Highly Efficient Production of Furfural from Corncob by Barley Hull Biochar-Based Solid Acid in Cyclopentyl Methyl Ether–Water System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Sn-NUS-BH
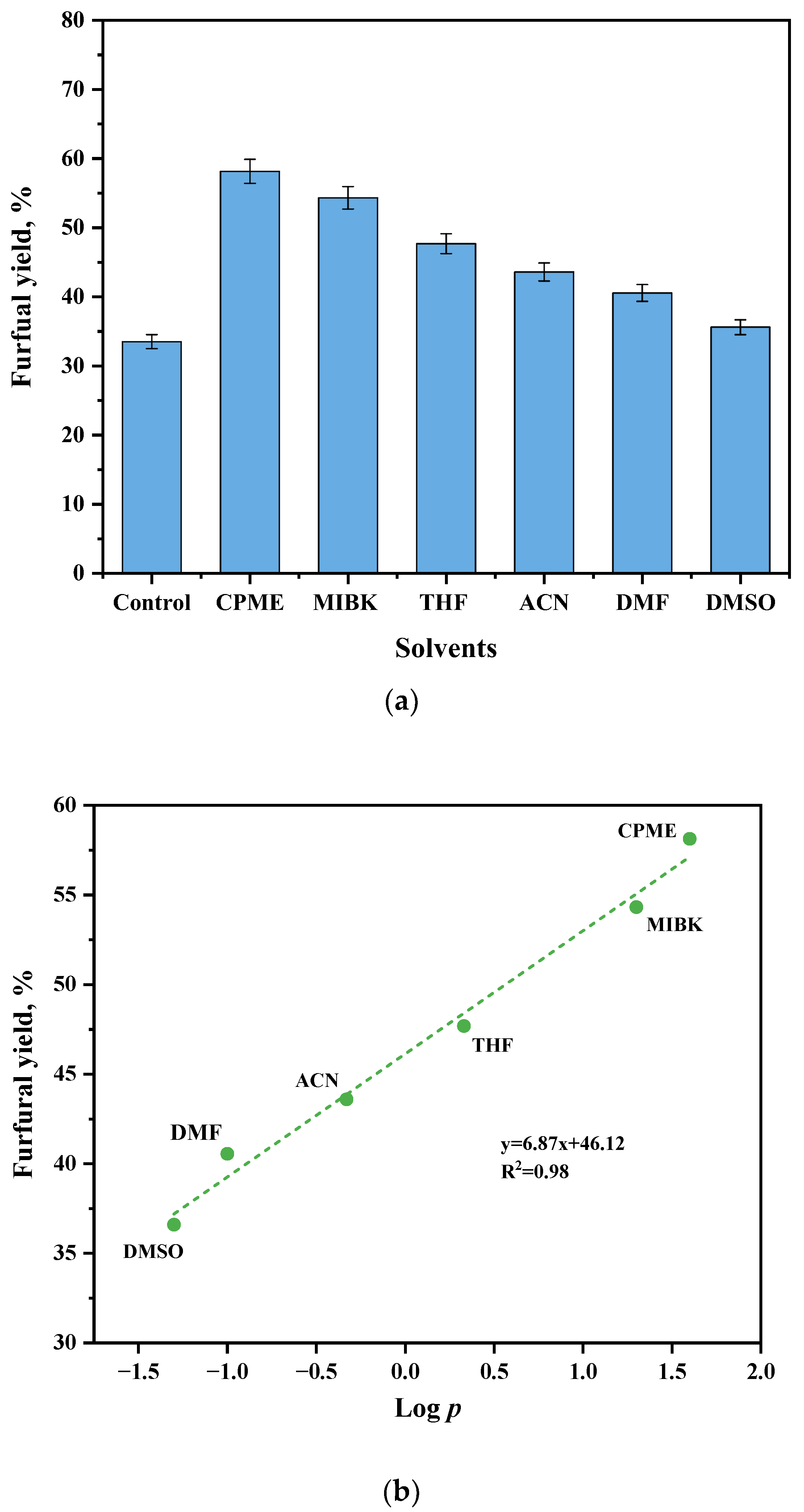
2.2. Effect of Organic Solvents on Furfural Yield
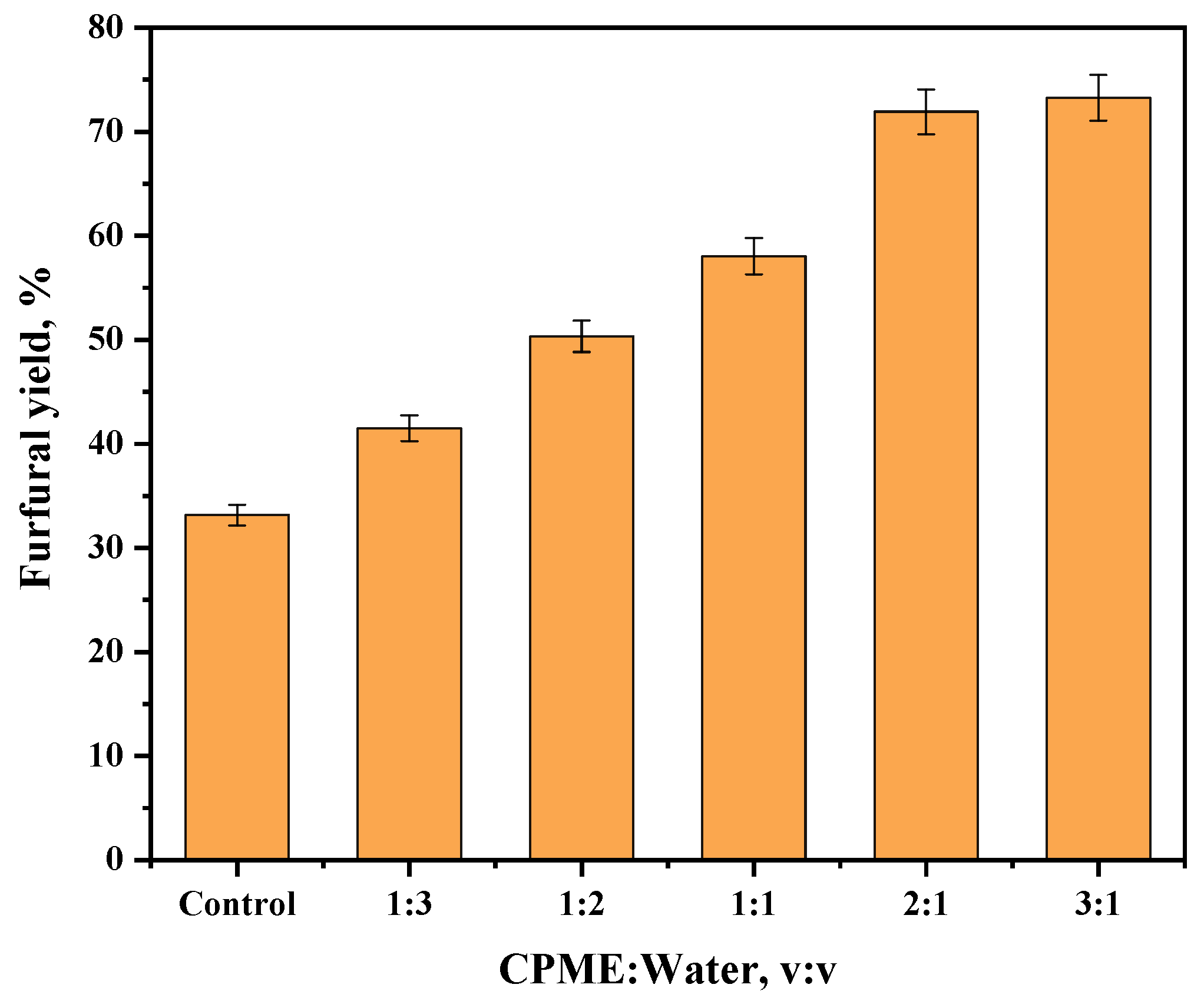
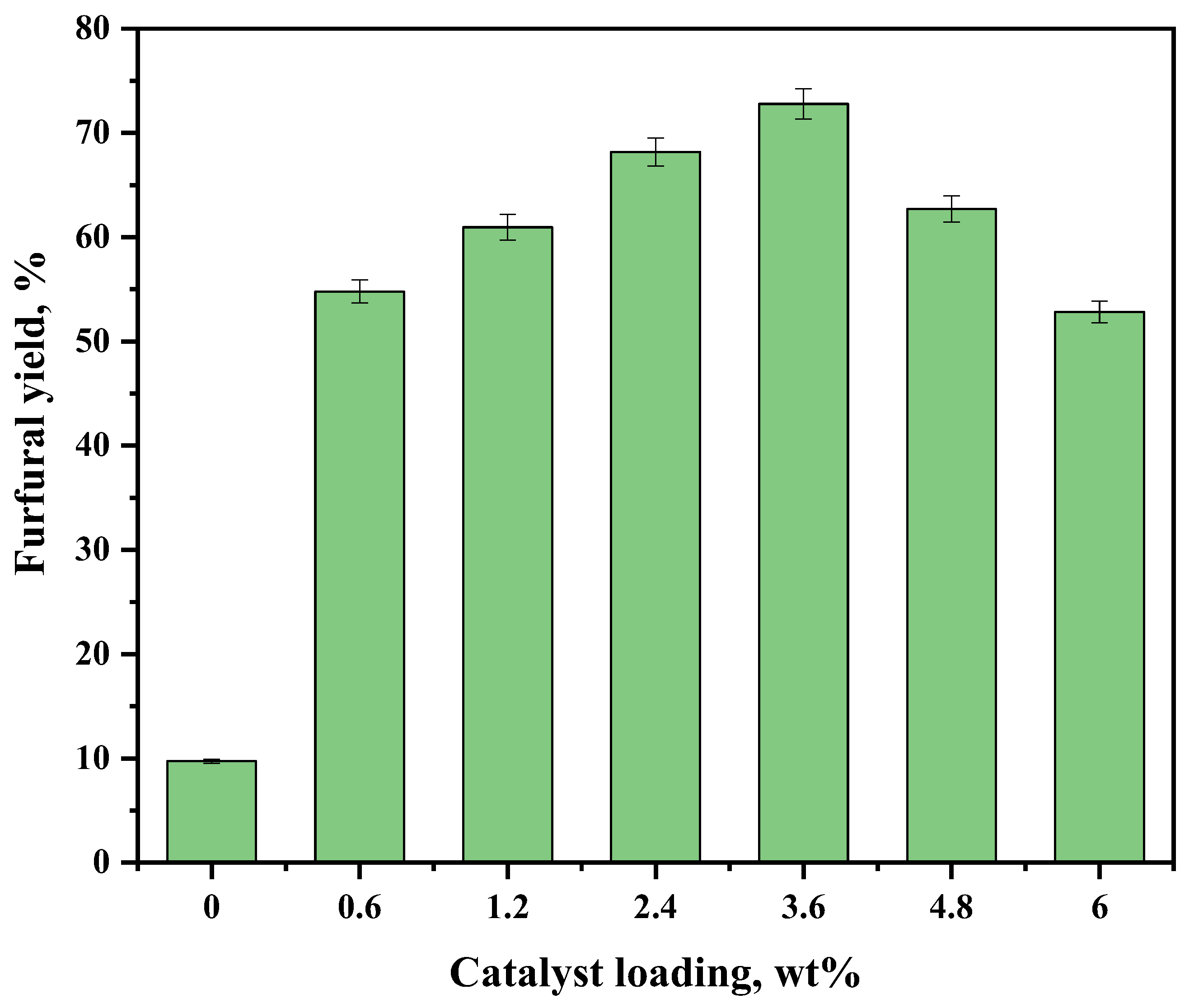
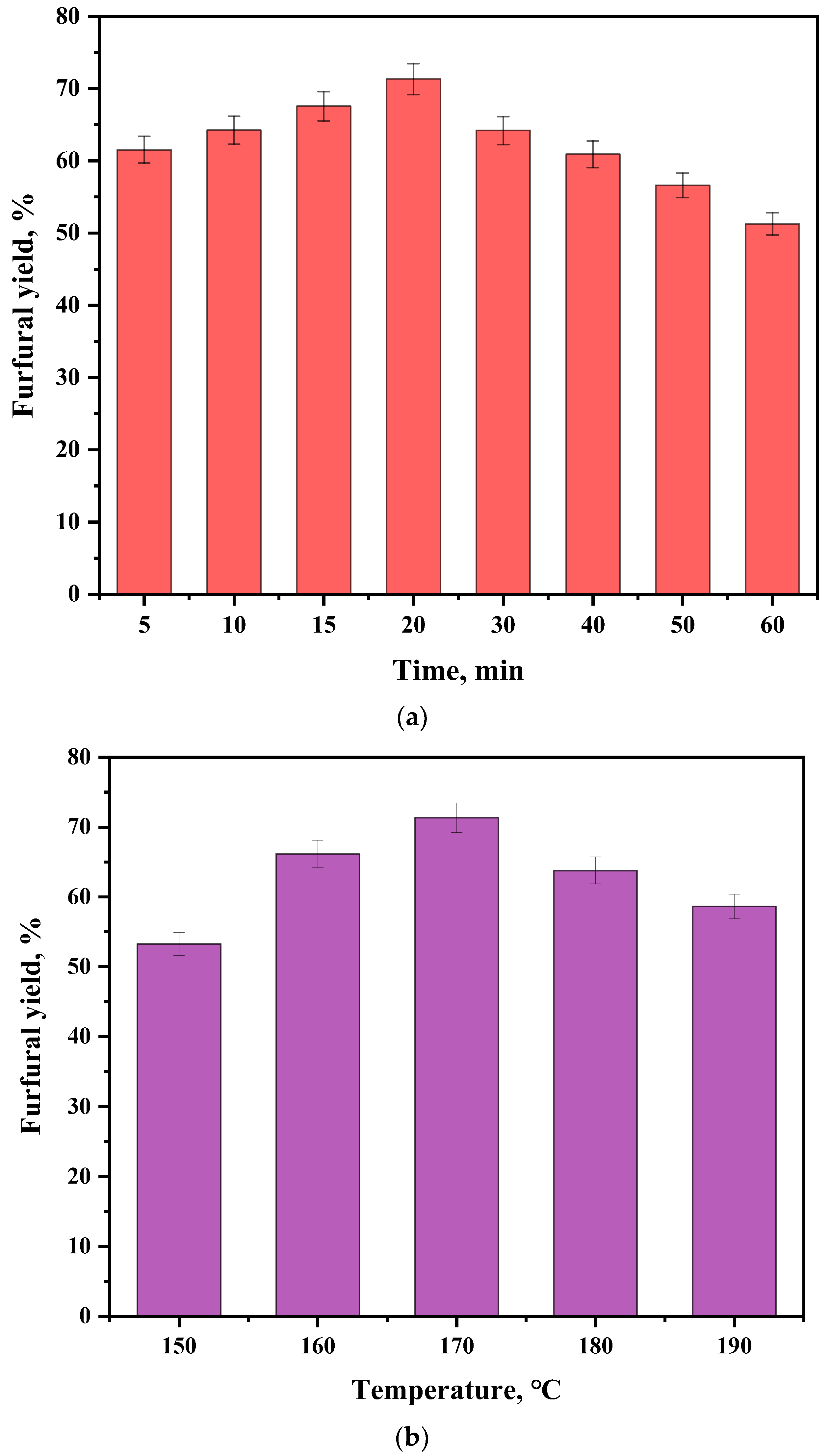
2.3. Synthesis of Furfural from Corncob by Sn-NUS-BH in CPME–Water Two-Phase System
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Solid Acid Sn-NUS-BH
3.3. Chemical Transformation of Corncob into Furfural by Sn-NUS-BH in Organic Solvent–Water System
3.4. Reuse of Sn-NUS-BH
3.5. Structural Features of Corncob and Sn-NUS-BH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, G.; Liu, X.; Du, H.; Zuo, J.; Wang, L. Way forward for alternative energy research: A bibliometric analysis during 1994–2013. Renew. Sustain. Energy Rev. 2015, 48, 276–286. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Len, T.; Urbanob, J.; Balu, A.; Luque, R. Transformation of furfural into dimorpholinocyclopentenone using Al-SBA-15 materials. Sustain. Chem. Pharm. 2024, 39, 101573. [Google Scholar] [CrossRef]
- He, Y.-C.; Jiang, C.-X.; Chong, G.-G.; Di, J.-H.; Wu, Y.-F.; Wang, B.-Q.; Xue, X.-X.; Ma, C.-L. Chemical-enzymatic conversion of corncob-derived xylose to furfuralcohol by the tandem catalysis with SO42−/SnO2-kaoline and E. coli CCZU-T15 cells in toluene–water media. Bioresour. Technol. 2017, 245, 841–849. [Google Scholar] [PubMed]
- Hu, S.L.; Liang, S.; Mo, L.Z.; Su, H.H.; Huang, J.S.; Zhang, P.J.; Qi, J.N. Conversion of biomass-derived monosaccharide into furfural over Cr–Mg-LDO@bagasse catalysts. Sustain. Chem. Pharm. 2023, 32, 101013. [Google Scholar] [CrossRef]
- Licursi, D.; Galletti, M.R.; Bertini, B.; Ardemani, L.; Scotti, N.; Fidio, N.D.; Fulignati, S.; Antonetti, C. Design approach for the sustainable synthesis of sulfonated biomass-derived hydrochars and pyrochars for the production of 5-(hydroxymethyl)furfural. Sustain. Chem. Pharm. 2023, 39, 101573. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and modification of hemicellulose from lignocellulosic biomass: A review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Yatis, R.G.; Kumar, D.H.; Chinnabhandar, R.K.; Raviraj, H.M.; Ravi Shankar, A.U. A review of the potential application of lignin in the production of bio-binder: Challenges and opportunities. J. Mater. Sci. 2024, 59, 3205–3224. [Google Scholar]
- Terrett, O.M.; Dupree, P. Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.; Yin, X.; Wei, M.; Xu, J.; Jiang, J.; Wang, K. Selective conversion of hemicellulose into furfural over low-cost metal salts in a γ-valerolactone/water solution. Ind. Crops Prod. 2020, 147, 112248. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Zhou, S.; Du, J.; Tao, Y.; Cheng, Y.; Wang, H. Bioconversion of cellulose and hemicellulose in reed sawdust to xylo-oligosaccharides and L-lactic acid. Ind. Crops Prod. 2022, 187, 115390. [Google Scholar] [CrossRef]
- Wang, Z.; Bhattacharyya, S.; Vlachos, D.G. Extraction of furfural and furfural/5-hydroxymethylfurfural from mixed lignocellulosic biomass-derived feedstocks. ACS Sustain. Chem. Eng. 2021, 9, 7489–7498. [Google Scholar] [CrossRef]
- Gong, L.; Zha, J.; Pan, L.; Ma, C.; He, Y.C. Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour. Technol. 2022, 351, 126945. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gao, R.; He, Y.-C.; Ma, C. Efficient synthesis of furfural from waste biomasses by sulfonated crab shell-based solid acid in a sustainable approach. Ind. Crops Prod. 2023, 202, 116989. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, W.; Ma, C.; He, Y.C. Efficient co-production of xylooligosaccharides, furfural and reducing sugars from yellow bamboo via the pretreatment with biochar-based catalyst. Bioresour. Technol. 2023, 387, 129637. [Google Scholar] [CrossRef]
- Sato, O.; Mimura, N.; Masuda, Y.; Shirai, M.; Yamaguchi, A. Effect of extraction on furfural production by solid acid-catalyzed xylose dehydration in water. J. Supercrit. Fluids 2019, 144, 14–18. [Google Scholar] [CrossRef]
- Gómez Millán, G.; Hellsten, S.; King, A.W.T.; Pokki, J.-P.; Llorca, J.; Sixta, H. A comparative study of water-immiscible organic solvents in the production of furfural from xylose and birch hydrolysate. J. Ind. Eng. Chem. 2019, 72, 354–363. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.-L.; Cao, D.-M.; Cao, X.-F.; Sun, S.-N. One-step conversion of corn stalk to glucose and furfural in molten salt hydrate/organic solvent biphasic system. Bioresour. Technol. 2023, 386, 129520. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, S.; Wang, T.; Tang, R.; Wang, Y.; Zhang, L. High conversion of xylose to furfural over corncob residue-based solid acid catalyst in water-methyl isobutyl ketone. Ind. Crops Prod. 2022, 180, 114781. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Luo, C.; Gu, X.; Lu, L.; Lu, X. Kinetics of furfural production from corn cob in γ-valerolactone using dilute sulfuric acid as catalyst. ACS Sustain. Chem. Eng. 2017, 5, 8587–8593. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Q.; Di, J.; Ma, C.; He, Y.C. An efficient chemoenzymatic cascade strategy for transforming biomass into furfurylamine with lobster shell-based chemocatalyst and mutated ω-transaminase biocatalyst in methyl isobutyl ketone-water. Bioresour. Technol. 2023, 369, 128424. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Varma, R.S.; Len, C. Comprehensive study on expeditious conversion of pre-hydrolyzed alginic acid to furfural in Cu(II) biphasic systems using microwaves. Mol. Catal. 2018, 445, 73–79. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L. Furfural production from biomass residues: Current technologies, challenges and future prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, G.; Wang, K. Efficient conversion of biomass derivatives to furfural with a novel carbon-based solid acid catalyst. Catal. Commun. 2023, 175, 106608. [Google Scholar] [CrossRef]
- Fúnez-Núñez, I.; García-Sancho, C.; Cecilia, J.A.; Moreno-Tost, R.; Serrano-Cantador, L.; Maireles-Torres, P. Recovery of pentoses-containing olive stones for their conversion into furfural in the presence of solid acid catalysts. Process Saf. Environ. Prot. 2020, 143, 1–13. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; An, S.; Huang, F.; Li, X.; Liu, J.; Pei, G.; Liu, Q. Efficient transformation of corn stover to furfural using p-hydroxybenzenesulfonic acid-formaldehyde resin solid acid. Bioresour. Technol. 2018, 264, 261–267. [Google Scholar] [CrossRef]
- Grant, K.R.; Brennan, M.; Hoad, S.P. The structure of the Barley husk influences its resistance to mechanical stress. Front. Plant Sci. 2020, 11, 614334. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xu, Z.; Liu, Q.; Ma, Q.; Jameel, H.; Chang, H.-M.; Ma, L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 2016, 209, 108–114. [Google Scholar] [CrossRef]
- Teng, X.; Si, Z.; Li, S.; Yang, Y.; Wang, Z.; Li, G.; Zhao, J.; Cai, D.; Qin, P. Tin-loaded sulfonated rape pollen for efficient catalytic production of furfural from corn stover. Ind. Crops Prod. 2020, 151, 112481. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Z.-Y.; Dong, J.-J.; Li, H.; Han, R.-Z.; Xu, G.-C.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Bioresour. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef]
- Yang, Q.; Fan, B.; He, Y.C. Combination of solid acid and solvent pretreatment for co-production of furfural, xylooligosaccharide and reducing sugars from Phyllostachys edulis. Bioresour. Technol. 2024, 395, 130398. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Zhong, L.; Sun, R.; Liang, L. Production of furfural from xylose, water-insoluble hemicelluloses and water-soluble fraction of corncob via a tin-loaded montmorillonite solid acid catalyst. Bioresour. Technol. 2015, 176, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Donggen, H.; Yu, D.; Su, Y.; Yuan, G. Preparation and characterization of ZnO/TiO2, SO42−/ZnO/TiO2 photocatalyst and their photocatalysis. J. Photochem. Photobiol. A Chem. 2004, 168, 7–13. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: Optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Luo, W.; Cao, W.; Bruijnincx, P.C.A.; Lin, L.; Wang, A.; Zhang, T. Zeolite-supported metal catalysts for selective hydrodeoxygenation of biomass-derived platform molecules. Green Chem. 2019, 21, 3744–3768. [Google Scholar] [CrossRef]
- Dietz, C.H.J.T.; Verra, M.; Verberkt, S.; Gallucci, F.; Kroon, M.C.; Neira D’Angelo, M.F.; Papaioannou, M.; van Sint Annaland, M. Sequential and in situ extraction of Ffurfural from reaction mixture and effect of extracting agents on furfural degradation. Ind. Eng. Chem. Res. 2019, 58, 16116–16125. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H.; Wang, K.; Lu, K.; Qu, Y.; Zhu, L.; Wang, S. Enhanced furfural production from biomass and its derived carbohydrates in the renewable butanone–water solvent system. Sustain. Energy Fuels 2019, 3, 3208–3218. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Huang, C.; Fu, Y.; Chang, J. Efficient conversion of xylose into furfural using sulfonic acid-functionalized metal–organic frameworks in a biphasic system. Ind. Eng. Chem. Res. 2018, 57, 16628–16634. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.-L.; Zhang, Z.-J.; Ma, C.; He, Y. Highly efficient chemoenzymatic cascade catalysis of biomass into furfurylamine by a heterogeneous shrimp shell-based chemocatalyst and an ω-transaminase biocatalyst in deep eutectic solvent–water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Zha, J.; Fan, B.; He, J.; He, Y.C.; Ma, C. Valorization of Biomass to Furfural by Chestnut Shell-based Solid Acid in Methyl Isobutyl Ketone-Water-Sodium Chloride System. Appl. Biochem. Biotechnol. 2022, 194, 2021–2035. [Google Scholar] [CrossRef]
- Hu, B.; Xie, W.-L.; Wu, Y.-T.; Liu, J.; Ma, S.-W.; Wang, T.-P.; Zheng, S.; Lu, Q. Mechanism study on the formation of furfural during zinc chloride-catalyzed pyrolysis of xylose. Fuel 2021, 295, 120656. [Google Scholar] [CrossRef]
- Ji, L.; Tang, Z.; Yang, D.; Ma, C.; He, Y.C. Improved one-pot synthesis of furfural from corn stalk with heterogeneous catalysis using corn stalk as biobased carrier in deep eutectic solvent-water system. Bioresour. Technol. 2021, 340, 125691. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhou, Y.; Zhang, X.; Peng, D.; Nie, X.; Chen, J.; Xiong, W. Conversion of carbohydrates into furfural and 5-hydroxymethylfurfural using furfuryl alcohol resin-based solid acid as catalyst. Cellulose 2022, 29, 1419–1433. [Google Scholar] [CrossRef]
- He, Y.; Huang, M.; Tang, W.; Ma, C. Improving enzymatic hydrolysis of sunflower straw pretreated by deep eutectic solvent with different carboxylic acids as hydrogen bond donors. Ind. Crops Prod. 2023, 193, 116157. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Yi, Y.; Lin, J.C.; Chen, D.; Ji, X.J. One-pot two-step biocatalytic upgrading of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid (FDCA) through a sequential oxidation process. Sustain. Chem. Pharm. 2024, 39, 101616. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Sun, Q.; Ma, J.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Elucidation of the interaction effects of cellulose, hemicellulose and lignin during degradative solvent extraction of lignocellulosic biomass. Fuel 2022, 327, 125141. [Google Scholar] [CrossRef]
- Nayak, R.; Cleveland, D.; Tran, G.; Joseph, F. Potential of bacterial cellulose for sustainable fashion and textile applications: A review. J. Mater. Sci. 2024, 59, 6685–6710. [Google Scholar] [CrossRef]
- Huang, C.; Lin, W.; Zheng, Y.; Zhao, X.; Ragauskas, A.; Meng, X. Evaluating the mechanism of milk protein as an efficient lignin blocker for boosting the enzymatic hydrolysis of lignocellulosic substrates. Green Chem. 2022, 24, 5263–5279. [Google Scholar] [CrossRef]
- Tang, W.; Tang, Z.; Qian, H.; Huang, C.; He, Y.C. Implementing dilute acid pretreatment coupled with solid acid catalysis and enzymatic hydrolysis to improve bioconversion of bamboo shoot shells. Bioresour. Technol. 2023, 381, 129167. [Google Scholar] [CrossRef]

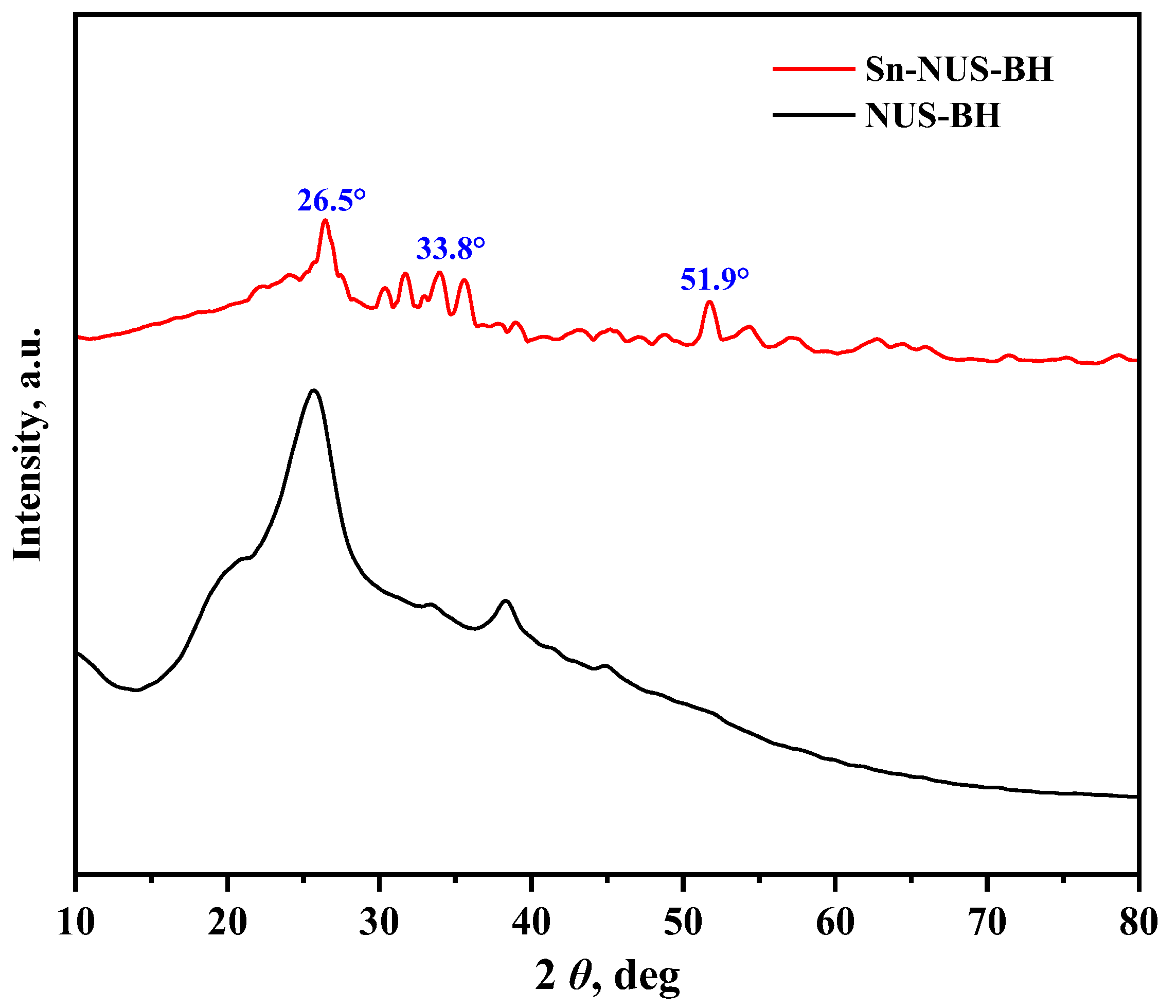
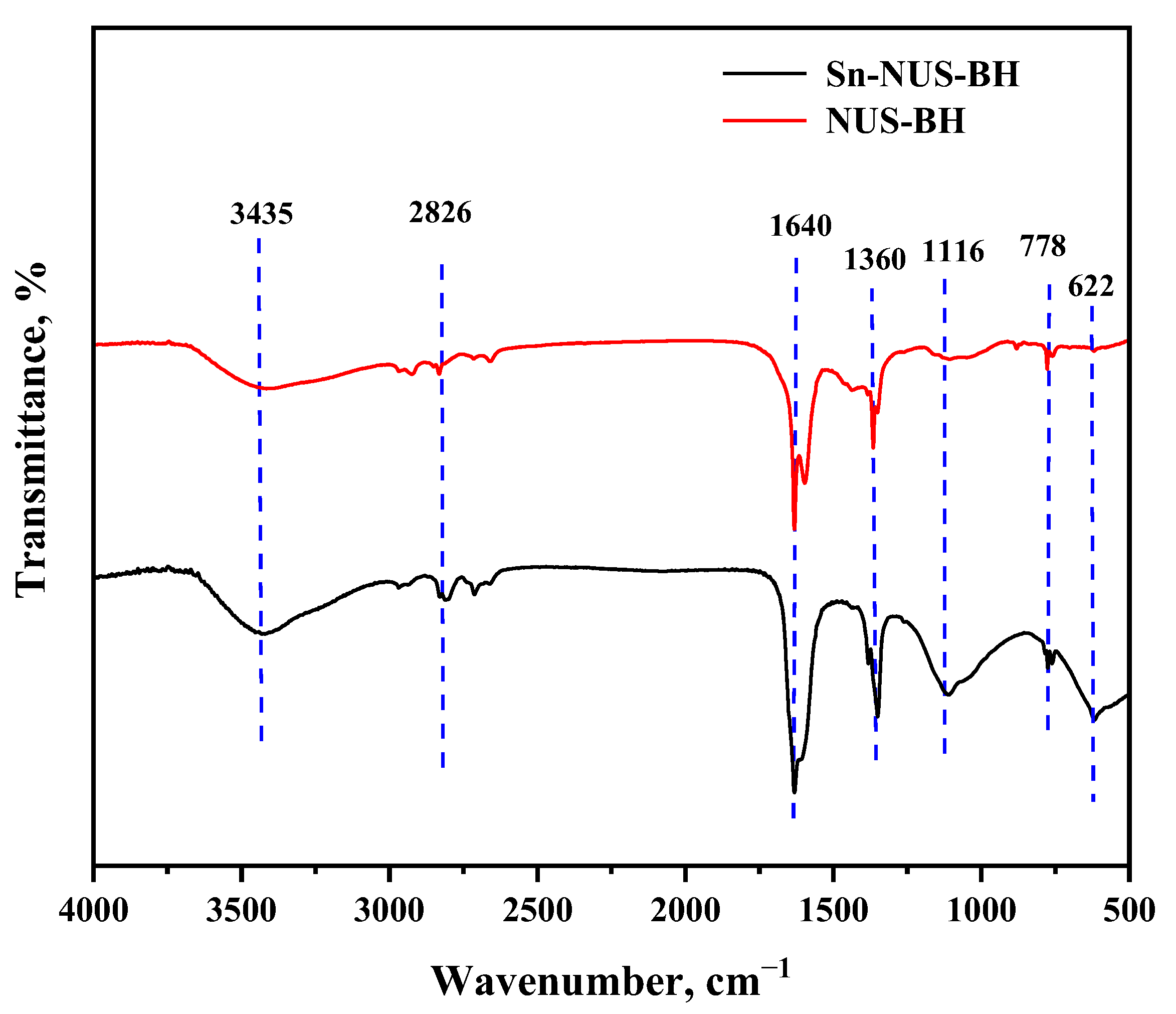
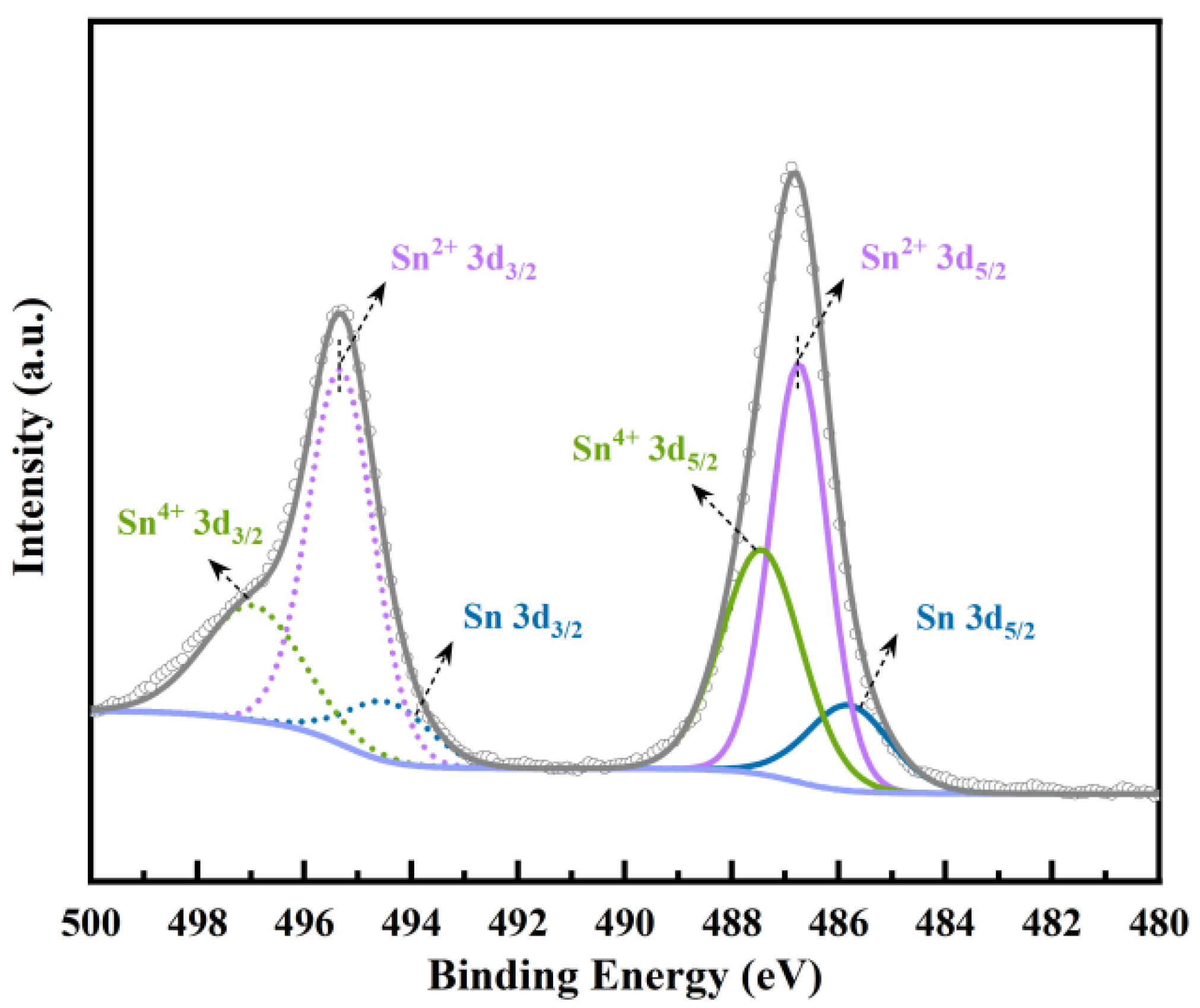
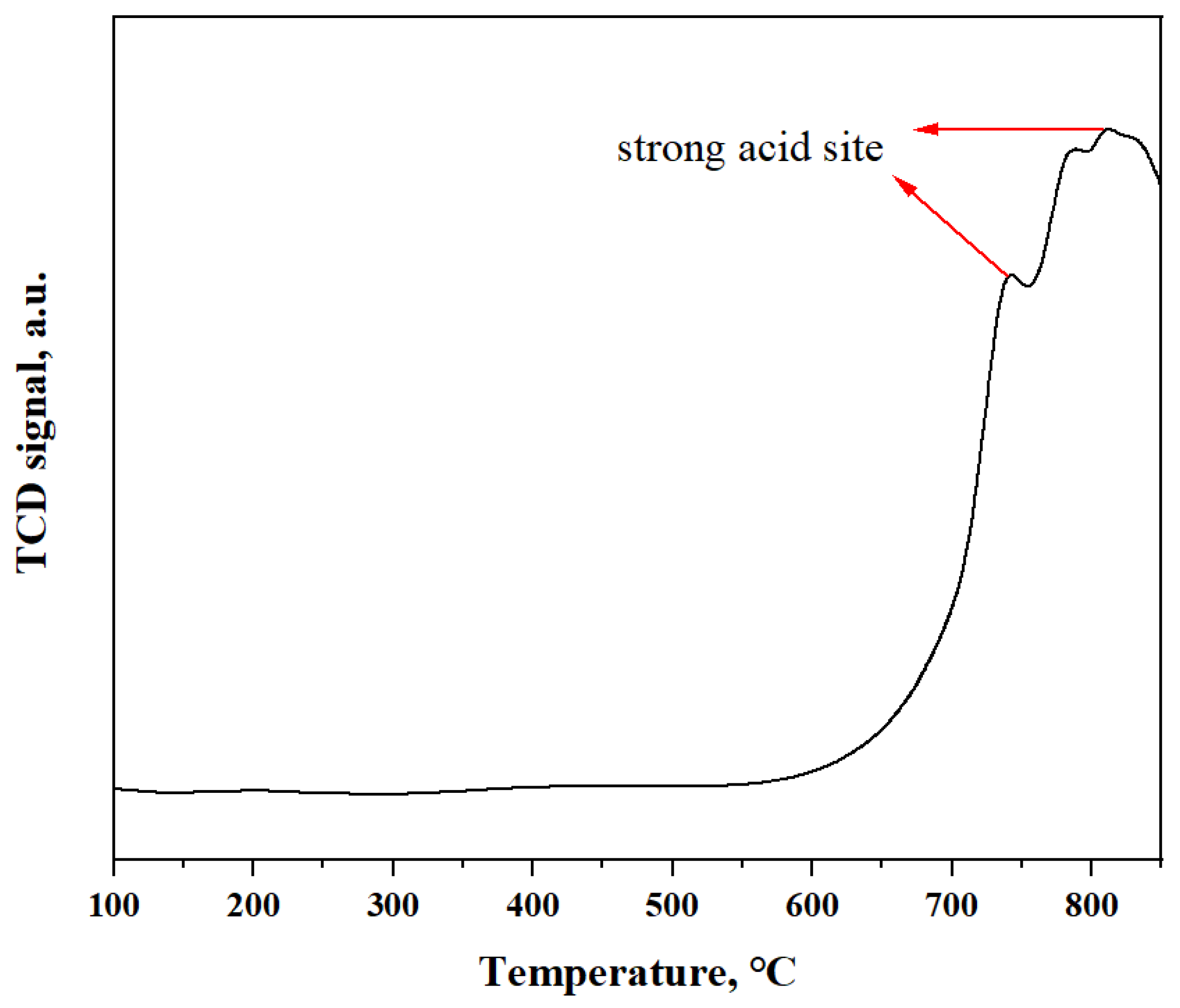
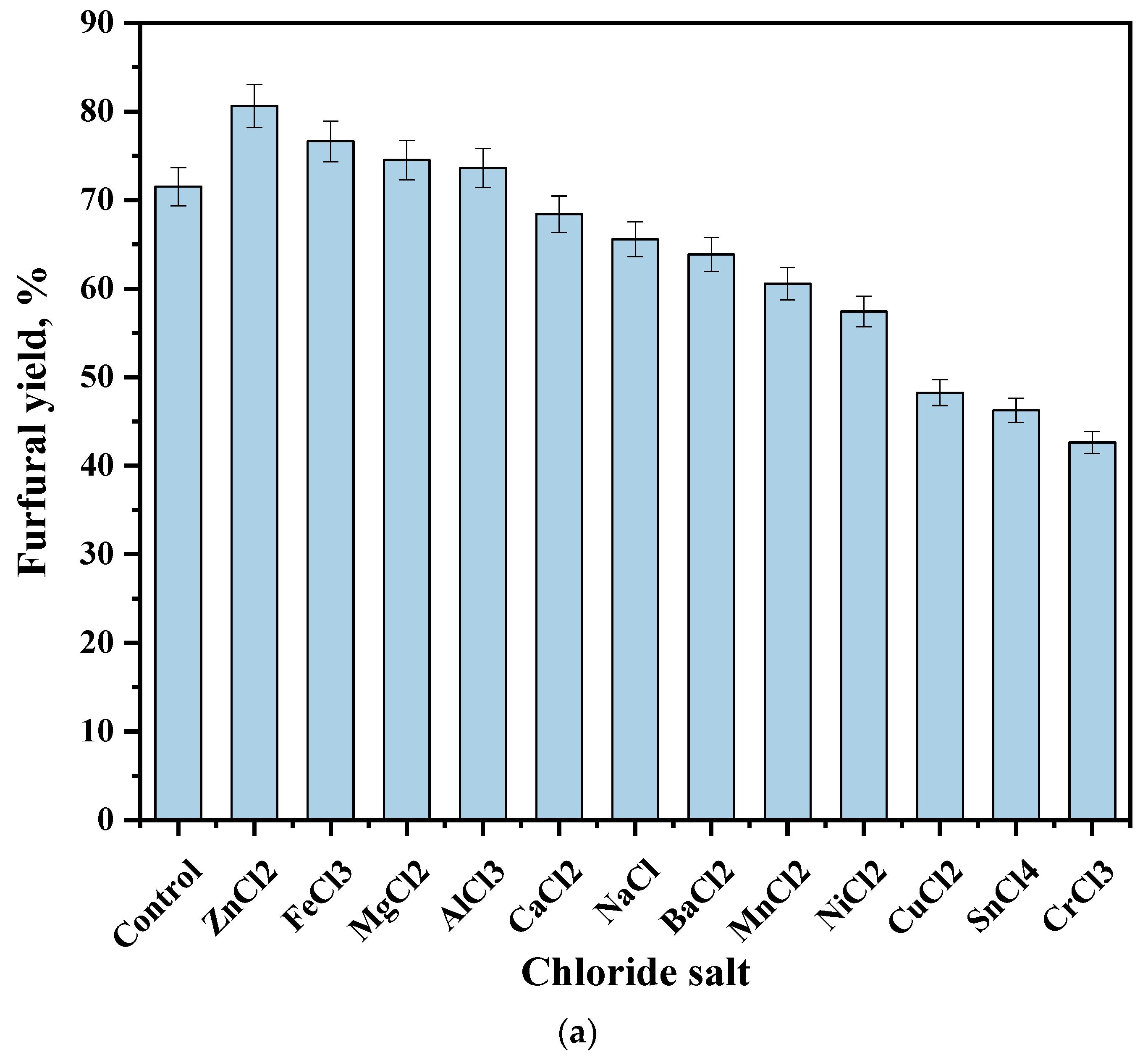
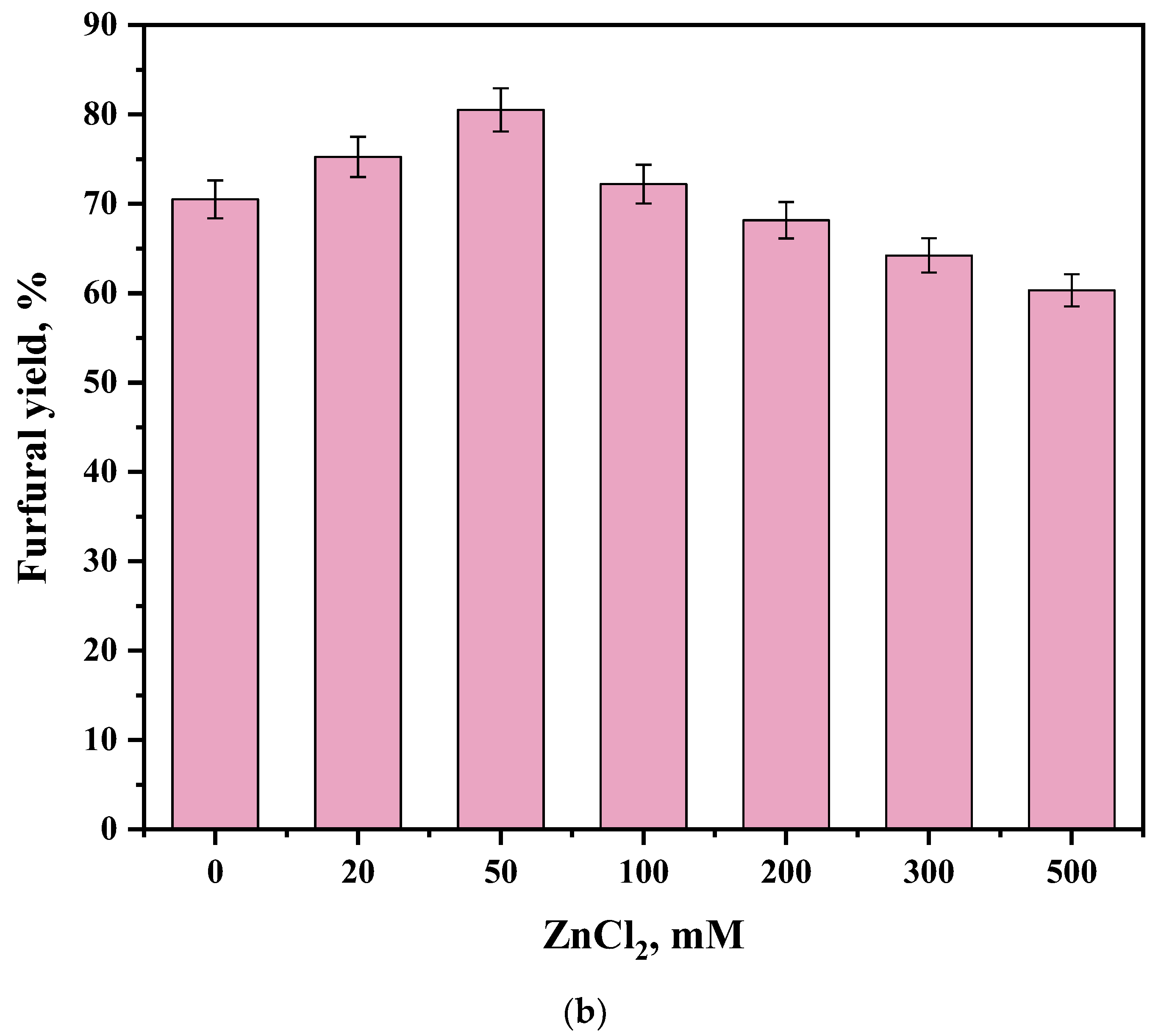

| Sample | Specific Surface Area, m2/g | Pore Volume, cm3/g | Pore Size, nm |
|---|---|---|---|
| NUS-BH | 0.6 | 0.04 | 11.8 |
| Sn-NUS-BH | 63.2 | 0.15 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Kong, L.; He, Y. Highly Efficient Production of Furfural from Corncob by Barley Hull Biochar-Based Solid Acid in Cyclopentyl Methyl Ether–Water System. Catalysts 2024, 14, 583. https://doi.org/10.3390/catal14090583
Fan B, Kong L, He Y. Highly Efficient Production of Furfural from Corncob by Barley Hull Biochar-Based Solid Acid in Cyclopentyl Methyl Ether–Water System. Catalysts. 2024; 14(9):583. https://doi.org/10.3390/catal14090583
Chicago/Turabian StyleFan, Bo, Linghui Kong, and Yucai He. 2024. "Highly Efficient Production of Furfural from Corncob by Barley Hull Biochar-Based Solid Acid in Cyclopentyl Methyl Ether–Water System" Catalysts 14, no. 9: 583. https://doi.org/10.3390/catal14090583






