Efficient and Robust Photodegradation of Dichlorvos Pesticide by BiOBr/WO2.72 Nanocomposites with Type-I Heterojunction under Visible Light Irradiation
Abstract
:1. Introduction
2. Results
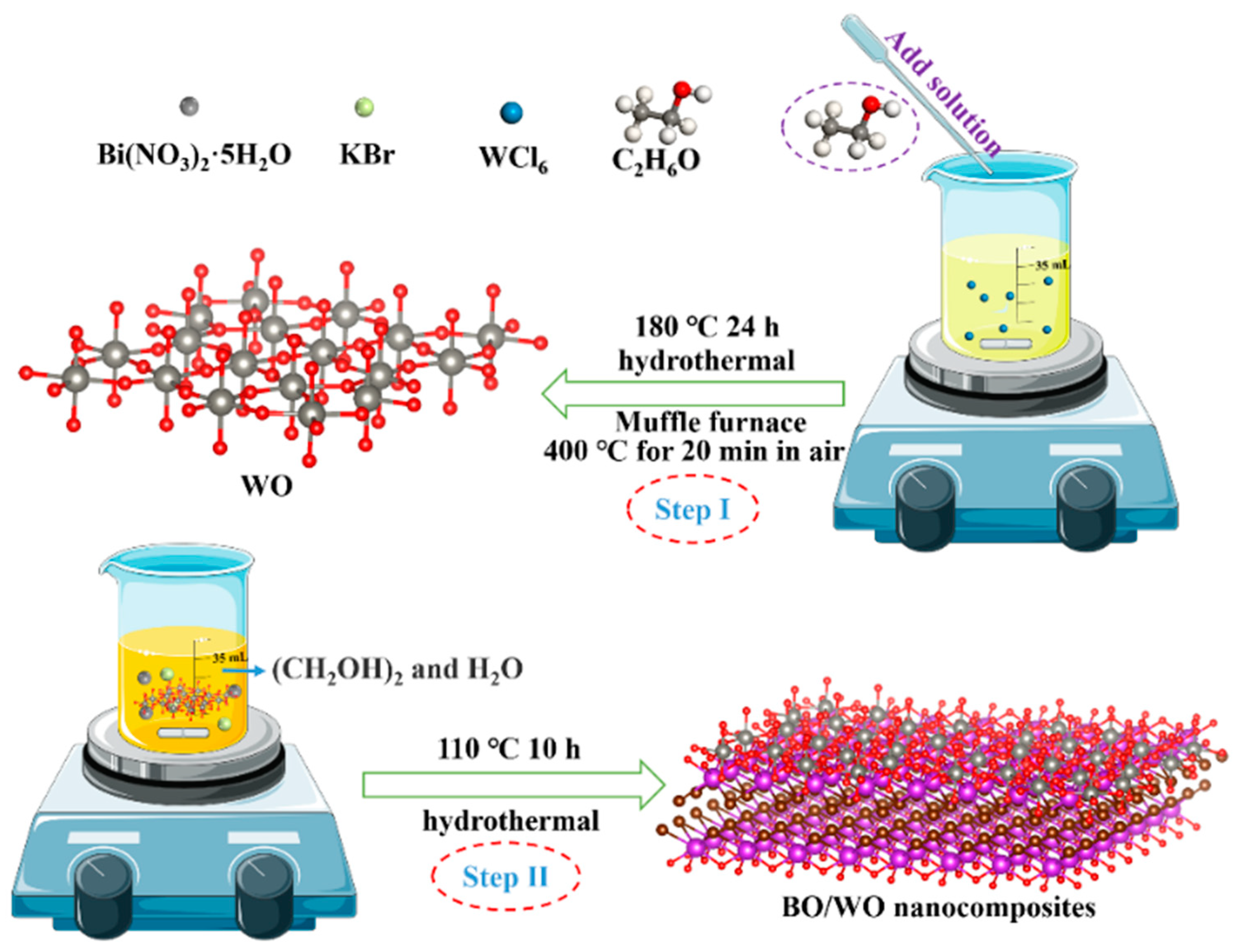
2.1. Diagram of Materials Synthesis Process
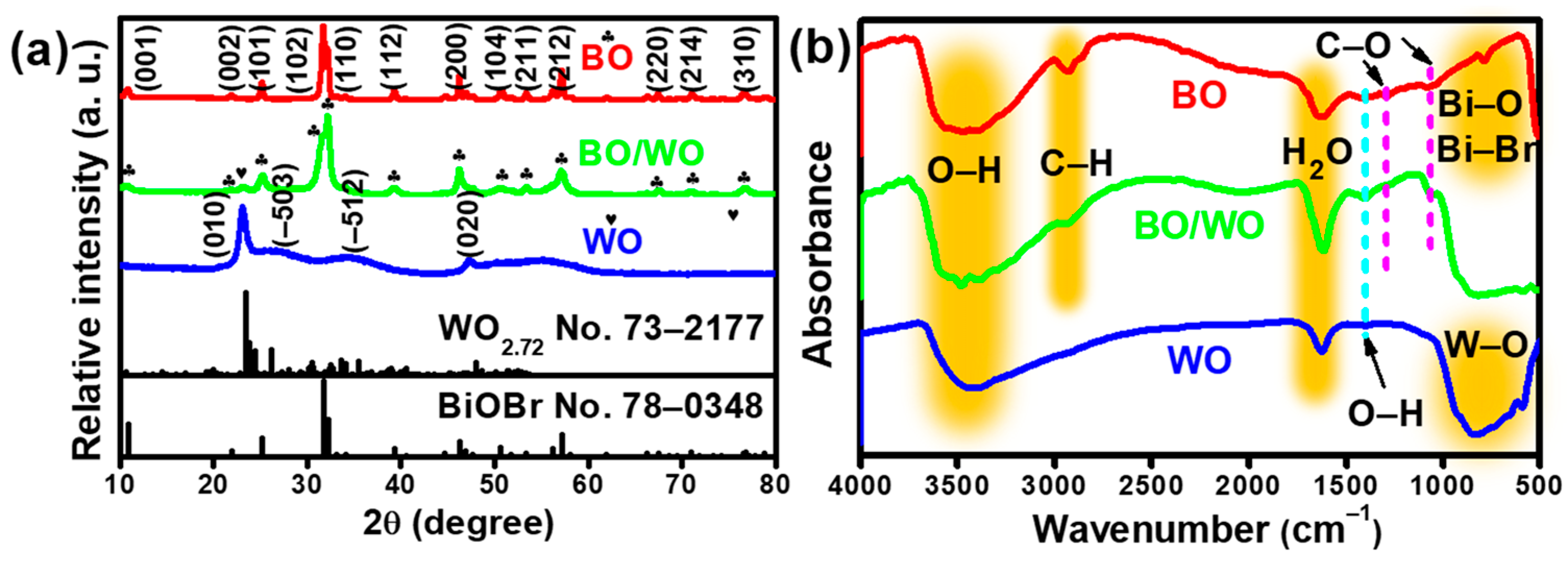
2.2. Analysis of Phase and Microscopic Morphology
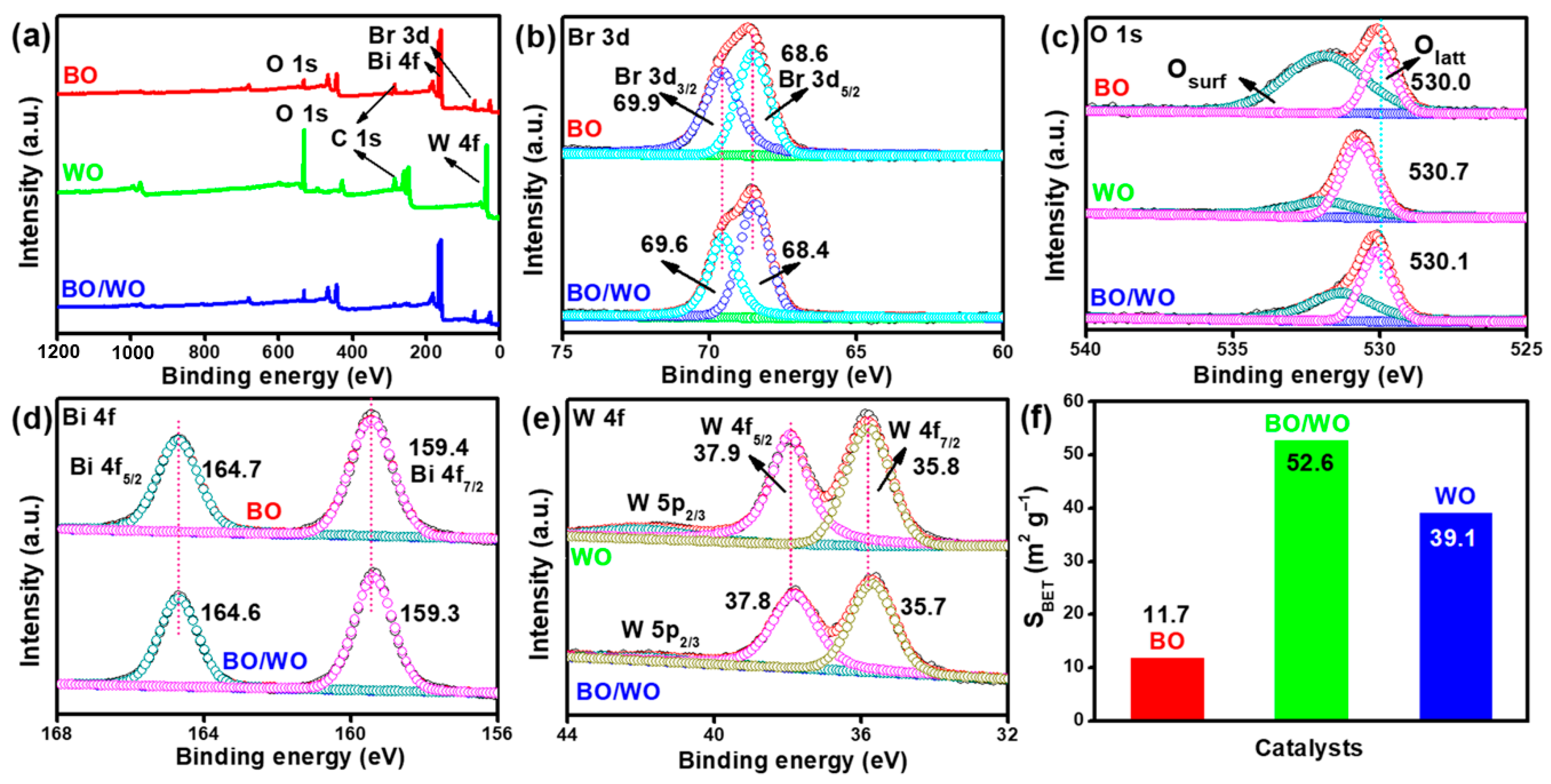
2.3. X-ray Photoelectron Spectroscopy (XPS) Analysis and Specific Surface Area
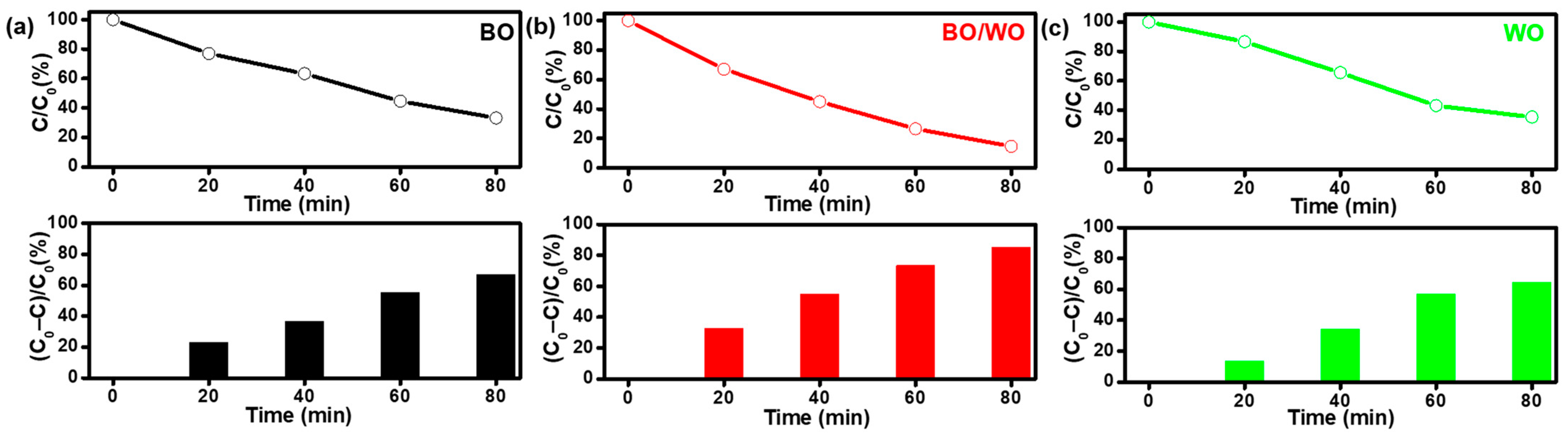
2.4. Photocatalytic Activity and Stability Analysis
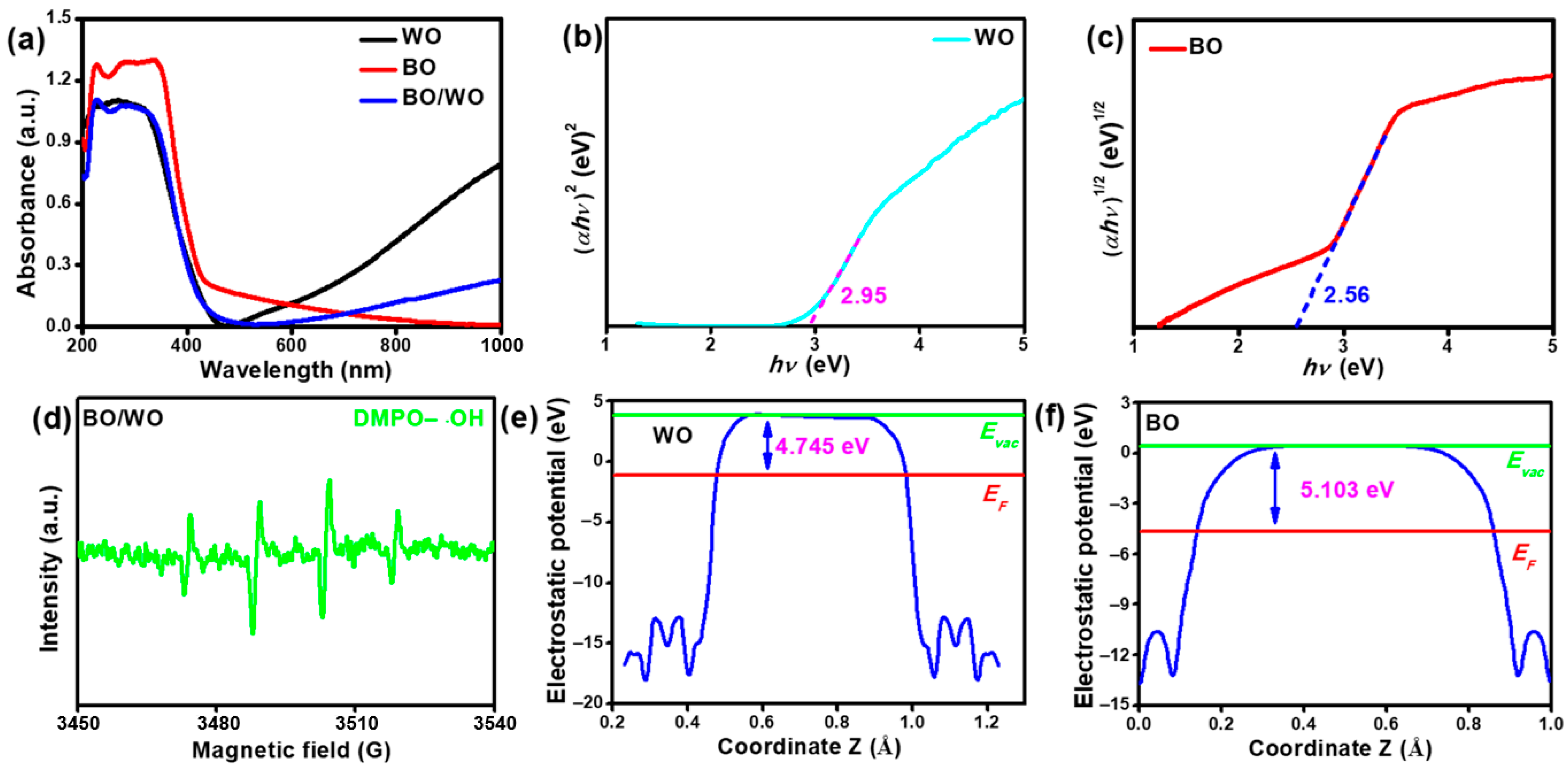
2.5. Optoelectronic Properties and Theoretical Calculations Analysis
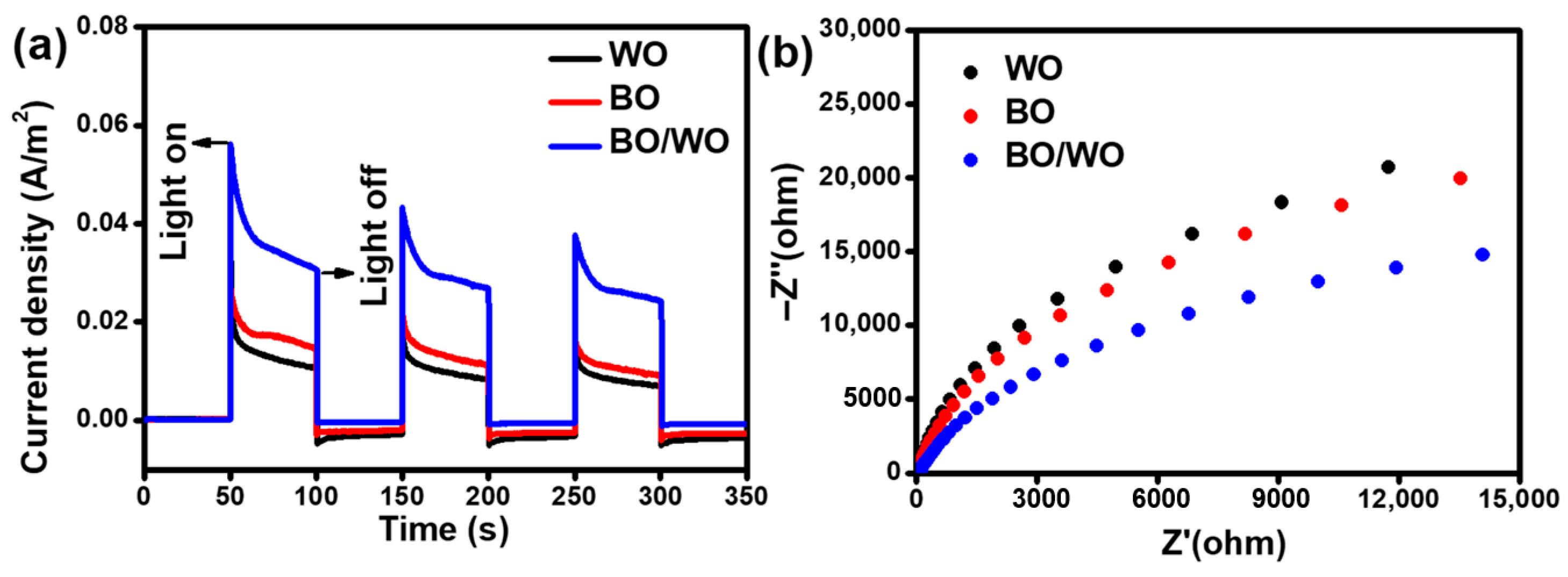
2.6. Photocatalytic Response and Electrochemical Impedance Spectroscopy Analysis
2.7. Photocatalytic Mechanism
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, S.; Zhang, J.; Li, H.; Du, Y.; Li, S.; Li, A.; Suo, X.; Wang, Y.; Sun, Q. Anti-inflammatory activity of the water extract of Chloranthus serratus roots in LPS-stimulated RAW264.7 cells mediated by the Nrf2/HO-1, MAPK and NF-κB signaling pathways. J. Ethnopharmacol. 2021, 271, 113880. [Google Scholar] [CrossRef]
- Wang, W.; Lu, H.; Liu, X.; Sun, G. Acute pancreatitis during lambda cyhalothrin poisoning. Toxin Rev. 2014, 33, 136–137. [Google Scholar]
- Wang, X.; Zhu, Y.; Lu, W.; Guo, X.; Chen, L.; Zhang, N.; Chen, S.; Ge, C.; Xu, S. Microcystin-LR-induced nuclear translocation of cGAS promotes mutagenesis in human hepatocytes by impeding homologous recombination repair. Toxicol. Lett. 2023, 373, 94–104. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, W.; Xia, Y.; Li, X.; Luan, J.; Chen, J. PEGylated niosomes as delivery systems efficiently prolonged the clearance time of 2-hydroxy-1,7-dimethoxyxanthone in vivo. Lat. Am. J. Pharm. 2015, 34, 1808–1814. [Google Scholar]
- Wu, T.; Xu, S.; Chen, B.; Bao, L.; Ma, J.; Han, W.; Xu, A.; Yu, K.; Wu, L.; Chen, S. Ambient PM2.5 exposure causes cellular senescence via DNA damage, micronuclei formation, and cGAS activation. Nanotoxicology 2022, 16, 757–775. [Google Scholar] [CrossRef]
- Zhu, W.; Li, C.; Dai, T.; Tao, F.; Wang, M.; Wang, C.; Han, Z.; Sun, N.; Zhao, Y.; Wang, D. Effects of allyl isothiocyanate on the expression, function, and its mechanism of ABCA1 and ABCG1 in pulmonary of COPD rats. Int. Immunopharmacol. 2021, 101, 108373. [Google Scholar] [CrossRef]
- Huang, R.; Sun, Y.; Zhang, X.; Sun, B.; Wang, Q.; Zhu, J. Biological evaluation of a novel herceptin-platinum (II) conjugate for efficient and cancer cell specific delivery. Biomed. Pharmacother. 2015, 73, 116–122. [Google Scholar] [CrossRef]
- Gao, J.; Yang, J.; Zhu, L.; Xu, C.; Nie, L. Acrylamide impairs the developmental potential of germinal vesicle oocytes by inducing mitochondrial dysfunction and autophagy/apoptosis in mice. Hum. Exp. Toxicol. 2021, 40, S370–S380. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, L.; Xu, Z.; Xu, H. Effect of drug-mediated cytoskeletal remodeling on osteogenic differentiation of human mesenchymal stem cells. Lat. Am. J. Pharm. 2019, 38, 1985–1992. [Google Scholar]
- Jia, Y.; Li, X.; Xie, H.; Shen, J.; Luo, J.; Wang, J.; Wang, K.; Liu, Q.; Kong, L. Analysis and pharmacokinetics studies of gastrodin and p-hydroxybenzyl alcohol in dogs using ultra fast liquid chromatography-tandem mass spectrometry method. J. Pharm. Biomed. Anal. 2014, 99, 83–88. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Zhai, L.; Meng, Q.; Wang, Z.; Zhang, J.; Li, Z. Highly efficient photocatalytic decomposition of triazophos using novel In2O3/WO3 nanocomposites with oxygen defects and S-Scheme heterojunctions. Int. J. Hydrogen Energy 2024, 57, 369–378. [Google Scholar]
- Hua, J.; Wang, Z.; Zhang, J.; Dai, K.; Shao, C.; Fan, K. A hierarchical Bi-MOF-derived BiOBr/Mn0.2Cd0.8S S-scheme for visible-light-driven photocatalytic CO2 reduction. J. Mater. Sci. Technol. 2023, 156, 64–71. [Google Scholar] [CrossRef]
- Anh, T.; Pham, T.; Viet, N.; Anh, D.; Cam, N.; Noi, N.; Nhiem, D.; Chau, C.; Ha, T.; Phuong, N.; et al. Synthesis of CoWO4/g-C3N4 Z-scheme heterojunction for the efficient photodegradation of diazinon with the addition of H2O2. J. Environ. Sci. Health B 2024, 59, 1–8. [Google Scholar] [CrossRef]
- Elhaddad, E.; Al-fawwaz, A.; Rehan, M. An effective stannic oxide/graphitic carbon nitride (SnO2 NPs@g-C3N4) nanocomposite photocatalyst for organic pollutants degradation under visible-light. J. Saudi Chem. Soc. 2023, 27, 101677. [Google Scholar] [CrossRef]
- Hao, P.; Chen, Z.; Yan, Y.; Shi, W.; Guo, F. Recent advances, application and prospect in g-C3N4-based S-scheme heterojunction photocatalysts. Sep. Purif. Technol. 2024, 330, 125302. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, L.; Zhang, Z.; Bie, C. A review on ZnO-based S-scheme heterojunction photocatalysts. Chin. J. Catal. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, S.; Wang, T.; Dhiman, P.; Sharma, G.; Du, B.; Stadler, F. Advances in S-scheme heterojunction semiconductor photocatalysts for CO2 reduction, nitrogen fixation and NOx degradation. Mater. Sci. Semicond. Process. 2023, 168, 107869. [Google Scholar] [CrossRef]
- Li, M.; Cui, H.; Zhao, Y.; Li, S.; Wang, J.; Ge, K.; Yang, Y. S-Scheme Heterojunction Photocatalysts for CO2 Reduction. Catalysts 2024, 14, 374. [Google Scholar] [CrossRef]
- Li, T.; Tsubaki, N.; Jin, Z. S-scheme heterojunction in photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 169, 82–104. [Google Scholar] [CrossRef]
- Lu, J.; Gu, S.; Li, H.; Wang, Y.; Guo, M.; Zhou, G. Review on multi-dimensional assembled S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2023, 160, 214–239. [Google Scholar] [CrossRef]
- Mansingh, S.; Das, K.; Priyadarshini, N.; Sahoo, D.; Prusty, D.; Sahu, J.; Mohanty, U.; Parida, K. Minireview Elaborating S-Scheme Charge Dynamic Photocatalysts: Journey from Z to S, Mechanism of Charge Flow, Characterization Proof, and H2O2 Evolution. Energy Fuels 2023, 37, 9873–9894. [Google Scholar] [CrossRef]
- Molaei, M. Principles, mechanism, and identification of S-scheme heterojunction for photocatalysis: A critical review. J. Am. Ceram. Soc. 2024, 107, 5695–5719. [Google Scholar] [CrossRef]
- Nazir, A.; Huo, P.; Rasool, A. Recent advances on graphitic carbon nitride-based S-scheme photocatalysts: Synthesis, environmental applications, and challenges. J. Organomet. Chem. 2024, 1004, 122951. [Google Scholar] [CrossRef]
- Nie, C.; Wang, X.; Lu, P.; Zhu, Y.; Li, X.; Tang, H. Advancements in S-scheme heterojunction materials for photocatalytic environmental remediation. J. Mater. Sci. Technol. 2024, 169, 182–198. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Pan, G.; Wang, N.; Xing, Y.; Zhang, Z. Recent progress in CdS-based S-scheme photocatalysts. J. Mater. Sci. Technol. 2024, 171, 162–184. [Google Scholar] [CrossRef]
- Sharma, K.; Hasija, V.; Malhotra, M.; Verma, P.; Le, Q.; Khan, A.; Thakur, S.; Quang, H.; Nguyen, V.; Singh, P.; et al. A review of CdS-based S-scheme for photocatalytic water splitting: Synthetic strategy and identification techniques. Int. J. Hydrogen Energy 2024, 52, 804–818. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Dai, K.; Zhang, J. Review on inorganic-organic S-scheme photocatalysts. J. Mater. Sci. Technol. 2023, 165, 187–218. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Cheng, B.; He, R.; Yu, J. S-Scheme Heterojunction Photocatalysts for H2O2 Production. J. Phys. Chem. Lett. 2023, 14, 4803–4814. [Google Scholar] [CrossRef]
- Wang, W.; Mei, S.; Jiang, H.; Wang, L.; Tang, H.; Liu, Q. Recent advances in TiO2-based S-scheme heterojunction photocatalysts. Chin. J. Catal. 2023, 55, 137–158. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, X.; Xiang, Q. MOFs-based S-scheme heterojunction photocatalysts. Coord. Chem. Rev. 2024, 504, 215674. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, X.; Chen, Y.; Xiao, X.; Chen, T.; Wang, Y. Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion. Materials 2023, 16, 3745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Du, P.; Yin, L.; Yao, J.; Wang, J.; Liu, C. Metal-organic framework-based S-scheme heterojunction photocatalysts. Nanoscale 2024, 16, 5487–5503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, J.; Zhao, Y.; Zhang, L.; Yu, J. Construction of 2D S-Scheme Heterojunction Photocatalyst. Adv. Mater. 2024, 36, 2310600. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.; Chen, C.; Ma, T.; Zhang, J.; Wang, Z. Transforming the Charge Transfer Mechanism in the In2O3/CdSe-DETA Nanocomposite from Type-I to S-Scheme to Improve Photocatalytic Activity and Stability During Hydrogen Production. Acta Phys.-Chim. Sin. 2023, 39, 2208030. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, A.; Li, W.; Li, Z.; Zhang, J. Efficient and Robust Photodegradation of Dichlorvos Pesticide by BiOBr/WO2.72 Nanocomposites with Type-I Heterojunction under Visible Light Irradiation. Catalysts 2024, 14, 548. https://doi.org/10.3390/catal14080548
Meng A, Li W, Li Z, Zhang J. Efficient and Robust Photodegradation of Dichlorvos Pesticide by BiOBr/WO2.72 Nanocomposites with Type-I Heterojunction under Visible Light Irradiation. Catalysts. 2024; 14(8):548. https://doi.org/10.3390/catal14080548
Chicago/Turabian StyleMeng, Aoyun, Wen Li, Zhen Li, and Jinfeng Zhang. 2024. "Efficient and Robust Photodegradation of Dichlorvos Pesticide by BiOBr/WO2.72 Nanocomposites with Type-I Heterojunction under Visible Light Irradiation" Catalysts 14, no. 8: 548. https://doi.org/10.3390/catal14080548




