Abstract
Biodiesel has received tremendous attention as a sustainable energy source. This review presents an overview of various catalysts utilized in biodiesel production and compares their potential for producing biodiesel. Presented here are the excellent features of the various catalysts while highlighting their drawbacks. For instance, production of biodiesel with homogeneous base catalysts is easy but it can only be used with refined oils having low levels of free fatty acid (FFAs). When homogeneous acid is used in esterification, it causes reactor corrosion. Water and FFAs do not affect heterogeneous acid catalysts. Thus, transesterification of triglycerides into biodiesel and converting FFAs into biodiesel through esterification can be catalyzed more efficiently using a heterogeneous acid catalyst. Biocatalysts are also being used to produce biodiesel from oils with high FFAs. However, heterogeneous acid catalysts and biocatalysts are not suitable for industrial application due to serious mass transfer limitations. Biodiesel yield and conversion were compared over various catalysts in this paper. Also presented are the effects of different reaction parameters on biodiesel yield over different catalysts. The correct interplay of factors like reaction temperature, time, alcohol-to-oil molar ratio, and catalyst loading produces optimal process conditions that give the highest biodiesel yield.
1. Introduction
The increased demand for fossil fuels and the need for a sustainable environment have become areas of great global concern. The world depends on the availability of fuels for its burgeoning population, which in turn desires a better quality of life. The yearly world consumption of primary energy is about 12.5 billion toe (ton of oil equivalent; 1 toe = 41.87 GJ), and with the growing population, the price of crude oil will increase and directly affect the global economy [1,2]. At present, petroleum-based fuels account for about 85% of primary energy consumption, 58% of which is utilized in the transport sector [3,4]. The mass utilization of non-renewable energy sources has raised concern for the option of renewable energies because of the detrimental environmental consequences, which incorporate global warming and serious climate changes [5,6]. The combustion of fossil-derived fuels produces approximately 98% of carbon emissions [7]. If the world continues to use non-sustainable energy sources for energy generation, it will not be wrong to assume there will be a time when this world will not be an ideal place to live. Therefore, in order to preserve our environment for future generations, it is necessary to take some measures in curtailing our demand for fossil fuels.
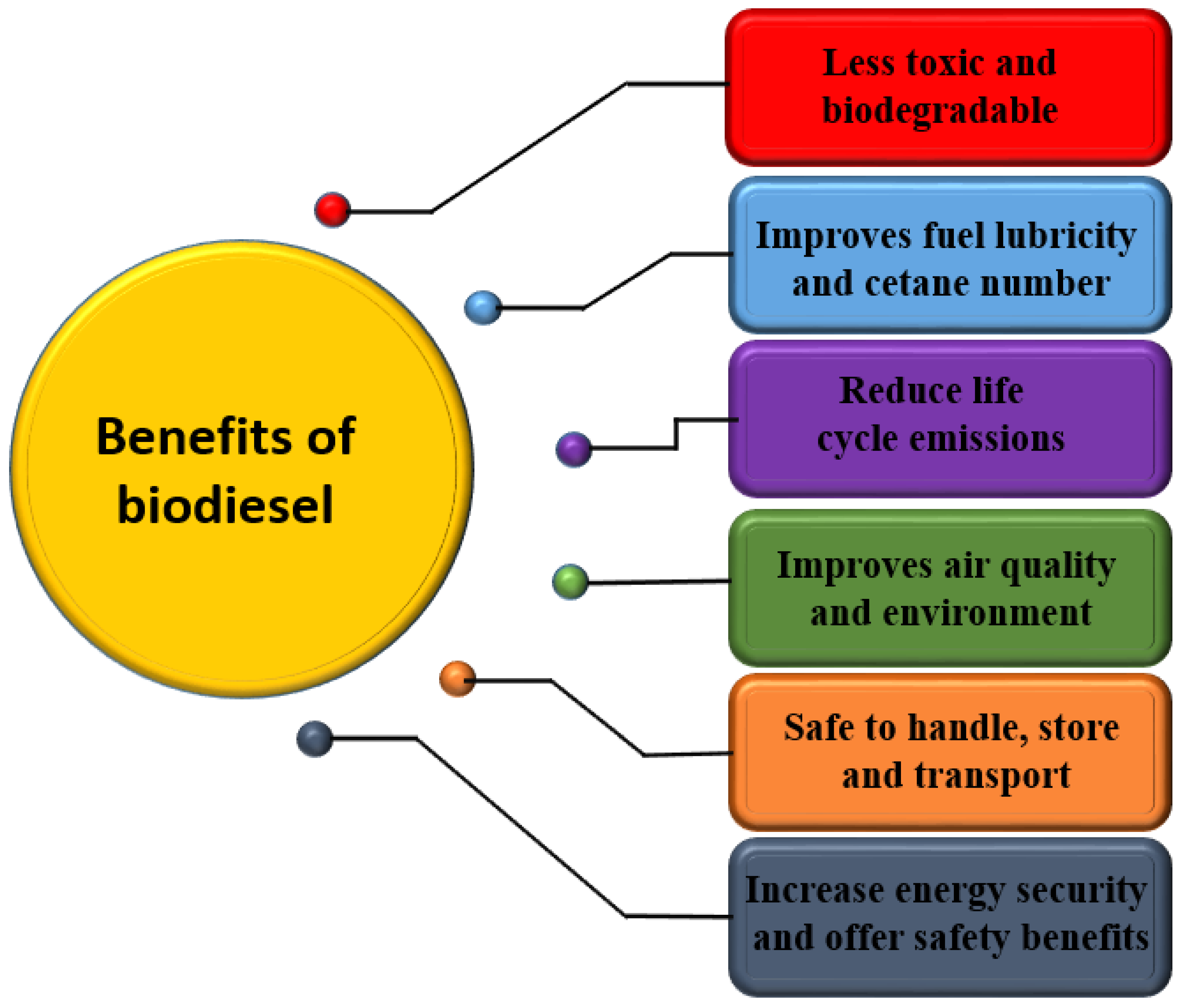
Sustainable development is the key issue, which has attracted significant attention and concern over the last few decades. Researchers are interested in developing potential renewable energy sources because of the declining fossil fuel reservoirs with an increase in crude oil prices and relatively rapid growth in the global population. For long-term environmental and economic growth, transitioning from energy dominated by fossil fuel to sustainable technologies based on renewable energy sources is now essential. This transition is not only challenging for researchers, but has economic and environmental implications [8]. The diversification of energy sources by transitioning from fossil-fuel-dominated energy to an energy portfolio covering a variety of renewable fuels would meet global energy demand [9]. Biodiesel has gotten a lot of attention as a green fuel because of its clean combustion characteristics and its sustainable sources [10,11]. In addition to being biodegradable, it is free of sulfur, has lower exhaust emission, and is free of aromatics [11]. Biodiesel production is a rapidly increasing biofuel technology since it provides a set of environmental advantages over traditional fossil fuels. Biofuel is employed as a first-generation fuel. According to a survey published by Zion Market Research, the global biofuel market is estimated to be USD 219 billion in 2022 [12]. Biodiesel is obtained from natural fats and oils and could be used in place of conventional fossil-based diesel without modifying the current diesel engines. Biodiesel offers several advantages over petroleum diesel, as illustrated in Figure 1.
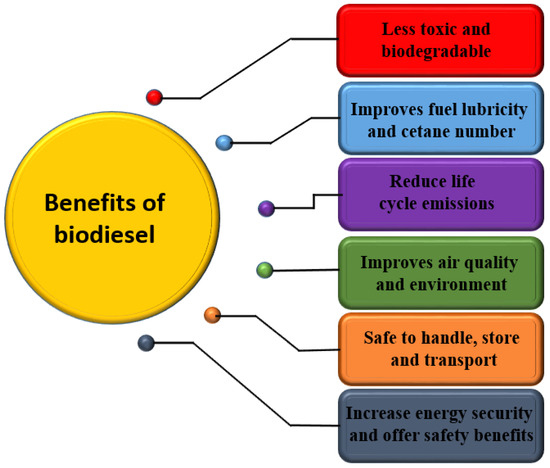
Figure 1.
Advantages of biodiesel over petrol-diesel.
Biodiesel is known for its higher lubricity compared to conventional diesel. Lubricity is crucial for reducing friction and wear in fuel injection systems, which is particularly beneficial for modern engines with high-pressure injection systems. The oxygen content in biodiesel enhances its lubricating properties, thereby protecting engine components and extending their lifespan. Regarding cetane number, which measures the combustion quality of diesel fuel, biodiesel typically has a higher cetane number than petroleum diesel. A higher cetane number means that biodiesel ignites more readily, leading to smoother engine operation, reduced noise, and lower emissions of unburned hydrocarbons and particulate matter [13]. Biodiesel also has positive effects on air quality and environmental sustainability. Due to its oxygen content, biodiesel burns more completely than fossil diesel, resulting in reduced emissions of carbon monoxide (CO), particulate matter (PM), and unburned hydrocarbons (HC). Additionally, biodiesel has lower sulfur content, leading to decreased sulfur oxide (SOx) emissions. Biodiesel is biodegradable and non-toxic, which reduces environmental impact in case of spills. Its use also lowers net carbon dioxide (CO2) emissions, as the CO2 released during combustion is offset by the CO2 absorbed by the plants used for biodiesel production, contributing to a lower overall carbon footprint.
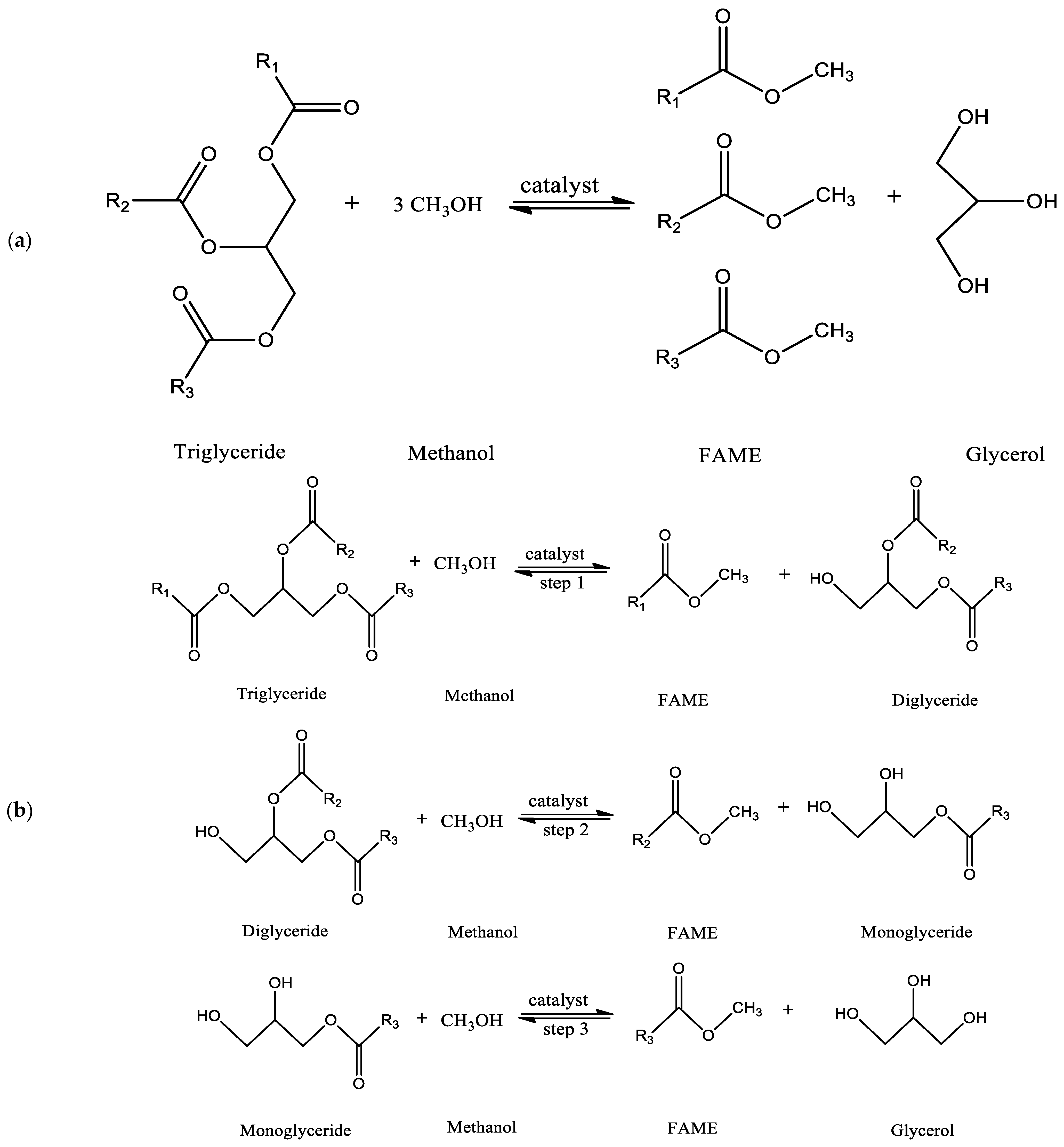
To produce biodiesel from fats and oils, four distinct methods are used: pyrolysis, hydrocarbon blending dilution, transesterification, and emulsification [9]. The transesterification method is mainly used to produce biodiesel from different kinds of fats and oils worldwide because the transesterification method produces fuel that is highly compatible with the diesel engines that are currently in use [8]. The ultimate effect of transesterification is to bring the oil’s viscosity down to the level of fossil diesel fuel [10]. Transesterification is a method of producing biodiesel that involves the reaction of plant-based triglycerides, recycled oil waste, or animal fats with alcohol in the presence of an effective catalyst to generate alkyl esters (biodiesel), with glycerol produced as a byproduct (Figure 2a) [14,15]. In general, three sequential reversible reaction steps occur during this transesterification reaction. First, diglyceride is formed through the conversion of triglyceride to diglyceride, and then diglyceride is converted to monoglyceride. The process of converting monoglyceride to glycerol follows. At each step of the reaction, biodiesel (a fatty acid methyl ester, FAME) is produced. Therefore, the method produces a total of three fatty acid methyl esters [14]. Figure 2b illustrates the complete chemical reaction used to produce biodiesel. In general, for triglyceride transesterification reaction, methanol is the favored alcohol because it is less costly and can react quickly with triglycerides owing to its advantages, which include high polarity and being the alcohol with the shortest alkyl chain [16]. The removal of glycerol is crucial for ensuring high-quality biodiesel. One common method is settling, which allows the biodiesel and glycerol to separate naturally over time due to their different densities, with the denser glycerol settling at the bottom and being removed by decantation. Centrifugation accelerates this separation process by using centrifugal force to effectively separate the biodiesel from the glycerol based on their density differences [17]. Filtration techniques can also be employed to remove impurities, including glycerol, from the biodiesel and can be used in combination with other separation methods [18]. Additionally, chemical treatment involves using chemical agents, such as acids or bases, to react with glycerol and facilitate its removal by altering its properties for easier separation [18,19]. These methods collectively ensure the effective removal of glycerol, resulting in high-quality biodiesel suitable for use.
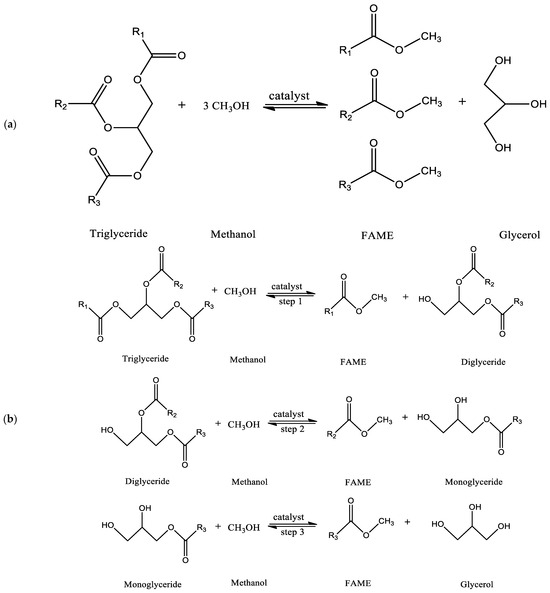
Figure 2.
(a) Overall transesterification reaction, and (b) transesterification reaction (sequential reaction).
2. Biodiesel Production Feedstock
A wide variety of feedstock can be used to produce biodiesel. The feedstock cost account for approximately 75% of the total biodiesel production costs [20]. The main issues surrounding biodiesel commercialization are feedstock prices and production costs [21]. Thus, in order to achieve low biodiesel production costs, it is important to choose suitable feedstock that are fairly cheap to produce biodiesel.
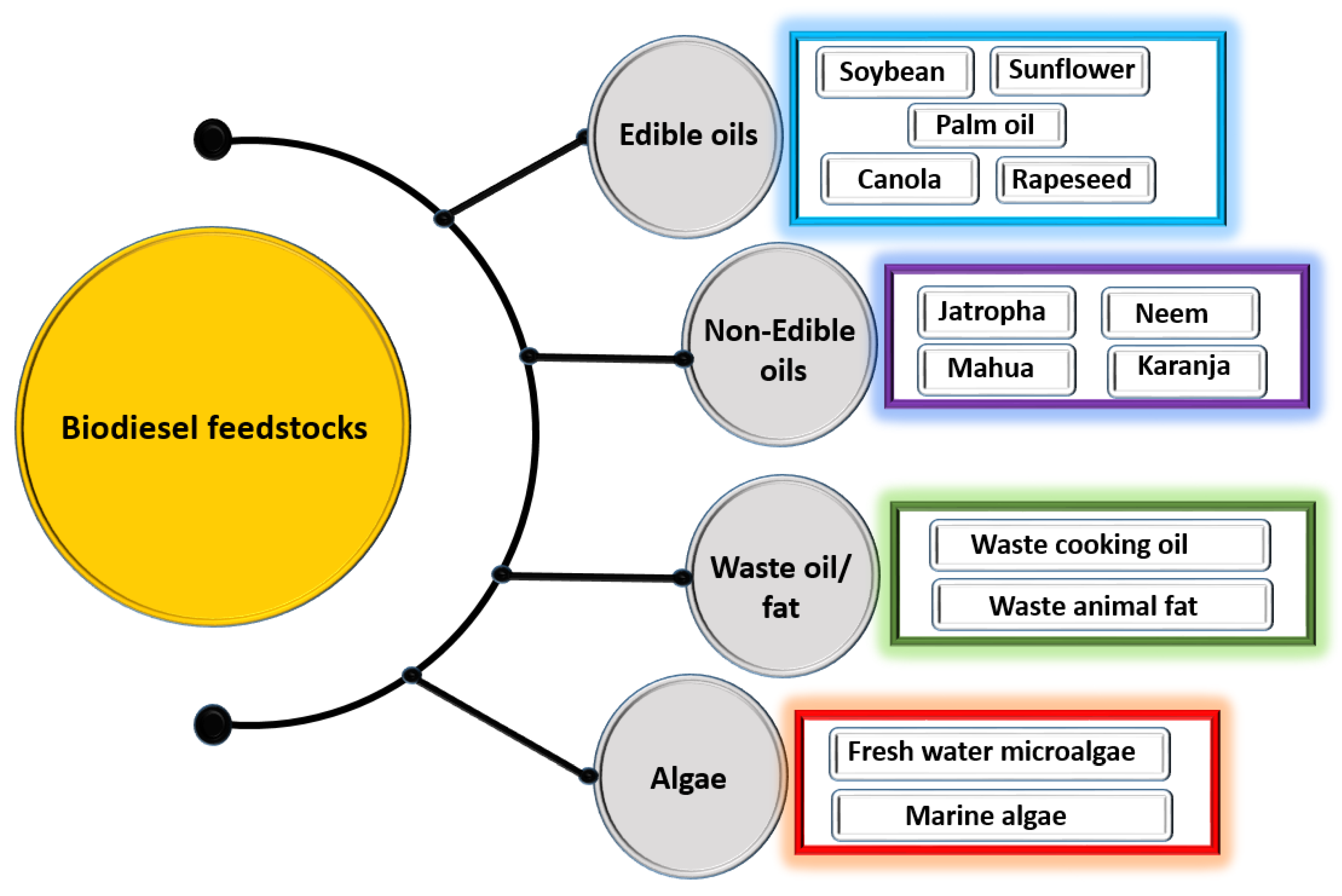
Biodiesel feedstock comes in a variety of forms: non-edible oil, edible oil, algal lipid, and waste cooking oil (Figure 3) [22,23,24,25]. Currently, almost all biodiesel production begins with edible oil as a feedstock because it is widely available from the established agricultural sector. In Malaysia, Europe, and the United States, edible oils are the main feedstock utilized in the production of biodiesel. However, extensive use of edible oil in biodiesel production can put the entire world at risk, in which the slightest planning error can have a significant impact on food supply and the economy [1]. The limited agricultural land typically available for growing food would be compromised when the fuel economy and the food economy are both in competition for the same resources. Based on these circumstances, the production of biodiesel from edible food crop feedstocks can cause serious food supply issues in developing countries due to competition for the same resources [26].
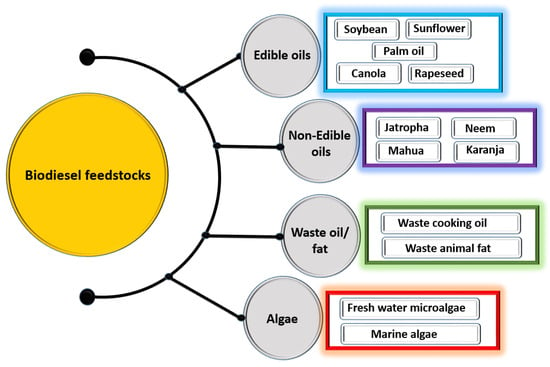
Figure 3.
Types of biodiesel feedstock.
Non-edible oils like Neem, Jatropha, Karanja, Castor, and Mahua have been identified as suitable feedstock for producing biodiesel [27]. Non-edible oil biodiesel may be deemed to have good prospects such as acceptable fuel quality, excellent engine efficiency, and desirable exhaust emission properties [28]. But biodiesel produced from non-edible oil incorporates both favorable and unfavorable attributes. Biodiesel is produced using non-edible oil as a feedstock because of its ready availability, lower aromatic content, biodegradability, and lower sulfur content [29]. However, non-edible oil is unfavorable as biodiesel feedstock due to the higher reactivity of its unsaturated fatty acid chains, lower volatility, higher viscosity, and carbon residue [29]. Micro algae are becoming increasingly popular as a feedstock for biodiesel production across the globe [30]. They have become a key feedstock for producing biodiesel because of its various benefits, including higher oil productivity, rapid growth rate than conventional crops, which are of course augmented by limited available land [30].
Waste cooking oil (WCO) could be used as a biodiesel feedstock instead of edible oil. WCO is released directly into the waterway or accumulated in the ground in most cafeterias, hotels, and other service industries [31]. Producing biodiesel from WCO presents three possible solutions: waste management, environmental, and economic [32]. It is environmentally friendly to produce biodiesel from WCO because it recycles used oil and also provides sustainable energy with lower emissions. It also reduces waste management costs. By utilizing WCO, the expense of producing biodiesel could be decreased by up to 70% [7]. Also, waste disposal concerns could be solved by producing biodiesel from WCO.
3. Types of Catalysts
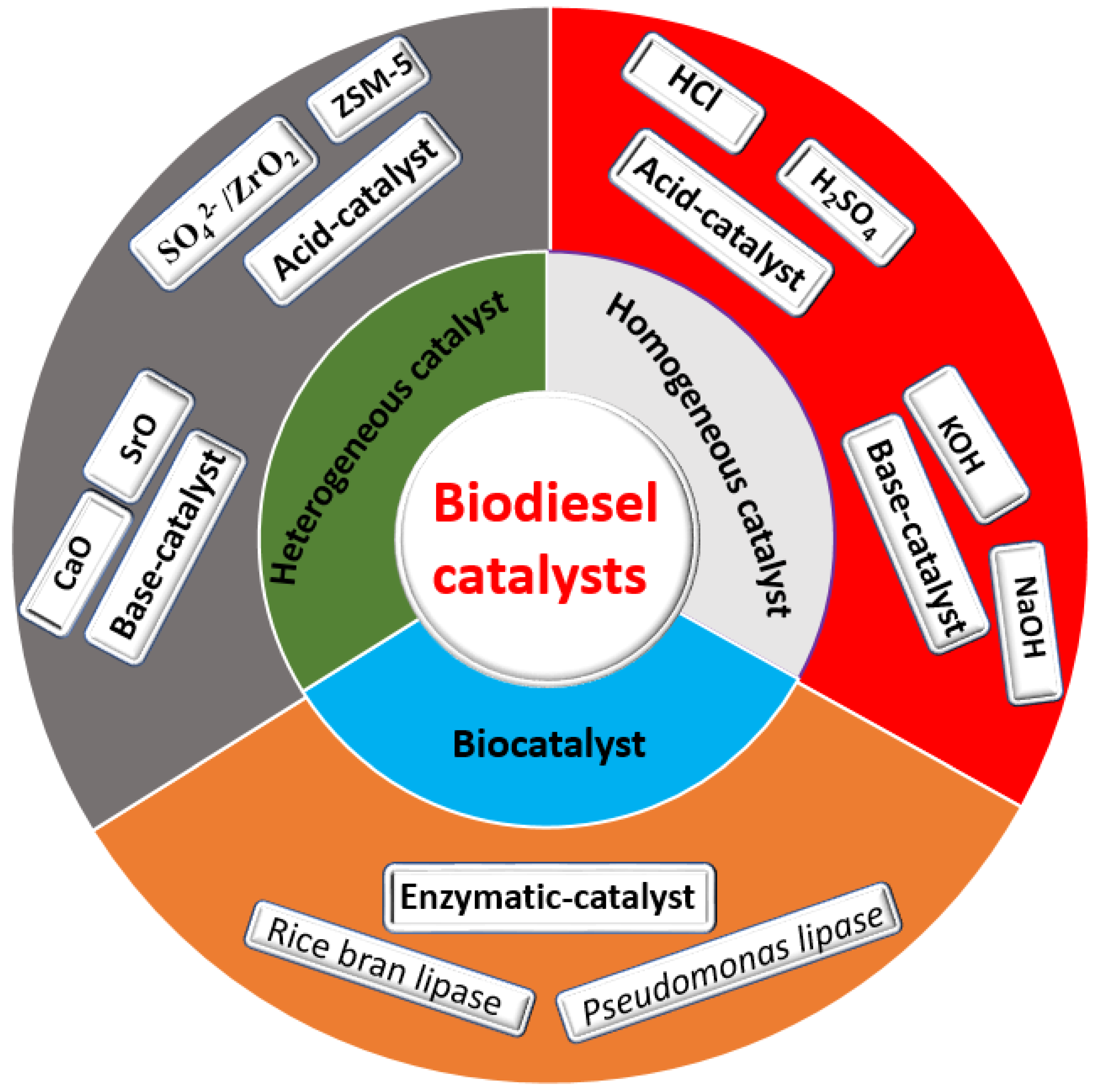
As mentioned previously, transesterification of vegetable oil, animal fat, and recycled waste oil with alcohol in the presence of a suitable catalyst produces methyl ester of long-chain fatty acid (biodiesel), with glycerol as a byproduct [33]. During the transesterification reaction, a catalyst plays a key role in providing an active site for the reactants and reduces the reaction’s total activation energy. In general, production of biodiesel involves the use of different kinds of catalysts harnessed for the transesterification of triglycerides. These include homogeneous and heterogeneous catalysts, as well as biocatalysts (Figure 4).
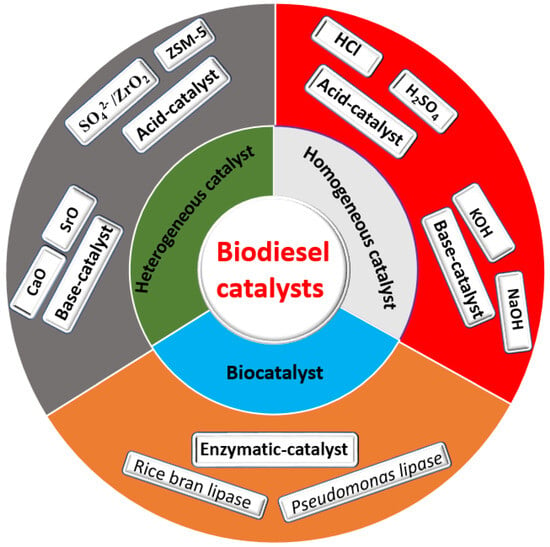
Figure 4.
Classification of biodiesel catalyst.
3.1. Biocatalysts
Catalysts that are derived from living organisms are known as biocatalysts and are often referred to as enzyme catalysts. Almost all biochemical reactions taking place in living cells are initiated by these catalysts. Each enzyme has a unique three-dimensional structure that corresponds to the reactant’s shape. In the study of biodiesel production, biocatalysts have lately become increasingly crucial [34]. The use of enzyme catalysts aims to address the problems posed by the use of synthetic catalysts, including a high amount of energy demands, feedstock pretreatment requirement, high costs, and complex procedures. Biocatalyst studies demonstrate an environmentally sustainable strategy for the production of biodiesel employing renewable material and polymer under moderate reaction conditions [34]. Typically, the enzyme normally utilized as biocatalyst is immobilized on inert materials that enable it to withstand the change in temperature and moisture content. Covalent bonding, cross-linking, and encapsulation were among the immobilization techniques documented in the preparation of biocatalysts [35]. However, in the typical transesterification reaction, the use of polar molecules like water and methanol limits the enzyme’s catalytic performance. This is alleviated via the stepwise addition of an organic solvent such as ethanol [36]. All biocatalysts do not require harsh conditions to function; however, strict hydration control is necessary since they are water-sensitive (they can be activated with small amount of water) [37]. The catalytic potential of enzymatic catalysts has been demonstrated in the transesterification process used to make biodiesel that employ a class of enzymes referred to as lipases, which are obtained from plants, microorganisms, and animals [38,39]. Lipases may be isolated from various bacterial species, including Rhizopus oryzae, Rhizomucor miehei, Pseudomonas fluorescens, Expansum lipase, and Pseudomonas cepacian [40]. Lipases are capable of catalyzing both the transesterification of triglycerides and hydrolysis under moderate conditions, making them suitable for biodiesel production [41,42,43]. Their special features, such as enantioselectivity, regioselectivity, and specificity, enable them to catalyze reactions at moderate pressures and temperatures while minimizing byproduct formation [44,45]. Enzymatic biodiesel is often promoted as a more environmentally friendly alternative to conventional biodiesel production methods due to its reduced energy consumption and lower production of chemical byproducts [42].
In the last few decades, the advantages associated with enzymatic transesterification, especially those using lipases, have made many researchers shift attention from chemical transesterification [40]. Several biocatalysts for transesterification have been reported in the literature over the last few decades. For instance, Shah et al. [36] examined the performance of immobilized Pseudomonas cepacia in the production of jatropha curcas oil biodiesel. It was observed that a 98% biodiesel yield was obtained using the enzyme at a temperature of 50 °C, 5 wt.%, 4:1 MREO in 8 h. Another study presented the use of immobilized Burkholderia cepacia for the production of jatropha curcas oil biodiesel, which under optimal conditions achieved a maximum biodiesel yield of 100% with 10:1 MREO, 5.25 g catalyst loading, and 35 °C in 24 h [37]. The catalytic transesterification of WCO over a pancreatic lipase catalyst was examined by Jayaraman et al. [46]. The optimum oil conversion using this catalyst was 88% with 3:1 MRMO, 1.5 wt.% catalyst amount, and 60 °C in 4 h. Marin-Suarez et al. [47] utilized Novozym 435 lipase as biocatalyst for transesterification of residual fish oil using ethanol. The authors reported that the Novozym 435 lipase catalyst showed excellent transesterification performance, where a maximum biodiesel yield of 82.91% was obtained for the reaction conditions of 50 wt.% catalyst and 36:1 MREO at 35 °C in 8 h. Choi et al. [48] used rice bran lipase as biocatalyst for the production of rice bran oil biodiesel. The authors found that the biocatalyst provided a yield of 83.4% with 3 wt.% catalyst and 6:1 MRMO at 40 °C in 12 days. Another research focused on the usage of Lipozyme Thermomyces lanuginose IM in the transesterification of corn oil, which under optimal process conditions gave a maximum yield of 98.95% with 6:1 MREO, 2.8 wt.% catalyst amount, and 35 °C in 12 h [49]. Arumugam et al. [50] transesterified Calophyllum inophyllum using Rhizopus oryzae lipase. They found that the Rhizopus oryzae lipase catalyst showed excellent transesterification performance, where a biodiesel yield of 92% was obtained using the following conditions: 20 wt.% catalyst amount, 35 °C reaction temperature, and 12:1 MRMO in 72 h. Li et al. [51] also produced biodiesel from Pistacia chinensis bge seed oil using Rhizopus oryzae lipase catalyst. The authors found that the Rhizopus oryzae lipase catalyst showed good performance in the transesterification of Pistacia chinensis bge seed oil and provided a maximum biodiesel yield of 94% at a temperature of 37 °C in 60 h, 5:1 MRMO, and 7 wt.% enzyme dosages. Caballero et al. [52] transesterified sunflower oil over immobilized Pig pancreatic lipase biocatalyst using ethanol under reaction conditions of temperature T= 60 °C, time t = 19 h, 0.1 wt.% of catalyst, and 2:1 MREO, giving a maximum biodiesel conversion of 57.2%. In another study, Rhizomucor miehei lipase was utilized in the biodiesel production from triolein, which under optimal process conditions (5.0 wt.% catalyst, 3:1 MREO, and 40 °C in 96 h) achieved maximum biodiesel yield of 77% [53]. Elsewhere, Kamini et al. [54] utilized Cryptococcus spp. S-2, as biocatalyst for transesterification of rice bran oil in the presence of methanol. The Cryptococcus spp. S-2 catalyst showed excellent transesterification performance, giving a maximum biodiesel yield of 80.2% under the following conditions: temperature T= 30 °C, time t = 120 h, and 4:1 MRMO. Martin Mittelbach [55] examined the catalytic performance of Pseudomonas lipase for the production of biodiesel from sunflower oil in the presence of methanol. The author found that a maximum biodiesel yield of 80% was obtained following the addition of oleic acid to sunflower oil using Pseudomonas lipase enzyme under the optimum process conditions (time t = 5 h, 10:1 MRMO, and temperature T= 45 °C). Alonazi et al. [56] transesterified coffee oil over CaCO3- immobilized Staphylococcus aureus and Bacillus stearothermophilus biocatalyst using methanol under reaction conditions of temperature T= 55 °C, time t = 12 h, 10 wt.% of enzyme catalyst, and 4:1 MRMO; a maximum biodiesel conversion of 97.66% was obtained using this combination of immobilized lipases. Table 1 depicts a comparison of various biocatalysts utilized in several research studies, in terms of biodiesel yield, process conditions, and feedstock.

Table 1.
Some studies on biodiesel production using biocatalysts.
3.1.1. Homogeneous Catalysts Utilized in Biodiesel Production
Conventionally, homogeneous catalysts are employed in biodiesel production from various oils. For industrial biodiesel production, the most commonly utilized catalyst is a homogeneous catalyst, as it is easy to use and the biodiesel product is obtained in less time. Homogeneous catalysts, which generally may be liquid or gaseous, and reactants are both in the same phase [57,58]. Homogeneous catalysts are classified into two categories: acidic catalysts and alkaline catalysts [59].
3.1.2. Homogeneous Base Catalysts
For triglyceride transesterification, homogeneous base catalysts are typically utilized. The base catalysts are metal-based oxide and alkaline liquids such as potassium methoxide, sodium methoxide, carbonates, sodium hydroxide, and barium hydroxide [60]. The catalytic activity of homogenous base catalysts is very high in transesterification [60]. Homogeneous base catalysts are currently the most widely used method for large-scale biodiesel production. This method offers several advantages, including high catalytic activity and the ability to achieve complete conversion of triglycerides to biodiesel [61]. Due to these benefits, homogeneous base catalysts have become the preferred choice in industrial settings, significantly contributing to the efficiency and scalability of biodiesel production processes [61]. Metallic hydroxide is often used as homogenous base catalysts in transesterification because of lower prices, but they usually have lower activity in transesterification than alkoxide [62]. In transesterification reactions, alkoxide species are highly basic and have a high activity which then attacks the triglyceride carbonyl group to form long-chain fatty acid (biodiesel) and glycerol [7].
Many researchers have studied the homogeneous base-catalyzed transesterification reactions of triglycerides. In 2008, Rashid et al. [63] used homogeneous base catalyst potassium hydroxide (KOH) to produce rapeseed oil biodiesel. The authors discovered that potassium hydroxide can achieve a yield of 96% with a 1 wt.% catalyst loading. Phan et al. showed that potassium hydroxide can produce promising results with maximum biodiesel yields of up to 90% at a 0.75 wt.% catalyst concentration [64]. However, when the catalyst amount was raised to 1.5 wt.% potassium hydroxide, only 75% of biodiesel yield was achieved. This declining trend is explained by saponification, which happens when catalyst loading is high, resulting in a lower biodiesel yield and increases reactant viscosity. The performance of different KOH catalyst loading in the transesterification of croton megalocarpus oil was examined by Kafuku et al. [65]. They observed that the use of a 0.50 wt.% catalyst loading produced a fairly low biodiesel conversion (50%). However, the biodiesel conversion increased significantly and reached a maximum value of 88% at a catalyst amount of 1.0 wt.%. Furthermore, they discovered that increasing the catalyst loading above the optimal catalyst concentration of 1.0 wt.% reduced biodiesel conversion, and soap was discovered in greater quantities due to excess catalysts that favor the saponification process.
Sivakumar et al. [66] carried out research to optimize biodiesel yield using KOH catalyst. Their results showed that 1.2 wt.% KOH catalyst at 75 °C can be considered as an optimum condition for transesterification of waste scum. A yield of 96.7% was achieved for these process conditions in 30 min. KOH catalyst was utilized by Dueso et al. in the catalytic transesterification of sunflower oil [67]. Under the reaction conditions of 60 °C temperature, 1 wt.% catalyst loading and 6:1 molar ratio of methanol to oil (MRMO), a biodiesel yield of 98.6% was obtained in 3 h with the KOH catalyst.
Transesterification of Elaeagnus angustifolia seed oil in methanol utilizing potassium methoxide catalyst was investigated by Kamran et al. [68]. At reaction conditions of temperature T= 60 °C, time t = 60 min, 1.0 wt.% of catalyst, and 9:1 MRMO, the optimal biodiesel yield of 95% was achieved. Also, in the transesterification of rice bran using sodium hydroxide (NaOH) catalyst, 72.8% yield was obtained at 0.9 wt.% NaOH catalyst, a temperature of 60 °C in 2 h at 6:1 MRMO [69].
When compared to homogeneous acid-catalyzed processes, the homogeneous base-catalyzed process with sodium methoxide and potassium methoxide catalyst and edible oils like sunflower, soybean, and canola is four thousand times faster than that of homogenous acid-catalyzed reaction [62]. The faster reaction rate is due to the higher efficiency of base catalysts in transesterification reactions, particularly with edible oils. Base catalysts enhance the reaction rate by facilitating the formation of reactive intermediates more rapidly, which accelerates the overall transesterification process compared to acid catalysts [62]. The base catalyst may facilitate a more efficient reaction pathway or lower activation energy compared to the acid catalyst, leading to a faster reaction rate. Unfortunately, this approach has a few drawbacks, including the fact that the catalyst is not recycled, and must be removed from the product streams [58]. The catalyst can also react with oil impurities to produce soap, especially free fatty acids [59]. The problem of soap production during base-catalyzed transesterification is a recognized challenge. To address this issue, several strategies can be employed. First, feedstocks with high free fatty acid (FFA) content are often pre-treated with acid catalysts. This pre-treatment converts FFAs into esters, thereby reducing the potential for soap formation in the subsequent base-catalyzed transesterification [70]. Additionally, the use of advanced base catalysts, which are designed to tolerate higher levels of FFAs, can effectively minimize soap production by enhancing the efficiency of the transesterification process and reducing undesired side reactions [71]. Optimizing reaction conditions, such as temperature, catalyst concentration, and the methanol-to-oil ratio, also helps in reducing soap formation [72]. Finally, post-reaction treatments, including washing the biodiesel with water or using acid washes, can remove soap and other impurities from the final biodiesel product, ensuring its purity and quality [61]. These strategies collectively address the issue of soap production and improve the overall efficiency of the transesterification process. The effective use of homogeneous base catalysts is confined to refined oils containing a FFAs of less than 0.5 weight percent [59]. In addition, these catalysts are removed after the reaction is complete by washing the biodiesel with water, which could lead to the loss of alkyl esters, consumption of energy, and the production of significant quantities of wastewater [73]. Therefore, it is important to explore the possibility of replacing homogeneous base catalysts with some other suitable transesterification reaction catalysts in order to establish a low-cost and environmentally friendly biodiesel production method.
3.1.3. Homogeneous Acid Catalysts
The homogeneous acid-catalyzed transesterification process offers a considerable advantage over homogeneous base-catalyzed transesterification because presence of FFAs in the feedstock has no effect on the acid catalyst and both esterification and transesterification can be catalyzed at the same time [74]. The most widely used catalysts for acid-catalyzed transesterification are sulfuric acids, hydrochloric acids, and sulfonic acids [74]. Homogeneous acid-catalysts produce very high alkyl ester yields. However, compared to base-catalyzed transesterification reactions, the reactions are slower, making the process costly as a result of high energy consumption [75]. Acid-catalyzed transesterification reaction has the same problems of separation as the base-catalyzed process.
To optimize biodiesel production, several researchers have made attempts to investigate the homogeneous acid-catalyzed transesterification of triglycerides. Freedman et al. [76] conducted extensive research on the catalytic activity of sulfuric acid (H2SO4) in the production of soybean oil biodiesel. They demonstrated that a biodiesel conversion of >90% can be obtained with a 1 mol % catalyst and 30:1 MRMO for 69 h. Using sulfuric acid and hydrochloric acid catalysts, biodiesel was produced from castor oil by Meneghetti et al. [77]. The authors found that biodiesel can be produced by transesterifying castor oil using ethanol or methanol. Using either ethanol or methanol, they obtained almost similar biodiesel yields, but they noticed that when methanol was used, the transesterification process proceeded very fast. Crabbe et al. [78] transesterified crude palm oil with methanol over H2SO4 catalyst. The authors found that a biodiesel yield of 97% can be achieved using a 5 wt.% catalyst and 40:1 MRMO at 95 °C in 9 h. Similarly, Wang et al. [79] used an H2SO4 catalyst for WCO transesterification. The authors observed that the sulfuric acid catalyst shows enhanced transesterification activity and provided a maximum methyl ester conversion of 90% at a reaction temperature of 95 °C, 20:1 MRMO, and sulfuric acid loading of 4 wt.% in 10 h. The homogeneous acid-catalyzed biodiesel production process is effective to convert low-cost feedstock to biodiesel, but not so economical because large quantities of catalysts and a long reaction time are required to obtain a high biodiesel yield. Homogeneous acid-catalysts are preferable for low-cost feedstock due to their insensitivity to the presence of FFAs and water, while FFAs and water, if present in the feedstock, have an effect on homogeneous base catalysts. Therefore, it is very important to search for a catalyst that has the potential of overcoming issues related to homogeneous catalyst biodiesel production for sustainable energy processes. Table 2 depicts a comparison of various homogeneous catalysts utilized by several research studies to convert different feedstock into biodiesel.

Table 2.
Some studies on biodiesel production utilizing homogeneous catalysts.
3.2. Heterogeneous Catalysts Used for Biodiesel Production
Heterogeneous catalysts are in different phases compared to the reactants and products [80]. Because of their availability and short reaction time, homogeneous catalysts are becoming more common in the biofuel industry [81]. However, when it comes to esterification and transesterification reactions, the use of a homogeneous catalyst causes many problems. For instance, when sulfuric acid is used in esterification, it causes reactor corrosion and high sulfur content in biodiesel products. For homogeneous base catalysts such as KOH and NaOH, product separation, liquid catalyst recovery, and product washing are required through the acid neutralization steps, which could lead to the production of a high amount of wastewater and higher energy consumption, thereby increasing the biodiesel production costs. It is expected that using heterogeneous catalysts as opposed to homogeneous catalysts will overcome the issues related to homogeneous catalysts. When compared to homogeneous catalysts, heterogeneous catalysts offer various benefits, including easy regeneration, elimination of product washing step, less corrosiveness, efficient conversion, environmental friendliness, and fewer disposal issues. Heterogeneous catalysts can handle multiple feedstocks in a single step [82]. However, the major problems of heterogeneous catalysis in biodiesel production include a higher ratio of alcohol to oil and higher temperature. Another disadvantage of heterogeneous catalysts is that they react with alcohol and oil to form three phases, which limits dispersion and hence reduces reaction rate [82]. The difficulty of mass transfer is resolved by the use of co-solvents such as heptane, ethanol, and tetrahydrofuran, which enables oil and alcohol miscibility, as a result of which the reaction rate is increased [83]. The use of catalyst supports is another way to overcome mass transfer difficulty [84]. The supports offer the active species increased pores and precise surface area in which they can attach and react with bulky triglycerides [84]. Heterogeneous catalysts are subdivided further into two categories: acidic catalysts and alkaline catalysts [80].
3.2.1. Heterogeneous Base Catalysts
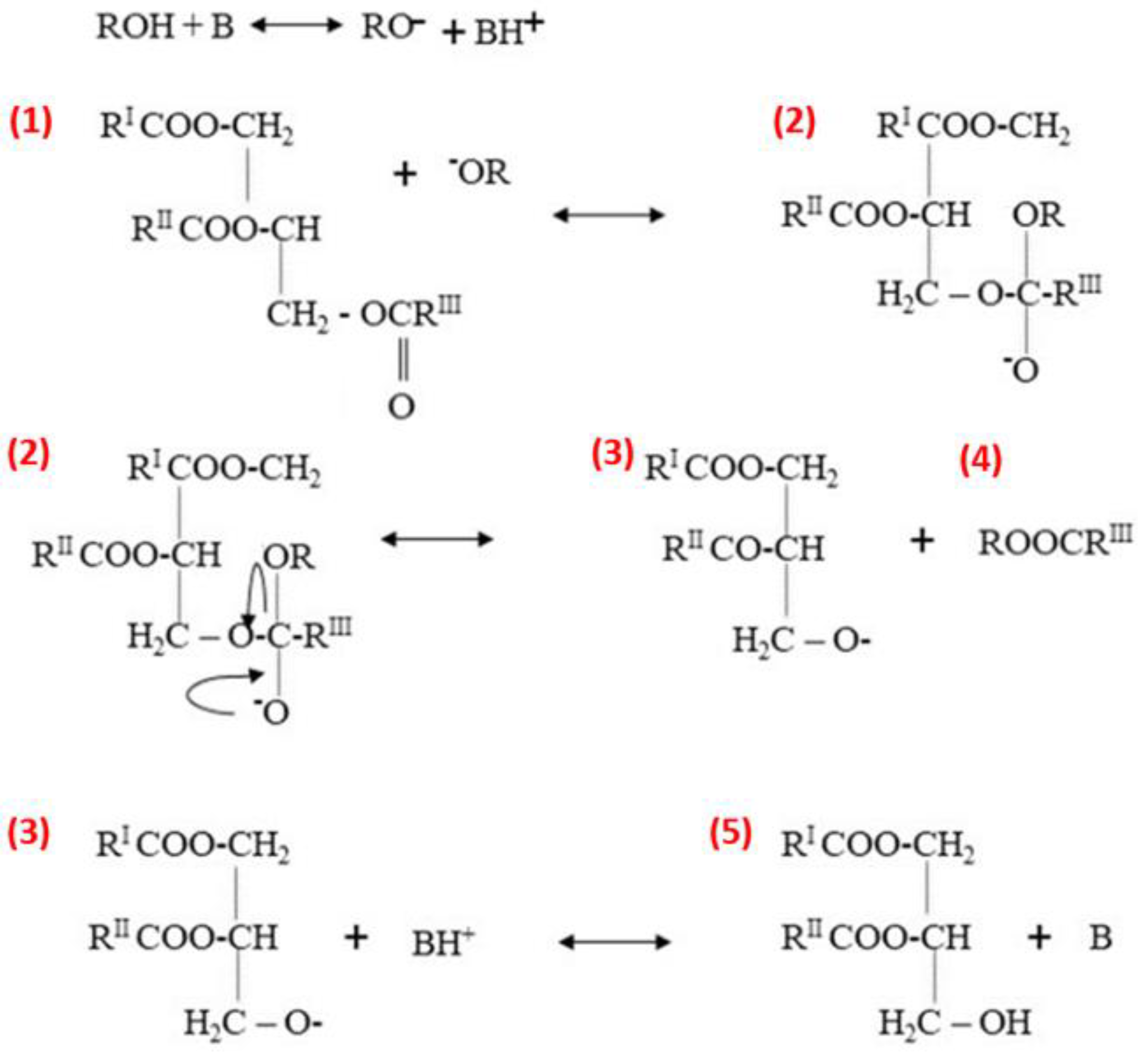
Heterogeneous base catalysts with alkaline properties on their surface are excellent candidates to substitute a homogeneous base catalyst for the transesterification process. Heterogeneous base catalysts have the tremendous potential to address the major challenges encountered in the biodiesel production industry. These catalysts offer several advantages, which include easy regeneration, environmental friendliness, and fewer disposal issues [85]. Furthermore, during heterogeneous base-catalyzed transesterification reactions, only a small amount of wastewater is produced. Heterogeneous base catalysts are typically more resistant to FFAs and water in the economical feedstock, which includes waste cooking oil and fat as compared to homogeneous base catalysts [86]. Longer catalyst life, renewability, selectivity, and higher activity can all be achieved with heterogeneous base catalysts [87]. Figure 5 illustrates the mechanism of base-catalyzed transesterification. Reactions involving heterogeneous bases proceed through interactions between the Lewis or Brønsted basic sites of the catalyst and a monohydric alcohol, typically methanol or ethanol. The resulting alkoxide mixture then reacts with the triglyceride esters in the oil, ultimately producing biodiesel and glycerol through a series of steps [88]. Heterogeneous base catalysts are further subdivided into six groups. Supported alkali earth metal or supported alkaline earth metal, hydrotalcite, zeolite, metal oxide, mixed metal oxide, and non-oxide are the six groups of heterogeneous base catalysts [89].
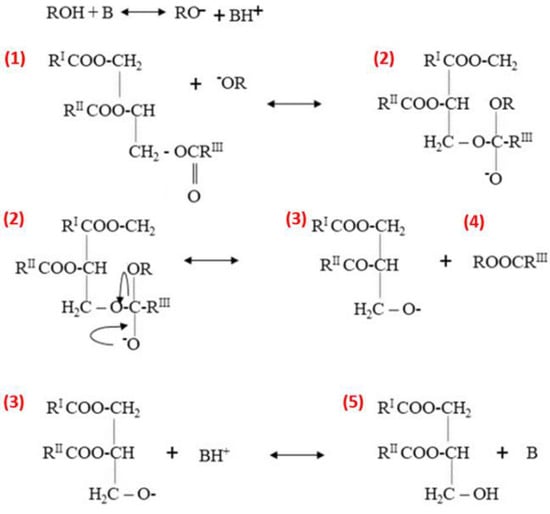
Figure 5.
Heterogeneous base catalysis reaction mechanism. Adapted from ref. [88].
Several heterogeneous base catalysts for biodiesel production have been documented in the literature over the last few decades. Liu et al. [90] transesterified soybean oil using strontium oxide (SrO) catalyst. The authors found that a biodiesel yield of 95.0% can be obtained with 12:1 MRMO and 3 wt.% catalyst at 65 °C for 30 min. Moreover, after ten cycles of reusing strontium oxide catalyst, the biodiesel yield was only reduced marginally. Kim et al. [91] transesterified soybean oil with methanol over Na/NaOH/γAl2O3 catalyst. The biodiesel yield achieved was 94% at 1 wt.% catalyst loading and 9:1 MRMO at 60 °C after 2 h. Liu et al. [87] documented the use of calcium oxide (CaO) catalyst in the production of biodiesel. The specific surface area (m2/g) of the catalyst was determined using the Brunner–Emmett–Teller (BET) technique. The total surface area of a solid catalyst was measured by BET surface area analyzers. A powdered analyte was suspended in an inert gaseous bath, and the surface adsorption of gas molecules was measured. The CaO catalyst BET surface area was 0.56 m2/g. At a process condition of T= 65 °C, t = 3 h, 8 wt.% of CaO catalyst, and 12:1 MRMO, a maximum biodiesel yield of 95% was achieved. They also investigated the comparative activity of calcium oxide with K2CO3/γAl2O3 and KF/γAl2O3 solid catalyst. They found that the CaO catalyst retained its activity after repeated use for 20 cycles and the yield remained unaffected, while the biodiesel yield was reduced when K2CO3/γAl2O3 and KF/γAl2O3 solid catalysts are subsequently reused for 20 cycles. This is due to the dissolution of alkali metal compounds in alcohol, which decreases the catalytic activity, and thus reduces the yield in the subsequent reaction.
The activity of a CaO catalyst in biodiesel production from sunflower oil was investigated by Granados et al. [92]. They demonstrated that with a catalyst amount and temperature of 1.0 wt.% and 60 °C, respectively, and 13:1 MRMO in 90 min, a biodiesel yield of 94% was achieved. The BET pore size diameter of the CaO catalyst was 30 nm and the surface area of the CaO catalyst was 32 m2/g. During their investigation, they found that the catalyst’s active site was poisoned by atmospheric gases (H2O and CO2). Consequently, the catalyst was subjected to high-temperature activation treatment before reaction to enhance its activity. Even though the key poison species, the carbonate group, was removed from the surface by this treatment, active species leaching was also observed. But the amount of leaching did not result in a substantial decrease in activity and the catalyst retained its activity after repeated use for eight cycles [59]. However, biodiesel conversion reduced from 94% in the first cycle to 80% in the second cycle, and the yield remained unaffected after that. Veljkovic et al. [93] studied the kinetics of calcium oxide catalyst in the transesterification of sunflower oil using methanol. A biodiesel yield of 98% was achieved with 1 wt.% catalyst content at 60 °C, and 6:1 MRMO in 2 h reaction time. Kaur et al. [94] used a lithium-doped calcium oxide (Li/CaO) catalyst in the production of jatropha and karanja oil biodiesel. X-ray diffraction, BET, and transmission electron microscopy were used to characterize the catalyst. Under optimized reaction conditions of 12:1 MRMO and 5 wt.% catalyst at 65 ℃, the catalyst produced the highest conversion of >99% in 1 h and 2 h reaction time for karanja and jatropha oil, respectively. Kouzu et al. [95] transesterified soybean oil utilizing CaO catalyst and methanol. The authors found that the catalyst shows excellent transesterification performance, where a biodiesel yield of 93% was obtained with 0.78 g of catalyst, a reaction time of 1 h, and a mixing speed of 500 rpm.
Mootabadi et al. [96] evaluated the performance of different catalysts in the production of palm oil biodiesel: calcium oxide (CaO), strontium oxide (SrO), and barium oxide (BaO). They recorded biodiesel yields of 95.2% (for BaO), 95.2% (for SrO), and 77.3% (for CaO) using a 3 wt.% catalyst in 1 h. The catalysts activity order was BaO > SrO > CaO. In another related study, Salamatinia et al. [97] studied the performance of BaO and SrO in the production of palm oil biodiesel. Biodiesel yields of 69.44% and 66.93% were achieved using 1 wt.% catalyst loading at 50 °C, and 9:1 MRMO in 30 min reaction time for BaO and SrO, respectively. They reported that the optimized biodiesel yields obtained with MRMO of 9:1 and catalyst loading of 3.0 wt.% in 50 min for SrO and BaO were >94% and 95.17%, respectively. Laskar et al. [98] employed waste snail shells as a heterogeneous base catalyst in the production of soybean oil biodiesel. This approach reduces the overall production costs and proves environmentally friendliness. The waste snail shells’ main component calcium carbonate (CaCO3) is transformed into calcium oxide (CaO) catalyst by calcination in air for 5 h at 900 °C [98]. They obtained 98% biodiesel yield from soybean oil using 6:1 MRMO at 28 °C and 3.5 wt.% catalyst amount in 7 h reaction time. Moreover, the catalyst retained its activity after reuse for eight reaction cycles. Also, Viriya-empikul et al. [99] used waste eggshells as a heterogeneous catalyst in biodiesel production from palm olein oil. The waste eggshells major component CaCO3 is transformed into CaO catalyst by calcination in the air for five hours at 800 °C. With a 10 wt.% catalyst and 18:1 MRMO, a biodiesel yield of >90% was achieved at 60 °C for 2 h. Nair et al. [100] investigated the use of clamshell as a catalyst in the transesterification of waste frying oil for biodiesel production. The CaO catalyst, which was synthesized from clamshell successfully transesterified waste frying oil, and >89% of biodiesel yield was obtained with 6.03:1 MRMO at 60 °C and 3 wt.% catalyst for 3 h. Birla et al. [101] reported an 87.28% biodiesel yield from WCO with 8.45:1 MRMO, 2 wt.% catalyst loading at 60 °C in 7 h using a heterogeneous base catalyst such as snail shells.
Xie et al. [102] transesterified soybean oil using NaX Zeolite/KOH heterogeneous solid base catalyst. The catalyst showed good transesterification performance, where a maximum biodiesel yield of 85.6% was obtained with 10:1 MRMO and 3.0 wt.% of catalyst loading at 65 °C for 8 h. In the transesterification of soybean oil with methanol, Fabbri et al. [103] employed a Na2PEG (300), obtained by treating PEG 300 (polyethylene glycol of molecular weigh 300 Da) with methanolic sodium methoxide solution, as a base catalyst in the production of soybean oil biodiesel with dimethyl carbonate. According to these authors, the catalyst provided a conversion of approximately 99% at 5 wt.% catalyst amounts and 90 °C reaction temperature after 5 h reaction time. Elsewhere, Tan et al. [104] transesterified used cooking oil over chicken eggshells and ostrich eggshells as a catalyst. A biodiesel yield of 94% and 96% were obtained with calcined chicken eggshells and calcined ostrich eggshells, respectively, using 1.5 wt.% catalyst loading at 65 °C, and 12:1 MRMO in 2 h reaction time. Also, Xu et al. [105] investigated the catalytic performance of KF/Zn(Al)O catalyst in making biodiesel from soybean oil. The synthesized catalyst is more reactive than that of Zn-Al hydrotalcite. In their study, the reaction was conducted for 3 h with 3 wt.% catalyst and 6:1 MRMO at 65 °C. A biodiesel yield of about 95% was achieved with these conditions within 3 h.
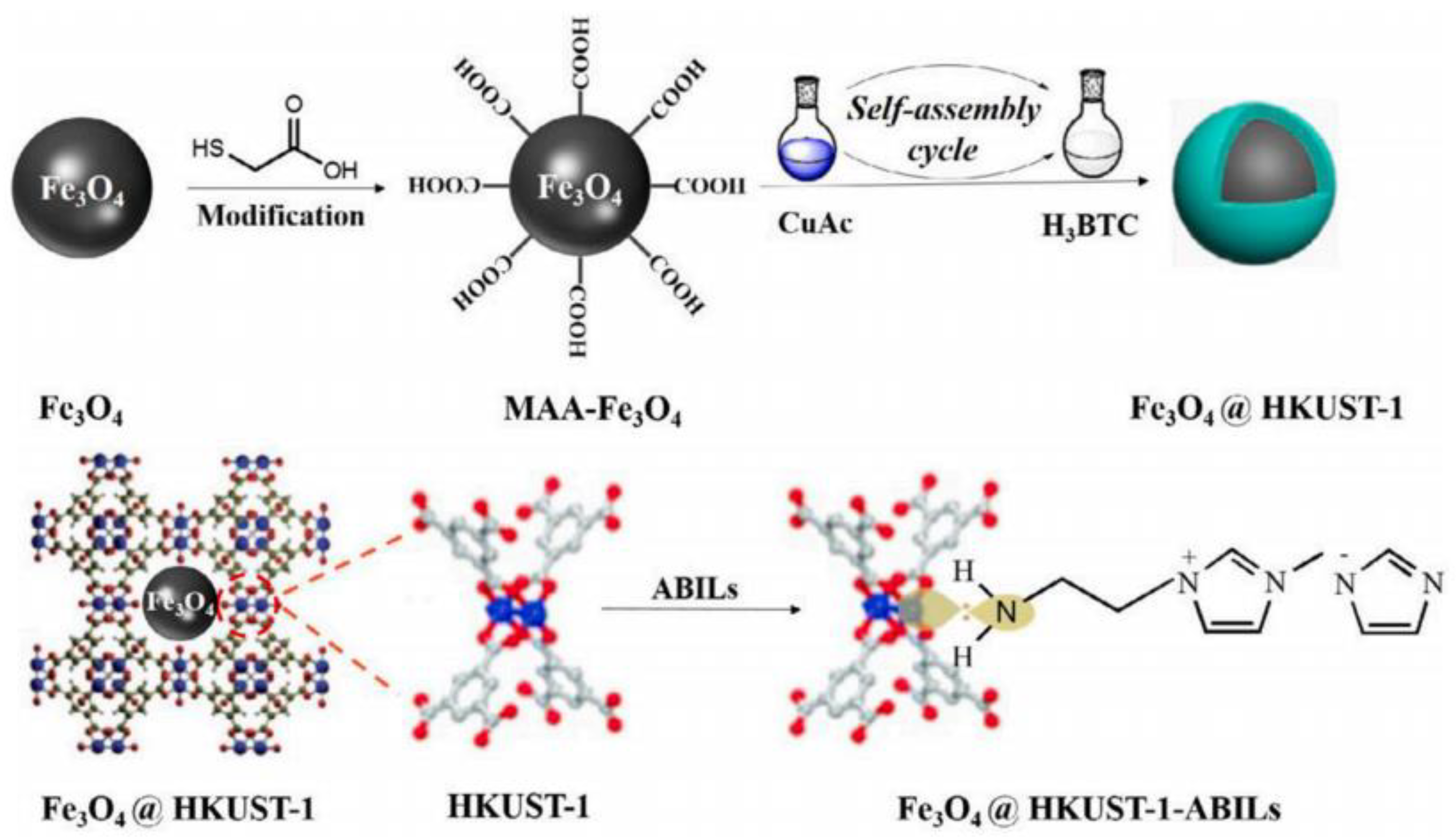
Navajas et al. [106] studied Mg-Al hydrotalcite catalytic activity in the transesterification of sunflower oil. The co-precipitation method was used to produce the catalyst with a Mg:Al molar ratio of 5. They obtained a 92% biodiesel yield at 60 °C temperature, 48:1 MRMO, and 2 wt.% catalyst loading for 24 h. They attributed the Mg-Al hydrotalcite catalytic activity to the existing basic sites at the crystal edge. Trakarnpruk et al. [107] reported a high biodiesel yield by utilizing a K-loaded Mg-Al hydrotalcite catalyst in palm oil biodiesel production. The authors conducted the reaction with 7.0 wt.% catalyst and 30:1 MRMO at 100 °C for 7 h. A biodiesel yield of approximately 86.6% was obtained with these conditions. Hernandez et al. [108] transesterified sunflower oil and WCO over Na-Mg-Al hydrotalcite catalyst. The optimum MRMO was 15:1 for sunflower oil and 9:1 for WCO. The catalyst produces the highest yield of 88% and 67% for sunflower oil and WCO, respectively, at 60 °C for 8 h using a 7 wt.% catalyst (hydrotalcite impregnated with 5% of sodium for sunflower oil and 10% of sodium for WCO). Yan et al. [109] used Zn3La1(Lanthanum modified ZnO) for biodiesel production from WCO. A yield of 93.7% was reported with 3 wt.% catalyst and 26:1 MRMO at 200 °C for 3 h. Agarwal et al. [110] transesterified WCO with KOH/Al2O3 catalyst. They reported that the catalyst showed excellent transesterification performance, where a yield of 96.8% was obtained with catalytic loading of 15 wt.% and 9:1 MRMO at 70 °C for 2 h. Moreover, when KOH/Al2O3 catalyst was subsequently reused for three cycles, the catalyst resulted in a reasonable conversion rate (83%). Dehkordi et al. [111] reported a 92.1% biodiesel yield from WCO with 10.0 wt.% catalyst and 30:1 MRMO at 65 °C in 2 h using CaO-ZrO2 catalyst. They observed that the stability of CaO-ZrO2 catalyst declined as the Ca/Zr molar ratio increased, such that high stability was attained with a catalyst prepared with a low concentration of CaO. Xie et al. [112] investigated the catalytic activity of a Fe3O4@HKUST-ABIL composite in producing biodiesel from soybean oil. The core–shell structured Fe3O4@HKUST-ABIL catalyst was prepared utilizing layer-by-layer assembly method (Figure 6). The highest biodiesel yield of 92.30% was achieved with a catalyst loading of 1.2 wt.% and 30:1 MRMO at 65 °C for 3 h. There is no significant change in the catalytic activity of the Fe3O4@HKUST-ABIL composite after five reaction cycles.
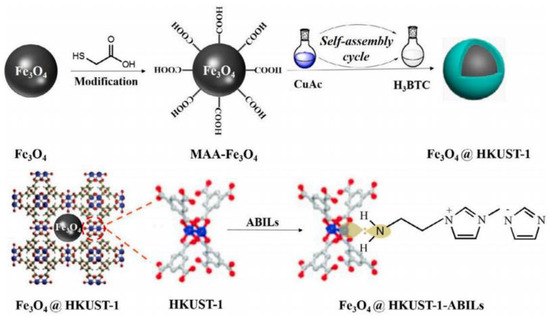
Figure 6.
Preparation of Fe3O4@HKUST-ABIL composite. Reprinted from Ref. [112].
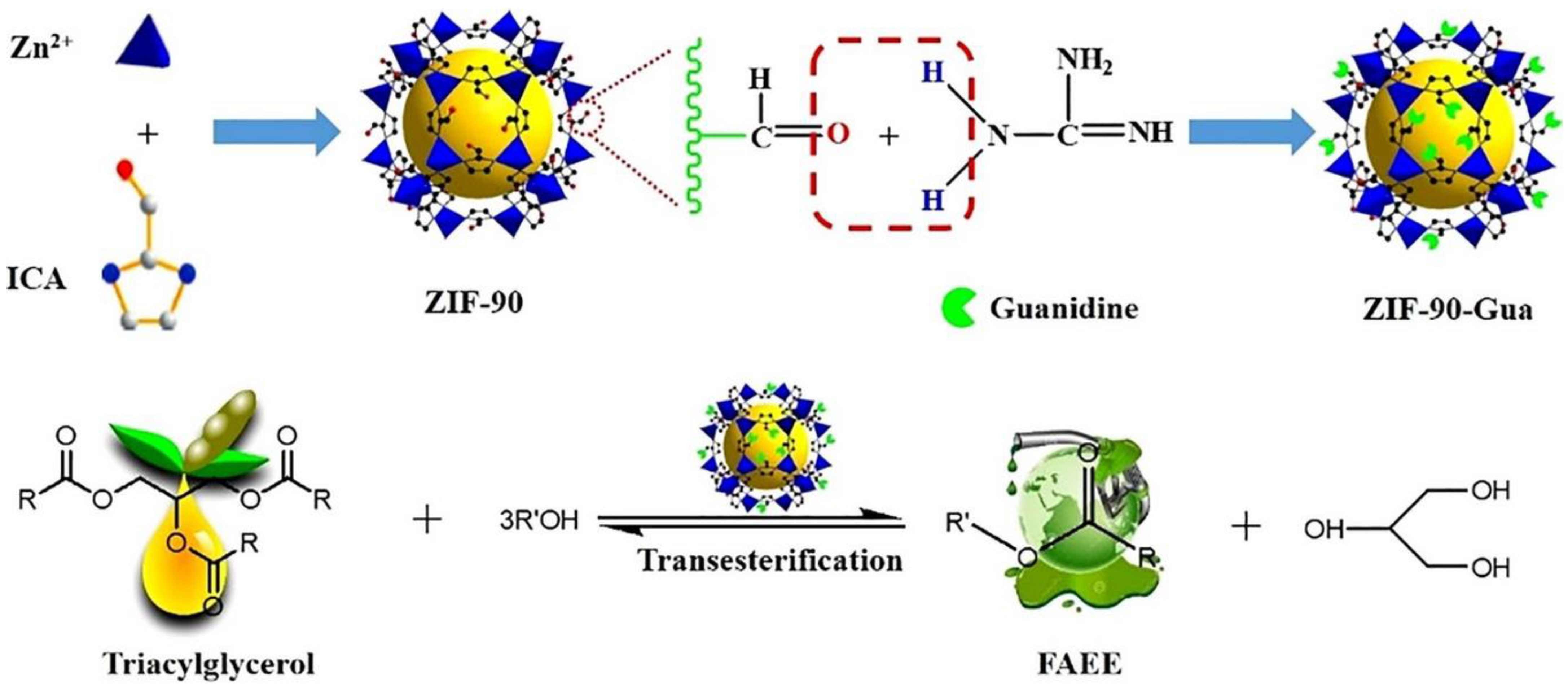
Similarly, Xie et al. [113] transesterified soybean oil over zeolitic imidazolate framework ZIF-90 with organic guanidine (ZIF-90-Gua) (Figure 7). The solid catalyst has long-term catalytic activity because guanidine is covalently bound to the support. The catalytic activity of the ZIF-90-Gua towards transesterification was evaluated under the optimum reaction conditions of T= 65 °C, t = 6 h, 1 wt.% of ZIF-90-Gua catalyst, and 15:1 MRMO, and a maximum biodiesel yield of 95.40% was achieved. The ZIF-90-Gua catalyst was easily recovered by filtration and reused five times without substantial loss of catalytic activity, indicating that it has great potential to be used as an efficient catalyst for biodiesel production.
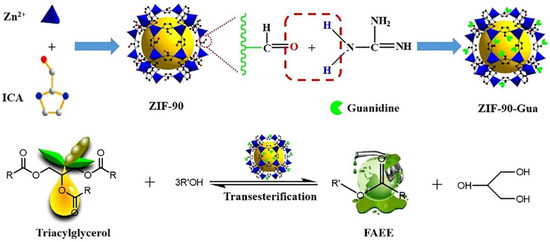
Figure 7.
Synthesis of ZIF-90-Gua catalyst. Reprinted from Ref. [113].
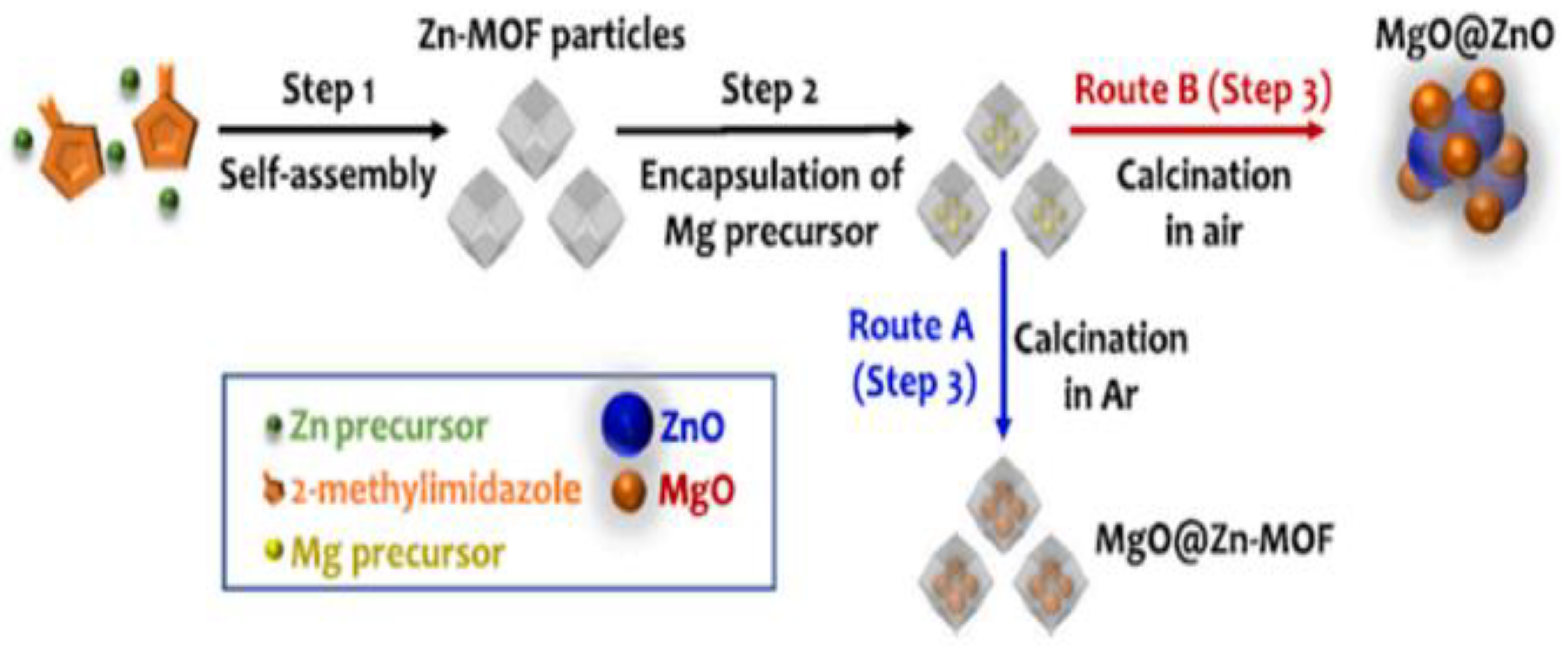
Abdelmigeed et al. [114] reported NaOH/magnetized ZIF-8 catalyst in synthesizing biodiesel from vegetable oil. A 99.80% vegetable oil yield was achieved with an MRMO of 21:1 and a catalyst loading of 3 wt.% at 65 °C for 1 h. Moreover, ASTM standard testing methods indicated that the produced biodiesel under optimal reaction conditions can be used as sustainable biodiesel fuel. In addition, the experimental data was well-fitted by the pseudo-second order kinetic model, as revealed by the kinetic study. Saeedi et al. [115] synthesized a sodium–zeolite imidazolate framework (ZIF-8) doped with potassium (KNa/ZIF-8) via sol–gel processing and investigated catalyst activity in the production of biodiesel from soybean oil. The addition of potassium to Na/ZIF-8 was aimed at enhancing its basicity, thereby leading to an improved catalytic performance in the production of biodiesel from soybean oil with methanol. The results show that KNa/ZIF-8 exhibited outstanding performance due to the highest number of basic sites in this sample and high surface area (around 1195 m2/g). The highest soybean oil yield of 98% was obtained using the KNa/ZIF-8 catalyst with potassium loading of 0.08% under an optimal reaction condition of MRMO of 10:1 and catalyst loading of 0.012 wt.% at 100 °C for 3.5 h. The catalyst demonstrated high stability and there was no significant loss in catalytic activity after reused for three cycles. Li et al. [116] prepared strontium oxide supported on MIL-100(Fe) catalyst [SrO-MIL-100(Fe)] using an in situ titration method (ST) and a mechanical mixing method (MM), and evaluated their performance in making palm oil biodiesel. The results show that MM-SrO exhibits the excellent activity, where a maximum biodiesel yield of 96.19% was achieved with an MRMO of 12:1 and a catalyst loading of 8 wt.% at 65 °C for 30 min. Moreover, when SrO-MIL-100(Fe)] catalyst was subsequently reused for three cycles, the catalyst resulted in a reasonable biodiesel yield (82.49%). Yang et al. [117] synthesized metal–organic framework-derived Mg-Zn hybrid catalysts (MgO@Zn-MOF) and investigated their performance in making biodiesel from soybean oil. As shown in Figure 8, Mg-Zn hybrid nanocatalysts were successfully synthesized under air and argon. The optimized reaction was performed with 1 wt.% catalyst and 3:1 MRMO at 210 °C for 2 h, and a maximum yield of 73.30% of the feedstock was obtained.
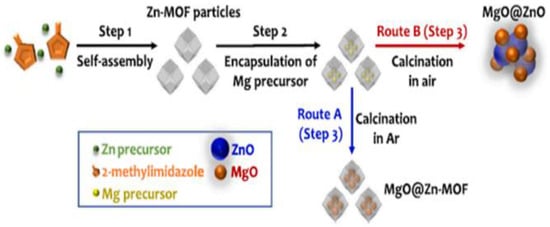
Figure 8.
Synthesis of two types of MOF-derived Mg-Zn hybrid nanostructures: (a) Route A (in Argon): MgO nanoparticles homogeneously encapsulated in Zn-MOF nanocrystal clusters (MgO@Zn-MOF). (b) Route B (in air): MgO nanoparticles uniformly decorated on ZnO nanoparticles (MgO@ZnO). Reprinted from Ref. [117].
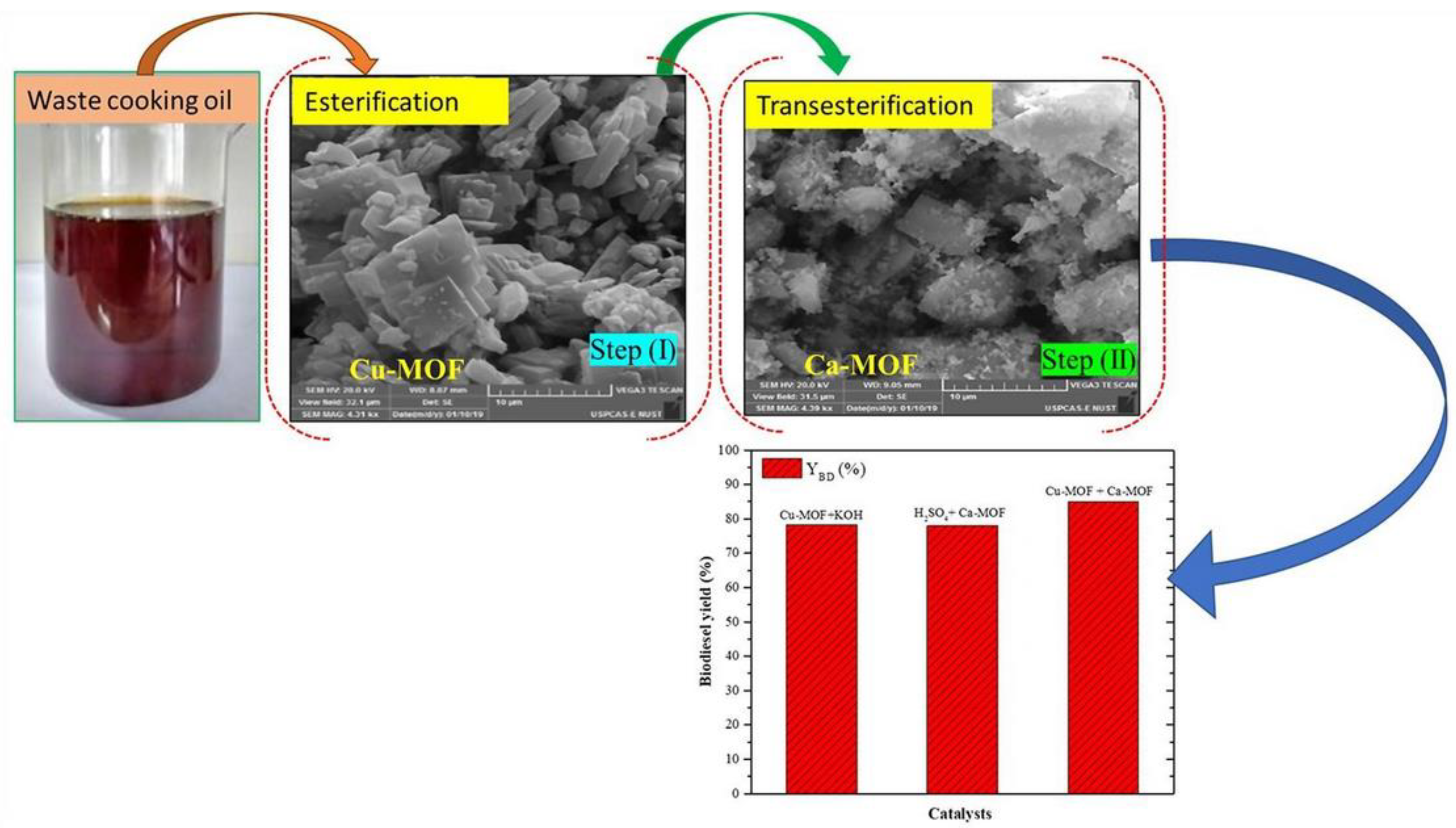
Fazaeli et al. [118] employed ZIF-8@GO doped with potassium and sodium (KNa/ZIF-8@GO) for the biodiesel transesterification process. The biodiesel yield reached 98% at 100 °C temperature, 18:1 MRMO, and 8 wt.% catalyst loading for 8 h. Furthermore, when the KNa/ZIF-8@GO catalyst was subsequently reused for three cycles, there was no appreciable loss in the catalytic activity of the KNa/ZIF-8@GO catalyst. Jamil et al. [119] transesterified WCO containing a high amount of FFA using Cu- and Ca-based metal–organic framework catalysts (Figure 9). The author found that a biodiesel yield of 84.5% can be obtained with 20:1 MRMO and 1 wt.% catalyst at 60 °C for 30 min. Moreover, after three cycles of reusing Cu- and Ca-based MOF catalysts, the biodiesel yield was reduced up to 7%.
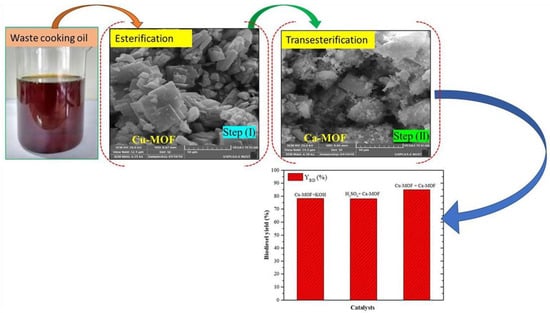
Figure 9.
Biodiesel transesterification process using Cu- and Ca-based MOF catalysts. Reprinted from Ref. [119].
In view of the literature presented above, it may therefore be argued that the heterogeneous base-catalyzed method is an efficient method of producing biodiesel with low cost and negligible environmental consequences. However, this process is sensitive to the FFA composition of feedstock used to produce biodiesel. Hence, the inability of heterogeneous base catalysts to withstand high FFAs of the economical feedstock under mild reaction conditions is the main limitation associated with their development. Table 3 shows a comparison of different heterogeneous base catalysts utilized by several research studies, in terms of biodiesel yield, process conditions, and feedstock.

Table 3.
Some studies on biodiesel production utilizing heterogeneous base catalysts.
3.2.2. Heterogeneous Acid-Catalysts
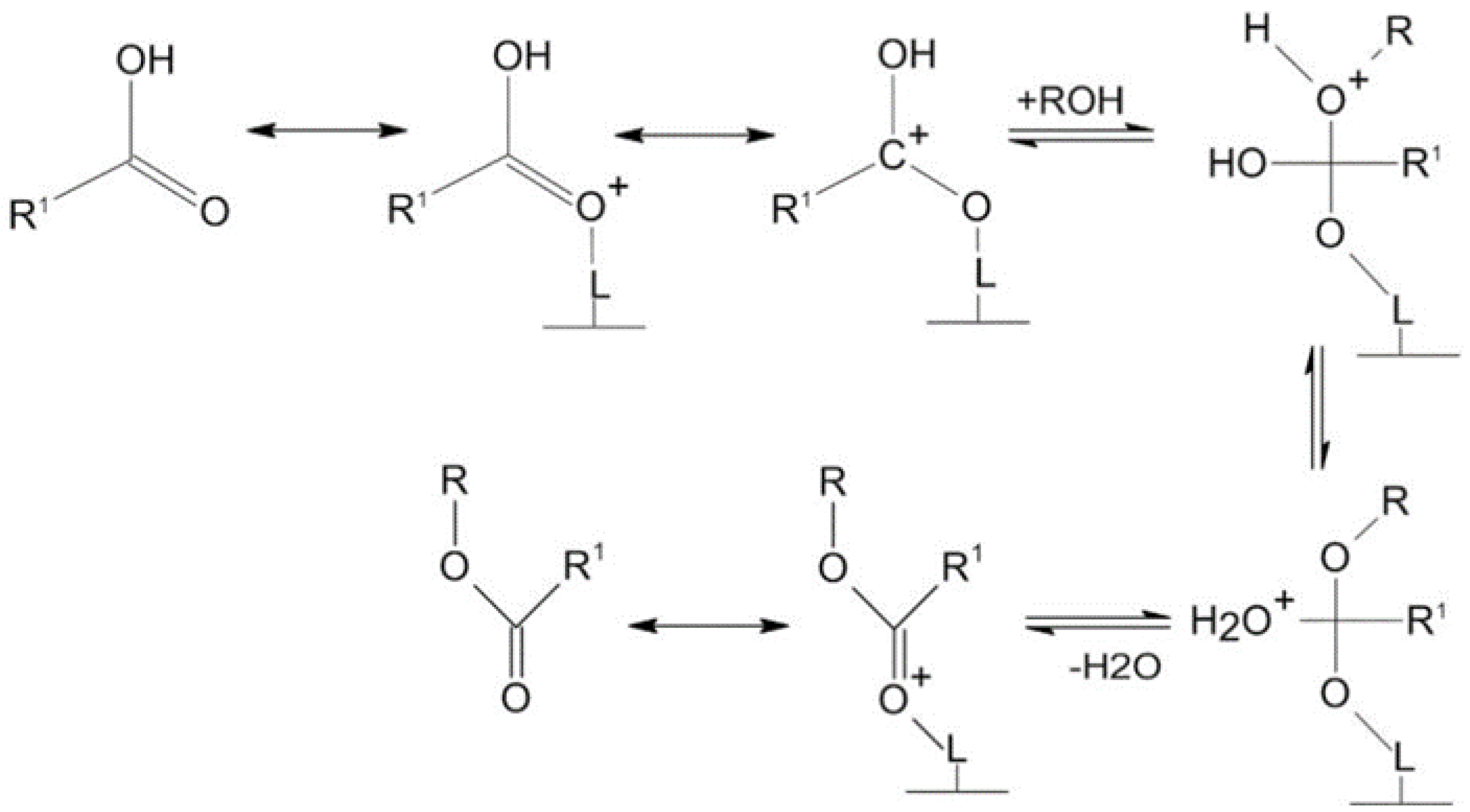
Heterogeneous acid catalysts have an enormous capacity to carry out both esterification of free fatty acid and transesterification of triglycerides simultaneously using low-cost feedstock, thus lowering the cost of producing biodiesel. Heterogeneous acid catalysts can usually withstand more extreme operating conditions and cause less corrosion problems compared to homogeneous acid catalysts [120]. These catalysts have many acid sites of various Brønsted or Lewis acidity strengths. Biodiesel is usually produced from feedstock with high FFAs using heterogeneous acid catalysts. However, the benefits of heterogeneous acid catalysts include easy separation from the finished product and their being recyclable for repeated use. Therefore, heterogeneous acid catalysts offer a more economical path to the biodiesel production process [121,122]. Nevertheless, to achieve good conversion yield, higher temperatures, higher catalyst loading, and longer reaction times are required for heterogeneous acidic catalysts [123]. Also, in some cases, soap formation is observed using heterogeneous acid catalysts, and purification is required in most cases to ensure the appropriate fuel quality. In addition, it is important to avoid poisoning, deactivation, and leaching of acid sites. Low-cost feedstocks, for example, waste cooking oils, have been described as promising feedstocks with the potential to decrease the cost of production, and thus enhance biodiesel production economic viability. Heterogeneous acid catalysts are usually non-sensitive to water and free fatty acid. Thus, free fatty acid esterification and triglycerides transesterification into biodiesel can be catalyzed more efficiently with a solid acid catalyst. In addition, the acid-catalyzed process is cost-effective and can also address waste disposal problems because heterogeneous acid catalysts can produce biodiesel directly from low-cost feedstocks [124]. In general, heterogeneous acid catalysts provide positively charged acid sites to which the FFAs or triglycerides in the oil adsorb. The carbonyl oxygen in the FFAs or triglycerides interacts with the Lewis acid sites (L*) on the catalyst surface, forming a carbocation that initiates the transesterification reaction between methanol and the adsorbed triglycerides, as shown in Figure 10 [125]. Nucleophiles, such as methanol, resulting from the deprotonation of the hydroxyl group, attack the electrophilic carbon. This leads to a rearrangement step, producing an intermediate that eliminates water molecules and forms methyl ester [61].
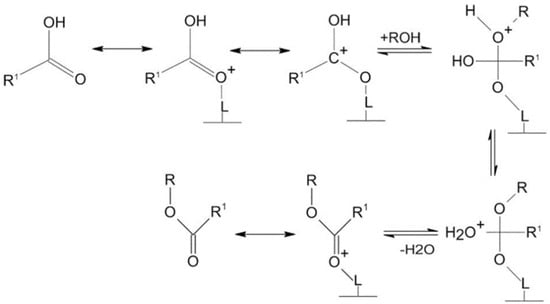
Figure 10.
Heterogeneous acid catalysis reaction mechanism [125].
The most effective catalysts from all the numerous areas of heterogeneous catalysis are metal oxide-based catalysts because mesoporous or porous metal oxides allow many catalytic processes. The importance of catalysts based on metal oxides lies in their successful catalysis of various processes, e.g., catalysis of acid–base, oxidation reactions, conversion of biomass, and photocatalysis. Due to their vast number of active acid sites, some metal oxides have higher catalytic activity, thus increasing the catalytic surface area. These properties improve the yield of reactions and reduce reaction times [126]. Most of these metal oxide-based catalysts originate from transition metals groups, such as titanium oxides (TiO2), zirconium oxides (ZrO2), tin oxides (SnO2), tungsten oxides (WO3), iron oxides (Fe2O4), and zinc oxides (ZnO). Among all these acid catalysts, sulfonated zirconia, tungsten–zirconia, and CaO–zirconia are suitable for transesterifying triglycerides into alkyl esters [127]. A suitable heterogeneous acid catalyst for transesterification reactions should have features including hydrophobic surface, interconnected large pores, and strong Brønsted/Lewis acid properties [128].
A myriad of studies on the utilization of the heterogeneous acid catalyst in biodiesel production have been conducted. Jitputti et al. [129] showed that sulfated zirconia (SO42− /ZrO2) can produce promising results with biodiesel yields of up to 86.3% and 90.3%, with crude coconut and palm kernel oils, respectively. However, biodiesel yields were only 48% (crude coconut oil) and 63% (palm kernel oil) when unsulfated zirconia was used as a catalyst instead of sulfated zirconia. The results showed that sulfated zirconia demonstrated the highest catalytic conversion (86.3% for coconut oil and 90.3% for palm kernel oil) in optimized conditions of 200 °C temperature, 6:1 MRMO, and 1.0 wt.% catalyst for 4 h. This essentially demonstrates that the main factor in achieving high triglyceride conversion is the modification of metal oxide surface acidity. Ibrahim et al. [130] explored the esterification of waste cooking oil using ZrO2 loaded on various supports (TiO2, SiO2, Fe2O3, and Al2O3). The hybrid sol–gel auto combustion approach was used to synthesize the catalyst. The esterification process was conducted with 120:1 MRMO and 0.1 wt.% catalyst at 120 °C for 3 h. Optimal biodiesel conversion (48.6%) was achieved with a ZrO2/SiO2 catalyst within 3 h. Park et al. [131] performed esterification of WCO with ZrO2, in which tungstate (WO3) was integrated into ZrO2 instead of sulfuric acid (H2SO4). WO3/ZrO2 was found to be more stable than SO42−/ZrO2, thus preventing acid sites from leaching into the reaction media. The authors discovered that after 2 h at 75 °C, 93% of FFAs conversion was achieved using 9:1 MRMO. Despite the high acidity of sulfated zirconia, during liquid-phase transesterification reaction, it is known to suffer substantial deactivation due to sulfate leaching. By dissolving the freshly prepared SO42−/ZrO2 catalyst in water, catalyst leaching has been tested [132]. Hydrolysis of the sulfate groups to H2SO4 and HSO4− rapidly lowers the pH of the suspension. This will induce transesterification through homogeneous acid catalysis, thereby obstructing heterogeneous catalytic activity. SO42−/ZrO2 was prepared using chlorosulfonic acid, HSO3Cl, rather than impregnation with H2SO4. The prepared catalyst (SO42−/ZrO2) exhibited a very high catalytic activity and no leaching of sulfates was noted. Chlorosulfonic acid is a very dangerous chemical, and exposure for a very short period may lead to death or serious trauma [133].
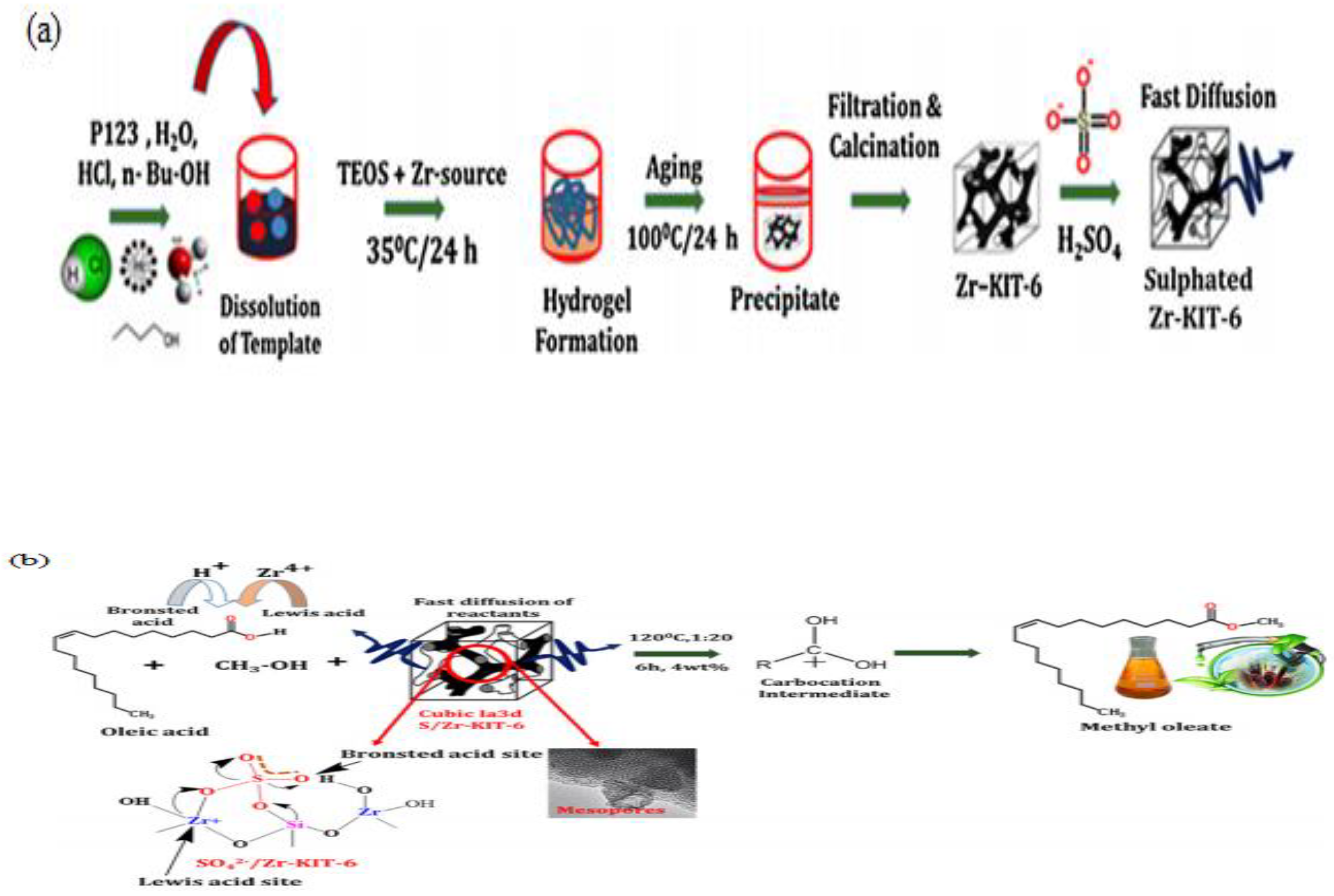
The esterification of methanol-induced FFA using SO42−/SnO2-ZrO catalyst was conducted by Enascuta et al. [134]. They reported that the esterification process involves Brønsted and Lewis acid sites. A biodiesel yield of 82.58% was achieved using the following conditions: 5.0 wt.% of catalyst and 6.5:1 of molar ratio of ethanol to oil (MREO) at 78 °C for 6 h. A number of new SO42/ZrO2-based catalysts were obtained from MCM-41 silica-supported calcined zirconium sulfate (ZrX-MCM-41, where X represents zirconia sulfate precursor weight percentage) have been studied by Jiménez et al. in the production of biodiesel using sunflower oil having high water and FFA content [135]. They reported that optimized ethyl esters yield of 91.5% was obtained with 12:1 MREO and 14.6 wt.% of catalyst at 200 °C in 6 h. In addition, as-prepared catalysts are stable (without sulfate species leaching) with consistent catalytic activity after three catalytic cycles. Elsewhere, Gopinath et al. [136] used mesoporous sulfated Zr-KIT-6 to produce biodiesel from oleic acid and jatropha oil. The catalyst was synthesized using a hydrothermal process. Sulfated Zr-KIT-6 catalyst is produced by loading various amount of zirconia into a fixed gel in an acidic medium and then sulfating it (see Figure 11). The authors found that biodiesel conversions of 85% and 96% were achieved from jatropha oil and oleic acid, respectively, with 4 wt.% catalyst and 20:1 MRMO at 120 °C in 6 h.
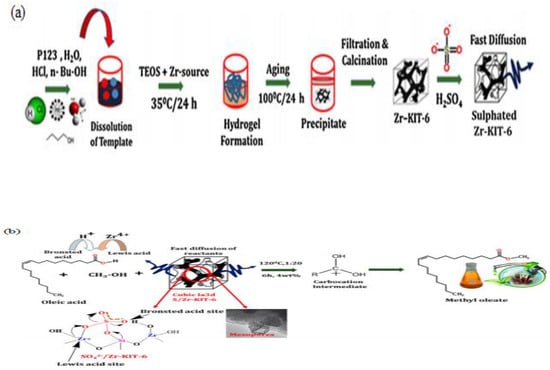
Figure 11.
(a) Schematic diagram of synthetic strategy of mesoporous sulfated Zr-KIT-6; (b) esterification of oleic acid by mesoporous sulfated Zr-KIT-6 (reprinted from ref. [136]).
Fatimah et al. [137] conducted transesterification of soybean oil with Zr2O-supported bamboo leaf ash (Zr2O/BLA) as a catalyst. The catalyst was synthesized using the impregnation technique and the catalyst had a 20 wt.% metal content. It is noted that the Zr2O/BLA catalyst showed excellent transesterification performance, where biodiesel yield of 89.99% was achieved under optimal process condition (12 wt.% of catalyst and 15:1 MRMO at 50 °C for 0.5 h). However, they obtained a yield of 95.99% after 1 h of reaction. Wang et al. [138] applied a zirconium-based carbonaceous (Zr-SO3H@CMC) catalyst to produce biodiesel from oleic acid. In this work, 99.1% biodiesel yield was obtained at 90 °C for 2h, using 5.0 wt.% catalyst amount, and 20:1 MRMO.
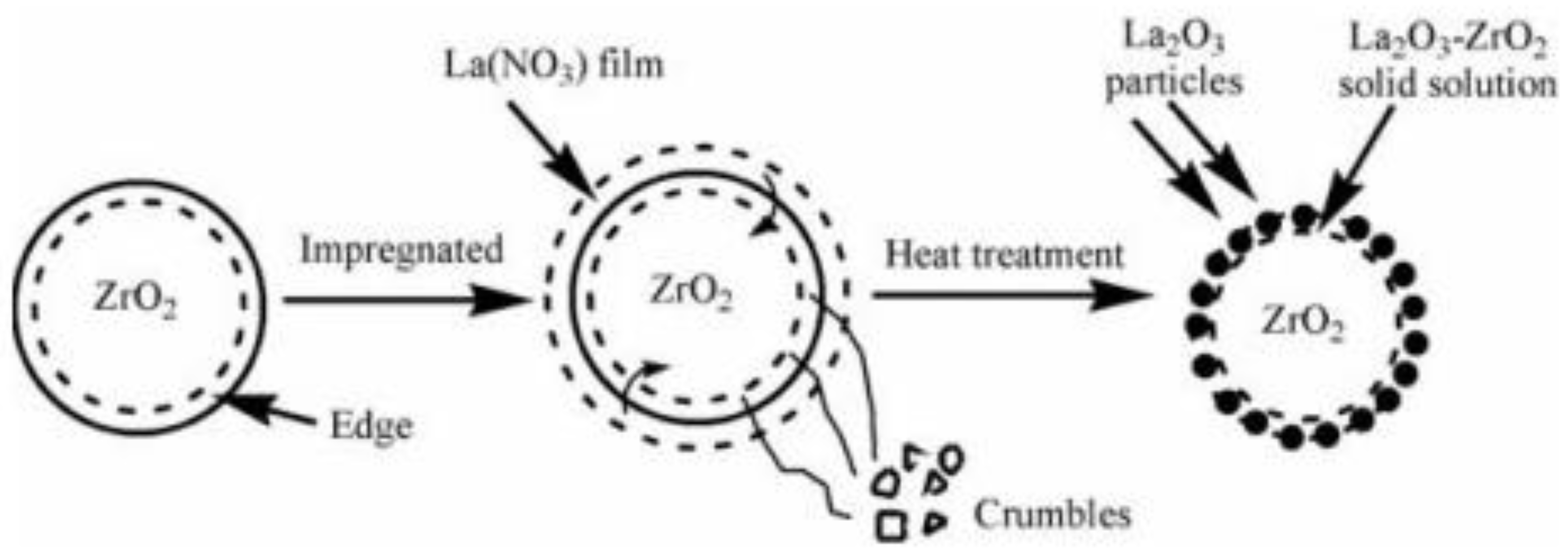
Guldhe et al. [139] evaluated the catalytic performance of tungstated zirconia (WO3/ZrO2) in the transesterification of S. Obliquus lipids with methanol. The Fourier transform infrared spectroscopy characterization of the WO3/ZrO2 indicated that Brønsted and Lewis acid sites are present. Transesterification of S. Obliquus lipids was carried out with 15 wt.% catalyst loading and 12:1 MRMO at 100 °C for 3 h. The maximum biodiesel conversion under this condition was 94.58%. Also, Sun et al. [140] explored the transesterification of sunflower oil using ZrO2 loaded on La2O3 support (ZrO2/La2O3) synthesized by wet impregnation, in the production of biodiesel (Figure 12). The authors reported optimum biodiesel conversion of 84.9% with 5 wt.% catalyst loading and 30:1 MRMO at 200 °C for 5 h.
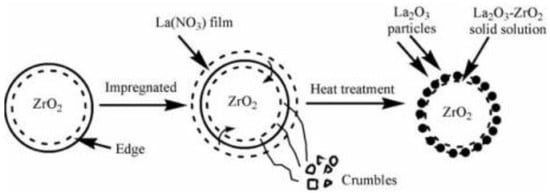
Figure 12.
Schematic of ZrO2/La2O3 catalyst surface solid-state reaction (reprinted from ref. [140]).
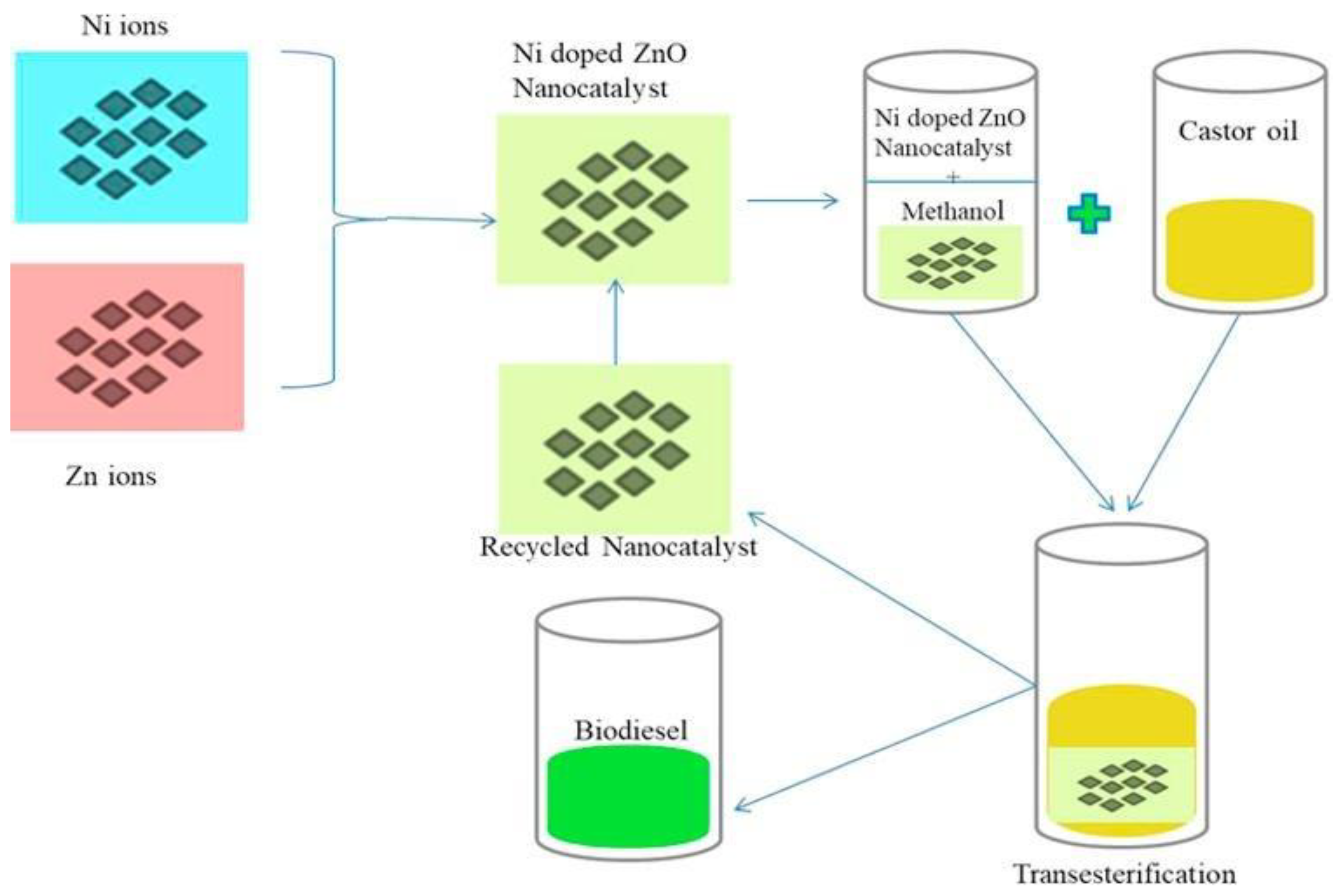
Malhotra et al. [141] used Sodium/ZnO-loaded SBA-15 in the production of virgin cotton seed oil biodiesel. This catalyst was synthesized under atmospheric conditions by the wet impregnation method. Cotton seed oil is converted to biodiesel via transesterification process using the following conditions: 12 wt.% catalyst amount, 65 °C temperature, and 24:1 MRMO in 4 h. A yield of 98% was obtained under these conditions. After reuse for five reaction cycles, the catalyst maintained activity and no substantial reduction in catalytic performance was noted [141]. Elsewhere, the application of manganese-doped zinc oxide was reported by Baskar et al. [142] to produce biodiesel using Mahua oil. It is noticed that 97% yield was achieved with 8 wt.% catalyst amounts and 7:1 MRMO at 50 °C in 50 min. Another study from the same research group focused on using nickel-doped zinc oxide in castor oil transesterification (Figure 13) [143]. They reported optimal biodiesel yield of 95.20% in 60 min for nickel-doped zinc oxide catalyst, at 11 wt.% catalyst loading, 8:1 MRMO at 55 °C.
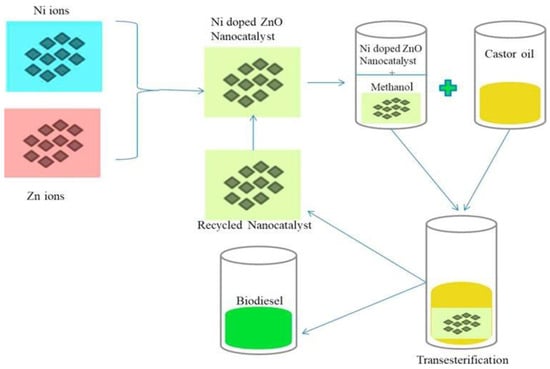
Figure 13.
Transesterification of castor oil by Ni-doped ZnO nanocatalyst (reprinted from ref. [143]).
Borah et al. [144] investigated transesterification of Mesua ferrea oil with cobalt-doped zinc oxide catalyst. The optimized reaction was conducted with 2.5 wt.% catalyst and 9:1 MRMO at 60 °C for 3 h, and a maximum conversion of 98.03% of the feedstock was obtained. Soltani et al. [145] explored the transesterification of palm fatty acid distillate using a mesoporous SO3H-ZnAl4O3 catalyst. A microwave irradiation heating system was used to synthesize the catalyst. They reported that the SO3H-ZnAl4O3 catalyst showed excellent esterification performance, where an optimal yield of 94.59% was achieved with 1.5 wt.% of catalyst and 9:1 MRMO at 60 °C in 20 min. AlSharifi et al. [146] used lithium/zinc composite supported by waste chicken bone, synthesized by the wet impregnation method, to produce biodiesel from waste canola oil. The authors reported that the optimum biodiesel conversion of 98% using different metal ratios and reaction conditions of 18:1MRMO and 4.0 wt.% catalyst loading at 60 °C in 3.5 h.
Tin oxide (SnO2) is a semiconductor material with a broad bandgap (approximately 3.7 eV). Generally, it is possible to obtain SnO2 in mesostructured form by hydrolyzing inorganic precursors in the presence of surfactants as a structure director [147]. During calcination, most of the SnO2 mesostructure collapses because they are not stable. An anionic surfactant with sulfate, such as that used in the preparation of mesoporous sulfated tin oxide, SO42−/SnO2, has been proposed to improve the stability of the mesostructure [148]. The sulfate groups are isolated on the SnO2 surface, which led to the stabilization of the mesostructure walls and ameliorates the stability. Additionally, SO42−/SnO2 has catalytic transesterification reaction potential because it is a strong super acid. In octanoic acid esterification at temperatures below 140 °C with methanol, SO42−/SnO2 showed superior catalytic activity to SO42−/ZrO2 [147]. Notwithstanding, the utilization of sulfated tin oxide in the biodiesel process is still very limited. The limited study carried out on sulfated tin oxide could be attributed to its complex method of preparation because it is difficult to achieve oxide gels from its salt in contrast with sulfated zirconia, which can easily be prepared [149]. Kafuku et al. [20] applied the silica-supported SO42−/SnO2 (SO42−/SnO2-SiO2) in the production of Moringa oleifera biodiesel. The optimized reaction was performed with 3 wt.% catalyst and 19.5:1 MRMO at 150 °C for 2.5 h, and a maximum yield of 84% of the feed stock was obtained. Similarly, Kafuku et al. [150] used SO42−/SnO2-SiO2 for transesterification of jatropha curcas. They reported an optimum methyl ester yield of 97% for a SO42−/SnO2-SiO2 catalyst and reaction conditions of 3.0 wt.% catalyst and 15:1 MRMO at 180 °C for 2 h.
Titanium dioxide (TiO2) has attracted considerable interest in biodiesel production because of its acidic properties. Furthermore, the sulfuric group on the surface of TiO2 would increase the catalyst strength [151]. He et al. [152] transesterified cottonseed oil with methanol using SO42−/ZrO2 and SO42/TiO2 catalysts. It was found that SO42/TiO2 could achieve a maximum biodiesel yield of >90% compared to SO42−/ZrO2, which could only achieve a yield of 85%. It was suggested that SO42/TiO2 reactivity could be improved by adding silica SiO2, to obtain SO42/TiO2–SiO2 [153]. The catalyst surface area increased when SO42/TiO2 was loaded onto SiO2 (from 99 m2/g to 258 m2/g). However, when compared to homogeneous catalysts temperatures between 50 and 100 °C, the temperature of the reaction is still high. Also, Gardy et al. [154] utilized TiO2/PrSO3H catalyst to produce WCO biodiesel and reported a 98.3% biodiesel yield with 2.5 wt.% catalyst and a 15:1 MRMO at 60 °C for 9 h. They reported that the catalyst was active in making biodiesel. They found that the catalyst retained activity, and there was no substantial loss in the activity after four cycles of reuse. In another work, Kaur et al. [155] studied a tungsten-supported TiO2/SiO2 catalyst in the transesterification of waste cottonseed oil. The sol–gel method was used to synthesize the catalyst. They reported an optimized biodiesel yield of 98% in 4 h at 5 wt.% catalyst loading and 30:1 MRMO at 65 °C reaction temperature.
Similarly, Dai et al. [156] examined the activity of Li2TiO3 on soybean oil treatment. The solid-state method was used to synthesize the catalyst, which involved the mixing and grinding of titanium oxide (TiO2) and lithium carbonate (Li2CO3), after which a two-hour calcination at 800 °C was performed. The authors stated that optimum biodiesel conversion of 98.5% was attained with 6 wt.% catalyst and 24:1 MRMO at 65 °C in 2 h. To prepare a solid Li/TiO2-based catalyst, a wet impregnation approach was employed. The authors reported that at 5.0 wt.% catalyst, 55 °C temperature, and 24:1 MRMO, they could achieve a yield of 98% in 3 h.
Furthermore, in oil refining and shape-selective petrochemical industries, zeolites are widely used as catalyst. Zeolite’s efficient catalytic activity is owing to its pore size, ion exchange, and chemical structure characteristics [62]. The zeolites framework structures contain equal-sized molecular pores and channels capable of absorbing molecules that fit within them while larger molecules are prevented. Zeolites have specific properties as a catalyst, such as shape selectivity, strong Brønsted acidity, the ability to maintain electro-neutrality, etc. [60]. However, due to the small pore size of zeolites, diffusion of reactant molecules into zeolite active sites may be a process constraint. In biodiesel production, zeolites such as H-USY, H-BETA, ZSM5, H-MOR have been discovered to have poor catalytic performance. This is because of the internal diffusion constraints of the larger reactant molecule into zeolites micropores. Thus, only the zeolite external surface catalyzes transesterification. The diffusion of bulky triglycerides molecules is severely limited; small-pore zeolites are not appropriate for transesterification [157,158]. Thus, zeolites with large pores are suitable heterogeneous acid catalysts.
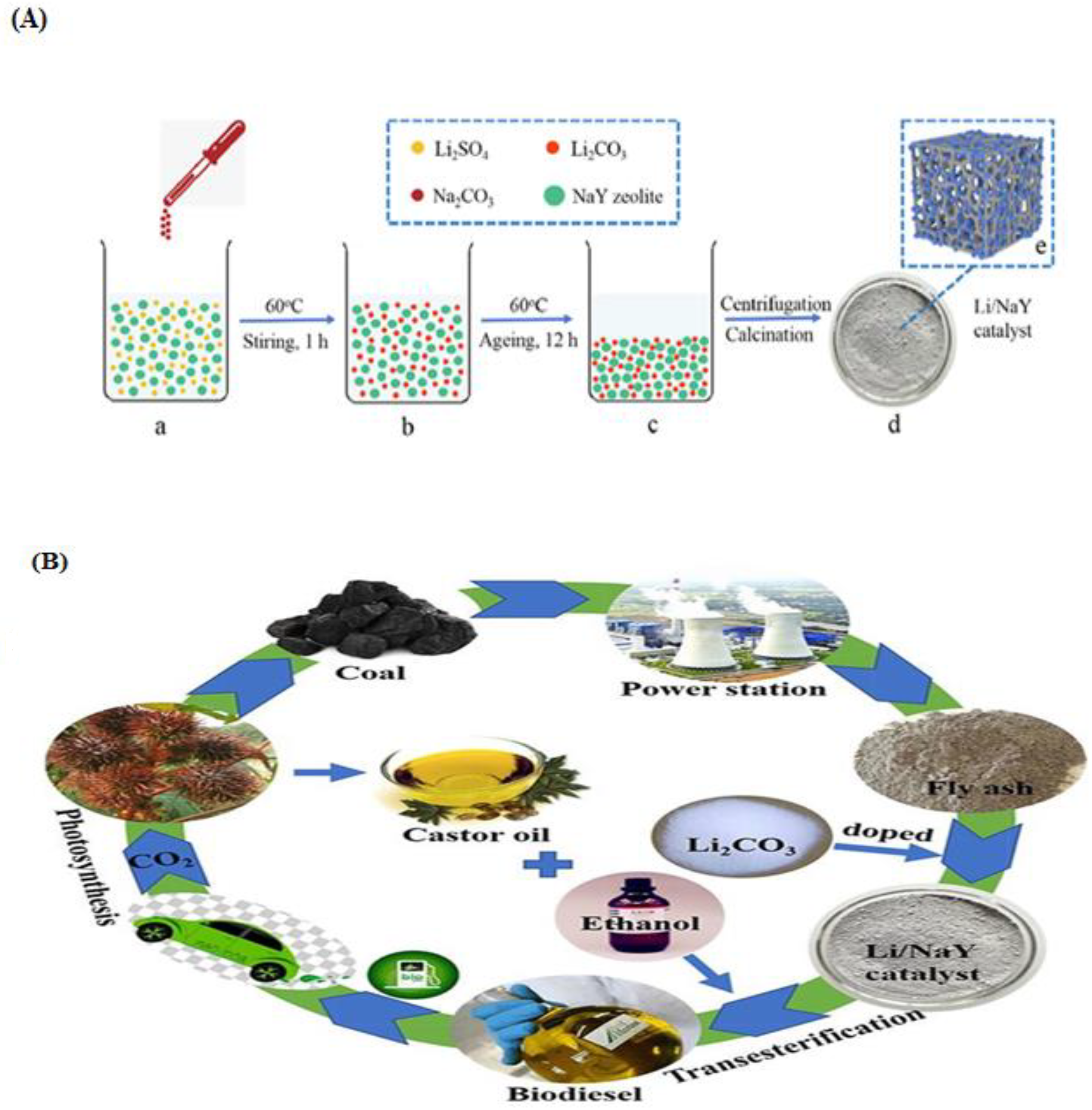
In the production of castor oil biodiesel, Li et al. [159] employed Li/NaY as a catalyst. The Li/NaY was synthesized using the co-precipitation method. In the co-precipitation method, LiCO3 and NaY zeolite support were mixed for some minutes and calcined at 750 °C for 4 h (Figure 14). They reported that an optimized oil yield of 98.6% was achieved at 3 wt.% catalyst, 18:1 MREO, at 75 °C in 2 h. Transesterification of oleic acid over zeolite Y with a Si/Al ratio of 3.1 was previously reported [160]. An optimal conversion of 85% was attained at 5 wt.% of Zeolite Y catalyst prepared from kaolin, 70 °C temperature, and 6:1 MREO in 1 h.
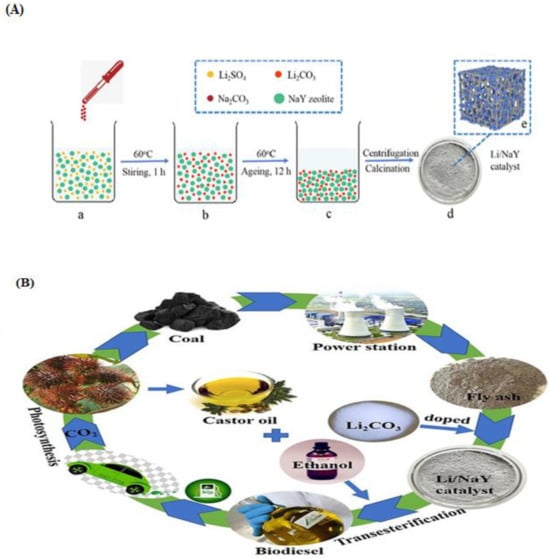
Figure 14.
(A) Schematic diagram for (a–d) synthesis of Li/NaY zeolite catalyst and (e) structure of Li/NaY zeolite catalyst. (B) Castor oil transesterification using a Li/NaY zeolite catalyst. (Reprinted from ref. [159]).
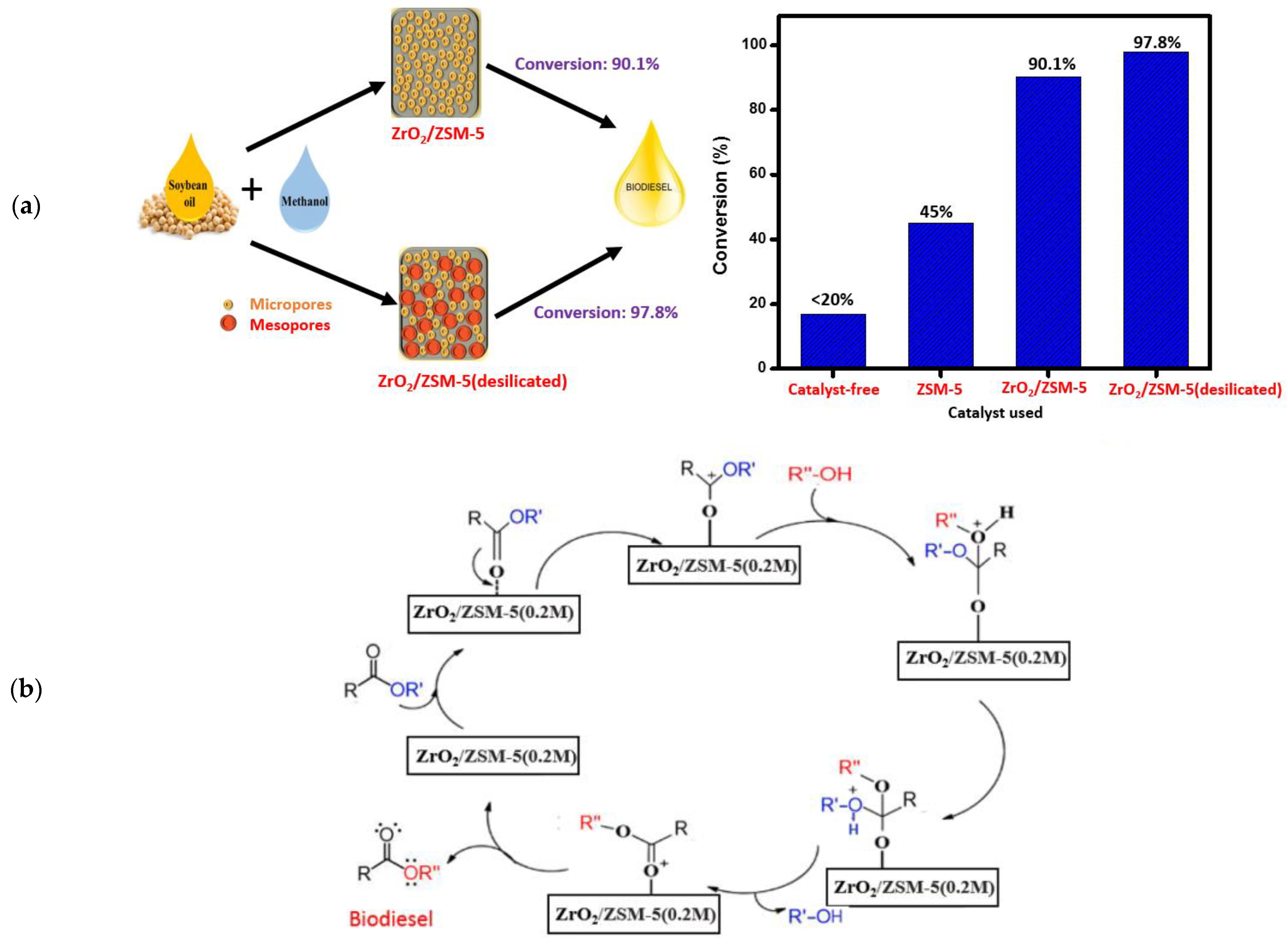
Yusuf et al. [161] prepared a series of desilicated zeolite-supported ZrO2 catalyst [ZrO2/ZSM-5(0.1–0.3 M)] via wet impregnation approach and they examined their catalytic activity in making biodiesel from soybean oil (Figure 15a). The as-prepared ZrO2/ZSM-5(0.2 M) catalyst exhibited better catalytic activity in the transesterification of soybean oil with methanol, compared to ZrO2/ZSM-5 catalyst, due to high surface area, large pore volume, and high dispersion of ZrO2 on the desilicated zeolite support with improved acidity and high number of active sites. It was found that optimized oil conversion of 97.8% was achieved at 1 wt.% desilicated zeolite-supported ZrO2 catalyst, 16:1 MRMO, and 200 °C temperature in 4 h. Moreover, the ZrO2/ZSM-5(0.2 M) catalyst exhibited excellent stability and reusability, giving a biodiesel conversion of 93.8% after three cycles of reuse. A plausible mechanism was proposed for the transesterification reaction over the ZrO2/ZSM-5(0.2 M) catalyst (Figure 15b).
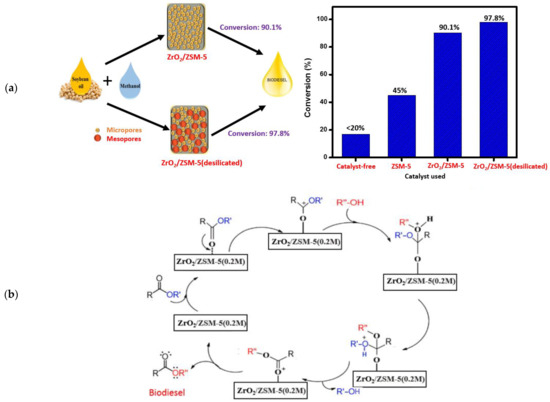
Figure 15.
(a) Soybean oil transesterification using a zeolite-supported ZrO2 catalyst and desilicated zeolite-supported ZrO2 catalyst. (b) Plausible mechanism of transesterification. Reprinted with permission from ref. [161].
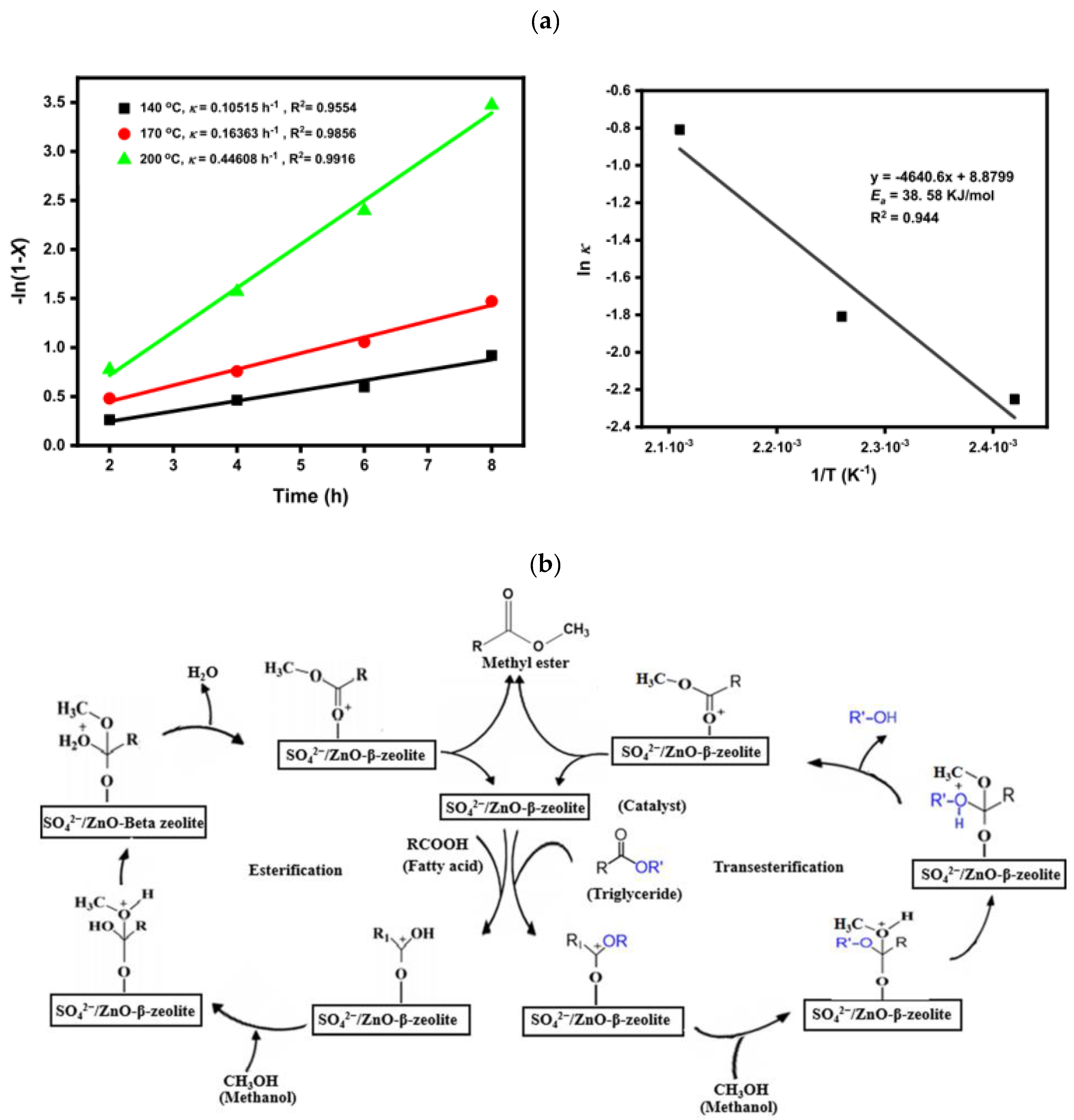
Another solid acid based on beta zeolite was synthesized via wet impregnation method and named as SO42−/ZnO-β-zeolite and was used for simultaneous transesterification and esterification of waste cooking oil to biodiesel [162]. The highest WCO conversion of 96.9% was achieved with 3 wt.% SO42−/ZnO-β-zeolite catalyst, 15:1 MRMO, and 200 °C temperature in 8 h. Furthermore, the evaluation of the catalyst stability and reusability revealed that the catalyst demonstrated good stability, maintaining biodiesel conversion of >80% after three synthesis cycles. Kinetic study showed that the reaction of WCO to biodiesel followed first-order kinetics (with 38.58 kJ/mol activation energy), allowing proposition of a plausible mechanism for the reactions (Figure 16).
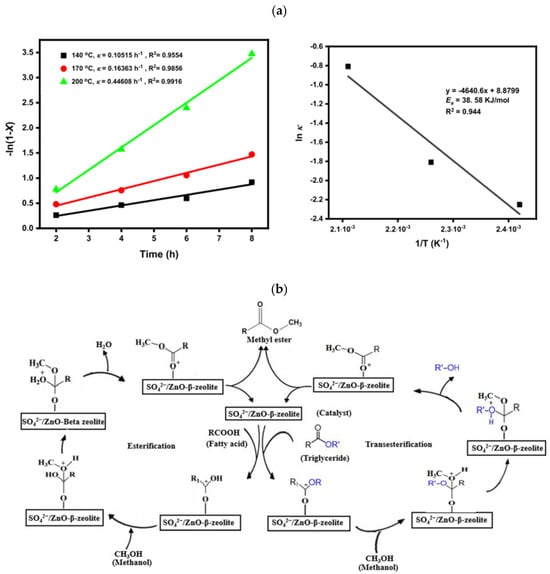
Figure 16.
(a) Plots of -ln(1-X) vs. time (left) and Arrhenius plot [ln K vs. 1/T] (right); (b) plausible mechanism for the reactions. Reprinted from ref. [162].
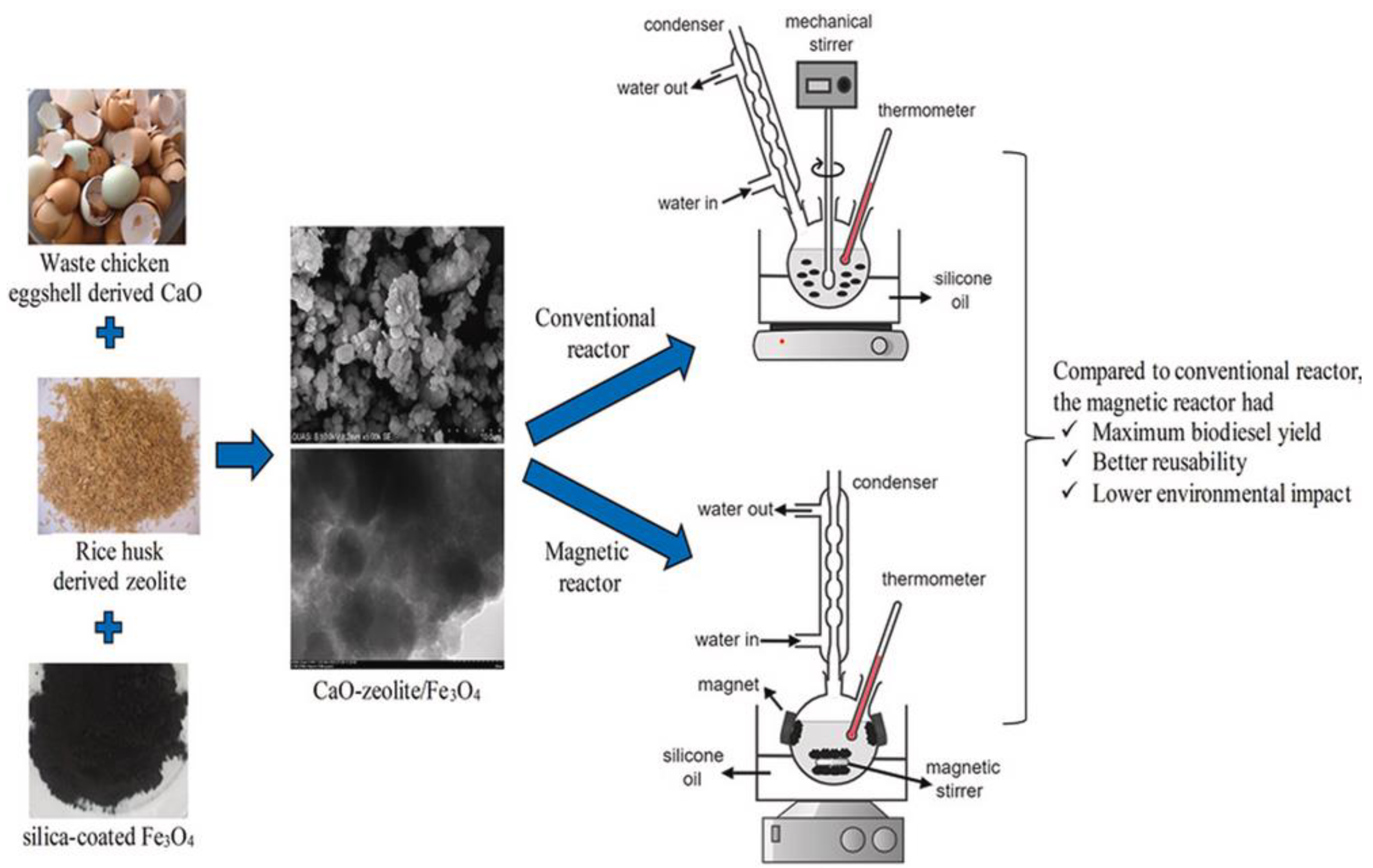
In the production of acidic soybean oil, Hou et al. [163] employed Mo/Ce/TiO2 composites as a catalyst. They reported that Mo/Ce/TiO2 catalyst exhibited better catalytic activity in the transesterification-esterfication of acidic soybean oil with methanol, due to the synergism of Brønsted and Lewis acid sites. They reported that an optimized oil yield of 93.8% was achieved at 5 wt.% catalyst, 30:1 MREO, at 140 °C in 8 h. In another study, Abati et al. [164] synthesize Al2O3 biobased heterogeneous catalysts using wet impregnation method. It was found that optimized oil conversion of 94.23% was achieved at 1 wt.% catalyst, and 90 °C temperature in 2 h. Elsewhere, the application of ZSM-5 was reported by Sathiyamoorthi et al. [165] to produce biodiesel using Jatropha curcas oil and a microchannel reactor. It is noticed that 99.7% yield was achieved with 10 wt.% catalyst amounts at 60 °C in 35 s. Another clay-based plasma-treated Co3O4/Kaolin catalyst was synthesized via the wet impregnation method and was used for the simultaneous transesterification and esterification of low-cost oil to biodiesel [166]. The highest oil conversion of 98.7% was achieved with 10 wt.% Co/Kaolin(400)-P(1000) catalyst, 20:1 MRMO, and 110 °C temperature in 3 h. Furthermore, the evaluation of the catalyst stability and reusability revealed that the catalyst demonstrated excellent stability, maintaining biodiesel conversion of 98.7% after five synthesis cycles. Elsewhere, Saadiah et al. [167] looked at the catalytic performance of CaO-zeolite/Fe3O4 catalyst in the synthesis of biodiesel from used cooking oil using conventional and magnetic reactors (Figure 17). According to the authors, the synthesized catalyst demonstrated excellent activity due to high magnetization, acid properties, and substantial surface area. In the magnetic reactor, the reaction was conducted for 300 min with 4 wt.% catalyst dosage and 5:1 MRMO at 55 °C. A maximum biodiesel yield of 96.91% was achieved with these conditions within 300 min. In addition, the CaO-zeolite/Fe3O4 catalyst exhibited excellent reusability, giving a biodiesel yield of 92.8% in the fourth run.
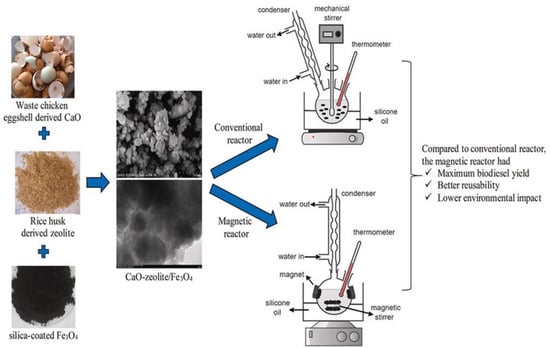
Figure 17.
Used cooking oil transesterification using a CaO-zeolite/Fe3O4 catalyst in conventional and magnetic reactors. Reprinted from ref. [167].
The most widely employed heterogeneous acid catalysts in liquid phase transesterification and esterification reaction are sulfonic ion exchange resins (Nafion resins, EBD resins, and Amberlyst resins) [168,169,170,171]. Sulfonic ion exchange resin has a cross-linking polymeric component where sulfonic groups bonded protons are the active sites of the transesterification and esterification process [172]. Furthermore, the swelling properties of sulfonic ion exchange resin influence their catalytic activity because swelling capacity limits reactant access to the acid site and therefore influences their activity [173]. Nafion SAC-13, EDS-100, and Amberlytes-15 are the most prevalent types of acidic ion exchange resins, and several researchers have made use of them [168,169,174]. The performance of these catalysts was observed to be good in free fatty acid esterification but poor in transesterification [175]. The performance of Amberlyst-15 as a catalyst for making biodiesel from sunflower oil was investigated by Vicente et al. [176]. It is observed that the Amberlyst-15 catalyst showed poor activity (0.7% oil conversion) with 6:1 MRMO and 1 wt.% catalyst at 60 °C for 8 h. Dos Reis et al. [177] utilized an Amberlytes-15 catalyst for biodiesel synthesis using Babassu coconut oil as substrate. Perhaps due to the high content of shorter-chain fatty acids in Babassu coconut oil and high MRMO of 300:1, Amberlytes-15 catalyst showed excellent transesterification performance, where oil conversion of 74% was obtained with 300:1 MRMO at 60 °C in 8 h. Therefore, if Amberlyte-15 is to be used, reaction temperature must be increased to 140–200 °C to achieve a faster reaction rate [171]. Amberlyte-15 and most of the ion exchange resin have poor thermal stability at a temperature above 150 °C. Therefore, this stability issue limits their applications.
Recently, biomass conversion-related reactions including transesterification and esterification have made substantial use of cellulose-derived solid acids. Among them, sulfonic (SO3-H)-bearing cellulose-derived solid acids materials have drawn special attention [178]. These materials could incorporate a significant amount of hydrophobic molecules into the bulk of carbon because the density of the hydrophobic functional group bound to carbon sheets is very high. Such incorporation offers excellent accessibility to the sulfonic group, resulting in high catalytic efficiency. Hara [178] investigated the catalytic activity of SO3-H-bearing carbon material in the production of oleic acid biodiesel. With a 0.307 g catalyst and 95 ° C temperature in 4 h, the optimal biodiesel yield using the SO3-H-bearing carbon material catalyst was 99.9%. The authors found that the catalyst is stable and maintains similar catalytic activity after ten reaction cycles. Zong et al. [179] utilized sulfonated D-glucose-derived sugar for transesterification of palmitic acid and oleic acid. They found that a biodiesel yield of 95% was obtained with 5 wt.% catalyst and 10:1 MRMO at 80 °C for 5 h.
To date, there are few studies on the use of sulfonated carbon-based catalyst in the transesterification of feedstock containing triglycerides, typically from used cooking oil. Lou et al. [180] utilized sulfonated carbon-based catalyst produced from sucrose, cellulose, and starch in the transesterification of WCO using methanol. They claimed that the carbon-based catalyst produced from starch has higher acid sites, higher pore size, and higher pore volume which allowed reactants to access the SO3-H sites. Furthermore, the optimum used cooking oil conversion using the carbon-based catalyst produced from starch was 92% with 30:1 MRMO and 10 wt.% catalyst at 80 °C in 8 h. Also, there was an insignificant change in performance of the catalyst even after fifty cycles, proving it to be a very stable catalyst.
By sulfonation of carbonized vegetable oil asphalt, Shu’s group developed a carbon-based catalyst and the catalyst was made up of a flexible carbon-based framework comprising sulfonic acid groups with polycyclic aromatic hydrocarbons that are highly dispersed [181]. They found that the carbon-based catalyst exhibited improved activity for the transesterification of WCO and provided an optimum conversion of 80.5% and 94.8% for triglycerides and FFA, respectively, with 0.2 wt.% catalyst and 16.8:1 MRMO at 220 °C in 4.5 h. They explained that optimum conversion exhibited by the catalyst is due to the hydrophobicity of the carbon sheet which prevented the hydroxyl group from being hydrated and bonded –SO3H hydrophilic groups that increased the contact of methanol with the protonated carboxylic group of triglycerides. The development of an effective carbon-based acid catalyst for biodiesel production at mild reaction conditions remains a challenge. Nonetheless, carbon-based catalysts need to be further improved, including transesterification reaction conditions and improvement on catalyst synthesis.
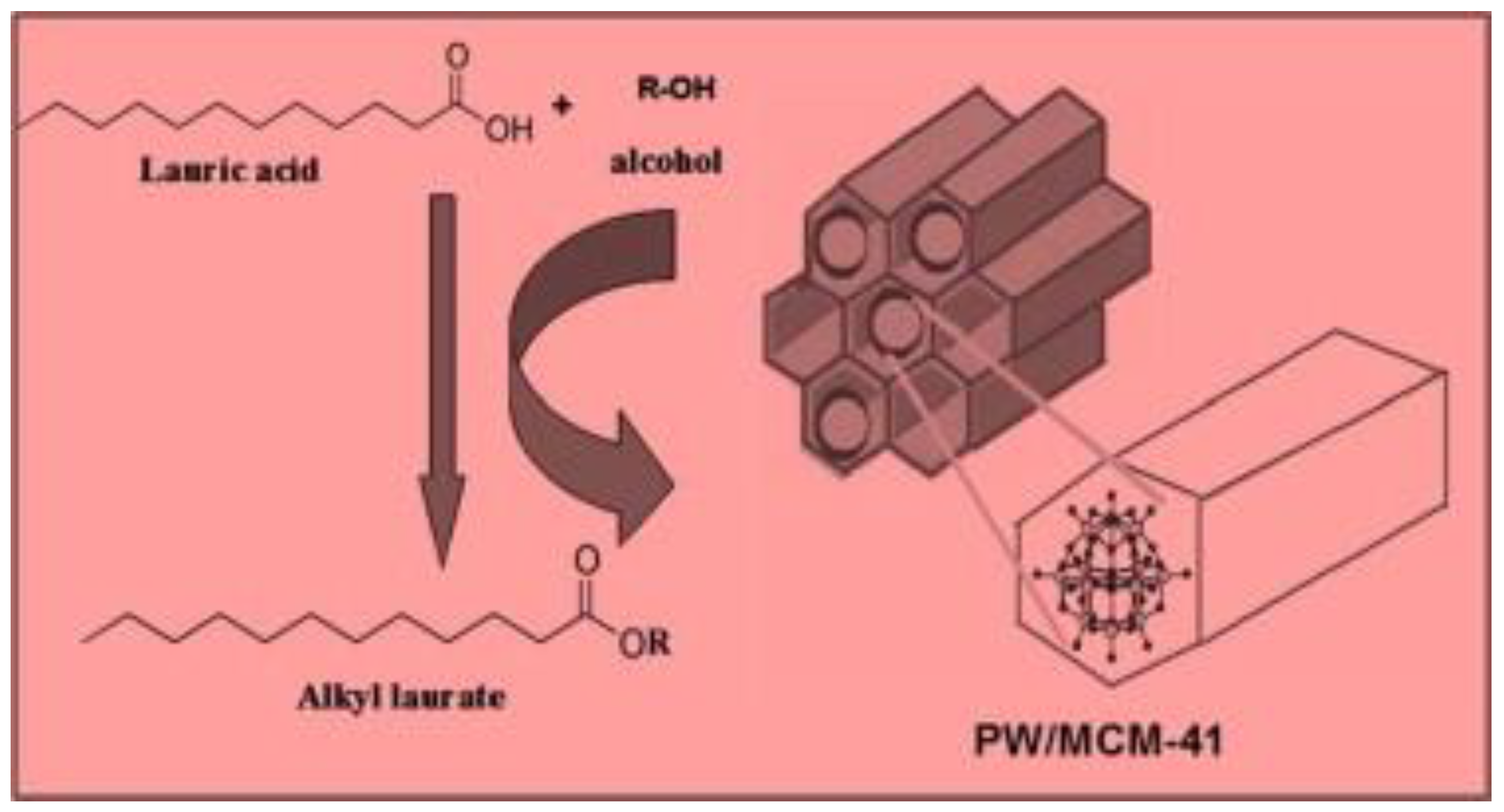
In the field of heterogeneous acid catalysts, heteropolyacids (HPAs) also have significant potential for transesterification of waste vegetable oils. HPAs are mostly a type of solid acid with a well-defined structure that consists of early transition metal-oxygen anion clusters. HPAs are considered to be a more environmentally friendly and cost-effective alternative to traditional acid catalysts because of their high stability, high mobility of proton, strong Brønsted acidity, and non-toxicity [182]. The most widely used HPA happens to be heteropoly tungstate (H3PW12O40), which has demonstrated outstanding performance in acid-catalyzed reaction including transesterification and esterification [183]. Cao et al. [184] evaluated the acidic activity of H3PW12O40·6H2O (PW12), for the transesterification of WCO. A biodiesel conversion of 87% was attained with 4.0 wt.% catalyst and 70:1 MRMO at 65 °C in 14 h. The authors found that the H3PW12O40·6H2O catalyst had a high FFA tolerance, and its catalytic activity was maintained after five catalytic cycles. However, the application of this catalyst on an industrial scale is restricted by the long reaction time and the high MRMO. In addition, if H3PW12O40·6H2O catalyst is to be used, the reaction temperature must not be more than 65 °C to achieve the stable catalytic performance [184]. They claimed that the transesterification must not be performed at a higher temperature because waste cooking oil contains a number of undesirable compounds that may induce side reactions. An attempt has been made to produce heteropolyacids with increased acidity by incorporating Lewis acid into them, resulting in heteropolyacids with Brønsted and Lewis acid sites [185]. Zhang et al. [185] transesterified used cooking oil over a Zr0.7H0.2PW12O40 (ZrHPW) catalyst. They found that the Zr0.7H0.2PW12O40 catalyst exhibited high acidity capacity compared to the original H3PW12O40 catalyst. The investigated Zr0.7H0.2PW12O40 catalyst displays unique properties and yielded an optimum biodiesel conversion of 98.8% with 2.1 wt.% catalyst and 20:1 MRMO at 65 °C in 8 h. In addition, after five catalytic cycles, a 95% yield was obtained. Elsewhere, another author used H3PW12O40/Nb2O5 catalyst in the transesterification process, which under optimum conditions produced a methyl ester yield of 92% with 18:1 MRMO and 3 wt.% catalyst at 200 °C in 20 h [186]. Brahmkhatri et al. [187] esterified lauric acid with H3PW12O40-supported MCM-41 (Figure 18). They reported that butyl laurate yield reached 95% with 0.2 g of H3PW12O40 loaded on MCM-41 supports catalyst and 1:2 oil to butanol ratio at 90 °C in 3h.
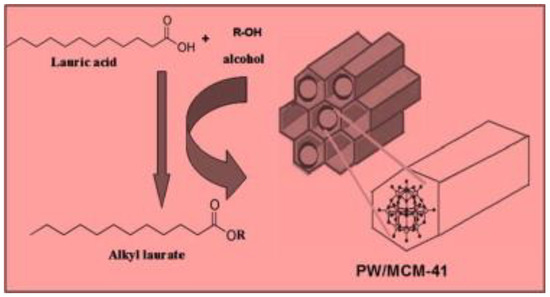
Figure 18.
Esterification of lauric acid by H3PW12O40 supported MCM-41 catalyst (reprinted from ref. [187].
Kulkarni et al. [188] utilized H3PW12O40 loaded on various supports (Al2O3, ZrO2, SiO2, and activated carbon) in the production of canola oil biodiesel, where the biodiesel yield reached 90% with 9:1 MRMO and 3.0 wt.% catalyst (H3PW12O40 loaded on ZrO2 supports) at 200 °C in 10 h. Alcañiz -Monge et al. [189] reported biodiesel production from esterification of palmitic acid using an activated carbon fiber-supported H3PW12O40 (H3PW12O40/ACF) catalyst. The authors obtained a biodiesel conversion of 89% with a 97:1 MRMO at 40 °C in 6 h.
Metal–organic framework-based acid catalysts have been extensively explored for the esterification or transesterification of edible/inedible oils to biodiesel [190,191]. Zhang et al. [192] synthesized nickel salt Keggin-type heteropolyacid on Zr(IV)-based metal–organic frameworks (NiHSiW/UiO-66) through hydrothermal method and investigated their catalytic performance in the production of biodiesel from oleic acid. The NiHSiW/UiO-66 catalyst was utilized as effective solid acid in the esterification of oleic acid to biodiesel with an optimal biodiesel conversion of 86.7% with 6 wt.% catalyst loading and 18:1 MRMO at 160 °C in 3 h. The high specific surface area of the NiHSiW/UiO-66 catalyst, as well as the synergistic effects of NiHSiW salts and the UiO-66 matrix, contributed to its exceptional activity. It was found that the NiHSiW/UiO-66 catalyst is relatively stable with around 50% biodiesel conversion after eight-times used. Similarly, Zhang et al. [193] explored the esterification of oleic acid using a Fe-BTC framework composite. A microwave irradiation heating system was used to synthesize the catalyst. They reported that the catalyst showed excellent esterification performance, where an optimal conversion of 72.3% was achieved with 10 wt.% catalyst loading and 16:1 MRMO at 160 °C in 3 h. Another solid acid based on metal–organic framework was synthesized via impregnation method and named as Sn1.5PW/Cu-BTC, and was used for the esterification of oleic acid to biodiesel with 87.7% conversion [194]. Amouhadi et al. [195] used the solvothermal method for preparing a heterogeneous MnO2@Mn(btc) catalyst for the esterification of oleic acid with biodiesel yield of 98% using the following reaction conditions: 5 wt.% catalyst loading and 30:1 MRMO at 200 °C for 5 h. Qian et al. [196] synthesized a series of MOF-808-R catalysts (R represents the volume ratio of N,N-dimethylformamide (DMF) to formic acid, ranging from 3/1 to 1/3) via solvothermal method and investigated their catalytic activity in microalgal lipids to FAME (Figure 19a). The as-prepared MOF-808-3/1 catalyst shows enhanced catalytic activity with a conversion efficiency of 96.24%. According to the authors, the increase in catalytic activity of MOF-808-3/1 catalyst is due to increased acidity, enhanced hierarchical porosity, and increased pore size from 1.63 nm to 5.34 nm. Moreover, the MOF-808-3/1 catalyst exhibited excellent reusability with a biodiesel conversion of 93.78% after six cycles. Through density functional theory, it was confirmed that the exposed Zr4+ sites serve as the active centers for the reaction, and a plausible reaction route was proposed (Figure 19b).
Figure 19.
(a) Synthesis of MOF-808; (b) probable reaction pathways for microalgal lipids conversion to FAME using MOF-808 catalyst. Adapted from ref. [196].
Taddeo et al. [197] prepared three different types of MOF-5 (T-MOF-5, C-MOF-5, and B-MOF-5, where T, C, and B represent tetragonal, cubic, and blend respectively), characterized and then investigated their catalytic activity in the production waste cooking oil biodiesel through simultaneous transesterification and esterification reaction. It was found that B-MOF-5 catalyst is suitable for the simultaneous transesterification and esterification of waste oil with a biodiesel yield of 70% using the following reaction conditions: 5 wt.% catalyst loading and 20:1 MRMO at 150 °C for 3 h. Elsewhere, Li et al. [198] reported the use of a biomass-derived hydrophobic metal–organic framework (FDCA/SA-Hf) for the esterification of oleic acid (Figure 20). Under optimum reaction conditions of 4.1 wt.% catalyst and 19:1 MRMO at 49 °C for 9.5 h, the oleic acid yield obtained was 98.6%. Moreover, after six runs, the biodiesel yield was 90% due to the high hydrophobicity and stability. It is noted that the [Hf-O-CH3] bond species observed in the presence of water is stabilized by the presence of a hydrophobic alkyl chain (SA) grafted on the acidic sites. This hydrophobic chain prevents the aggregation and adsorption of water at the acidic sites, which otherwise would lead to hydrolysis. As a result, the stability of the [Hf-O-CH3] bond species is maintained despite the presence of water.
Figure 20.
Esterification of oleic acid using FDCA/SA-Hf catalyst. (Reprinted from ref [198].).
Oghabi et al. [199] used MOF nanocatalyst for the conversion of oleic acid in the presence of methanol. Their results suggested high capability of the developed catalyst with a biodiesel conversion of 95% under reaction conditions of 3 wt.% catalyst dosage and 10:1 MRMO at 160 °C in 6 h. Also, the developed nanocatalyst was highly reusable, with a biodiesel conversion of 87% after six sequence cycles. Narenji-Sani et al. [200] studied biodiesel production from free fatty acids using ZIF-like grafted H6P2W18O62 catalyst (Figure 21). They reported the highest conversion of 92% at 3 wt.% catalyst loading and 60:1 MRMO at 80 °C in 4 h.
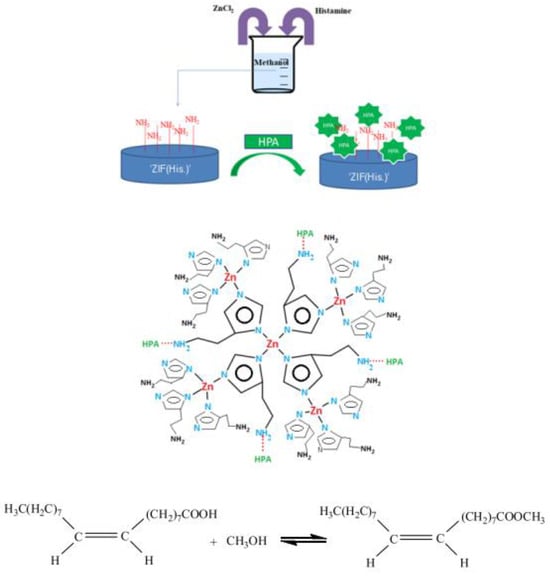
Figure 21.
Synthesis pathway and the proposed molecular structure for ZIF-like grafted H6P2W18O62 catalyst. Adapted from ref. [200].
Zhang et al. [201] prepared ZrSiW/Fe-BTC and ZrSiW/UiO-66 catalysts via hydrothermal method and investigated their catalytic performance in the production of biodiesel from oleic acid. In comparison with ZrSiW/Fe-BTC, the ZrSiW/UiO-66 catalyst exhibited better catalytic performance in the conversion of oleic acid to biodiesel in the presence of methanol, due to its high surface area, large pore size, and high acidity. They reported an oleic acid conversion of 98% using 0.24 g ZrSiW/UiO-66 catalyst, 20:1 MRMO, and 160 °C temperature in 4 h. Moreover, the catalyst showed excellent stability and reusability, with a biodiesel conversion of 88.9% after six consecutive runs. The authors also proposed a plausible mechanism for the esterification reaction involved (Figure 22).
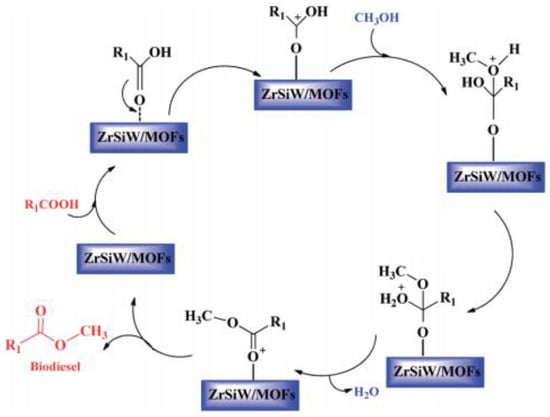
Figure 22.
Plausible mechanism for transesterification reactions. Adapted from ref. [201].
Similarly, Pangestu et al. [202] employed a CuBTc-MOF catalyst prepared through solvothermal method for transesterification of palm oil. An optimum biodiesel yield of 91% was reported for CuBTc-MOF catalyst and reaction conditions of 0.04 g catalyst and 5:1 MRMO at 60 °C for 4 h. The plausible mechanism for transesterification reaction was proposed by the authors (Figure 23). They also reported that CuBTc-MOF catalyst is reusable over several cycles with no significant loss in activity.
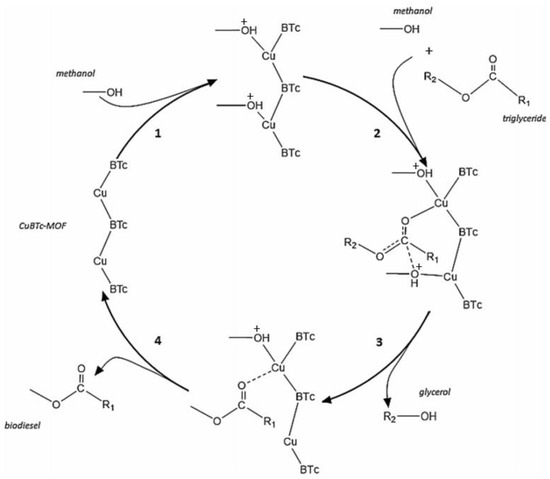
Figure 23.
Transesterification of palm oil to biodiesel using Cu-BTc-MOF. Adapted from ref. [202].
Yusuf et al. [203] prepared CuO/UiO-66 metal–organic frameworks via a wet impregnation approach and examined their catalytic activity in making biodiesel from waste cooking oil. The as-prepared 7%CuO/UiO-66 catalyst exhibited better catalytic activity in the transesterification of waste cooking oil with methanol, due to the synergistic effect between UiO-66 and copper oxide in the composite. It was found that optimized oil conversion of 90.1% was achieved at 6 wt.% 7%CuO/UiO-66 catalyst, 20:1 MRMO, and 160 °C temperature in 4 h. Moreover, the 7%CuO/UiO-66 catalyst exhibited excellent stability and reusability, giving a biodiesel conversion of 80% after three cycles of reuse.
Table 4 Comparison of different heterogeneous acid catalysts used in several research studies to convert different feedstock into biodiesel.

Table 4.
Some studies on biodiesel production using heterogeneous acid catalysts.
4. Process Conditions/Parameters for Biodiesel Production
Transesterification reactions occur under various reaction process conditions, including reaction time, molar ratio of alcohol to oil, reaction temperature, and catalyst amount, all of which have a significant effect on biodiesel yield. To achieve a complete reaction and high biodiesel yield that meets regulatory specifications, these conditions have to be optimized during the transesterification reaction. The effects of these reaction conditions are explained in detail in the sections that follow, as depicted in Figure 24.
Figure 24.
Various process conditions to be optimized during the transesterification reaction.
4.1. Effect of Methanol to Oil Molar Ratio (MRMO)
MRMO is vital in both transesterification and esterification reaction of triglycerides. Excess methanol is necessary to achieve high biodiesel yield during the reversible esterification of high FFA triglycerides to biodiesel [204]. When triglyceride interacts with the active site of the catalyst, it is converted into fatty acid methyl ester (FAME). At the carbonyl group, triglycerides are protonated to form carbocation, that could undergo a reaction to produce an ester [205]. However, excess methanol is flooded into the active sites of the catalyst, preventing formation of protonated triglycerides. During methanolysis, excess methanol is problematic during the separation of glycerol and ester layer because of the solubility of glycerol layer in the ester layer. Methanol functions as an emulsifier due to its polar nature, aiding emulsion formation [56]. Nevertheless, it is believed that using a large amount of methanol improved the interaction between triglycerides and catalyst and enhanced biodiesel yield [206].
Several attempts have been made to investigate the optimum MRMO required to obtain higher biodiesel yield. Li et al. [207] investigated the effect of process parameters on the biodiesel yield using MRMO in the range of 10:1 to 30:1. An optimal biodiesel yield of 90% was obtained using 20:1 MRMO at 65 °C with 3 wt.% catalyst amounts. Furthermore, a significant decrease in biodiesel yield was observed when changing the MRMO from 20:1 to 30:1. Hence, for economics and ease of methanol regeneration, 20:1 MRMO was adopted. Atapour et al. [208] found that a 90.8% biodiesel yield was obtained using 7:1 MRMO. The authors further observed that the biodiesel yield decreased by increasing the MRMO from 7:1 to 11:1 for the given transesterification reaction.
Amani et al. [209] investigated the impact of the MRMO on waste cooking palm oil biodiesel production. A biodiesel yield of 93% was obtained from the transesterification using 14:1 MRMO. Furthermore, when the MRMO was raised from 14:1 to 18:1, the biodiesel yield decreased. Similarly, Hoque et al. [210] explored the impact of methanol on biodiesel yield during WCO transesterification. They reported that maximum biodiesel yield was obtained with a 6:1 MRMO in 2 h. Birla et al. [101] utilized a heterogeneous catalyst obtained from snail shells to examine the effect of different variables on the production of biodiesel. An optimum biodiesel yield of 87.28% was obtained using 2.0 wt.% catalyst and optimal MRMO of 8.45:1 in 7 h. Furthermore, the authors observed no increase in biodiesel yield while the MRMO was increased beyond the optimum.
Phan et al. [64] studied the effect of methanol on biodiesel yield during transesterification of WCO using KOH catalyst. The authors found that methyl ester yield increased as MRMO increased from 5:1 to 8:1, and an optimum yield of 90% was obtained using 8:1 MRMO after about 90 min. They observed that biodiesel yield decreased when MRMO increased beyond 9:1 under the same process conditions. The authors further explained that the decrease in product yield was because separation of glycerol becomes difficult at higher MRMO and the yield of methyl ester reduced since a part of the byproduct, which is glycerol, remains in the biodiesel phase.
Patil et al. [211] investigated the effect of MRMO on biodiesel yield from WCO. They found that biodiesel yield increased with MRMO. However, once the optimal MRMO of 9:1 was reached, the effect became insignificant, and the yield remained essentially unchanged. Similarly, the influence of methanol amount on biodiesel conversion from transesterification reaction of castor and linseed oils was investigated by Varma et al. [212]. They found that biodiesel yield increased with MRMO and maximum biodiesel yield was realized at an optimal MRMO of 40:1 from transesterification of each feedstock. Further, biodiesel yield remained constant when the MRMO increased from 40:1 to 70:1. Song et al. [213] studied the effect of methanol amount on biodiesel conversion from transesterification reaction of RBD (refined, bleached, and deodorized) palm oil. The results showed that biodiesel yield increased with increasing MRMO and maximum biodiesel yield was attained at an optimum MRMO of 30:1 from transesterification of the feedstock. Further, it was observed that biodiesel yield remained constant when MRMO increased beyond the optimum. He et al. [214] investigated the impact of methanol/oil molar ratio on biodiesel production from soybean oil. The authors claimed that biodiesel yield increased as the MRMO increased, and maximum yield was obtained with MRMO of 40:1. Moreover, the authors further reported that no substantial increase in the biodiesel yield was observed when MRMO increased beyond the optimal molar ratio (40:1). Using a 12:1 molar ratio of methanol to vegetable oil, Kataria et al. [215] reported the maximum biodiesel yield of 98.5%. However, they observed a reduction in biodiesel yield beyond the optimum ratio due to excess methanol, resulting in the production of excess glycerol that could potentially block the active sites in the catalyst. Kant et al. [216] also reported similar results, achieving a conversion of 75.3% when using MRMO of 25:1. However, increasing the MRMO beyond the optimal ratio led to a decrease in the conversion. Furthermore, it was noted that an excess methanol had the potential to deactivate enzymatic catalyst, thereby affecting the overall biodiesel conversion [217].
In principle, it is generally concluded that the MRMO is one of the most important parameters that influence biodiesel yield during transesterification of oil to biodiesel. During transesterification of triglycerides to biodiesel, excess alcohol is required to push the reversible reaction process to the product side in order to increase reaction rate and biodiesel yield. However, as some studies have shown, using too much alcohol slowed the formation of the biodiesel products due to dilution of the system [218]. The use of excess amounts of alcohol also poses problems in processing, equipment cost, and energy consumption for methanol recovery [218,219]. Moreover, because of the increased solubility of glycerol in the ester phase, a considerable amount of methanol interferes with glycerol separation [220]. When glycerol is present in an ester solution, it aids in the shift of the reversible reaction process to the reactant’s side, reducing the ester yield [221]. Therefore, for transesterification of the selected feedstock, MRMO must be optimized in order to reduce biodiesel production cost and obtain the highest biodiesel yield possible.
4.2. Effect of Temperature
Another important process parameter that influences biodiesel yield and reaction rate during transesterification reaction is the temperature [48,49]. In principle, the rate of reaction increases as the temperature increases. The transesterification reaction can occur at various temperatures; however, a higher temperature is favorable, as it pushes the reaction to the product side due to its endothermic nature [222]. The use of high temperature reduces oil viscosities and mass transfer problems between the phases, which in turn increases biodiesel yield, reduces reaction time, and increases the rate of reaction [40,46].
Several experiments have been carried out to investigate the effect of temperature on biodiesel yield in order to achieve optimum temperature for the transesterification reaction. Kusdiana et al. [223] investigated the optimum reaction temperature to obtain higher biodiesel yield using supercritical methanol. They found that the conversion increases as the reaction temperature rises from 200 to 400 ℃. The authors noted that after 4 min at 350 °C, rapeseed oil was completely converted to biodiesel, yielding 95.0 wt.% biodiesel. However, the rapeseed oil conversion decreased as the temperature was increased above 350 °C. This is because biodiesel thermal decomposition takes place at a temperature above 350 °C. Varma et al. [212] reported similar findings. According to these authors, the conversion of linseed oil and castor oil to biodiesel increased with an increase in temperature, but at a temperature above 350 °C, thermal decomposition of biodiesel was observed. In addition, they also found that conversion of palm oil and groundnut oil to biodiesel increased with temperature, and biodiesel yield reached a maximum at 350 °C after 40 min. Miao et al. [224] explored the role of reaction temperature on biodiesel yield. They used methanol to transesterify soybean oil at temperatures ranging from 100 to 200 °C. A high biodiesel yield of 98.4% was recorded by the authors at 120 °C, which was considered to be the reaction’s optimal temperature. In the presence of an alkaline catalyst, Phan et al. evaluated the impact of temperature on the methyl ester yield utilizing used cooking oil as a feedstock [64]. They reported that methyl ester content increased as the temperature was increased from 30 to 50 °C, and the methyl ester content slightly decreased as the temperature was increased up to 70 °C because both saponification and transesterification reactions were enhanced at high temperatures. Lam et al. [225] evaluated the transesterification of WCO over a SO42−/SnO2–SiO2 catalyst to produce biodiesel. They found that temperature significantly influences biodiesel yield. The biodiesel yield increased with temperature, with an optimal yield of 81.4% obtained at 150 °C after 1 h. Furthermore, as stated by Saka et al. [226], at a higher temperature, typically around 400 °C, thermal decomposition of triglycerides occurred. Bahar et al. [227] reported high biodiesel yield when the reaction was conducted at room temperature. However, it was observed that beyond optimal temperature, there is a decrease in biodiesel yield. This decrease was attributed to the necessity to keeping the reaction temperature within the boiling point of methanol to prevent the evaporation of methanol. Gunay et al. [228] observed that high temperature promoted saponification reaction, which in turn decrease the biodiesel yield. Therefore, it is essential to optimize the reaction temperature to achieve a high biodiesel yield.
4.3. Effect of Reaction Time
Reaction time plays a crucial role in determining the reaction kinetics of oil conversion to biodiesel under supercritical conditions [229]. The conversion of triglycerides to biodiesel was observed to increase with time in the transesterification of triglycerides [229]. This was attributed to an increase in the reaction rate constant based on the Arrhenius equation. Also, owing to the mass transfer limitation between the oil–methanol catalyst, the conversion rate increases with reaction time. Nevertheless, exceeding the optimum reaction time can result in lower biodiesel yield due to backward transesterification reaction, which in turn reduces the ester content [230,231].
The reaction time is one of the important factors that influence triglyceride conversion to biodiesel; therefore, various attempts have been made to examine the impact of reaction time on biodiesel yield and to determine the optimal time for the transesterification reaction. Rashid et al. [63] studied the influence of reaction time on biodiesel production. The authors reported gradual increases in ester yield with time, obtaining a maximum biodiesel yield of 97.81% in about 70 min. Similarly, Lam et al. [225] investigated the influence of biodiesel yield during transesterification of WCO in the presence of SO42−/SnO2 catalyst using a methanol–ethanol mixture. They reported that a maximum biodiesel yield of 81.40% was obtained with an MREO of 9:6:1, 6.0 wt.% catalyst, reaction temperature of 150 °C, and 60 min reaction time. In addition, the reaction temperature has a direct impact on the reaction time, where a higher reaction temperature assists in the reduction of reaction time for biodiesel production and vice versa [232,233]. The influence of temperature on reaction time during the production of used cooking oil biodiesel was evaluated by Fu et al. [234]. The authors observed that at 65 °C, 80% biodiesel yield was obtained in 11 h reaction time, while at 120 °C, the same biodiesel yield was achieved within just 2 h. Furthermore, the biodiesel yield increased to 95.2% when the reaction at 120 °C was continued for 5 h. Similarly, Kapilakarn et al. [235] obtained a fatty acid methyl ester (FAME) yield of 97% in 50 min at 55 °C, whereas the same FAME yield was obtained in only 20 min at 70 °C.
Thus, it can be inferred that reaction time is crucial in the production of biodiesel from triglyceride transesterification reactions. Since the transesterification reaction is reversible, reaction time must be enhanced to prevent backward reaction and achieve the maximum biodiesel yield for sustainable energy production.
4.4. Effect of Catalyst Loading
A catalyst is believed to increase the rate of a reaction by lowering the overall reaction activation energy. Therefore, the presence of catalysts in the conversion of triglycerides to biodiesel speeds up the reaction process by reducing the required activation energy. Typically, the reaction will be initiated with small catalyst loading, and it will be gradually increased, while other conditions are kept constant, until an optimum biodiesel yield is obtained. Bhatti et al. [236] reported a high biodiesel yield on chicken tallow at 40 °C when H2SO4 catalyst loading was increased from 1 to 3 g. Biodiesel yield decreased when H2SO4 catalyst loading was increased above 2.5 g at the same temperature. Dependence of biodiesel yield on catalyst loading differs slightly at other temperatures. Similarly, in the presence of methanol, Amani et al. [237] evaluated the impact of catalyst loading on biodiesel yield. The authors reported a maximum biodiesel yield of 90% using 3 wt.% catalyst loading in 3 h. However, it was observed that further increasing the catalyst loading to 4 wt.% lowered the biodiesel yield from 90% to 83%. It must also be mentioned that low catalyst amounts can lead to methanol solubility in low-cost feedstock such as waste cooking oil [238]. Santya et al. [239] reported that the optimal catalyst loading in the production of biodiesel from WCO was 1.5 wt.%. It was observed that increasing the catalyst loading beyond the optimum level resulted in a decrease in biodiesel conversion [240]. They identified mass transfer limitations as the primary reason for the decline in biodiesel conversion. They observed that increasing the catalyst loading beyond the optimum level (optimum level: 4.5 wt.%) resulted in a decrease in conversion rate. Therefore, the interdependence of reaction parameters necessitates their optimization, which should be tailored to the specific feedstocks and catalyst employed for the production of biodiesel [241].
5. Conclusions and Future Outlook
Among the various biodiesel production processes from oils and fats, transesterification is the most appealing process because transesterification produces fuel that is highly compatible with the diesel engines that are currently in use. The transesterification process is designed to reduce the viscosity of fats and natural oils. Although microemulsion of oils reduces viscosity, there are still issues with engine performance. This review presents a discussion of the biodiesel production process and various catalysts used in laboratory-scale biodiesel production to date. The following conclusions may be drawn from this review.
1. The feedstock used in biodiesel production is an essential parameter to consider when estimating the overall cost of biodiesel production. Therefore, many studies of various non-food crops feedstock, including non-edible oils, algal lipid, and WCO, have been carried out to reduce the cost of biodiesel production, and also to prevent competition between food and fuel for the same resources.
2. A base homogeneous catalysts process requires mild reaction conditions, has high biodiesel yield, is inexpensive, and has a rapid reaction rate. Nevertheless, the conversion is only effective with refined oils because the catalyst is sensitive to the FFA content of the oil, and produces glycerol and soap as byproducts. As a result, a substantial amount of water is required during the purification process.
3. The acid homogeneous catalyst is suitable for low-cost oil feedstock and it is insensitive to free fatty acid content of oils. Nevertheless, there are a few drawbacks to this process, such as slow reaction rate, corrosion problems, and difficulty in separation of catalyst from the products.
4. A heterogeneous catalyst offers several benefits over a homogeneous catalyst, including fewer disposal issues, elimination of product washing step, less corrosiveness, efficient conversion, faster reaction rate, environmental friendliness, mild reaction conditions, and easy regeneration. However, some issues are associated with heterogeneous catalysts compared with homogeneous catalysts; these include expensive catalyst preparation, being time-consuming, and a mass transfer problem.
5. The biocatalyst process requires a lower reaction temperature. However, this process has a few drawbacks such as slow reaction rate and the high cost of enzyme preparation. Furthermore, the enzyme activity is reduced due to glycerin solubility in biodiesel. The adaptability of the enzyme and further improvement of the current process must be studied in order to reduce cost and improve the reaction rate.
Overall, future work should focus on addressing the following aspects:
- Development of economically viable heterogeneous catalysts with both high activity and selectivity suitable for use in the industry;
- Investigating novel catalyst supports characterized by a selective surface area and an interconnected system of suitable pore sizes;
- Further investigation into biomass or waste materials as catalyst sources in order to develop new catalysts, reduce the cost of biodiesel and enhance the sustainability of commercially available solid catalysts;
- Improving hydrotalcite-based catalyst preparation routes and treatment steps in order to facilitate their transition from laboratory-scale applications to industrial-scale utilization;
- Continuing research and exploration of industrial enzymatic biodiesel production as a prospective and sustainable option for the future;
- Careful design of one-step catalyst preparation routes, avoiding complex multistep grafting methods, to enhance the interaction between acid sites and the catalyst framework structure, thus preventing the leakage of active phases;
- Research focused on investigating the phase behavior of mixtures and enhancing product purification processes;
- The technical viability of the integrated biodiesel–glycerol processing plant should be reevaluated by conducting a techno-economic analysis using real-world experimental and pilot-scale data.
Author Contributions
Conceptualization, S.A.O.; formal analysis, B.O.Y.; investigation, B.O.Y.; methodology, B.O.Y., S.A.O. and S.A.G.; project administration, S.A.O. and S.A.G.; resources, S.A.O. and S.A.G.; supervision, S.A.O.; validation, B.O.Y. and S.A.O.; writing—original draft, B.O.Y.; writing—review and editing, B.O.Y., S.A.O. and S.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
No funding source is reported for this review article.
Data Availability Statement
Data available upon request.
Acknowledgments
The authors gratefully acknowledge Khalid Alhooshani, the Chemistry Department, and King Fahd University of Petroleum and Minerals for making available the resources used for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| °C | Degree Celsius |
| eV | Electronvolt |
| GJ | Gigajoule |
| toe | Ton of oil equivalent |
| BET | Brunauer–Emmett–Teller |
| FAME | Fatty acid methyl ester |
| FFAs | Free fatty acids |
| HPAs | Heteropolyacids |
| MOF | Metal–organic framework |
| MREO | Molar ratio of ethanol to oil |
| MRMO | Molar ratio of methanol to oil |
| RBD | Refined, bleached, and deodorized |
| WCO | Waste cooking oil |
References
- Jess, A. What Might Be the Energy Demand and Energy Mix to Reconcile the World’s Pursuit of Welfare and Happiness with the Necessity to Preserve the Integrity of the Biosphere? Energy Policy 2010, 38, 4663–4678. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Lai, K.K. Global Economic Activity and Crude Oil Prices: A Cointegration Analysis. Energy Econ. 2010, 32, 868–876. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, Technology and Food Security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of Liquid Biofuels from Renewable Resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Bao, Z.; Xiao, H.; Liang, J.; Zhang, L.; Xiong, X.; Sun, N.; Si, T.; Zhao, H. Homology-Integrated CRISPR-Cas (HI-CRISPR) System for One-Step Multigene Disruption in Saccharomyces Cerevisiae. In Proceedings of the Systems Biology 2014–Topical Conference at the 2014 AIChE Annual Meeting, Atlanta, GA, USA, 16–21 November 2014; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 86, pp. 3–12. [Google Scholar]
- Ngomade, S.B.L.; Fotsop, C.G.; Bhonsle, A.K.; Rawat, N.; Gupta, P.; Singh, R.; Tchummegne, I.K.; Singh, R.K.; Atray, N. Pilot-Scale Optimization of Enhanced Biodiesel Production from High FFA Podocarpus Falcatus Oil via Simultaneous Esterification and Transesterification Assisted by Zirconia-Supported ZSM-5. Chem. Eng. Res. Des. 2024, 209, 52–66. [Google Scholar] [CrossRef]
- Balat, M. Potential Alternatives to Edible Oils for Biodiesel Production—A Review of Current Work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, C.; Vittayapadung, S.; Shen, X.; Dong, M. Opportunities and Challenges for Biodiesel Fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel Production from Waste Cooking Oil Using Bifunctional Heterogeneous Solid Catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of Biodiesel as Transportation Fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Muanruksa, P.; Winterburn, J.; Kaewkannetra, P. A Novel Process for Biodiesel Production from Sludge Palm Oil. MethodsX 2019, 6, 2838–2844. [Google Scholar] [CrossRef]
- Sarasota, F. Biofuels Market Analysis by Type (Bioethanol, Biodiesel), and by Form (Solid, Liquid, and Gaseous)—Global Industry Perspective, Comprehensive Analysis, and Forecast 2016–2022. 2017. Available online: https://reportocean.com/industry-verticals/any-question?report_id=13299 (accessed on 22 July 2024).
- Mekonnen, K.D.; Endris, Y.A.; Abdu, K.Y. Alternative Methods for Biodiesel Cetane Number Valuation: A Technical Note. ACS Omega 2024, 9, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Amais, R.S.; Garcia, E.E.; Monteiro, M.R.; Nogueira, A.R.A.; Nóbrega, J.A. Direct Analysis of Biodiesel Microemulsions Using an Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2010, 96, 146–150. [Google Scholar] [CrossRef]
- De Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel Production from Mixtures of Waste Fish Oil, Palm Oil and Waste Frying Oil: Optimization of Fuel Properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Use of Vegetable Oils as I.C. Engine Fuels—A Review. Renew. Energy 2004, 29, 727–742. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel and Renewable Diesel: A Comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel Separation and Purification: A Review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. In Vitro Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Kafuku, G.; Lam, M.K.; Kansedo, J.; Lee, K.T.; Mbarawa, M. Heterogeneous Catalyzed Biodiesel Production from Moringa Oleifera Oil. Fuel Process. Technol. 2010, 91, 1525–1529. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of Transesterification Methods for Production of Biodiesel from Vegetable Oils and Fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Chen, K.S.; Lin, Y.C.; Hsu, K.H.; Wang, H.K. Improving Biodiesel Yields from Waste Cooking Oil by Using Sodium Methoxide and a Microwave Heating System. Energy 2012, 38, 151–156. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V. V Biodiesel Production from Renewable Feedstocks: Status and Opportunities. Renew. Sustain. Energy Rev. 2012, 16, 4763–4784. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A Review on Novel Processes of Biodiesel Production from Waste Cooking Oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Mortimer, S.R. Waste Cooking Oil as an Energy Resource: Review of Chinese Policies. Renew. Sustain. Energy Rev. 2012, 16, 5225–5231. [Google Scholar] [CrossRef]
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakub, Z. Overview on the Current Trends in Biodiesel Production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Azam, M.M.; Waris, A.; Nahar, N.M. Prospects and Potential of Fatty Acid Methyl Esters of Some Non-Traditional Seed Oils for Use as Biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-Edible Vegetable Oils: A Critical Evaluation of Oil Extraction, Fatty Acid Compositions, Biodiesel Production, Characteristics, Engine Performance and Emissions Production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- No, S.Y. Inedible Vegetable Oils and Their Derivatives for Alternative Diesel Fuels in CI Engines: A Review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of Biodiesel: Current Scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651. [Google Scholar] [CrossRef]
- Owolabi, R.U.; Osiyemi, N.A.; Amosa, M.K.; Ojewumi, M.E. Biodiesel from Household/Restaurant Waste Cooking Oil (WCO). J. Chem. Eng. Process Technol. 2011, 2, 2–5. [Google Scholar] [CrossRef]
- Knothe, G. Introduction. In The Biodiesel Handbook, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 90, pp. 1–3. ISBN 9780983507260. [Google Scholar]
- Sankaranarayanan, S.; Antonyraj, C.A.; Kannan, S. Transesterification of Edible, Non-Edible and Used Cooking Oils for Biodiesel Production Using Calcined Layered Double Hydroxides as Reusable Base Catalysts. Bioresour. Technol. 2012, 109, 57–62. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ling, F.W.; Jun, L.S. Proposed Kinetic Mechanism of the Production of Biodiesel from Palm Oil Using Lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel Production Using Heterogeneous Catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gupta, M.N. Lipase Catalyzed Preparation of Biodiesel from Jatropha Oil in a Solvent Free System. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Abdulla, R.; Ravindra, P. Immobilized Burkholderia Cepacia Lipase for Biodiesel Production from Crude Jatropha curcas L. Oil. Biomass Bioenergy 2013, 56, 8–13. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel Production Using Enzymatic Transesterification–Current State and Perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Szczesna Antczak, M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic Biodiesel Synthesis—Key Factors Affecting Efficiency of the Process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel Production–Current State of the Art and Challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef]
- Gonçalves, A.P.V.; Lopes, J.M.; Lemos, F.; Ramôa Ribeiro, F.; Prazeres, D.M.F.; Cabral, J.M.S.; Aires-Barros, M.R. Zeolites as Supports for Enzymatic Hydrolysis Reactions. Comparative Study of Several Zeolites. J. Mol. Catal. B Enzym. 1996, 1, 53–60. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the Time Finally Come for Green Oleochemicals and Biodiesel Production Using Large-Scale Enzyme Technologies? Current Status and New Developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Faisal, S.; Sadek, M.S.; Pastore, C.; di Bitonto, L.; Alshammari, S.O.; Mussagy, C.U.; El-Bahy, S.M.; Abdellatief, T.M.M.; El-Bahy, Z.M.; Mustafa, A. Sustainable Synthesis of 2-Ethyl Hexyl Oleate via Lipase-Catalyzed Esterification: A Holistic Simulation and Cost Analysis Study. Sustain. Chem. Pharm. 2024, 41, 101726. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Brask, J.; Fjerbaek, L. Enzymatic Biodiesel Production: Technical and Economical Considerations. Eur. J. Lipid Sci. Technol. 2008, 110, 692–700. [Google Scholar] [CrossRef]
- Mustafa, A. Lipase Catalyzed Reactions: A Promising Approach for Clean Synthesis of Oleochemicals. In Sustainable Solutions for Environmental Pollution; Wiley: Hoboken, NJ, USA, 2021; pp. 417–447. [Google Scholar]
- Jayaraman, J.; Alagu, K.; Appavu, P.; Joy, N.; Jayaram, P.; Mariadoss, A. Enzymatic Production of Biodiesel Using Lipase Catalyst and Testing of an Unmodified Compression Ignition Engine Using Its Blends with Diesel. Renew. Energy 2020, 145, 399–407. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of Immobilized Lipases in the Transesterification of Waste Fish Oil for the Production of Biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Choi, N.; No, D.S.; Kim, H.; Kim, B.H.; Kwak, J.; Lee, J.S.; Kim, I.H. In Situ Lipase-Catalyzed Transesterification in Rice Bran for Synthesis of Fatty Acid Methyl Ester. Ind. Crops Prod. 2018, 120, 140–146. [Google Scholar] [CrossRef]
- Mata, T.M.; Sousa IR, B.G.; Vieira, S.S.; Caetano, N.S. Biodiesel Production from Corn Oil via Enzymatic Catalysis with Ethanol. Energy Fuels 2012, 26, 3034–3041. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Biodiesel Production from Calophyllum Inophyllum Oil Using Lipase Producing Rhizopus Oryzae Cells Immobilized within Reticulated Foams. Renew. Energy 2014, 64, 276–282. [Google Scholar] [CrossRef]
- Li, X.; He, X.Y.; Li, Z.L.; Wang, Y.D.; Wang, C.Y.; Shi, H.; Wang, F. Enzymatic Production of Biodiesel from Pistacia Chinensis Bge Seed Oil Using Immobilized Lipase. Fuel 2012, 92, 89–93. [Google Scholar] [CrossRef]
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, G. Sustainable Preparation of a Novel Glycerol-Free Biofuel by Using Pig Pancreatic Lipase: Partial 1,3-Regiospecific Alcoholysis of Sunflower Oil. Process Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Macario, A.; Moliner, M.; Corma, A.; Giordano, G. Increasing Stability and Productivity of Lipase Enzyme by Encapsulation in a Porous Organic-Inorganic System. Microporous Mesoporous Mater. 2009, 118, 334–340. [Google Scholar] [CrossRef]
- Kamini, N.R.; Iefuji, H. Lipase Catalyzed Methanolysis of Vegetable Oils in Aqueous Medium by Cryptococcus spp. S-2. Process Biochem. 2001, 37, 405–410. [Google Scholar] [CrossRef]
- Mittelbach, M. Lipase Catalyzed Alcoholysis of Sunflower Oil. J. Am. Oil Chem. Soc. 1990, 67, 168–170. [Google Scholar] [CrossRef]
- Alonazi, M.; Al-diahan, S.K.; Alzahrani, Z.R.A.; Ben, A. Combined Immobilized Lipases for Effective Biodiesel Production from Spent Coffee Grounds. Saudi J. Biol. Sci. 2023, 30, 103772. [Google Scholar] [CrossRef] [PubMed]
- de Boer, K.; Moheimani, N.R.; Borowitzka, M.A.; Bahri, P.A. Extraction and Conversion Pathways for Microalgae to Biodiesel: A Review Focused on Energy Consumption. J. Appl. Phycol. 2012, 24, 1681–1698. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern Heterogeneous Catalysts for Biodiesel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Balat, M. Biodiesel Fuel from Triglycerides via Transesterificationa Review. Energy Sources Part A Recover. 2009, 31, 1300–1314. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic Heterogeneous Catalysts for Biodiesel Production from Vegetable Oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- De Lima, A.L.; Ronconi, C.M.; Mota, C.J.A. Heterogeneous Basic Catalysts for Biodiesel Production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of Biodiesel through Optimized Alkaline-Catalyzed Transesterification of Rapeseed Oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel Production from Waste Cooking Oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Biodiesel Production from Croton Megalocarpus Oil and Its Process Optimization. Fuel 2010, 89, 2556–2560. [Google Scholar] [CrossRef]
- Sivakumar, P.; Anbarasu, K.; Renganathan, S. Bio-Diesel Production by Alkali Catalyzed Transesterification of Dairy Waste Scum. Fuel 2011, 90, 147–151. [Google Scholar] [CrossRef]
- Dueso, C.; Muñoz, M.; Moreno, F.; Arroyo, J.; Gil-Lalaguna, N.; Bautista, A.; Gonzalo, A.; Sánchez, J.L. Performance and Emissions of a Diesel Engine Using Sunflower Biodiesel with a Renewable Antioxidant Additive from Bio-Oil. Fuel 2018, 234, 276–285. [Google Scholar] [CrossRef]
- Kamran, E.; Mashhadi, H.; Mohammadi, A.; Ghobadian, B. Biodiesel Production from Elaeagnus angustifolia. L Seed as a Novel Waste Feedstock Using Potassium Hydroxide Catalyst. Biocatal. Agric. Biotechnol. 2020, 25, 101578. [Google Scholar] [CrossRef]
- Wakil, M.A.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R. Rice Bran: A Prospective Resource for Biodiesel Production in Bangladesh. Int. J. Green Energy 2016, 13, 497–504. [Google Scholar] [CrossRef]
- Photaworn, S.; Tongurai, C.; Kungsanunt, S. Process Development of Two-Step Esterification plus Catalyst Solution Recycling on Waste Vegetable Oil Possessing High Free Fatty Acid. Chem. Eng. Process. Process Intensif. 2017, 118, 1–8. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Moheimani, N.R.; Ennaceri, H. Graphene-Based Catalysts for Biodiesel Production: Characteristics and Performance. Sci. Total Environ. 2023, 859, 160000. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the Art of Biodiesel Production Processes: A Review of the Heterogeneous Catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Tran, H.L.; Ryu, Y.J.; Seong, D.H.; Lim, S.M.; Lee, C.G. An Effective Acid Catalyst for Biodiesel Production from Impure Raw Feedstocks. Biotechnol. Bioprocess Eng. 2013, 18, 242–247. [Google Scholar] [CrossRef]
- Schuchardt, U.; Sercheli, R.; Matheus, R. Transesterification of Vegetable Oils: A Review General Aspects of Transesterification Transesterification of Vegetable Oils Acid-Catalyzed Processes Base-Catalyzed Processes. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview Properties of Biodiesel Diesel Blends from Edible and Non-Edible Feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Freedman, B.E.H.P.; Pryde, E.H.; Mounts, T.L. Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; De Coimbra, M.A.; Soletti, J.I.; Carvalho, S.H.V. Ethanolysis of Castor and Cottonseed Oil: A Systematic Study Using Classical Catalysts. J. Am. Oil Chem. Soc. 2006, 83, 819–822. [Google Scholar] [CrossRef]
- Crabbe, E.; Nolasco-Hipolito, C.; Kobayashi, G.; Sonomoto, K.; Ishizaki, A. Biodiesel Production from Crude Palm Oil and Evaluation of Butanol Extraction and Fuel Properties. Process Biochem. 2001, 37, 65–71. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of Two Different Processes to Synthesize Biodiesel by Waste Cooking Oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A Review on Latest Developments and Future Prospects of Heterogeneous Catalyst in Biodiesel Production from Non-Edible Oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha curcas L. ): A Review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Shanks, B.H. Conversion of Oils and Fats Using Advanced Mesoporous Heterogeneous Catalysts. J. Am. Oil Chem. Soc. 2006, 83, 79–91. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, R.; Sinha, R.; Sarin, R.; Malhotra, R.; Verma, R.; Raghunath, N. Process for Producing Biodiesel and the Product Thereof. US20060094890A1, 4 May 2006. [Google Scholar]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of Solid Catalysts for Biodiesel Production: A Review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a New Biodiesel That Integrates Glycerol, by Using CaO as Heterogeneous Catalyst, in the Partial Methanolysis of Sunflower Oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.P.; Pomonis, P.J.; Kontominas, M.G. Transesterification of Soybean Frying Oil to Biodiesel Using Heterogeneous Catalysts. Fuel Process. Technol. 2009, 90, 671–676. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of Soybean Oil to Biodiesel Using CaO as a Solid Base Catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Lee, D.W.; Park, Y.M.; Lee, K.Y. Heterogeneous Base Catalysts for Transesterification in Biodiesel Synthesis. Catal. Surv. from Asia 2009, 13, 63–77. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of Soybean Oil to Biodiesel Using SrO as a Solid Base Catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, B.S.; Kim, M.J.; Park, Y.M.; Kim, D.K.; Lee, J.S.; Lee, K.Y. Transesterification of Vegetable Oil to Biodiesel Using Heterogeneous Base Catalyst. Proc. Catal. Today 2004, 93–95, 315–320. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from Sunflower Oil by Using Activated Calcium Oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Veljković, V.B.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Skala, D.U. Kinetics of Sunflower Oil Methanolysis Catalyzed by Calcium Oxide. Fuel 2009, 88, 1554–1562. [Google Scholar] [CrossRef]
- Kaur, M.; Ali, A. Lithium Ion Impregnated Calcium Oxide as Nano Catalyst for the Biodiesel Production from Karanja and Jatropha Oils. Renew. Energy 2011, 36, 2866–2871. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Yamanaka, S.; Hidaka, J. Active Phase of Calcium Oxide Used as Solid Base Catalyst for Transesterification of Soybean Oil with Refluxing Methanol. Appl. Catal. A Gen. 2008, 334, 357–365. [Google Scholar] [CrossRef]
- Mootabadi, H.; Salamatinia, B.; Bhatia, S.; Abdullah, A.Z. Ultrasonic-Assisted Biodiesel Production Process from Palm Oil Using Alkaline Earth Metal Oxides as the Heterogeneous Catalysts. Fuel 2010, 89, 1818–1825. [Google Scholar] [CrossRef]
- Salamatinia, B.; Mootabadi, H.; Bhatia, S.; Abdullah, A.Z. Optimization of Ultrasonic-Assisted Heterogeneous Biodiesel Production from Palm Oil: A Response Surface Methodology Approach. Fuel Process. Technol. 2010, 91, 441–448. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste Snail Shell Derived Heterogeneous Catalyst for Biodiesel Production by the Transesterification of Soybean Oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef] [PubMed]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste Shells of Mollusk and Egg as Biodiesel Production Catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Nair, P.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Synthesis of Biodiesel from Low FFA Waste Frying Oil Using Calcium Oxide Derived from Mereterix Mereterix as a Heterogeneous Catalyst. J. Clean. Prod. 2012, 29–30, 82–90. [Google Scholar] [CrossRef]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics Studies of Synthesis of Biodiesel from Waste Frying Oil Using a Heterogeneous Catalyst Derived from Snail Shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Li, H. Soybean Oil Methyl Esters Preparation Using NaX Zeolites Loaded with KOH as a Heterogeneous Catalyst. Bioresour. Technol. 2007, 98, 936–939. [Google Scholar] [CrossRef]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a Potential Biofuel Obtained from Soybean Oil by Transmethylation with Dimethyl Carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste Ostrich- and Chicken-Eggshells as Heterogeneous Base Catalyst for Biodiesel Production from Used Cooking Oil: Catalyst Characterization and Biodiesel Yield Performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Xu, C.; Sun, J.; Zhao, B.; Liu, Q. On the Study of KF/Zn(Al)O Catalyst for Biodiesel Production from Vegetable Oil. Appl. Catal. B Environ. 2010, 99, 111–117. [Google Scholar] [CrossRef]
- Navajas, A.; Campo, I.; Moral, A.; Echave, J.; Sanz, O.; Montes, M.; Odriozola, J.A.; Arzamendi, G.; Gandía, L.M. Outstanding Performance of Rehydrated Mg-Al Hydrotalcites as Heterogeneous Methanolysis Catalysts for the Synthesis of Biodiesel. Fuel 2018, 211, 173–181. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Porntangjitlikit, S. Palm Oil Biodiesel Synthesized with Potassium Loaded Calcined Hydrotalcite and Effect of Biodiesel Blend on Elastomer Properties. Renew. Energy 2008, 33, 1558–1563. [Google Scholar] [CrossRef]
- del Remedio Hernandez, M.; Reyes-Labarta, J.A.; Valdes, F.J. New Heterogeneous Catalytic Transesterification of Vegetable and Used Frying Oil. Ind. Eng. Chem. Res. 2010, 49, 9068–9076. [Google Scholar] [CrossRef]
- Yan, S.; Mohan, S.; Dimaggio, C.; Kim, M.; Ng, K.Y.S.; Salley, S.O. Long Term Activity of Modified ZnO Nanoparticles for Transesterification. Fuel 2010, 89, 2844–2852. [Google Scholar] [CrossRef]
- Agarwal, M.; Chauhan, G.; Chaurasia, S.P.; Singh, K. Study of Catalytic Behavior of KOH as Homogeneous and Heterogeneous Catalyst for Biodiesel Production. J. Taiwan Inst. Chem. Eng. 2012, 43, 89–94. [Google Scholar] [CrossRef]
- Molaei Dehkordi, A.; Ghasemi, M. Transesterification of Waste Cooking Oil to Biodiesel Using Ca and Zr Mixed Oxides as Heterogeneous Base Catalysts. Fuel Process. Technol. 2012, 97, 45–51. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic Ionic Liquid Functionalized Magnetically Responsive Fe3O4@ HKUST-1 Composites Used for Biodiesel Production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Guanidine Post-Functionalized Crystalline ZIF-90 Frameworks as a Promising Recyclable Catalyst for the Production of Biodiesel via Soybean Oil Transesteri Fi Cation. Energy Convers. Manag. 2019, 198, 111922. [Google Scholar] [CrossRef]
- Abdelmigeed, M.O.; Al-sakkari, E.G.; Hefney, M.S.; Ismail, F.M.; Ahmed, T.S.; Ismail, I.M. Biodiesel Production Catalyzed by NaOH/Magnetized ZIF-8: Yield Improvement Using Methanolysis and Catalyst Reusability Enhancement. Renew. Energy 2021, 174, 253–261. [Google Scholar] [CrossRef]
- Saeedi, M.; Fazaeli, R.; Aliyan, H. Nanostructured Sodium–Zeolite Imidazolate Framework (ZIF-8) Doped with Potassium by Sol–Gel Processing for Biodiesel Production from Soybean Oil. J. Sol-Gel Sci. Technol. 2016, 77, 404–415. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Ma, X.; Wu, Z.; Li, Y.; Zhang, L. Catalytic Performance of Strontium Oxide Supported by MIL–100 (Fe) Derivate as Transesteri Fi Cation Catalyst for Biodiesel Production. Energy Convers. Manag. 2019, 180, 401–410. [Google Scholar] [CrossRef]
- Yang, C.; Viet, M.; Liang, T.; Khoa, T.; Kieu, T.; Huynh, X. Metal-Organic Framework-Derived Mg-Zn Hybrid Nanocatalyst for Biodiesel Production. Adv. Powder Technol. 2022, 33, 103365. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H. Production of Biodiesel through Transesteri Fi Cation of Soybean Oil Using ZIF-8@GO Doped with Sodium and Potassium Catalyst. Russ. J. Appl. Chem. 2015, 88, 1701–1710. [Google Scholar] [CrossRef]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and Calcium-Based Metal Organic Framework (MOF) Catalyst for Biodiesel Production from Waste Cooking Oil: A Process Optimization Study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A Review of Current Technology for Biodiesel Production: State of the Art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Amin, A. Review of Diesel Production from Renewable Resources: Catalysis, Process Kinetics and Technologies. Ain Shams Eng. J. 2019, 10, 821–839. [Google Scholar] [CrossRef]
- Meher, L.C.; Churamani, C.P.; Arif, M.; Ahmed, Z.; Naik, S.N. Jatropha Curcas as a Renewable Source for Bio-Fuels—A Review. Renew. Sustain. Energy Rev. 2013, 26, 397–407. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of Heterogeneous Solid Acid Catalyst Performance on Low Grade Feedstocks for Biodiesel Production: A Review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized Polymeric Sulfonated Ionic Liquid on Core-Shell Structured Fe3O4/SiO2 Composites: A Magnetically Recyclable Catalyst for Simultaneous Transesterification and Esterifications of Low-Cost Oils to Biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-based Heterogeneous Catalysts for Biodiesel Production: A Comprehensive Review. Int. J. Energy Res. 2022, 46, 3782–3809. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science–Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Clark, J.H.; Monks, G.L.; Nightingale, D.J.; Price, P.M.; White, J.F. A New Solid Acid-Based Route to Linear Alkylbenzenes. J. Catal. 2000, 193, 348–350. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in Solid Acid Catalysts for Biodiesel Production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of Crude Palm Kernel Oil and Crude Coconut Oil by Different Solid Catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, H.R.; El-Molla, S.A. Influence of Support on Physicochemical Properties of ZrO2 Based Solid Acid Heterogeneous Catalysts for Biodiesel Production. Catal. Commun. 2019, 122, 10–15. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, J.Y.; Chung, S.H.; Park, I.S.; Lee, S.Y.; Kim, D.K.; Lee, J.S.; Lee, K.Y. Esterification of Used Vegetable Oils Using the Heterogeneous WO3/ZrO2 Catalyst for Production of Biodiesel. Bioresour. Technol. 2010, 101, S59–S61. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Omota, F.; Kiss, A.A. Innovative Process for Fatty Acid Esters by Dual Reactive Distillation. Comput. Chem. Eng. 2009, 33, 743–750. [Google Scholar] [CrossRef]
- Yadav, G.D.; Murkute, A.D. Preparation of a Novel Catalyst UDCaT-5: Enhancement in Activity of Acid-Treated Zirconia–Effect of Treatment with Chlorosulfonic Acid Vis-à-Vis Sulfuric Acid. J. Catal. 2004, 224, 218–223. [Google Scholar] [CrossRef]
- Enascuta, C.E.; Stepan, E.; Bolocan, I.; Bombos, D.; Calin, C.; Oprescu, E.E.; Lavric, V. Simultaneous Production of Oil Enriched in ω-3 Polyunsaturated Fatty Acids and Biodiesel from Fish Wastes. Waste Manag. 2018, 75, 205–214. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Calcined Zirconium Sulfate Supported on MCM-41 Silica as Acid Catalyst for Ethanolysis of Sunflower Oil. Appl. Catal. B Environ. 2011, 103, 91–98. [Google Scholar] [CrossRef]
- Gopinath, S.; Kumar, P.S.M.; Arafath, K.A.Y.; Thiruvengadaravi, K.V.; Sivanesan, S.; Baskaralingam, P. Efficient Mesoporous SO42−/Zr-KIT-6 Solid Acid Catalyst for Green Diesel Production from Esterification of Oleic Acid. Fuel 2017, 203, 488–500. [Google Scholar] [CrossRef]
- Fatimah, I.; Rubiyanto, D.; Taushiyah, A.; Najah, F.B.; Azmi, U.; Sim, Y.L. Use of ZrO2 Supported on Bamboo Leaf Ash as a Heterogeneous Catalyst in Microwave-Assisted Biodiesel Conversion. Sustain. Chem. Pharm. 2019, 12, 100129. [Google Scholar] [CrossRef]
- Wang, Y.T.; Fang, Z.; Zhang, F. Esterification of Oleic Acid to Biodiesel Catalyzed by a Highly Acidic Carbonaceous Catalyst. Catal. Today 2019, 319, 172–181. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel Synthesis from Microalgal Lipids Using Tungstated Zirconia as a Heterogeneous Acid Catalyst and Its Comparison with Homogeneous Acid and Enzyme Catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Sun, H.; Ding, Y.; Duan, J.; Zhang, Q.; Wang, Z.; Lou, H.; Zheng, X. Transesterification of Sunflower Oil to Biodiesel on ZrO2 Supported La2O3 Catalyst. Bioresour. Technol. 2010, 101, 953–958. [Google Scholar] [CrossRef]
- Malhotra, R.; Ali, A. 5-Na/ZnO Doped Mesoporous Silica as Reusable Solid Catalyst for Biodiesel Production via Transesterification of Virgin Cottonseed Oil. Renew. Energy 2019, 133, 606–619. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and Kinetics of Biodiesel Production from Mahua Oil Using Manganese Doped Zinc Oxide Nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel Production from Castor Oil Using Heterogeneous Ni Doped ZnO Nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and Application of Co Doped ZnO as Heterogeneous Nanocatalyst for Biodiesel Production from Non-Edible Oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Nehdi, I.A.; Al-Resayes, S.I.; Al-Muhtaseb, A.H. Sulfonated Mesoporous Zinc Aluminate Catalyst for Biodiesel Production from High Free Fatty Acid Feedstock Using Microwave Heating System. J. Taiwan Inst. Chem. Eng. 2017, 70, 219–228. [Google Scholar] [CrossRef]
- AlSharifi, M.; Znad, H. Transesterification of Waste Canola Oil by Lithium/Zinc Composite Supported on Waste Chicken Bone as an Effective Catalyst. Renew. Energy 2020, 151, 740–749. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Catalytic Action of Sulfated Tin Oxide for Etherification and Esterification in Comparison with Sulfated Zirconia. Appl. Catal. A Gen. 2004, 269, 187–191. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Sulfated Tin Oxide as Solid Superacid Catalyst for Transesterification of Waste Cooking Oil: An Optimization Study. Appl. Catal. B Environ. 2009, 93, 134–139. [Google Scholar] [CrossRef]
- Khder, A.S.; El-Sharkawy, E.A.; El-Hakam, S.A.; Ahmed, A.I. Surface Characterization and Catalytic Activity of Sulfated Tin Oxide Catalyst. Catal. Commun. 2008, 9, 769–777. [Google Scholar] [CrossRef]
- Kafuku, G.; Lee, K.T.; Mbarawa, M. The Use of Sulfated Tin Oxide as Solid Superacid Catalyst for Heterogeneous Transesterification of Jatropha Curcas Oil. Chem. Pap. 2010, 64, 734–740. [Google Scholar] [CrossRef]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- He, C.; Baoxiang, P.; Dezheng, W.; Jinfu, W. Biodiesel Production by the Transesterification of Cottonseed Oil by Solid Acid Catalysts. Front. Chem. Eng. China 2007, 1, 571–575. [Google Scholar] [CrossRef]
- Peng, B.X.; Shu, Q.; Wang, J.F.; Wang, G.R.; Wang, D.Z.; Han, M.H. Biodiesel Production from Waste Oil Feedstocks by Solid Acid Catalysis. Process Saf. Environ. Prot. 2008, 86, 441–447. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel Production from Used Cooking Oil Using a Novel Surface Functionalised TiO2 Nano-Catalyst. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef]
- Kaur, M.; Malhotra, R.; Ali, A. Tungsten Supported Ti/SiO2 Nanoflowers as Reusable Heterogeneous Catalyst for Biodiesel Production. Renew. Energy 2018, 116, 109–119. [Google Scholar] [CrossRef]
- Dai, Y.M.; Kao, I.H.; Chen, C.C. Evaluating the Optimum Operating Parameters of Biodiesel Production Process from Soybean Oil Using the Li2TiO3 Catalyst. J. Taiwan Inst. Chem. Eng. 2017, 70, 260–266. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid Acid Catalysts for Biodiesel Production–Towards Sustainable Energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Omar, B.M.; Bita, M.; Louafi, I.; Djouadi, A. Esterification Process Catalyzed by ZSM-5 Zeolite Synthesized via Modified Hydrothermal Method. MethodsX 2018, 5, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY Zeolite as a Heterogeneous Alkaline Catalyst for Biodiesel Production: Process Optimization and Kinetics Study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel Production by Esterification of Oleic Acid over Zeolite Y Prepared from Kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Oladepo, S.A.; Ganiyu, S.A. Zr-Modified Desilicated ZSM-5 Catalysts as Highly Active and Recyclable Catalysts for Production of Biodiesel from Soybean Oil: Insight into Improved Catalyst Properties, Acidity and Dispersion through Desilication. Fuel 2023, 351, 128729. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Oladepo, S.A.; Ganiyu, S.A. Biodiesel Production from Waste Cooking Oil via β– Zeolite- Supported Sulfated Metal Oxide Catalyst Systems. ACS Omega 2023, 8, 23720–23732. [Google Scholar] [CrossRef]
- Hou, S.; Xie, W. Three-Dimensional Hierarchical Meso/Macroporous Mo/Ce/TiO2 Composites Enhances Biodiesel Production from Acidic Soybean Oil by Transesterification-Esterifiications. Energy Convers. Manag. 2024, 305, 118273. [Google Scholar] [CrossRef]
- Modupe Abati, S.; Bamisaye, A.; Abidemi Adaramaja, A.; Rapheal Ige, A.; Adesina Adegoke, K.; Olurotimi Ogunbiyi, E.; Abidemi Idowu, M.; Olabintan, A.B.; Saleh, T.A. Biodiesel Production from Spent Vegetable Oil with Al2O3 and Fe2O3-Biobased Heterogenous Nanocatalysts: Comparative and Optimization Studies. Fuel 2024, 364, 130847. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Lee, J.; Devanesan, S.; Priya, S.D.; Shanmuganathan, R. Catalytic Biodiesel Production from Jatropha Curcas Oil: A Comparative Analysis of Microchannel, Fixed Bed, and Microwave Reactor Systems with Recycled ZSM-5 Catalyst. Environ. Res. 2024, 258, 119474. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Ghasemi, I.; Bekhradinassab, E. Design of Highly Recoverable Clay-Foundation Composite of Plasma-Treated Co3O4/Kaolin to Produce Biodiesel from Low-Cost Oil. Fuel 2024, 366, 131267. [Google Scholar] [CrossRef]
- Saadiah, N.; Ngadi, N.; Mohammed, I.; Anako, L.; Yamani, Z.; Haron, S. A Cleaner Approach with Magnetically Assisted Reactor Setup over CaO-Zeolite/Fe3O4 Catalyst in Biodiesel Production: Evaluation of Catalytic Performance, Reusability and Life Cycle Assessment Studies. J. Clean. Prod. 2023, 419, 138329. [Google Scholar] [CrossRef]
- Russbueldt, B.M.E.; Hoelderich, W.F. New Sulfonic Acid Ion-Exchange Resins for the Preesterification of Different Oils and Fats with High Content of Free Fatty Acids. Appl. Catal. A Gen. 2009, 362, 47–57. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.E.; Holmen, A.; Keurentjes, J.T.F. Comparison of Commercial Solid Acid Catalysts for the Esterification of Acetic Acid with Butanol. Appl. Catal. A Gen. 2006, 297, 182–188. [Google Scholar] [CrossRef]
- Park, J.Y.; Wang, Z.M.; Kim, D.K.; Lee, J.S. Effects of Water on the Esterification of Free Fatty Acids by Acid Catalysts. Renew. Energy 2010, 35, 614–618. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G.; Bruce, D.A.; Lotero, E. Transesterification of Triacetin with Methanol on Solid Acid and Base Catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Tesser, R.; Casale, L.; Verde, D.; Di Serio, M.; Santacesaria, E. Kinetics and Modeling of Fatty Acids Esterification on Acid Exchange Resins. Chem. Eng. J. 2010, 157, 539–550. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha Cultivation in Southern India: Assessing Farmers’ Experiences. Biofuels, Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G. A Comparison of the Esterification of Acetic Acid with Methanol Using Heterogeneous versus Homogeneous Acid Catalysis. J. Catal. 2006, 242, 278–286. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Okuhara, T. Liquid Phase Esterification of Acrylic Acid with 1-Butanol Catalyzed by Solid Acid Catalysts. Appl. Catal. A Gen. 1999, 180, 261–269. [Google Scholar] [CrossRef]
- Vicente, G.; Coteron, A.; Martinez, M.; Aracil, J. Application of the Factorial Design of Experiments and Response Surface Methodology to Optimize Biodiesel Production. Ind. Crops Prod. 1998, 8, 29–35. [Google Scholar] [CrossRef]
- Dos Reis, S.C.M.; Lachter, E.R.; Nascimento, R.S.V.; Rodrigues, J.A.; Reid, M.G. Transesterification of Brazilian Vegetable Oils with Methanol over Ion-Exchange Resins. J. Am. Oil Chem. Soc. 2005, 82, 661–665. [Google Scholar] [CrossRef]
- Nakajima, K.; Hara, M. Amorphous Carbon with SO3H Groups as a Solid Brønsted Acid Catalyst. ACS Catal. 2012, 2, 1296–1304. [Google Scholar] [CrossRef]
- Zong, M.H.; Duan, Z.Q.; Lou, W.Y.; Smith, T.J.; Wu, H. Preparation of a Sugar Catalyst and Its Use for Highly Efficient Production of Biodiesel. Green Chem. 2007, 9, 434–443. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient Production of Biodiesel from High Free Fatty Acid-Containing Waste Oils Using Various Carbohydrate-Derived Solid Acid Catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of Biodiesel from Waste Vegetable Oil with Large Amounts of Free Fatty Acids Using a Carbon-Based Solid Acid Catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Misono, M. Unique Acid Catalysis of Heteropoly Compounds (Heteropolyoxometalates) in the Solid State. Chem. Commun. 2001, 1, 1141–1153. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A. 12-Tungstophosphoric Acid Supported on Mesoporous Molecular Material: Synthesis, Characterization and Performance in Biodiesel Production. J. Clean. Prod. 2014, 72, 46–56. [Google Scholar] [CrossRef]
- Cao, F.; Chen, Y.; Zhai, F.; Li, J.; Wang, J.; Wang, X.; Wang, S.; Zhu, W. Biodiesel Production from High Acid Value Waste Frying Oil Catalyzed by Superacid Heteropolyacid. Biotechnol. Bioeng. 2008, 101, 93–100. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Chen, Y.; Wang, J.; Feng, L.; Wang, X.; Cao, F. Heteropolyacid Nanoreactor with Double Acid Sites as a Highly Efficient and Reusable Catalyst for the Transesterification of Waste Cooking Oil. Energy Fuels 2009, 23, 4640–4646. [Google Scholar] [CrossRef]
- Srilatha, K.; Issariyakul, T.; Lingaiah, N.; Sai Prasad, P.S.; Kozinski, J.; Dalai, A.K. Efficient Esterification and Transesterification of Used Cooking Oil Using 12-Tungstophosphoric Acid (TPA)/Nb2O5 Catalyst. Energy Fuels 2010, 24, 4748–4755. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. Esterification of Lauric Acid with Butanol-1 over H3PW12O40 Supported on MCM-41. Fuel 2012, 102, 72–77. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid Acid Catalyzed Biodiesel Production by Simultaneous Esterification and Transesterification. Green Chem. 2006, 8, 1056–1062. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Trautwein, G.; Marco-Lozar, J.P. Biodiesel Production by Acid Catalysis with Heteropolyacids Supported on Activated Carbon Fibers. Appl. Catal. A Gen. 2013, 468, 432–441. [Google Scholar] [CrossRef]
- Prasad, S.; Dhakshinamoorthy, A.; Lalthazuala, S. Metal-Organic Framework as a Heterogeneous Catalyst for Biodiesel Production: A Review. Chem. Eng. J. Adv. 2022, 12, 100415. [Google Scholar] [CrossRef]
- Cong, W.; Nanda, S.; Li, H.; Fang, Z. Metal–organic framework-based functional catalytic materials for biodiesel production: A review. Green Chem. 2021, 23, 2595–2618. [Google Scholar] [CrossRef]
- Zhang, Q.; Ling, D.; Lei, D.; Deng, T.; Zhang, Y.; Ma, P. Synthesis and Catalytic Properties of Nickel Salts of Keggin-Type Heteropolyacids Embedded Metal-Organic Framework Hybrid Nanocatalyst. Green Process. Synth. 2020, 9, 131–138. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Yang, T.; Yue, C.; Pu, Q.; Zhang, Y. Facile Synthesis of Polyoxometalates Tethered to Post Fe-BTC Frameworks for Esterification of Free Fatty Acids to Biodiesel. RSC Adv. 2019, 9, 8113–8120. [Google Scholar] [CrossRef]
- Zhang, Q.; Ling, D.; Lei, D.; Wang, J.; Liu, X.; Zhang, Y.; Ma, P. Green and Facile Synthesis of Metal-Organic Framework Cu-BTC-Supported Sn(II)-Substituted Keggin Heteropoly Composites as an Esterification Nanocatalyst for Biodiesel Production. Front. Chem. 2020, 8, 129. [Google Scholar] [CrossRef]
- Amouhadi, E.; Fazaeli, R.; Aliyan, H. Biodiesel Production via Esterification of Oleic Acid Catalyzed by MnO2@Mn (Btc) as a Novel and Heterogeneous Catalyst. J. Chin. Chem. Soc. 2019, 66, 608–613. [Google Scholar] [CrossRef]
- Qian, L.; Cheng, J.; Xin, K.; Guo, H.; Mao, Y.; Tu, J.; Yang, W. Bioresource Technology Enhancing Catalytic Activity and Pore Structure of Metal–Organic Framework-808 via Ligand Competition for Biodiesel Production from Microalgal Lipids at Reduced Temperatures. Bioresour. Technol. 2023, 386, 129533. [Google Scholar] [CrossRef] [PubMed]
- Taddeo, F.; Vitiello, R.; Russo, V.; Tesser, R.; Turco, R.; Di Serio, M. Biodiesel Production from Waste Oil Catalysed by and Mechanism. Catalysts 2023, 13, 503. [Google Scholar]
- Li, Y.; Zhu, K.; Jiang, Y.; Chen, L.; Zhang, H.; Li, H.; Yang, S. Biomass-Derived Hydrophobic Metal-Organic Frameworks Solid Acid for Green Efficient Catalytic Esterification of Oleic Acid at Low Temperatures. Fuel Process. Technol. 2023, 239, 107558. [Google Scholar] [CrossRef]
- Oghabi, M.; Rostamizadeh, M. Sulfation of Metal-Organic Framework (MOF) Nanocatalyst for Esterification of Oleic Acid with Methanol to Produce Biodiesel. Environ. Health Eng. Manag. J. 2023, 10, 321–329. [Google Scholar] [CrossRef]
- Narenji-sani, F.; Tayebee, R.; Chahkandi, M. New Task-Speci Fi c and Reusable ZIF-like Grafted H6P2W18O62 Catalyst for the E Ff Ective Esteri Fi Cation of Free Fatty Acids. ACS Omega 2020, 5, 9999–10010. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Luo, Q.; Wang, J.; Deng, T.; Zhang, Y.; Ma, P. Efficient Biodiesel Production from Oleic Acid Using Metal-Organic Framework Encapsulated Zr-Doped Polyoxometalate Nano-Hybrids. RSC Adv. 2020, 10, 8766–8772. [Google Scholar] [CrossRef]
- Pangestu, T.; Kurniawan, Y.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Hartono, S.B.; Ismadji, S. The Synthesis of Biodiesel Using Copper Based Metal-Organic Framework as a Catalyst. J. Environ. Chem. Eng. 2019, 7, 103277. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Oladepo, S.A.; Ganiyu, S.A.; Peedikakkal, A.M.P. Synthesis and Evaluation of CuO/UiO-66 Metal-Organic Frameworks as a Solid Catalyst for Biodiesel Production from Waste Cooking Oil. Mol. Catal. 2024, 564, 114313. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of Neat and Used Frying Oil: Optimization for Biodiesel Production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-Derived Solid Acid Catalysts for Biodiesel Production from Low-Cost Feedstocks: A Review. Catal. Rev.–Sci. Eng. 2014, 56, 187–219. [Google Scholar]
- Yujaroen, D.; Goto, M.; Sasaki, M.; Shotipruk, A. Esterification of Palm Fatty Acid Distillate (PFAD) in Supercritical Methanol: Effect of Hydrolysis on Reaction Reactivity. Fuel 2009, 88, 2011–2016. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.D.; Sun, L.; Zhang, J.; Xu, H.P. Fatty Acid Methyl Ester Synthesis Catalyzed by Solid Superacid Catalyst SO42/ZrO2-TiO2/La3+. Appl. Energy 2010, 87, 156–159. [Google Scholar] [CrossRef]
- Atapour, M.; Kariminia, H.R. Characterization and Transesterification of Iranian Bitter Almond Oil for Biodiesel Production. Appl. Energy 2011, 88, 2377–2381. [Google Scholar] [CrossRef]
- Amani, H.; Ahmad, Z.; Hameed, B.H. Highly Active Alumina-Supported Cs-Zr Mixed Oxide Catalysts for Low-Temperature Transesterification of Waste Cooking Oil. Appl. Catal. A Gen. 2014, 487, 16–25. [Google Scholar] [CrossRef]
- Hoque, M.E.; Singh, A.; Chuan, Y.L. Biodiesel from Low Cost Feedstocks: The Effects of Process Parameters on the Biodiesel Yield. Biomass Bioenergy 2011, 35, 1582–1587. [Google Scholar] [CrossRef]
- Patil, P.; Deng, S.; Isaac Rhodes, J.; Lammers, P.J. Conversion of Waste Cooking Oil to Biodiesel Using Ferric Sulfate and Supercritical Methanol Processes. Fuel 2010, 89, 360–364. [Google Scholar] [CrossRef]
- Varma, M.N.; Madras, G. Synthesis of Biodiesel from Castor Oil and Linseed Oil in Supercritical Fluids. Ind. Eng. Chem. Res. 2007, 46, 1–6. [Google Scholar] [CrossRef]
- Song, E.S.; won Lim, J.; Lee, H.S.; Lee, Y.W. Transesterification of RBD Palm Oil Using Supercritical Methanol. J. Supercrit. Fluids 2008, 44, 356–363. [Google Scholar] [CrossRef]
- He, H.; Wang, T.; Zhu, S. Continuous Production of Biodiesel Fuel from Vegetable Oil Using Supercritical Methanol Process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Kataria, J.; Mohapatra, S.K.; Kundu, K. Biodiesel Production from Waste Cooking Oil Using Heterogeneous Catalysts and Its Operational Characteristics on Variable Compression Ratio CI Engine. J. Energy Inst. 2019, 92, 275–287. [Google Scholar] [CrossRef]
- Kant, S.; Gurav, R.; Choi, T.; Joong, H.; Yang, S.; Song, H.; Young, J.; Park, Y.; Han, Y.; Choi, Y. Bioresource Technology Conversion of Waste Cooking Oil into Biodiesel Using Heterogenous Catalyst Derived from Cork Biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.; He, Y.; Zeng, L.; Han, X.; Yan, Y. Burkholderia Cepacia Lipase Immobilized on Heterofunctional Magnetic Nanoparticles and Its Application in Biodiesel Synthesis. Sci. Rep. 2017, 7, 16473. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wang, B.; Zeng, J.; Yang, C.; Wang, Y.; Fang, T. Esterification of Free Fatty Acids with Supercritical Methanol for Biodiesel Production and Related Kinetic Study. RSC Adv. 2015, 5, 52072–52078. [Google Scholar] [CrossRef]
- De Boer, K.; Bahri, P.A. Supercritical Methanol for Fatty Acid Methyl Ester Production: A Review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar] [CrossRef]
- Lee, J.S.; Saka, S. Biodiesel Production by Heterogeneous Catalysts and Supercritical Technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Lin, H.; Lee, M.J. Biodiesel Production with Continuous Supercritical Process: Non-Catalytic Transesterification and Esterification with or without Carbon Dioxide. Bioresour. Technol. 2013, 145, 362–369. [Google Scholar] [CrossRef]
- Tomic, M.; Micic, R.; Kiss, F.; Dedovic, N.; Simikic, M. Economic and Environmental Performance of Oil Transesterification in Supercritical Methanol at Different Reaction Conditions: Experimental Study with a Batch Reactor. Energy Convers. Manag. 2015, 99, 8–19. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of Transesterification in Rapeseed Oil to Biodiesel Fuel as Treated in Supercritical Methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Miao, S.; Shanks, B.H. Esterification of Biomass Pyrolysis Model Acids over Sulfonic Acid-Functionalized Mesoporous Silicas. Appl. Catal. A Gen. 2009, 359, 113–120. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Mixed Methanol-Ethanol Technology to Produce Greener Biodiesel from Waste Cooking Oil: A Breakthrough for SO42-/SnO2-SiO2 Catalyst. Fuel Process. Technol. 2011, 92, 1639–1645. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel Fuel from Rapeseed Oil as Prepared in Supercritical Methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Bahar, I.; Deshmukhya, T.; Bhanja, P.; Paul, B. Transesteri Fi Cation of Soybean Oil at Room Temperature Using Biowaste as Catalyst; an Experimental Investigation on the Effect of Co-Solvent on Biodiesel Yield. Renew. Energy 2020, 162, 98–111. [Google Scholar] [CrossRef]
- Günay, M.E.; Türker, L.; Tapan, N.A. Significant Parameters and Technological Advancements in Biodiesel Production Systems. Fuel 2019, 250, 27–41. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A Review on Biodiesel Production Using Catalyzed Transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel Production Process Optimization and Characterization to Assess the Suitability of the Product for Varied Environmental Conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical Aspects of Biodiesel Production by Transesterification—A Review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lee, J.S.; Park, J.Y.; Wu, C.Z.; Yuan, Z.H. Novel Biodiesel Production Technology from Soybean Soapstock. Korean J. Chem. Eng. 2007, 24, 1027–1030. [Google Scholar] [CrossRef]
- Jordanov, D.I.; Dimitrov, Y.K.; Petkov, P.S.T.; Ivanov, S.K. Biodiesel Production by Sunflower Oil Transesterification. Oxid. Commun. 2007, 30, 300–305. [Google Scholar]
- Fu, B.; Gao, L.; Niu, L.; Wei, R.X.G. Biodiesel from Waste Cooking Oil via Heterogeneous Superacid Catalyst SO42−/ZrO2. No Title. Energy Fuels 2008, 23, 569–572. [Google Scholar] [CrossRef]
- Kapilakarn, K.; Peugtong, A. A Comparison of Costs of Biodiesel Production from Transesterication. Int. Energy J. 2007, 8, 1–6. [Google Scholar]
- Bhatti, H.N.; Hanif, M.A.; Qasim, M. Ata-ur-Rehman Biodiesel Production from Waste Tallow. Fuel 2008, 87, 2961–2966. [Google Scholar] [CrossRef]
- Amani, H.; Ahmad, Z.; Asif, M.; Hameed, B.H. Transesterification of Waste Cooking Palm Oil by MnZr with Supported Alumina as a Potential Heterogeneous Catalyst. J. Ind. Eng. Chem. 2014, 20, 4437–4442. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, D.W.; Kim, D.K.; Lee, J.S.; Lee, K.Y. The Heterogeneous Catalyst System for the Continuous Conversion of Free Fatty Acids in Used Vegetable Oils for the Production of Biodiesel. Catal. Today 2008, 131, 238–243. [Google Scholar] [CrossRef]
- Santya, G.; Maheswaran, T.; Fei, K. Optimization of Biodiesel Production from High Free Fatty Acid River Catfish Oil (Pangasius Hypothalamus) and Waste Cooking Oil Catalyzed by Waste Chicken Egg Shells Derived Catalyst. SN Appl. Sci. 2019, 1, 152. [Google Scholar] [CrossRef]
- Al-muhtaseb, A.H.; Jamil, F.; Al-haj, L.; Tay, M.; Myint, Z.; Mahmoud, E.; Ahmad, M.N.M.; Hasan, A.O.; Ra, S. Biodiesel Production over a Catalyst Prepared from Biomass-Derived Waste Date Pits. Biotechnol. Rep. 2018, 20, e00284. [Google Scholar] [CrossRef]
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative Effect of Reaction Time on Biodiesel Production from Low Free Fatty Acid Beef Tallow: A Definition of Product Yield. SN Appl. Sci. 2019, 1, 140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).