Unveiling New Product Formations beyond Conventional Pathways in De-Halogenation of Halo-Acetic Acids Using Ni-Encapsulated Sol-Gel Catalysts †
Abstract
:1. Introduction
2. Results and Discussion
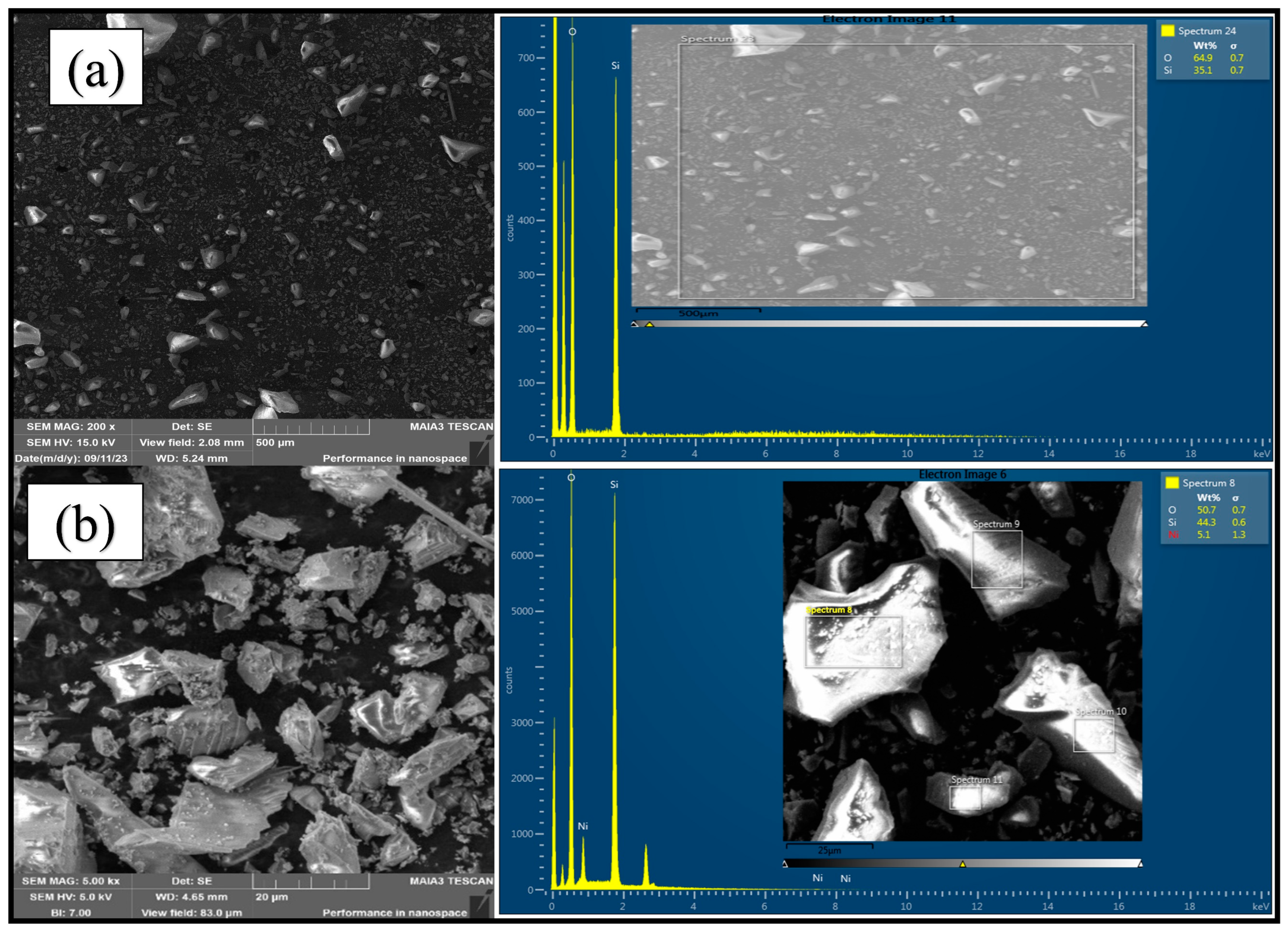
2.1. Catalyst Characterization
2.2. Catalytic Activity and Mechanism
2.2.1. MBAA Reduction Reaction
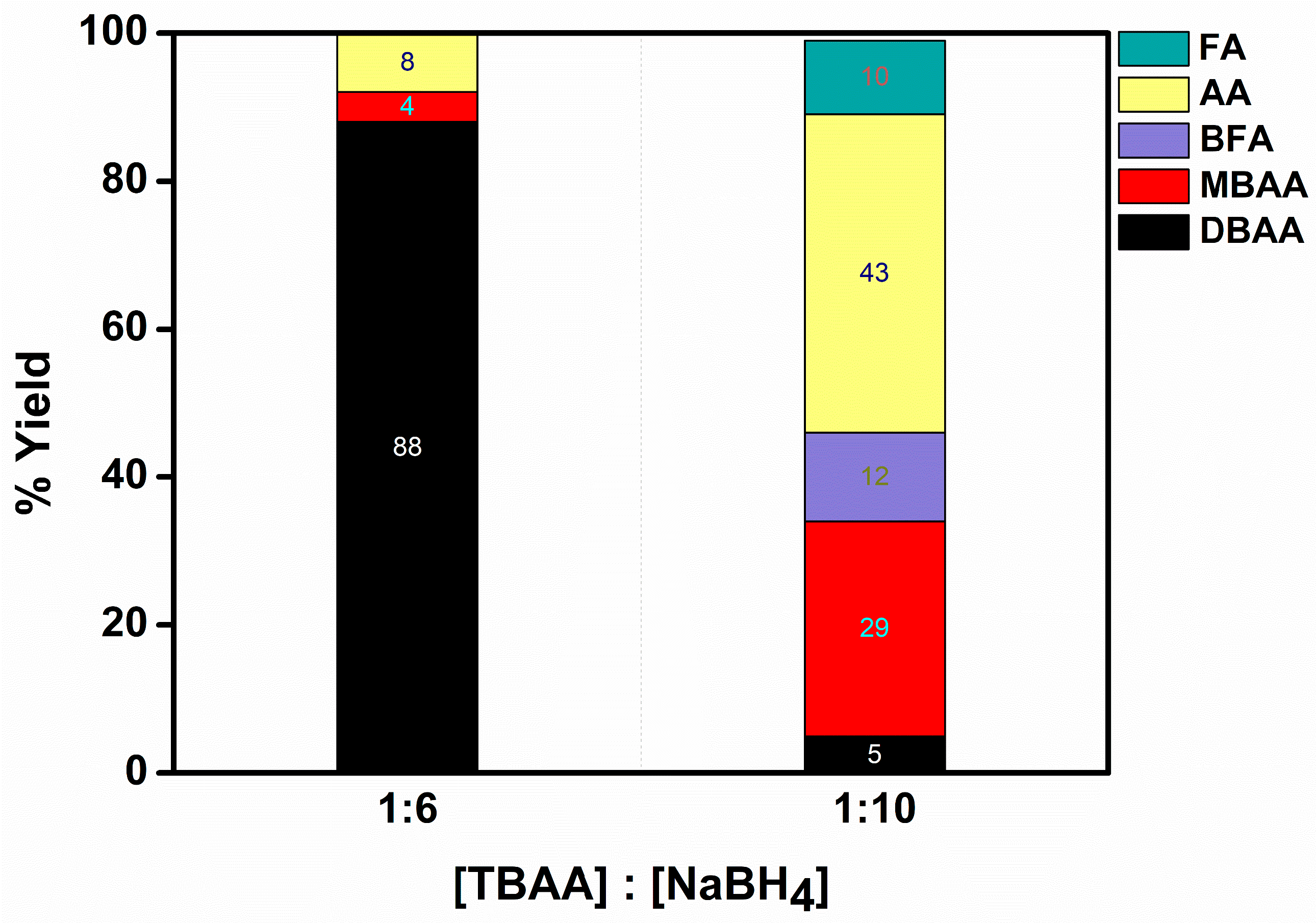
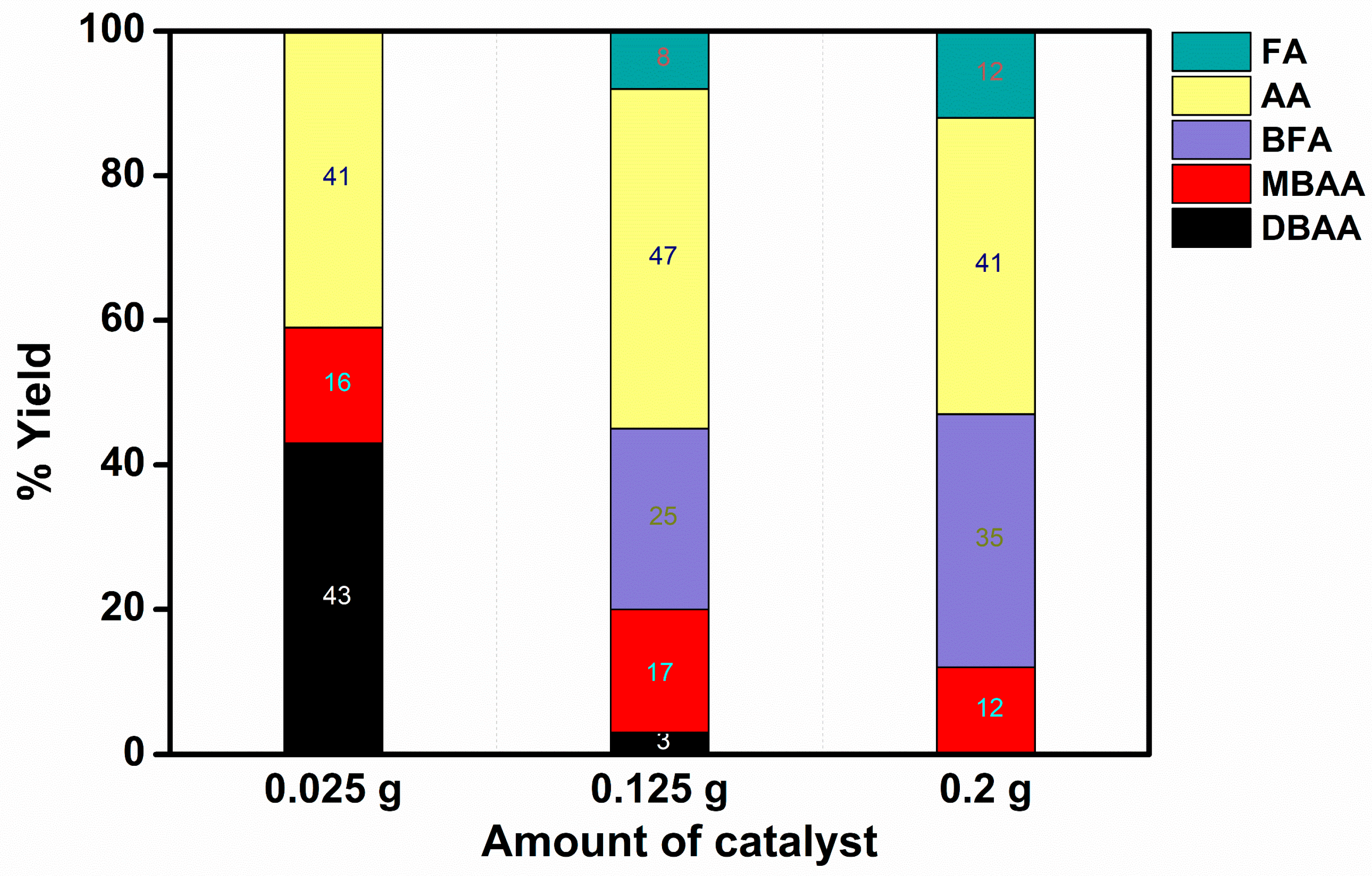
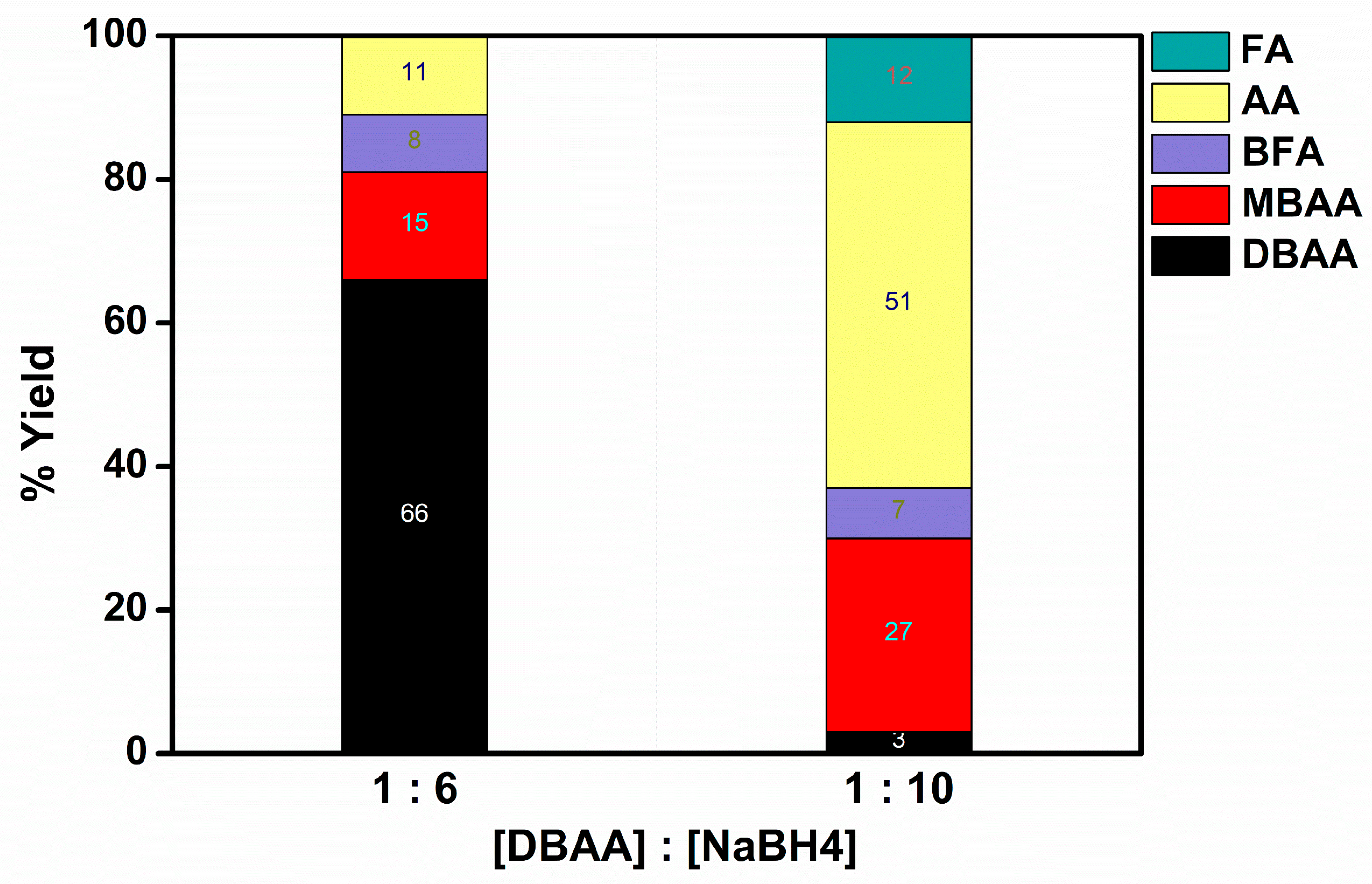
2.2.2. TBAA Reduction Reaction
- In all of the experiments we conducted, no traces of TBAA were detected. The findings indicate that sodium borohydride is capable of fully converting TBAA into DBAA. Previous studies have highlighted the rapid hydrolysis of TBAA in the presence of water, leading to the immediate formation of DBAA [12].
- The de-bromination increases with the increase in the catalyst used, at constant concentrations of TBAA and BH4−. This suggests that the competition between de-bromination and hydrogen evolution is dramatically affected by the amount of BH4−/H adsorbed by each Ni(0) nanoparticle. More concisely, the notable reduction in the amount of DBAA as well as MBAA suggests higher de-bromination which eventually leads to less available H atoms on the surface for evolution. Meanwhile, the improved ratio of BFA/FA to AA indicates that the radicals react with each other leading to dimerization, rather than reacting with hydrogen leading to AA.
- The formation of FA and bromo-fumaric acid (BFA) proves that CBrkH2−kCO2− radicals are involved in the process. It is of interest to note that analogous results were reported for Au(0)@ORMOSIL [3,13] but not for Ag(0)@ORMOSIL [3] and Fe(0)@ORMOSIL [4]. Interestingly, in the catalytic de-bromination of TBAA by Au(0)@ORMOSIL, succinic acid is the final product, whereas for Ni(0)@ORMOSIL, the final products are FA, MA, and BFA.
- All these catalysts induce de-bromination, forming the same products but in different ratios. The yields of bromide were measured using ion chromatography. The results that corroborate the organic products are summarized in Table S1.
- The de-bromination with Ni(H2O)62+, as measured by the combined amounts of DBAA and MBAA, is somewhat better than that with Ni(II)@ORMOSIL, and Ni0-NPs suspended in water surpasses that with Ni(0)@ORMOSIL. This is tentatively attributed to the location of the Ni(0)-NPs in the pores of the ORMOSIL matrices. Thus, BH4− probably reacts with the Ni(0)-NPs when no substrate is around, increasing the hydrogen evolution reaction (HER) yield [14].
- The yield of FA by the ORMOSIL catalysts is lower than that observed in the aqueous solutions. This is attributed to the lower chance that two TBAA substrates will be present near a Ni(0) entrapped in a pore when it is reduced.
- The ORMOSIL catalysts are still preferred due to the possibility of recycling them.
2.2.3. DBAA Reduction Reaction
2.2.4. TCAA, MCAA, and CAA Reduction Reactions
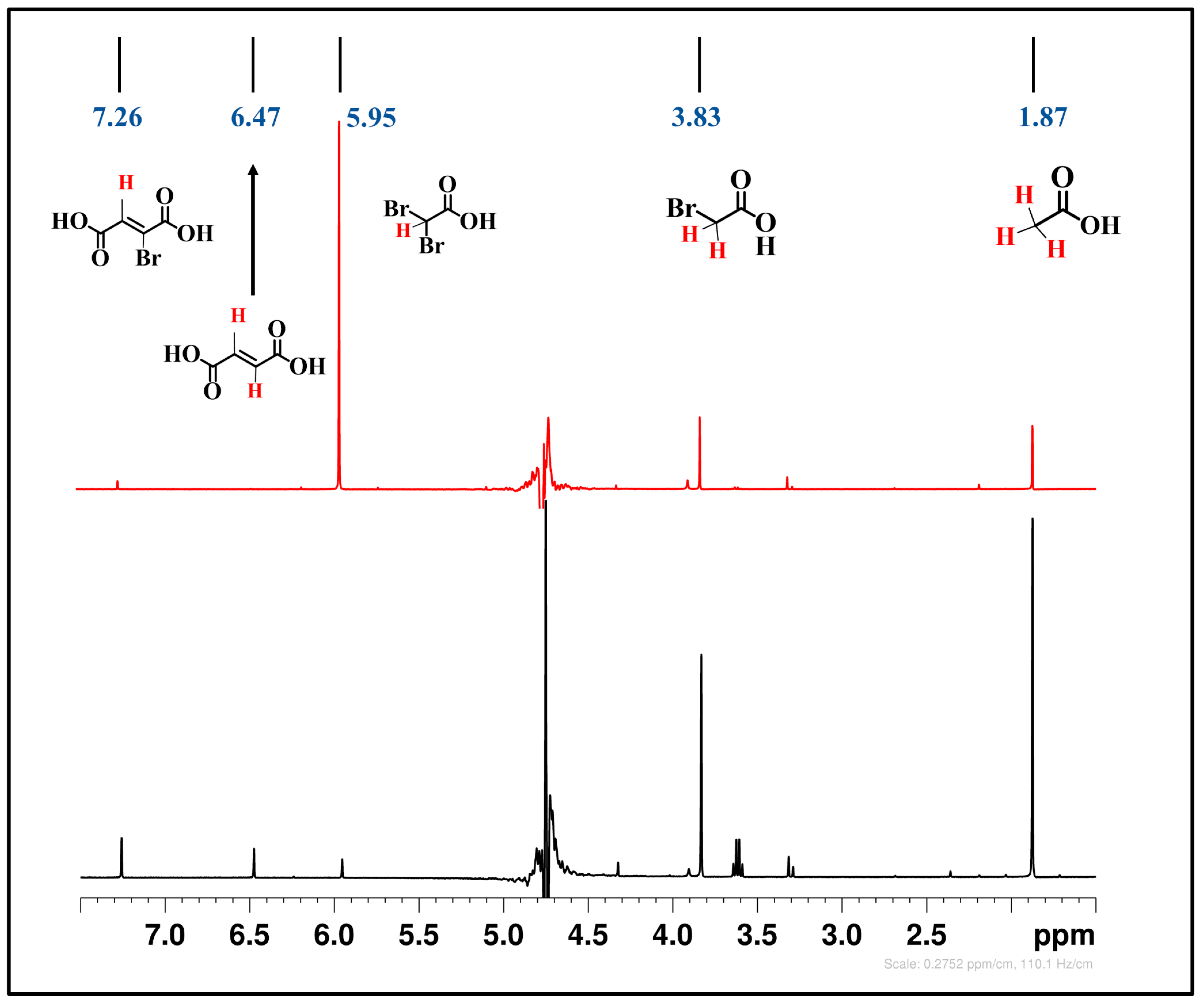
2.2.5. Mechanistic Analysis
{(M0-NP)-Hn+m−1}(n−m)− + Xk−1CH3−kCO2∙− + X− + H+ (k = 1; 2; 3 X = Br or Cl)
Xk−1CH3−kCO2∙−
{(M0-NP)-Hn+m}-BH3(n−m+1)− + Xk−1CH3−kCO2∙− + X− + H+
Xk−1CH3−kCO2∙− + X−
CXk−1H4−kCO2H
{(M0-NP)-Hn+m}(n−m−1)− + CXk−1H4−kCO2H + OH−
[{(M0-NP)-Hn+m}-BH3](n−m+1)− + CXk−1H4−kCO2H
{(Ni0-NP)-Hn+m}- (Xk−1CH3−kCO2H)l−2(n−m)− + (-CXk−1H3−kCO2H)2
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Measurements
3.3. Syntheses
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pals, J.A.; Ang, J.K.; Wagner, E.D.; Plewa, M.J. Biological Mechanism for the Toxicity of Haloacetic Acid Drinking Water Disinfection Byproducts. Environ. Sci. Technol. 2011, 45, 5791–5797. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Postigo, C. Drinking Water Disinfection By-Products BT—Emerging Organic Contaminants and Human Health; Barceló, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–137. ISBN 978-3-642-28132-7. [Google Scholar]
- Adhikary, J.; Meyerstein, D.; Marks, V.; Meistelman, M.; Gershinsky, G.; Burg, A.; Shamir, D.; Kornweitz, H.; Albo, Y. Sol-Gel Entrapped Au0- and Ag0-Nanoparticles Catalyze Reductive de-Halogenation of Halo-Organic Compounds by BH4−. Appl. Catal. B Environ. 2018, 239, 450–462. [Google Scholar] [CrossRef]
- Neelam; Meyerstein, D.; Adhikary, J.; Burg, A.; Shamir, D.; Albo, Y. Zero-Valent Iron Nanoparticles Entrapped in SiO2 Sol-Gel Matrices: A Catalyst for the Reduction of Several Pollutants. Catal. Commun. 2020, 133, 105819. [Google Scholar] [CrossRef]
- Su, B.; Cao, Z.C.; Shi, Z.J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896. [Google Scholar] [CrossRef]
- Meistelman, M.; Meyerstein, D.; Burg, A.; Shamir, D.; Albo, Y. “Doing More with Less”: Ni(II)@ORMOSIL, a Novel Sol-Gel Pre-Catalyst for the Reduction of Nitrobenzene. Catalysts 2021, 11, 1391. [Google Scholar] [CrossRef]
- Osby, J.O.; Heinzman, S.W.; Ganem, B. Studies on the Mechanism of Transition-Metal-Assisted Sodium Borohydride and Lithium Aluminum Hydride Reductions. J. Am. Chem. Soc. 1986, 108, 67–72. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. The Chemical Effects of Molecular Sol–Gel Entrapment. Chem. Soc. Rev. 2007, 36, 932–940. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of “sol-Gel” Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the Alkyl-Alkoxide Precursor on the Structure and Catalytic Properties of Hybrid Sol-Gel Catalysts. Chem. Mater. 2005, 17, 6686–6694. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Ma, Y.C.; Chiang, C.Y. Evaluation of the Effects of Various Gas Chromatographic Parameters on Haloacetic Acids Disinfection By-Products Analysis. J. Chromatogr. A 2005, 1076, 216–219. [Google Scholar] [CrossRef]
- Adhikary, J.; Meistelman, M.; Burg, A.; Shamir, D.; Meyerstein, D.; Albo, Y. Reductive Dehalogenation of Monobromo- and Tribromoacetic Acid by Sodium Borohydride Catalyzed by Gold Nanoparticles Entrapped in Sol–Gel Matrices Follows Different Pathways. Eur. J. Inorg. Chem. 2017, 2017, 1510–1515. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, X.; Zi, Q.; Zhang, R.; Dou, T.; Yip, A.C.K. Mechanism and Kinetics of Sodium Borohydride Hydrolysis over Crystalline Nickel and Nickel Boride and Amorphous Nickel-Boron Nanoparticles. J. Power Sources 2014, 268, 596–603. [Google Scholar] [CrossRef]
- De Jong, G.T.; Bickelhaupt, F.M. Catalytic Carbon-Halogen Bond Activation: Trends in Reactivity, Selectivity, and Solvation. J. Chem. Theory Comput. 2007, 3, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Meistelman, M.; Adhikary, J.; Burg, A.; Shamir, D.; Gershinsky, G.; Meyerstein, D.; Albo, Y. Ag0 and Au0 Nanoparticles Encapsulated in Sol-Gel Matrices as Catalysts in Reductive de-Halogenation Reactions. Chim. Oggi 2017, 35, 23–26. [Google Scholar]
- Raju Karimadom, B.; Varshney, S.; Zidki, T.; Meyerstein, D.; Kornweitz, H. DFT Study of the BH4− Hydrolysis on Au(111) Surface. ChemPhysChem 2022, 23, e202200069. [Google Scholar] [CrossRef]
- Karimadom, B.R.; Kornweitz, H. The Effectiveness of Silver and Gold in Catalytic Homogenous and Heterogenous Borohydride Hydrolysis—A DFT Study. ChemPhysChem 2024, 25, e202400253. [Google Scholar] [CrossRef]
- Karimadom, B.R.; Meyerstein, D.; Kornweitz, H. Calculating the Adsorption Energy of a Charged Adsorbent in a Periodic Metallic System—The Case of BH4− Hydrolysis on the Ag(111) Surface. Phys. Chem. Chem. Phys 2021, 23, 25667–25678. [Google Scholar] [CrossRef]
- Zidki, T.; Cohen, H.; Meyerstein, D. Reactions of Alkyl-Radicals with Gold and Silver Nanoparticles in Aqueous Solutions. Phys. Chem. Chem. Phys. 2006, 8, 3552–3556. [Google Scholar] [CrossRef]
- Bar-Ziv, R.; Zilbermann, I.; Zidki, T.; Cohen, H.; Meyerstein, D. Reactions of Alkyl Peroxyl Radicals with Metal Nanoparticles in Aqueous Solutions. J. Phys. Chem. C 2009, 113, 3281–3286. [Google Scholar] [CrossRef]
- Goia, D.V.; Matijević, E. Preparation of Monodispersed Metal Particles. New J. Chem. 1998, 22, 1203–1215. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidyadharan, K.; Meyerstein, D.; Marks, V.; Burg, A.; Meistelman, M.; Albo, Y. Unveiling New Product Formations beyond Conventional Pathways in De-Halogenation of Halo-Acetic Acids Using Ni-Encapsulated Sol-Gel Catalysts. Catalysts 2024, 14, 596. https://doi.org/10.3390/catal14090596
Vidyadharan K, Meyerstein D, Marks V, Burg A, Meistelman M, Albo Y. Unveiling New Product Formations beyond Conventional Pathways in De-Halogenation of Halo-Acetic Acids Using Ni-Encapsulated Sol-Gel Catalysts. Catalysts. 2024; 14(9):596. https://doi.org/10.3390/catal14090596
Chicago/Turabian StyleVidyadharan, Kavya, Dan Meyerstein, Vered Marks, Ariela Burg, Michael Meistelman, and Yael Albo. 2024. "Unveiling New Product Formations beyond Conventional Pathways in De-Halogenation of Halo-Acetic Acids Using Ni-Encapsulated Sol-Gel Catalysts" Catalysts 14, no. 9: 596. https://doi.org/10.3390/catal14090596










