Abstract
The increasing concentration of carbon dioxide (CO2) in the atmosphere is a significant contributor to global warming and climate change. Effective CO2 capture and storage technologies are critical to mitigating these impacts. This review explores various materials used for CO2 capture, focusing on the latest advancements and their applications. The review categorizes these materials into chemical and physical absorbents, highlighting their unique properties, advantages, and limitations. Chemical absorbents, such as amine-based solutions and hydroxides, have been widely used due to their high CO2 absorption capacities and established technological frameworks. However, they often suffer from high energy requirements for regeneration and potential degradation over time. Recent developments in ionic liquids (ILs) and polymeric ionic liquids (PILs) offer promising alternatives, providing tunable properties and lower regeneration energy. Physical absorbents, including advanced solvents like nanofluids and ionic liquids as well as industrial processes like selexol, rectisol, and purisol, demonstrate enhanced CO2 capture efficiency under various conditions. Additionally, adsorbents like activated carbon, zeolites, metal-organic frameworks (MOFs), carbon nanotubes (CNTs), and layered double hydroxides (LDHs) play a crucial role by providing high surface areas and selective CO2 capture through physical or chemical interactions. This paper summarizes the state of research on different materials and discusses their advantages and limitations while being used in CO2 capture technologies. This review also discussed multiple studies examining the use of catalysts and absorption mechanisms in combination with different sorbents, focusing on how these approaches enhance the efficiency of absorption and desorption processes. Through a comprehensive analysis, this review aims to provide valuable insights into the type of materials that are most suitable for CO2 capture and also provides directions for future research in this area.
1. Introduction
The increasing concentration of greenhouse gases (GHGs) in the earth’s atmosphere has emerged as a pivotal environmental concern, directly impacting climate change and air quality. This increase leads to extreme weather conditions which negatively impact human health, food and water security, and economic and social structures, leading to substantial loss and damage to natural ecosystems [1,2,3]. To limit the increase in global average temperature to 2 °C or less, it is essential to cut global CO2 emissions by 50% to 85% by mid-century [4,5,6].
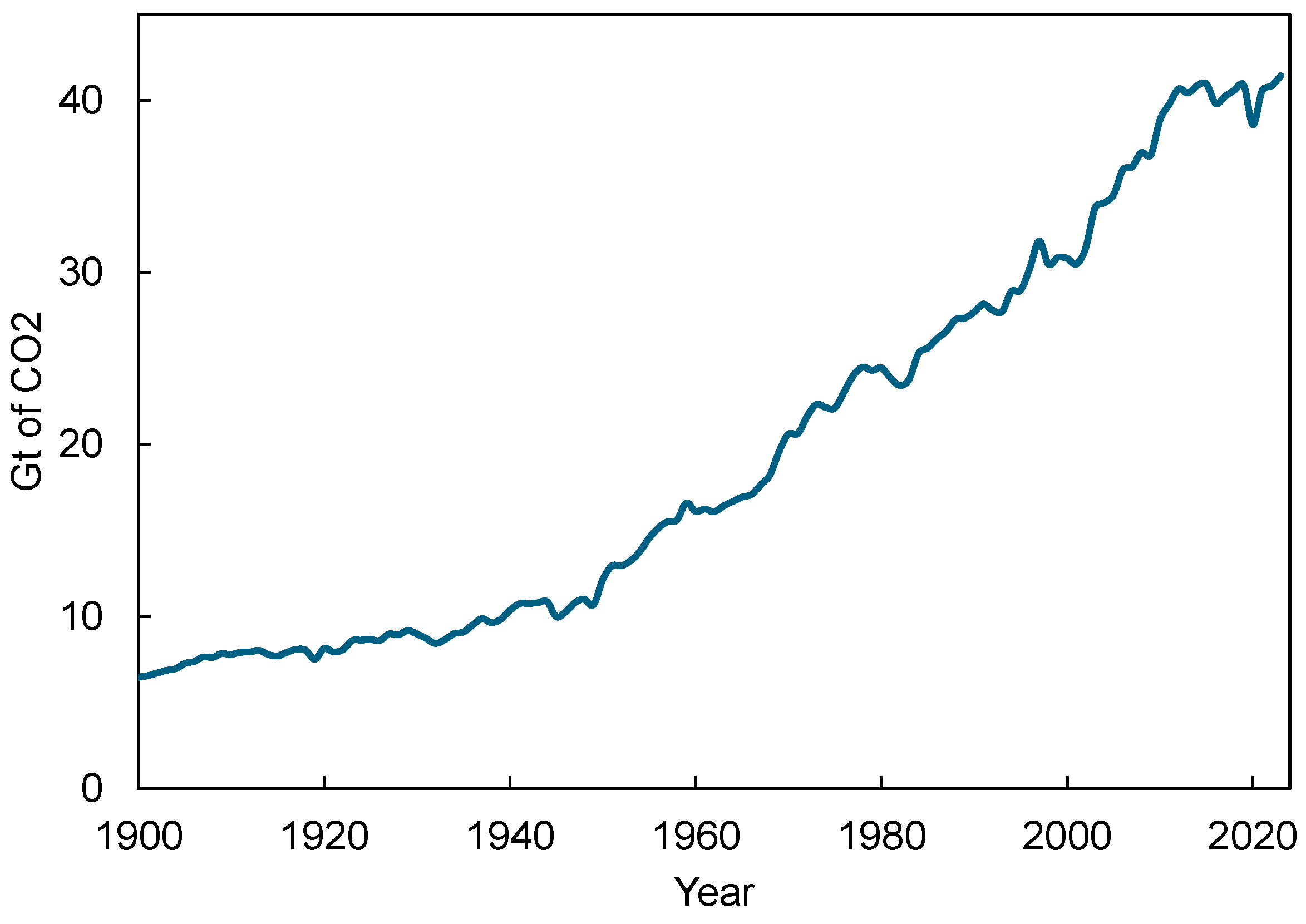
It is imperative to curb the level of CO2 in the atmosphere to stabilize the increase in average global temperature because it is the primary greenhouse gas responsible for global warming and climate change. The use of effective CO2 capture technologies can also enable the utilization of captured CO2 in various industrial processes, leading to a circular carbon cycle [7]. Despite global efforts to reduce CO2 emissions, CO2 levels have been rising continuously over the years as can be seen in Figure 1. Apart from the drop in the pandemic year 2020, CO2 emissions in 2023 were the highest ever at 41.4 Gt CO2 [8] as shown in Figure 1. This increase is mostly driven by the continued reliance on fossil fuels for energy and transportation, industrial activities, and the expansion of urban areas.
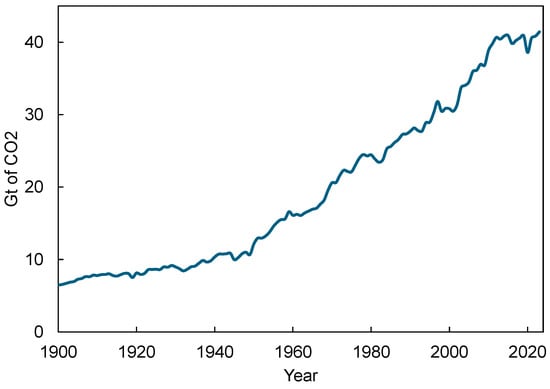
Figure 1.
Historical global CO2 emissions till 2023. Data source: Global Carbon Budget (2024) [8].
Several methods have been undertaken to capture CO2 and mitigate its impact on the environment. Carbon Capture and Storage (CCS) technology captures CO2 emissions directly from sources such as power plants and industrial sources and stores them underground in geological formations. Apart from CO2 capture from the sources, direct air capture (DAC) technologies are used to remove CO2 directly from the ambient air to lower atmospheric CO2 levels [9]. The captured CO2 can be converted into useful products such as chemicals, fuels, and building materials, creating a circular carbon economy [10,11]. To capture CO2, materials such as various forms of amine-based solvents, ionic liquids, solid adsorbents like activated carbon, zeolites, metal-organic frameworks (MOFs), metal oxides, and membranes for separation have been utilized for different carbon removal processes.
This review paper aims to provide a comprehensive overview of the current state and future prospects of materials used for CO2 capture. Through this paper, we aim to highlight the potential of various materials to mitigate CO2 emissions. The goal is to inform researchers, industry stakeholders, and policymakers, about the latest developments and encourage further innovation in the design and implementation of efficient CO2 capture technologies.
2. Sources of Carbon Dioxide (CO2)
The CO2 emissions from different sectors are shown in Figure 2. To ensure consistent categorization and avoid overlap, we adhered to the methodology from Climate Watch (climatewatchdata.com) [12], ensuring that sources are accurately classified and not counted multiple times. The sources of CO2 from each sector are indicated subsequently.
1. Electricity and heat: The major contributor to CO2 emissions is the electricity and heat required in residential and commercial spaces. Most of the present energy demand is met by burning fossil fuels. Power plants, which burn coal, oil, or natural gas, dominate global emissions, particularly in developing economies with limited opportunities for renewable sources of energy due to higher costs. Almost 70% of the emissions from this sector come from burning coal to produce electricity [13]. Apart from coal combustion, natural gas plants, oil-fired plants, and heat production contribute to the emissions [14].
2. Transportation: The transportation sector, which includes cars, trucks, ships, and planes, contributes 20.8% of total CO2 emissions due to the burning of fuels like gasoline and diesel, despite a shift toward electric vehicles. The main reason for the rise in emissions is the rise in the number of personal vehicles [15]. Aviation and maritime transportation also contribute significantly due to the use of high-density fuels that produce greater amounts of CO2 emissions per unit of fuel [16].
3. Manufacturing and construction: The third sector that produces CO2 in high quantities is the manufacturing and construction sector, which contributes about 18% of the total CO2 emissions. The CO2 emissions during construction are from energy use for manufacturing materials from iron, steel, cement, and other chemical reactions necessary for production. CO2 emissions from the iron and steel sector come due to the high temperatures required for production and the use of carbon as a reducing agent [17,18].
4. Buildings: The buildings sector contributes 7.6% of global CO2 emissions [19]. These emissions include energy used for cooling, heating, and lighting for residential and commercial activities [20,21,22]. This energy and heat are primarily generated from fossil fuels. The emissions from this sector can be reduced by enhancing building energy efficiency and switching to renewable energy sources such as solar [23].
5. Industry: The industrial sector contributes to CO2 emissions primarily by energy-intensive activities such as refining and processing of materials. The main industries include cement and steel, which account for large portions of emissions due to their reliance on fossil fuels. The cement industry emits substantial CO2 during the calcination process (CaCO3 (limestone)→CaO (lime) + CO2) [24,25], while the steel industry generates emissions through the use of carbon as a reducing agent in high-temperature processes necessary for converting iron ore to steel [17,18]. In China, heavy industries such as iron and steel, chemical, and machinery are prominent sources of industrial CO2 emissions [26]. Several studies have shown that increases in industrial activities and energy intensity significantly drive emissions growth.
6. Land-use change and forestry: The land-use and forestry sector contribute to about 3.14% of the total global CO2 emissions. Deforestation and land clearing for agriculture reduce the number of trees that can absorb CO2 from the atmosphere. The burning of biomass for land clearing and crop residues also contributes to emissions [27,28]. Some studies show that a 1% increase in forest area can lead to a 0.11% decline in emissions [29]. Although forestry aids in carbon sequestration, it is not sufficient to stabilize atmospheric carbon levels and requires additional CO2 reduction measures [27].
7. Other fuel combustion: Emissions from this sector come from the burning of coal, oil, natural gas, biomass, and waste in various sectors such as agriculture, fishing, and military fuel use [19]. This combustion makes up about 1.6% of the total CO2 emissions [19].
8. Fugitive emissions: These include emissions from leaks from fossil fuel plants, coal mining, flaring, storage facilities, and transporting fuels. These leaks account for up to about 0.74% of the total CO2 emissions around the world [19].
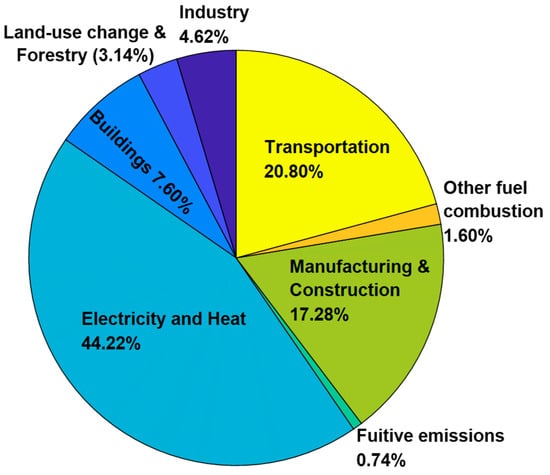
Figure 2.
Sources of CO2 by sector for the year 2021. Developed from data by climatewatch.com [19].
Figure 2.
Sources of CO2 by sector for the year 2021. Developed from data by climatewatch.com [19].
3. Methods to Capture Carbon Dioxide (CO2)
The methods to capture CO2 can be divided broadly into two categories based on the concentration of the CO2 in the gaseous mixture, namely (i) high-concentration capture (CO2 5–50%), and low-concentration capture (CO2 < 1%). The high-concentration capture methods include pre-combustion capture and oxy-fuel combustion capture with CO2 concentration between 15–50% (150,000–500,000 ppm) [30,31] and post-combustion capture with a CO2 concentration of 5–15% (50,000–150,000 ppm) [30,32]. The low-concentration capture includes direct air capture methods from air with a CO2 concentration of around 400 ppm [31,32].
3.1. High Concentration Capture Methods
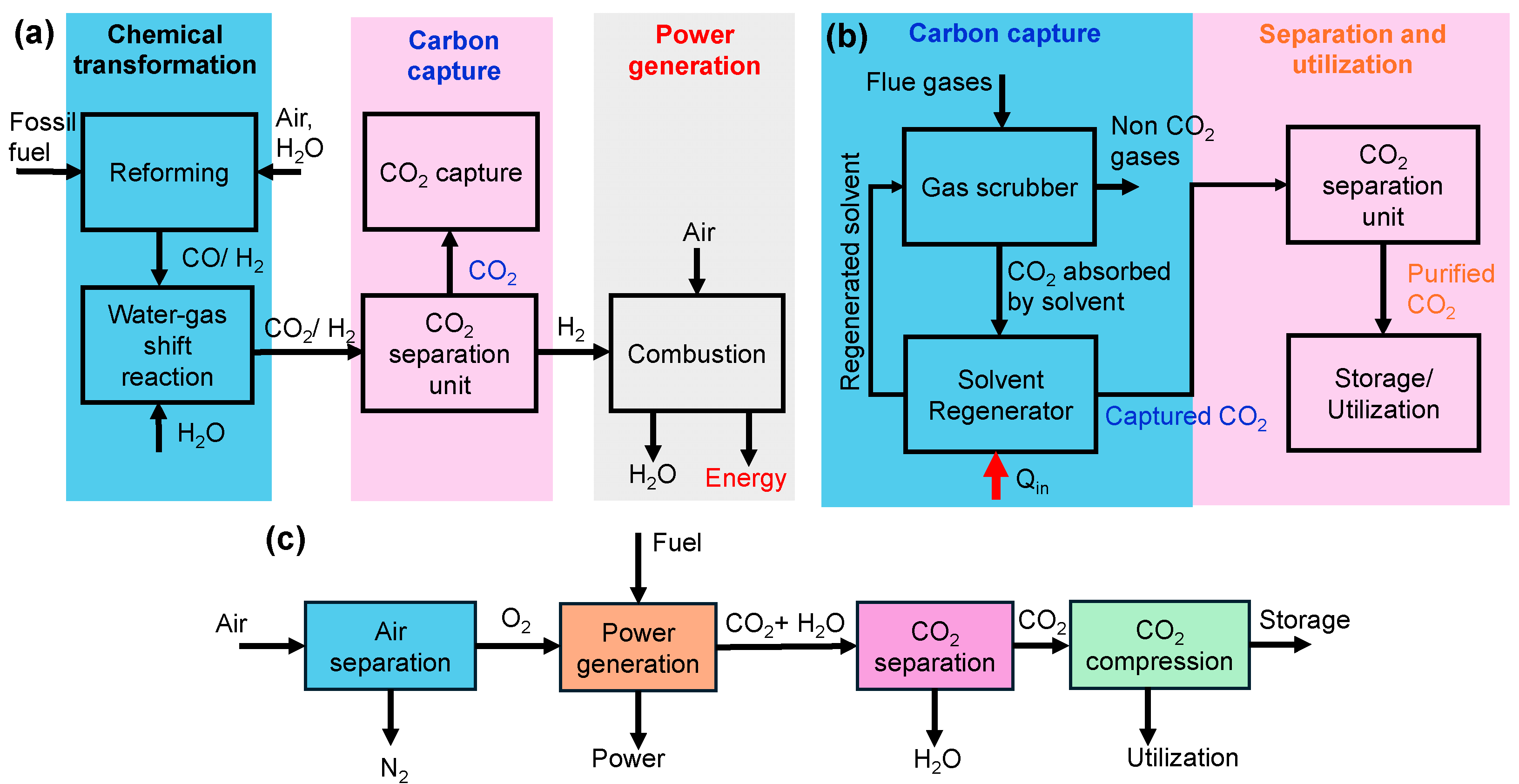
In pre-combustion CO2 capture, CO2 is removed before combustion through a gasification process, where fossil fuels are converted into syngas (CO + H2) under high temperature and pressure. The water–gas shift reaction (CO + H2O ⇄ CO2 + H2) produces CO2 and hydrogen. The resulting gas mixture typically has a CO2 concentration of 15–50% (150,000–500,000 ppm) [30,31], which is then separated using various techniques such as absorption [33,34], adsorption [35], or membrane separation [34,36]. This process is commonly used in Integrated Gasification Combined Cycle (IGCC) power plants, as shown in Figure 3a.
In post-combustion CO2 capture, CO2 is captured from flue gases after fossil fuel combustion. These flue gases typically contain CO2 concentrations of 13–15% for coal-fired power plants and 3–4% for natural gas plants [30,32]. CO2 is absorbed or adsorbed by liquid or solid sorbents, with the solvent being regenerated for reuse. The desorbed CO2 is purified and stored, as depicted in Figure 3b.
In oxy-fuel combustion, fuel is burned in pure oxygen instead of air as shown in Figure 3c. This method results in a flue gas with a CO2 concentration as high as 70 vol% (~700,000 ppm), making CO2 capture more efficient [37,38]. The first step is oxygen production (air separation) which utilizes technologies like cryogenic distillation, pressure swing adsorption (PSA), or membrane technology to produce high-purity oxygen from atmospheric air. This oxygen is then used in the combustion of fuels like coal, oil, or natural gas, producing flue gases predominantly composed of CO2 and water vapor, minimizing the presence of nitrogen and other gases. Following combustion, the flue gases undergo treatment where water vapor is condensed and removed, resulting in a concentrated CO2 stream that can be captured efficiently for storage or further processed for utilization in applications such as enhanced oil recovery or other carbon utilization strategies [39]. This integrated process streamlines the capture of CO2, enhancing the efficiency of emissions reduction from fossil fuel combustion.
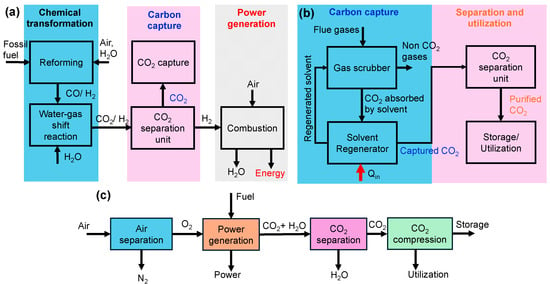
Figure 3.
Schematic showing the steps in (a) pre-combustion capture (adapted from Chung et al. [40] (open access under CC BY 4.0) (b) post-combustion capture and, (c) oxy-fuel combustion method of carbon capture and separation (adapted from Feron and Hendriks [41]) (open access under CC BY 4.0)).
Figure 3.
Schematic showing the steps in (a) pre-combustion capture (adapted from Chung et al. [40] (open access under CC BY 4.0) (b) post-combustion capture and, (c) oxy-fuel combustion method of carbon capture and separation (adapted from Feron and Hendriks [41]) (open access under CC BY 4.0)).
3.2. Direct Air Capture (DAC)
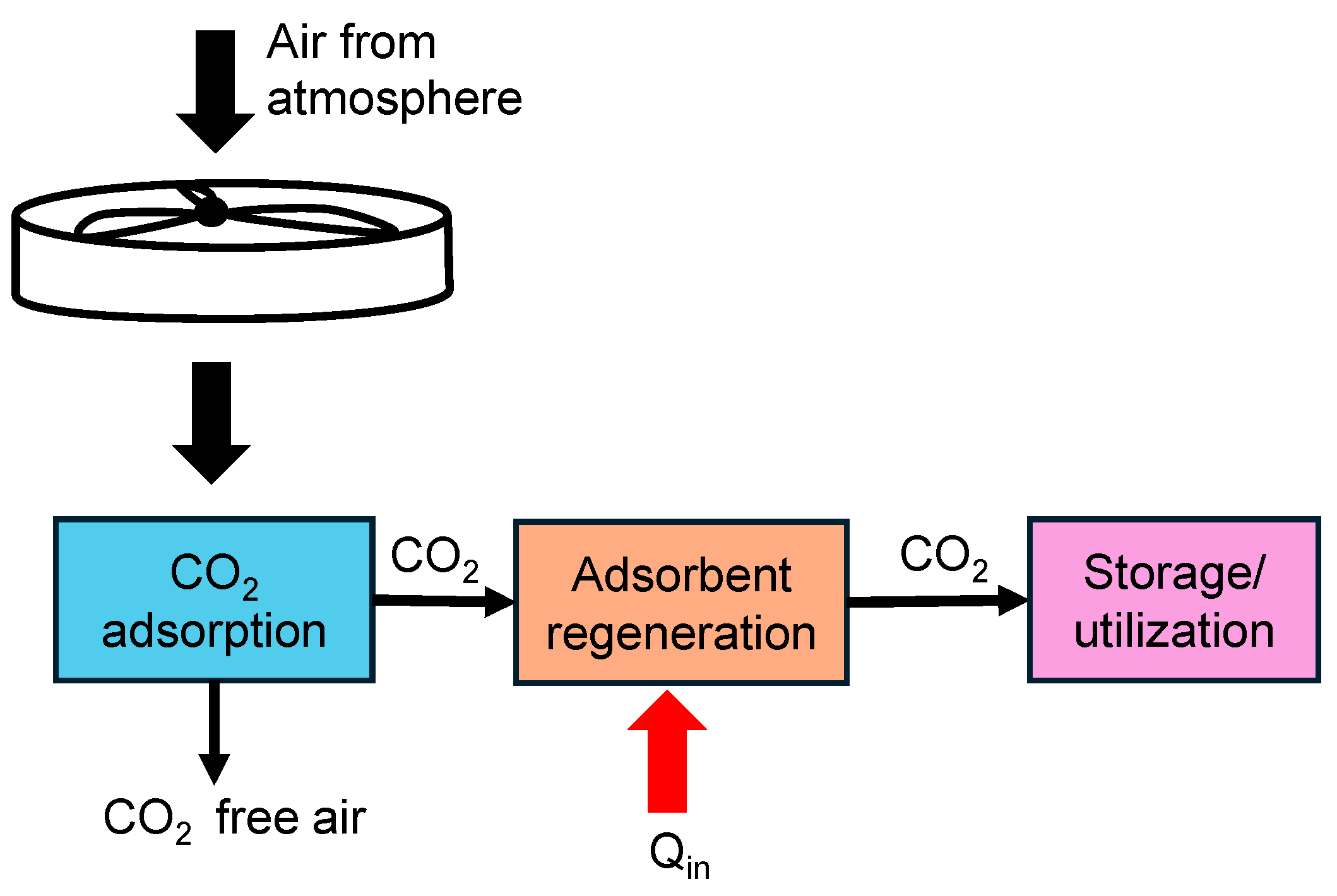
Direct Air Capture (DAC) is a technology that extracts CO2 directly from the atmospheric air, regardless of the source of emissions, providing a means to reduce global atmospheric CO2 levels. Direct air capture differs from the other methods in terms of the concentration of CO2. The concentration of CO2 in air is much lower (around 400 ppm) [31,32] than other methods, thereby making this method more challenging to implement. The schematic shown in Figure 4 illustrates the steps involved in direct air capture of CO2 from the atmosphere.
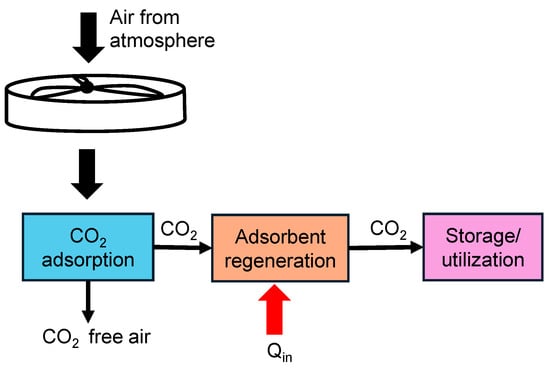
Figure 4.
Schematic showing direct air capture process of CO2 with heat added (Qin) to aid in regenerating the adsorbent.
The chart in Figure 5 shows various CO2 separation techniques and links them to capture methods that utilize them, highlighting their use and the materials involved. Absorption is the most widely implemented, especially in industrial settings like power plants, where amines such as monoethanolamine (MEA) are commonly used to chemically bind with CO2. Adsorption is employed in a range of applications from industrial to portable systems, using materials like activated carbon, zeolites, and metal-organic frameworks (MOFs) for their ability to capture CO2 through physical or chemical means.
Cryogenic distillation [42], though less common due to its high energy demands, is used in scenarios requiring very pure CO2, operating by cooling and condensing gases at low temperatures. Chemical looping offers a promising future in large-scale applications, using metal oxides to cyclically capture and release CO2. Lastly, gas hydrates represent the least utilized method, still mostly at the experimental stage, forming solid clathrates under specific high-pressure and low-temperature conditions to trap CO2. This hierarchical presentation reflects both the maturity and the scope of adoption of each technology in tackling CO2 emissions.
Although membranes do not capture CO2 but rather separate it from a mixture of gases, membranes have been widely used for CO2 separation by leveraging the selectivity permeability of CO2 as compared to other gases [42,43]. Membranes are best known due to their high energy efficiency. Membrane technology has an advantage over absorption/adsorption technologies due to lower energy consumption, good weight, and no regeneration requirements [44]. Luis et al. [45] have reviewed the advancements in CO2 separation that have been made using membrane technologies, including how the membranes have been used for CO2 separation in pre- and post-combustion capture. They have also tabulated the material the membranes are made of, and other specifications such as membrane permeance, thickness, etc., as well as the mixtures of gases from which CO2 can be separated. Dai et al. [46] have reviewed the advancements in composite membranes used for CO2 capture by the use of 2D nanomaterials as fillers to enhance membrane performance. Some of the materials discussed include graphene oxide (GO), MXene, graphite carbon nitride (g-C3N4), metal-organic frameworks (MOFs), and layered double hydroxides (LDHs). The findings emphasize that these 2D nanomaterials significantly improve the permeability and selectivity of composite membranes, making them highly effective for CO2 capture. Ozkan et al. [47] have summarized the different kinds of membrane materials that are commonly used for CO2 capture along with membrane properties such as CO2 selectivity, performance, and thickness. They have also summarized the fabrication method used to synthesize the membranes and the test conditions employed during the CO2 absorption studies.
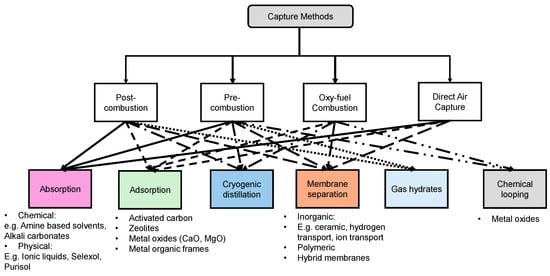
Figure 5.
Materials for CO2 capture using different methods. Adapted with permission from D’Alessandro et al. [48].
Figure 5.
Materials for CO2 capture using different methods. Adapted with permission from D’Alessandro et al. [48].
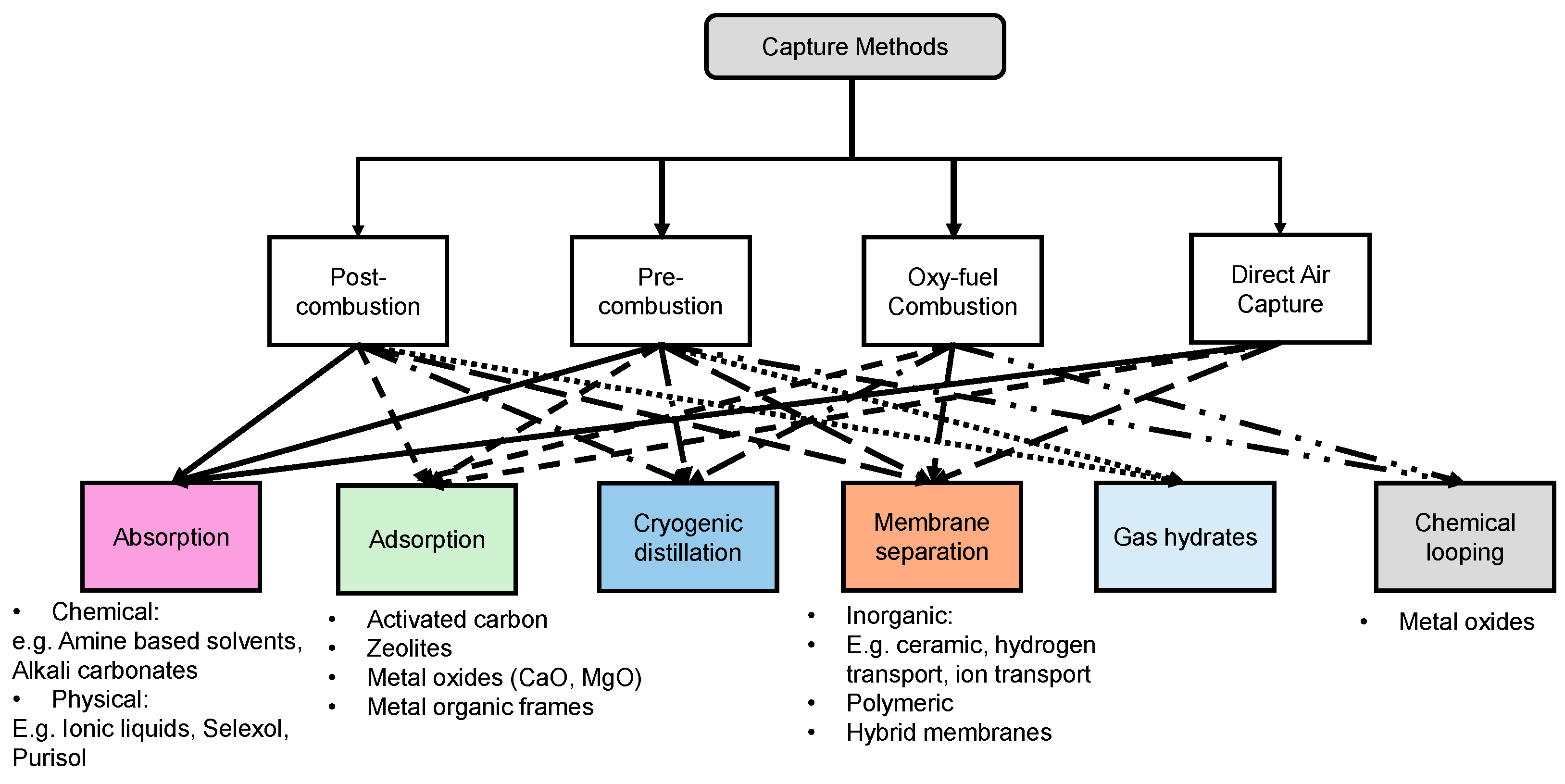
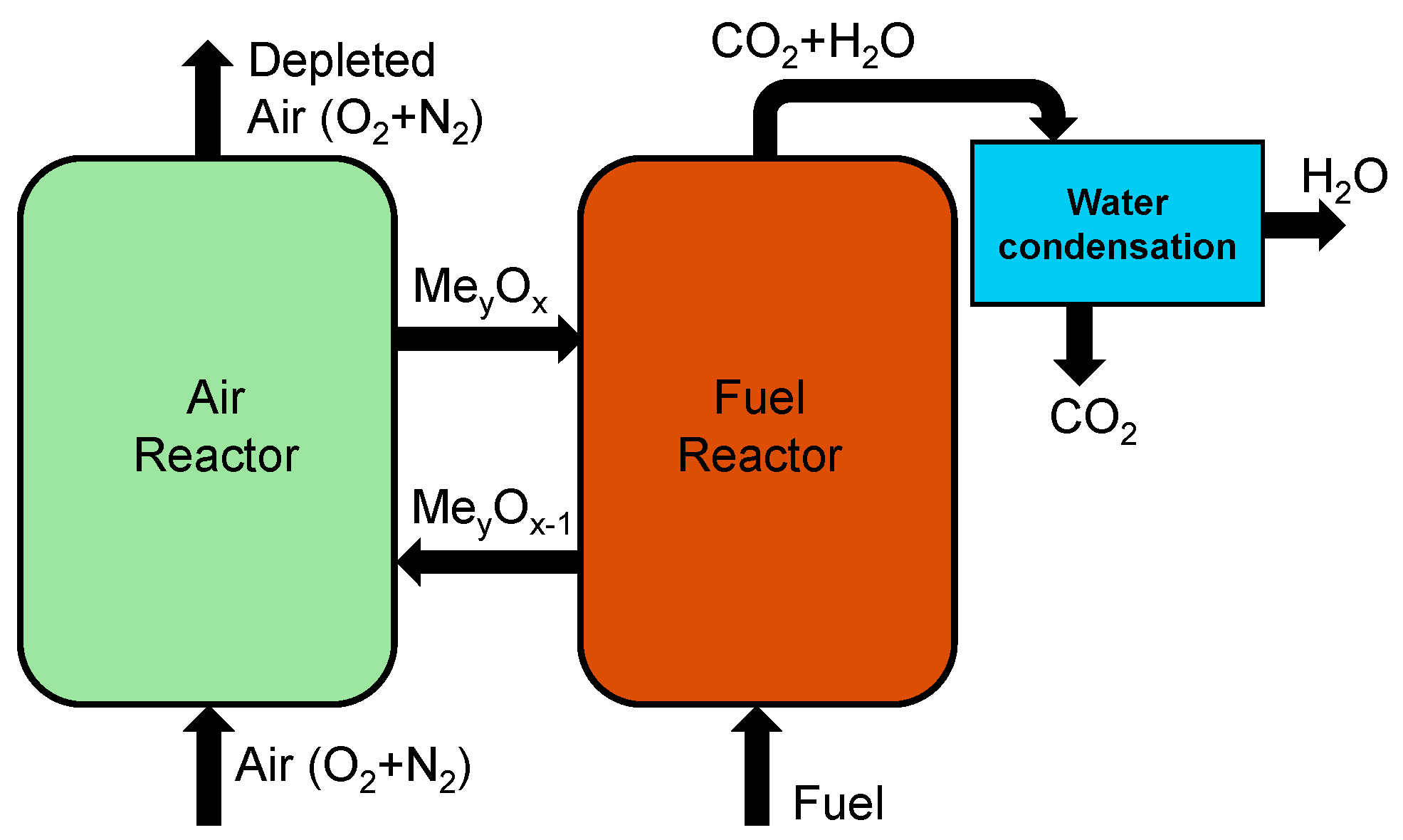
Chemical looping involves combustion technology designed for power and heat generation with CO2 capture capabilities with two separate reactors, as shown in Figure 6. The process involves metal oxide acting as an oxygen carrier to transfer oxygen from the air to the fuel, without direct contact between air and fuel, resulting in a nearly pure CO2 stream along with water vapor which can be condensed and pure CO2 can be sequestered or utilized [49,50]. The reduced metal oxide is sent to the air reactor for oxidation so it can be used again in the fuel reactor. Many studies on chemical looping combustion have used different materials as oxygen carriers. Manović and Anthony [51] have utilized copper-based materials like composite CaO/CuO as the oxygen carriers from the air reactor to the fuel reactor. Other materials such as iron-based carriers like Co-doped Fe2O3, have also shown enhanced reactivity and stability [52,53]. Matzen et al. [54] have reviewed the use of natural ores like ilmenite, hematite, chryscolla, malachite, anhydrite, etc., and suggested that they could offer a cost-effective alternative to synthetic metal oxides. Ksepko [55] studied the use of perovskite materials like Sr(Fe1−xCux)O3−δ and suggested them as oxygen carriers in chemical looping due to their high-temperature stability and high mechanical strength.
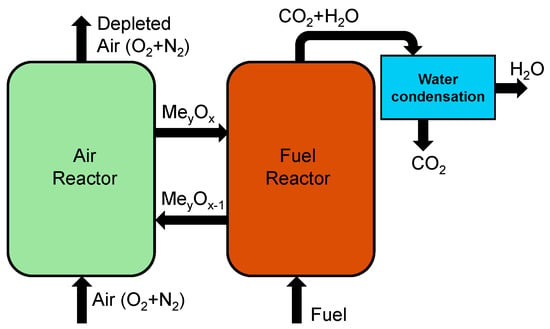
Figure 6.
Schematic showing chemical looping combustion of a hydrocarbon fuel.
4. Materials for CO2 Capture
4.1. Liquid Absorbents
Liquid sorbents can mainly be categorized, based on the adsorption mechanism, into chemical and physical absorbents. In chemical absorption, the absorption of CO2 occurs by the breaking of bonds and formation of new compounds with CO2 often forming carbonates. On the other hand, in physical absorption, CO2 dissolves into a solvent through weak interactions like Van der Waals or dipole-dipole interactions that are driven by pressure and temperature. In this process, CO2 is captured by the solvent/absorbent at low temperature and increasing pressure and is desorbed by heating the solvent and lowering the pressure. Chemical absorbents perform better at low partial pressure and the loading capacity saturates as the partial pressure of CO2 increases, whereas for physical absorbents, the loading capacity increases linearly with the partial pressure of CO2 in accordance with Henry’s law () [56]. Since there is no chemical bond formation in physical absorption, the regeneration energy required during desorption is much smaller than in chemical absorption. The most common applications for physical absorbents are in pre-combustion CO2 capture to form syngas and Integrated Gasification Combined Cycle (IGCC) power plants [57,58].
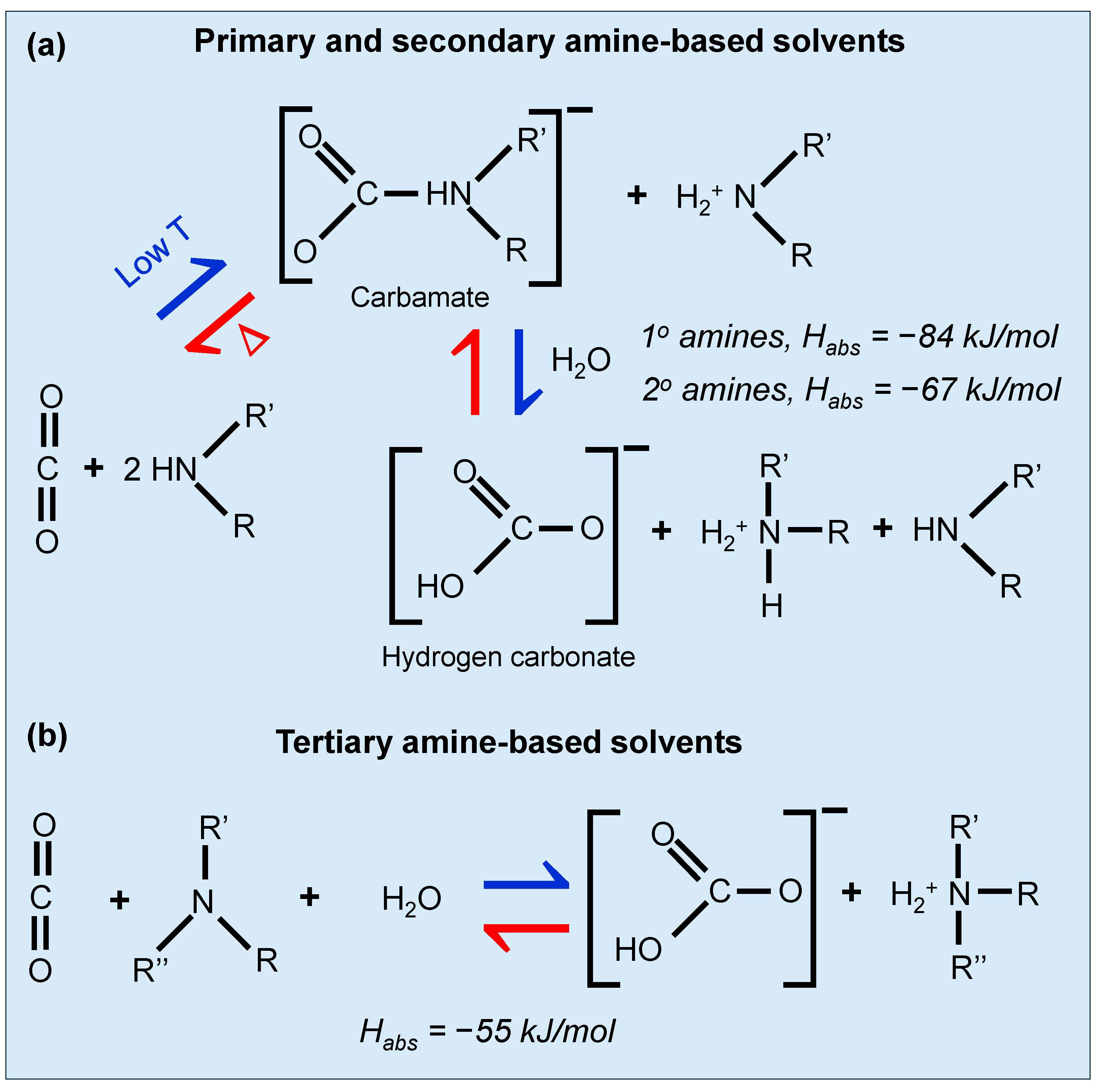
Chemical absorption using amines has been a staple in CO2 capture for over 50 years, primarily utilizing monoethanolamine (MEA). In this process, an aqueous amine-based solution flows down an absorption tower while CO2-rich flue gas is introduced from below [59,60,61]. CO2 reacts with the amine at around 40 °C to form carbamates. The mechanism of primary and tertiary amines is well-studied at this temperature by Vaidya et al. [62]. The CO2 reaction of primary and tertiary amine-containing solvents with CO2 is shown in Figure 7. Primary or secondary amines have a more favorable absorption energy with Habs = −84 kJ/mol [63,64] and −67 kJ/mol [64,65], respectively, and hence can absorb CO2 easily. However, the favorable absorption makes it harder to desorb the CO2, thereby requiring a high regeneration energy. For the tertiary amines, the absorption energy is less favorable (Habs = −55 kJ/mol [64,66]) and desorbtion/regeneration is somewhat easier. After absorption, the CO2-rich amine solution is heated in a stripping tower to release the CO2, a step requiring considerable energy due to the strong bond formed in the carbamate. The system recycles the amine solution back to the absorber after regeneration. Despite its high affinity for CO2 and the maturity of the technology, it has some limitations like high energy costs for amine regeneration and material degradation from impurities like SOx and NOx [48]. Innovations aim to reduce these drawbacks by using amines that require less energy to regenerate, such as diethanolamine (DEA) [67] and N-methyldiethanolamine (MDEA) [68], which require lower heat reactions and higher CO2 loading capacities. Other recent studies on alkanolamines like MEA, DEA, and MDEA, have substantiated their use due to their effective and reversible interaction with CO2, forming stable carbamate compounds [69].
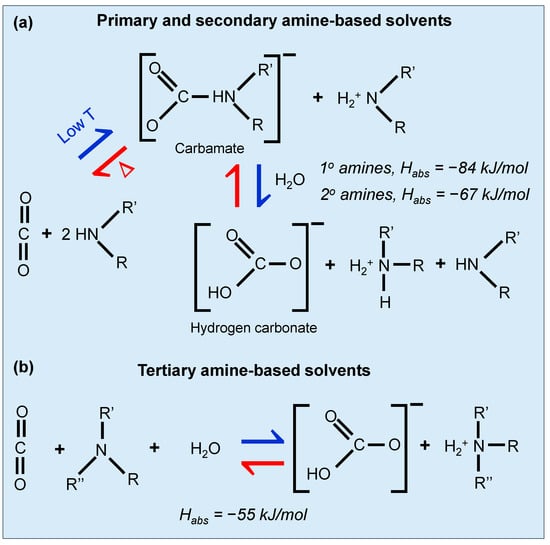
Figure 7.
Reaction of (a) primary, secondary, and (b) tertiary amines containing solvents with CO2. Adapted with permission from D’Alessandro et al. [48].
Apart from the linear amines, sterically hindered amines have also been studied for CO2 removal from gases. When a bulky group is placed adjacent to the amino nitrogen site, steric hindrance is introduced. This bulky substituent lowers carbamate stability which favors carbamate reversion to bicarbonate ion and free amine leading to thermodynamic loadings nearing one mole of CO2 per mole of amine [70] and provides greater binding of CO2 per molecule as compared to primary, secondary, or tertiary amines [56]. Hindered amines, providing even lower regeneration costs and improved absorption rates, represent another advancement in enhancing the efficiency and cost-effectiveness of this CO2 capture method [62,70,71,72,73]. Amino acids are explored for their safety and lower environmental impact, with studies like those by Yang et al. [74] highlighting the specific capabilities of amino-acid ionic liquids.
Catalysts accelerate the rate-limiting steps in absorption by facilitating the formation of intermediates such as carbamates [75]. They help in the electrophilic addition where the amine acts as a nucleophile and CO2 as an electrophile, forming a carbamate via a zwitterion intermediate. Metal oxides such as K/MgO and TiO(OH)2 provide free O2− ions that bind with CO2, increasing its reactivity and improving mass transfer [76]. The structure of catalysts like SBA-15 improves mass transfer due to their large pore sizes, allowing faster interaction between CO2 and amines [77]. These catalysts provide sites that promote the formation and stabilization of intermediate zwitterions, resulting in a faster and more efficient CO2 capture process.
In the desorption process, catalysts enhance the breakdown of carbamate and bicarbonate intermediates and improve this step kinetically. Catalysts accelerate the deprotonation of carbamate ions enabling CO2 release with less energy. Metal oxides, such as γ-Al2O3 and TiO2, are used as catalysts due to the presence of Bronsted and Lewis acid sites which stabilize the transition states and intermediate compounds and enable the separation of CO2 [78]. Other catalysts like SO42−/ZrO2 have strong acid sites that break down carbamate bonds more easily by creating favorable sites for the reversible binding of CO2 and stabilizing the amine in its neutral form post-CO2 release [79]. Hence, the use of catalysts reduces the energy required for solvent regeneration and improves the kinetics of the desorption process.
Inorganic chemical absorbents have also been used for CO2 capture due to their effective and diverse mechanisms of action. These absorbents primarily involve reactions between CO2 and inorganic compounds, forming stable, non-volatile products that can be easily handled or converted into useful materials. Some of these include aqueous solutions of metal hydroxides, such as calcium hydroxide and sodium hydroxide that react with CO2 to form carbonates. Materials like calcium oxide and zirconium oxides can be synthetically tailored for enhanced CO2 uptake and thermal stability [80]. Other solvents like potassium carbonate (K2CO3) have also been used with promoters due to their low cost compared to amines and low regeneration energy [81]. Ammonia solution has also been used in pre-combustion capture of CO2 due to its high absorption capacity. However, this solution requires careful handling due to the toxicity of ammonia [82,83].
Ionic liquids (ILs) are another class of materials that have been widely studied as absorbents for CO2 capture. These substances offer advantages such as low vapor pressure and high chemical stability [74,84,85,86]. ILs can be tailored to enhance specific aspects of absorption efficiency. The absorption process can be chemical or physical, depending on the nature of the IL’s interaction with CO2 [87,88]. Ionic liquids undergoing only physisorption are suitable for use in pre-combustion capture (high CO2 partial pressure) whereas the ones undergoing chemical absorption are used in post-combustion capture (low CO2 partial pressure) [87].
ILs can be functionalized with various anionic and cationic components to optimize their CO2-capturing abilities. Studies such as those by Li et al. [89] demonstrate the potential of dianionic-functionalized ionic liquids for efficient and reversible CO2 chemisorption. Ionic liquids are valued for their adjustable structural properties and high CO2 solubility, as detailed by Hu et al. [74]. Lastly, innovations in ionic liquids and hybrid techniques continue to evolve, providing new pathways to increase the efficiency and sustainability of CO2 capture technologies as mentioned in Wu et al. [90]. Each of these materials offers a strategic avenue for advancing CO2 capture technology, aiming to balance high loading capacity and lower regeneration energy. Zhao and Baker [84], explored the structural variation of ILs by functionalizing them and studied how that affects the selective interaction of the IL with CO2. Meanwhile, Yang et al. [91], enhanced the CO2 loading capacities of fluorinated ILs, focusing on increasing both solubility and absorption kinetics, which are crucial for industrial applications. Gu et al. [92], developed hydrophobic deep eutectic solvents that efficiently capture CO2 even at low partial pressures of CO2. These solvents can maintain hydrophobicity after CO2 absorption and hence require less desorption energy than would be required to desorb the absorbed water.
Some ionic liquids such (EMmim)(NTf2) have been used as homogeneous catalyst for CO2 capture, particularly in monoethanolamine (MEA) systems during both absorption and desorption [93]. Adding even 2000 ppm of [EMmim][NTf2] to MEA improved the desorption kinetics and hence considerably reduced energy costs by allowing CO2 desorption at lower temperatures. During absorption, the IL acts as a proton acceptor, facilitating the formation of carbamate or bicarbonate intermediates in the MEA solution thereby promoting CO2 absorption. During desorption, the IL alters the pH of the MEA solution enhancing the hydrolysis of the carbamate intermediates which results in quicker release of CO2 from the carbamate. An improvement in desorption rates by 791% at 85 °C when using the IL in the MEA system as compared to when the MEA solution was used without this IL catalyst. The MEA solution was found to be stable without degradation with use of [EMmim][NTf2] for over 50 cycles.
Yang et al. [94] used task-specific ionic liquids (TSILs) such as amino-functionalized ILs and superbase-derived ILs for CO2 capture and catalytic conversion. The absorption and desorption mechanism are similar to that of amine-based solutions. The amino groups in TSILs react with CO2 to form carbamate species and stable intermediates, which makes CO2 available for catalytic conversion.
In cycloaddition reactions with epoxides, the capture mechanism involves zinc halides (ZnCl2, ZnBr2) as co-catalysts, which coordinate with epoxides to activate the oxygen atom. The TSILs’ anionic and cationic components stabilize the CO2-epoxide complex helping the reaction happen under mild conditions as shown in Equation (1). The coupling of capture and conversion made the desorption process more effective.
Zhang et al. [95] studied imidazolium-based ILs such as [bmim][PF6] and [emim][BF4] for their potential in CO2 fixation and conversion, especially in reactions with epoxides and amines. ILs with nucleophilic anions like Cl− can act as catalyst. In imidazolium ILs, CO2 interacts with the anions (e.g., PF6− or BF4−) via weak Lewis acid-base interactions, resulting in high CO2 solubility and facilitating its activation. Upon dissolution, CO2 is in a high concentration, allowing it to participate in catalytic reactions with other substrates. Similarly Aquino et al. [96] explored imidazolium-based ILs combined with ZnBr2 or ZnCl2 as catalysts in CO2 capture and conversion. The IL/ZnBr2 systems resulted in 90% yield and 82% selectivity in cyclic carbonate formation under mild conditions, and the ILs demonstrated stability over several cycles. The presence of halide ions in the ILs act as CO2 activators as well as nucleophiles, thereby improving the efficiency of CO2 conversion.
Another class of ionic liquids called polymeric ionic liquids (PILs) comprise of cations and anions attached to a large polymeric chain and have shown significant improvement in CO2 capture. Zulfiqar et al. [97] have reviewed various PILs and studied the role of anions, and cations towards the solubility of CO2 in PIL and the selectivity of PIL towards CO2. Sadeghpour et al. [98] have also reviewed various PILs for CO2 including their synthesis and application as absorbents, adsorbents, and membranes. Jiang et al. [99] developed bi-functional PILs that efficiently capture, CO2 and catalyze subsequent chemical reactions, demonstrating the potential for integrated capture and utilization processes. These studies highlight the innovative approaches to enhancing CO2 capture using polymeric liquids.
Another type of solvent called nanofluids has emerged as a promising solution for enhancing CO2 capture efficiency. Nanofluids are comprised of nanoparticles dispersed in base fluids such as water or salt solutions. They provide a greater area for CO2 absorption due to the presence of nanoparticles. Zhang et al. [100], provide a comprehensive review of the mechanisms enhancing CO2 absorption in nanofluids, emphasizing the role of nanoparticle size and concentration. Devakki et al. [101], demonstrate significant improvements in CO2 uptake and absorption rates through experimental investigations. They dispersed TiO2 and Al2O3 nanoparticles in DI water at concentrations of 0.02–0.14 wt.%. They found that the optimum concentration gave the maximum absorption of CO2 and absorption decreased beyond that concentration. Lee et al. [102], explore nano emulsion absorbents, highlighting increased surface area for faster absorption processes. Other studies like Yu et al. [103], review liquid nano-absorbents that increase desorption rates and hence reduce energy costs for solvent regeneration.
There are some industrial processes such as selexol, rectisol, and purisol that have been used widely to capture CO2 using physical solvents due to their established high efficiency. Selexol [104,105] uses dimethyl ethers of polyethylene glycols as solvent. Rectisol [104,106,107] employs methanol at low temperatures, which is ideal for coal gasification applications. Purisol [106,108] processes utilize N-methyl-2-pyrrolidone as the solvent for CO2 removal. The processes are relatively inexpensive, costing approximately $0.2–0.5 per gram [109] of solvent. Fully commercial with a Technology Readiness Level (TRL) of 9 [110], the selexol, rectisol, and purisol processes are on par with amine-based solvent systems in terms of deployment and maturity.
In Table 1, we have summarized the different chemical and physical absorbents based on their advantages, disadvantages, and applications in which they are used.

Table 1.
Summary of relevant literature on various chemical and physical absorbents for CO2 capture.
4.2. Solid Adsorbents
Adsorbents play a crucial role in CO2 capture by providing surface areas where CO2 molecules can adhere through physical or chemical interactions [42,118,119]. The primary mechanisms include physisorption, where CO2 is held by weak van der Waals forces, and chemisorption, involving stronger chemical bonds. Lu et al. [120] compared carbon nanotubes, zeolites, and activated carbon, finding that modified carbon nanotubes showed the highest CO2 adsorption capacity. Nie et al. [121] discussed various adsorbents, including activated carbons, highlighting their promising capacities and applications in industrial CO2 capture. Since most adsorbents exhibit physisorption capture mechanism, they have relatively lower selectivity for CO2 compared to amine-based solvents [7].
Activated carbon is a prominent adsorbent due to its high surface area, porosity, and high absorption capacity [122]. Its surface can also be modified by adding functional groups to enhance CO2 adsorption. Studies such as those by Diyuk et al. [123], show how activated carbon functionalized with diethylamine groups can improve adsorption capacity significantly, enhancing CO2 capture efficiency by 30–40% compared to unmodified counterparts. Maniarasu et al. [124], explore the use of biomass-based activated carbon, highlighting its sustainability and effectiveness in capturing CO2 from various gas streams, showcasing its potential for large-scale applications. Khalili et al. [125] synthesized activated carbon/polyaniline nanocomposites, which showed significantly enhanced CO2 adsorption capacities.
Bhatti et al. [126] investigated metal-impregnated activated carbons (ACs) using metals like Fe, Ni, and Mo as catalysts to improve desorption rates in amine-based solution systems, especially with 5M monoethanolamine solution (MEA). They found that the Mo/AC catalyst performed the best and was able to reduce the solvent regeneration energy by about 21.2% compared to the non-catalytic MEA solution. The role of metal-impregnated AC catalysts is to provide Lewis acid sites that stabilize intermediates making the bond breaking between amines and C to allow release of CO2 at a lower temperature. In addition to lower regeneration energy, the use of catalysts also increased the desorption rate by 113% making them cost-effective and scalable for industrial CO2 capture applications.
Another study by Bhatti et al. [127] used activated carbon (AC) treated with sulfuric acid (H2SO4) and phosphoric acid (H3PO4) as catalysts to increase the desorption rates of CO2 from MEA solutions. Acid treatment increases the surface acidity and introduces oxygenated functional groups that facilitate CO2 desorption. They found that due to this increased surface acidity, CO2 desorption could happen at 86 °C as compared to 120–140 °C in the non-catalytic case. Asa result studies showed a 20% reduction in regeneration heat input.
Malini et al. [128] reviewed methods utilizing activated carbon (AC) from biomass, and modifying its basicity to improve CO2 capture efficiency. Techniques such as nitrogen doping and metal oxide impregnation which create basic sites that promote chemisorption of CO2 were reviewed. Nitrogen doping introduces amine and pyridine-like groups that act as basic sites, as shown in Equation (2), enhancing interaction between CO2 and the AC surface.
CO2 + N-site → N-COO−
When metal oxides like MgO and CaO are impregnated onto the AC, they also increase the basic sites, thus enhancing CO2 chemisorption through the formation of carbonate and bicarbonate species. The study concludes that by optimizing basicity and surface characteristics, these ACs become highly effective CO2 adsorbents.
Zeolites have also been widely used as adsorbents for CO2 capture. Zeolites are crystalline, porous aluminosilicate minerals known for their structured pores which can selectively adsorb gases. Kumar et al. [129] comprehensively reviews the zeolites that have been used for CO2 capture. They reported on the integration of nano-sized metals into the zeolite structure, which significantly improves the adsorption kinetics and capacity under various temperature conditions. Rahmah et al. [130] have reviewed the use of small-pore zeolites for CO2 by adsorption and zeolite membranes. Cecilia et al. [131] discuss the synthesis of zeolites from kaolinite, a clay mineral, and their efficacy in CO2 capture. This study demonstrates how reaction conditions during zeolite synthesis affect CO2 adsorption capacity. Dabbawala et al. [132] synthesized and examined the performance of hierarchical zeolite-Y for CO2 capture and found their CO2 adsorption capacity to be higher than conventional microporous zeolites-Y.
Zhang et al. [133] used 5% Ni/Zeolite 4A catalyst that improves methanol decomposition for syngas (H2 and CO) production as well as enhancing CO2 capture. Methanol decomposes on the Ni surface in a multi-step process and produces syngas, as shown in Equation (3).
CH3OH → CH3O → CH2O → CHO → CO + 2H2
This reaction sequence leverages the strong interactions between Ni and the zeolite 4A support. The presence of Ni in various oxidation states helps in effective methanol decomposition and limits some of the byproducts like CO2 and CH4.
The presence of zeolite 4A allows for selective capturing of CO2 during the reaction. The microporous structure of Zeolite 4A adsorbs CO2 and maintains catalytic activity, which suppresses the reverse reaction. Adding water vapor aids in stabilizing Ni active sites and increases CO selectivity by promoting decomposition and preventing CO2 formation as shown in Equation (4).
The Ni/Zeolite 4A catalyst presents a low-cost and efficient alternative to precious metal-based catalysts for methanol decomposition integrated with CO2 capture.
Alivand et al. [134] studied solid acid-base catalysts such as γ-Al2O3 and ZrO2 for enhancing solvent regeneration in amine-based systems saving energy costs needed for desorption. The presence of Bronsted and Lewis acid sites on metal oxides enhance proton transfer and stabilize the intermediate bicarbonate species, respectively, allowing easier release of CO2 at lower temperatures. The use of these catalysts can decrease regeneration temperatures from 120–140 °C to 90 °C, reducing regeneration energy by 27–48%.
Similarly Zhang et al. [135] used zeolite Hβ as a solid acid catalyst with a tri-solvent blend of MEA, MDEA, and PZ for CO2 capture that was able to enhance CO2 desorption kinetics and hence reduced the solvent regeneration energy. The Bronsted acid sites on the Hβ zeolite enabled proton exchange, making CO2 desorption faster at 98 °C. The mesoporous structure of the zeolite essentially improved the desorption process by stabilizing the intermediate bicarbonate and carbamate ions, accelerating CO2 release from the amine solution. The Hβ catalyst with the tri-blend amine system lowered regeneration energy by 66.1% compared to standard MEA regeneration showing the importance of a catalyst.
Metal-organic framework (MOFs) is another class of materials that have been found to be useful in capturing CO2. Ding et al. [136] reviewed various metal-organic frameworks and MOF-based materials for CO2 capture. They have highlighted the unique properties of MOFs, such as high surface areas and chemical tunability, which are crucial for effective CO2 capture and conversion processes. Hu et al. [137] examine the performance of MOFs in repeated CO2 adsorption-desorption cycles, and found that certain MOFs, like UiO-66(Zr), show high resilience in multi-cycle tests, maintaining structural integrity and performance over numerous cycles, which is vital for their use in pressure swing adsorption processes. Liu et al. [138] investigated the effects of water on CO2 adsorption in MOFs, particularly how water impacts the selectivity and capacity of MOFs in capturing CO2 from flue gas. Trickett et al. [139] explored the use of MOFs in photocatalytic CO2 reduction, by converting CO2 into useful chemicals using light. They reported how modifying MOF structures can improve their light absorption and catalytic activity.
Wei et al. [140] investigate a heteropolyacid-modified cerium-based MOF (CeM-HPW) to enhance desorption in an amine-based system for CO2 capture to reduce MEA regeneration. The combination of CeO2 and HPW forms this catalyst and provides a high density of Lewis and Bronsted acid sites, respectively. The Bronsted sites facilitate proton transfer making carbamate decomposition faster whereas the Lewis acid sites help stabilize intermediates during the carbamate breakdown, lowering the energy barrier for solution. This setup enables desorption to occur at lower temperatures (~88 °C), improves the desorption rate by 166%, and decreases the desorption energy by about 29.4%.
Trickett et al. [139] reviewed various studies on MOFs and identified the structural and chemical properties that make them effective for each stage of CO2 management—capture, regeneration, and conversion. They identified that MOFs with coordinately unsaturated metal sites and alkylamine-functionalized MOFs can enhance CO2 adsorption and catalysis. MOFs with unsaturated metal sites such as Mg-MOF-74 have strong Lewis acid-base interactions with CO2 allowing for CO2 adsorption through electrostatic interactions. Some Mg2+ in the MOF acts as the Lewis acid and forms a coordination bond with the partially negative O in CO2, as seen in Equation (5).
For catalytic conversion, MOFs like UiO-66 functionalized with alkylamines or metal sites can activate CO2 for reactions such as cyclic carbonate synthesis from epoxides. In such reactions, a Lewis acidic metal center in the MOF activates the epoxide, while a nucleophilic site opens the epoxide ring, facilitating CO2 insertion and cyclic carbonate formation as shown in Equation (6).
The tunability of MOFs for various functionalities can make them selectively capture CO2 from mixed gas streams even under mild conditions.
Tyagi et al. [141] reviewed various metal-based catalysts within MOFs for CO2 capture and its conversion to cyclic carbonates using epoxides. They discuss bifunctional catalysts that use both Lewis acid and base sites to enable cycloaddition of CO2 and epoxides as shown above in Equation (6). The metal within the MOFs acts as the Lewis acid that coordinates with the epoxide and polarizes the C-O bond present in the epoxide making it prone to nucleophilic attack by a Lewis base within the MOF such as a halide or amine. The polarized epoxide ring opens, creating an intermediate that reacts with CO2 to form a cyclic carbonate.
Similarly Zhang et al. [142] used Ag(I)-embedded sulfonate-based MOF designed for ambient CO2 capture and catalytic conversion. The absorption mechanism was similar to other MOFs shown above forming cyclic carbonates and oxazolidinones. The Ag(I) ions serve as Lewis acid sites to polarize CO2, enhancing its reactivity. And the alkyne facilitates the formation of cyclic carbonates. The reaction mechanism is as shown in the following Equation (7). They showed that this MOF system achieved high yields under ambient conditions as well as being reused for multiple cycles.
Carbon nanotubes (CNTs) have emerged as a powerful adsorbent for CO2 capture due to their unique tubular structure and large surface area. Several studies [143,144,145] have shown that functionalizing CNTs with amine groups can significantly enhance their CO2 adsorption capabilities. Keller et al. [143] synthesized porous multi-walled carbon nanotube-based (MWCNT) microtubes functionalized with polyethylenimine (PEI) and found that PEI impregnation enhanced the CO2 sorption capacity of the CNT microtubes. Irani et al. [144] developed a CO2 sorbent by immobilizing tetraethylenepentamine (TEPA) onto modified carbon nanotubes. Their kinetic and thermodynamic adsorption studies found activation energies for CO2 desorption of MCNTs/TEPA to be low and that could greatly enhance desorption speed and thereby concluded that it could reduce the CO2 capture costs.
Layered Double Hydroxides (LDHs) are versatile adsorbents due to their tunable interlayer chemistry and the ability to undergo ion exchange, which can be exploited to capture CO2. Adsorption by LDHs is chemical in nature and particularly useful for post-combustion adsorption from flue gases [122]. Dewangan et al. [146] have reviewed the recent progress that has been made in using LDH-derived metal-based catalysts for the conversion of CO2 into useful chemicals. Li et al. [147] have summarized the recent progress in the design and synthesis of LDH-based nanomaterials, which have been applied in the fields of catalysis and adsorption, and the different ways they have been used for CO2 conversion.
Polymeric ionic liquids (PILs) have also been used as adsorbents for CO2 capture. Zulfiqar et al. [97] have reviewed the role of various polymeric ionic liquids (PILs) as adsorbents for CO2 capture. PILs offer a highly versatile platform for fabricating various sorbents tailored for specific applications, including flue gas separation (CO2/N2) and natural gas purification (CO2/CH4). It explores the recent advancements in PIL research, particularly as solid sorbents for CO2 capture and separation, and discusses the influence of various factors such as cations, anions, polymer backbones, and porosity on CO2 sorption efficiency. Additionally, the review outlines future strategies to enhance the CO2 capture performance of PILs, providing valuable insights into the ongoing research efforts in this field.
Table 2 summarizes the different adsorbents along with their advantages, disadvantages, and the applications in which they are used.

Table 2.
Summary of different types of adsorbents and their advantages, disadvantages, and the applications in which they are used.
Table 2.
Summary of different types of adsorbents and their advantages, disadvantages, and the applications in which they are used.
| Material | Advantages | Disadvantages | Applications | Refs |
|---|---|---|---|---|
| Activated carbon | High surface area, cost-effective, | good adsorption capacity, may require modification to improve performance | Industrial emissions, power plants | [120,124,125,148,149,150] |
| Zeolites | High selectivity, good thermal stability | High cost, sensitivity to moisture | Industrial and commercial applications, natural gas processing | [120,129,131,151,152,153] |
| Metal-organic frameworks (MOFs) | Extremely high surface areas, tunable pore sizes and functionalities | High cost, can be fragile and sensitive to moisture | Flue gases from industries, direct air capture | [40,136,137,138,139] |
| Carbon nanotubes (CNTs) | High mechanical strength, very large surface area, can be functionalized | High production cost, complex synthesis process | Flue gases, direct air capture | [120,143,144,145] |
| Layered double hydroxides (LDHs) | Chemical nature allows for ion exchange, tunable interlayer chemistry | Lower stability compared to other adsorbents, slower kinetics | Flue gas treatment, post-combustion power plants | [146,147,154,155,156] |
| Polymeric ionic liquids (PILs) | Tailorable for specific separations, can be regenerated, high thermal stability | High viscosity, may require specific conditions for optimal performance | Specific industrial applications, flue gas treatment | [97,98] |
4.3. Comparison of Materials
Table 3 summarizes the various materials used for CO2 capture based on criteria such as absorption capacity, material cost, and applications in which they are most commonly used. CO2 absorption capacity (mol of CO2/kg of sorbent) is defined as the maximum amount of CO2 that can be absorbed by the material per unit mass of the material. We see that amine-based solutions, such as MEA, can absorb 8.2 mol CO2/kg, with hindered amines offering even higher capacities. Inorganic solvents like K2CO3 (7.24 mol CO2/kg), NaOH (3.21 mol CO2/kg), and NH3 (27 mol CO2/kg) show varied capacities. Ionic liquids can absorb up to 3.6 mol CO2/kg, while activated carbon captures 2–4 mol CO2/kg, though it performs poorly at low pressures. Zeolites exhibit a high capacity of over 8 mol CO2/kg, remaining effective at low pressures, while metal-organic frameworks (MOFs) and carbon nanotubes (CNTs) capture around 4 and 5 mol CO2/kg, respectively, but are less effective at lower pressures.
The materials cost is obtained from Sigma-Aldrich (St. Louis, MO, USA, sigmaaldrich.com) [109], highlighting their price per gram of sorbent. Amine-based solutions and blends are among the more expensive options, with costs ranging from $200 to $500 per gram. Inorganic chemical solvents, such as potassium carbonate (K2CO3), sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2), and ammonia (NH3) solutions, offer a broad spectrum of prices, from as low as $0.5 per gram for Ca(OH)2 to $7 per gram for K2CO3. Ionic liquids and metal-organic frameworks (MOFs) fall in the mid-range, costing between $20 and $50 per gram, balancing performance with affordability. Advanced materials like graphene and carbon nanotubes (CNTs), are significantly more expensive, costing between $600 and $800 per gram. More economical options include activated carbon and zeolites, with zeolites costing as little as $0.4 per gram.
To gauge the technology readiness level (TRL) of the different materials, we have utilized the International Energy Agency Greenhouse Gas (IEAGHG) TRL model as described by Menmuir et al. [157] and Bukar et al. [158] that categories TRL into three main phases: Research (TRL 1–3), where basic principles are observed, and initial experiments are conducted; Development (TRL 4–6), where technologies undergo laboratory and pilot-scale validation in relevant environments; and Demonstration and Commercialization (TRL 7–9), where systems are tested in operational settings, with TRL 9 indicating full commercial deployment. According to Bukar et al. [158], amine-based solvents have achieved Technology Readiness Level (TRL) 9, indicating full commercial deployment. This method is extensively used in power plants, refineries, and chemical industries for large-scale CO2 separation. The Chilled Ammonia Process (CAP) has reached TRL 7, with a fully functional prototype demonstrated. An ammonia (NH3) and potassium carbonate (K2CO3) mixed solvent system is at TRL 4, validated in a lab but requiring further development. Ionic liquid solvents have a low TRL of 3, with demonstration only at the lab scale. Kearns et al. [110] have listed TRL for solid sorbents based on the adsorption technology used rather than the material itself. Pressure Swing Adsorption (PSA) is fully commercial (TRL 9) and widely deployed by Air Liquide, Air Products, and UOP in industrial gas separation. Temperature Swing Adsorption (TSA) is at TRL 5–7, with large pilot-scale demonstrations by Svante and Kawasaki CO2 Capture.
The table also lists their most suitable applications. Amine-based solvents are identified as the most suitable for carbon scrubbing from flue gases in post-combustion power plants. However, they require high energy for regeneration and have higher operational costs. Other inorganic chemical absorbents, such as K2CO3, NaOH, Ca(OH)2, and NH3 solutions, are primarily used in pre-combustion CO2 capture, such as in IGCC plants. Although these absorbents, particularly NaOH and Ca(OH)2, are less expensive than amine-based solvents, their lower absorption capacities and largely non-regenerable nature make them less practical for cyclic use. Physical absorbents like ionic liquids are versatile and can be used in various applications, including pre-combustion capture, CO2 capture from flue gases, and even direct air capture. Solid adsorbents such as activated carbon, zeolites, metal-organic frameworks (MOFs), and graphene-based materials like carbon nanotubes are also summarized in the table, along with their potential applications. Various previous studies utilizing these different materials for CO2 capture are cited in the last column.

Table 3.
Summary of various materials used for CO2 capture based on criteria such as absorption capacity, material cost, and applications.
Table 3.
Summary of various materials used for CO2 capture based on criteria such as absorption capacity, material cost, and applications.
| Materials | Absorption Capacity (mol CO2/kg Sorbent) | Cost ($/g Sorbent) | Application | TRL (Companies) | Refs |
|---|---|---|---|---|---|
| Amine based solutions/amine blends | 8.2 mol CO2/kg of MEA [159] (can be higher for hindered amines) | 200–500 | -Flue gas -Direct air capture | 9 (Mitsubishi Heavy Industries (Tokyo, Japan), Fluor (Irving, TX, USA), Aker Solutions (Fornebu, Norway), BASF (Ludwigshafen, Germany)) | [70,71,73,90,111,160,161,162] |
| Inorganic chemical solvents (K2CO3, NaOH, NH3 solutions) | 7.24 (K2CO3) [163], 3.21 (NaOH) [164], 27 (NH3) [165] | 7 (K2CO3), 0.6 (NaOH), 3.8 (NH3) | -Pre-combustion capture (IGCC plants) -Direct air capture | 4–7 (SRI International (Menlo Park, CA, USA), Baker Hughes (Houston, TX, USA) and University of Illinois (Urbana-Champaign, IL, USA)) | [81,82,83,112,113,114,115,116,117] |
| Ionic liquids | As high as 3.6 mol CO2/kg IL [166] | 20–50 | -Pre-combustion capture (IGCC plants) -Flue gas treatment -Direct air capture | 3 (R&D Lab scale) | [34,74,84,90,91] |
| Activated carbon | 2–4 mol CO2/kg [167] (poor at low pressures) | 18 | -Flue gas with high concentration of CO2 | 1. Pressure Swing Adsorption- 9 (Air Liquide (Paris, France), Air Products (Allentown, PA, USA), UOP (Des Plaines, IL, USA)) 2. Temperature Swing Adsorption-5–7 (Svante (Burnaby, BC, Canada), Kawasaki CO2 Capture (Tokyo, Japan)) | [120,124,125,148,149,150] |
| Zeolites | Over 8 mol CO2/kg [167,168] (good even at low pressures) | 0.2–5 | -Flue gas treatment -Direct air capture | [120,129,131,151,152,153] | |
| Metal-organic frameworks (MOFs) | 4 mol CO2/kg [169] (poor at low pressures) | 20–50 | -Flue gas treatment -Direct air capture | [40,136,137,138,139] | |
| Graphene/carbon nanotubes (CNTs) | Up to 5 mol CO2/kg [144] (poor at low pressures) | 600–800 | -Flue gas treatment -Direct air capture | [120,143,144,145] |
5. Conclusions
In conclusion, this review extensively investigates the diverse range of materials employed in the capture of carbon dioxide, emphasizing their pivotal role in addressing the escalating issue of global warming. Through a detailed analysis of various absorption, adsorption, and membrane technologies, the paper underlines the evolution and effectiveness of different materials, from traditional amines to advanced metal-organic frameworks and innovative ionic liquids.
The effectiveness of these materials in CO2 capture technologies varies significantly, with each offering unique advantages in terms of selectivity, capacity, and energy efficiency. For instance, amine-based solvents continue to be the cornerstone for post-combustion capture due to their high efficiency and capacity. Meanwhile, materials like zeolites and activated carbon stand out in adsorption processes for their high surface areas and tunability. Versatile materials like ionic liquids and metal-organic frameworks demonstrate exceptional potential due to their customizable structures and ability to be tailored for specific capture mechanisms, which can significantly enhance CO2 capture efficiency and selectivity. Moreover, the development of polymeric ionic liquids and nanomaterial-enhanced membranes showcases the ongoing innovation in this field, aiming to reduce energy requirements and operational costs. We reviewed multiple studies examining the use of catalysts and absorption mechanisms in combination with different sorbents, focusing on how these approaches enhance the efficiency of absorption and desorption processes.
We have also summarized the various materials in a table to compare them based on different properties such as absorption capacity, cost of the materials, as well as their application so as to help future researchers/industry professionals choose the best materials for a particular application. Future research should aim to further enhance the regeneration efficiency, durability, and economic viability of these materials to facilitate their adoption on a larger scale.
Author Contributions
A.R.: Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing. J.M.A.: Conceptualization, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Arizona State University, Office of the Provost.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Intergovernmental Panel On Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2023; ISBN 978-1-00-915789-6. [Google Scholar]
- Wang, P.; Liu, Z.; Pan, Z.; González-Arias, J.; Shang, L.; Wang, Y.; Zhang, Z. Advances in life cycle assessment of chemical absorption-based carbon capture technologies. Sep. Purif. Technol. 2024, 346, 127252. [Google Scholar] [CrossRef]
- Yan, Y.; Borhani, T.N.; Subraveti, S.G.; Pai, K.N.; Prasad, V.; Rajendran, A.; Nkulikiyinka, P.; Asibor, J.O.; Zhang, Z.; Shao, D.; et al. Harnessing the power of machine learning for carbon capture, utilisation, and storage (CCUS)—A state-of-the-art review. Energy Environ. Sci. 2021, 14, 6122–6157. [Google Scholar] [CrossRef]
- IEA—International Energy Agency. Available online: https://www.iea.org/search/products (accessed on 23 April 2024).
- Van Den Broek, M.; Berghout, N.; Rubin, E.S. The potential of renewables versus natural gas with CO2 capture and storage for power generation under CO2 constraints. Renew. Sustain. Energy Rev. 2015, 49, 1296–1322. [Google Scholar] [CrossRef]
- United Nations. Paris Agreement; 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 22 April 2024).
- Ozkan, M.; Custelcean, R. Guest Editors The status and prospects of materials for carbon capture technologies. MRS Bull. 2022, 47, 390–394. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J. Global Carbon Budget 2024. Earth Syst. Sci. Data Discuss. 2024; 1–133, preprint. [Google Scholar] [CrossRef]
- Lian, X.; Xu, L.; Chen, M.; Wu, C.; Li, W.; Huang, B.; Cui, Y. Carbon Dioxide Captured by Metal Organic Frameworks and Its Subsequent Resource Utilization Strategy: A Review and Prospect. J. Nanosci. Nanotechnol. 2019, 19, 3059–3078. [Google Scholar] [CrossRef]
- Kothandaraman, A. Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2010. [Google Scholar]
- Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G.A.; Surya Prakash, G.K. CO2 capture by amines in aqueous media and its subsequent conversion to formate with reusable ruthenium and iron catalysts. Green Chem. 2016, 18, 5831–5838. [Google Scholar] [CrossRef]
- Climate Watch Methodology for GHGs Emissions. Available online: https://wri-sites.s3.us-east-1.amazonaws.com/climatewatch.org/www.climatewatch.org/climate-watch/wri_metadata/CW_GHG_Method_Note.pdf (accessed on 20 February 2025).
- Foster, V.; Bedrosyan, D. Understanding CO2 Emissions from the Global Energy Sector; World Bank Group: Washington, DC, USA, 2014. [Google Scholar]
- Garg, A.; Shukla, P.R.; Kankal, B.; Mahapatra, D. CO2 emission in India: Trends and management at sectoral, sub-regional and plant levels. Carbon Manag. 2017, 8, 111–123. [Google Scholar] [CrossRef]
- Daziano, R.A.; Waygood, E.O.D.; Patterson, Z.; Braun Kohlová, M. Increasing the influence of CO2 emissions information on car purchase. J. Clean. Prod. 2017, 164, 861–871. [Google Scholar] [CrossRef]
- Gray, N.; McDonagh, S.; O’Shea, R.; Smyth, B.; Murphy, J.D. Decarbonising ships, planes and trucks: An analysis of suitable low-carbon fuels for the maritime, aviation and haulage sectors. Adv. Appl. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Kim, Y.; Worrell, E. International comparison of CO2 emission trends in the iron and steel industry. Energy Policy 2002, 30, 827–838. [Google Scholar] [CrossRef]
- Xu, C.C.; Cang, D. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Climate Watch. Washington, DC: World Resources Institute. 2024. Available online: https://www.climatewatchdata.org (accessed on 3 April 2024).
- Li, Y.L.; Han, M.Y.; Liu, S.Y.; Chen, G.Q. Energy consumption and greenhouse gas emissions by buildings: A multi-scale perspective. Build. Environ. 2019, 151, 240–250. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Greenough, R.; Taylor, S.; Ozawa-Meida, L.; Acquaye, A. Operational vs. embodied emissions in buildings—A review of current trends. Energy Build. 2013, 66, 232–245. [Google Scholar] [CrossRef]
- Röck, M.; Saade, M.R.M.; Balouktsi, M.; Rasmussen, F.N.; Birgisdottir, H.; Frischknecht, R.; Habert, G.; Lützkendorf, T.; Passer, A. Embodied GHG emissions of buildings—The hidden challenge for effective climate change mitigation. Appl. Energy 2020, 258, 114107. [Google Scholar] [CrossRef]
- US EPA, O. Sources of Greenhouse Gas Emissions. Overviews and Factsheets. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 18 June 2024).
- Shen, W.; Cao, L.; Li, Q.; Zhang, W.; Wang, G.; Li, C. Quantifying CO2 emissions from China’s cement industry. Renew. Sustain. Energy Rev. 2015, 50, 1004–1012. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. CARBON DIOXIDE EMISSIONS FROM THE GLOBAL CEMENT INDUSTRY. Annu. Rev. Energy. Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, G.; Ye, Z.; Gao, P. CO 2 emissions from industrial sector in Fujian Province, China: A decomposition analysis. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012039. [Google Scholar] [CrossRef]
- Lasocki, T.J. Climate Change Mitigation Through Forestry: Theory and Practice. J. Sustain. For. 2001, 14, 147–166. [Google Scholar] [CrossRef]
- Mendelsohn, R.; Sohngen, B. The Net Carbon Emissions fromHistoric Land Use and Land UseChange. JfE 2019, 34, 263–283. [Google Scholar] [CrossRef]
- Parajuli, R.; Joshi, O.; Maraseni, T. Incorporating Forests, Agriculture, and Energy Consumption in the Framework of the Environmental Kuznets Curve: A Dynamic Panel Data Approach. Sustainability 2019, 11, 2688. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Energy.gov Office of Fossil Energy and Carbon Management. Pre-Combustion Carbon Capture Research. Energy.gov. Available online: https://www.energy.gov/fecm/pre-combustion-carbon-capture-research (accessed on 9 September 2024).
- National Energy Technology Laboratory. Carbon Dioxide Capture Approaches. netl.doe.gov. Available online: https://netl.doe.gov/research/carbon-management/energy-systems/gasification/gasifipedia/capture-approaches (accessed on 9 September 2024).
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Paramio, C.; Palomar, J. Aspen plus supported design of pre-combustion CO2 capture processes based on ionic liquids. Sep. Purif. Technol. 2022, 290, 120841. [Google Scholar] [CrossRef]
- Sohaib, Q.; Muhammad, A.; Younas, M.; Rezakazemi, M. Modeling pre-combustion CO2 capture with tubular membrane contactor using ionic liquids at elevated temperatures. Sep. Purif. Technol. 2020, 241, 116677. [Google Scholar] [CrossRef]
- Program Plan-carbon Capture. US Department of Energy 2013. Available online: https://netl.doe.gov/sites/default/files/2020-11/Program-Plan-Carbon-Capture-2013.pdf (accessed on 22 April 2024).
- Beavis, R. The EU FP6 CACHET project—Final results. Energy Procedia 2011, 4, 1074–1081. [Google Scholar] [CrossRef]
- Jurado, N.; Darabkhani, H.G.; Anthony, E.J.; Oakey, J.E. Oxy-fuel Combustion for Carbon Capture and Sequestration (CCS) from a Coal/Biomass Power Plant: Experimental and Simulation Studies. In Progress in Clean Energy, Volume 2: Novel Systems and Applications; Dincer, I., Colpan, C.O., Kizilkan, O., Ezan, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 177–192. ISBN 978-3-319-17031-2. [Google Scholar]
- National Energy Technology Laboratory. Oxy-Combustion. Available online: https://www.netl.doe.gov/node/7477 (accessed on 9 September 2024).
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Chung, Y.G.; Gómez-Gualdrón, D.A.; Li, P.; Leperi, K.T.; Deria, P.; Zhang, H.; Vermeulen, N.A.; Stoddart, J.F.; You, F.; Hupp, J.T.; et al. In silico discovery of metal-organic frameworks for precombustion CO 2 capture using a genetic algorithm. Sci. Adv. 2016, 2, e1600909. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Hendriks, C.A. CO2 Capture Process Principles and Costs. Oil Gas Sci. Technol.—Rev. D’ifp Energ. Nouv. 2005, 60, 451–459. [Google Scholar] [CrossRef]
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control 2015, 40, 126–166. [Google Scholar] [CrossRef]
- Wu, J.; Chung, T.-S. Supramolecular Polymer Network Membranes with Molecular-Sieving Nanocavities for Efficient Pre-Combustion CO2 Capture. Small Methods 2022, 6, 2101288. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, J.; Huang, K.; Zhu, X.; Yang, W. The current status of high temperature electrochemistry-based CO2 transport membranes and reactors for direct CO2 capture and conversion. Prog. Energy Combust. Sci. 2021, 82, 100888. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van Der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Luo, W.; Wang, Y.; Mu, P.; Li, J. A review on the recent advances in composite membranes for CO2 capture processes. Sep. Purif. Technol. 2023, 307, 122752. [Google Scholar] [CrossRef]
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current status and pillars of direct air capture technologies. iScience 2022, 25, 103990. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H. CO2 capturing methods: Chemical looping combustion (CLC) as a promising technique. Sci. Total Environ. 2021, 788, 147850. [Google Scholar] [CrossRef] [PubMed]
- Chemical Looping Combustion. netl.doe.gov. Available online: https://netl.doe.gov/node/7478 (accessed on 21 June 2024).
- Manovic, V.; Anthony, E.J. Integration of Calcium and Chemical Looping Combustion using Composite CaO/CuO-Based Materials. Environ. Sci. Technol. 2011, 45, 10750–10756. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.; Long, D.T.; Cheng, W.L.; Dong, C.Q.; Zhang, J.J. Research on Chemical Materials with Characteristic on Chemical Looping Combustion of Co-Doped Fe2O3 Oxygen Carrier with CO. AMM 2014, 540, 30–34. [Google Scholar] [CrossRef]
- Chen, L.; Kong, L.; Bao, J.; Combs, M.; Nikolic, H.S.; Fan, Z.; Liu, K. Experimental evaluations of solid-fueled pressurized chemical looping combustion—The effects of pressure, solid fuel and iron-based oxygen carriers. Appl. Energy 2017, 195, 1012–1022. [Google Scholar] [CrossRef]
- Matzen, M.; Pinkerton, J.; Wang, X.; Demirel, Y. Use of natural ores as oxygen carriers in chemical looping combustion: A review. Int. J. Greenh. Gas Control 2017, 65, 1–14. [Google Scholar] [CrossRef]
- Ksepko, E. Perovskite Sr(Fe1-xCux)O3-δ materials for chemical looping combustion applications. Int. J. Hydrogen Energy 2018, 43, 9622–9634. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Single solvents, solvent blends, and advanced solvent systems in CO2 capture by absorption: A review. Int. J. Glob. Warm. 2015, 7, 184–225. [Google Scholar] [CrossRef]
- NETL DOE. Selexol. netl.doe.gov. Available online: https://netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/selexol (accessed on 16 September 2024).
- Kohl, A.L.; Nielsen, R. Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; ISBN 978-0-08-050720-0. [Google Scholar]
- Pellegrini, G.; Strube, R.; Manfrida, G. Comparative study of chemical absorbents in postcombustion CO2 capture. Energy 2010, 35, 851–857. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases 2018, 8, 998–1031. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-Alkanolamine Reaction Kinetics: A Review of Recent Studies. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Xiao, M.; Cui, D.; Liu, H.; Tontiwachwuthikul, P.; Liang, Z. A new model for correlation and prediction of equilibrium CO2 solubility in N-methyl-4-piperidinol solvent. AIChE J. 2017, 63, 3395–3403. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. The solubility of H2 S and CO2 in aqueous monoethanolamine solutions. Can. J. Chem. Eng. 1974, 52, 803–805. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. Solubility of carbon dioxide in aqueous diethanolamine solutions at high pressures. J. Chem. Eng. Data 1972, 17, 465–468. [Google Scholar] [CrossRef]
- Jou, F.Y.; Mather, A.E.; Otto, F.D. Solubility of hydrogen sulfide and carbon dioxide in aqueous methyldiethanolamine solutions. Ind. Eng. Chem. Proc. Des. Dev. 1982, 21, 539–544. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, Y.; Ge, B.; He, Z.; Zhu, X.; Li, J.; Liu, S.; Yu, L. Mixed Diethanolamine and Polyethyleneimine with Enhanced CO2 Capture Capacity from Air. Adv. Sci. 2023, 10, 2207253. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Samdani, S.; Berrouk, A.S.; Kroon, M.C.; Peters, C.J. Effect of Additives on the CO2 Absorption in Aqueous MDEA Solutions. Ind. Eng. Chem. Res. 2014, 53, 20032–20035. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Sartori, G.; Savage, D.W. Sterically hindered amines for carbon dioxide removal from gases. Ind. Eng. Chem. Fund. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Fragale, C.; Narracci, M. Reaction of silylalkylmono- and silylalkyldi-amines with carbon dioxide: Evidence of formation of inter- and intra-molecular ammonium carbamates and their conversion into organic carbamates of industrial interest under carbon dioxide catalysis. Green Chem. 2002, 4, 439–443. [Google Scholar] [CrossRef]
- Yeh, J.T.; Pennline, H.W.; Resnik, K.P. Study of CO2 Absorption and Desorption in a Packed Column. Energy Fuels 2001, 15, 274–278. [Google Scholar] [CrossRef]
- Mimura, T.; Suda, T.; Iwaki, I.; Honda, A.; Kumazawa, H. Kinetics of Reaction Between Carbon Dioxide and Sterically Hindered Amines for Carbon Dioxide Recovery from Power Plant Flue Gases. Chem. Eng. Commun. 1998, 170, 245–260. [Google Scholar] [CrossRef]
- Hu, H.; Li, F.; Xia, Q.; Li, X.; Liao, L.; Fan, M. Research on influencing factors and mechanism of CO2 absorption by poly-amino-based ionic liquids. Int. J. Greenh. Gas Control 2014, 31, 33–40. [Google Scholar] [CrossRef]
- De Meyer, F.; Bignaud, C. The use of catalysis for faster CO2 absorption and energy-efficient solvent regeneration: An industry-focused critical review. Chem. Eng. J. 2022, 428, 131264. [Google Scholar] [CrossRef]
- Afari, D.B.; Coker, J.; Narku-Tetteh, J.; Idem, R. Comparative Kinetic Studies of Solid Absorber Catalyst (K/MgO) and Solid Desorber Catalyst (HZSM-5)-Aided CO2 Absorption and Desorption from Aqueous Solutions of MEA and Blended Solutions of BEA-AMP and MEA-MDEA. Ind. Eng. Chem. Res. 2018, 57, 15824–15839. [Google Scholar] [CrossRef]
- Shi, H.; Huang, M.; Huang, Y.; Cui, L.; Zheng, L.; Cui, M.; Jiang, L.; Ibrahim, H.; Tontiwachwuthikul, P. Eley–Rideal model of heterogeneous catalytic carbamate formation based on CO2–MEA absorptions with CaCO3, MgCO3 and BaCO3. R. Soc. Open Sci. 2019, 6, 190311. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: Advancement of amine regeneration using metal modified MCM-41. Chem. Eng. J. 2020, 383, 123077. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Zhang, X.; Bairq, Z.A.S.; Huang, Y.; Tontiwachwuthikul, P.; Liang, Z. Catalytic performance and mechanism of SO42−/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoethanolamine solution. Appl. Energy 2020, 259, 114179. [Google Scholar] [CrossRef]
- Abanades, J.C.; Criado, Y.A.; Fernández, J.R. An air CO2 capture system based on the passive carbonation of large Ca(OH)2 structures. Sustain. Energy Fuels 2020, 4, 3409–3417. [Google Scholar] [CrossRef]
- Isa, F.; Zabiri, H.; Ng, N.K.S.; Shariff, A.M. CO2 removal via promoted potassium carbonate: A review on modeling and simulation techniques. Int. J. Greenh. Gas Control 2018, 76, 236–265. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A comparative review of potential ammonia-based carbon capture systems. J. Environ. Manag. 2021, 287, 112357. [Google Scholar] [CrossRef]
- Chehrazi, M.; Moghadas, B.K. A review on CO2 capture with chilled ammonia and CO2 utilization in urea plant. J. CO2 Util. 2022, 61, 102030. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Functionalized ionic liquids for CO2 capture under ambient pressure. Green Chem. Lett. Rev. 2023, 16, 2149280. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Yu, G.; Ji, X. Reviewing and screening ionic liquids and deep eutectic solvents for effective CO2 capture. Front. Chem. 2022, 10, 951951. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, J.; Luo, M.; Liu, X.; Zhao, Y.; Fei, W. Molecular Simulation and Experimental Study on Low-Viscosity Ionic Liquids for High-Efficient Capturing of CO2. Energy Fuels 2022, 36, 1604–1613. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Kammerer, S.; Borho, I.; Jung, J.; Schmidt, M.S. Review: CO2 capturing methods of the last two decades. Int. J. Environ. Sci. Technol. 2023, 20, 8087–8104. [Google Scholar] [CrossRef]
- Li, F.; Zeng, S.; Bai, Y.; Dong, H.; Wang, H.; Ji, X.; Zhang, X. Efficient and Reversible Chemisorption of Carbon Dioxide with Dianionic-Functionalized Ionic Liquid-Based Solvents. Energy Fuels 2020, 34, 8526–8533. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Mumford, K.; Stevens, G.W.; Fei, W.; Wang, Y. Recent advances in carbon dioxide capture and utilization with amines and ionic liquids. Green Chem. Eng. 2020, 1, 16–32. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Ma, N.; Li, X.; Huang, Z. CO2 absorption enhancement of fluorinated ionic liquids on nonaqueous biphasic absorbents: Experimental and theoretical study. Carbon Capture Sci. Technol. 2023, 9, 100147. [Google Scholar] [CrossRef]
- Gu, Y.; Hou, Y.; Ren, S.; Sun, Y.; Wu, W. Hydrophobic Functional Deep Eutectic Solvents Used for Efficient and Reversible Capture of CO2. ACS Omega 2020, 5, 6809–6816. [Google Scholar] [CrossRef]
- He, X.; Gao, Y.; Shi, Y.; Zhang, X.; Liang, Z.; Zhang, R.; Song, X.; Lai, Q.; Adidharma, H.; Russell, A.G.; et al. [EMmim][NTf2]—A Novel Ionic Liquid (IL) in Catalytic CO2 Capture and ILs’ Applications. Adv. Sci. 2023, 10, 2205352. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Zhao, Y.-N.; He, L.-N. CO2 chemistry: Task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 2011, 1, 545. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Peng, H.; Liu, Y.; Tao, D.-J.; Wu, P.; Fan, J.-P.; Huang, K. Highly Efficient CO2 Capture by Polyethylenimine Plus 1-Ethyl-3-Methylimidazolium Acetate Mixed Absorbents. ACS Sustain. Chem. Eng. 2019, 7, 9369–9377. [Google Scholar] [CrossRef]
- Aquino, A.S.; Bernard, F.L.; Vieira, M.O.; Borges, J.V.; Rojas, M.F.; Vecchia, F.D.; Ligabue, R.A.; Seferin, M.; Menezes, S.; Einloft, S. A New Approach to CO2 Capture and Conversion Using Imidazolium Based-Ionic Liquids as Sorbent and Catalyst. J. Braz. Chem. Soc. 2014, 25, 2251–2257. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Sadeghpour, M.; Yusoff, R.; Aroua, M.K. Polymeric ionic liquids (PILs) for CO2 capture. Rev. Chem. Eng. 2017, 33, 183–200. [Google Scholar] [CrossRef]
- Jiang, D.; He, Y.; Zhang, J.; Yin, J.; Ding, J.; Wang, S.; Li, H. Conjugate acid-base bi-functional polymeric ionic liquids (CAB-PILs) as efficient catalysts for CO2 capture and subsequent glycidol cycloaddition reaction. J. Mol. Liq. 2023, 375, 121284. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Devakki, B.; Thomas, S. Experimental Investigation on Absorption Performance of Nanofluids for CO2 Capture. Int. J. Air-Cond. Ref. 2020, 28, 2050017. [Google Scholar] [CrossRef]
- Lee, W.; Xu, R.; Kim, S.; Park, J.H.; Kang, Y.T. Nanofluid and nanoemulsion absorbents for the enhancement of CO2 absorption performance. J. Clean. Prod. 2021, 291, 125848. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nano-absorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, S.-M.; Hung, C.-I. Carbon dioxide capture by single droplet using Selexol, Rectisol and water as absorbents: A theoretical approach. Appl. Energy 2013, 111, 731–741. [Google Scholar] [CrossRef]
- Tsunatu, D.; Mohammed-Dabo, I.; Waziri, S. Technical Evaluation of Selexol-Based CO2 Capture Process for a Cement Plant. BJECC 2015, 5, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hochgesand, G. Rectisol and Purisol. Ind. Eng. Chem. 1970, 62, 37–43. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Song, D. Conceptual design, optimization and thermodynamic analysis of a CO2 capture process based on Rectisol. Energy 2022, 244, 122566. [Google Scholar] [CrossRef]
- Schütz, M.; Daun, M.; Weinspach, P.-M.; Krumbeck, M.; Hein, K.R.G. Study on the CO2-recovery from an ICGCC-plant. Energy Convers. Manag. 1992, 33, 357–363. [Google Scholar] [CrossRef]
- MilliporeSigma|Life Science Products & Service Solutions. Available online: https://www.sigmaaldrich.com/US/en (accessed on 30 September 2024).
- Kearns, D.D.; Liu, H.; Consoli, C.; Technology Readiness And Costs Of CCS. Global CCS Institute. 2021. Available online: https://scienceforsustainability.org/w/images/b/bc/Technology-Readiness-and-Costs-for-CCS-2021-1.pdf (accessed on 21 February 2025).
- Karlov, S.P.; Kazenin, D.A.; Baranov, D.A.; Volkov, A.V.; Polyanin, D.A.; Vyaz’min, A.V. Interphase effects and macrokinetics of chemisorption in the absorption of CO2 by aqueous solutions of alkalis and amines. Russ. J. Phys. Chem. 2007, 81, 665–679. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.; Wu, Y.; Kentish, S.; Stevens, G. Recent Progress on the Performance of Different Rate Promoters in Potassium Carbonate Solvents for CO2 Capture. Energy Procedia 2017, 114, 2279–2286. [Google Scholar] [CrossRef]
- Hu, G.; Nicholas, N.J.; Smith, K.H.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into promoted potassium carbonate solutions: A review. Int. J. Greenh. Gas Control 2016, 53, 28–40. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Wan Alwi, S.R.; Manan, Z.A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Int. J. Greenh. Gas Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Chen, Z.; Gao, R.; Yu, H.; Yuan, T.; Liu, Z.; Maeder, M. Heterogeneous catalysts for the hydrogenation of amine/alkali hydroxide solvent captured CO2 to formate: A review. Greenh. Gases 2021, 11, 807–823. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Du, J.; Yang, W.; Xu, L.; Bei, L.; Lei, S.; Li, W.; Liu, H.; Wang, B.; Sun, L. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia. Chem. Eng. J. 2024, 488, 150954. [Google Scholar] [CrossRef]
- Akeeb, O.; Wang, L.; Xie, W.; Davis, R.; Alkasrawi, M.; Toan, S. Post-combustion CO2 capture via a variety of temperature ranges and material adsorption process: A review. J. Environ. Manag. 2022, 313, 115026. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Nie, L.; Mu, Y.; Jin, J.; Chen, J.; Mi, J. Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas. Chin. J. Chem. Eng. 2018, 26, 2303–2317. [Google Scholar] [CrossRef]
- Wei, J.W.; Zhao, S.S. Capture of Carbon Dioxide by Adsorption—A Review. AMR 2012, 538–541, 2240–2245. [Google Scholar] [CrossRef]
- Diyuk, V.E.; Zaderko, A.N.; Grischenko, L.M.; Tsapyuk, G.G.; Vakaliuk, A.V.; Lisnyak, V.V.; Mariychuk, R. CO2 adsorption on pristine, oxidized, and diethylamine-functionalized activated carbon sorbents. E3S Web Conf. 2020, 154, 07001. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Potential of using biomass based activated carbon for carbon dioxide capture. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1130, 012022. [Google Scholar] [CrossRef]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. Synthesis of activated carbon/polyaniline nanocomposites for enhanced CO2 adsorption. RSC Adv. 2016, 6, 35692–35704. [Google Scholar] [CrossRef]
- Bhatti, A.H.; Waris, M.; Kazmi, W.W.; Bhatti, U.H.; Min, G.H.; Park, B.C.; Kweon, S.; Baek, I.H.; Nam, S.C. Metal impregnated activated carbon as cost-effective and scalable catalysts for amine-based CO2 capture. J. Environ. Chem. Eng. 2023, 11, 109231. [Google Scholar] [CrossRef]
- Bhatti, A.H.; Waris, M.; Kazmi, W.W.; Kang, K.-H.; Bhatti, U.H. Acid-treated activated carbon as simple and inexpensive catalyst to accelerate CO2 desorption from aqueous amine solution. Carbon Capture Sci. Technol. 2023, 8, 100131. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Rahmah, W.; Kadja, G.T.M.; Mahyuddin, M.H.; Saputro, A.G.; Dipojono, H.K.; Wenten, I.G. Small-pore zeolite and zeotype membranes for CO2 capture and sequestration—A review. J. Environ. Chem. Eng. 2022, 10, 108707. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Morales-Ospino, R.; Finocchio, E.; Busca, G.; Sapag, K.; Villarroel-Rocha, J.; Bastos-Neto, M.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Kaolinite-based zeolites synthesis and their application in CO2 capture processes. Fuel 2022, 320, 123953. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, X.; Xue, Z.; Shi, H.; Meng, X.; Feng, B.; Wang, X.; Yang, N. Water enhanced methanol decomposition for simultaneous heat recovery and ready-to-use synthesis gas production over CO2 capture enhanced Ni/zeolite 4A catalyst. Int. J. Hydrogen Energy 2023, 48, 21701–21711. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Catalytic Solvent Regeneration for Energy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 18755–18788. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Gao, H.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Zeolite catalyst-aided tri-solvent blend amine regeneration: An alternative pathway to reduce the energy consumption in amine-based CO2 capture process. Appl. Energy 2019, 240, 827–841. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal–Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Wei, K.; Xing, L.; Li, Y.; Xu, T.; Li, Q.; Wang, L. Heteropolyacid modified Cerium-based MOFs catalyst for amine solution regeneration in CO2 capture. Sep. Purif. Technol. 2022, 293, 121144. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, D.; Malik, N.; Kumar, S.; Singh Malik, R. Metal catalyst for CO2 capture and conversion into cyclic carbonate: Progress and challenges. Mater. Today 2023, 65, 133–165. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, H.; Fei, H. Unusual Missing Linkers in an Organosulfonate-Based Primitive–Cubic (pcu)-Type Metal–Organic Framework for CO2 Capture and Conversion under Ambient Conditions. ACS Catal. 2018, 8, 2519–2525. [Google Scholar] [CrossRef]
- Keller, L.; Ohs, B.; Lenhart, J.; Abduly, L.; Blanke, P.; Wessling, M. High capacity polyethylenimine impregnated microtubes made of carbon nanotubes for CO2 capture. Carbon 2018, 126, 338–345. [Google Scholar] [CrossRef]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified carbon nanotubes/tetraethylenepentamine for CO2 capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Ngoy, J.M.; Wagner, N.; Riboldi, L.; Bolland, O. A CO2 Capture Technology Using Multi-walled Carbon Nanotubes with Polyaspartamide Surfactant. Energy Procedia 2014, 63, 2230–2248. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef]
- García, S.; Gil, M.V.; Martín, C.F.; Pis, J.J.; Rubiera, F.; Pevida, C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem. Eng. J. 2011, 171, 549–556. [Google Scholar] [CrossRef]
- Lahuri, A.H.; Ling, M.N.K.; Rahim, A.A.; Nordin, N. Adsorption Kinetics for CO2 Capture using Cerium Oxide Impregnated on Activated carbon. Acta Chim. Slov. 2020, 67, 570–580. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, S.; He, S.; Wu, C. Direct air capture of CO2 by KOH-activated bamboo biochar. J. Energy Inst. 2022, 105, 399–405. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Zhao, Z.-J.; Siahrostami, S.; Nørskov, J.K.; Studt, F. Cation-exchanged zeolites for the selective oxidation of methane to methanol. Catal. Sci. Technol. 2018, 8, 114–123. [Google Scholar] [CrossRef]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Román-Leshkov, Y. Catalytic Oxidation of Methane into Methanol over Copper-Exchanged Zeolites with Oxygen at Low Temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef]
- Stuckert, A.N.; Yang, R.T. CO2 Capture from the Atmosphere and Simultaneous Concentration Using Zeolites and Amine-Grafted SBA-15. Environ. Sci. Technol. 2011, 45, 10257–10264. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz Da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.G.; Maroto-Valer, M.M.; Chen, Y.; Garcia, S. Layered Double Hydroxides-Based Mixed Metal Oxides: Development of Novel Structured Sorbents for CO2 Capture Applications. ACS Appl. Mater. Interfaces 2021, 13, 11805–11813. [Google Scholar] [CrossRef]
- Santamaría, L.; Korili, S.A.; Gil, A. Layered double hydroxides for CO2 adsorption at moderate temperatures: Synthesis and amelioration strategies. Chem. Eng. J. 2023, 455, 140551. [Google Scholar] [CrossRef]
- Menmuir, D.; Florence, S.; Taylor, K. Next Generation Carbon Capture Technology: Technology Review Work Package 2. 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1079540/aecom-next-gen-carbon-capture-technology-technology-review-annex-1.pdf (accessed on 21 February 2025).
- Bukar, A.M.; Asif, M. Technology readiness level assessment of carbon capture and storage technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Freguia, S.; Rochelle, G.T. Modeling of CO2 capture by aqueous monoethanolamine. AIChE J. 2003, 49, 1676–1686. [Google Scholar] [CrossRef]
- Closmann, F.; Nguyen, T.; Rochelle, G.T. MDEA/Piperazine as a solvent for CO2 capture. Energy Procedia 2009, 1, 1351–1357. [Google Scholar] [CrossRef]
- Gomes, J.; Santos, S.; Bordado, J. Choosing amine-based absorbents for CO2 capture. Environ. Technol. 2015, 36, 19–25. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Jayakumar, A.; Gomez, A.; Mahinpey, N. Post-combustion CO2 capture using solid K2CO3: Discovering the carbonation reaction mechanism. Appl. Energy 2016, 179, 531–543. [Google Scholar] [CrossRef]
- Helmi, M.; Moazami, F.; Ghaemi, A.; Hemmati, A. Synthesis, characterization and performance evaluation of NaOH@Chitosan-Fe3O4 as an adsorbent for CO2 capture. Fuel 2023, 338, 127300. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Mitigating NH3 Vaporization from an Aqueous Ammonia Process for CO2 Capture. Int. J. Chem. React. Eng. 2011, 9. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on Molecular Sieves and Activated Carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).