Improvement in the Photocatalytic Hydrogen Production of Flower-Shaped ZnIn2S4 by Surface Modification with Amino Silane
Abstract
:1. Introduction
2. Results
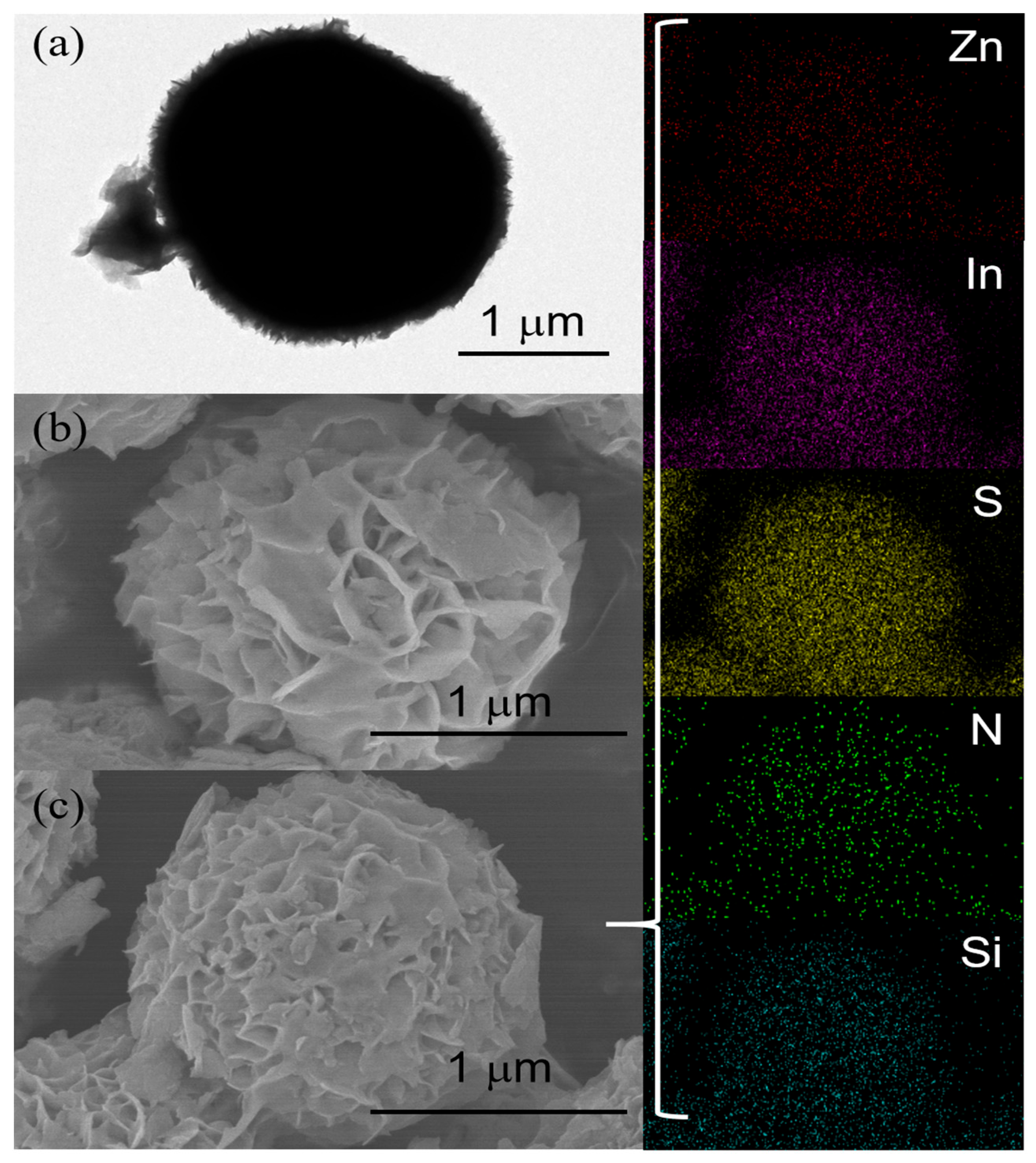
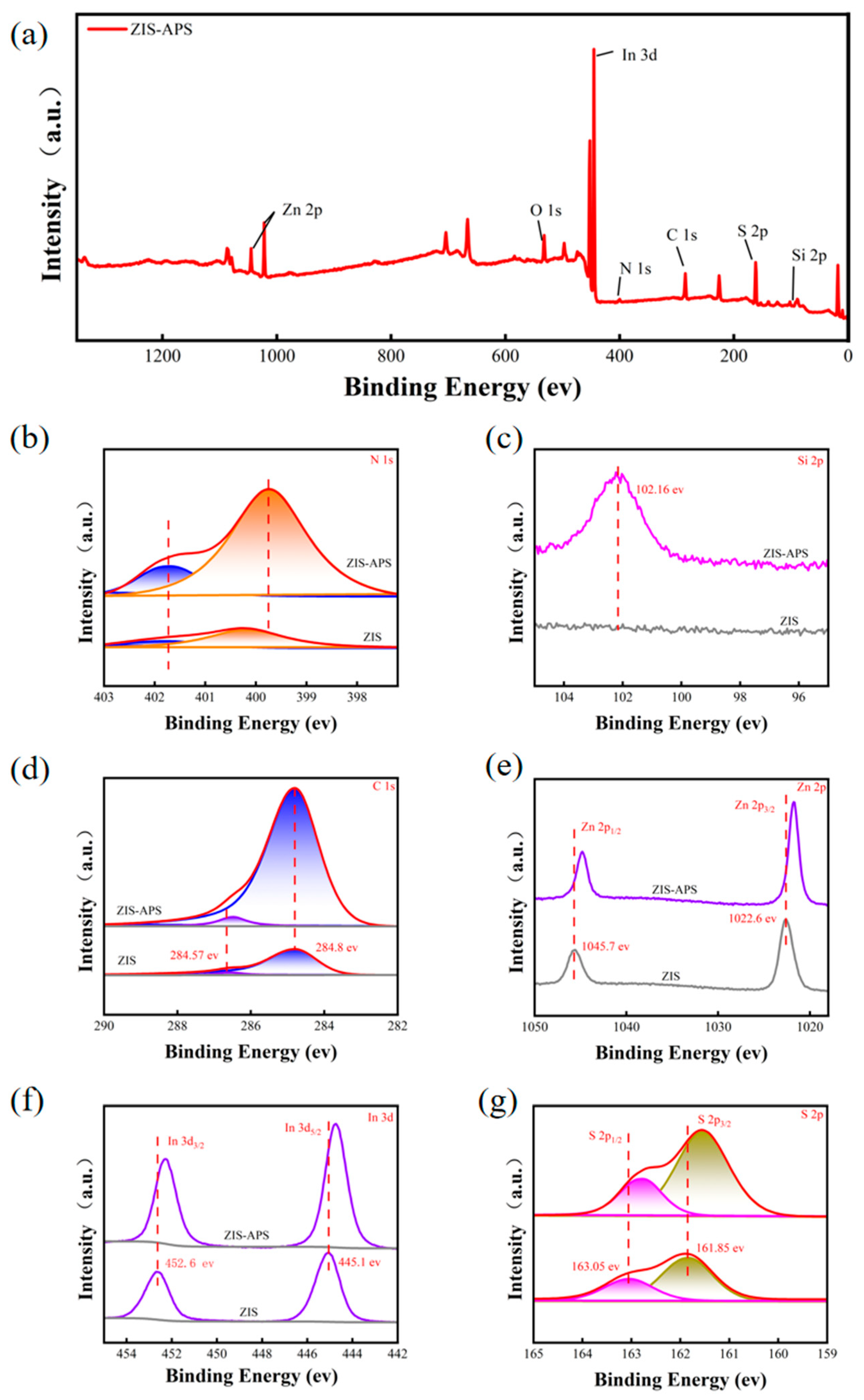
2.1. Morphology and Structure
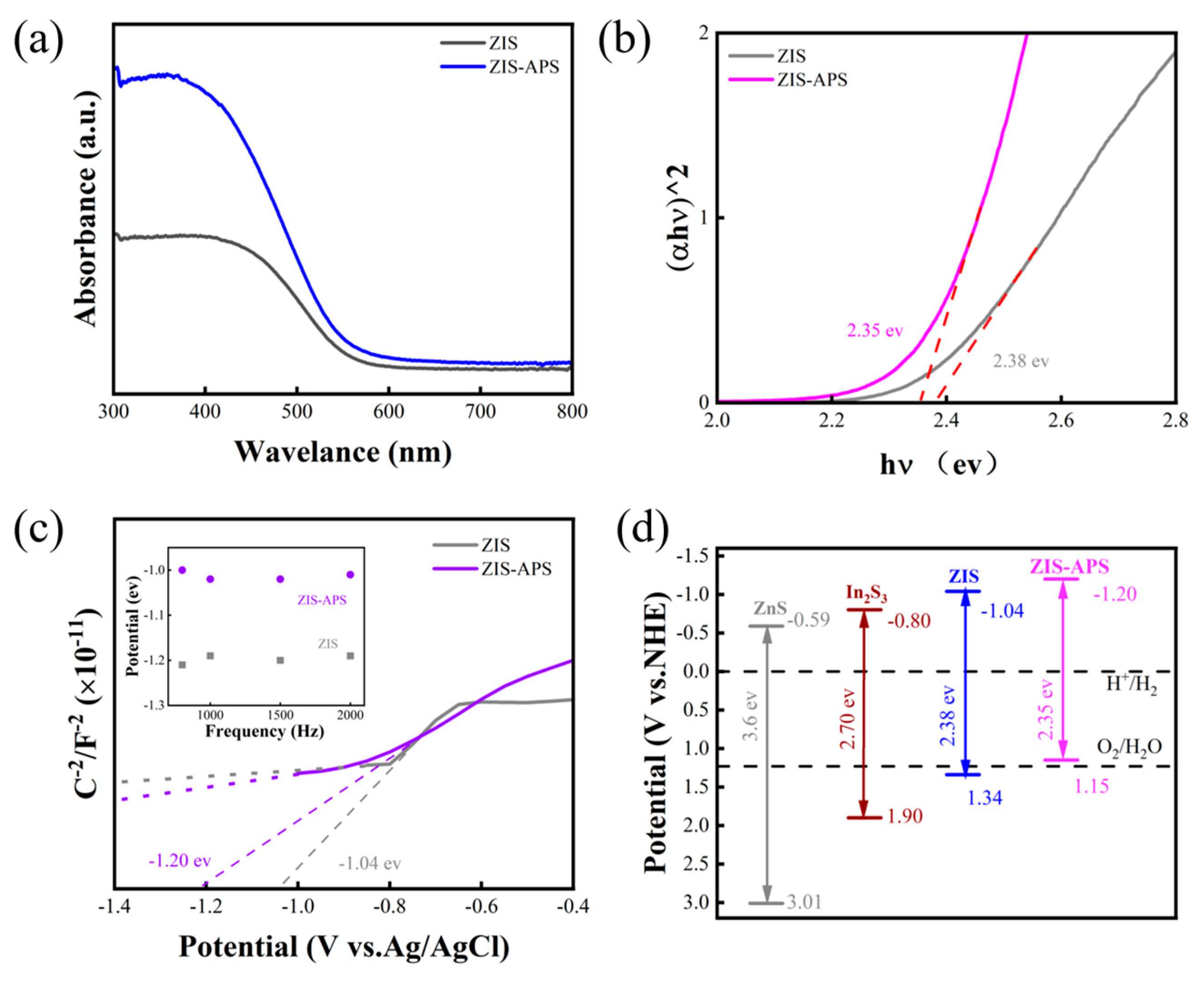
2.2. Optical Properties and Band Gap
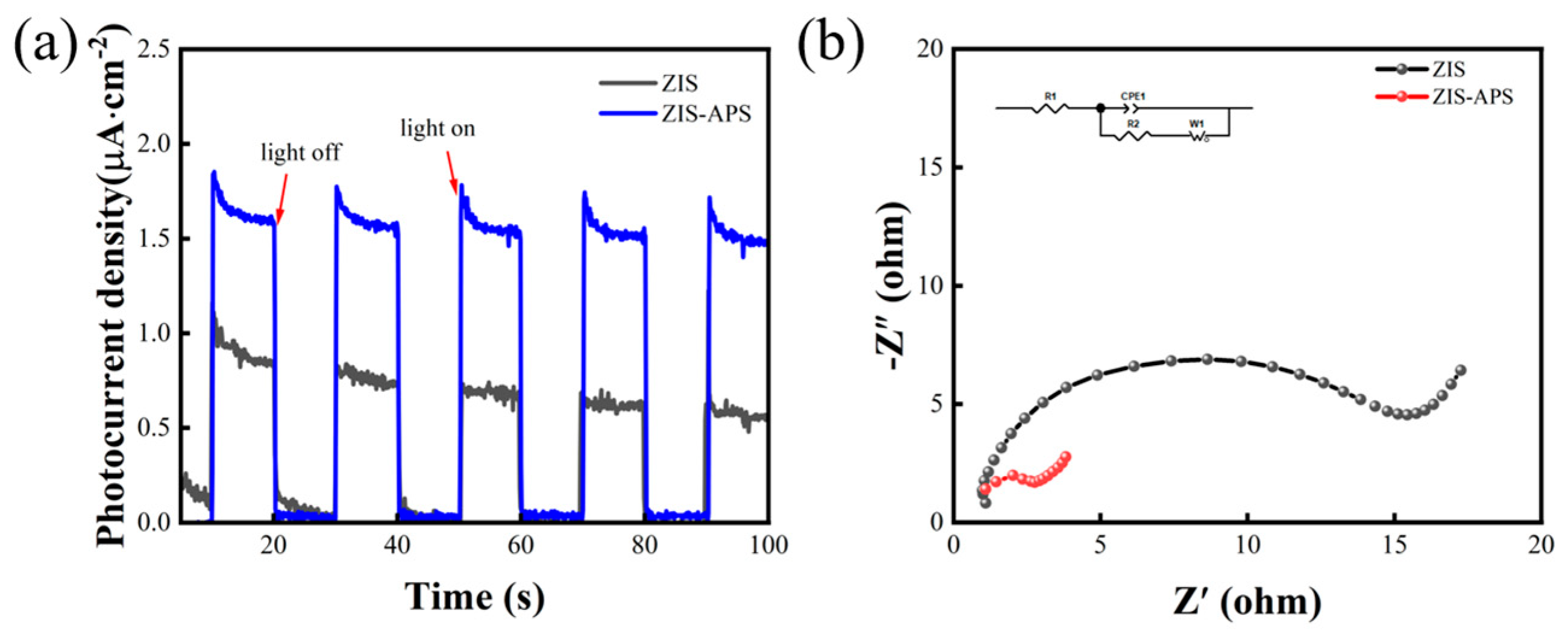
2.3. Photocatalytic H2 Evolution
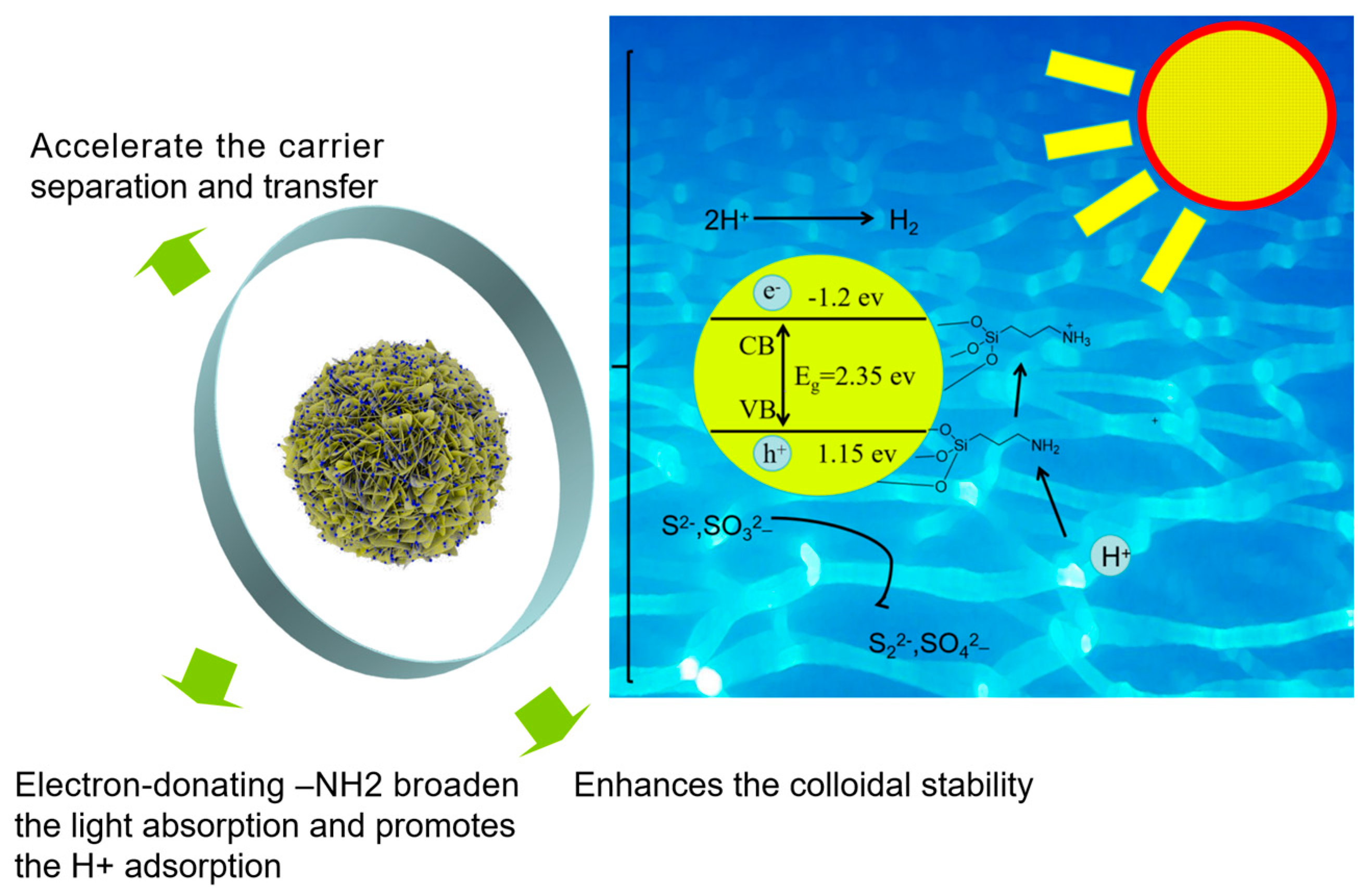
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ipatieff, V.N.; Monroe, G.S.; Fischer, L.E. Low temperature hydrogen production. Ind. Eng. Chem. 1950, 42, 92–94. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shang, Q.; Wan, Y.; Cheng, Q.; Liao, G.; Pan, Z. Self-template synthesis of double-shell TiO2@ ZiF-8 hollow nanospheres via Ssonocrystallization with enhanced photocatalytic activities in hydrogen generation. Appl. Catal. B Environ. 2019, 241, 149–158. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, P.; Saxena, N.; Chen, Z.; Tyagi, A.; Zhang, G.; Sun, S. CdS Based 3D Nano/microarchitectures: Formation mechanism, tailoring of visible light activities and emerging applications in photocatalytic H2 production, CO2 reduction and organic pollutant degradation. J. Mater. Chem. A 2023, 11, 10015–10064. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, B.; Wang, S.; Wang, L. Solar energy conversion on g-C3N4 photocatalyst: Light harvesting, charge separation, and surface kinetics. J. Energy Chem. 2018, 27, 1111–1123. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Bi, R.; Han, B.; Shen, Y.; Liu, J.; Wang, Z. Enhancing photocatalytic overall water splitting activity of SrTiO3 nanoparticles by a synergetic Pt/CrOx dual cocatalyst system. ACS Appl. Energy Mater. 2023, 6, 10600–10609. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, W.; Huang, B.; Dai, Y. Plasmon-enhanced solar water splitting on metal semiconductor photocatalysts. Chem.-A Eur. J. 2018, 24, 18322–18333. [Google Scholar] [CrossRef]
- Shi, R.; Ye, H.F.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.; Lin, Z.; Fu, W.; Che, C.; Chen, Y. Interstitial P-doped CdS with long-lived photogenerated electrons for photocatalytic water splitting without sacrificial Agents. Adv. Mater. 2018, 30, 1705941. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Cui, X.; Zheng, W. Highly carbon-doped TiO2 derived from mxene boosting the photocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2018, 6, 13480–13486. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Guo, L.; Yao, X. In situ photochemical synthesis of Zn-doped Cu2O hollow microcubes for highly efficient photocatalytic H2 production. ACS Sustain. Chem. Eng. 2014, 2, 1446–1452. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.P.; Yu, J.S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Hang, K.; Zu, D. Tuning defects in oxides at room temperature by lithium reduction. Nat. Commun. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, X.; Zhang, C.; Li, R.; Liu, J.; Wang, Y.; Fan, C.; Li, Z. Original self-assembled S-Scheme BiOBr-(001)/Bi2SiO5/Bi heterojunction photocatalyst with rich oxygen vacancy for boosting CO2 reduction performance. J. Colloid Interface Sci. 2023, 644, 426–436. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.; Liu, Q.; Yang, X.; Tang, H.; Yang, J. Accelerating photocatalytichydrogen evolution and pollutant degradation by coupling organic co-catalystswith TiO2. Chin. J. Catal. 2019, 40, 380–389. [Google Scholar] [CrossRef]
- Feng, C.; Wu, Z.P.; Huang, K.W.; Ye, J.; Zhang, H. surface modification of 2D photocatalysts for solar energy conversion. Adv. Mater. 2022, 34, 2200180. [Google Scholar] [CrossRef]
- Lin, X.; Xu, S.; Wei, Z.Q.; Hou, S.; Xiao, F.X. Selective Organic Transformation over a Self-Assembled AllSolid-State Z-Scheme Core-Shell Photoredox System. J. Mater. Chem. A 2020, 8, 20151–20161. [Google Scholar] [CrossRef]
- Wu, J.-W.; Wei, Y.C.; Torimoto, T.; Chien, Y.N.; Chen, C.Y.; Chang, T.M.; Sone, M.; Hsieh, P.Y.; Hsu, Y.I. Yolk@Shell Nanostructures for Water Splitting: Current Development and Future Prospects. ACS Materials Lett. 2024, 6, 4066–4089. [Google Scholar] [CrossRef]
- Hu, J.; Xie, J.; Jia, W.; Zhang, S.; Cao, Y. Interesting molecule adsorption strategy induced energy band tuning: Boosts 43 times photocatalytic water splitting ability for commercial TiO2. Appl. Catal. B 2020, 268, 118753. [Google Scholar] [CrossRef]
- Wanag, A.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Influence of modification of titanium dioxide by silane coupling agents on the photocatalytic activity and stability. J. Environ. Chem. Eng. 2020, 8, 103917. [Google Scholar] [CrossRef]
- Lei, W.; Wang, H.; Khan, S.; Suzuki, N.; Takagi, K.; Katsumata, K.; Teshima, K.; Terashima, C.; Fujishima, A. Interfacial molecular regulation of TiO2 for enhanced and stable cocatalyst-free photocatalytic hydrogen production. J. Colloid Interface Sci. 2023, 645, 219–226. [Google Scholar] [CrossRef]
- Shao, W.; Wang, L.; Wang, H.; Zhao, Z.; Xie, Y. Efficient exciton dissociation in heterojunction interface realizing enhanced photoresponsive performance. J. Phys. Chem. Lett. 2019, 10, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Peng, T.; Zeng, P.; Zhang, X.; Liu, X. Template-free hydrothermal synthesis of ZnIn2S4 floriated microsphere as an efficient photocatalyst for H2 production under visible-light irradiation. J. Phys. Chem. C 2011, 115, 6149–6155. [Google Scholar] [CrossRef]
- Xue, C.; An, H.; Yan, X.; Li, J.; Yang, B.; Wei, J.; Yang, G. Spatial charge separation and transfer in ultrathin CdIn2S4/rGO nanosheet arrays decorated by ZnS quantum dots for efficient visible-light-driven hydrogen evolution. Nano Energy 2017, 39, 513–523. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Xie, J.; Zhang, X.; Liu, Q.; Yao, T.; Wei, S.; Zhang, Q.; Xie, Y. Enhanced photoexcited carrier separation in oxygen-doped ZnIn2S4 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 6716–6720. [Google Scholar] [CrossRef]
- Yu, M.S.; Lv, X.Y.; Idris, A.M.; Li, S.H.; Lin, J.Q.; Lin, H.; Wang, J.; Li, Z.Q. Upconversion nanoparticles coupled with hierarchical ZnIn2S4 nanorods as a near-infrared responsive photocatalyst for photocatalytic CO2 reduction. J. Colloid. Interf. Sci. 2022, 612, 782–791. [Google Scholar] [CrossRef]
- Sun, L.L.; Liu, X.S.; Jiang, X.H.; Feng, Y.B.; Ding, X.L.; Jiang, N.; Wang, J.G. An internal electric field and interfacial S–C bonds jointly accelerate S-scheme charge transfer achieving efficient sunlight-driven photocatalysis. J. Mater. Chem. A 2022, 10, 25279–25294. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Zhu, Y.; Yang, J.; Tang, B.; Zhang, T.; Yang, H. Cu-MOF modified ZnIn2S4 nanosheet composite catalyst for photocatalytic hydrogen production. Renew. Energy 2024, 228, 120672. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, M.; Lv, Y.; Rao, Y.; Huang, Y. A short review on research progress of ZnIn2S4-based S-scheme heterojunction:Improvement strategies. Chin. Chem. Lett. 2024, in press. [CrossRef]
- Zhang, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; Lu, J. Fabricating 1D/2D Co3O4/ZnIn2S4 core–shell heterostructures with boosted charge transfer for photocatalytic hydrogen production. Appl. Surf. Sci. 2023, 610, 155272. [Google Scholar] [CrossRef]
- Cai, X.; Zang, Y.; Zang, S.; Tang, S.; Jing, F.; Mo, L.; Teng, D.; Lin, W.; Zhang, G. Binary pyrene-benzene polymer/Znln2S4 S-scheme photocatalyst for enhanced hydrogen evolution and antibiotics degradation. Appl. Surf. Sci. 2023, 637, 157871. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Wang, I.L. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Kumar, Y.; Sudhaik, A.; Sharma, K.; Sonu; Raizada, P.; Khan, A.A.P.; Nguyen, Y.H.; Ahamad, T.; Singh, P.; Asiri, A.M. Construction of magnetically separable novel arrow down dual S-scheme ZnIn2S4/BiOCl/FeVO4 heterojunction for improved photocatalytic activity. J. Photochem. Photobiol. A Chem. 2023, 435, 114326. [Google Scholar] [CrossRef]
- Liu, X.T.; Jiang, Z.J.; Cao, X.; Shen, Z.; Zhao, W.; Wanf, F.; Cui, M.; Liang, C. Fabrication of S-scheme g-C3N4/Zn4In2S7 heterojunction photocatalyst for enhancing selective cleavage of β-O-4 bond in lignin model compounds and lignin. ACS Sustain. Chem. Eng. 2023, 11, 14947–14959. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sun, L.L.; Li, X.L. Ultrasonic-assistant hydrothermal synthesis of ZnIn2S4/graphene-quantum-dots nanowires and the sono-effect improved catalytic activity. J. Water Process Eng. 2024, 65, 105807. [Google Scholar] [CrossRef]
- Demir, R.; Göde, F.; Güneri, E.; Mehmet, E.F. The effect of nanoparticle sizes on the structural, optical and electrical properties of indium sulfide thin films consisting of In2S3 and In6S7 phases. J. Mol. Struct. 2021, 1227, 129565. [Google Scholar] [CrossRef]
- Duan, S.; Li, A.; Wang, Y.; Chen, X.; Liu, B.; Liang, B.; Dai, C.; Yan, L.; Guo, J. Fabrication and performance of a 3D porous graphene aerogel-supported Ni–ZnS composite photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132948. [Google Scholar] [CrossRef]
- Huang, W.X.; Li, Z.P.; Wu, C.; Zhang, H.J.; Sun, J.; Lin, Q. Delaminating Ti3C2 MXene by blossom of ZnIn2S4 microflowers for noble-metal-free photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 120, 89–98. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Liu, S.; Chen, D.M.; Zhang, S.S.; Carabineiro, S.A.C.; Lv, K.L. A novel S-scheme 3D ZnIn2S4/WO3 heterostructure for improved hydrogen production under visible light irradiation. Chin. J. Catal. 2022, 43, 2615–2624. [Google Scholar] [CrossRef]
- Zhou, D.X. ZnIn2S4 Photocatalysts: Structural Design, Controllable Preparation and Their Photocatalytic Hydrogen Evolution Performance. Ph.D. Thesis, Beijing University of Science and Technology, Beijing, China, 2022. [Google Scholar]
- Yuan, Y.J.; Tu, J.R.; Ye, Z.J.; Chen, D.Q.; Hu, B.; Huang, Y.W.; Chen, T.T.; Cao, D.P.; Yu, Z.T.; Zou, Z.G. MoS2-graphene/ZnIn2S4 hierarchical microarchitectures with an electron transport bridge between light-harvesting semiconductor and cocatalyst: A highly efficient photocatalyst for solar hydrogen generation. Appl. Catal. B Environ. 2016, 188, 13–22. [Google Scholar] [CrossRef]
- Wang, L.F.; Zeng, Q.R.; Gan, Y.F.; Wei, Y.Z.; Wang, X.P.; Zeng, D.Q. Insight into the role of nickel carbide nanoparticles in improving photocatalytic H2 generation over ZnIn2S4 under visible light. FlatChem 2024, 47, 100711. [Google Scholar] [CrossRef]
- Ji, M.X.; Wen, J.; Xu, Q.Y. Highly efficient visible-light-driven water splitting for H2 evolution and degradation of ECs using CdS/ZnIn2S4 S-scheme heterojunction with built-in electric field. Fuel 2024, 374, 132444. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.Y.; Yan, B.; Xia, C.X.; Yang, G.W. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liang, B.; Yan, L. Improvement in the Photocatalytic Hydrogen Production of Flower-Shaped ZnIn2S4 by Surface Modification with Amino Silane. Catalysts 2024, 14, 607. https://doi.org/10.3390/catal14090607
Chen X, Liang B, Yan L. Improvement in the Photocatalytic Hydrogen Production of Flower-Shaped ZnIn2S4 by Surface Modification with Amino Silane. Catalysts. 2024; 14(9):607. https://doi.org/10.3390/catal14090607
Chicago/Turabian StyleChen, Xiangyu, Benliang Liang, and Luting Yan. 2024. "Improvement in the Photocatalytic Hydrogen Production of Flower-Shaped ZnIn2S4 by Surface Modification with Amino Silane" Catalysts 14, no. 9: 607. https://doi.org/10.3390/catal14090607





