Comparative Study on Ethanol-Based Oxygenate Synthesis via Syngas over Monometallic Rh Catalysts Supported on Different Zr-MOFs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Texture Characterization of Supports
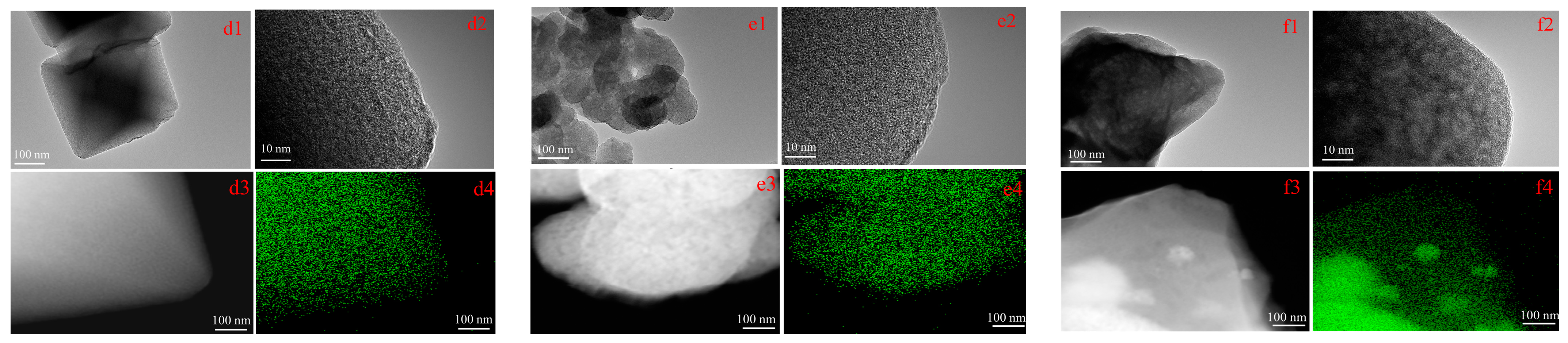
2.2. Texture and Structure Characterization of Catalysts
2.3. Catalyst Performances
2.4. Surface Chemical States
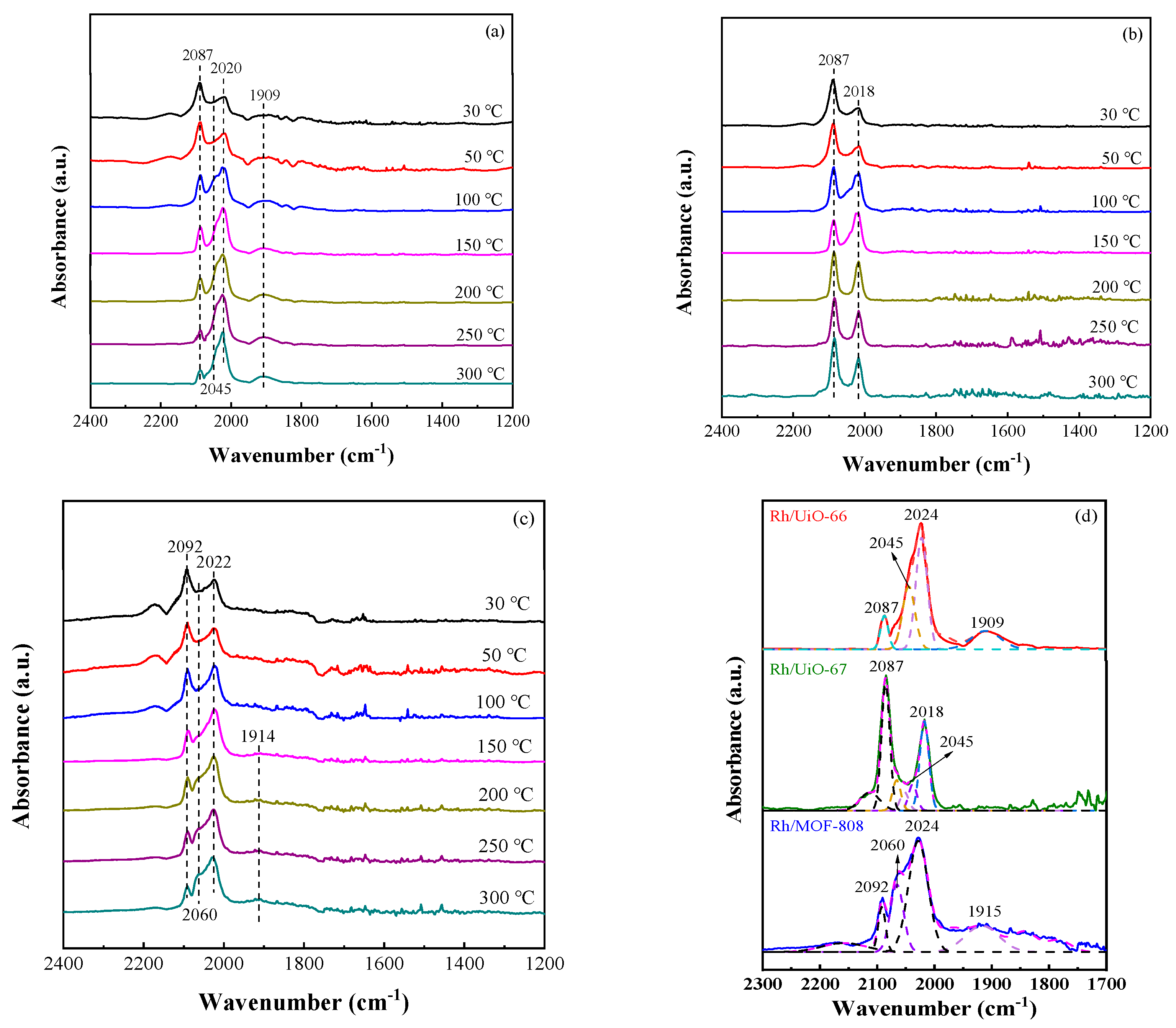
2.5. DRIFTS Study
2.6. Stability of the Rh@UiO-67 Catalyst
3. Materials and Method
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spivey, J.J.; Egbebi, A.; Kim, S.; Dale, B.E.; Park, B.G.; Andries, J.; Buhre, B.; Lampenius, H.; Zimmerman, W.H.; Campbell, C.N. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007, 36, 1514–1528. [Google Scholar] [CrossRef]
- Subramani, V.; Gangwal, S.K. A Review of Recent Literature to Search for an Efficient Catalytic Process for the Conversion of Syngas to Ethanol. Energy Fuels 2008, 22, 814–839. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.; Stewart, J.A.; Pérez-Ramírez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef]
- Ao, M.; Pham, G.H.; Sunarso, J.; Tade, M.O.; Liu, S.M. Active Centers of Catalysts for Higher Alcohol Synthesis from Syngas: A Review. ACS Catal. 2018, 8, 7025–7050. [Google Scholar] [CrossRef]
- Gupta, M.; Smith, M.L.; Spivey, J.J. Heterogeneous Catalytic Conversion of Dry Syngas to Ethanol and Higher Alcohols on Cu-Based Catalysts. ACS Catal. 2011, 1, 641–656. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, S.; Guo, L. Ethanol synthesis catalyzed by single Ni atom supported on Mo6S8 support. Appl. Catal. A Gen. 2018, 553, 52–64. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co–Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Xiang, Y.; Chitry, V.; Liddicoat, P.; Felfer, P.; Cairney, J.; Ringer, S.; Kruse, N. Long-Chain Terminal Alcohols through Catalytic CO Hydrogenation. J. Am. Chem. Soc. 2013, 135, 7114–7117. [Google Scholar] [CrossRef]
- Lim, J.; Park, H.-G.; Kim, T.-W.; Kim, D.; Ha, K.-S. Promoted Rh nanocrystal-incorporated carbon sphere catalysts for higher alcohol synthesis. Fuel 2016, 169, 25–32. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, T.-W.; Chae, H.-J.; Kim, C.-U.; Jeong, S.-Y.; Kim, J.-R.; Ha, K.-S. Mesoporous Carbon Supported Rh Nanoparticle Catalysts for the Production of C2+ Alcohol from Syngas. J. Nanosci. Nanotechnol. 2016, 16, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Ma, H.; Qian, W.; Jin, F.; Ying, W. Direct Conversion of Syngas to Ethanol over Rh–Fe/γ-Al2O3 Catalyst: Promotion Effect of Li. Catal. Lett. 2018, 148, 691–698. [Google Scholar] [CrossRef]
- Wang, Y.; Luo H., Y.; Liang D., B.; Bao X., H. Different Mechanisms for the Formation of Acetaldehyde and Ethanol on the Rh–Based Catalysts. J. Catal. 2000, 196, 46–55. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Ma, H.; Qian, W.; Ying, W. Effect of Ce promoter on Rh-Fe/TiO2 catalysts for ethanol synthesis from syngas. Catal. Commun. 2017, 98, 90–93. [Google Scholar] [CrossRef]
- Yang, N.; Yoo, J.S.; Schumann, J.; Bothra, P.; Singh, J.A.; Valle, E.; Abild-Pedersen, F.; Nørskov, J.K.; Bent, S.F. Rh-MnO interface sites formed by atomic layer deposition promote syngas conversion to higher oxygenates. ACS Catal. 2017, 7, 5746–5757. [Google Scholar] [CrossRef]
- Chen, W.M.; Ding, Y.J.; Jiang, D.H.; Pan, Z.D.; Luo, H.Y. An Effective Method of Controlling Metal Particle Size on Impregnated Rh-Mn-Li/SiO2 Catalyst. Catal. Lett. 2005, 104, 177–180. [Google Scholar] [CrossRef]
- Prieto, G.; Concepción, P.; Martínez, A.; Mendoza, E. New insights into the role of the electronic properties of oxide promoters in Rh-catalyzed selective synthesis of oxygenates from synthesis gas—ScienceDirect. J. Catal. 2011, 280, 274–288. [Google Scholar] [CrossRef]
- Mei, D.; Rousseau, R.; Kathmann, S.M.; Glezakou, V.A.; Engelhard, M.H.; Jiang, W.; Wang, C.; Gerber, M.A.; White, J.F.; Stevens, D.J. Ethanol synthesis from syngas over Rh-based/SiO2 catalysts: A combined experimental and theoretical modeling study. J. Catal. 2010, 271, 325–342. [Google Scholar] [CrossRef]
- Da Won, B.; Jeong, M.H.; Kim, M.H.; Chung, C.H.; Moon, D.J.; Suh, Y.W.; Baik, J.H.; Bae, J.W. Rh-Mn/tungsten carbides for direct synthesis of mixed alcohols from syngas: Effects of tungsten carbide phases. Microporous Mesoporous Mater. 2018, 255, 44–52. [Google Scholar] [CrossRef]
- Wang, S.R.; Guo, W.W.; Wang, H.X.; Zhu, L.J.; Qiu, K.Z. Influence of Mn Promotion on CO Hydrogenation over Rh/CNTs Catalyst. Catal. Lett. 2014, 144, 1305–1312. [Google Scholar] [CrossRef]
- Huang, X.; Teschner, D.; Dimitrakopoulou, M.; Fedorov, A.; Frank, B.; Kraehnert, R.; Rosowski, F.; Kaiser, H.; Schunk, S.; Kuretschka, C. Inside Cover: Atomic-Scale Observation of the Metal–Promoter Interaction in Rh-Based Syngas-Upgrading Catalysts. Angew. Chem. Int. Ed. 2019, 58, 8709–8713. [Google Scholar] [CrossRef]
- Schwartz, V.; Campos, A.; Egbebi, A.; Spivey, J.J.; Overbury, S.H. EXAFS and FT-IR Characterization of Mn and Li Promoted Titania-Supported Rh Catalysts for CO Hydrogenation. Acs Catal. 2011, 1, 1298–1306. [Google Scholar] [CrossRef]
- Trevino, H.; Lei, G.D.; Sachtler, W. CO Hydrogenation to Higher Oxygenates over Promoted Rhodium: Nature of the Metal-Promoter Interaction in Rhmn/NaY. J. Catal. 1995, 154, 245–252. [Google Scholar] [CrossRef]
- He, Z.L.; Jiang, H.Q.; Zang, S.Y. An excellent Rh,F,P-tridoped TiO2 photocatalyst with efficient carrier separation. Mater. Lett. 2019, 252, 38–41. [Google Scholar] [CrossRef]
- Zhu, C.X.; Xu, K.Z.; Fang, Y.; Lv, Q.; Jiang, K.; Zhao, H.B.; Chen, Y.; Wang, P.; Yang, H.; Wu, L.Z.; et al. Synergetic effect of Ce/Zr for ethanol synthesis from syngas over Rh-based catalyst. Fuel 2023, 334, 126770. [Google Scholar] [CrossRef]
- Wei, W.; Gong, H.Y.; Sheng, L.; Zhu, S.G.; Feng, L. Loading Rh single atoms onto hollow cubic C2MoS4 nanoparticles for decreased electron/hole recombination and increased photocatalytic performance. J. Alloys Compd. 2022, 896, 162832. [Google Scholar] [CrossRef]
- Zhang, Y.; Ligthart, D.; Quek, X.Y.; Gao, L.; Hensen, E.J.M. Influence of Rh nanoparticle size and composition on the photocatalytic water splitting performance of Rh/graphitic carbon nitride. Int. J. Hydrogen Energy 2014, 39, 11537–11546. [Google Scholar] [CrossRef]
- Liu, G.; Fang, H.; Wang, G.; Liu, N.; Liu, J.; Huang, L.; Liang, X.; Yuan, Y. Dispersion of Rh–WxC nanocomposites on carbon nanotubes by one-pot carburization for synthesis of higher alcohols from syngas. Fuel 2021, 305, 121533. [Google Scholar] [CrossRef]
- Chen, G.; Guo, C.-Y.; Zhang, X.; Huang, Z.; Yuan, G. Direct conversion of syngas to ethanol over Rh/Mn-supported on modified SBA-15 molecular sieves: Effect of supports. Fuel Process. Technol. 2011, 92, 456–461. [Google Scholar] [CrossRef]
- Dong, J.; Cui, P.; Shi, P.-F.; Cheng, P.; Zhao, B. Ultrastrong Alkali-Resisting Lanthanide-Zeolites Assembled by [Ln60] Nanocages. J. Am. Chem. Soc. 2015, 137, 15988–15991. [Google Scholar] [CrossRef]
- Kossev, K.; Koseva, N.; Troev, K. Calcium chloride as co-catalyst of onium halides in the cycloaddition of carbon dioxide to oxiranes. Mol. Catal. 2003, 194, 29–37. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.-T.; Liu, X.-H.; Zou, K.; Deng, W.-Q. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Wang, B.J.; Guo, W.S.; Zhang, R.G.; Ling, L.X.; Li, Z.Q. C2 Oxygenates Formation from Syngas over the Cu-Rich and Rh-Rich Surfaces of Rh-Cu Bimetallic Catalysts: Probing into the Effects of the Surface Structure and Composition on the Catalytic Performance. J. Phys. Chem. C 2019, 123, 19528–19539. [Google Scholar] [CrossRef]
- Carrillo, P.; Shi, R.; Teeluck, K.; Senanayake, S.D.; White, M.G. In situ formation of FeRh nanoalloys for oxygenate synthesis. ACS Catal. 2018, 8, 7279–7286. [Google Scholar] [CrossRef]
- Asundi, A.S.; Hoffman, A.S.; Bothra, P.; Boubnov, A.; Vila, F.D.; Yang, N.; Singh, J.A.; Zeng, L.; Raiford, J.A.; Pedersen, F.A.; et al. Understanding structure–property relationships of MoO3-promoted Rh catalysts for syngas conversion to alcohols. J. Am. Chem. Soc. 2019, 141, 19655–19668. [Google Scholar] [CrossRef]
- Gutterod, E.S.; Lazzarini, A.; Fjermestad, T.; Kaur, G.; Manzoli, M.; Bordiga, S.; Svelle, S.; Lillerud, K.P.; Skúlason, E.; Oien-Odegaard, S.; et al. Hydrogenation of CO2 to Methanol by Pt Nanoparticles Encapsulated in UiO-67: Deciphering the Role of the Metal-Organic Framework. J. Am. Chem. Soc. 2020, 142, 999–1009. [Google Scholar] [CrossRef]
- Duflot, M.; Marchal, C.; Caps, V.; Artero, V.; Christoforidis, K.; Keller, V. Optimization of NH2-UiO-66/TiO2/Au composites for enhanced gas-phase CO2 photocatalytic reduction into CH4. Catal. Today 2023, 413–415, 114018. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Rodríguez-Pereira, J.; Baldovino-Medrano, V.G.; Ramírez-Caballero, G.E. An analysis of the effect of zirconium precursors of MOF-808 on its thermal stability, and structural and surface properties. CrystEngComm 2019, 21, 1407–1415. [Google Scholar] [CrossRef]
- Siu, P.W.; Brown, Z.J.; Farha, O.K.; Hupp, J.T.; Scheidt, K.A. A mixed dicarboxylate strut approach to enhancing catalytic activity of a de novo urea derivative of metal–organic framework UiO-67. Chem. Commun. 2013, 49, 10920–10922. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Bruss, A.J.; Gelesky, M.A.; Machado, G.; Dupont, J. Rh (0) nanoparticles as catalyst precursors for the solventless hydroformylation of olefins. J. Mol. Catal. A Chem. 2006, 252, 212–218. [Google Scholar] [CrossRef]
- Egbebi, A.; Schwartz, V.; Overbury, S.H.; Spivey, J.J. Effect of Li Promoter on titania-supported Rh catalyst for ethanol formation from CO hydrogenation. Catal. Today 2010, 149, 91–97. [Google Scholar] [CrossRef]
- Chen, W.; Song, X.; Ning, L.; Ding, Y. Ammonia Hydrothermally Treated SiO2 Supported Rh-Based Catalyst for CO Hydrogenation to C2 Oxygenates: Remarkable Effect of Support Pore Size. Ind. Eng. Chem. Res. 2020, 59, 18798–18807. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; Such-Basañez, I.; Illán-Gómez, M.; De Lecea, C.S.-M.; Bueno-López, A. Study by isotopic gases and in situ spectroscopies (DRIFTS, XPS and Raman) of the N2O decomposition mechanism on Rh/CeO2 and Rh/γ-Al2O3 catalysts. J. Catal. 2010, 276, 390–401. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Poston Jr, J.A.; Spivey, J.J. Synthesis, characterization, and catalytic activity of Rh-based lanthanum zirconate pyrochlores for higher alcohol synthesis. Catal. Today 2013, 207, 65–73. [Google Scholar] [CrossRef]
- Solymosi, F.; Pasztor, M. An infrared study of the influence of carbon monoxide chemisorption on the topology of supported rhodium. J. Phys. Chem. 1985, 89, 4789–4793. [Google Scholar] [CrossRef]
- Rice, C.; Worley, S.; Curtis, C.; Guin, J.; Tarrer, A. The oxidation state of dispersed Rh on Al2O3. J. Chem. Phys. 1981, 74, 6487–6497. [Google Scholar] [CrossRef]
- Xue, X.Y.; Yu, J.; Han, Y.; Xiao, X.Z.; Shi, Z.P.; Mao, H.F.; Mao, D.S. Zr-based metal-organic frameworks drived Rh-Mn catalysts for highly selective CO hydrogenation to C2 oxygenates. J. Ind. Eng. Chem. 2020, 86, 220–231. [Google Scholar] [CrossRef]
- Han, X.Y.; Li, M.S.; Chang, X.; Hao, Z.W.; Chen, J.Y.; Pan, Y.T.; Kawi, S.; Ma, X.B. Hollow structured Cu@ZrO2 derived from Zr-MOF for selective hydrogenation of CO2 to methanol. J. Energy Chem. 2022, 71, 277–287. [Google Scholar] [CrossRef]
- Chen, G.Q.; Yu, J.; Li, G.H.; Zheng, X.; Mao, H.F.; Mao, D.S. Cu thorn-ZrO2 interfacial sites with highly dispersed copper nanoparticles derived from Cu@UiO-67 hybrid for efficient CO2 hydrogenation to methanol. Int. J. Hydrogen Energy 2023, 48, 2605–2616. [Google Scholar] [CrossRef]
- Yu, J.; Mao, D.S.; Ding, D.; Guo, X.M.; Lu, G.Z. New insights into the effects of Mn and Li on the mechanistic pathway for CO hydrogenation on Rh-Mn-Li/SiO2 catalysts. J. Mol. Catal. A Chem. 2016, 423, 151–159. [Google Scholar] [CrossRef]




| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | ||||
|---|---|---|---|---|---|---|
| Total | Micropore | External | Total | Micropore | Mesopore | |
| UiO-66 | 1179.1 | 1004.7 | 174.4 | 0.69 | 0.51 | 0.18 |
| Rh@UiO-66 | 903.9 | 622.8 | 281.1 | 0.53 | 0.32 | 0.21 |
| UiO-67 | 1057.0 | 912.4 | 144.6 | 0.61 | 0.46 | 0.15 |
| Rh@UiO-67 | 805.7 | 573.6 | 232.1 | 0.54 | 0.30 | 0.24 |
| MOF-808 | 662.4 | 455.4 | 107.0 | 0.33 | 0.23 | 0.10 |
| Rh@MOF-808 | 145.2 | 43.4 | 101.8 | 0.15 | 0.02 | 0.13 |
| Catalyst | CO Conv. (%) | Selectivity (%) | C2+ Oxy Productivity (mol/molRh·h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | CH4 | MeOH | HAC | EtOH | C2+ H a | C2+ Oxy b | |||
| Rh@UiO-66 | 28.2 | 2.0 | 22.2 | 9.6 | 19.2 | 31.1 | 16.0 | 50.2 | 88.7 |
| Rh@UiO-67 | 36.5 | 1.9 | 20.2 | 4.9 | 17.5 | 32.1 | 14.5 | 58.5 | 136.8 |
| Rh@MOF-808 | 18.0 | 2.3 | 23.8 | 9.5 | 17.7 | 23.5 | 19.9 | 44.5 | 51.9 |
| Catalyst | a Rh+ | a Rh0 | Rh+/Rh0 | b CO (gdc) | b CO (l) | CO (gdc)/CO (l) |
|---|---|---|---|---|---|---|
| Rh@UiO-66 | 2978.3 | 4659.8 | 0.64 | 6.81 | 3.09 | 2.24 |
| Rh@UiO-67 | 2530.8 | 3711.9 | 0.68 | 6.76 | 2.50 | 2.62 |
| Rh@MOF-808 | 1556.1 | 6529.1 | 0.24 | 4.75 | 3.61 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Duan, X.; Yu, X.; Zheng, X.; Mao, H.; Yu, J. Comparative Study on Ethanol-Based Oxygenate Synthesis via Syngas over Monometallic Rh Catalysts Supported on Different Zr-MOFs. Catalysts 2024, 14, 566. https://doi.org/10.3390/catal14090566
Yu R, Duan X, Yu X, Zheng X, Mao H, Yu J. Comparative Study on Ethanol-Based Oxygenate Synthesis via Syngas over Monometallic Rh Catalysts Supported on Different Zr-MOFs. Catalysts. 2024; 14(9):566. https://doi.org/10.3390/catal14090566
Chicago/Turabian StyleYu, Ruiqi, Xiangjiang Duan, Xuanwang Yu, Xiang Zheng, Haifang Mao, and Jun Yu. 2024. "Comparative Study on Ethanol-Based Oxygenate Synthesis via Syngas over Monometallic Rh Catalysts Supported on Different Zr-MOFs" Catalysts 14, no. 9: 566. https://doi.org/10.3390/catal14090566
APA StyleYu, R., Duan, X., Yu, X., Zheng, X., Mao, H., & Yu, J. (2024). Comparative Study on Ethanol-Based Oxygenate Synthesis via Syngas over Monometallic Rh Catalysts Supported on Different Zr-MOFs. Catalysts, 14(9), 566. https://doi.org/10.3390/catal14090566






