Catalytic Application of POSS–COF-[(Co(acetate)2] for Selective Reduction of Nitriles to Amines
Abstract
:1. Introduction
2. Results and Discussion
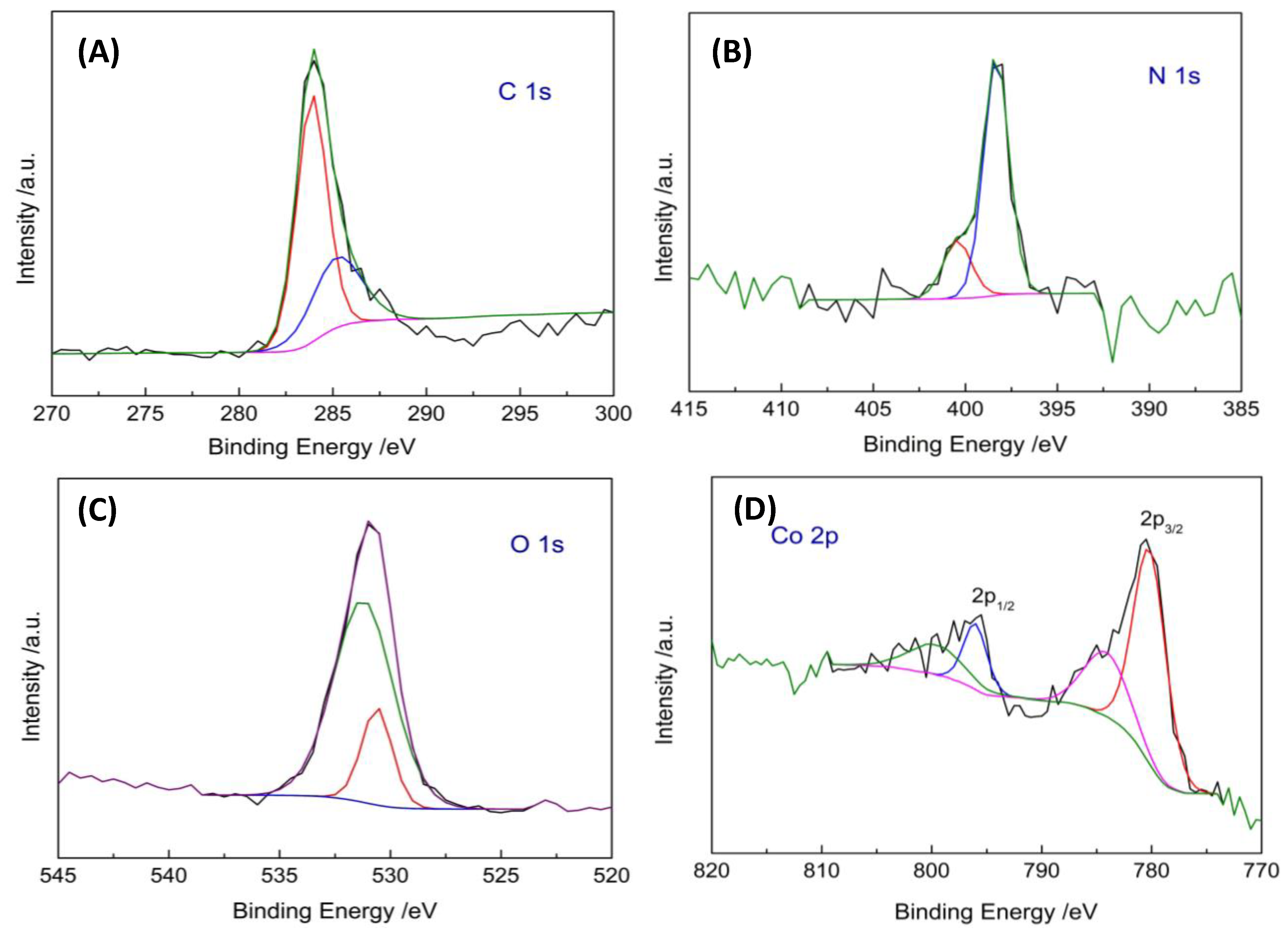
2.1. Chemical and Morphological Properties of POSS–COF (Co) Catalyst
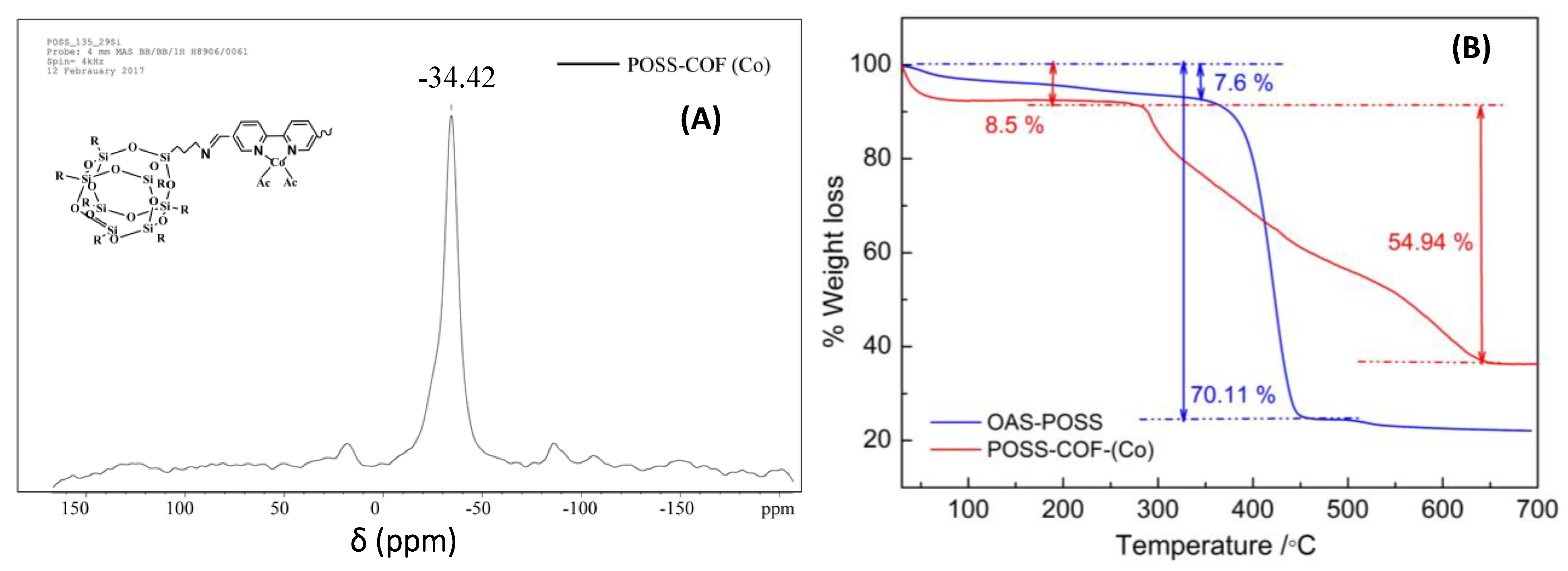
2.2. 29Si Solid-State NMR Spectroscopy of POSS–COF (Co) Catalyst
2.3. Thermal Properties of POSS–COF (Co) Catalyst
2.4. Catalytic Activity of POSS–COF (Co) Catalyst for the Reduction of Hetero (Aryl)nitriles
3. Materials and Methods
3.1. Experimental Supplies
3.2. Synthesis of POSS–COF (Co) Framework
3.3. Plausible Mechanism for the Hydrogenation of Aromatic Nitriles Using POSS–COF (Co) Catalyst
3.4. General Procedure for Catalysis Experiment
3.5. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Janeta, M.; John, Ł.; Ejfler, J.; Szafert, S. High-Yield Synthesis of Amido-Functionalized Polyoctahedral Oligomeric Silsesquioxanes by Using Acyl Chlorides. Chem. Eur. J. 2014, 20, 15966–15974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Janeta, M.; Lis, T.; Szafert, S. Zinc Imine Polyhedral Oligomeric Silsesquioxane as a Quattro-Site Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Low-Pressure CO2. Chem. Eur. J. 2020, 26, 13686–13697. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Schottner, G. Hybrid sol− gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid inorganic–organic mesoporous silicates—Nanoscopic reactors coming of age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Wang, W.-J.; Hai, X.; Mao, Q.-X.; Chen, M.-L.; Wang, J.-H. Polyhedral oligomeric silsesquioxane functionalized carbon dots for cell imaging. ACS Appl. Mater. Interfaces 2015, 7, 16609–16616. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, E.A.; Basset, J.-M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Sakugawa, S.; Wada, K.; Inoue, M. Ti-bridged silsesquioxanes as precursors of silica-supported titanium oxide catalysts for the epoxidation of cyclooctene. J. Catal. 2010, 275, 280–287. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Czaban, J.; Krishnan, G.R.; Malińska, M.; Woźniak, K.; Siddique, H.; Peeva, L.G.; Livingston, A.G.; Grela, K. Batchwise and continuous nanofiltration of POSS-Tagged Grubbs–Hoveyda-Type olefin metathesis catalysts. ChemSusChem 2013, 6, 182–192. [Google Scholar] [CrossRef]
- Leng, Y.; Liu, J.; Zhang, C.; Jiang, P. A polyhedral oligomeric silsesquioxane (POSS)-bridged oxo-molybdenum Schiff base complex with enhanced heterogeneous catalytic activity in epoxidation. Catal. Sci. Technol. 2014, 4, 997–1004. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tsujimoto, M.; Packwood, D.; Duong, N.T.; Nishiyama, Y.; Kadota, K.; Kitagawa, S.; Horike, S. Construction of a Hierarchical Architecture of Covalent Organic Frameworks via a Postsynthetic Approach. J. Am. Chem. Soc. 2018, 140, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis. Appl. Catal. B Environ. 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Yusran, Y.; Fang, Q.; Valtchev, V. Electroactive Covalent Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2020, 32, 2002038. [Google Scholar] [CrossRef]
- Rasheed, T. Covalent organic frameworks as promising adsorbent paradigm for environmental pollutants from aqueous matrices: Perspective and challenges. Sci. Total Environ. 2022, 833, 155279. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Chowdhury, I.H.; Das, S.; Islam, S.M. Recent trends in covalent organic frameworks (COFs) for carbon dioxide reduction. Mater. Adv. 2022, 3, 8063–8080. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, X.; Zhang, H.-Y.; Zhang, R.; Liu, Y.-H.; Zhang, F.-M.; Lu, M.; Yang, Z.-D.; Lan, Y.-Q. Engineering β-ketoamine covalent organic frameworks for photocatalytic overall water splitting. Nat. Commun. 2023, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-B.; Xie, K.-H.; Xu, H.-P.; Wang, Y.-J.; Zhao, F.; Geng, Y.; Dong, Y.-B. Covalent organic frameworks and their composites as multifunctional photocatalysts for efficient visible-light induced organic transformations. Coord. Chem. Rev. 2022, 472, 214774. [Google Scholar] [CrossRef]
- Yang, H.; Shi, R.; Shang, L.; Zhang, T. Recent Advancements of Porphyrin-Like Single-Atom Catalysts: Synthesis and Applications. Small Struct. 2021, 2, 2100007. [Google Scholar] [CrossRef]
- Antonangelo, A.; Westrup, K.; Burt, L.; Bezzu, C.G.; Malewschik, T.; Machado, G.; Nunes, F.; McKeown, N.; Nakagaki, S. Synthesis, crystallographic characterization and homogeneous catalytic activity of novel unsymmetric porphyrins. RSC Adv. 2017, 7, 50610–50618. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Ziolek, M. Catalytic liquid-phase oxidation in heterogeneous system as green chemistry goal—Advantages and disadvantages of MCM-41 used as catalyst. Catal. Today 2004, 90, 145–150. [Google Scholar] [CrossRef]
- Rubab, A.; Sharif, M.; Razzaq, R.; Jackstell, R.; Nafady, A.; Weiß, J.; Sohail, M. Highly active iron oxide@NC catalyst derived from the iron acetate/polyacrylonitrile threads: Driving nitroarene conversion to value-added amines. J. Appl. Polym. Sci. 2024, e56062. [Google Scholar] [CrossRef]
- Venezia, A.M.; La Parola, V.; Liotta, L.F. Structural and surface properties of heterogeneous catalysts: Nature of the oxide carrier and supported particle size effects. Catal. Today 2017, 285, 114–124. [Google Scholar] [CrossRef]
- Downing, R.S.; Kunkeler, P.J.; van Bekkum, H. Catalytic syntheses of aromatic amines. Catal. Today 1997, 37, 121–136. [Google Scholar] [CrossRef]
- Ono, N. The Nitro Group in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-0-471-45846-3. [Google Scholar]
- Sun, Z.; Bottari, G.; Barta, K. Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles. Green Chem. 2015, 17, 5172–5181. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Banerjee, D.; Arockiam, P.B.; Junge, H.; Junge, K.; Pohl, M.-M.; Radnik, J.; Bruckner, A.; Beller, M. Highly selective transfer hydrogenation of functionalised nitroarenes using cobalt-based nanocatalysts. Green Chem. 2015, 17, 898–902. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332. [Google Scholar] [CrossRef] [PubMed]
- Aller, B. Raney cobalt hydrogenation catalysts. I. the preparation of the catalyst. J. Appl. Chem. 2007, 7, 130–134. [Google Scholar] [CrossRef]
- Adkins, H. Reactions of Hydrogen with Organic Compounds over Copper-Chromium Oxide and Nickel Catalysts. Nature 1938, 141, 811. [Google Scholar]
- Bagal, D.B.; Bhanage, B.M. Recent Advances in Transition Metal-Catalyzed Hydrogenation of Nitriles. Adv. Synth. Catal. 2015, 357, 883–900. [Google Scholar] [CrossRef]
- Reguillo, R.; Grellier, M.; Vautravers, N.; Vendier, L.; Sabo-Etienne, S. Ruthenium-catalyzed hydrogenation of nitriles: Insights into the mechanism. J. Am. Chem. Soc. 2010, 132, 7854–7855. [Google Scholar] [CrossRef]
- Yoshida, T.; Okano, T.; Otsuka, S. Catalytic hydrogenation of nitriles and dehydrogenation of amines with the rhodium(I) hydrido compounds [RhH(PPri3)3] and [Rh2H2(µ-N2){P(cyclohexyl)3}4]. J. Chem. Soc. Chem. Commun. 1979, 870–871. [Google Scholar] [CrossRef]
- Rajesh, K.; Dudle, B.; Blacque, O.; Berke, H. Homogeneous hydrogenations of nitriles catalyzed by rhenium complexes. Adv. Synth. Catal. 2011, 353, 1479–1484. [Google Scholar] [CrossRef]
- Elangovan, S.; Topf, C.; Fischer, S.; Jiao, H.; Spannenberg, A.; Baumann, W.; Ludwig, R.; Junge, K.; Beller, M. Selective Catalytic Hydrogenations of Nitriles, Ketones, and Aldehydes by Well-Defined Manganese Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 8809–8814. [Google Scholar] [CrossRef]
- Mukherjee, A.; Srimani, D.; Chakraborty, S.; Ben-David, Y.; Milstein, D. Selective Hydrogenation of Nitriles to Primary Amines Catalyzed by a Cobalt Pincer Complex. J. Am. Chem. Soc. 2015, 137, 8888–8891. [Google Scholar] [CrossRef] [PubMed]
- Schneekönig, J.; Tannert, B.; Hornke, H.; Beller, M.; Junge, K. Cobalt pincer complexes for catalytic reduction of nitriles to primary amines. Catal. Sci. Technol. 2019, 9, 1779–1783. [Google Scholar] [CrossRef]
- Tokmic, K.; Jackson, B.J.; Salazar, A.; Woods, T.J.; Fout, A.R. Cobalt-Catalyzed and Lewis Acid-Assisted Nitrile Hydrogenation to Primary Amines: A Combined Effort. J. Am. Chem. Soc. 2017, 139, 13554–13561. [Google Scholar] [CrossRef]
- Lange, S.; Elangovan, S.; Cordes, C.; Spannenberg, A.; Jiao, H.; Junge, H.; Bachmann, S.; Scalone, M.; Topf, C.; Junge, K.; et al. Selective catalytic hydrogenation of nitriles to primary amines using iron pincer complexes. Catal. Sci. Technol. 2016, 6, 4768–4772. [Google Scholar] [CrossRef]
- Chakraborty, S.; Leitus, G.; Milstein, D. Selective hydrogenation of nitriles to primary amines catalyzed by a novel iron complex. Chem. Commun. 2016, 52, 1812–1815. [Google Scholar] [CrossRef]
- Lévay, K.; Hegedűs, L. Recent achievements in the hydrogenation of nitriles catalyzed by transitional metals. Curr. Org. Chem. 2019, 23, 1881–1900. [Google Scholar] [CrossRef]
- Cho, J.H.; Byun, S.; Cho, A.; Kim, B.M. One-pot, chemoselective synthesis of secondary amines from aryl nitriles using a PdPt–Fe3O4 nanoparticle catalyst. Catal. Sci. Technol. 2020, 10, 4201–4209. [Google Scholar] [CrossRef]
- Chandrashekhar, V.G.; Senthamarai, T.; Kadam, R.G.; Malina, O.; Kašlík, J.; Zbořil, R.; Gawande, M.B.; Jagadeesh, R.V.; Beller, M. Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives. Nat. Catal. 2022, 5, 20–29. [Google Scholar] [CrossRef]
- Sharma, D.M.; Punji, B. Selective Synthesis of Secondary Amines from Nitriles by a User-Friendly Cobalt Catalyst. Adv. Synth. Catal. 2019, 361, 3930–3936. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Sohail, M.; Alshammari, A.S.; Pohl, M.-M.; Beller, M.; Jagadeesh, R.V. Cobalt-based nanoparticles prepared from MOF–carbon templates as efficient hydrogenation catalysts. Chem. Sci. 2018, 9, 8553–8560. [Google Scholar] [CrossRef]
- Liu, W.; Sahoo, B.; Junge, K.; Beller, M. Cobalt Complexes as an Emerging Class of Catalysts for Homogeneous Hydrogenations. Acc. Chem. Res. 2018, 51, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yang, Y.; Lü, C. Quaternized POSS modified rGO-supported Pd nanoparticles as a highly efficient catalyst for reduction and Suzuki coupling reactions. New J. Chem. 2019, 43, 18601–18610. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, X.; Tong, J.; Li, X.; Wang, W.; Cheng, B.; Cai, Z. Solution-blown SPEEK/POSS nanofiber–nafion hybrid composite membranes for direct methanol fuel cells. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sun, J.; Jia, L.; Chen, X. Efficient Adsorption and Extraction of Glutathione S-Transferases with Glutathione-Functionalized Graphene Oxide–Polyhedral Oligomeric Silsesquioxane Composite. Molecules 2023, 28, 340. [Google Scholar] [CrossRef]
- Lai, Y.S.; Tsai, C.W.; Yang, H.W.; Wang, G.P.; Wu, K.H. Structural and electrochemical properties of polyurethanes/polyhedral oligomeric silsesquioxanes (PU/POSS) hybrid coatings on aluminum alloys. Mater. Chem. Phys. 2009, 117, 91–98. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Yu, K.; Zhou, X.; Xie, X. Synthesis, thermal stability and photoresponsive behaviors of azobenzene-tethered polyhedral oligomeric silsesquioxanes. New J. Chem. 2011, 35, 2781–2792. [Google Scholar] [CrossRef]
- Janeta, M.; Rajczakowska, M.; Ejfler, J.; Łydżba, D.; Szafert, S. Synthesis and microstructural properties of the scaffold based on a 3-(trimethoxysilyl) propyl methacrylate–POSS hybrid towards potential tissue engineering applications. RSC Adv. 2016, 6, 66037–66047. [Google Scholar]
- Kovacic, J.E. The C-N stretching frequency in the infrared spectra of Schiff’s base complexes—I. Copper complexes of salicylidene anilines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1967, 23, 183–187. [Google Scholar] [CrossRef]
- Danilova, J.S.; Avdoshenko, S.M.; Karushev, M.P.; Timonov, A.M.; Dmitrieva, E. Infrared spectroscopic study of nickel complexes with salen-type ligands and their polymers. J. Mol. Struct. 2021, 1241, 130668. [Google Scholar] [CrossRef]
- Chaudhary, R.G.; Tanna, J.A.; Gandhare, N.V.; Bagade, M.B.; Bhuyar, S.S.; Gharpure, M.P.; Juneja, H.D. Synthesis and characterization of metal coordination polymers with fumaroyl bis (paramethoxyphenylcarbamide) by using FTIR, XRD, SEM and TG techniques. J. Chin. Adv. Mater. Soc. 2015, 3, 177–187. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Q.; Jin, S.; Wang, Y.; Yuan, Z.; Chi, Q.; Zhang, Z. Mild and selective hydrogenation of nitriles into primary amines over a supported Ni catalyst. New J. Chem. 2020, 44, 549–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Chi, Q.; Zhang, Z. Nitrogen-Doped Carbon-Supported Nickel Nanoparticles: A Robust Catalyst to Bridge the Hydrogenation of Nitriles and the Reductive Amination of Carbonyl Compounds for the Synthesis of Primary Amines. ChemSusChem 2019, 12, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Formenti, D.; Mocci, R.; Atia, H.; Dastgir, S.; Anwar, M.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A State-of-the-Art Heterogeneous Catalyst for Efficient and General Nitrile Hydrogenation. Chem. Eur. J. 2020, 26, 15589–15595. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, Y.-N.; Qian, Y.; Tang, W.; Zhang, R.; Wen, J.; Zhang, X. Cobalt-Catalyzed Hydrogenative Transformation of Nitriles. ACS Catal. 2021, 11, 13761–13767. [Google Scholar] [CrossRef]




 | |||
|---|---|---|---|
| Entry | Catalyst | % Conversion | % Yield |
| 1 | POSS–COF (Co) | >99 | 99 |
| 2 | POSS | <1 | <1 |
| 3 | POSS–COF | <1 | <1 |
| 4 | Co-2,2′ Bipyridine | <2 | <1 |
| Entry | Solvent | % Conversion |
|---|---|---|
| 1 | Isopropyl Alcohol | >99 |
| 2 | Ethanol | 76 |
| 3 | Methanol | 72 |
| 4 | n-Butanol | 54 |
| 5 | Toluene | 40 |
| 6 | Tetrahydrofuran | 10 |
| Entry | Substrate | Products | Conv. (%) | Sel. (%) | S.D. * |
|---|---|---|---|---|---|
| 2a. |  |  | 95 | 82 | 3 |
| 2b. |  | 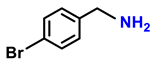 | 90 | 85 | 3 |
| 2c. |  |  | 90 | 88 | 4 |
| 2d. |  | 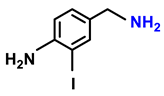 | 95 | 92 | 4 |
| 2e. | 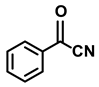 |  | 98 | 95 | 3 |
| 2f. |  | 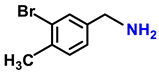 | 85 | 80 | 5 |
| 2g. |  | 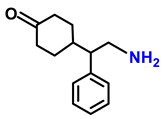 | 65 | 82 | 2 |
| 2h. | 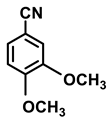 | 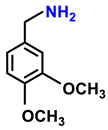 | 99 | 90 | 2 |
| 2i. | 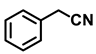 | 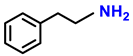 | 98 | 94 | 1.5 |
| 2j. |  |  | 65 | 83 | 4 |
| 2k. | 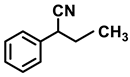 | 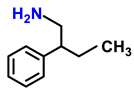 | 92 | 88 | 2 |
| 2l. | 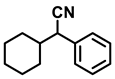 | 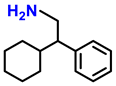 | 95 | 93 | 2 |
| 2m. | 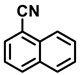 |  | 95 | 92 | 1 |
| 2n. | 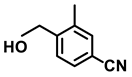 |  | 95 | 92 | 2 |
| 2o. |  |  | 95 | 92 | 2 |
| Catalyst | Nitrile | Reaction Conditions | % Yield | Ref |
|---|---|---|---|---|
| Ni/Al2O3-600 | Benzonitrile (1 mmol) | H2, NH3·H2O, EtOH, 60 °C, 6 h | 95 | [61] |
| MC/Ni | Benzonitrile (1 mmol) | H2 (2.5 bar), MeOH, 60 °C, 4 h | 99 | [62] |
| (MesCCC)-CoCl2py (py = pyridine) | Benzonitrile | NaHBEt3, KOtBu, toluene, 115 °C, 8 h, H2 | >99 | [43] |
| Co-NC-MgO-700 | PhCN (0.5 mmol) | H2 (20 bar), NH3, iPrOH, 24 h | 98 | [63] |
| PdPt–Fe3O4 | PhCN (0.5 mmol) | NaBH4 (1 mmol), MeOH, 25 °C, 8 h | 98 | [47] |
| POSS–COF (Co) | Benzonitrile | H2 (4.5 MPa), IPA, 130 °C, 0.5 MPa NH3, 20 h | >99 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubab, A.; Sohail, M.; Alshammari, R.H.; Nafady, A.; Wahab, M.A.; Abdala, A. Catalytic Application of POSS–COF-[(Co(acetate)2] for Selective Reduction of Nitriles to Amines. Catalysts 2024, 14, 557. https://doi.org/10.3390/catal14090557
Rubab A, Sohail M, Alshammari RH, Nafady A, Wahab MA, Abdala A. Catalytic Application of POSS–COF-[(Co(acetate)2] for Selective Reduction of Nitriles to Amines. Catalysts. 2024; 14(9):557. https://doi.org/10.3390/catal14090557
Chicago/Turabian StyleRubab, Anosha, Manzar Sohail, Riyadh H. Alshammari, Ayman Nafady, Md. A. Wahab, and Ahmed Abdala. 2024. "Catalytic Application of POSS–COF-[(Co(acetate)2] for Selective Reduction of Nitriles to Amines" Catalysts 14, no. 9: 557. https://doi.org/10.3390/catal14090557








