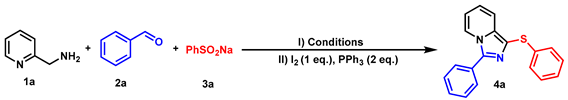
Abstract
In this report, we developed an efficient one-pot method for the synthesis of 3-phenyl-1-(phenylthio)imidazo[1,5-a]pyridine analogs starting from 2-aminomethylpyridines, benzaldehydes, and sodium benzenesulfinates, which constructed C-N and C-S bonds simultaneously. The method features mild reaction conditions, a wide range of substrates, high atom utilization, and convenient and easily accessible starting materials.
1. Introduction
As an important nitrogen-containing heterocyclic compound, imidazo[1,5-a]pyridine has a wide range of applications in various fields, especially in medicinal chemistry [1,2,3,4,5,6,7]. For example, imidazo[1,5-a]pyridine derivative (Figure 1a) shows good anti-inflammatory effects as NIK inhibitors [8], compound b (Figure 1b) demonstrates excellent anti-cancer activity in biomedical fields [9], and compound c (Figure 1c) shows good antitumor activity in vivo and vitro [10]. In addition, compound d (Figure 1d) shows promising results in treating brain injury, such as Alzheimer’s disease [11].
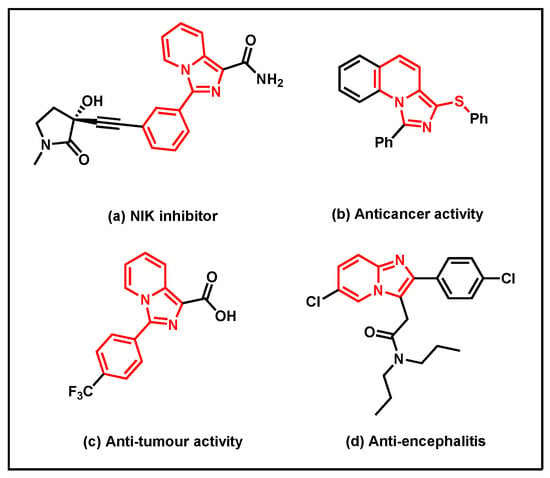
Figure 1.
Imidazopyridine-based drugs and biologically relevant molecules.
Imidazo[1,5-a]pyridine is considered to be a special backbone with a wide range of applications in medicine and chemistry [12,13,14], and efforts have been made to modify this skeleton over the past years [15,16]. In recent years, sulfur-containing compounds have also attracted significant attention due to their widespread presence in various natural products and drugs [17]. Therefore, the modification of imidazopyridine by the construction of C(sp2)–S bonds is highly desirable [18,19,20,21,22,23,24,25,26,27]. Conventional methods for the synthesis of imidazo[1,5-a]pyridines are generated by cyclic dehydration or arylation reactions initiated by using trifluoromethic anhydride (Tf2O) and 2-methoxypyridine (2-MeO-Py) (Scheme 1a) [28]. In recent years, relevant literature reports have been published on the synthesis of 3-sulfinylimidazo[1,5-a]quinoline derivatives using iodine-catalyzed imidazo[1,5-a]quinolines and disulfides as sulfinylation reagents (Scheme 1b). [9] In 2018, Song’s group used sodium sulfite as the sulfur source to prepare 3-sulfinylimidazolo [1,2-a] pyridine derivatives under high temperatures (Scheme 1c) [29]. Similarly, 3-sulfinylimidazo[1,5-a]pyridine can also be synthesized by C–H functionalization using disulfide esters, 2-methylaminopyridine, and sulfonylhydrazine (Scheme 1d) [30]. These methods are important for the C–S modification of imidazo[1,5-a]pyridines, while they still suffer from safety issues, harsh reaction conditions, long reaction time, the use of toxic starting materials, and expensive substrates.
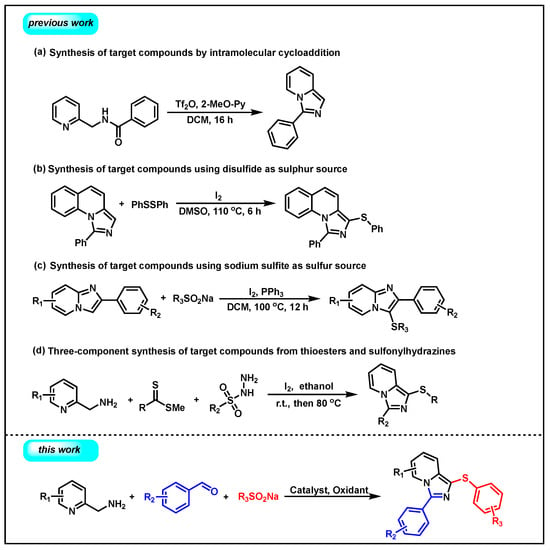
Scheme 1.
Strategies for the synthesis of imidazo[1,5-a]pyridines and 3-sulfinylimidazo[1,5-a]pyridines.
Inspired by our longstanding interest in organosulfur chemistry [31,32,33,34,35,36], we would like to report an efficient method for the synthesis of 3-phenyl-1-(phenylthio)imidazo[1,5-a]pyridine by using sodium benzenesulfinates, 2-aminomethylpyridines, and benzaldehydes as starting materials, which might pave an alternative way for the preparation of this important backbone (Scheme 1, this work).
2. Results and Discussion
Initially, 2-aminomethylpyridine (1a), benzaldehyde (2a), and sodium benzenesulfinate (3a) were chosen as starting materials for the synthesis of 3-phenyl-1-(phenylthio)imidazo-[1,5-a]pyridine analogs, and the reaction conditions are summarized in Table 1. Since the second step of the reaction using sodium benzenesulfinate is essentially quantitative in yields, we mainly focused on the optimization in the first step. Inspired by the previous literature, we used TBHP (tert-Butyl hydroperoxide) as the oxidant, I2 as the catalyst, and DMF (N,N-Dimethylformamide) as the solvent at a temperature of 70 °C, obtaining the target compound 4a with a 60% yield (entry 1). Firstly, a variety of oxidants (m-CPBA (3-Chloroperoxybenzoic acid), IBX (2-Iodoxybenzoic acid), PIDA (Iodobenzene diacetate), and K2S2O8) were screened, and it was found that TBHP was the best oxidant. The reaction did not occur without the addition of oxidants (entries 2–6). Furthermore, when the catalyst was replaced with NaI or NIS, it had a negative impact on the results (entries 8–9). Additionally, a series of solvents such as DCM (Dichloromethane), Et2O (Diethyl ether), EtOH (Ethanol), and 1,4-Dioxane were screened, and the best results were obtained with DMF (entries 10–13). Moreover, further optimization on the reaction temperature showed that 100 °C was the best, giving the target product with a 70% yield (entry 16). Subsequently, the oxidant loading was optimized, and the results showed that the optimal oxidant loading was 1.0 equiv. (entries 18–19). Finally, the screening on the ratio (entries 16, 20-21) of 1a and 2a showed that the best one is 1a:2a=2:1. Therefore, the optimal reaction conditions are summarized in entry 16.

Table 1.
Optimization of the reaction conditions a,b.
Based on the above optimal reaction conditions, the substrate range of substituted benzaldehyde 1a and sodium sulfite 3a was explored (Figure 2). Firstly, aryl aldehydes containing electron-withdrawing groups (–Cl, –Br, –NO2) were used to carry out the reaction in moderate to good yields (4e–4g). When changing the position of chlorine, the yields were significantly reduced, such as 4h and 4i, which may be due to the influence of steric hindrance. In addition, the yield of the substrate containing electron-donating groups (–CH3, –tBu, –Ph) decreased considerably (4b–4d). To our delight, when naphthalene formaldehyde, pyridine formaldehyde, and thiophene formaldehyde were used, the corresponding target compounds were obtained smoothly (4m–4o). In addition, the substrate range of sodium benzenesulfinates was also surveyed. The experimental results showed that the target compounds bearing Me–, Cl–, and F– were obtained in moderate to good yields (4j–4l). Unfortunately, no target compounds were obtained when sodium alkylsulfinate was tested. Subsequently, the target compounds (4p–4za) were obtained in high yield when the substituents on the benzene ring of aryl aldehydes and sodium benzenesulfinates were adjusted simultaneously.
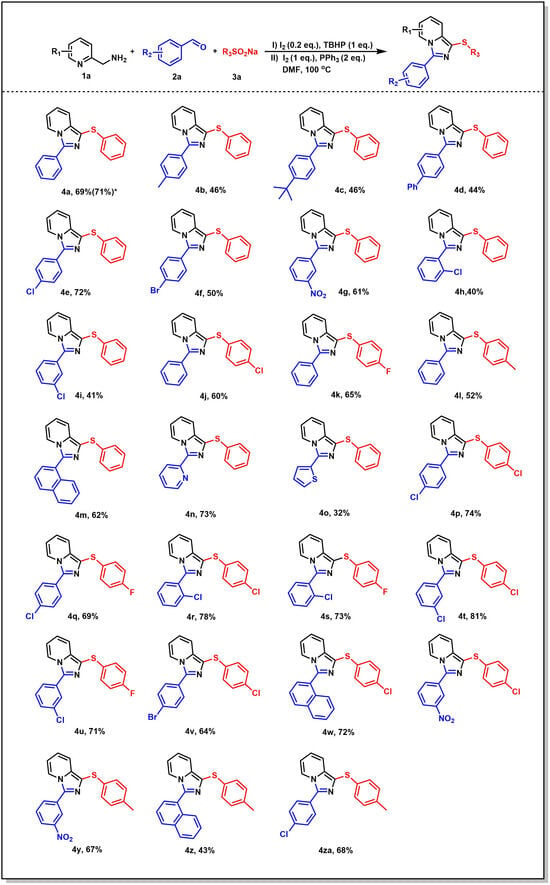
Figure 2.
Substrate scope for various benzaldehydes and sodium benzenesulfinates. Reaction conditions: 1a (1 mmol), 2a (0.5 mmol), 3a (1 mmol), I2 (0.1 mmol), TBHP (0.5 mmol), DMF (2 mL). The mixture in the sealed tube was stirred at 100 °C for 2 h in the first step and was stirred for 2 h in the second step. Isolated yield. * For a scaled-up reaction, 1a (20 mmol, 2.16 g), 2a (10 mmol, 1.06 g), iodine (2 mmol, 0.5 g) in DMF (30 mL) were added into the reaction tube, then TBHP (10 mmol, 1.29 g) was added, and the mixture was stirred at 100 °C for 2 h. Then, 3a (20 mmol, 3.28 g), iodine (10 mmol, 2.54 g), PPh3 (Triphenylphosphine) (20 mmol, 5.25 g) was added, and the mixture was stirred at 100 °C and monitored by TLC (Thin Layer Chromatography) until the starting material (1a or 2a) was consumed. The crude product was purified by column chromatography to give 4a (71%, 2.15 g).
To explore the reaction mechanism, some control experiments were performed. Initially, 3 equiv. of BHT (2,6-di-tert-butyl-4-methylphenol) was added to the reaction system under standard conditions, giving the target product 4a in a 68% yield (Scheme 2a). This indicated that a radical pathway was excluded. Furthermore, sodium benzenesulfinate (3a) could be converted to diphenyl disulfide in 75% in the presence of triphenylphosphine and iodine (Scheme 2b). Interestingly, the yield of diphenyl disulfide remained basically unchanged when 3 equiv. of BHT was added under these conditions. Finally, the target product 4a could be obtained by using diphenyl disulfide in the presence of iodine, giving the target product in 72% yield (Scheme 2c). The product yield remained essentially unchanged even with the addition of 3 equiv. of BHT (Scheme 2d).
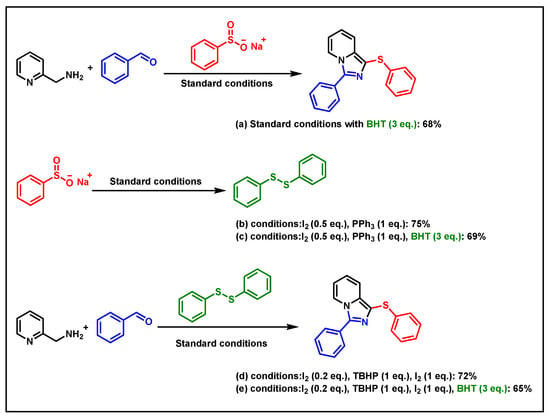
Scheme 2.
Control experiments.
Based on the results of control experiments and related literature [37,38,39,40], we proposed a possible mechanism for the model reaction (Scheme 3). Initially, 2-aminomethylpyridine (1a) and benzaldehyde (2a) reacted with iodine/TBHP to form intermediate A. Subsequently, A reacted with I2 to produce intermediate B. Meanwhile, sodium benzenesulfinate (3a) generated diphenyl disulfide C in the presence of PPh3 and I2. Thus, B reacted with diphenyl disulfide C to form intermediate D, which gave the target product 4a via the removal of HI.
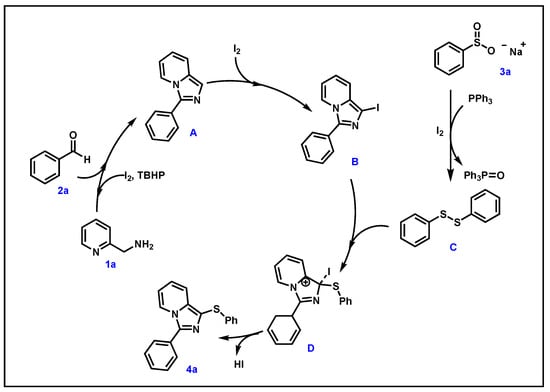
Scheme 3.
Plausible reaction mechanism.
3. Experimental Section
- General Information. See the details in the Supplementary Materials.
- General procedure for the synthesis of 3-sulfinylimidazo[1,5-a]pyridines.
A mixture of pyridin-2-ylmethanamine (1a, 1 mmol), benzaldehyde (2a, 0.5 mmol), and iodine (0.1 mmol) in DMF (3 mL) was added into the reaction tube, then TBHP (1.0 eq., based on 2a) was added, and the mixture was stirred at 100 °C for 2 h. Then, sodium benzenesulfinate (3a, 1 mmol), iodine (0.5 mmol), and PPh3 (2.0 eq., based on 2a) were added, and the mixture was stirred at 100 °C and monitored by TLC until the starting material (1a or 2a) was consumed. The reaction was then quenched with saturated Na2S2O3 solution (about 5 mL), and extracted with ethyl acetate. The original solution was dried with anhydrous Na2SO4 and evaporated in vacuo. The crude product was purified by column chromatography to give 4a.
3-phenyl-1-(phenylthio)imidazo[1,5-a]pyridine (4a): 104 mg (yield: 69%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.32 (d, J = 7.2 Hz, 1H), 7.85–7.83 (m, 2H), 7.65 (d, J = 9.1 Hz, 1H), 7.55–7.51 (m, 2H), 7.46 (t, J = 7.4 Hz, 1H), 7.23–7.17 (m, 4H), 7.09 (t, J = 6.9 Hz, 1H), 6.87 (dd, J = 9.2, 6.4 Hz, 1H), 6.67 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 139.3, 138.4, 135.1, 129.5, 129.2, 129.0, 128.8, 128.3, 127.1, 125.5, 122.1, 121.1, 120.1, 118.5, 113.9. HRMS (ESI) m/z [(M + H)+] Calcd for C19H15N2S+ (303.0950), found 303.0953.
1-(phenylthio)-3-(p-tolyl)imidazo[1,5-a]pyridine (4b): 72 mg (yield: 46%), a white solid. M.P.: 142-146 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.29 (d, J = 7.2 Hz, 1H), 7.74–7.72 (m, 2H), 7.64 (d, J = 9.2 Hz, 1H), 7.35–7.33 (m, 2H), 7.23–7.16 (m, 4H), 7.08 (t, J = 7.0 Hz, 1H), 6.85 (dd, J = 9.2, 6.4 Hz, 1H), 6.65 (t, J = 6.8 Hz, 1H), 2.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 139.5, 139.3, 138.4, 135.0, 129.7, 128.8, 128.2, 127.1, 126.6, 125.5, 122.2, 121.0, 119.7, 118.5, 113.8, 21.5. HRMS (ESI) m/z [(M + H)+] Calcd for C20H17N2S+ (317.1107), found 317.1104.
3-(4-(tert-butyl)phenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4c): 82 mg (yield: 46%), a green solid. M.P.: 140-148 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.31 (d, J = 7.2 Hz, 1H), 7.79–7.77 (m, 2H), 7.62 (d, J = 9.2 Hz, 1H), 7.56–7.54 (m, 2H), 7.23–7.16 (m, 4H), 7.07 (t, J = 7.1 Hz, 1H), 6.82 (dd, J = 9.2, 6.4 Hz, 1H), 6.62 (t, J = 6.8 Hz, 1H), 1.38 (s, 9H). 13C NMR (100 MHz, CDCl3) δ (ppm): 152.4, 139.5, 138.5, 135.0, 128.8, 128.0, 127.0, 126.7, 126.0, 125.5, 122.2, 121.0, 119.8, 118.4, 113.7, 34.9, 31.3. HRMS (ESI) m/z [(M + H)+] Calcd for C23H23N2S+ (359.1576), found 359.1572.
3-([1,1′-biphenyl]-4-yl)-1-(phenylthio)imidazo[1,5-a]pyridine (4d): 84 mg (yield: 44%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.39 (d, J = 7.3 Hz, 1H), 7.96 (s, 1H), 7.94 (s, 1H), 7.80 (s, 1H), 7.77 (s, 1H), 7.70–7.67 (m, 3H), 7.52–7.48 (m, 2H), 7.41 (t, J = 7.3 Hz, 1H), 7.28–7.20 (m, 4H), 7.12 (t, J = 7.1 Hz, 1H), 6.90 (dd, J = 9.2, 6.4 Hz, 1H), 6.71 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 141.8, 140.3, 139.0, 138.4, 135.2, 129.0, 128.9, 128.6, 128.4, 127.8, 127.7, 127.1, 127.1, 125.6, 122.2, 121.2, 120.3, 118.5, 114.0. HRMS (ESI) m/z [(M + H)+] Calcd for C25H19N2S+ (379.1263), found 379.1260.
3-(4-chlorophenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4e): 121 mg (yield: 72%), a yellow solid. M.P.: 104–1110 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.25 (d, J = 7.2 Hz, 1H), 7.79 (s, 1H), 7.77 (s, 1H), 7.65 (d, J = 9.1 Hz, 1H), 7.50 (s, 1H), 7.48 (s, 1H), 7.22–7.17 (m, 4H), 7.09 (t, J = 6.7 Hz, 1H), 6.88 (dd, J = 9.2, 6.5 Hz, 1H), 6.69 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.1, 135.3, 135.0, 129.4, 129.3, 128.9, 128.0, 127.2, 125.6, 122.4, 121.9, 121.3, 120.5, 118.6, 114.3. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14ClN2S+ (337.0561), found 337.0565.
3-(4-bromophenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4f): 95 mg (yield: 50%), a yellow solid. M.P.: 118–126 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.25 (d, J = 7.2 Hz, 1H), 7.73–7.70 (m, 2H), 7.65–7.73 (m, 3H), 7.22–7.16 (m, 4H), 7.09 (t, J = 6.7 Hz, 1H), 6.88 (dd, J = 9.0, 6.6 Hz, 1H), 6.69 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.1, 135.3, 132.3, 129.6, 128.9, 128.5, 127.2, 125.6, 123.2, 121.9, 121.3, 120.6, 118.6, 114.3. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13BrN2S+ (381.0056), found 381.0053.
3-(3-nitrophenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4g): 105 mg (yield: 61%), a yellow solid. M.P.: 116–120 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.70 (s, 1H), 8.34 (d, J = 7.5 Hz, 1H), 8.27–8.21 (m, 2H), 7.72–7.68 (m, 2H), 7.22–7.16 (m, 4H), 7.09 (t, J = 6.6 Hz, 1H), 6.95 (dd, J = 8.9, 6.7 Hz, 1H), 6.79 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.6, 137.8, 136.6, 135.7, 134.0, 131.3, 130.3, 128.9, 127.3, 125.8, 123.5, 122.3, 121.9, 121.6, 121.5, 118.7, 115.1. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14N3O2S+ (348.0801), found 348.0805.
3-(2-chlorophenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4h): 67 mg (yield: 40%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.69–7.65 (m, 3H), 7.55 (d, J = 7.7 Hz, 1H), 7.48–7.40 (m, 2H), 7.20–7.19 (m, 4H), 7.11–7.06 (m, 1H), 6.92 (dd, J = 9.9, 6.6 Hz, 1H), 6.69 (t, J = 7.3 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.5, 136.9, 134.7, 134.3, 133.4, 131.1, 130.0, 128.8, 128.8, 127.3, 127.0, 125.5, 122.9, 121.3, 119.6, 118.1, 113.5. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14ClN2S+ (337.0561), found 337.0564.
3-(3-chlorophenyl)-1-(phenylthio)imidazo[1,5-a]pyridine (4i): 69 mg (yield: 41%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.32 (d, J = 7.2 Hz, 1H), 7.86 (s, 1H), 7.74 (d, J = 7.3 Hz, 1H), 7.68 (d, J = 9.2 Hz, 1H), 7.49–7.44 (m, 2H), 7.24–7.17 (m, 4H), 7.12–7.08 (m, 1H), 6.91 (dd, J = 9.1, 6.4 Hz, 1H), 6.74 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.9, 137.7, 135.3, 135.1, 131.0, 130.3, 129.3, 128.9, 128.2, 127.4, 126.2, 125.7, 121.9, 121.5, 118.7, 114.5. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14ClN2S+ (337.0561), found 337.0565.
1-((4-chlorophenyl)thio)-3-phenylimidazo[1,5-a]pyridine (4j): 101 mg (yield: 60%), a white solid. M.P.: 104–110 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.32 (d, J = 7.3 Hz, 1H), 7.84–7.82 (m, 2H), 7.64 (d, J = 9.2 Hz, 1H), 7.56–7.52 (m, 2H), 7.47 (t, J = 7.3 Hz, 1H), 7.15 (s, 4H), 6.90 (dd, J = 9.1, 6.4 Hz, 1H), 6.69 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 139.4, 136.9, 135.0, 131.5, 129.3, 129.1, 128.9, 128.5, 128.3, 122.2, 121.5, 119.5, 118.3, 114.1. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14ClN2S+ (337.0561), found 337.0563.
1-((4-fluorophenyl)thio)-3-phenylimidazo[1,5-a]pyridine (4k): 104 mg (yield: 65%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.32 (d, J = 7.2 Hz, 1H), 7.85–7.83 (m, 2H), 7.67 (d, J = 9.2 Hz, 1H), 7.56–7.52 (m, 2H), 7.47 (t, J = 7.4 Hz, 1H), 7.28–7.25 (m, 2H), 6.94–6.87 (m, 3H), 6.68 (t, J = 6.8 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3) δ (ppm): 161.4 [d, J(C–F) = 244.0 Hz], 139.3, 134.9, 133.2, 129.5, 129.5 [d, J(C-F) = 8.0 Hz], 129.2, 129.1, 128.3, 122.1, 121.3, 120.5, 118.3, 115.9 [d, J(C-F) = 21.0 Hz], 113.9. 19F NMR (377 MHz, CDCl3) δ –117.0. HRMS (ESI) m/z [(M + H)+] Calcd for C19H14FN2S+ (321.0856), found 321.0853.
3-phenyl-1-(p-tolylthio)imidazo[1,5-a]pyridine (4l): 82 mg (yield: 52%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.29 (d, J = 7.2 Hz, 1H), 7.84–7.82 (m, 2H), 7.64 (d, J = 9.2 Hz, 1H), 7.54–7.50 (m, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.18–7.16 (m, 2H), 7.02–7.00 (m, 2H), 6.84 (dd, J = 9.2, 6.4 Hz, 1H), 6.64 (t, J = 6.8 Hz, 1H), 2.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 139.1, 136.5, 135.5, 134.8, 134.6, 129.6, 129.6, 129.1, 129.0, 128.3, 127.8, 122.0, 121.0, 118.5, 113.9, 21.0. HRMS (ESI) m/z [(M + H)+] Calcd for C20H17N2S+ (317.1107), found 317.1104.
3-(naphthalen-1-yl)-1-(phenylthio)imidazo[1,5-a]pyridine (4m): 109 mg (yield: 62%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.01 (d, J = 8.2 Hz, 1H), 7.95 (d, J = 8.1 Hz, 1H), 7.79 (d, J = 7.1 Hz, 1H), 7.74–7.70 (m, 3H), 7.63–7.59 (m, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.31–7.29 (m, 2H), 7.24–7.20 (m, 2H), 7.11 (t, J = 7.3 Hz, 1H), 6.89 (dd, J = 9.6, 6.4 Hz, 1H), 6.58–6.54 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.6, 138.1, 134.7, 134.0, 131.8, 130.3, 129.0, 128.9, 128.7, 127.2, 126.6, 126.5, 125.5, 125.4, 125.3, 122.5, 121.3, 119.8, 118.3, 113.5. HRMS (ESI) m/z [(M + H)+] Calcd for C23H17N2S+ (353.1107), found 353.1104.
1-(phenylthio)-3-(pyridin-2-yl)imidazo[1,5-a]pyridine (4n):. 110 mg (yield: 73%), a white solid. M.P.: 100-110 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 10.00 (d, J = 7.3 Hz, 1H), 8.61 (d, J = 4.0 Hz, 1H), 8.43 (d, J = 8.1 Hz, 1H), 7.73 (t, J = 7.8 Hz, 1H), 7.64 (d, J = 9.1 Hz, 1H), 7.20–7.15 (m, 5H), 7.09–7.0 (m, 1H), 6.93 (dd, J = 9.0, 6.5 Hz, 1H), 6.76 (t, J = 6.9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 150.6, 148.1, 138.3, 136.6, 136.4, 136.3, 128.9, 126.9, 126.8, 125.5, 122.4, 122.2, 120.3, 117.6, 114.2. HRMS (ESI) m/z [(M + H)+] Calcd for C18H14N3S+ (304.0903), found 304.0906.
1-(phenylthio)-3-(thiophen-2-yl)imidazo[1,5-a]pyridine (4o): 49 mg (yield: 32%), a yellow solid. M.P.: 112–116 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.40 (d, J = 7.2 Hz, 1H), 7.65 (d, J = 9.1 Hz, 1H), 7.60 (d, J = 3.7 Hz, 1H), 7.45 (d, J = 5.1 Hz, 1H), 7.21–7.16 (m, 5H), 7.10–7.07 (m, 1H), 6.89 (dd, J = 9.1, 6.4 Hz, 1H), 6.76 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.2, 135.1, 134.0, 131.5, 128.8, 127.7, 127.1, 126.6, 125.8, 125.6, 122.4, 121.1, 120.5, 118.5, 114.4. HRMS (ESI) m/z [(M + H)+] Calcd for C17H13N2S2+ (309.0515), found 309.0518.
3-(4-chlorophenyl)-1-((4-chlorophenyl)thio)imidazo[1,5-a]pyridine (4p): 138 mg (yield: 74%), a yellow solid. M.P.: 140–148 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.26 (d, J = 7.3 Hz, 1H), 7.78–7.76 (m, 2H), 7.64 (d, J = 9.2 Hz, 1H), 7.51–7.49 (m, 2H), 7.14 (s, 4H), 6.91 (dd, J = 9.2, 6.5 Hz, 1H), 6.71 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.3, 136.7, 135.3, 135.2, 131.5, 129.4, 129.4, 128.9, 128.5, 127.9, 121.9, 121.6, 112.0, 118.4, 114.3. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13Cl2N2S+ (371.0171), found 371.0175.
3-(4-chlorophenyl)-1-((4-fluorophenyl)thio)imidazo[1,5-a]pyridine (4q): 122 mg (yield: 69%), a white oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.27 (d, J = 7.2 Hz, 1H), 7.81 (s, 1H), 7.79 (s, 1H), 7.69 (d, J = 9.2 Hz, 1H), 7.54 (s, 1H), 7.52 (s, 1H), 7.29–7.25 (m, 2H), 6.95 –6.91 (m, 3H), 6.73 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 161.5 [d, J(C-F) = 244.0 Hz], 138.1, 135.2, 135.0, 132.9, 129.7 [d, J(C-F) = 7.0 Hz], 129.5, 129.4, 127.8, 121.9, 121.5, 120.9, 118.5, 115.9 [d, J(C-F) = 22.0 Hz], 114.3. 19F NMR (377 MHz, CDCl3) δ -116.7. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13ClFN2S+ (355.0467), found 355.0465.
3-(2-chlorophenyl)-1-((4-chlorophenyl)thio)imidazo[1,5-a]pyridine (4r): 145 mg (yield: 78%), a yellow solid. M.P.: 150–152 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.69–7.64 (m, 3H), 7.55 (d, J = 7.8 Hz, 1H), 7.49–7.40 (m, 2H), 7.15–7.12 (m, 4H), 6.94 (dd, J = 9.1, 6.5 Hz, 1H), 6.70 (t, J = 6.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.1, 134.7, 134.3, 133.3, 131.4, 131.3, 130.0, 128.9, 128.6, 128.3, 127.3, 123.0, 121.7, 119.1, 117.9, 113.6. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13Cl2N2S+ (371.0171), found 371.0174.
3-(2-chlorophenyl)-1-((4-fluorophenyl)thio)imidazo[1,5-a]pyridine (4s): 130 mg (yield: 73%), a white solid. M.P.: 80–92°C. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.68–7.62 (m, 3H), 7.54 (s, 1H), 7.53 (s, 1H), 7.47–7.39 (m, 2H), 7.24 -7.21 (m, 2H), 6.94–6.87 (m, 3H), 6.69–6.66 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 161.4 [d, J(C–F) = 244.0 Hz], 136.9, 134.5, 134.3, 133.3, 131.2, 130.0, 129.3 [d, J(C–F) = 8.0 Hz], 128.7, 127.3, 122.9, 121.5, 120.0, 118.0, 115.9 [d, J(C–F) = 22.0 Hz], 113.5. 19F NMR (377 MHz, CDCl3) δ –117.1. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13ClFN2S+ (355.0467), found 355.0464.
3-(3-chlorophenyl)-1-((4-chlorophenyl)thio)imidazo[1,5-a]pyridine(4t): 150 mg (yield: 81%), a yellow solid. M.P.: 98–102 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.30 (d, J = 7.2 Hz, 1H), 7.84 (s, 1H), 7.72 (d, J = 7.3 Hz, 1H), 7.64 (d, J = 9.2 Hz, 1H), 7.48–7.41 (m, 2H), 7.17–7.12 (m, 4H), 6.92 (dd, J = 9.2, 6.5 Hz, 1H), 6.73 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.9, 136.7, 135.4, 135.1, 131.6, 131.1, 130.4, 129.3, 128.9, 128.5, 128.2, 126.1, 122.0, 121.8, 120.2, 118.4, 114.5. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13Cl2N2S+ (371.0171), found 371.0175.y6
3-(3-chlorophenyl)-1-((4-fluorophenyl)thio)imidazo[1,5-a]pyridine (4u): 126 mg (yield: 71%), a yellow solid. M.P.: 98–100°C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.30 (d, J = 7.3 Hz, 1H), 7.85 (s, 1H), 7.74–7.67 (m, 2H), 7.49–7.42 (m, 2H), 7.27–7.24 (m, 2H), 6.95–6.89 (m, 3H), 6.74 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 160.5 [d, J(C-F) = 243.0 Hz], 137.7, 135.1, 132.9, 131.1, 130.3, 129.7 [d, J(C-F) = 8.0 Hz], 129.3, 128.2, 126.1, 121.9, 121.6, 121.1, 118.5, 115.9 [d, J(C–F) = 23.0 Hz], 114.4. 19F NMR (377 MHz, CDCl3) δ –116.7. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13ClFN2S+ (355.0467), found 355.0463.
3-(4-bromophenyl)-1-((4-chlorophenyl)thio)imidazo[1,5-a]pyridine (4v): 133 mg (yield: 64%), a white solid. M.P.:150–156°C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.26 (d, J = 7.2 Hz, 1H), 7.72 (s, 1H), 7.70 (s, 1H), 7.66–7.62 (m, 3H), 7.14 (s, 4H), 6.91 (dd, J = 9.2, 6.5 Hz, 1H), 6.71 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.3, 136.7, 135.3, 132.3, 131.5, 129.6, 128.9, 128.5, 128.3, 123.4, 121.9, 121.6, 120.0, 118.4, 114.4. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13BrClN2S+ (414.9666), found 414.9669.
1-((4-chlorophenyl)thio)-3-(naphthalen-1-yl)imidazo[1,5-a]pyridine (4w): 140 mg (yield: 72%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.02 (d, J = 8.2 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 7.1 Hz, 1H), 7.73–7.67 (m, 3H), 7.64–7.60 (m, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.50–7.46 (m, 1H), 7.24–7.17 (m, 4H), 6.92 (dd, J = 9.2, 6.4 Hz, 1H), 6.59 (t, J = 6.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 138.3, 137.0, 134.7, 134.0, 131.7, 131.5, 130.4, 129.0, 128.9, 128.7, 128.6, 127.3, 126.5, 126.3, 125.3, 125.3, 122.6, 121.6, 119.3, 118.1, 113.6. HRMS (ESI) m/z [(M + H)+] Calcd for C23H16ClN2S+ (387.0717), found 387.0720.
1-((4-chlorophenyl)thio)-3-(3-nitrophenyl)imidazo[1,5-a]pyridine (4x): 144 mg (yield: 75%), a yellow solid. M.P.:140–144 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.68 (s, 1H), 8.34 (d, J = 7.2 Hz, 1H), 8.26 (d, J = 8.2 Hz, 1H), 8.20 (d, J = 8.1 Hz, 1H), 7.72–7.65 (m, 2H), 7.13 (s, 4H), 6.97 (dd, J = 9.2, 6.5 Hz, 1H), 6.81 (t, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.6, 136.7, 136.4, 135.7, 134.0, 131.7, 131.2, 130.3, 129.0, 128.6, 123.6, 122.4, 122.2, 121.7, 120.9, 118.5, 115.1. HRMS (ESI) m/z [(M + H)+] Calcd for C19H13ClN3O2S+ (382.0412), found 382.0416.
3-(3-nitrophenyl)-1-(p-tolylthio)imidazo[1,5-a]pyridine (4y): 120 mg (yield: 67%), a yellow solid. M.P.:140–144°C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.70 (s, 1H), 8.33 (d, J = 7.2 Hz, 1H), 8.28–7.26 (m, 2H), 7.71–7.68 (m, 2H), 7.18 (s, 1H), 7.16 (s, 1H), 7.02–7.01 (m, 2H), 6.94 (dd, J = 9.2, 6.4 Hz, 1H), 6.78 (t, J = 6.8 Hz, 1H), 2.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.6, 136.3, 135.9, 135.4, 134.0, 133.9, 131.3, 130.2, 129.7, 128.1, 123.4, 122.5, 122.3, 121.7, 121.5, 118.8, 115.0, 21.0. HRMS (ESI) m/z [(M + H)+] Calcd for C20H16N3O2S+ (362.0958), found 362.0955.
3-(naphthalen-1-yl)-1-(p-tolylthio)imidazo[1,5-a]pyridine (4z): 78 mg (yield: 43%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.03 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 7.5 Hz, 1H), 7.80 (d, J = 7.1 Hz, 1H), 7.75–7.71 (m, 3H), 7.65–7.61 (m, 1H), 7.56 (t, J = 7.5 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 7.28 (s,1H), 7.26 (s,1H), 7.08 (s, 1H), 7.06 (s, 1H), 6.90 (dd, J = 9.1, 6.3 Hz, 1H), 6.59–6.56 (m, 1H), 2.30 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.8, 135.6, 134.7, 134.4, 134.0, 131.7, 130.3, 129.6, 129.0, 128.7, 128.0, 127.2, 126.5, 126.4, 125.4, 125.3, 122.5, 121.1, 120.7, 118.4, 113.5, 21.0. HRMS (ESI) m/z [(M + H)+] Calcd for C24H19N2S+ (367.1263), found 367.1266.
3-(4-chlorophenyl)-1-(p-tolylthio)imidazo[1,5-a]pyridine (4za): 120 mg (yield: 68%), a yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.22 (d, J = 7.3 Hz, 1H), 7.77 (m, 1H), 7.75 (m, 1H), 7.64 (d, J = 9.1 Hz, 1H), 7.48 (s, 1H), 7.46 (s, 1H), 7.16 (s, 1H), 7.14 (s, 1H),, 7.01 (s, 1H), 6.99 (s, 1H), 6.85 (dd, J = 9.2, 6.4 Hz, 1H), 6.66 (t, J = 6.8 Hz, 1H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.9, 135.7, 135.0, 134.9, 134.4, 129.6, 129.4, 129.3, 128.1, 127.8, 121.8, 121.4, 121.1, 118.6, 114.2, 21.0. HRMS (ESI) m/z [(M + H)+] Calcd for C20H16ClN2S+ (351.0717), found 351.0714.
4. Conclusions
In conclusion, we developed a one-pot strategy for the efficient synthesis of sulfinylimidazo[1,5-a]pyridine derivatives starting from 2-aminomethylpyridines, benzaldehydes, and sodium benzenesulfinates, which constructed C–N and C–S bonds simultaneously. The method is characterized by a short reaction time, mild reaction conditions, high atom efficiency, and good yields. This method demonstrates significant potential for the preparation of a variety of biologically or pharmaceutically active compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14090601/s1, General Information, Typical procedure, NMR spectra of all of the products.
Author Contributions
Conceptualization, Z.D. and Z.G.; methodology, L.H.; writing—original draft preparation, L.H.; writing—review and editing, Y.L.; supervision, Z.D.; project administration, Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from the National Natural Science Foundation of China (22401223), the Natural Science Foundation of Hubei Province (2024AFB315), the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD02), the Open and Innovation Fund of Hubei Three Gorges Laboratory (SC240004), Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (CSPC202306), Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110), and Knowledge Innovation Program of Wuhan -Basic Research (2023020201010142) is greatly appreciated.
Data Availability Statement
The data supporting this article have been included as part of the Supplementary Materials.
Acknowledgments
L. H. is thankful for the support of Postgraduate Innovation Foundation from Wuhan Institute of Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mennie, K.M.; Reutershan, M.H.; White, C.; Adams, B.; Becker, B.; Deng, J.; Katz, J.D.; LaBlue, E.; Margrey, K.; Divergent, J.S. Divergent and Regioselective Synthesis of Pyrazolo[1,5-a]pyridines and Imidazo[1,5-a]pyridines. Org. Lett. 2021, 23, 4694–4698. [Google Scholar] [CrossRef]
- Meena, N.; Dhiman, S.; Rangan, K.; Kumar, A. Cobalt-catalyzed Tandem One-pot Synthesis of Polysubstituted Imidazo[1,5-a]pyridines and Imidazo[1,5-a]isoquinolines. Org. Biomol. Chem. 2022, 20, 4215–4223. [Google Scholar] [CrossRef]
- Suhas, G.; Jagannath, S.; Sagar, T. Mg3N2-assisted One-pot Synthesis of 1,3-disubstituted Imidazo[1,5-a]pyridine. RSC Adv. 2020, 10, 11808–11815. [Google Scholar]
- Wang, L.H.; Zheng, X.S.; Zheng, Q.Z.; Li, Z.L.; Wu, J.; Gao, G. Thioether-Assisted Cu-Catalyzed C5–H Arylation of Imidazo[1,5-a]pyridines. Org. Lett. 2022, 24, 3834–3838. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.R.; Darapaneni, C.M.; Patil, R.D.; Kumari, H. Recent Synthetic Methodologies for Imidazo[1,5-a]pyridines and Related Heterocycles. Org. Biomol. Chem. 2022, 20, 3440–3468. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Yu, S.; Li, X. Rh(III)-Catalyzed Oxidative Annulation of 2-Phenylimidazo[1,2-a]pyridines with Alkynes: Mono versus Double C–H Activation. J. Org. Chem. 2015, 80, 3471–3479. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Fan, X. Synthesis of Naphtho[1′,2′:4,5]imidazo[1,2-a]pyridines and Imidazo[5,1,2-cd]indolizines through Pd-Catalyzed Cycloaromatization of 2-Phenylimidazo[1,2-a]pyridines with Alkynes. J. Org. Chem. 2015, 80, 7508–7518. [Google Scholar] [CrossRef] [PubMed]
- Blaquiere, N.; Castanedo, G.M.; Burch, J.D.; Berezhkovskiy, L.M.; Brightbill, H.; Brown, S.; Chan, C.; Chiang, P.C.; Crawford, J.J.; Dong, T.; et al. Scaffold-Hopping Approach To Discover Potent, Selective, and Efficacious Inhibitors of NF-κB Inducing Kinase. J. Med. Chem. 2018, 61, 6801–6813. [Google Scholar] [CrossRef]
- Wu, S.S.; Feng, C.T.; Hu, D.; Huang, Y.K.; Li, Z.; Luo, Z.G.; Ma, S.T. Iodine-Catalyzed Direct C-H Thiolation of Imidazo[1,5-a]quinolines for the Synthesis of 3-Sulfenylimidazo[1,5-a]quinolines. Org. Biomol. Chem. 2017, 15, 1680–1685. [Google Scholar] [CrossRef]
- Kamal, A.; Ramakrishna, G.; Ramaiah, M.J.; Viswanath, A.; Rao, A.S.; Bagul, C.; Mukhopadyay, D.; Pushpavalli, S.N.C.V.L.; Pal-Bhadra, M. Design, Synthesis and Biological Evaluation of Imidazo[1,5-a]pyridine–PBD Conjugates as Potential DNA-directed Alkylating Agents. Med. Chem. Commun. 2013, 4, 697. [Google Scholar] [CrossRef]
- Trapani, G.; Franco, M.; Ricciardi, L.; Latrofa, A.; Genchi, G.; Sanna, E.; Tuveri, F.; Cagetti, E.; Biggio, G.; Liso, G. Synthesis and Binding Affinity of 2-Phenylimidazo[1,2-a]pyridine Derivatives for both Central and Peripheral Benzodiazepine Receptors. A New Series of High-Affinity and Selective Ligands for the Peripheral Type. J. Med. Chem. 1997, 40, 3109–3118. [Google Scholar] [CrossRef]
- Zeng, K.; Ye, J.; Meng, X.; Dechert, S.; Simon, M.; Gong, S.; Mata, R.A.; Zhang, K. Anomeric Stereoauxiliary Cleavage of the C–N Bond of d-Glucosamine for the Preparation of Imidazo[1,5-a]pyridines. Chem. Eur. J. 2022, 28, e202200648. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Qin, M.; Chen, Y.; Liu, Y.; Tian, Y.; Chen, B. Diiodine-Mediated Oxidative Reaction for the Construction of Imidazo[1,5-a]pyridines under Metal-Free Conditions. Synlett 2020, 31, 695–698. [Google Scholar] [CrossRef]
- Tang, J.; Lu, F.; Sun, Y.; Yang, Z.; Zhang, E.; Lu, J. Relay Copper-Catalyzed Synthesis of Imidazo[1,5-a]pyridine Scaffolds from Phenylalanine and Halohydrocarbon. J. Org. Chem. 2023, 88, 17499–17504. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Lace, B.; Garino, C.; Priola, E.; Artuso, E.; Vioglio, P.C.; Barolo, C.; Fin, A.; Genre, A.; Prandi, C. New Substituted Imidazo[1,5-a]pyridine and Imidazo[5,1-a]isoquinoline Derivatives and their Application in Fluorescence Cell Imaging. Dye Pigment. 2018, 157, 298–304. [Google Scholar] [CrossRef]
- Albano, S.; Olivo, G.; Mandolini, L.; Massera, C.; Ugozzoli, F.; Di Stefano, S. Formation of Imidazo[1,5-a]pyridine Derivatives Due to the Action of Fe2+ on Dynamic Libraries of Imines. J. Org. Chem. 2017, 82, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Hu, M.; Hu, J. Good Partnership Between Sulfur and Fluorine: Sulfur-Based Fluorination and Fluoroalkylation Reagents for Organic Synthesis. Chem. Rev. 2015, 115, 765–825. [Google Scholar] [CrossRef]
- Wang, W.; Niu, J.L.; Liu, W.B.; Shi, T.H.; Hao, X.Q.; Song, M.P. Rhodium(III)-Catalyzed Annulation of 2-arylimidazo[1,2-a]pyridines and Alkynes Via Direct Double C–H Activation. Tetrahedron 2015, 71, 8200–8207. [Google Scholar] [CrossRef]
- Li, K.; Zhao, X.M.; Yang, F.L.; Hou, X.H.; Xu, Y.; Guo, Y.C.; Hao, X.Q.; Song, M.P. Catalyst-free Friedel–Crafts Hydroxyalkylation of Imidazo[1,2-α]pyridines with Ethyl Trifluoropyruvate. RSC Adv. 2015, 5, 90478–90481. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, X.; Li, K.; Guo, Y.J.; Wang, M.D.; Zhao, X.M.; Hao, X.Q.; Song, M.P. Reactivity of p-Toluenesulfonylmethyl Isocyanide: Iron-Involved C–H Tosylmethylation of Imidazopyridines in Nontoxic Media. J. Org. Chem. 2016, 81, 8370–8377. [Google Scholar] [CrossRef]
- Zhu, M.; Han, X.; Fu, W.; Wang, Z.; Ji, B.; Hao, X.Q.; Song, M.P.; Xu, C. Regioselective 2,2,2-Trifluoroethylation of Imidazopyridines by Visible Light Photoredox Catalysis. J. Org. Chem. 2016, 81, 7282–7287. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Q.; Liu, W.B.; Shen, X.J.; Wang, W.; Liang, Z.K.; Zhu, X.; Song, M.P. Nitrosylation of Imidazo[1,2-a]pyridines in Metal Free System. Saudi Chem. Soc. 2017, 21, 91–94. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, X.J.; Tian, Z.Y.; Lu, S.; Tian, L.L.; Liu, W.B.; Song, B.; Hao, X.Q. Rhodium-Catalyzed Direct Bis-cyanation of Arylimidazo [1, 2-α] pyridine Via Double C–H activation. J. Org. Chem. 2017, 82, 6022–6031. [Google Scholar] [CrossRef] [PubMed]
- Desaintjean, A.; Haupt, T.; Bole, L.J.; Judge, N.R.; Hevia, E.; Knochel, P. Regioselective Bromine/Magnesium Exchange for the Selective Functionalization of Polyhalogenated Arenes and Heterocycles. Angew. Chem. Int. Ed. 2021, 60, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W.; Jiang, K.; Li, J.X.; Luo, S.H.; Wang, Z.Y.; Jiang, H.F. 1,1-Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation-Caused-Quenching Molecule 1,1-Diphenylethene through Simple Thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Rahaman, R.; Barman, P. Iodine-Catalyzed Mono- and Disulfenylation of Indoles in PEG400 through a Facile Microwave-Assisted Process. Eur. J. Org. Chem. 2017, 42, 6327–6334. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, W.; Zhao, W.; Li, Y.P.; Huang, Y.; Liu, Y.; Zhou, A. Generation of Thioethers Via Direct C–H Functionalization with Sodium Benzenesulfinate as a Sulfur Source. Org. Biomol. Chem. 2016, 14, 1428–1431. [Google Scholar] [CrossRef]
- Pelletier, G.; Charette, A.B. Triflic Anhydride Mediated Synthesis of Imidazo[1,5-a]azines. Org. Lett. 2013, 15, 2290–2293. [Google Scholar] [CrossRef]
- Guo, Y.J.; Lu, S.; Tian, L.L.; Huang, E.L.; Hao, X.Q.; Zhu, X.; Song, M.P. Iodine-Mediated Difunctionalization of Imidaz opyridines with Sodium Sulfinates: Synthesis of Sulfones and Sulfides. J. Org. Chem. 2018, 83, 338–349. [Google Scholar] [CrossRef]
- Pavan Kumar, C.S. Tandem Approach for the Synthesis of 3-sulfenylimidazo[1,5-a]pyridines from Dithioesters. RSC Adv. 2016, 6, 48375–48378. [Google Scholar]
- Dong, Z.B.; Liu, X.; Bolm, C. Copper-catalyzed C(sp2)-S Coupling Reactions for the Synthesis of Aryl Dithiocarbamates with Thiuram Disulfide Reagents. Org. Lett. 2017, 19, 5916–5919. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, M.; Xu, W.; Zeng, M.T.; Zhu, H.; Chang, C.Z.; Dong, Z.B. An Environmentally Benign and Efficient Synthesis of Substituted Benzothiazole-2-thiols, Bnzoxazole-2-thiols, and Benzimidazoline-2-thiones in Water. Green Chem. 2017, 19, 5591–5598. [Google Scholar] [CrossRef]
- Dong, Z.B.; Balkenhohl, M.; Tan, E.; Knochel, P. Synthesis of Functionalized Diaryl Sulfides by Cobalt-catalyzed Coupling between Arylzinc Pivalates and Diaryl Disulfides. Org. Lett. 2018, 20, 7581–7584. [Google Scholar] [CrossRef]
- Yang, C.L.; Gao, X.J.; Jiang, X.Y.; Shi, Z.; Hao, E.J.; Dong, Z.B. Synthesis of Unsymmetric Thiosulfonates Starting from N-substituted O-thiocarba- mates: Easy Access to the S-SO2 bond. J. Org. Chem. 2022, 87, 11656–11668. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Wu, Y.X.; Ye, L.; Cui, J.J.; Dong, Z.B. An Efficient and Practical Construction of S-N Bond from Aryl Thioureas and Amines under Metal-free Conditions. Eur. J. Org. Chem. 2022, 2022, e202200419. [Google Scholar] [CrossRef]
- Feng, R.; Ma, J.; Dong, Z.B. One-pot Synthesis of N-benzyl/allyl-N-phenyl-2-benzothiazolamines from 1-(2-Iodophenyl)-3-phenylthioureas and Benzyl/allyl Halides. Eur. J. Org. Chem. 2023, 26, e202201145. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, R.; Lin, S.; Yan, Z.; Guo, S. Iodine-mediated Thiolation of Phenol/Phenylamine Derivatives and Sodium Arylsulfinates in Neat Water. RSC Adv. 2015, 5, 108030–108033. [Google Scholar] [CrossRef]
- Feng, R.; Li, Z.Y.; Liu, Y.J.; Dong, Z.B. Selective Synthesis of Sulfonamides and Sulfenamides from Sodium Sulfinates and Amines. J. Org. Chem. 2024, 89, 1736–1747. [Google Scholar] [CrossRef]
- Van Phuc, B.; Nguyen, N.T.; Van, N.T.H.; Nguyen, T.L.; Nguyen, V.H.; Tran, C.M.; Nguyen, H.; Nguyen, M.T.; Hung, T.Q.; Dang, T.T. Facile Iodine-promoted Synthesis of bis(1-imidazo[1,5-a]pyridyl)arylmethanes and Exploration of Applications. Chem. Commun. 2023, 59, 1947. [Google Scholar] [CrossRef]
- Mahajan, S.; Sawant, S.D. Iodine/TBHP-Mediated One-Pot Multicomponent Protocol for Tandem C–N and C–S Bond Formation To Access Sulfenylimidazo[1,5-a]pyridines via C–H Functionalization. J. Org. Chem. 2022, 87, 11387–11398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).