SrTiO3/TiO2 Composites: Performance and Mechanism Study. Water 2024, 16, 210. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Duan, L.; Zhang, Y.; Rodrigues, L.A.; Reddy, D.A.; Zhu, H. Construction of SrTiO3/TiO2 Heterojunction by in-Situ Ion Exchange Method to Enhance Photochemical Performance. Mater. Sci. Eng. B 2024, 299, 117010. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Wu, J.; Li, N.; He, N.; Zhao, H.; Wu, Q.; Li, X. Perovskite-SrTiO3/TiO2/PDA as Photoelectrochemical Glucose Biosensor. Ceram. Int. 2021, 47, 29807–29814. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. A Heterogeneous TiO2/SrTiO3 Coating on Titanium Alloy with Excellent Photocatalytic Antibacterial, Osteogenesis and Tribocorrosion Properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]
- Qian, K.; Fang, F.; E, Y.; Xu, Y.; Tong, X.; Chen, P.; Han, L.; Li, Z. Bimetallic Bi and Ni Doped LTA Zeolite as Synergy Electrocatalyst towards High Concentration of Methanol Oxidation Reaction. Int. J. Hydrogen Energy 2023, 48, 6998–7003. [Google Scholar] [CrossRef]
- Su, C.; Duan, X.; Miao, J.; Zhong, Y.; Zhou, W.; Wang, S.; Shao, Z. Mixed Conducting Perovskite Materials as Superior Catalysts for Fast Aqueous-Phase Advanced Oxidation: A Mechanistic Study. ACS Catal. 2017, 7, 388–397. [Google Scholar] [CrossRef]
- Oanh, L.T.M.; Huy, N.X.; Phuong, D.T.T.; Bich, D.D.; Van Minh, N. A Study on Structure, Morphology, Optical Properties, and Photocatalytic Ability of SrTiO3/TiO2 Granular Composites. Phys. B Condens. Matter 2018, 532, 37–41. [Google Scholar] [CrossRef]
- Cao, T.; Xu, J.; Chen, M. Construction of 2D/0D Direct Z-Scheme Bi4O5I2/Bi3TaO7 Heterojunction Photocatalysts with Enhanced Activity for Levofloxacin Degradation under Visible Light Irradiation. Sep. Purif. Technol. 2022, 291, 120896. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, A.; Sharma, G.; Naushad, M.; Ubaidullah, M.; García-Peñas, A. Developing a G-C3N4/NiFe2O4 S-Scheme Hetero-Assembly for Efficient Photocatalytic Degradation of Cephalexin. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129968. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Fan, L.; Wang, X.; Fu, X.; Long, J. Heteroatomic Ni, Sn Clusters-Grafted Anatase TiO2 Photocatalysts: Structure, Electron Delocalization, and Synergy for Solar Hydrogen Production. J. Phys. Chem. C 2015, 119, 10478–10492. [Google Scholar] [CrossRef]
- Lv, Z.; Li, W.; Cheng, X.; Liu, B.; Guo, Z.; Zhuang, T.; Zhang, C. Constructing Internal Electric Field in CdS via Bi, Ni Co-Doping Strategy for Enhanced Visible-Light H2 Production. Appl. Surf. Sci. 2021, 556, 149758. [Google Scholar] [CrossRef]
- Nematollahi, R.; Ghotbi, C.; Khorasheh, F.; Larimi, A. Ni-Bi Co-doped TiO2 as Highly Visible Light Response Nano-photocatalyst for CO2 Photo-reduction in a Batch Photo-reactor. J. CO2 Util. 2020, 41, 101289. [Google Scholar] [CrossRef]
- Peng, H.; Luo, M.; Yang, R.; Dong, L.; Zheng, X. Insights into the solar-light activated adsorption-photocatalysis of ZnLaxBi2−xO4 heterojunctions for tetracycline hydrochloride removal. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133523. [Google Scholar] [CrossRef]
- Maggu, C.; Singla, S.; Basu, S. Unleashing the Power of Sunlight: Bi2O3/Sb2S3 Photocatalysis for Sustainable Wastewater Remediation of Tetracycline and Rhodamine-B. J. Environ. Manag. 2024, 349, 119424. [Google Scholar] [CrossRef]
- Duan, X.; Yang, C.; Han, H.; Song, Z.; Ma, C.; Feng, S. Construction of Bi2Sn2O7/Cu2O Z-type Photocatalyst with Enhanced Tetracycline Removal under Visible Light. J. Mol. Liq. 2024, 403, 124693. [Google Scholar] [CrossRef]
- Ni, Q.; Ke, X.; Qian, W.; Yan, Z.; Luan, J.; Liu, W. Insight into Tetracycline Photocatalytic Degradation Mechanism in a Wide pH Range on BiOI/BiOBr: Coupling DFT/QSAR Simulations with Experiments. Appl. Catal. B: Environ. 2024, 340, 123226. [Google Scholar] [CrossRef]
- Liu, H.; Huo, W.; Zhang, T.C.; Ouyang, L.; Yuan, S. Photocatalytic Removal of Tetracycline by a Z-Scheme Heterojunction of Bismuth Oxyiodide/Exfoliated g-C3N4: Performance, Mechanism, and Degradation Pathway. Mater. Today Chem 2022, 23, 100729. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Peng, Y.; Li, X.; Zhou, C.; Xu, R.; Zhang, Y. One-Step Synthesis of Co-Doped UiO-66 Nanoparticle with Enhanced Removal Efficiency of Tetracycline: Simultaneous Adsorption and Photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Chen, X.; Wang, Z.; Ji, H.; Chen, L.; Liu, W.; Wang, C.-C. Bifunctional Bi12O17Cl2/MIL-100(Fe) Composites toward Photocatalytic Cr(VI) Sequestration and Activation of Persulfate for Bisphenol A Degradation. Sci. Total Environ. 2021, 752, 141901. [Google Scholar] [CrossRef]
- Girotto, G.Z.S.; Thill, A.; Matte, L.P.; Vogt, M.A.H.; Machado, T.V.P.; Dick, L.F.; Mesquita, F.; Bernardi, F. Ni/SrTiO3Nanoparticles for Photodegradation of Methylene Blue. ACS Appl. Nano Mater. 2022, 5, 13295–13307. [Google Scholar] [CrossRef]
- He, H.; Wang, W.; Xu, C.; Yang, S.; Sun, C.; Wang, X.; Yao, Y.; Mi, N.; Xiang, W.; Li, S.; et al. Highly Efficient Degradation of Iohexol on a Heterostructured Graphene-Analogue Boron Nitride Coupled Bi2MoO6 Photocatalyst under Simulated Sunlight. Sci. Total Environ. 2020, 730, 139100. [Google Scholar] [CrossRef]
- Yu, C.; Lu, Y.; Zhang, Y.; Qian, A.; Zhang, P.; Tong, M.; Yuan, S. Significant Contribution of Solid Organic Matter for Hydroxyl Radical Production during Oxygenation. Environ. Sci. Technol. 2022, 56, 11878–11887. [Google Scholar] [CrossRef]
- Lv, X.; Lam, F.L.K.; Hu, X. Developing SrTiO3/TiO2 Heterostructure Nanotube Array for Photocatalytic Fuel Cells with Improved Efficiency and Elucidating the Effects of Organic Substrates. Chem. Eng. J. 2022, 427, 131602. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, N.; Wang, H.; Mao, L. Sacrificial Template Synthesis of Core-Shell SrTiO3/TiO2 Heterostructured Microspheres Photocatalyst. Ceram. Int. 2017, 43, 4807–4813. [Google Scholar] [CrossRef]



| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| TiO2 | 50 | 0.18 | |
| S-TO | 63.3 | 0.33 | 20.8 |
| Bi/S-TO | 59.8 | 0.30 | 20.4 |
| Ni/S-TO | 65.6 | 0.32 | 20.1 |
| Bi/Ni/S-TO | 63.3 | 0.29 | 18.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhao, N.; Hu, M.; Liu, X.; Deng, B. Strengthened Removal of Tetracycline by a Bi/Ni Co-Doped SrTiO3/TiO2 Composite under Visible Light. Catalysts 2024, 14, 539. https://doi.org/10.3390/catal14080539
Chen W, Zhao N, Hu M, Liu X, Deng B. Strengthened Removal of Tetracycline by a Bi/Ni Co-Doped SrTiO3/TiO2 Composite under Visible Light. Catalysts. 2024; 14(8):539. https://doi.org/10.3390/catal14080539
Chicago/Turabian StyleChen, Weifang, Na Zhao, Mingzhu Hu, Xingguo Liu, and Baoqing Deng. 2024. "Strengthened Removal of Tetracycline by a Bi/Ni Co-Doped SrTiO3/TiO2 Composite under Visible Light" Catalysts 14, no. 8: 539. https://doi.org/10.3390/catal14080539
APA StyleChen, W., Zhao, N., Hu, M., Liu, X., & Deng, B. (2024). Strengthened Removal of Tetracycline by a Bi/Ni Co-Doped SrTiO3/TiO2 Composite under Visible Light. Catalysts, 14(8), 539. https://doi.org/10.3390/catal14080539







