Rational Engineering of Nanostructured NiS/GO/PVA for Efficient Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Results and Discussion
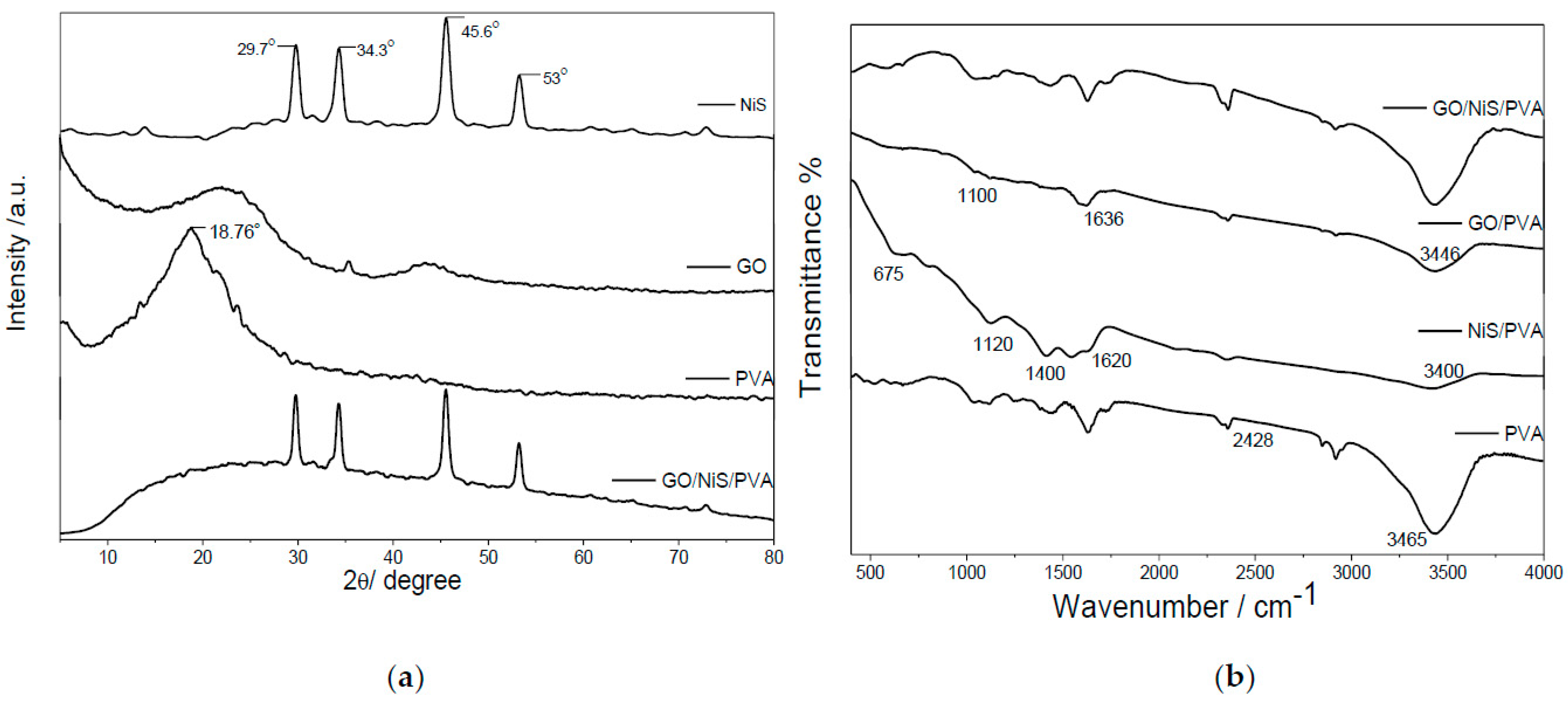
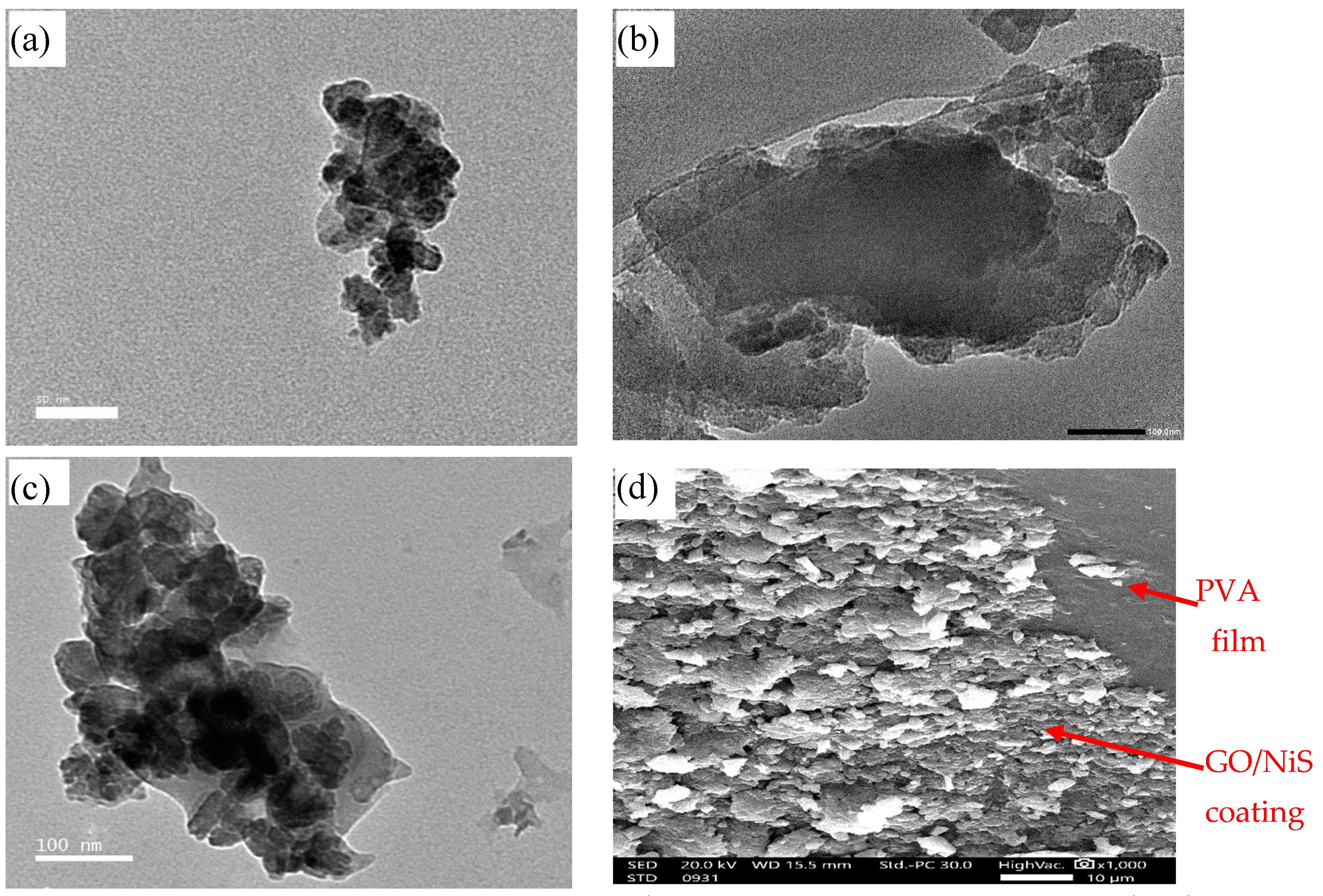
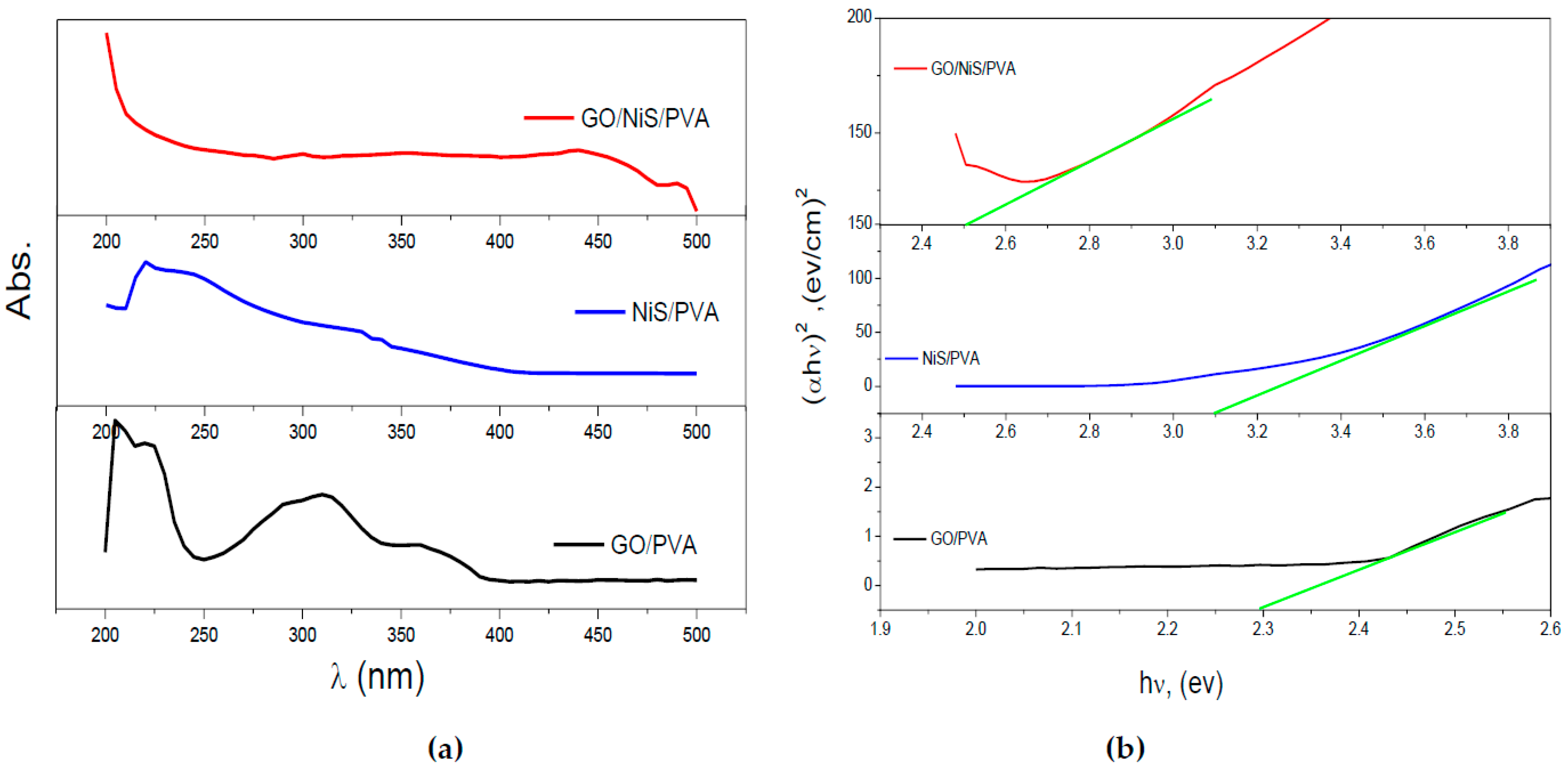
2.1. Characterization of Prepared GO, NiS, and Coated Films
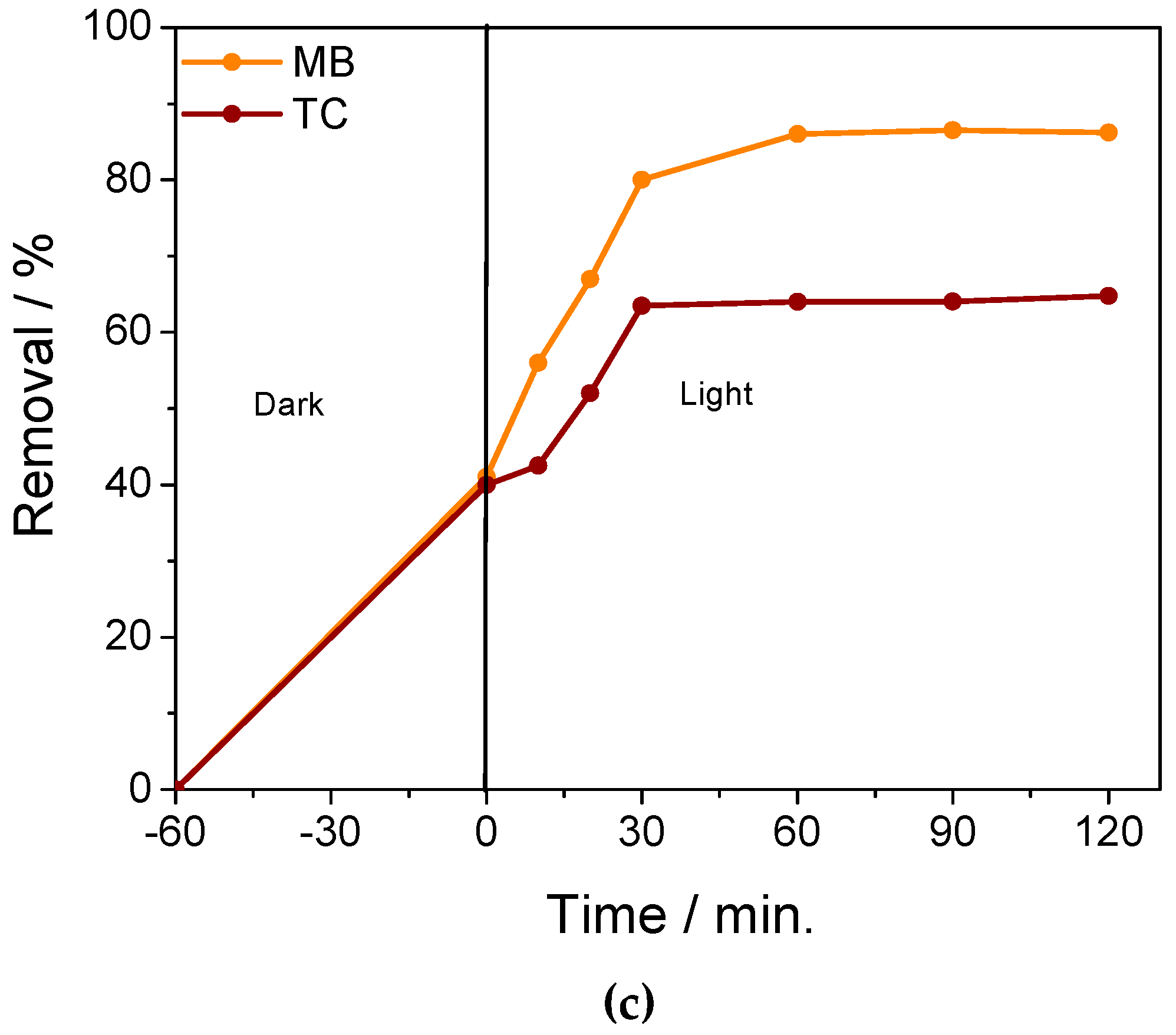
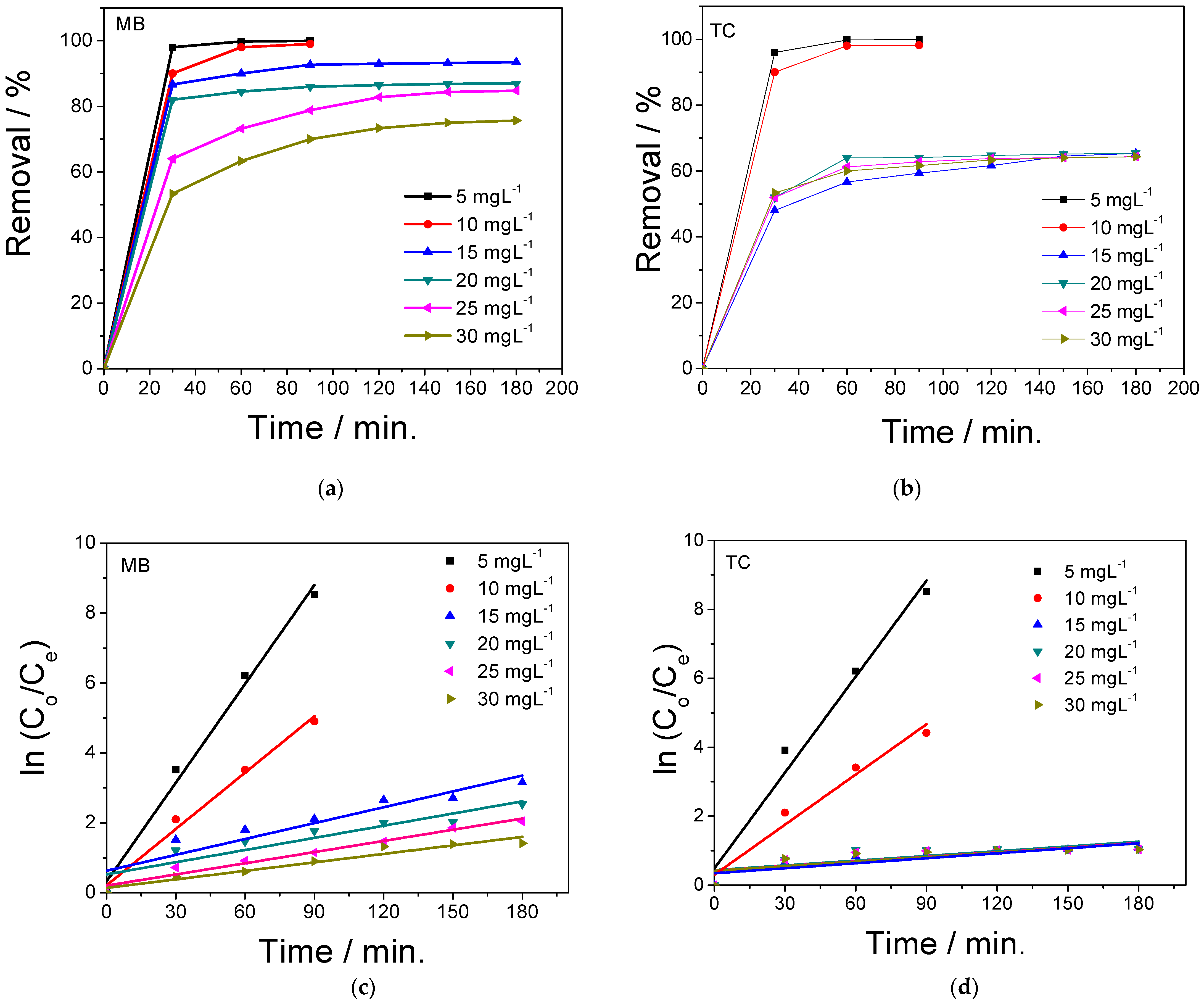
2.2. Adsorption and Photocatalytic Performance of GO/NiS/PVA-Coated Film
2.3. Photocatalytic Mechanism
2.4. Kinetics of the Photocatalytic Degradation
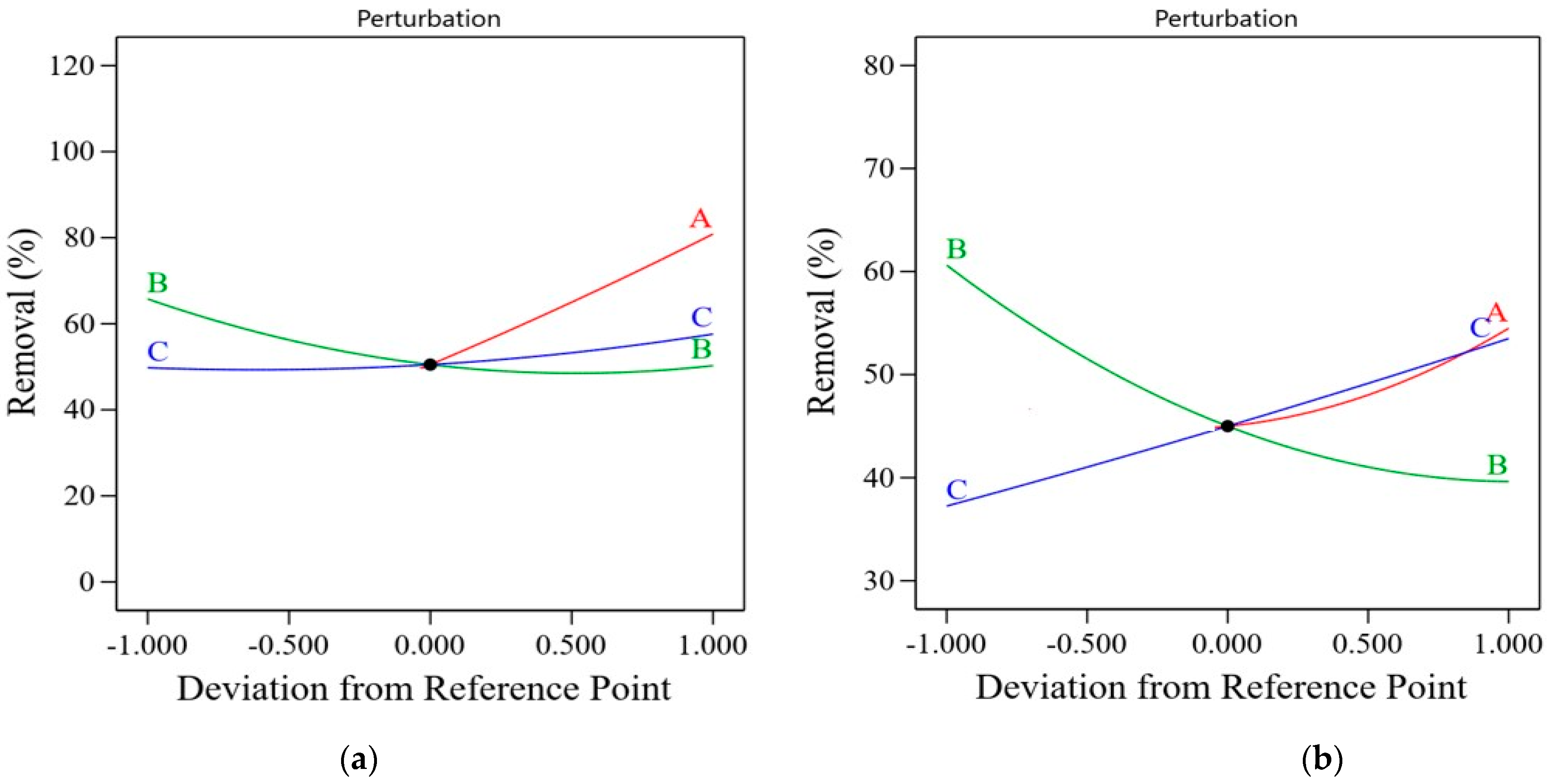
2.5. Optimization of the Photocatalytic Process Conditions Using the RSM Model
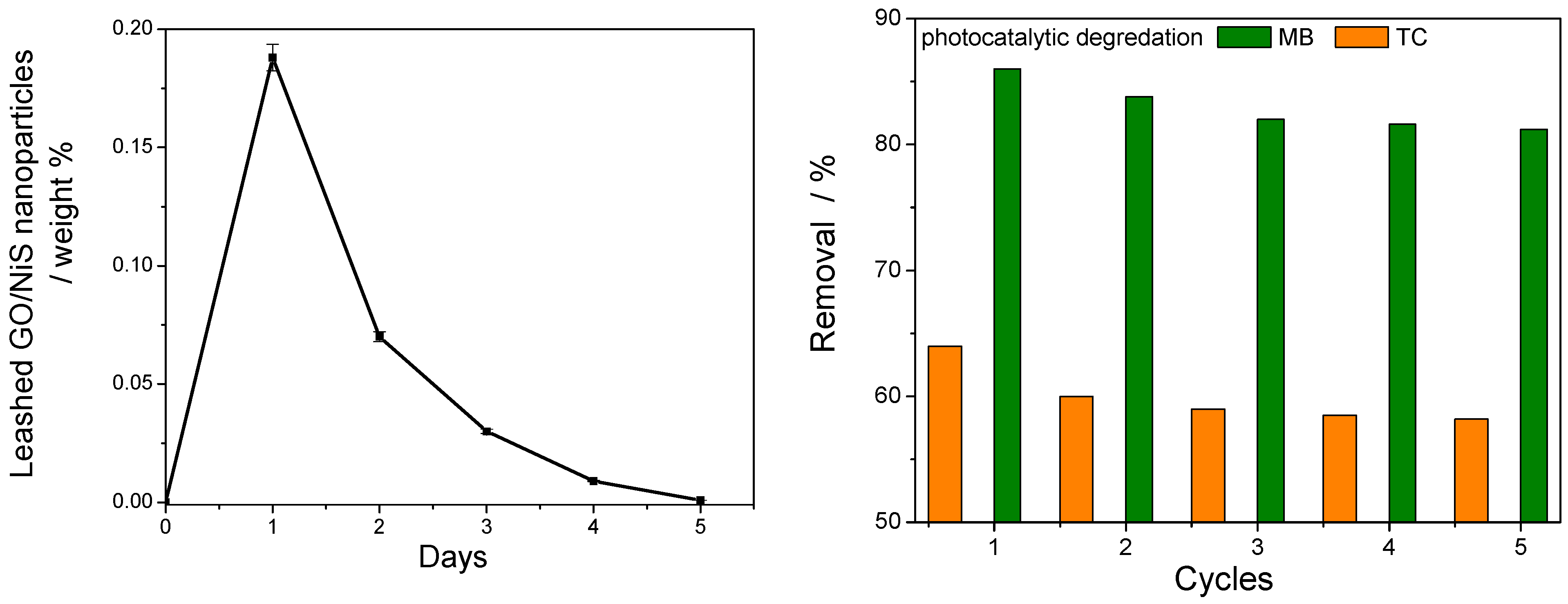
2.6. Stability and Reusability of GO/NiS/PVA-Coated Film
3. Materials and Methods
3.1. Preparation of Graphene Oxide (GO)
3.2. Preparation of Nickel Sulfide (NiS)
3.3. Preparation of GO/NiS/PVA-Coated Films
3.4. Characterization of Prepared Materials
3.5. Adsorption/Photocatalytic Tests
3.6. Optimization of the Degradation Process
3.7. Stability and Recycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Lee, J.; Siengchin, S. Ecofriendly and low-cost bio adsorbent for efficient removal of methylene blue from aqueous solution. Sci. Rep. 2022, 12, 20580. [Google Scholar] [CrossRef]
- Ahmed, T.; Noor, W.; Faruk, O.; Bhoumick, M.C.; Uddin, M.T. Removal of methylene blue (MB) from waste water by adsorption on jackfruit leaf powder (JLP) in continuously stirred tank reactor. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1086, 012012. [Google Scholar] [CrossRef]
- Weber, E.J.; Wolfe, N.L. Kinetics studies of reduction of aromatic azo compounds in anerobic sediment/water systems. Environ. Toxicol. Chem. 1987, 6, 911–919. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Tian, Y.; Liu, C.; Zhang, S.; Cao, L.; Zhou, Y.; Zhang, S. Effective removal of tetracycline antibiotics from water by magnetic functionalized biochar derived from rice waste. Environ. Pollut. 2023, 330, 121681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in food chain: The consequences for antibiotic resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G.; Pan, M.; Lyu, T.; Zhan, L.; et al. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Alhamzani, A.G.; Abou-Krisha, M.M.; Aljlil, S. Developing a new sustainable eco-adsorbent film from flexographic printing plate waste to remove cationic organic and inorganic pollutants. RSC Adv. 2024, 14, 24373–24383. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Toghan, A.; Gouda, M.H.; Zahran, H.F.; Alakhras, A.I.; Sanad, M.M.; Elessawy, N.A. Development of a new promising nanocomposite photocatalyst of polyaniline/carboxylated graphene oxide supported on PVA film to remove different ecological pollutants. Diam. Relat. Mater. 2023, 139, 110400. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent advances in cellulose- and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Haile, C.T.; Ahmad, N.; Chiu, C.-W.; Kuo, C.-F.J. Highly photoactive novel NiS/BiOI nanocomposite photocatalyst towards efficient visible light organic pollutant degradation and carcinogenetic Cr (VI) reduction for environmental remediation. Chemosphere 2023, 323, 138108. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Shahzadi, T.; Riaz, T.; Zaib, M.; Khan, S.; Habila, M.A.; Sillanpaa, M. Photocatalytic Degradation Studies of Organic Dyes over Novel Cu/Ni Loaded Reduced Graphene Oxide Hybrid Nanocomposite: Adsorption, Kinetics and Thermodynamic Studies. Molecules 2023, 28, 6474. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, Q.; Zhang, X.; Zhou, H. Enhanced photocatalytic hydrogen production activity of Ni modified TiO2/GO nanosheet composites. New J. Chem. 2023, 47, 15694–15700. [Google Scholar] [CrossRef]
- Kandhasamy, N.; Murugadoss, G.; Kannappan, T.; Kirubaharan, K.; Manavalan, R.K.; Devanesan, S.; AlSalhi, M.S.; Mythili, R.; Shinde, S.; Yadav, H.M. Synthesis of nickel–manganese sulfide decorated with reduced graphene oxide nanocomposite for ultra-fast photocatalytic degradation of organic dye molecules. Carbon Lett. 2024, 34, 827–840. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Wang, X.-T.; Li, Y.; Zhang, X.-Q.; Li, J.-F.; Li, X.; Wang, C.-W. Design and fabrication of NiS/LaFeO3 heterostructures for high efficient photodegradation of organic dyes. Appl. Surf. Sci. 2020, 504, 144363. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Liu, X.; Li, H.; Yu, G.; Wang, D. Ni7S6 decorated g-C3N4/graphene oxide composites for enhanced visible light photocatalytic hydrogen evolution. Diam. Relat. Mater. 2024, 143, 110869. [Google Scholar] [CrossRef]
- Ghosh, N.; Sen, S.; Biswas, G.; Singh, L.R.; Chakdar, D.; Haldar, P.K. A comparative study of CuO nanoparticle and CuO/PVA-PVP nanocomposite on the basis of dye removal performance and antibacterial activity in wastewater treatment. Int. J. Environ. Anal. Chem. 2022, 104, 2234–2254. [Google Scholar] [CrossRef]
- Gouda, M.H.; Khowdiary, M.; Alsnani, H.; Roushdy, N.; Youssef, M.E.; Elnouby, M.; Elessawy, N.A. Adsorption and antibacterial studies of a novel hydrogel adsorbent based on ternary eco-polymers doped with sulfonated graphene oxide developed from upcycled plastic waste. J. Contam. Hydrol. 2024, 264, 104362. [Google Scholar] [CrossRef]
- Baruah, S.; Kumar, S.; Nayak, B.; Puzari, A. Optoelectronically suitable graphene oxide-decorated titanium oxide/polyaniline hybrid nanocomposites and their enhanced photocatalytic activity with methylene blue and rhodamine B dye. Polym. Bull. 2021, 78, 1703–1720. [Google Scholar] [CrossRef]
- Mitra, M.; Ahamed, S.T.; Ghosh, A.; Mondal, A.; Kargupta, K.; Ganguly, S.; Banerjee, D. Polyaniline/Reduced Graphene Oxide Composite-Enhanced Visible-Light-Driven Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Rathore, B.S.; Chauhan, N.P.S.; Rawal, M.K.; Ameta, S.C.; Ameta, R. Chitosan–polyaniline–copper(II) oxide hybrid composite for the removal of methyl orange dye. Polym. Bull. 2020, 77, 4833–4850. [Google Scholar] [CrossRef]
- Abdul Razak, M.F.S.; Salleh, M.N.; Azani, A.; Zakir, N.I.M. Decolorization of reactive red 2 dye by using pva- polyaniline immobilized system. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012013. [Google Scholar] [CrossRef]
- Gemeay, A.H.; Elsharkawy, R.G.; Aboelfetoh, E.F. Graphene Oxide/Polyaniline/Manganese Oxide Ternary Nanocomposites, Facile Synthesis, Characterization, and Application for Indigo Carmine Removal. J. Polym. Environ. 2018, 26, 655–669. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Da Oh, W.; Shopware, N.F.; Gasim, M.F.; Okoye, P.; Ul-Hamid, A.; Mohamed, A.R. Tunable band structure of synthesized carbon dots modified graphitic carbon nitride/bismuth oxychlorobromide heterojunction for photocatalytic degradation of tetracycline in water. J. Colloid Interface Sci. 2023, 629, 189–205. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Barroga, S.Q.; Mathew, R.A.; Peña-Bahamonde, J.; Louie, S.M.; Perez, J.V.D.; Rodrigues, D.F. Use of polyaniline coating on magnetic MoO3 and its effects on material stability andvisible-light photocatalysis of tetracycline. J. Environ. Chem. Eng. 2022, 10, 107635. [Google Scholar] [CrossRef]
- Reddy, P.L.; Deshmukh, K.; Kovářík, T.; Reiger, D.; Nambiraj, N.A.; Lakshmipathy, R.; Khadheer Pasha, S.K. Enhanced dielectric properties of green syn-thesized Nickel Sulphide (NiS) nanoparticles integrated polyvinylalcohol nanocomposites. Mater. Res. Express 2020, 7, 064007. [Google Scholar]
- Shalaby, A.; Nihtianova, D.; Markov, P.; Staneva, A.D.; Iordanova, R.S.; Dimitriev, B.Y. Structural analysis of reduced graphene oxide by transmission electron microscopy. Bulg. Chem. Commun. 2015, 47, 291–295. [Google Scholar]
- Fazli, Y.; Pourmortazavi, S.M.; Kohsari, I.; Sadeghpur, M. Electrochemical synthesis and structure characterization of nickel sulfide nanoparticles. Mater. Sci. Semicond. Process 2014, 27, 362–367. [Google Scholar] [CrossRef]
- Balavijayalakshmi, J.; Sonia, D. Impact of iron doping on structural and optical properties of nickel sulfide nanoparticles. Mater. Today Proc. 2022, 50, 117–122. [Google Scholar] [CrossRef]
- Shriber, P.; Tkachev, M.; Atkins, A.; Perelshtein, I.; Bretler, S.; Schmerling, B.; Mariotto, G.; Giarola, M.; Fleger, Y.; Nessim, G.D. Synthesis of nickel sulfide dendrites from nickel foil using thermal annealing. Materialia 2022, 21, 101316. [Google Scholar] [CrossRef]
- Abu Hurayra–Lizu, K.; Bari, W.; Gulshan, F.; Islam, M.R. GO based PVA nanocomposites: Tailoring of optical and structural properties of PVA with low percentage of GO nanofillers. Heliyon 2021, 7, e06983. [Google Scholar] [CrossRef]
- Zainab, S.; Azeem, M.; Awan, S.U.; Rizwan, S.; Iqbal, N.; Rashid, J. Optimization of bandgap reduction in 2-dimensional GO nanosheets and nanocomposites of GO/iron-oxide for electronic device applications. Sci. Rep. 2023, 13, 6954. [Google Scholar] [CrossRef]
- Luo, Y.-N.; Li, Y.; Qian, L.-L.; Wang, X.-T.; Wang, J.; Wang, C.-W. Excellent photocatalytic performance from NiS decorated TiO2 nanoflowers with exposed {001} facets. Mater. Res. Bull. 2020, 130, 110945. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.-T.; Lim, T.-T.; Yan, X. Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci. Rep. 2015, 5, 16369. [Google Scholar] [CrossRef]
- Muninathan, S.; Arumugam, S. Enhanced photocatalytic activities of NiS decorated reduced graphene oxide for hydrogen production and toxic dye degradation under visible light irradiation. Int. J. Hydrog. Energy 2021, 46, 6532–6546. [Google Scholar] [CrossRef]
- Box, G.; Behnken, D. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Al Naim, A.F.; El-Shamy, A.G. A new reusable adsorbent of polyvinyl alcohol/magnesium peroxide (PVA/MgO2) for highly selective adsorption and dye removal. Mater. Chem. Phys. 2021, 270, 124820. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Toghan, A.; Elnouby, M.S.; Alakhras, A.I.; Hamad, H.A.; Youssef, M.E. Development and activity enhancement of zirconium/vanadium oxides as micro-heterogeneous ceramic electrocatalyst for ORR in low temperature fuel cell. Ceram. Int. 2023, 49, 4313–4321. [Google Scholar] [CrossRef]



| Nanocomposite Film | Contact Angle |
|---|---|
| PVA | ∼49.5° ± 3 |
| NiS/PVA | ∼114° ± 5 |
| GO/PVA | ∼31.5° ± 2.5 |
| GO/NiS/PVA | ∼81° ± 4.5 |
| Initial Concentration (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | |
| MB | 0.094 | 0.054 | 0.015 | 0.012 | 0.011 | 0.008 |
| TC | 0.093 | 0.049 | 0.0048 | 0.0045 | 0.0044 | 0.0044 |
| Symbol | Independent Variables | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | light intensity, lm | 0 | 1 | 2 |
| B | initial concentration/mg L−1 | 10 | 20 | 30 |
| C | lumination time/min. | 15 | 30 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toghan, A.; Roushdy, N.; Alhussain, H.; Elessawy, N.A. Rational Engineering of Nanostructured NiS/GO/PVA for Efficient Photocatalytic Degradation of Organic Pollutants. Catalysts 2024, 14, 567. https://doi.org/10.3390/catal14090567
Toghan A, Roushdy N, Alhussain H, Elessawy NA. Rational Engineering of Nanostructured NiS/GO/PVA for Efficient Photocatalytic Degradation of Organic Pollutants. Catalysts. 2024; 14(9):567. https://doi.org/10.3390/catal14090567
Chicago/Turabian StyleToghan, Arafat, Naglaa Roushdy, Hanan Alhussain, and Noha A. Elessawy. 2024. "Rational Engineering of Nanostructured NiS/GO/PVA for Efficient Photocatalytic Degradation of Organic Pollutants" Catalysts 14, no. 9: 567. https://doi.org/10.3390/catal14090567
APA StyleToghan, A., Roushdy, N., Alhussain, H., & Elessawy, N. A. (2024). Rational Engineering of Nanostructured NiS/GO/PVA for Efficient Photocatalytic Degradation of Organic Pollutants. Catalysts, 14(9), 567. https://doi.org/10.3390/catal14090567







