Abstract
The complete anodic oxidation of ammonia is an important part of direct ammonia fuel cells. Fabricating a high-performance electrocatalyst for ammonia oxidation reaction is meaningful for developing a direct ammonia fuel cell. Herein, we designed one platinum-decorated NiCo-layered double hydroxide nanoflower on Ni foam (Pt-NiCo-LDH-Ni foam) and measured the electrocatalytic performance via the cyclic voltammetry (CV) technique. The experimental results demonstrated that the optimized Pt-NiCo-LDH-Ni foam showed great electrocatalytic performance, with a low overpotential with a value of −0.573 V, a high current density of 17.75 mA cm−2 for the ammonia oxidation reaction, and good stability.
1. Introduction
Owing to increasing energy demands and consequent environmental pollution, fossil fuels do not meet the requirements of energy and environmental protection [1]. Therefore, recently, developing clean energy sources has been given more attention [2]. Ammonia exhibits two obvious advantages [3]. First, ammonia can be used as green fuel for direct ammonia fuel cells. As the anodic reaction, the thermodynamically required potential of the electrochemical ammonia oxidation reaction is 0.06 V, which is lower than that of the overpotential of water splitting (1.23 V). In addition, the final production of electrochemical ammonia oxidation reactions is N2 and H2O, achieving zero carbon emissions. Because of the above advantages, ammonia has received more attention for direct ammonia fuel cells.
However, the ammonia oxidation reaction is a multistep process, and the intermediates have also shown poisoning toward the electrocatalysts of the ammonia oxidation reaction process. Therefore, designing high-performance electrocatalysts to meet the requirements is important. Today, the electrocatalysts for ammonia oxidation reactions have been reported and researched including noble metals, metal oxides, layered double hydroxides, and so forth. Noble metals with high electrocatalytic performances, including Pt [4], Ir [5], and noble metal-based composites [6,7,8], have been reported. For example, Su et al. successfully designed carbon paper-supported Pt with various morphologies via the electrodepositing process under different electrodeposition potentials. The measured results proved that the cauliflower-like Pt catalyst exhibited better catalytic activity and durability 4.7 times than that of commercial Pt black [9]. Kim and their group reported on PtZn alloy nanoparticles that were prepared via the one-pot polyol process. The electrocatalytic performances of PtZn alloy nanoparticles were discussed via four different ammonia solutions, and synthesized PtZn electrocatalysts exhibited enhanced electrocatalytic performances in terms of the current density by 2.5–3.3, 2.3–2.7, 2.2–2.8, and 3.9–4.8 fold for NH4OH, NH4Cl, NH4NO3, and (NH4)2SO4, respectively, compared with Pt/C [8]. In addition, layered double hydroxides have also been used as electrocatalysts for ammonia oxidation reactions owing to the great advantages of their unique three-dimensional structure, good conductivity, high surface area, and low cost [10,11,12]. Using a facile hydrothermal coupled with electroreduction method, Xu et al. successfully constructed amorphous NiFe-layered double hydroxide nanosheets modified with boron nanoclusters (B-NiFe-LDH/NF), and this B-NiFe-LDH/NF showed the great electrocatalytic performance for the ammonia oxidation reaction [13]. Nickel–copper-layered double hydroxide has also been used as a catalyst for the electrochemical oxidation reaction of ammonia. Mao et al. prepared one NiCu-layered double hydroxide (NiCu-LDH) on nickel foam for ammonia oxidation via a facile hydrothermal reaction. Compared with Ni(OH)2/NF, the obtained Ni0.9Cu0.1-LDH/NF exhibited smaller and denser nanosheets, providing more electrochemical active areas and active sites. The electrochemical measurements indicated that Ni0.9Cu0.1-LDH/NF exhibited great electrocatalytic activities [14]. However, it was difficult to find related reports for ammonia oxidation reactions based on Pt and layered double hydroxide composites that acted as electrocatalysts.
In this work, to enhance the kinetic process of ammonia oxidation reaction, we first grew NiCo-layered double hydroxide nanoflowers on Ni foam and then electrodeposited platinum to decorate NiCo-layered double hydroxide nanoflowers (Pt-NiCo-LDH-Ni foam). The optimized Pt-NiCo-LDH-Ni foam was characterized and measured by transmission electron microscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, and an electrochemical station. In addition, the catalytic process for ammonia oxidation reaction is discussed.
2. Results and Discussion
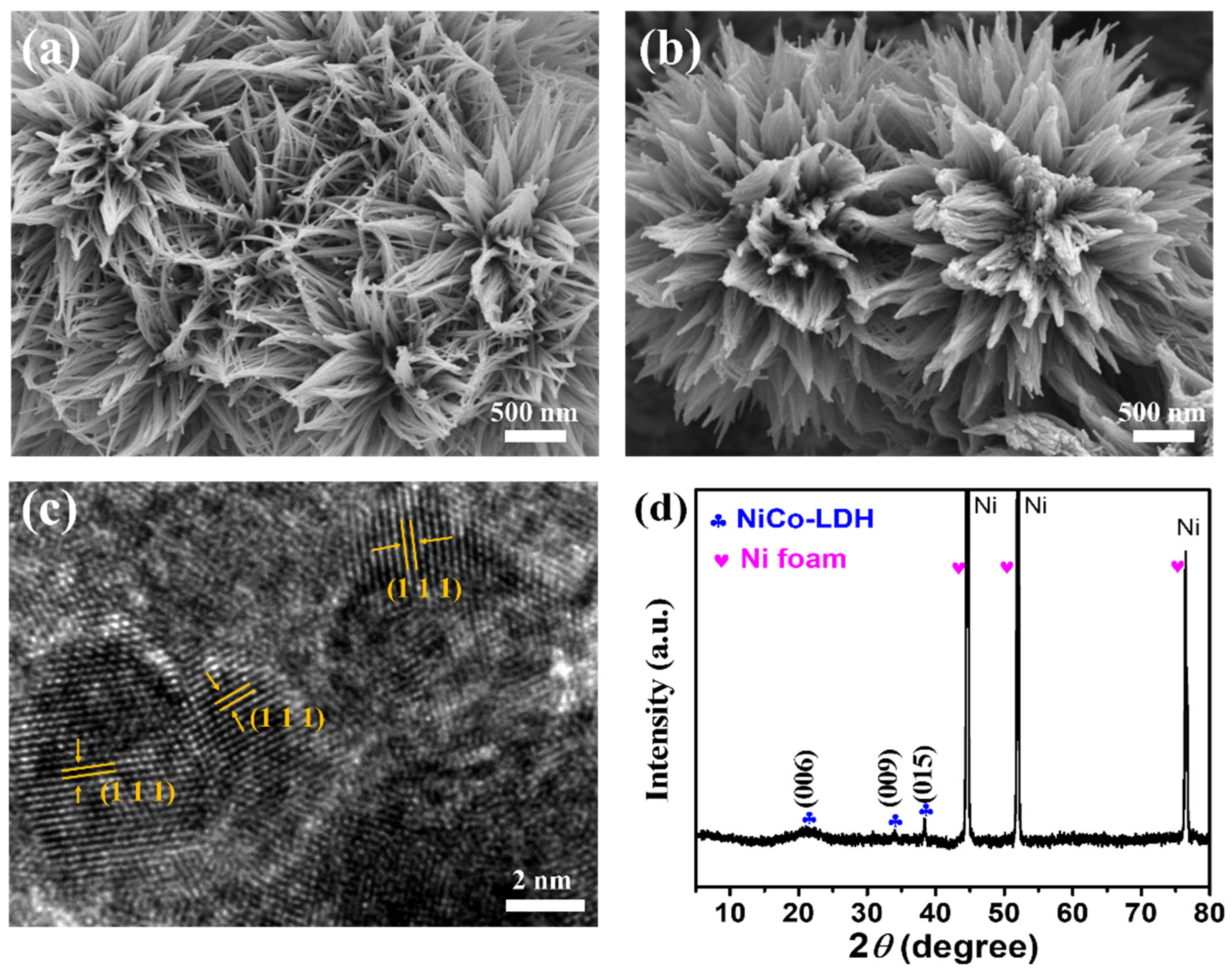
To prove the synthesis of NiCo-LDH-Ni foam nanoflowers and Pt-NiCo-LDH-Ni foam, SEM, TEM, XRD, and XPS were used to characterize the morphology and composition. Figure 1a shows the SEM image of the NiCo-LDH-Ni foam. Many nanowires could be found, and these nanowires were assembled into nanoflowers. After depositing Pt, these nanowires gathered further, as shown in Figure 1b. The HRTEM of Pt-NiCo-LDH-Ni foam is shown in Figure 1c; the obvious lattice stripe with a distance of 0.227 nm was found to belong to the crystal plane (111) of Pt [15], indicating that Pt nanoparticles were loaded on the NiCo-LDH nanoflowers. Figure 1d shows the XRD pattern of the Pt-NiCo-LDH-Ni foam; three strong diffraction peaks belonging to the Ni foam could be found, and three diffraction peaks, which were located at 20.89, 34.11, and 38.32, could be ascribed to the crystal plane (006), (009), and (015) of NiCo-LDH, indicating the formation of NiCo-LDH. However, no obvious diffraction peaks that could be attributed to Pt nanoparticles could be found. A possible reason is that the diffraction peaks were covered because of the strong diffraction peaks of the Ni foam.
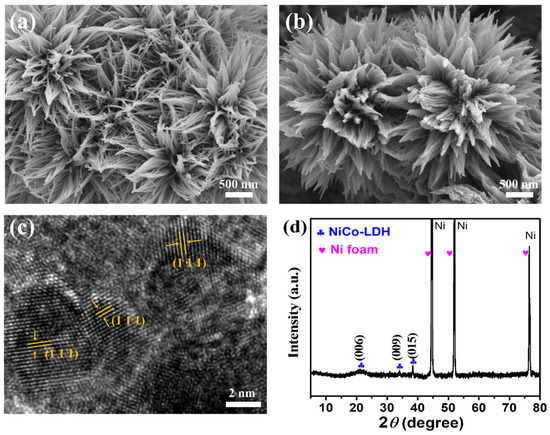
Figure 1.
The SEM image of (a) NiCo-LDH-Ni foam and (b) Pt-NiCo-LDH-Ni foam-3; (c) the HRTEM image of Pt-NiCo-LDH-Ni foam-3; (d) the XRD spectrum of Pt-NiCo-LDH-Ni foam-3.
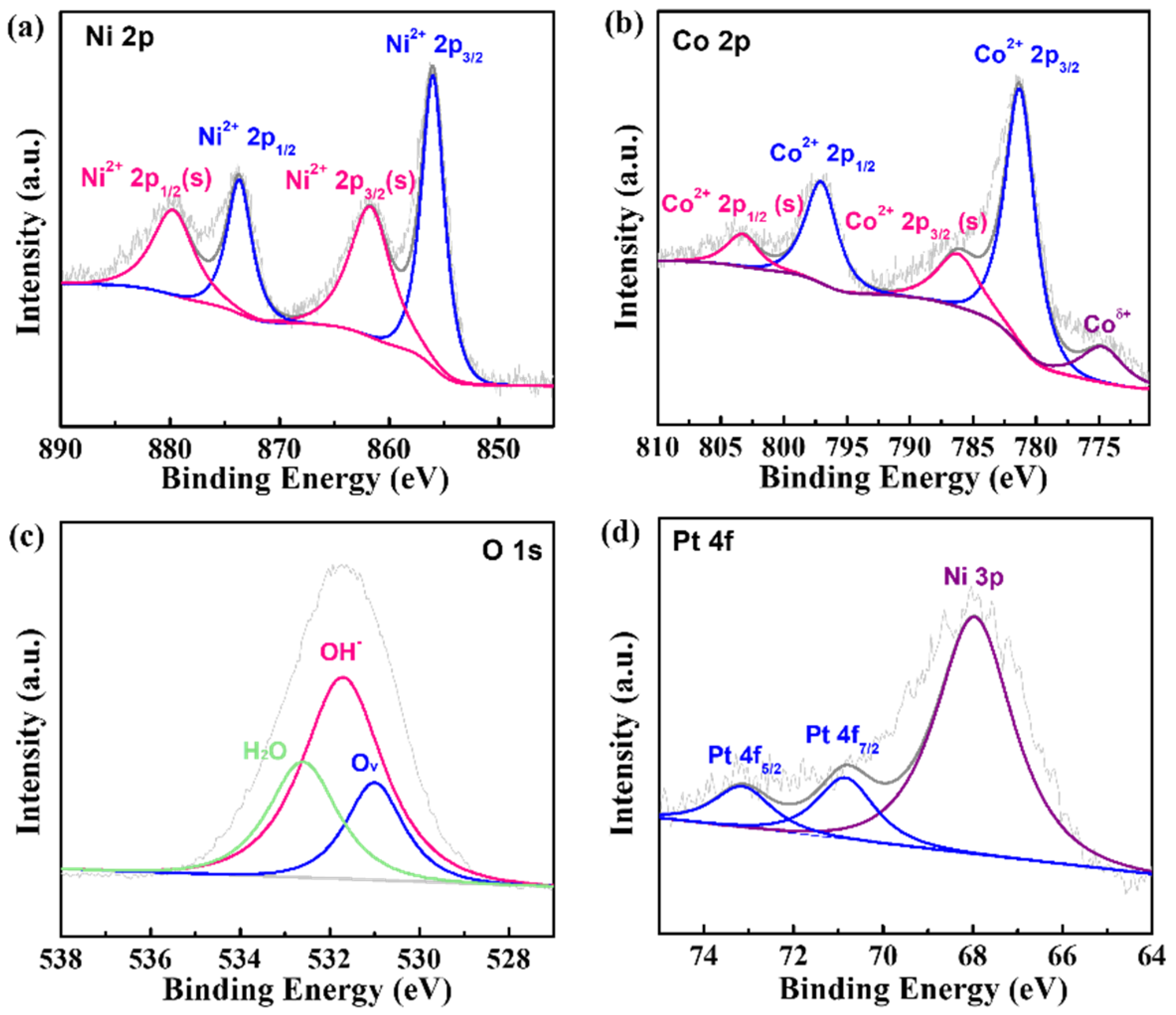
To further analyze the composition of the Pt-NiCo-LDH-Ni foam, XPS measurement was conducted, and the XPS results are shown in Figure 2. As shown in Figure 2a, four fitted peaks could be found. Two peaks at binding energies of 855.99 eV and 873.67 eV belonged to Ni2+ 2p3/2 and Ni2+ 2p1/2. In addition, two peaks at 861.70 eV and 879.71 eV were the satellite peaks of Ni 2p3/2 and Ni 2p1/2 [16]. The XPS spectrum of the Co element is given in Figure 2b. The fitted peaks, which were located at 781.28 eV and 797.04 eV, could be attributed to Co2+ 2p3/2 and Co2+ 2p1/2, and the two satellite peaks could be detected at binding energies of 786.18 eV and 803.29 eV. In addition, one fitted peak at 774.74 eV could be ascribed to Coδ+(δ = 0~2) [16]. As shown in Figure 2c, the XPS spectrum of the O element could be fitted to one peak, which was located at 531.71 eV and could be attributed to OH- [16]. The fitted results of the XPS spectra of the Ni, Co, and O elements further proved the formation of NiCo-LDH. Finally, the XPS spectrum of the Pt element is shown in Figure 2d. Two peaks at 70.85 eV and 73.14 eV could be attributed to Pt 4f7/2 and Pt 4f5/2 [17], indicating that Pt was zero-valent. In addition, the fitted peak at 67.96 eV could be ascribed to Ni 3p. The above results indicate that Pt nanoparticles were deposited on NiCo-LDH.
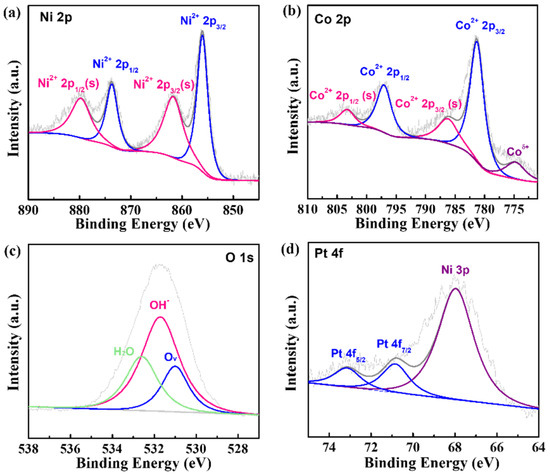
Figure 2.
The XPS spectra of (a) N 2p, (b) Co 2p, (c) O 1s, and (d) Pt 4f in Pt-NiCo-LDH-Ni foam-3.
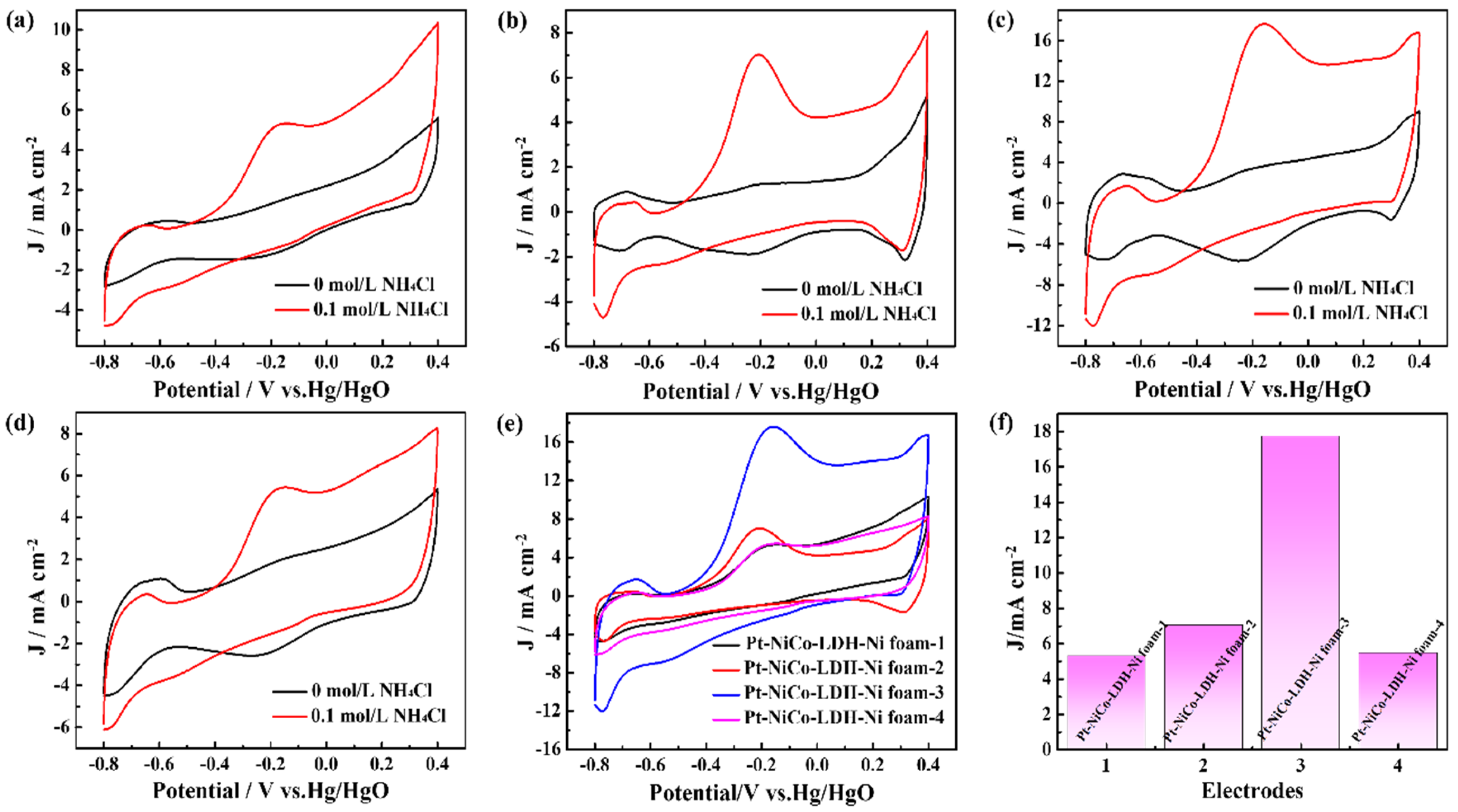
To obtain the best electrocatalyst for ammonia oxidation reaction, the deposition cycles of the Pt nanoparticles were controlled. Figure 3 shows the CV curves of the Pt-NiCo-LDH-Ni foam-1, Pt-NiCo-LDH-Ni foam-2, Pt-NiCo-LDH-Ni foam-3, and Pt-NiCo-LDH-Ni foam-4 electrodes in 1 M KOH with the presence and absence of 0.1 M NH4Cl. As shown in Figure 3a, the CV curves of the Pt-NiCo-LDH-Ni foam-1 electrode in 1 M KOH with the presence and absence of 0.1 M NH4Cl exhibited obvious changes. A clear oxidation peak could be found at a potential of about −0.2 V in 1 M KOH with the presence of 0.1 M NH4Cl, indicating that the Pt-NiCo-LDH-Ni foam-1 electrode showed electrocatalytic activity for the ammonia oxidation reaction. Figure 3b–d show the CV curves of the Pt-NiCo-LDH-Ni foam-2, Pt-NiCo-LDH-Ni foam-3, and Pt-NiCo-LDH-Ni foam-4 electrodes in 1 M KOH with the presence and absence of 0.1 M NH4Cl, and similar oxidation peaks at about −0.2 V could be found. Differently, with the increasing deposition cycles from 5 to 15 cycles, on one hand, the onset potentials gradually shifted in a negative direction, and the onset potential of the Pt-NiCo-LDH-Ni foam-3 electrode achieved −0.573 V. On the other hand, the oxidation peak current densities increased from 5.51 to 17.75 mA cm−2 (Figure 3e,f). However, when the deposition cycles achieved 20 cycles, the Pt-NiCo-LDH-Ni foam-4 electrode was etched during the deposition process, resulting in a larger onset potential and smaller oxidation peak current density than that of the Pt-NiCo-LDH-Ni foam-3 electrode. The above results proved that when the deposition cycle was 15, the Pt-NiCo-LDH-Ni foam-3 electrode showed the best electrocatalytic performance.
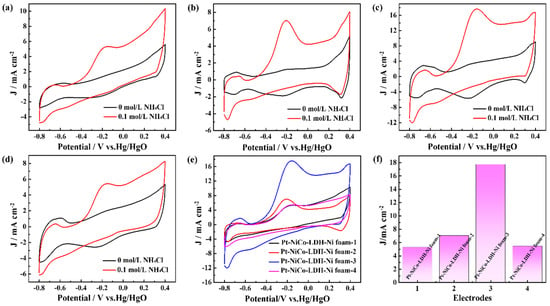
Figure 3.
The CV curves of (a) Pt-NiCo-LDH-Ni foam−1, (b) Pt-NiCo-LDH-Ni foam−2, (c) Pt-NiCo-LDH-Ni foam−3, and (d) Pt-NiCo-LDH-Ni foam−4 electrodes in the presence and absence of 0.1 M NH4Cl; (e) the curves of Pt-NiCo-LDH-Ni foam−1, Pt-NiCo-LDH-Ni foam−2, Pt-NiCo-LDH-Ni foam−3, and Pt-NiCo-LDH-Ni foam−4 electrodes in the presence of 0.1 M NH4Cl; and (f) the oxidation peak current densities of Pt-NiCo-LDH-Ni foam−1, Pt-NiCo-LDH-Ni foam−2, Pt-NiCo-LDH-Ni foam−3, and (d) Pt-NiCo-LDH-Ni foam−4 electrodes.
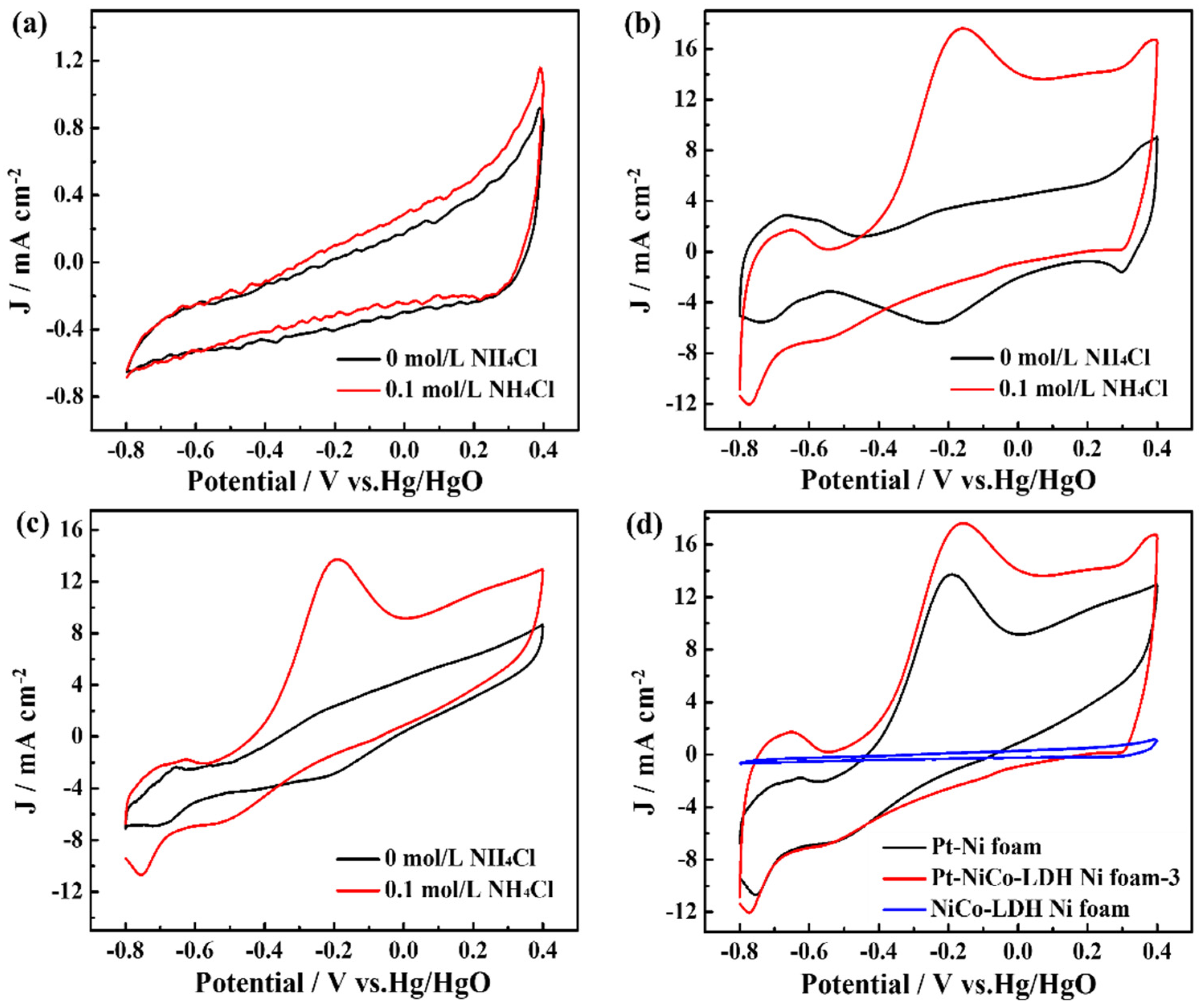
In comparison, the electrochemical activity of the NiCo-LDH-Ni foam and the Pt-Ni foam for the catalytic ammonia oxidation reaction was also measured, as shown in Figure 4. As shown in Figure 4a, the CV curves of the NiCo-LDH-Ni foam have clear current changes after being added into 0.1 M NH4Cl; this obvious current change could be attributed to the electrocatalytic ability of NiCo-LDH according to Equations (1)–(4). Differently, the Pt-Ni foam electrode exhibited an obvious oxidation peak, and the oxidation peak current achieved 13.85 mA cm−2 after adding 0.1 M NH4Cl (Figure 4b) according to Equations (5)–(9). The above results indicate that Pt was the main factor in enhancing the electrocatalytic performances and that NiCo-LDH nanoflowers provided a large surface for the deposition of Pt nanoparticles, resulting in Pt-NiCo-LDH-Ni foam-3 electrodes with better electrocatalytic performances than those of Pt-Ni foam.
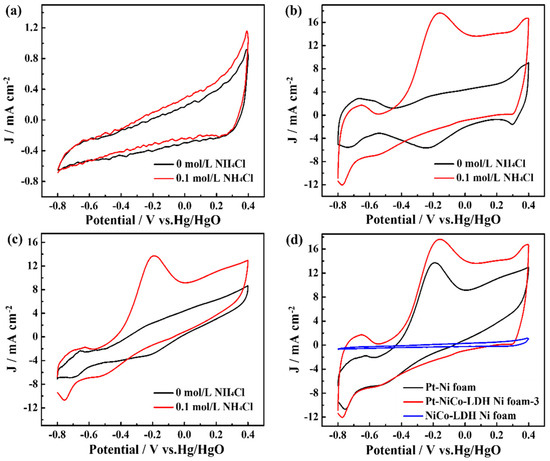
Figure 4.
The CV curves of (a) NiCo-LDH-Ni foam, (b) Pt-NiCo-LDH-Ni foam−3, and (c) Pt-Ni foam electrodes in the presence and absence of 0.1 M NH4Cl and (d) the curves of Pt-Ni foam, NiCo-LDH-Ni foam, and Pt-NiCo-LDH-Ni foam−3 electrodes in the presence of 0.1 M NH4Cl.
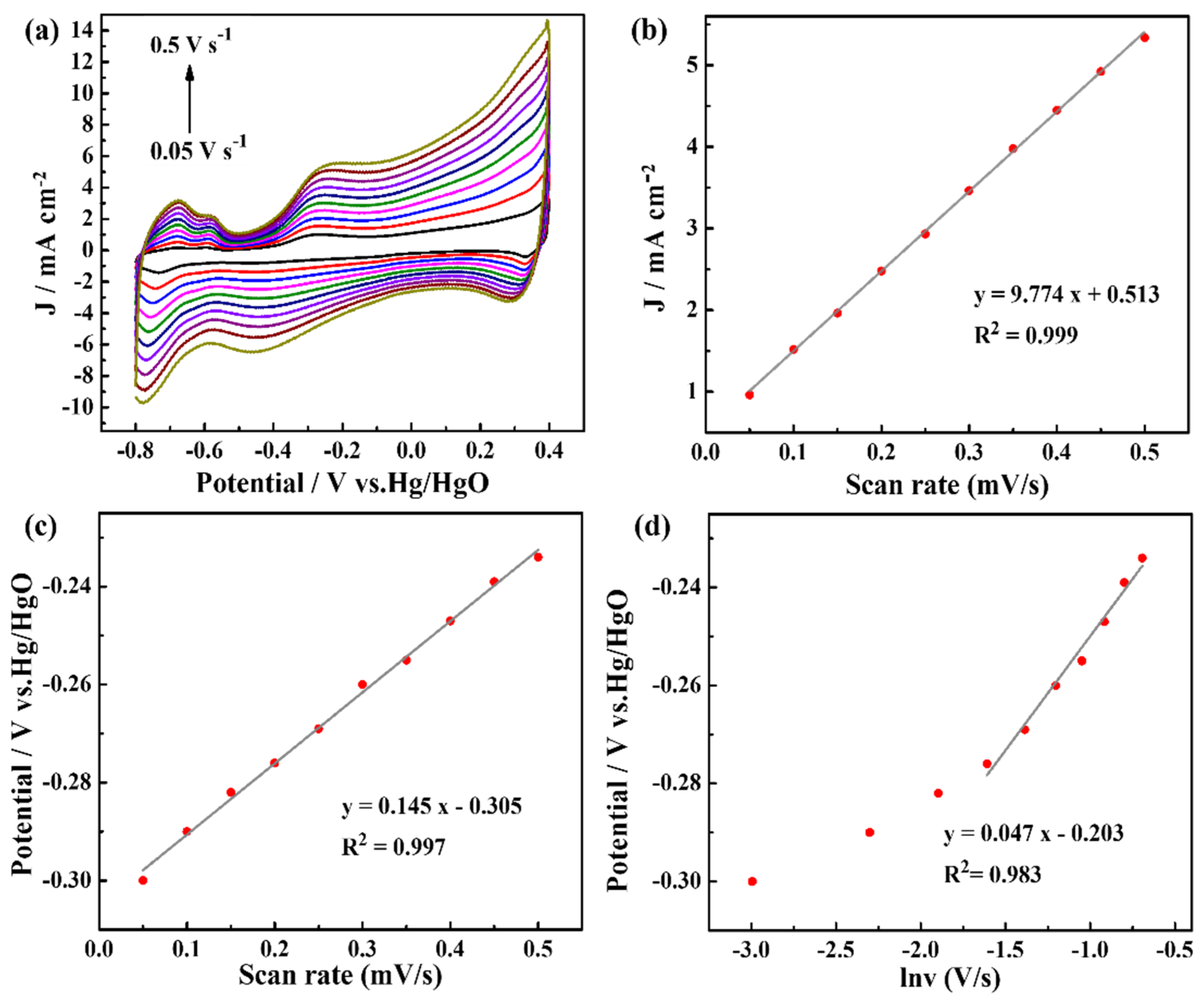
To analyze the kinetics of ammonia oxidation reaction, the scan rates were controlled, and the CV curves are shown in Figure 5a; with increased scan rates, the oxidation peak current densities increased. In addition, the oxidation peak potentials showed slight shifts to positive potentials. Figure 5b shows a fitted curve of the current densities and scan rates. The fitted curve shows a good linear relationship with the linear regression values (R2) of 0.999, indicating that the electrochemical oxidation of ammonia on the Pt-NiCo-LDH-Ni foam-3 electrode is dominated by a surface-controlled process. In addition, when the scan rate increased, the oxidation peak potential shifted slightly in the positive direction. There is also a good linear relationship between the oxidation peak potentials and scanning rates (R2 = 0.997) (Figure 5c), indicating an irreversible process of the ammonia oxidation reaction on the Pt-NiCo-LDH-Ni foam-3 electrode. The oxidation peak potential (Epa) and the logarithm of the scan rate (ln ν) (ν ≥ 0.2 V s−1) exhibited a good linear relationship (Figure 5d). The equation can be fitted by Laviron’s theoretical model, as shown in Equation (10), and the number of electrons () involved in the rate-determining step of ammonia oxidation reaction on the Pt-NiCo-LDH-Ni foam-3 electrode can be calculated [18].
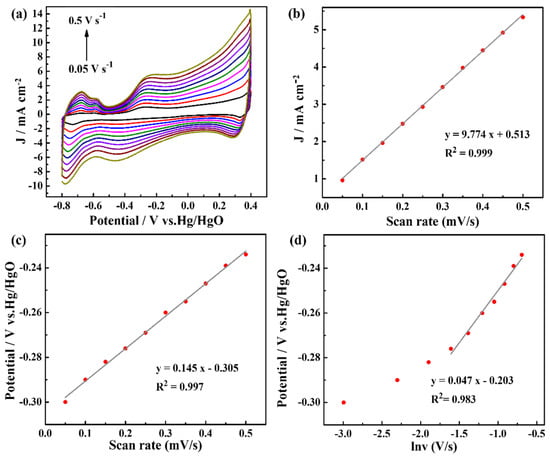
Figure 5.
(a) CV curves of the Pt-NiCo-LDH-Ni foam−3 electrode in 1 M KOH with 1 mM NH4Cl at different scan rates from 50 mV s−1 to 500 mV s−1; (b) oxidation peak current densities vs. the scan rates; (c) oxidation peak potentials vs. the scan rate; (d) the effect of the scan rate on peak potentials.
The parameters in Equation (1) are given in the supporting information. The value was determined by the slope of Epa versus ln ν as 0.552. Meanwhile, the value of can be computed by Equation (11):
In Equation (11), is the potential of a half peak. was calculated to be 0.530, and then the number of transfer electrons in the rate-determining step of the ammonia oxidation reaction was determined to be 1.042 1.
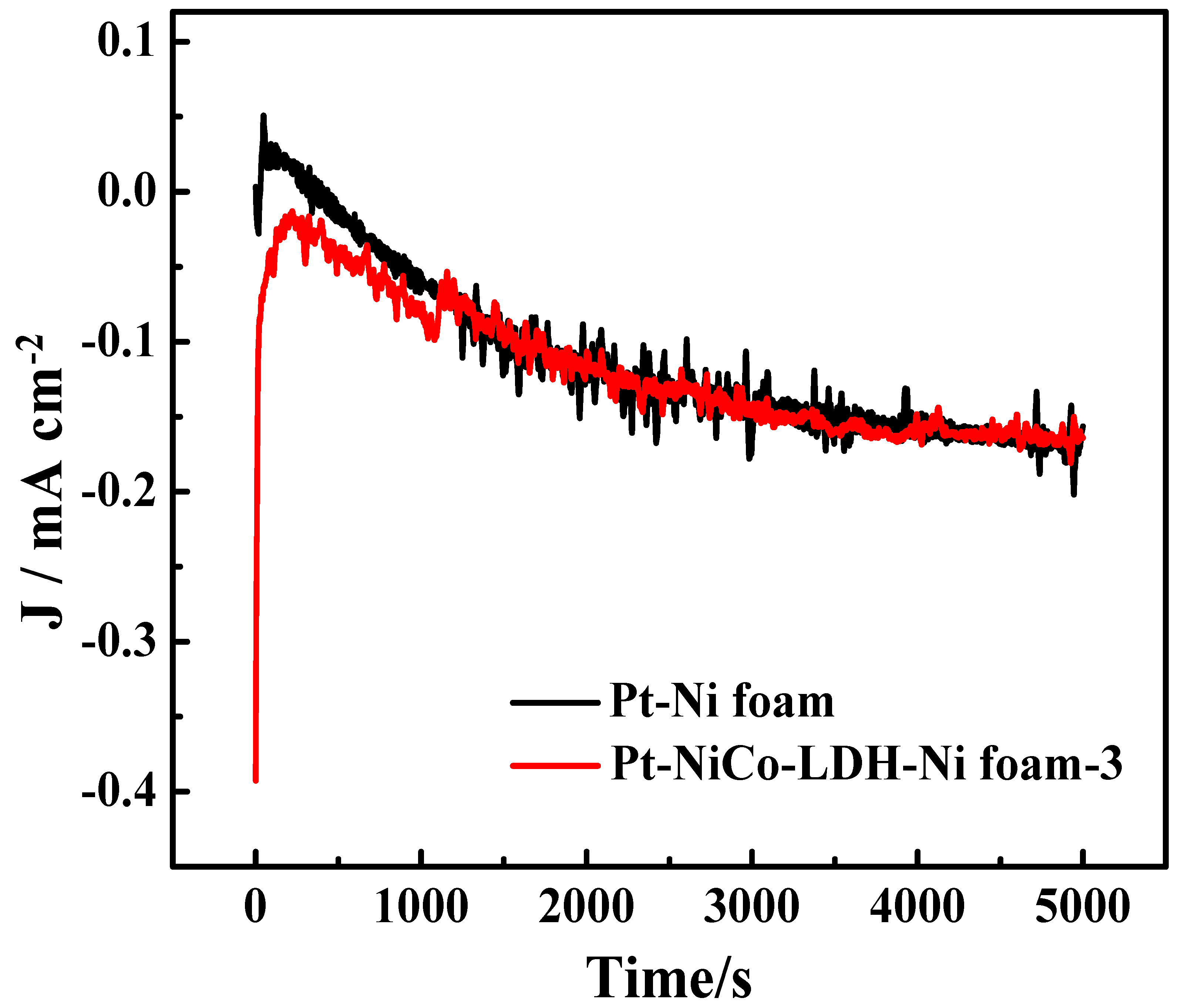
Figure 6 shows the stability measurements of the Pt-NiCo-LDH-Ni foam-3 electrode and the Pt-Ni foam electrode for 5000 s. The Pt-NiCo-LDH-Ni foam-3 electrode showed better stability than the Pt-Ni foam electrode. In addition, Table 1 shows the ammonia oxidation reaction test condition and the activity of reported catalysts, indicating that the introduction of NiCo-LDH nanoflowers enhanced the dispersibility of Pt and further enhanced the electrochemical performance of the Pt-NiCo-LDH-Ni foam-3 electrode.
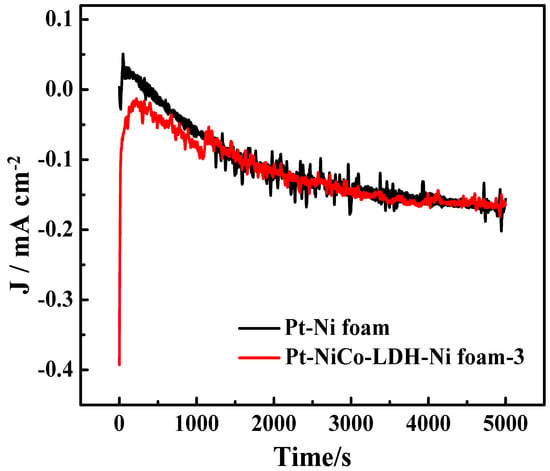
Figure 6.
The stability measurements of Pt-Ni foam and Pt-NiCo-LDH-Ni foam−3 electrodes in the presence of 0.1 M NH4Cl for 5000 s.

Table 1.
The ammonia oxidation reaction test conditions and the activity of reported catalysts.
3. Experimental
The Synthesis of Pt-NiCo-LDH-Ni Foam
Ni foam with a size of 1 cm × 2.5 cm was washed with acetone, HCl (1 M), and deionized water and dried before use. Next, 2.4 mM NiCl2·6H2O and 0.8 mM CoCl2·6H2O were used to prepare the mixed solution with the volume of 50 mL deionized water. A total of 0.3 g urea was added to the above solution and stirred for 1 h. The obtained suspension and the Ni foam slice were moved into Teflon-lined stainless autoclaves to be further heated at 120 °C for 12 h. NiCo-LDH could grow on the surface of Ni foam during the above process. For the synthesis of Pt-NiCo-LDH, a three-electrode system was used with the NiCo-LDH-Ni foam as the working electrode, a Pt sheet as the counter electrode, and the Saturated Calomel Electrode (SCE) as the reference electrode in a mixed solution (30 mL) including 0.25 M H2SO4 and 1.25 mM H2PtCl6·6H2O. The potential was from −0.3 V to 0.4 V, and the scan rate was 0.25 mV s−1. The obtained samples were named Pt-NiCo-LDH-Ni foam-1, Pt-NiCo-LDH-Ni foam-2, Pt-NiCo-LDH-Ni foam-3, and Pt-NiCo-LDH-Ni foam-4 with 5, 10, 15, and 20 deposition cycles. In addition, when there were 15 deposition cycles, the Ni foam acted as the working electrode, and the obtained sample was named as Pt-Ni foam.
4. Conclusions
In this work, we successfully fabricated a platinum-decorated NiCo-layered double hydroxide on Ni foam via a hydrothermal reaction and an electrodeposition technique. Under optimal preparation conditions, Pt-NiCo-LDH-Ni foam showed the best electrocatalytic performance, with a low overpotential and a high oxidation peak current density. The great catalytic performances could be attributed to (1) the good electrocatalytic activity of the Pt- and NiCo-layered double hydroxide for the ammonia oxidation reaction, (2) the NiCo-layered double hydroxide nanoflowers providing large surface for depositing Pt, and (3) a good synergistic effect between the Pt- and NiCo-layered double hydroxide nanoflowers.
Author Contributions
Methodology, X.W., C.Z. and L.Z.; investigation, X.W. and C.Z.; resources, L.Z. and J.G.; data curation, Y.G., Y.H. and H.C.; writing—original draft preparation, X.W., C.Z. and L.Z.; writing—review and editing, X.W., C.Z. and L.Z.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the PhD Start-up Fund of Bohai University (05013/0522bs010, 05015/0522xn070).
Data Availability Statement
Research data can be obtained via corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lyu, Z.H.; Fu, J.J.; Tang, T.; Zhang, J.N.; Hu, J.S. Design of ammonia oxidation electrocatalysts for efficient direct ammonia fuel cells. EnergyChem 2023, 5, 1000093. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.; Kim, H.; Jun, Y.J.; Kim, S.Y.; Ahn, S.H. Recent progress in Pt-based electrocatalysts for ammonia oxidation reaction. Appl. Mater. Today 2022, 29, 101640. [Google Scholar] [CrossRef]
- Tran, D.T.; Nguyen, T.H.; Jeong, H.; Tran, P.K.L.; Malhotra, D.; Jeong, K.U.; Kim, N.H.; Lee, J.H. Recent engineering advances in nanocatalysts for NH3-to-H2 conversion technologies. Nano Energy 2022, 94, 106929. [Google Scholar] [CrossRef]
- Park, D.C.; Hwang, S.; Kim, D.H. Designing ammonia oxidation catalysts via physical mixing of Pt/SiO2 and Cu/ZSM-5. Catal. Today 2024, 437, 114769. [Google Scholar] [CrossRef]
- Reddy, K.S.S.V.P.; Chung, J.S.; Kang, S.G. Electrochemical Ammonia Oxidation Reaction over Mn/CeO2 (M = Pt, Ir; n = 3, 4) Catalysts. J. Phys. Chem. C 2024, 128, 10317–10323. [Google Scholar] [CrossRef]
- Tang, H.; Gu, Y.F.; Luo, J.B.; Zhang, W.; Zhang, J.; Zhou, Y. Controlled synthesis of bead-like Pt as an efficient electrocatalyst for electrocatalytic ammonia oxidation. ChemistrySelect 2024, 9, e202400482. [Google Scholar] [CrossRef]
- Fang, H.H.; Liao, C.; Cai, Q.Y.; Zhong, F.L.; Lin, L.; Chen, C.Q.; Luo, Y.; Jiang, L.L. Tuning surficial atomic configuration of Pt-Ir catalysts for efficient ammonia oxidation and low-temperature direct ammonia fuel cells. Chem. Eng. Sci. 2023, 280, 118836. [Google Scholar] [CrossRef]
- Matin, M.A.; Kim, S.; Kim, Y.Y.; Han, C.; Byun, S.; Kim, H.C. PtZn alloy nanoparticles with enhanced activity and stability synthesized by a simplified polyol process for electro-oxidation of ammonia. J. Power Sources 2024, 591, 233885. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Wang, X.C.; Tan, M.H.; Liu, H.Y.; Ma, Q.; Xu, Q.; Pollet, B.G.; Su, H.N. Electrodeposited platinum with various morphologies on carbon paper as efficient and durable self-supporting electrode for methanol and ammonia oxidation reactions. Int. J. Hydrogen Energy 2023, 48, 2617–2627. [Google Scholar] [CrossRef]
- Liu, Y.X.; Bai, Y.; Yang, W.W.; Ma, J.H.; Sun, K.N. Self-supported electrode of NiCo-LDH/NiCo2S4/CC with enhanced performance for oxygen evolution reaction and hydrogen evolution reaction. Electrochim. Acta 2021, 367, 137534. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Du, X.Q.; Zhang, X.S.; Wang, Y.H. Ni3S2/MxSy–NiCo LDH (M = Cu, Fe, V, Ce, Bi) heterostructure nanosheet arrays on Ni foam as high-efficiency electrocatalyst for electrocatalytic overall water splitting and urea splitting. Dalton Trans. 2023, 52, 763. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Kumar, A.; Peela, N.R. Activated carbon supported Ni-Co layered double hydroxides nanowires: An effective and low-cost electrocatalyst for ethanol electro-oxidation in alkaline media. J. Electrochem. Soc. 2023, 170, 034509. [Google Scholar] [CrossRef]
- Wang, J.Y.; Qing, S.J.; Tong, X.L.; Zhang, K.; Luo, G.N.; Ding, J.; Xu, L.J. Boron nanoclusters endowed NiFe layered double hydroxides with efficient bifunction toward ammonia oxidation reaction and hydrogen evolution reaction. Appl. Surf. Sci. 2023, 640, 158330. [Google Scholar] [CrossRef]
- Wang, H.M.; Yuan, Y.Q.; Wang, G.Q.; Miao, Z. Preparation of nickel-copper layered double hydroxide on nickel foam as a non-noble catalyst for efficient electrooxidation of ammonia. J. Phys. Conf. Ser. 2022, 2390, 012072. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zheng, T.; Yu, C.L.; Zheng, J.B.; Tang, Z.B.; Zhang, N.W.; Shu, Q.; Chen, B.H. Platinum-nickel alloy nanoparticles supported on carbon for 3-pentanone hydrogenation. Applied Surface Science. 2017, 409, 29–34. [Google Scholar] [CrossRef]
- Wang, J.M.; Wei, X.F.; Wang, P.P.; Miao, J.; Zhang, R.C.; Zhang, N.; Zhou, X.Q.; Xu, H.; Zhang, J.; Li, H.S.; et al. Insights into the enhanced performance of NiCo-LDH modified Pd/NF cathode for electrocatalytic hydrodechlorination. Fuel 2023, 341, 127689. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.Y.; Liu, J.L.; Gu, J.L.; Suo, H.; Zhao, C.; Wang, X.Y. Achieving Ni@(Pt/Ni(OH)2) ternary nanoflowers derived from Ni nanoflowers for electrochemical ammonia-nitrogen detection in the aqueous environment. Microchem. J. 2024, 200, 110401. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, M.; Wang, X.Y.; Gu, J.L. Enhanced electrocatalytic oxidation activity of platinum-poly(methylene blue) as a sensitive sensor for ammonia-nitrogen detection. Microchem. J. 2024, 199, 110238. [Google Scholar] [CrossRef]
- Zhang, C.; Hwang, S.Y.; Peng, Z. Shape-enhanced ammonia electro-oxidation property of a cubic platinum nanocrystal catalyst prepared by surfactant-free synthesis. J. Mater. Chem. A 2013, 1, 14402–14408. [Google Scholar] [CrossRef]
- Jin, H.; Lee, S.; Sohn, Y.; Lee, S.H.; Kim, P.; Yoo, S.J. Capping agent-free synthesis of surface engineered Pt nanocube for direct ammonia fuel cell. Int. J. Energy Res. 2021, 45, 18281–18291. [Google Scholar] [CrossRef]
- Cunci, L.; Velez, C.A.; Perez, I.; Suleiman, A.; Larios, E.; Jose-Yacaman, M.; Watkins, J.J.; Cabrera, C.R. Platinum electrodeposition at unsupported electrochemically reduced nanographene oxide for enhanced ammonia oxidation. ACS Appl. Mater. Interfaces 2014, 6, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Achrai, B.; Zhao, Y.; Wang, T.; Tamir, G.; Abbasi, R.; Setzler, B.P.; Page, M.; Yan, Y.; Gottesfeld, S. A direct ammonia fuel cell with a KOH-free anode feed generating 180 mW cm−2 at 120 °C. J. Electrochem. Soc. 2020, 167, 134518. [Google Scholar] [CrossRef]
- Silva, J.C.M.; Ntais, S.; Teixeira-Neto, É.; Spinacé, E.V.; Cui, X.; Neto, A.O.; Baranova, E.A. Evaluation of carbon supported platinum-ruthenium nanoparticles for ammonia electro-oxidation: Combined fuel cell and electrochemical approach. Int. J. Hydrogen Energy 2017, 42, 193–201. [Google Scholar] [CrossRef]
- Endo, K.; Nakamura, K.; Katayama, Y.; Miura, T. Pt-Me (Me = Ir, Ru, Ni) binary alloys as an ammonia oxidation anode. Electrochim. Acta. 2004, 49, 2503–2509. [Google Scholar] [CrossRef]
- Xue, Q.; Zhao, Y.; Zhu, J.; Ding, Y.; Wang, T.; Sun, H.; Li, F.; Chen, P.; Jin, P.; Yin, S.; et al. PtRu nanocubes as bifunctional electrocatalysts for ammonia electrolysis. J. Mater. Chem. A 2021, 9, 8444–8451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).