Advanced XPS-Based Techniques in the Characterization of Catalytic Materials: A Mini-Review
Abstract
1. Introduction
2. Conventional XPS Technology and Functional Accessories (Analysis at Different Depths)
2.1. ISS (The Analysis Depth Is Less than 0.5 nm)
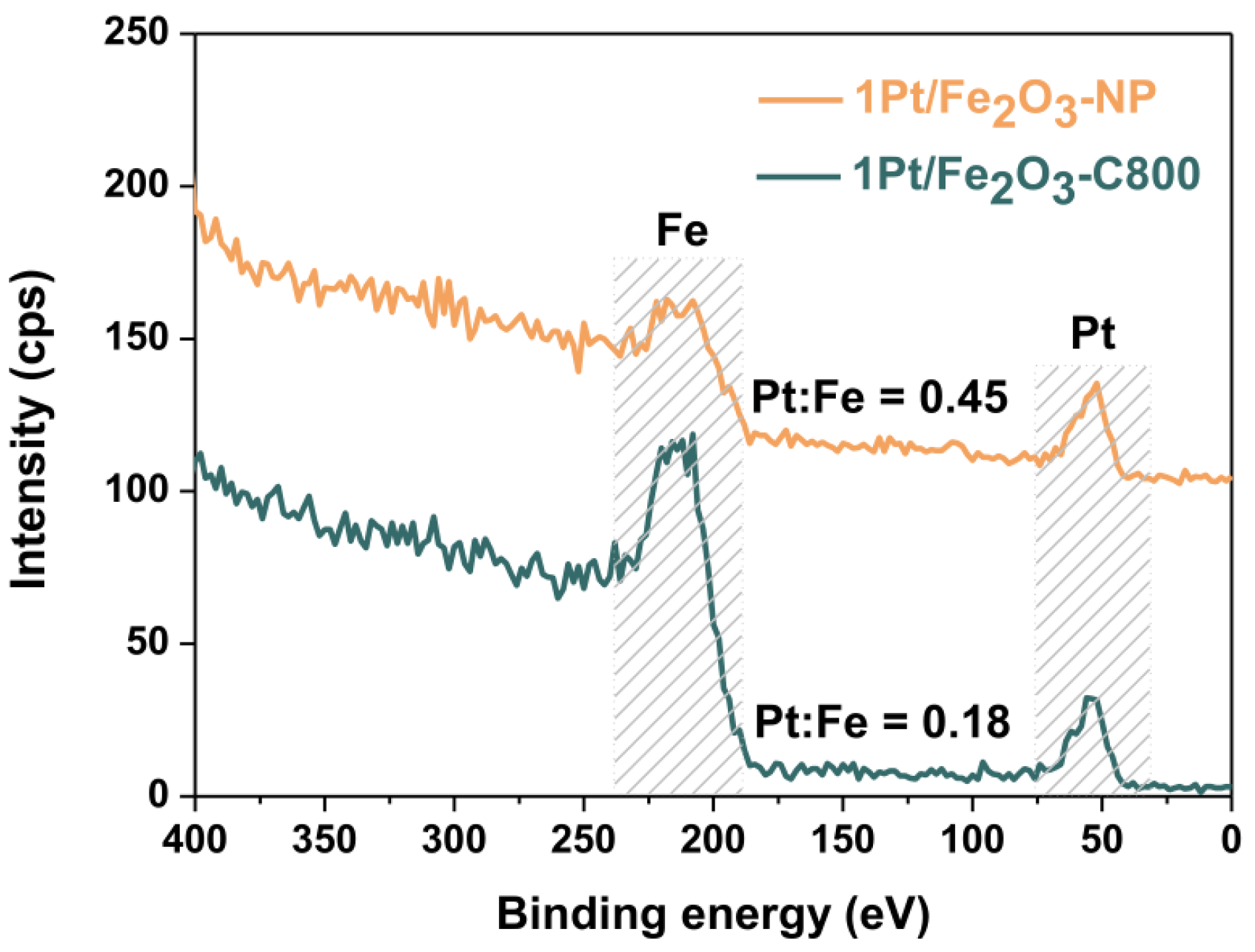
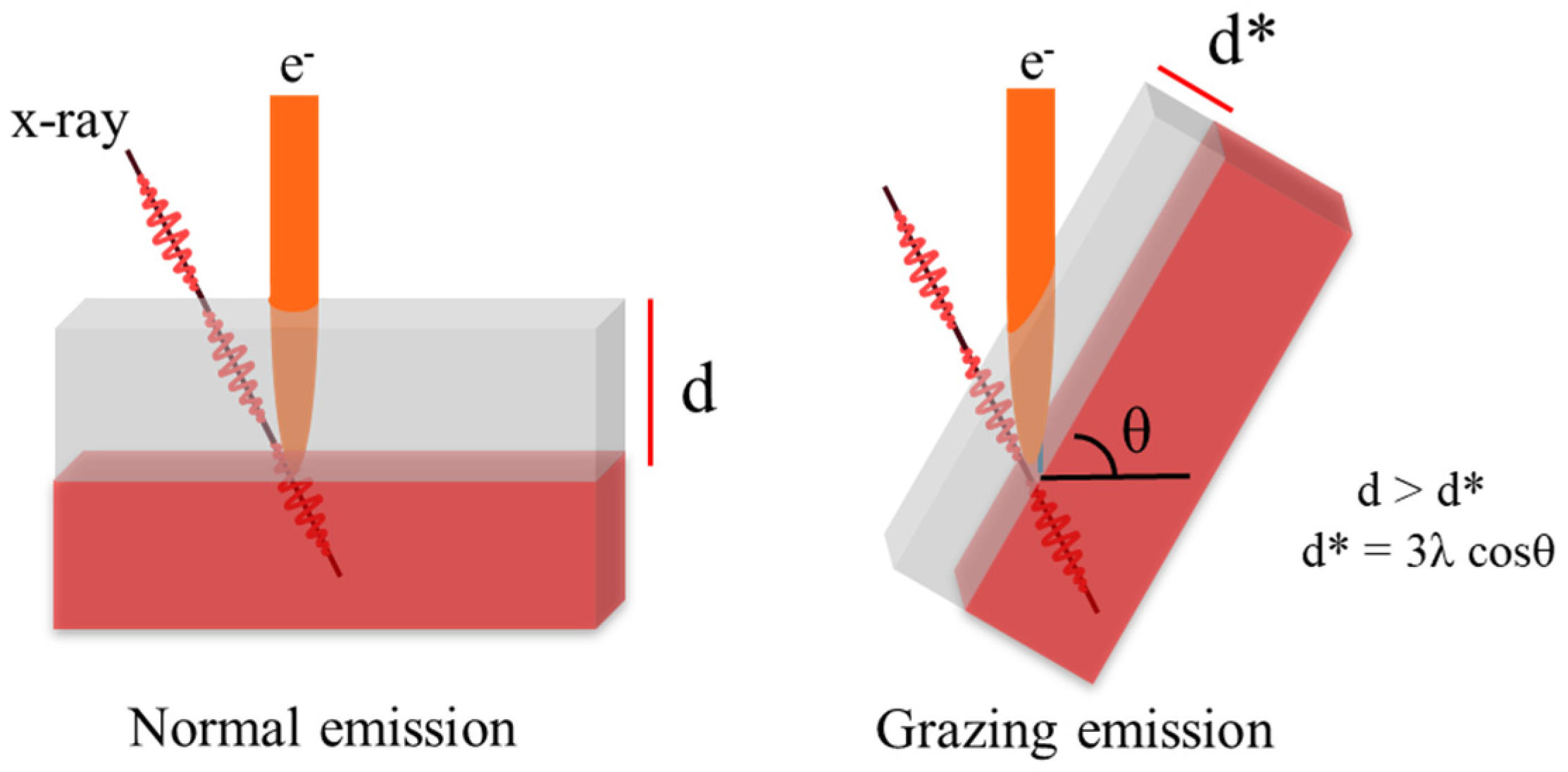
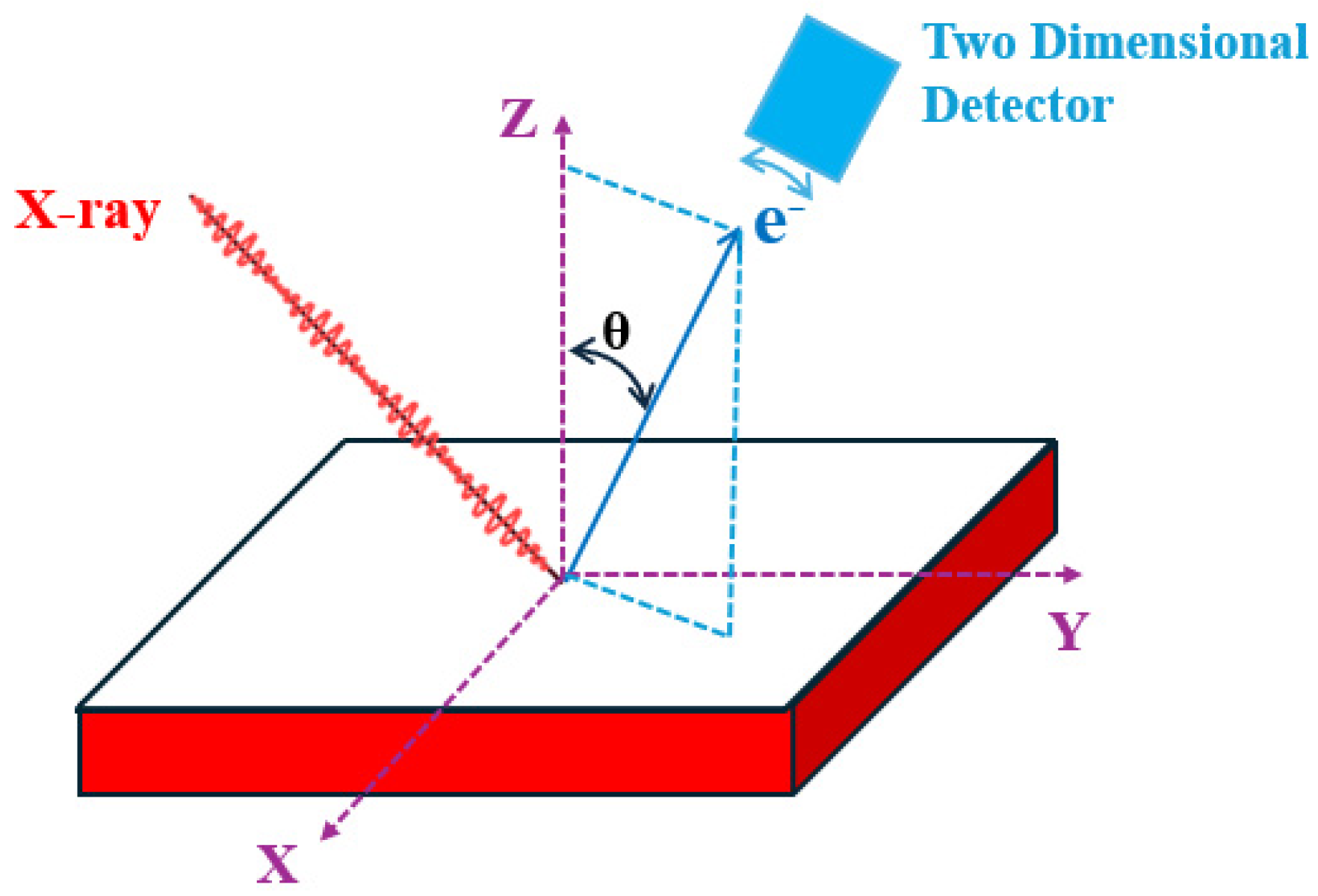
2.2. Angle-Resolved XPS (The Analysis Depth Is about 1~10 nm)
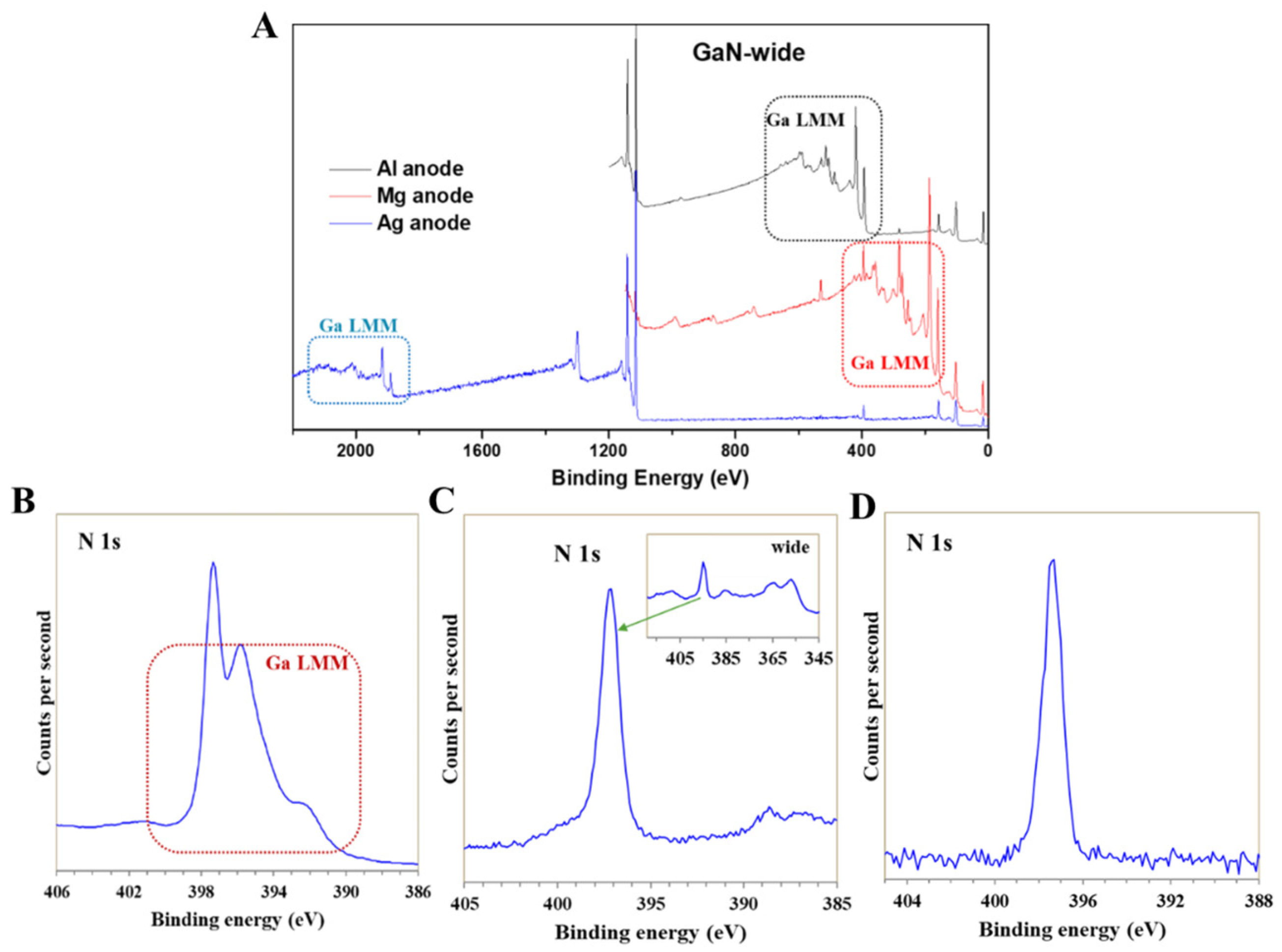
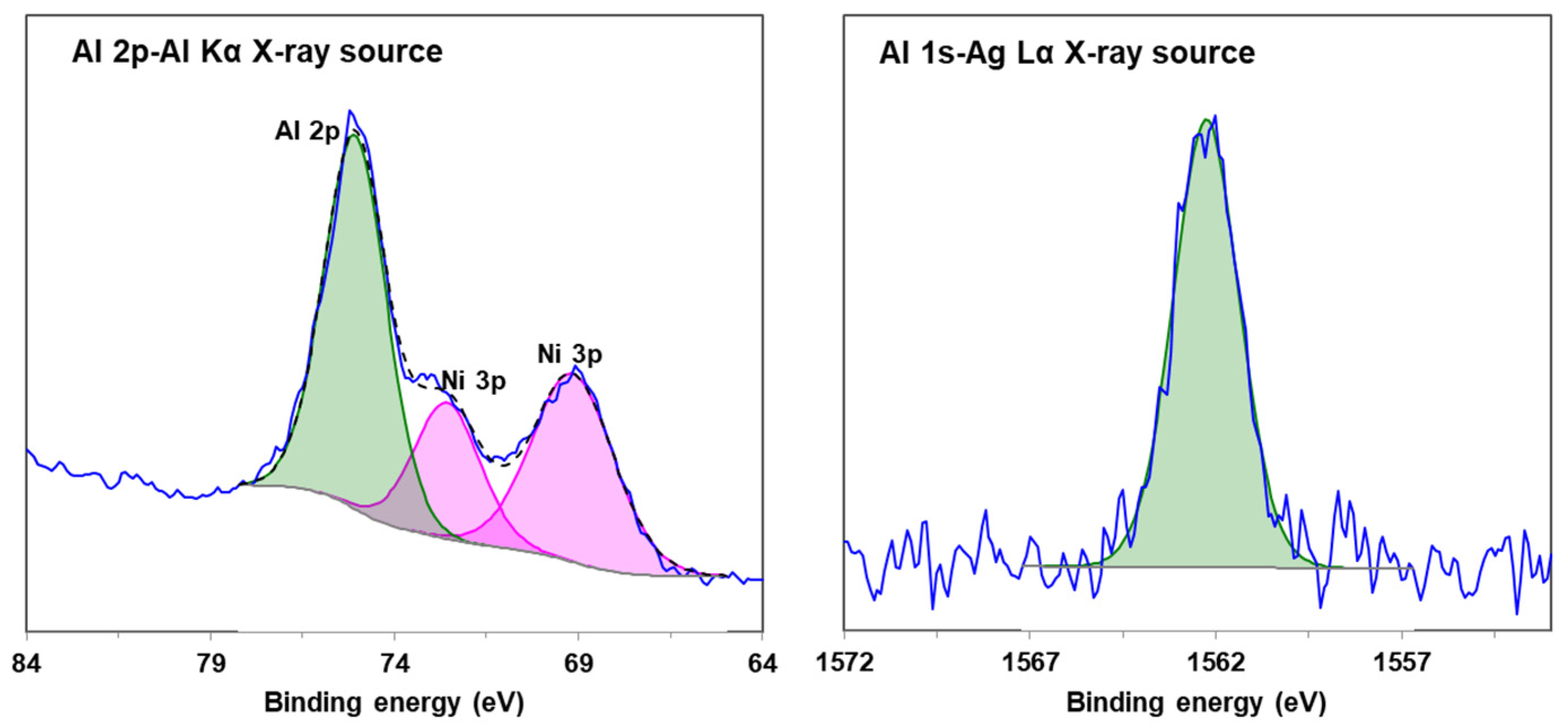
2.3. High Energy X-ray Source (X-ray Sources Involved in This Article Have an Analysis Depth of Less than 50 nm)
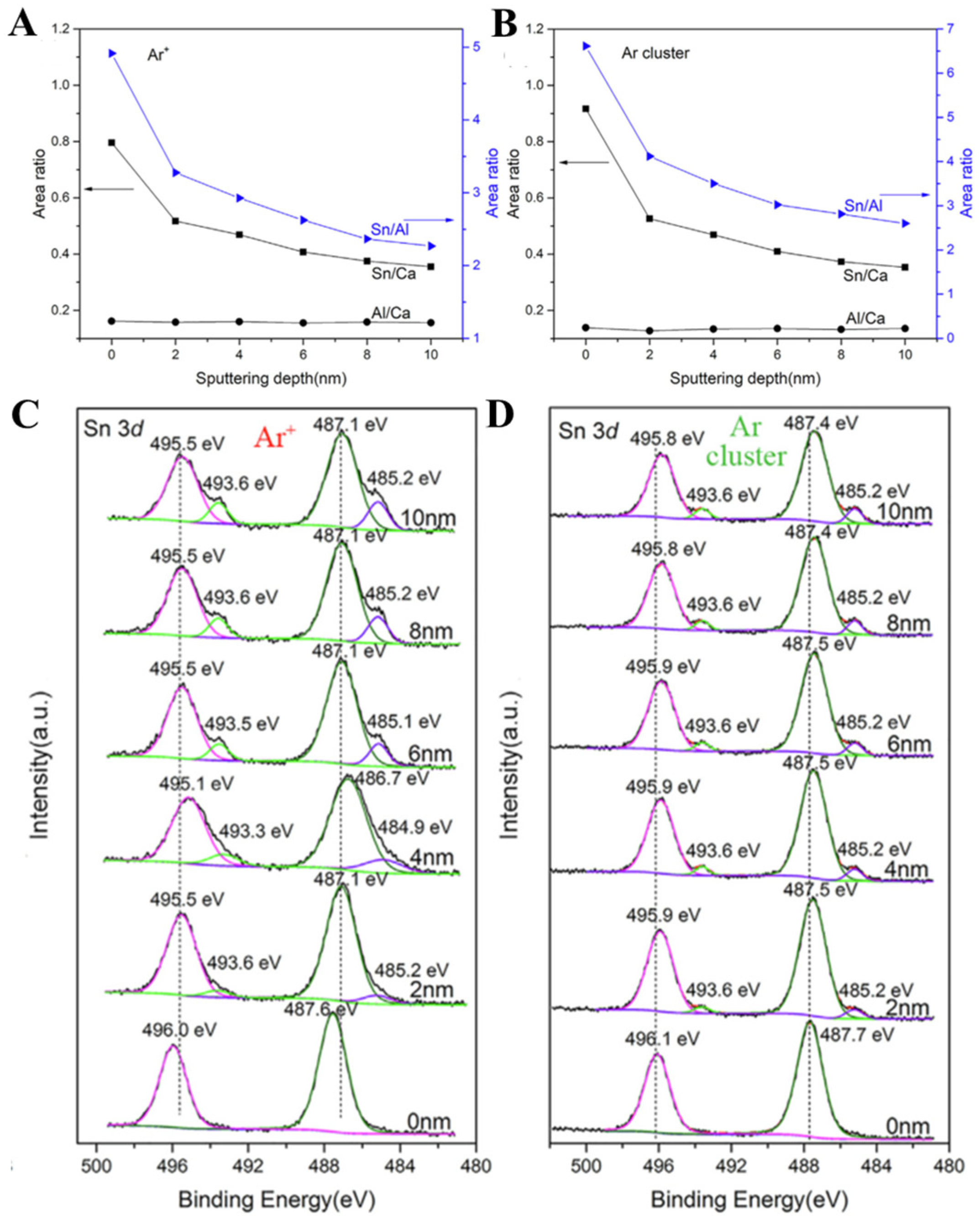
2.4. Mono Argon Ion and Argon Cluster Ion Etching (The Analysis Depth Is about 10 nm~2 μm)
3. Quasi In Situ XPS
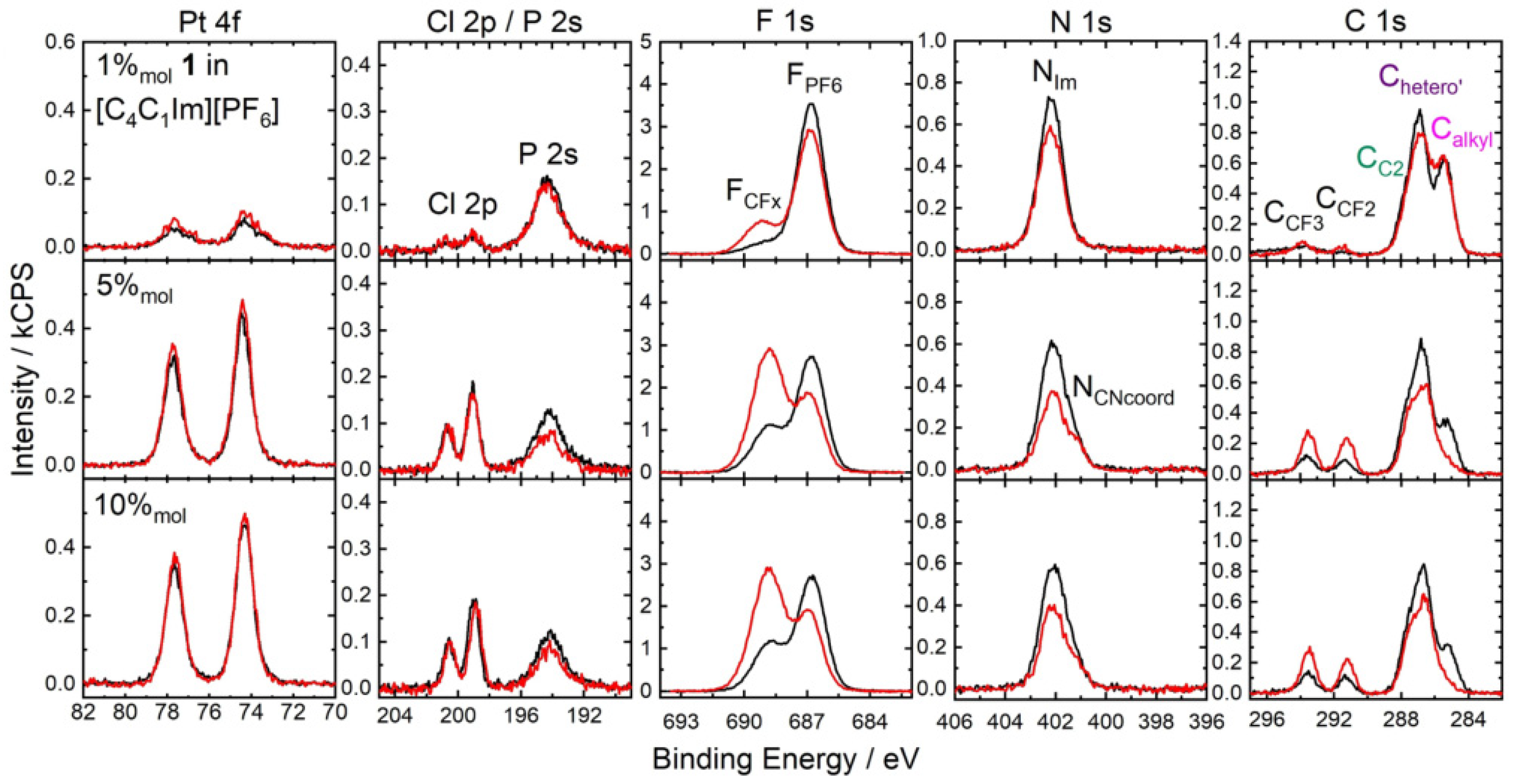
3.1. Transfer of Samples under Inert Atmosphere
3.2. High Temperature Cat Cell
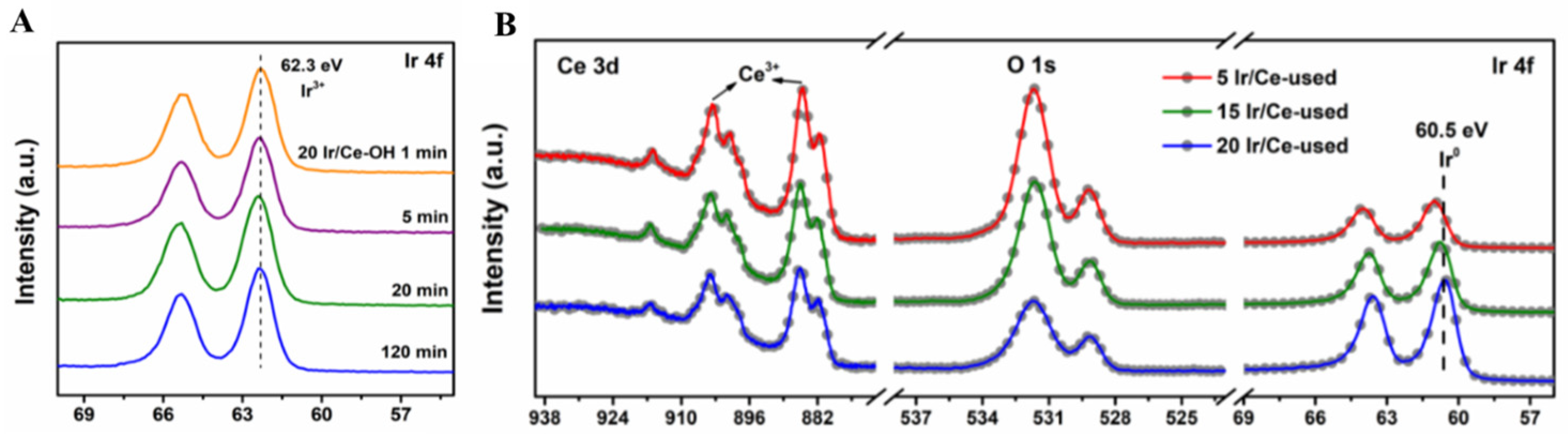
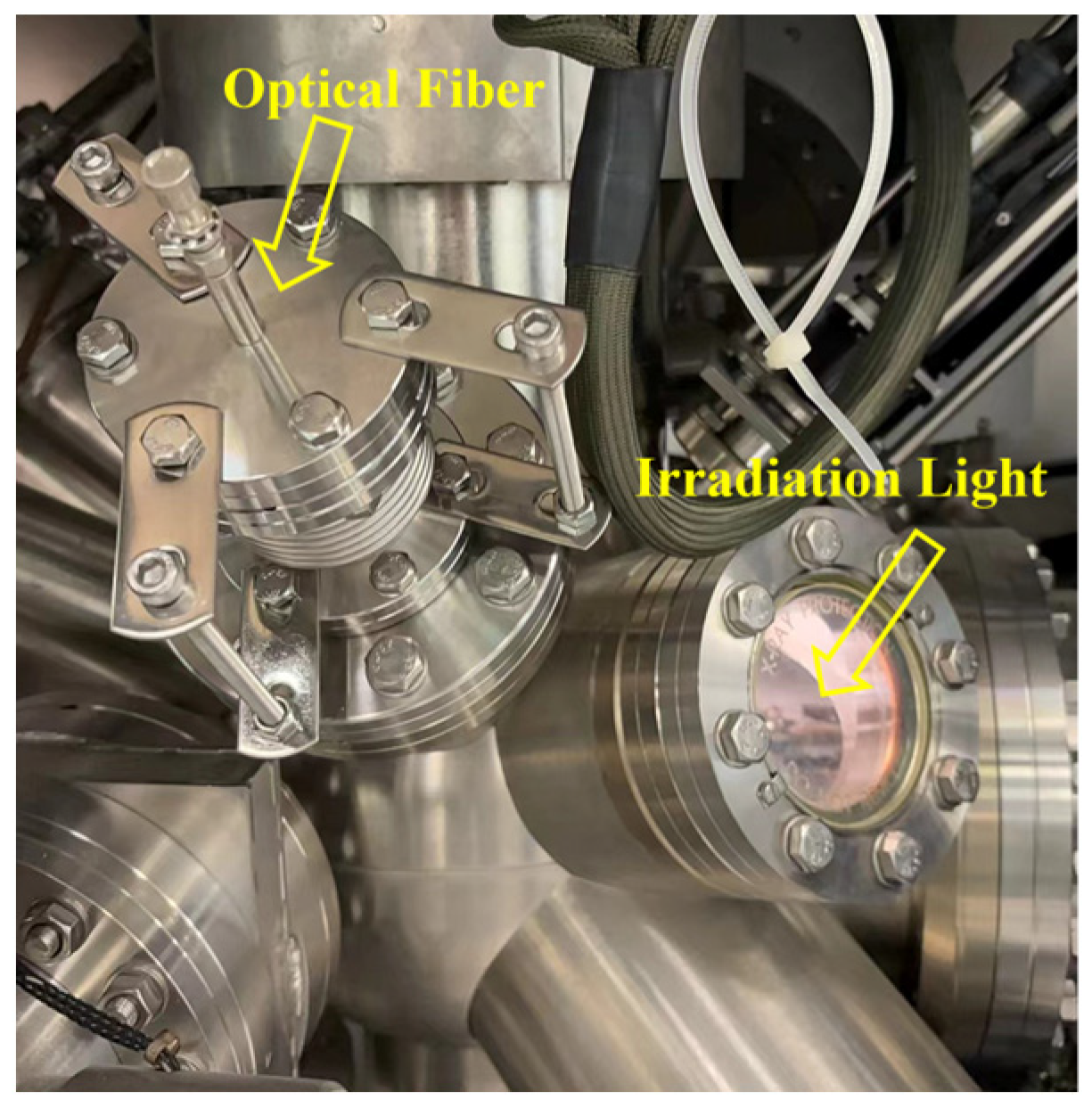
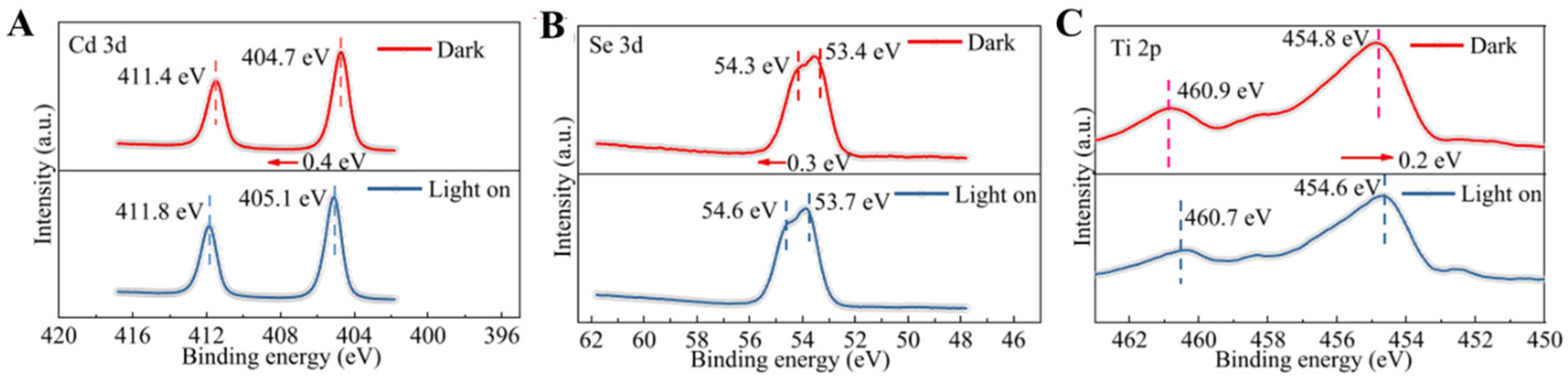
4. In Situ Irradiated XPS (ISI-XPS)
5. Conclusions and Prospective
5.1. Improve the Quality of XPS Data Analysis
5.2. Further Study the Shift and Intensity Change of Spectra Peak in ISI-XPS Experiments
5.3. The Measurement of Electrical Band Gap
5.4. Further Optimizing In Situ XPS to Deepen the Study of Catalytic Reaction Mechanisms
Author Contributions
Funding
Conflicts of Interest
References
- Siegbahn, K. Electron spectroscopy for atoms, molecules, and condensed matter. Rev. Mod. Phys. 1982, 54, 709. [Google Scholar] [CrossRef]
- Penn, D.R. Quantitative chemical analysis by ESCA. J. Electron Spectrosc. Relat. Phenom. 1976, 9, 29–40. [Google Scholar] [CrossRef]
- Jain, V.; Biesinger, M.C.; Linford, M.R. The Gaussian-Lorentzian Sum, Product, and Convolution (Voigt) functions in the context of peak fitting X-ray photoelectron spectroscopy (XPS) narrow scans. Appl. Surf. Sci. 2018, 447, 548–553. [Google Scholar] [CrossRef]
- Lubenchenko, A.V.; Batrakov, A.A.; Pavolotsky, A.B.; Lubenchenko, O.I.; Ivanov, D.A. XPS study of multilayer multicomponent films. Appl. Surf. Sci. 2018, 427, 711–721. [Google Scholar] [CrossRef]
- Metson, J.B.; Hyland, M.M.; Gillespie, A.; Hemmingsen-Jensen, M. X-ray photoelectron spectroscopy applications to corrosion and adhesion at metal oxide surfaces. Colloids Surf. A Physicochem. Eng. Asp. 1994, 93, 173–180. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Shard, A.G.; Counsell, J.D.P.; Cant, D.J.H.; Smith, E.F.; Navabpour, P.; Zhang, X.; Blomfield, C.J. Intensity calibration and sensitivity factors for XPS instruments with monochromatic Ag Lα and Al Kα sources. Surf. Interface Anal. 2019, 51, 763–773. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Parlett, C.M.A.; Robinson, N.; Durndell, L.J.; Manayil, J.C.; Beaumont, S.K.; Jiang, S.; Hondow, N.S.; Lamb, A.C.; Jampaiah, D.; et al. A spatially orthogonal hierarchically porous acid–base catalyst for cascade and antagonistic reactions. Nat. Catal. 2020, 3, 921–931. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Prosvirin, I.P.; Saraev, A.A.; Klyushin, A.Y.; Knop-Gericke, A.; Bukhtiyarov, V.I. In situ formation of the active sites in Pd–Au bimetallic nanocatalysts for CO oxidation: NAP (near ambient pressure) XPS and MS study. Faraday Discuss. 2018, 208, 255–268. [Google Scholar] [CrossRef]
- Alayoglu, S.; Somorjai, G.A. Ambient Pressure X-ray Photoelectron Spectroscopy for Probing Monometallic, Bimetallic and Oxide-Metal Catalysts Under Reactive Atmospheres and Catalytic Reaction Conditions. Top. Catal. 2015, 59, 420–438. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. XPS/STM study of model bimetallic Pd–Au/HOPG catalysts. Appl. Surf. Sci. 2016, 367, 214–221. [Google Scholar] [CrossRef]
- Languille, M.A.; Ehret, E.; Lee, H.C.; Jeong, C.K.; Toyoshima, R.; Kondoh, H.; Mase, K.; Jugnet, Y.; Bertolini, J.C.; Aires, F.J.C.S.; et al. In-situ surface analysis of AuPd(110) under elevated pressure of CO. Catal. Today 2016, 260, 39–45. [Google Scholar] [CrossRef]
- Erickson, N.C.; Raman, S.N.; Hammond, J.S.; Holmes, R.J. Depth profiling organic light-emitting devices by gas-cluster ion beam sputtering and X-ray photoelectron spectroscopy. Org. Electron. 2014, 15, 2988–2992. [Google Scholar] [CrossRef]
- Zemek, J.; Jiricek, P.; Houdkova, J.; Jurek, K.; Gedeon, O. Lead-silicate glass surface sputtered by an argon cluster ion beam investigated by XPS. J. Non-Cryst. Solids 2017, 469, 1–6. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, J.; Wang, M.; Yu, S.; Xu, Y.; Tian, S.; Gao, Z.; Xiao, D.; Liu, G.; Zhou, W.; et al. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustain. 2023, 6, 712–719. [Google Scholar] [CrossRef]
- Xu, X.; Lan, T.; Zhao, G.; Nie, Q.; Jiang, F.; Lu, Y. Interface-hydroxyl enabling methanol steam reforming toward CO-free hydrogen production over inverse ZrO2/Cu catalyst. Appl. Catal. B Environ. 2023, 334, 122839. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Y.; Ou, P.; Ye, Z.; Li, X.-Y.; Vanka, S.; Ma, T.; Sun, H.; Wang, P.; Zhou, P.; et al. Light-driven synthesis of C2H6 from CO2 and H2O on a bimetallic AuIr composite supported on InGaN nanowires. Nat. Catal. 2023, 6, 987–995. [Google Scholar] [CrossRef]
- Zhang, J.; Su, B.-J.; Wu, K.-H.; Xia, Q.; Knibbe, R.; Gentle, I. Low-coordinated surface nickel oxide as electrocatalyst for efficient water oxidation. J. Catal. 2024, 429, 115278. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, Y.; Chen, R.; Dong, C.-L.; Huang, Y.-C.; Ma, M.; Shi, Z.; Liu, J.; Zhang, Z.; Qiu, M.; et al. Alloyed Pt Single-Atom Catalysts for Durable PEM Water Electrolyzer. Adv. Funct. Mater. 2023, 33, 2214795. [Google Scholar] [CrossRef]
- Huang, Q.; Xia, G.-J.; Huang, B.; Xie, D.; Wang, J.; Wen, D.; Lin, D.; Xu, C.; Gao, L.; Wu, Z.; et al. Activating lattice oxygen by a defect-engineered Fe2O3–CeO2 nano-heterojunction for efficient electrochemical water oxidation. Energy Environ. Sci. 2024, 17, 5260–5272. [Google Scholar] [CrossRef]
- Fedorov, A.Y.; Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Zubavichus, Y.V.; Bukhtiyarov, V.I. Thermally Induced Surface Structure and Morphology Evolution in Bimetallic Pt-Au/HOPG Nanoparticles as Probed Using XPS and STM. Nanomaterials 2024, 14, 57. [Google Scholar] [CrossRef]
- Horrell, B.A.; Cocke, D.L. Application of Ion-Scattering Spectroscopy to Catalyst Characterization. Catal. Rev. 1987, 29, 447–491. [Google Scholar] [CrossRef]
- Smith, D.P. Scattering of Low-Energy Noble Gas Ions from Metal Surfaces. J. Appl. Phys. 1967, 38, 340–347. [Google Scholar] [CrossRef]
- Brongersmal, H.H.; Mu, P.M. Analysis of the outermost atomic layer of a surface by low-energy ion scattering. Surf. Sci. 1973, 35, 393–412. [Google Scholar] [CrossRef]
- Brongersma, H.H.; Draxler, M.; de Ridder, M.; Bauer, P. Surface composition analysis by low-energy ion scattering. Surf. Sci. Rep. 2007, 62, 63–109. [Google Scholar] [CrossRef]
- Cushman, C.V.; Brüner, P.; Zakel, J.; Major, G.H.; Lunt, B.M.; Smith, N.J.; Grehl, T.; Linford, M.R. Low energy ion scattering (LEIS). A practical introduction to its theory, instrumentation, and applications. Anal. Methods 2016, 8, 3419–3439. [Google Scholar] [CrossRef]
- ter Veen, H.R.J.; Kim, T.; Wachs, I.E.; Brongersma, H.H. Applications of High Sensitivity-Low Energy Ion Scattering (HS-LEIS) in heterogeneous catalysis. Catal. Today 2009, 140, 197–201. [Google Scholar] [CrossRef]
- Lang, R.; Xi, W.; Liu, J.-C.; Cui, Y.-T.; Li, T.; Lee, A.F.; Chen, F.; Chen, Y.; Li, L.; Li, L.; et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 2019, 10, 234. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zhen, Y.; Chen, M.; Wan, H. In situ FTIR and ex situ XPS/HS-LEIS study of supported Cu/Al2O3 and Cu/ZnO catalysts for CO2 hydrogenation. Chin. J. Catal. 2021, 42, 367–375. [Google Scholar] [CrossRef]
- Caporali, S.; Pedio, M.; Chiappe, C.; Pomelli, C.S.; Acres, R.G.; Bardi, U. Surface study of metal-containing ionic liquids by means of photoemission and absorption spectroscopies. Surf. Sci. 2016, 648, 360–365. [Google Scholar] [CrossRef]
- Hemmeter, D.; Kremitzl, D.; Schulz, P.S.; Wasserscheid, P.; Maier, F.; Steinrück, H.-P. The Buoy Effect: Surface Enrichment of a Pt Complex in IL Solution by Ligand Design. Chem. –A Eur. J. 2023, 29, e202203325. [Google Scholar] [CrossRef]
- Hemmeter, D.; Paap, U.; Maier, F.; Steinrück, H.-P. Structure and Surface Behavior of Rh Complexes in Ionic Liquids Studied Using Angle-Resolved X-ray Photoelectron Spectroscopy. Catalysts 2023, 13, 871. [Google Scholar] [CrossRef]
- Hemmeter, D.; Paap, U.; Taccardi, N.; Mehler, J.; Schulz, P.; Wasserscheid, P.; Maier, F.; Steinrück, H.P. Formation and Surface Behavior of Pt and Pd Complexes with Ligand Systems Derived from Nitrile-functionalized Ionic Liquids Studied by XPS. ChemPhysChem 2023, 24, e202200391. [Google Scholar] [CrossRef]
- Ma, W.; Fan, W.; Li, Q.; Zhang, H.; Zhu, K.; Sun, C.; Wang, L.; Zong, X. Chloroplast-mimicking nanoreactor for enhanced CO2 electrocatalysis. Sci. Bull. 2024. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Dai, W. XPS Analysis of Semiconductor GaN with Different Anodes. J. Fudan Univ. Nat. Sci. 2023, 62, 9–15. [Google Scholar] [CrossRef]
- Bure, T.R.; Renault, O.; Nolot, E.; Lardin, T.; Robert-Goumet, C.; Pauly, N. Assessing advanced methods in XPS and HAXPES for determining the thicknesses of high-k oxide materials: From ultra-thin layers to deeply buried interfaces. Appl. Surf. Sci. 2023, 609, 155317. [Google Scholar] [CrossRef]
- Vanleenhove, A.; Hoflijk, I.; Zborowski, C.; Vaesen, I.; Artyushkova, K.; Conard, T. High-energy X-ray photoelectron spectroscopy spectra of TiO2 measured by Cr Kα. Surf. Sci. Spectra 2022, 29, 014017. [Google Scholar] [CrossRef]
- Zborowski, C.; Conard, T.; Vanleenhove, A.; Hoflijk, I.; Vaesen, I. Reference survey spectra of elemental solid measured with Cr Kα photons as a tool for Quases analysis (1): Transition metals period 4 elements (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn). Surf. Sci. Spectra 2022, 29, 024002. [Google Scholar] [CrossRef]
- Matsuo, J.; Toyoda, N.; Akizuki, M.; Yamada, I. Sputtering of elemental metals by Ar cluster ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 121, 459–463. [Google Scholar] [CrossRef]
- Yamada, I. Historical milestones and future prospects of cluster ion beam technology. Appl. Surf. Sci. 2014, 310, 77–88. [Google Scholar] [CrossRef]
- Popok, V.N.; Barke, I.; Campbell, E.E.B.; Meiwes-Broer, K.-H. Cluster–surface interaction: From soft landing to implantation. Surf. Sci. Rep. 2011, 66, 347–377. [Google Scholar] [CrossRef]
- Yamada, I.; Matsuo, J.; Toyoda, N.; Aoki, T.; Seki, T. Progress and applications of cluster ion beam technology. Curr. Opin. Solid State Mater. Sci. 2015, 19, 12–18. [Google Scholar] [CrossRef]
- Aoki, T. Molecular dynamics simulations of cluster impacts on solid targets: Implantation, surface modification, and sputtering. J. Comput. Electron. 2014, 13, 108–121. [Google Scholar] [CrossRef]
- Zemek, J.; Jiricek, P.; Houdkova, J.; Artemenko, A.; Jelinek, M. Diamond-like carbon and nanocrystalline diamond film surfaces sputtered by argon cluster ion beams. Diam. Relat. Mater. 2016, 68, 37–41. [Google Scholar] [CrossRef]
- Hu, Q.; Tan, R.; Yao, W.; Cui, Y.; Li, J.; Song, W. Preparation and X-ray photoelectron spectroscopic characterization of Sn-doped C12A7:e− electride nanoparticles. Appl. Surf. Sci. 2020, 508, 145244. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Y.; Yang, F.; Zhang, Q.; Zhang, J.; Cao, M.; Dai, W.-L. Electroless-hydrothermal construction of nickel bridged nickel sulfide@mesoporous carbon nitride hybrids for highly efficient noble metal-free photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 45, 176–186. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, H.; Huang, Y.; Wang, X.; Gao, L.; Li, Q.; Li, Y.; Zhang, Y.; Cui, Y.; Gao, R.; et al. Rational design of covalent organic frameworks/NaTaO3 S-scheme heterostructure for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 664, 916–927. [Google Scholar] [CrossRef]
- Hueso, J.L.; Martínez-Martínez, D.; Caballero, A.; González-Elipe, A.R.; Mun, B.S.; Salmerón, M. Near-ambient X-ray photoemission spectroscopy and kinetic approach to the mechanism of carbon monoxide oxidation over lanthanum substituted cobaltites. Catal. Commun. 2009, 10, 1898–1902. [Google Scholar] [CrossRef][Green Version]
- Bentrup, U. Combining in situ characterization methods in one set-up: Looking with more eyes into the intricate chemistry of the synthesis and working of heterogeneous catalysts. Chem. Soc. Rev. 2010, 39, 4718–4730. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Li, B.-Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Trotochaud, L.; Head, A.R.; Karslıoğlu, O.; Kyhl, L.; Bluhm, H. Ambient pressure photoelectron spectroscopy: Practical considerations and experimental frontiers. J. Phys. Condens. Matter 2017, 29, 053002. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, D.; Zafeiratos, S. A mini review of in situ near-ambient pressure XPS studies on non-noble, late transition metal catalysts. Catal. Sci. Technol. 2019, 9, 3851–3867. [Google Scholar] [CrossRef]
- Isaacs, M.; Davies-Jones, J.; Davies, P.; Guan, S.; Lee, R.; Morgan, D.; Palgrave, R. Advanced XPS Characterization: XPS-based multi-technique analyses for comprehensive understanding of functional materials. Mater. Chem. Front. 2021, 5, 7931–7963. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Chen, Y.; Li, W.; Lin, L.; Li, M.; Deng, Y.; Wang, X.; Ge, B.; Yang, C.; et al. Tuning the Selectivity of Catalytic Carbon Dioxide Hydrogenation over Iridium/Cerium Oxide Catalysts with a Strong Metal–Support Interaction. Angew. Chem. Int. Ed. 2017, 56, 10761–10765. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, G.; Yang, L.; Li, F. Tuning surface-interface structures of ZrO2 supported copper catalysts by in situ introduction of indium to promote CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2020, 605, 117805. [Google Scholar] [CrossRef]
- Dong, C.; Gao, Z.; Li, Y.; Peng, M.; Wang, M.; Xu, Y.; Li, C.; Xu, M.; Deng, Y.; Qin, X.; et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 2022, 5, 485–493. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Deng, Y.; Xu, M.; Artiglia, L.; Wen, W.; Gao, R.; Chen, B.; Yao, S.; Zhang, X.; et al. A stable low-temperature H2-production catalyst by crowding Pt on α-MoC. Nature 2021, 589, 396–401. [Google Scholar] [CrossRef]
- Wang, H.; Fan, S.; Guo, S.; Wang, S.; Qin, Z.; Dong, M.; Zhu, H.; Fan, W.; Wang, J. Selective conversion of CO2 to isobutane-enriched C4 alkanes over InZrOx-Beta composite catalyst. Nat. Commun. 2023, 14, 2627. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A Highly Stable Copper-Based Catalyst for Clarifying the Catalytic Roles of Cu0 and Cu+ Species in Methanol Dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, G.; Xi, Y.; Wang, J.; Sun, P.; Liu, Q.; Yan, W.; Cui, Y.; Jiang, Z.; Li, F. Selective and stable upgrading of biomass-derived furans into plastic monomers by coupling homogeneous and heterogeneous catalysis. Chem 2022, 8, 1034–1049. [Google Scholar] [CrossRef]
- Jiao, Z.; Shang, M.; Liu, J.; Lu, G.; Wang, X.; Bi, Y. The charge transfer mechanism of Bi modified TiO2 nanotube arrays: TiO2 serving as a “charge-transfer-bridge”. Nano Energy 2017, 31, 96–104. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-ray Photoelectron Spectroscopy Investigation on a Direct Z-Scheme TiO2/CdS Composite Film Photocatalyst. Adv. Mater. 2019, 31, 1807920. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ Irradiated XPS Investigation on S-Scheme TiO2@ZnIn2S4 Photocatalyst for Efficient Photocatalytic CO2 Reduction. Small 2021, 17, e2103447. [Google Scholar] [CrossRef]
- Filippov, T.N.; Kovalevskiy, N.S.; Solovyeva, M.I.; Chetyrin, I.A.; Prosvirin, I.P.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. In situ XPS data for the uranyl-modified oxides under visible light. Data Brief 2018, 19, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Sezen, H.; Suzer, S. XPS for chemical- and charge-sensitive analyses. Thin Solid Film. 2013, 534, 1–11. [Google Scholar] [CrossRef]
- Sezen, H.; Rockett, A.A.; Suzer, S. XPS Investigation of a CdS-Based Photoresistor under Working Conditions: Operando–XPS. Anal. Chem. 2012, 84, 2990–2994. [Google Scholar] [CrossRef]
- Costantini, R.; Grazioli, C.; Cossaro, A.; Floreano, L.; Morgante, A.; Dell’Angela, M. Pump–Probe X-ray Photoemission Reveals Light-Induced Carrier Accumulation in Organic Heterojunctions. J. Phys. Chem. C 2020, 124, 26603–26612. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Ding, B.; Shao, G.; Li, N.; Zhang, P. Electrospinning Photocatalysis Meet In Situ Irradiated XPS: Recent Mechanisms Advances and Challenges. Small 2023, 19, 2303867. [Google Scholar] [CrossRef]
- Mu, C.; Lv, C.; Meng, X.; Sun, J.; Tong, Z.; Huang, K. In Situ Characterization Techniques Applied in Photocatalysis: A Review. Adv. Mater. Interfaces 2023, 10, 2201842. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Wang, X. Robust construction of CdSe nanorods@Ti3C2 MXene nanosheet for superior photocatalytic H2 evolution. Appl. Catal. B Environ. Int. J. Devoted Catal. Sci. Its Appl. 2023, 328, 122537. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Zhang, S.; Shen, J.; Zhu, X.; Cui, Y.; Li, P.; Lin, C.; Li, X.; Xiao, Q.; et al. Ice-Templated Synthesis of Atomic Cluster Cocatalyst with Regulable Coordination Number for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2024, 36, 2400764. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, H.; Zhang, H.; Wang, X.; Gao, L.; Cui, Y.; Zong, B.; Li, H.; Dai, W.-L. Synergistic V–Nb Sites Modulate Selective Alkene Epoxidation with In Situ Photogenerated H2O2 over COF@MXene Heterostructures. ACS Catal. 2024, 14, 12541–12550. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, F.; Zhang, W.; Shao, G.; Zhang, P. Detecting and Quantifying Wavelength-Dependent Electrons Transfer in Heterostructure Catalyst via In Situ Irradiation XPS. Adv. Sci. 2023, 10, e2205020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Hou, R.; Ai, Y.; Cai, M.; Shi, Z.; Zhang, P.; Shao, G. Revealing electron numbers-binding energy relationships in heterojunctions via in-situ irradiated XPS. Appl. Catal. B Environ. Energy 2024, 356, 124223. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. A step-by-step guide to perform X-ray photoelectron spectroscopy. J. Appl. Phys. 2022, 132, 011101. [Google Scholar] [CrossRef]
- Major, G.H.; Avval, T.G.; Moeini, B.; Pinto, G.; Shah, D.; Jain, V.; Carver, V.; Skinner, W.; Gengenbach, T.R.; Easton, C.D.; et al. Assessment of the frequency and nature of erroneous X-ray photoelectron spectroscopy analyses in the scientific literature. J. Vac. Sci. Technol. A 2020, 38, 061204. [Google Scholar] [CrossRef]
- Major, G.H.; Pinder, J.W.; Austin, D.E.; Baer, D.R.; Castle, S.L.; Čechal, J.; Clark, B.M.; Cohen, H.; Counsell, J.D.P.; Herrera-Gomez, A.; et al. Perspective on improving the quality of surface and material data analysis in the scientific literature with a focus on X-ray photoelectron spectroscopy (XPS). J. Vac. Sci. Technol. A 2023, 41, 038501. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Volkringer, C.; Loiseau, T.; Tejeda, A.; Hureau, M.; Moissette, A. Band gap analysis in MOF materials: Distinguishing direct and indirect transitions using UV–vis spectroscopy. Appl. Mater. Today 2024, 37, 102094. [Google Scholar] [CrossRef]
- Rotonnelli, B.; Fernandes, M.-S.D.; Bournel, F.; Gallet, J.-J.; Lassalle-Kaiser, B. In situ/operando X-ray absorption and photoelectron spectroscopies applied to water-splitting electrocatalysis. Curr. Opin. Electrochem. 2023, 40, 101314. [Google Scholar] [CrossRef]
- Weingarth, D.; Foelske-Schmitz, A.; Wokaun, A.; Kötz, R. In situ electrochemical XPS study of the Pt/[EMIM][BF4] system. Electrochem. Commun. 2011, 13, 619–622. [Google Scholar] [CrossRef]
- Ogletree, D.F.; Bluhm, H.; Lebedev, G.; Fadley, C.S.; Hussain, Z.; Salmeron, M. A differentially pumped electrostatic lens system for photoemission studies in the millibar range. Rev. Sci. Instrum. 2002, 73, 3872–3877. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liao, Y.; Sun, Y.; Wang, W.; Wu, J.; Dai, W.; Huang, T. Advanced XPS-Based Techniques in the Characterization of Catalytic Materials: A Mini-Review. Catalysts 2024, 14, 595. https://doi.org/10.3390/catal14090595
Cui Y, Liao Y, Sun Y, Wang W, Wu J, Dai W, Huang T. Advanced XPS-Based Techniques in the Characterization of Catalytic Materials: A Mini-Review. Catalysts. 2024; 14(9):595. https://doi.org/10.3390/catal14090595
Chicago/Turabian StyleCui, Yuanyuan, Yifan Liao, Youbao Sun, Wenchang Wang, Jinqi Wu, Weilin Dai, and Taohong Huang. 2024. "Advanced XPS-Based Techniques in the Characterization of Catalytic Materials: A Mini-Review" Catalysts 14, no. 9: 595. https://doi.org/10.3390/catal14090595
APA StyleCui, Y., Liao, Y., Sun, Y., Wang, W., Wu, J., Dai, W., & Huang, T. (2024). Advanced XPS-Based Techniques in the Characterization of Catalytic Materials: A Mini-Review. Catalysts, 14(9), 595. https://doi.org/10.3390/catal14090595







