Biodiesel Synthesis from Coconut Oil Using the Ash of Citrus limetta Peels as a Renewable Heterogeneous Catalyst
Abstract
:1. Introduction
2. Result and Discussion
2.1. Optimization of Reaction Parameters
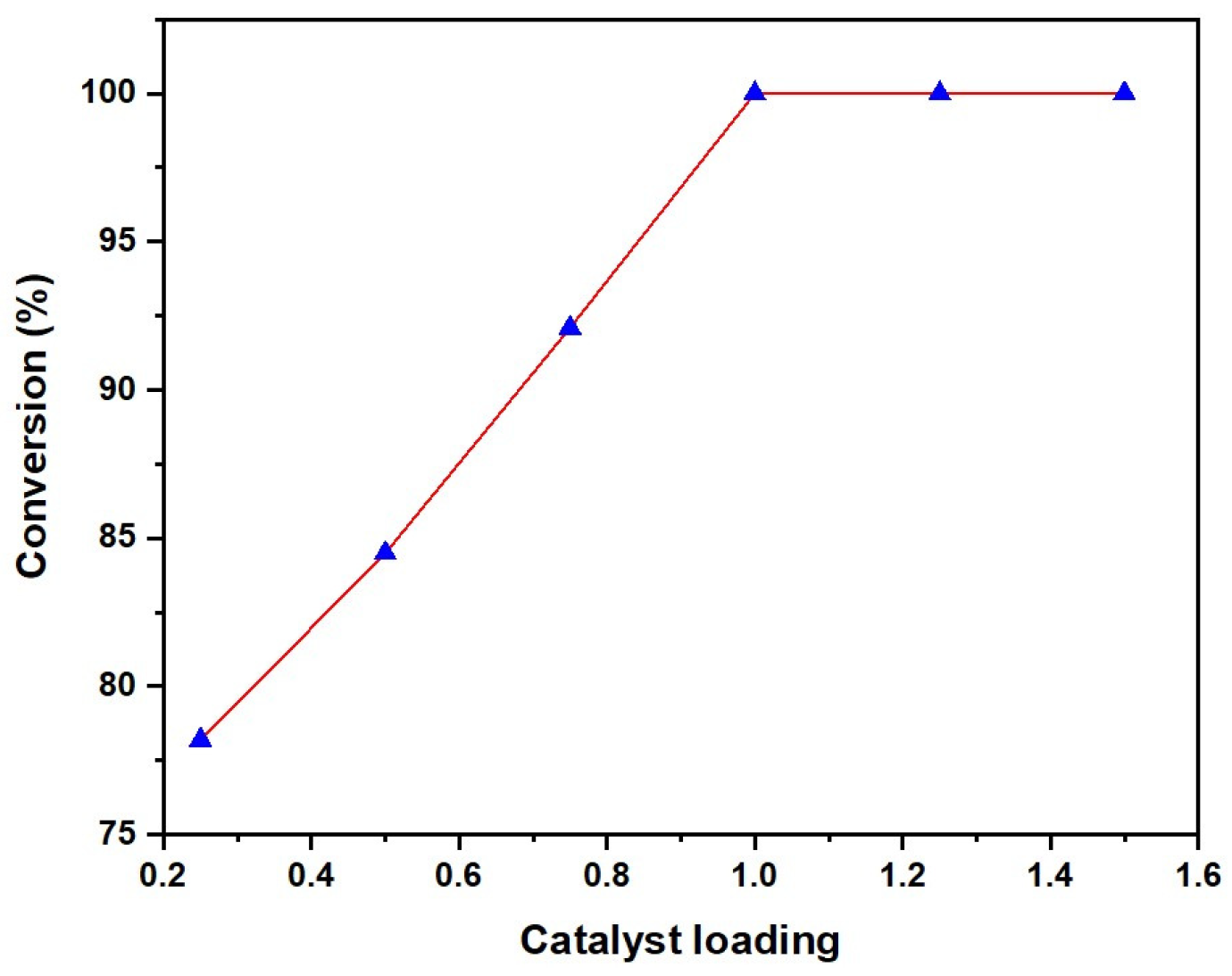
2.1.1. Catalyst Amount Effect
2.1.2. Methanol/Oil Molar Ratio Effect
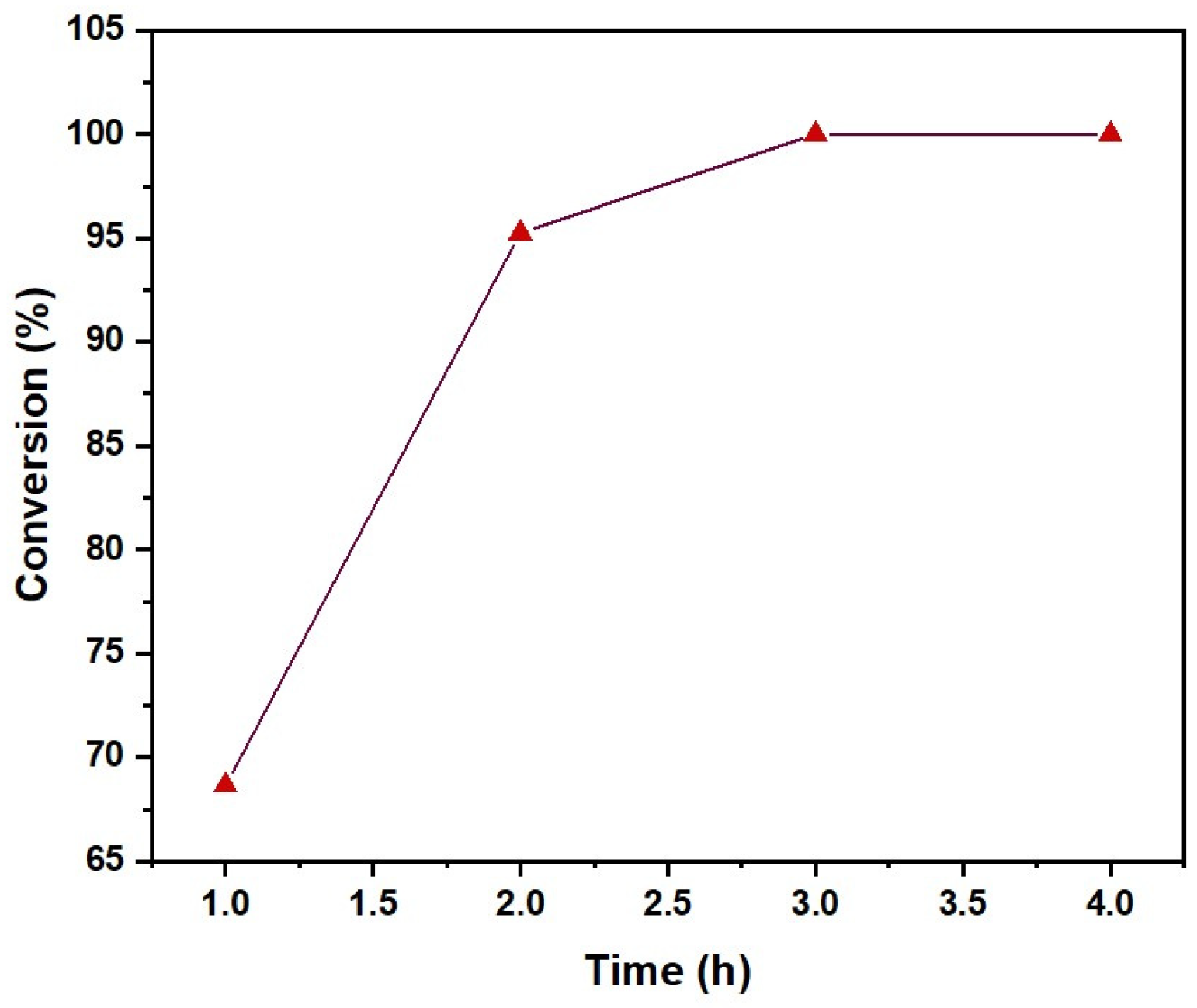
2.1.3. Reaction Time Effect
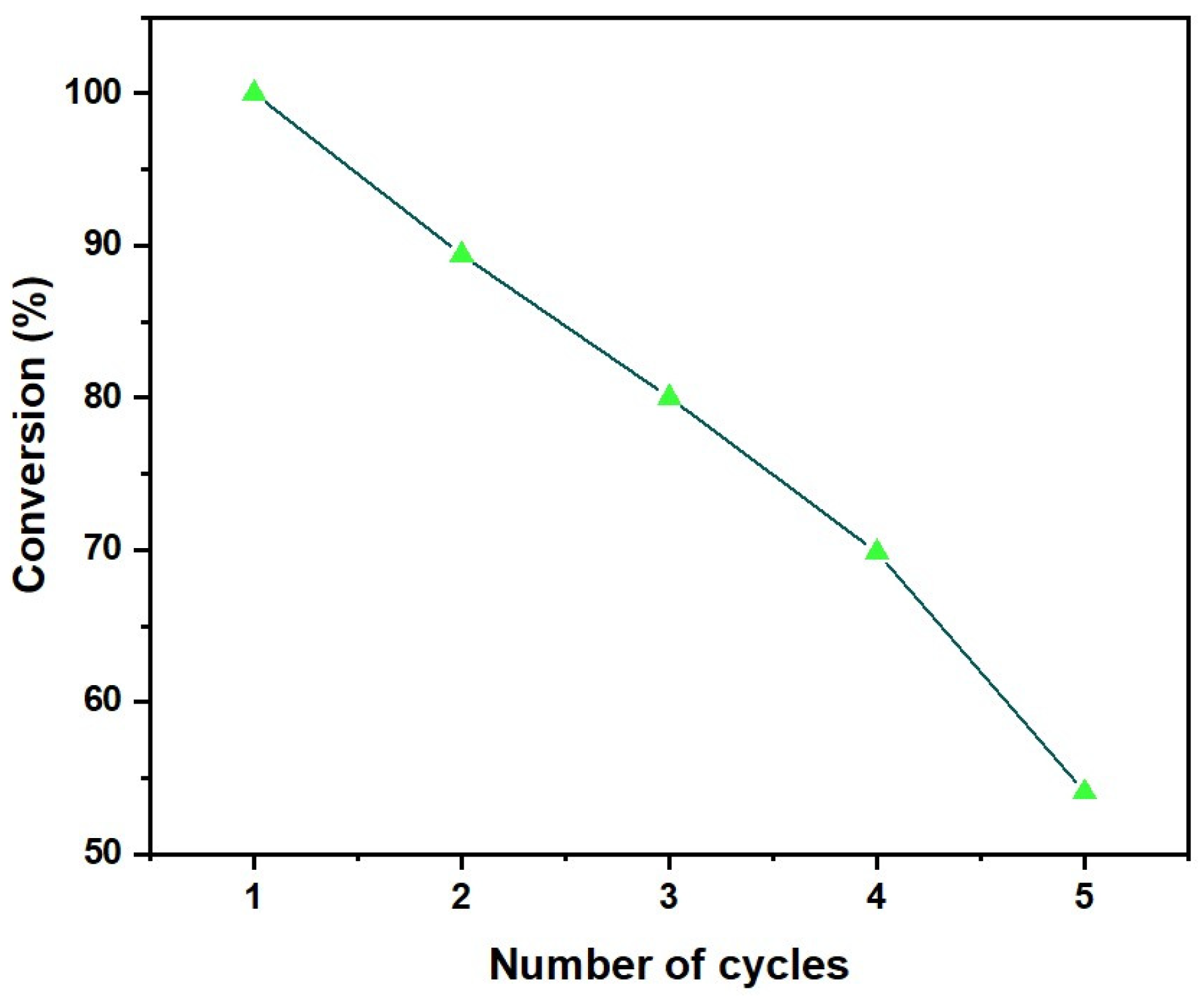
2.1.4. Heterogeneity Effect
3. Experimental
3.1. Materials and Methodology
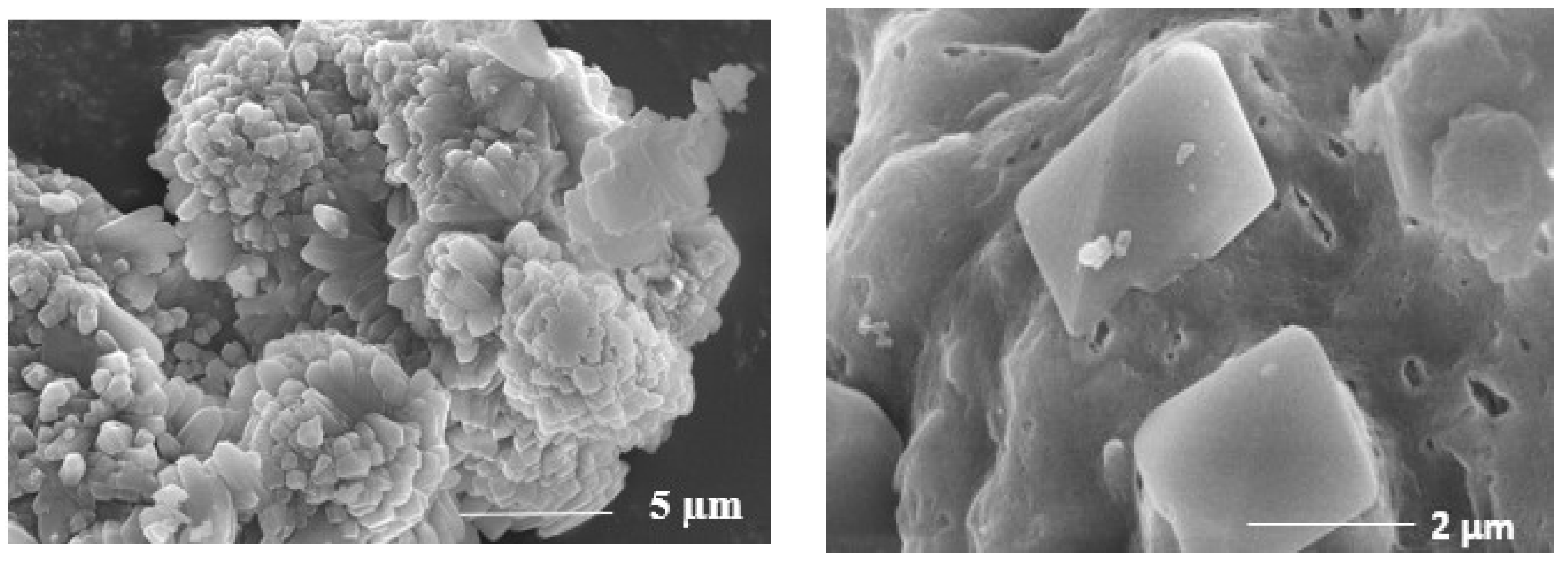
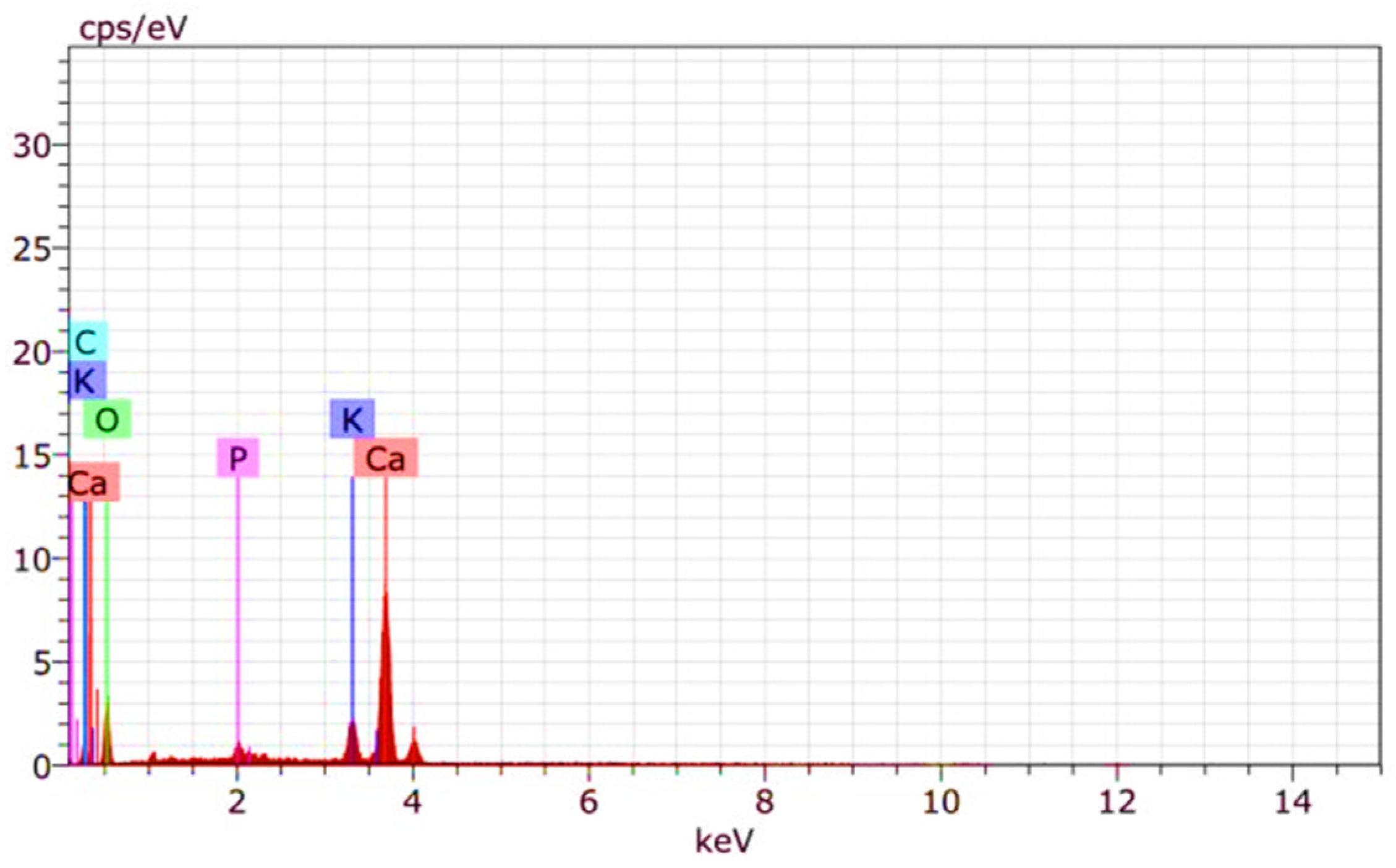
3.2. Catalyst Preparation
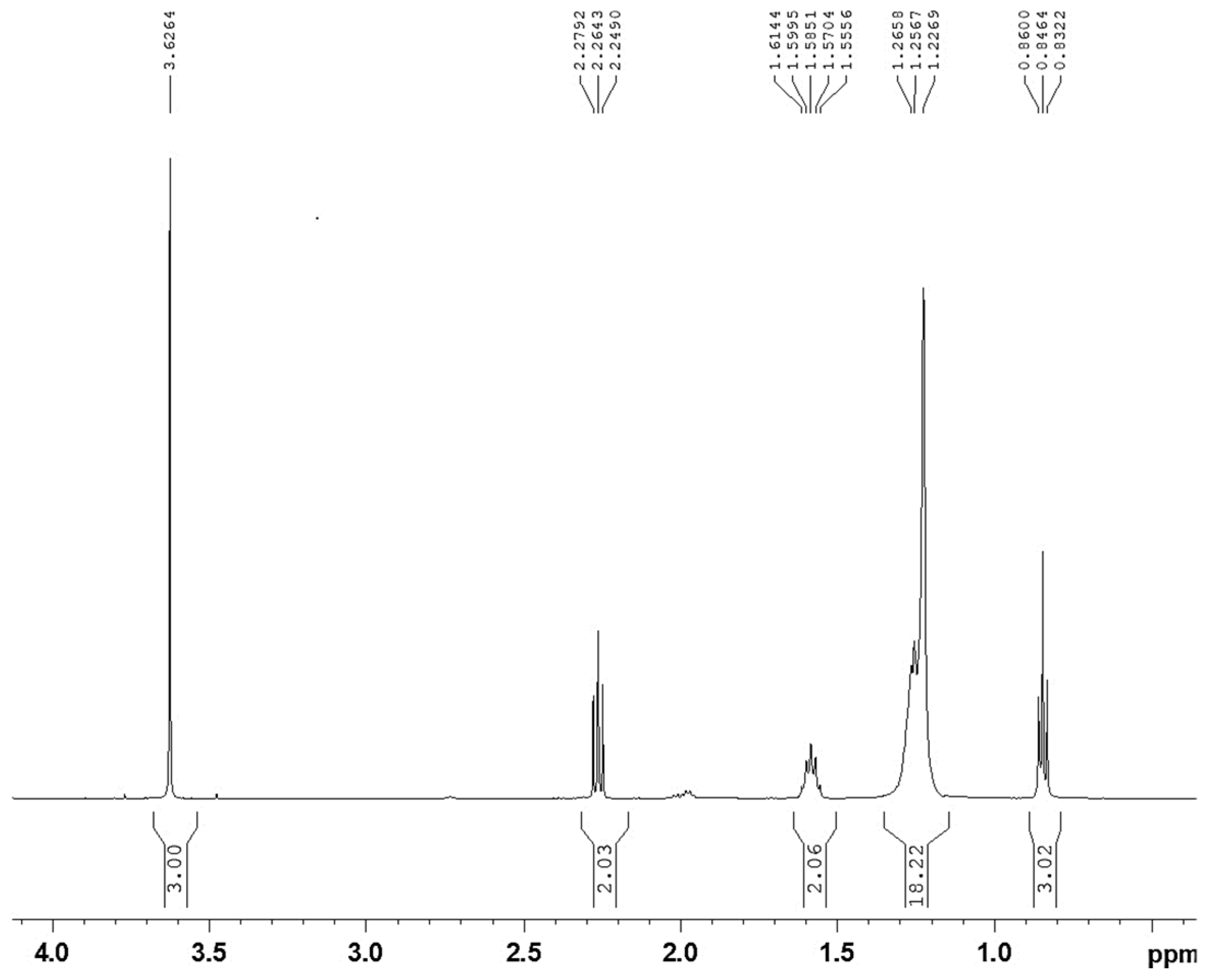
3.3. Procedure for the Synthesis of Biodiesel
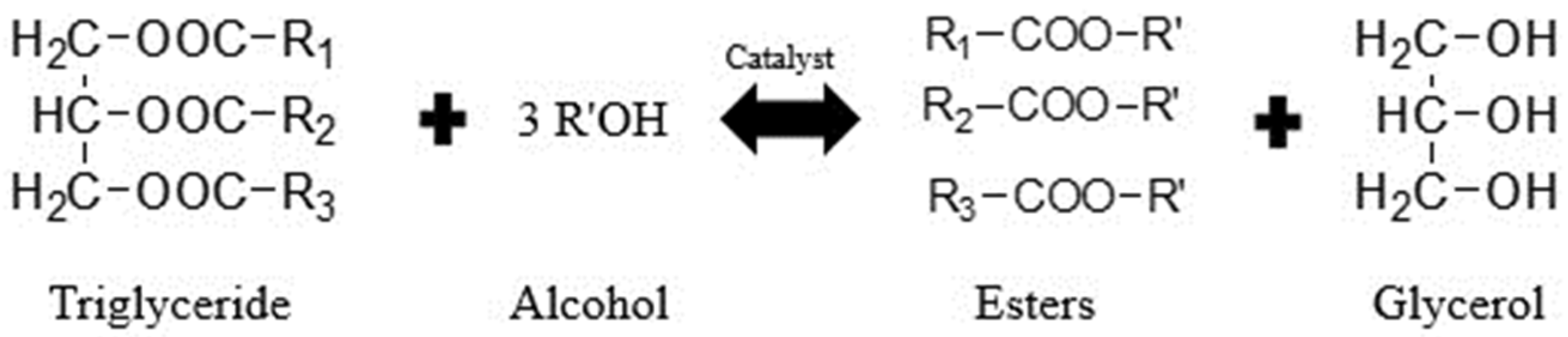
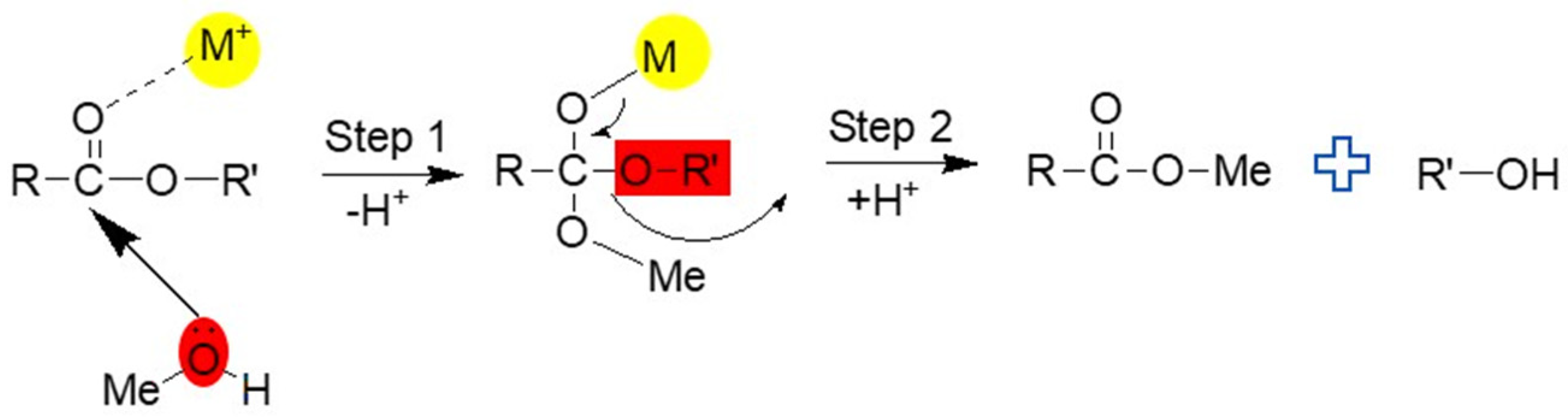
3.4. Plausible Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arumugam, A.; Ponnusami, V. Biodiesel production from Calophyllum inophyllum oil a potential non-edible feedstock: An overview. Renew. Energy 2019, 131, 459–471. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z.; Asfar, J. Preparation and performance investigation of a lignin-based solid acid catalyst manufactured from olive cake for biodiesel production. Renew. Energy 2019, 132, 667–682. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Téllez, M.M.; Pérez-Díaz, L.M.; Zeferino-Díaz, R.; Hilario-Martínez, J.C.; Sandoval-Ramírez, J. Biodiesel production under mild reaction conditions assisted by high shear mixing. Renew. Energy 2019, 130, 174–181. [Google Scholar] [CrossRef]
- Eldiehy, K.S.H.; Bardhan, P.; Borah, D.; Gohain, M.; Rather, M.A.; Deka, D.; Mandal, M. A comprehensive review on microalgal biomass production and processing for biodiesel production. Fuel 2022, 324, 124773. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, H.; Wu, X.; Shan, R. Exploiting the Waste Biomass of Durian Shell as a Heterogeneous Catalyst for Biodiesel Production at Room Temperature. Int. J. Environ. Res. Public Health 2023, 20, 1760. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; Lião, L.M.; Ferreira, A.G. Critical review on analytical methods for biodiesel characterization. Talanta 2008, 77, 593–605. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; da Silva Santos, M.; Boffo, E.F.; Pereira-Filho, E.R.; Lião, L.M.; Ferreira, A.G. Evaluation of biodiesel–diesel blends quality using 1H NMR and chemometrics. Talanta 2009, 78, 660–664. [Google Scholar] [CrossRef]
- Gonzaga, F.B.; Sobral, S.P. A new method for determining the acid number of biodiesel based on coulometric titration. Talanta 2012, 97, 199–203. [Google Scholar] [CrossRef]
- Hamza, M.; Ayoub, M.; Shamsuddin, R.B.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2021, 21, 101200. [Google Scholar] [CrossRef]
- Shinde, K.; Kaliaguine, S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering 2019, 3, 18. [Google Scholar] [CrossRef]
- Sree, J.V.; Chowdary, B.A.; Kumar, K.S.; Anbazhagan, M.P.; Subramanian, S. Optimization of the biodiesel production from waste cooking oil using homogeneous catalyst and heterogeneous catalysts. Mater. Today Proc. 2021, 46, 4900–4908. [Google Scholar] [CrossRef]
- Wang, J.; Mu, M.; Liu, Y. Recycled cement. Constr. Build. Mater. 2018, 190, 1124–1132. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rokhum, L.; Gupta, R.; Chatterjee, S. Zinc oxide supported silver nanoparticles as a heterogeneous catalyst for production of biodiesel from palm oil. Environ. Prog. Sustain. Energy 2020, 39, e13369. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Fouad, Y.O. Utilization of Ficus carica leaves as a heterogeneous catalyst for production of biodiesel from waste cooking oil. Environ. Sci. Pollut. Res. 2019, 26, 32804–32814. [Google Scholar] [CrossRef] [PubMed]
- Sahani, S.; Roy, T.; Sharma, Y.C. Clean and efficient production of biodiesel using barium cerate as a heterogeneous catalyst for the biodiesel production; kinetics and thermodynamic study. J. Clean. Prod. 2019, 237, 117699. [Google Scholar] [CrossRef]
- Jayaraju, R.M.; Gaddam, K.; Ravindiran, G.; Palani, S.; Paulraj, M.P.; Achuthan, A.; Saravanan, P.; Muniasamy, S.K. Biochar from waste biomass as a biocatalyst for biodiesel production: An overview. Appl. Nanosci. 2022, 12, 3665–3676. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Mares, E.K.L.; Gonçalves, M.A.; da Luz, P.T.S.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Acai seed ash as a novel basic heterogeneous catalyst for biodiesel synthesis: Optimization of the biodiesel production process. Fuel 2021, 299, 120887. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Parametric study and optimization of microwave assisted biodiesel synthesis from Argemone Mexicana oil using response surface methodology. Chem. Eng. Process.-Process Intensif. 2022, 170, 108665. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. Citrus limetta Peel-Derived Catalyst for Sustainable Production of Biodiesel. ACS Omega 2022, 7, 28534–28544. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Ahmad, M.; Zafar, M.; Gaafar, A.-R.Z.; Hodhod, M.S.; Sultana, S.; Athar, M.; Ozdemir, F.A.; Makhkamov, T.; Yuldashev, A.; et al. Novel Copper Oxide Phyto-Nanocatalyst Utilized for the Synthesis of Sustainable Biodiesel from Citrullus colocynthis Seed Oil. Processes 2023, 11, 1857. [Google Scholar] [CrossRef]
- Thakur, A.; Devi, P.; Saini, S.; Jain, R.; Sinha, R.K.; Kumar, P. Citrus limetta Organic Waste Recycled Carbon Nanolights: Photoelectro Catalytic, Sensing, and Biomedical Applications. ACS Sustain. Chem. Eng. 2019, 7, 502–512. [Google Scholar] [CrossRef]
- Hossain, M.A.; Chowdhury, S.M.; Rekhu, Y.; Faraz, K.S.; Islam, M.U. Biodiesel from Coconut Oil: A RenewableAlternative Fuel for Diesel Engine. World Acad. Sci. Eng. Technol. 2012, 68, 1289–1293. [Google Scholar]
- Lugo-Méndez, H.; Sánchez-Domínguez, M.; Sales-Cruz, M.; Olivares-Hernández, R.; Lugo-Leyte, R.; Torres-Aldaco, A. Synthesis of biodiesel from coconut oil and characterization of its blends. Fuel 2021, 295, 120595. [Google Scholar] [CrossRef]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef]
- Betiku, E.; Ajala, S.O. Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology. Ind. Crops Prod. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Sádaba, I.; Granados, M.L.; Riisager, A.; Taarning, E. Deactivation of solid catalysts in liquid media: The case of leaching of active sites in biomass conversion reactions. Green Chem. 2015, 17, 4133–4145. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using Napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous Production of Biodiesel via Transesterification from Vegetable Oils in Supercritical Methanol. Energy Fuels 2006, 20, 812–817. [Google Scholar] [CrossRef]
- ASTM D 6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuel. ASTM International: West Conshohocken, PA, USA, 2023.
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana Colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Saad, M.; Siyo, B.; Alrakkad, H. Preparation and characterization of biodiesel from waste cooking oils using heterogeneous Catalyst(Cat.TS-7) based on natural zeolite. Heliyon 2023, 9, e15836. [Google Scholar] [CrossRef]
- Hangun-Balkir, Y. Green Biodiesel Synthesis Using Waste Shells as Sustainable Catalysts with Camelina sativa Oil. J. Chem. 2016, 2016, 6715232. [Google Scholar] [CrossRef]






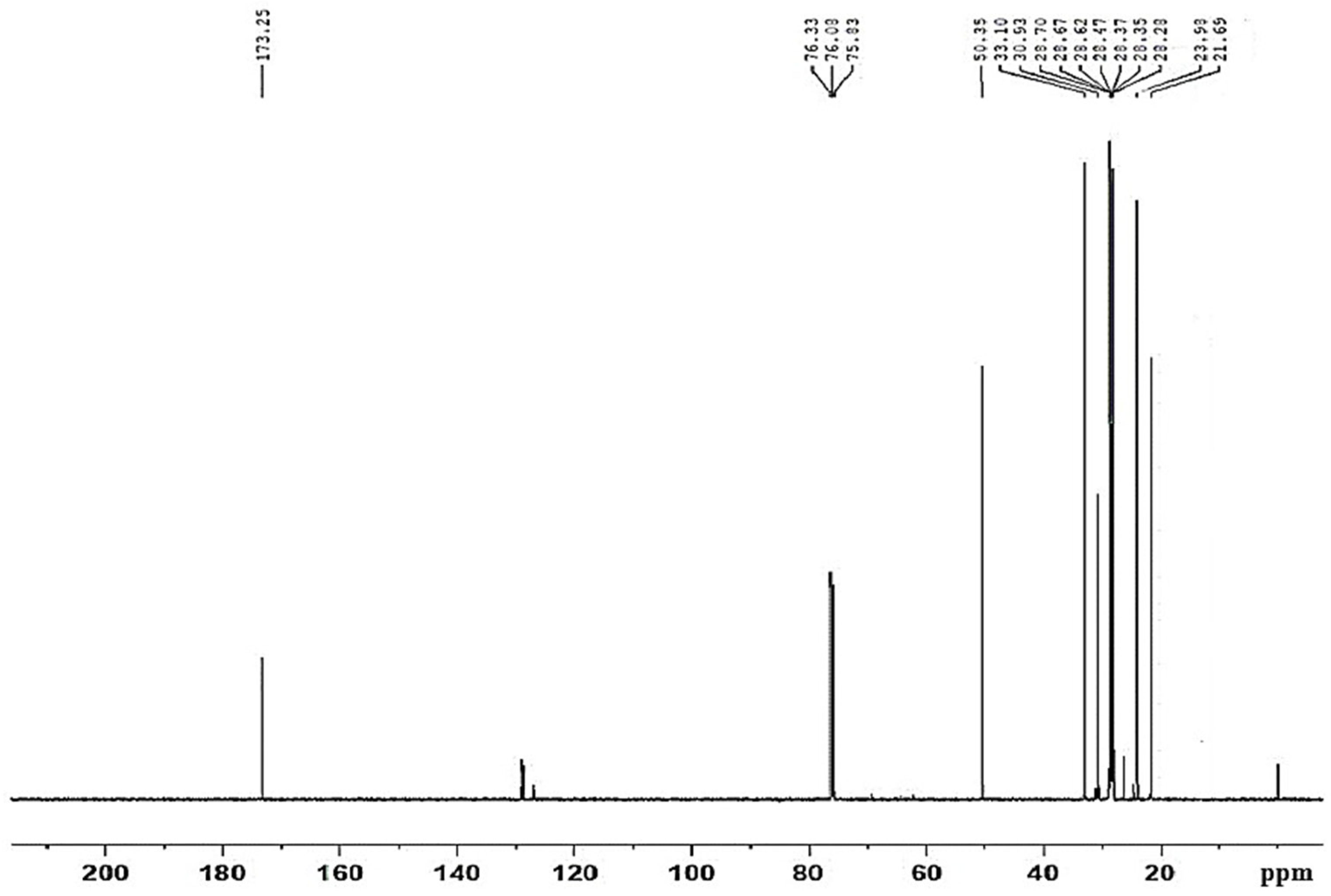


| Properties | Biodiesel | ASTM D 6751 [34] |
|---|---|---|
| Density (g) | 0.87 | 0.86–0.90 |
| Viscosity (mm2/s) | 2.5 | 1.9–6.0 |
| Flashpoint (°C) | 142 | 93 min |
| Cetane number | 54 | Above 46 |
| Heating value (MJ/kg) | 43.1 | - |
| Boiling point (°C) | 294 | - |
| Oxidation stability | 3 h | 3 h |
| Acid value (mg KOH−1 g) | 0.09 | <0.80 |
| Feedstock Oil | Catalyst | Catalyst Loading (wt%) | Methanol: Oil | Time (Hours) | Temperature (°C) | Conversion (%) | References |
|---|---|---|---|---|---|---|---|
| Waste cooking oil | Zeolites | 1.5 | 9:1 | 2 | 60 | 93 | [36] |
| Camelina sativa oil | Waste shell | 1 | 12:1 | 3 | 65 | Lobster shell—90% Eggshell—97.2% | [37] |
| Waste cooking oil | Citrus limetta peels | 5 | 20:1 | 3 | 70 | 96 | [24] |
| Coconut oil | CCLP | 1 | 6:1 | 3 | 60 | 100 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, P.; Kaur, G.; Hasan, I. Biodiesel Synthesis from Coconut Oil Using the Ash of Citrus limetta Peels as a Renewable Heterogeneous Catalyst. Catalysts 2024, 14, 549. https://doi.org/10.3390/catal14080549
Kaushik P, Kaur G, Hasan I. Biodiesel Synthesis from Coconut Oil Using the Ash of Citrus limetta Peels as a Renewable Heterogeneous Catalyst. Catalysts. 2024; 14(8):549. https://doi.org/10.3390/catal14080549
Chicago/Turabian StyleKaushik, Priyal, Gurmeet Kaur, and Imran Hasan. 2024. "Biodiesel Synthesis from Coconut Oil Using the Ash of Citrus limetta Peels as a Renewable Heterogeneous Catalyst" Catalysts 14, no. 8: 549. https://doi.org/10.3390/catal14080549






