Green Synthesis of LaMnO3-Ag Nanocomposites Using Citrus limon (L.) Burm Peel Aqueous Extract: Photocatalytic Degradation of Rose Bengal Dye and Antibacterial Applications
Abstract
:1. Introduction
2. Results and Discussion
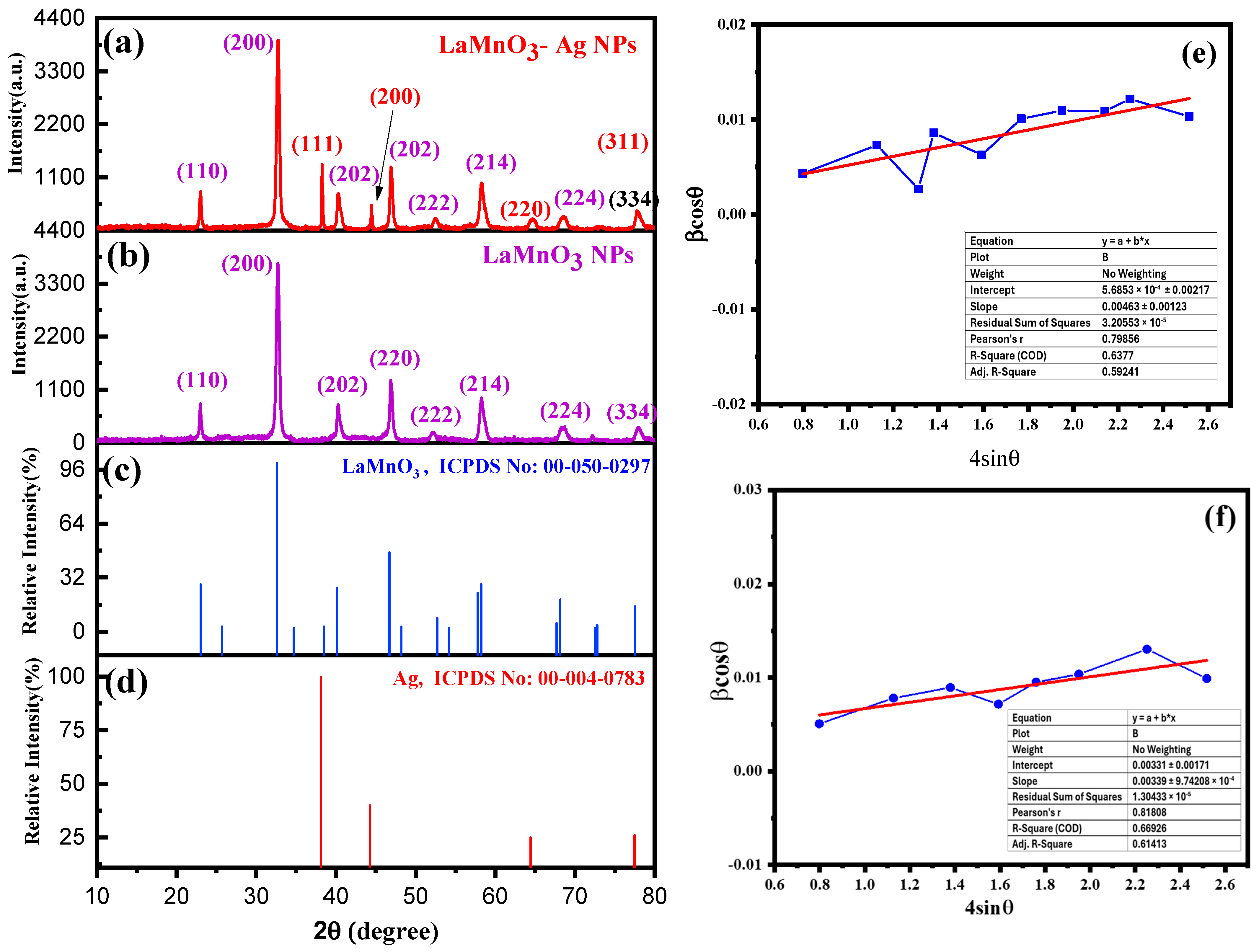
2.1. X-ray Diffraction
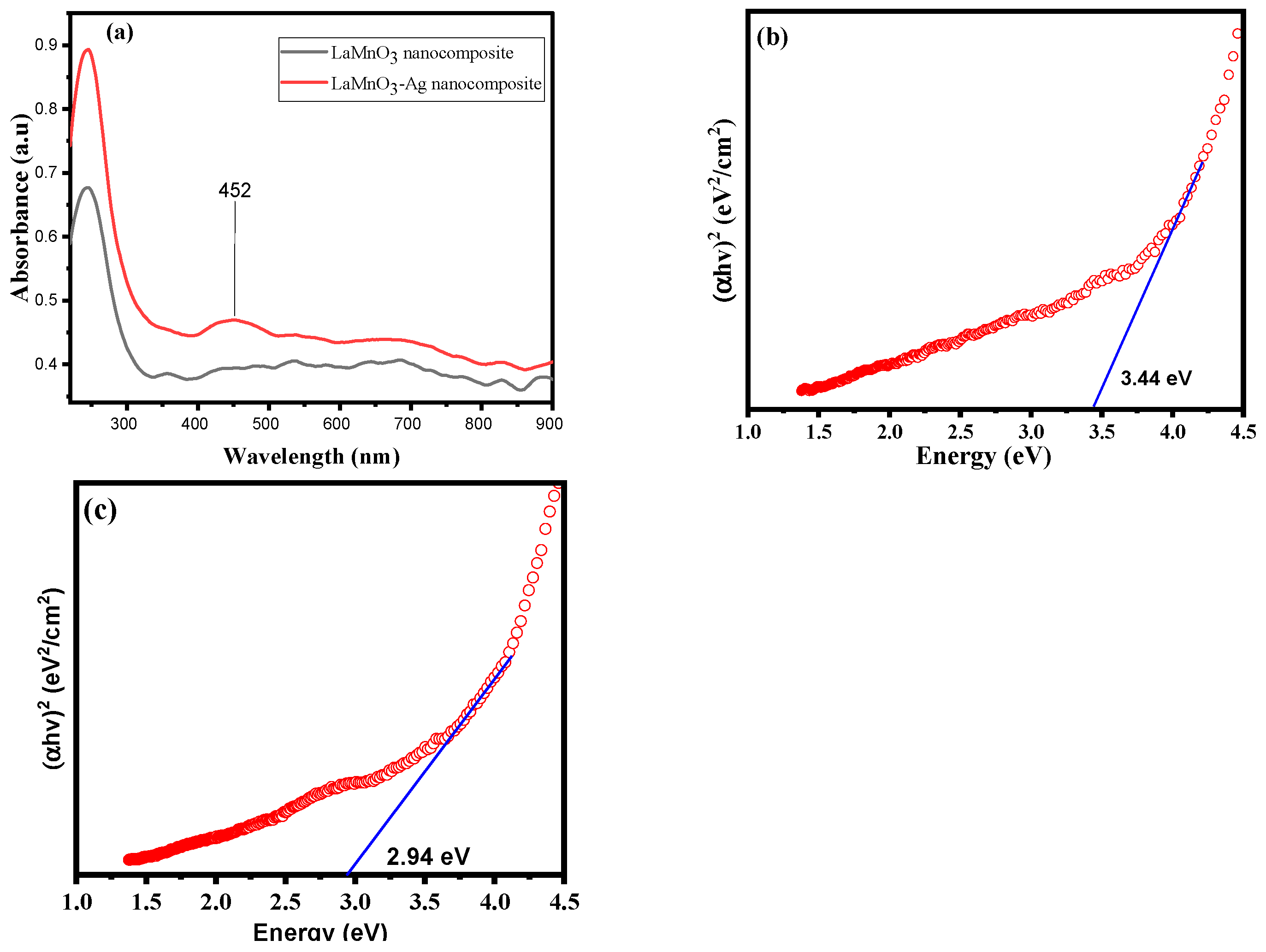
2.2. UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS)
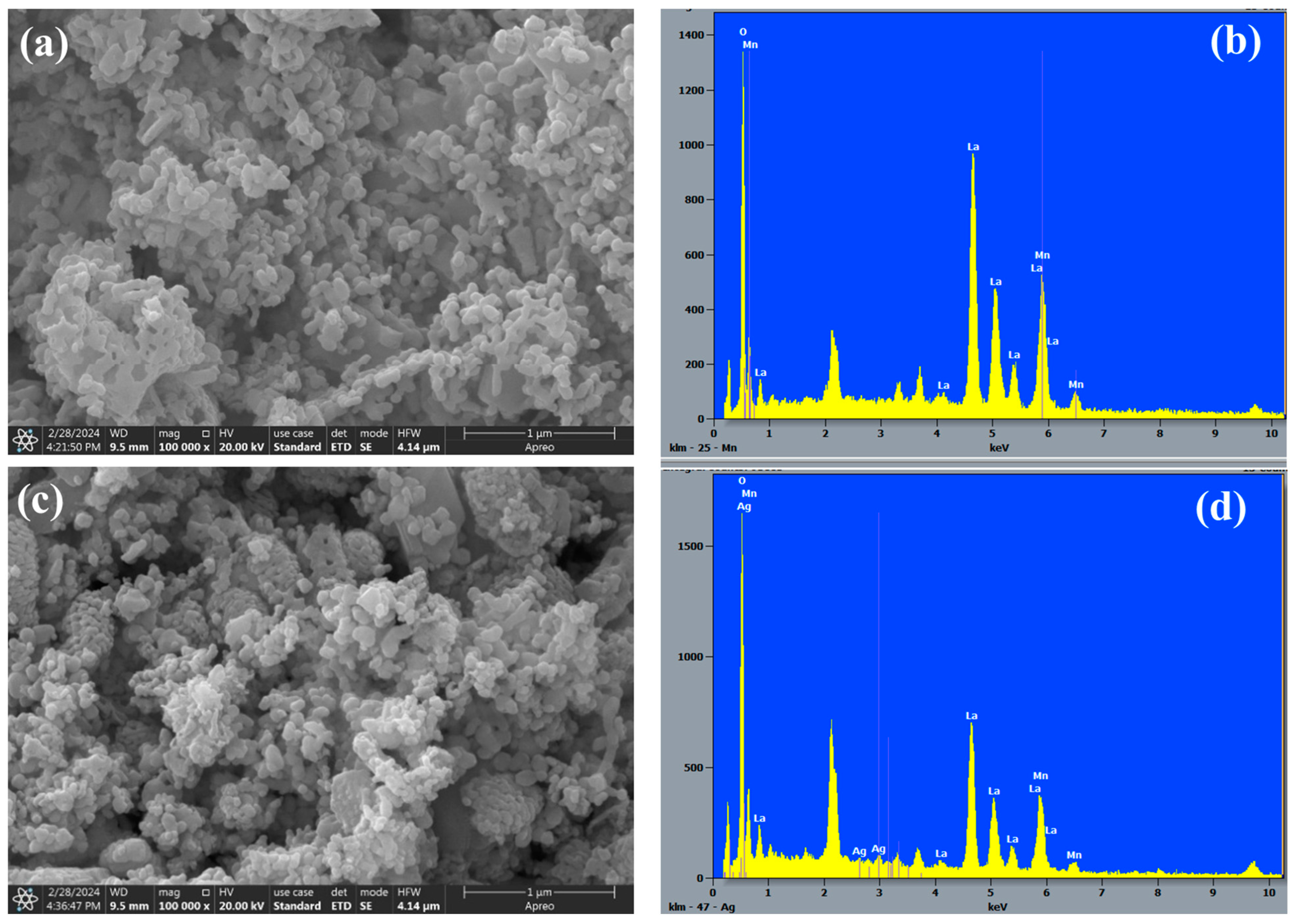
2.3. Morphological Analysis
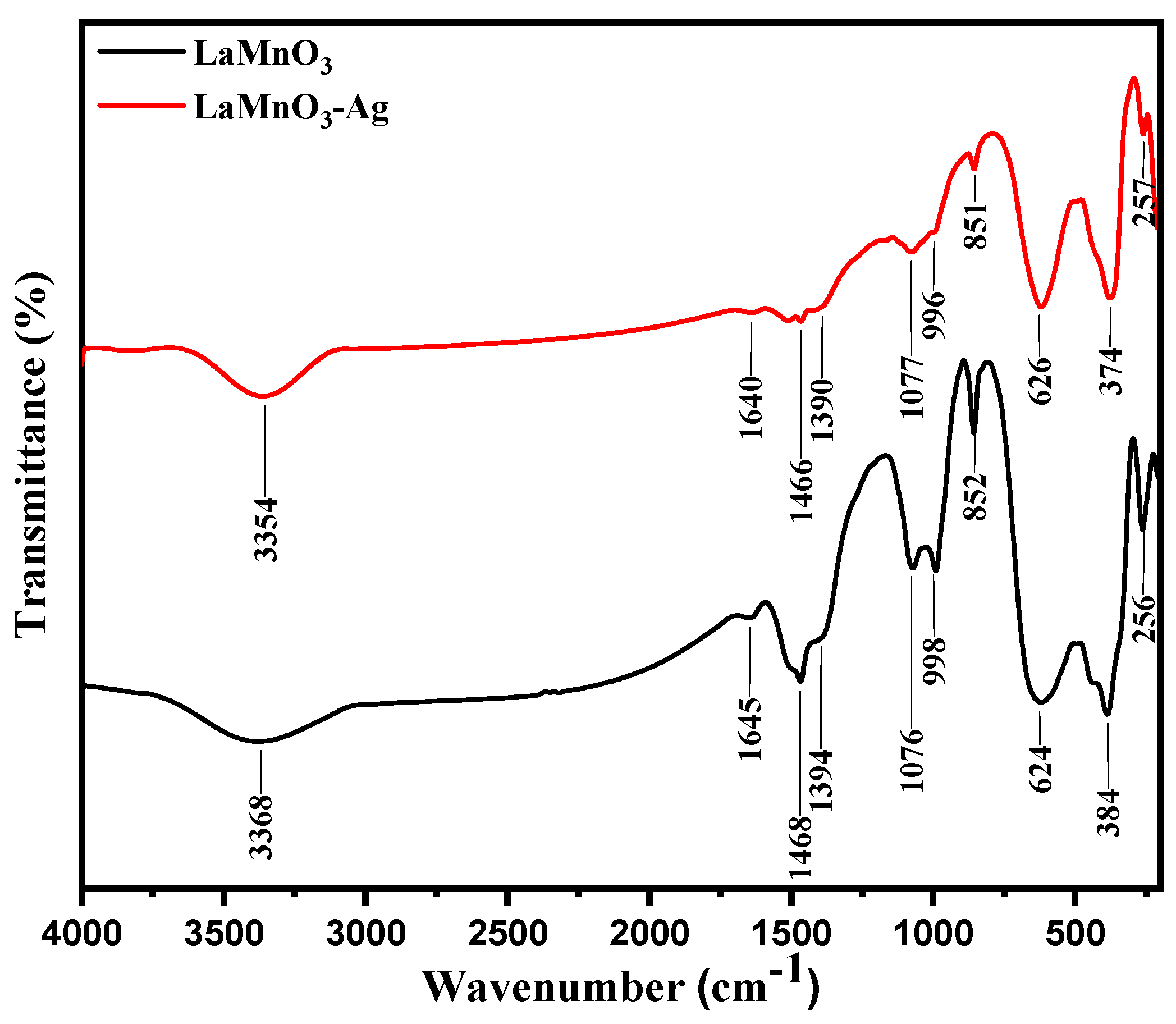
2.4. FT-IR Analysis
2.5. BET Surface Area
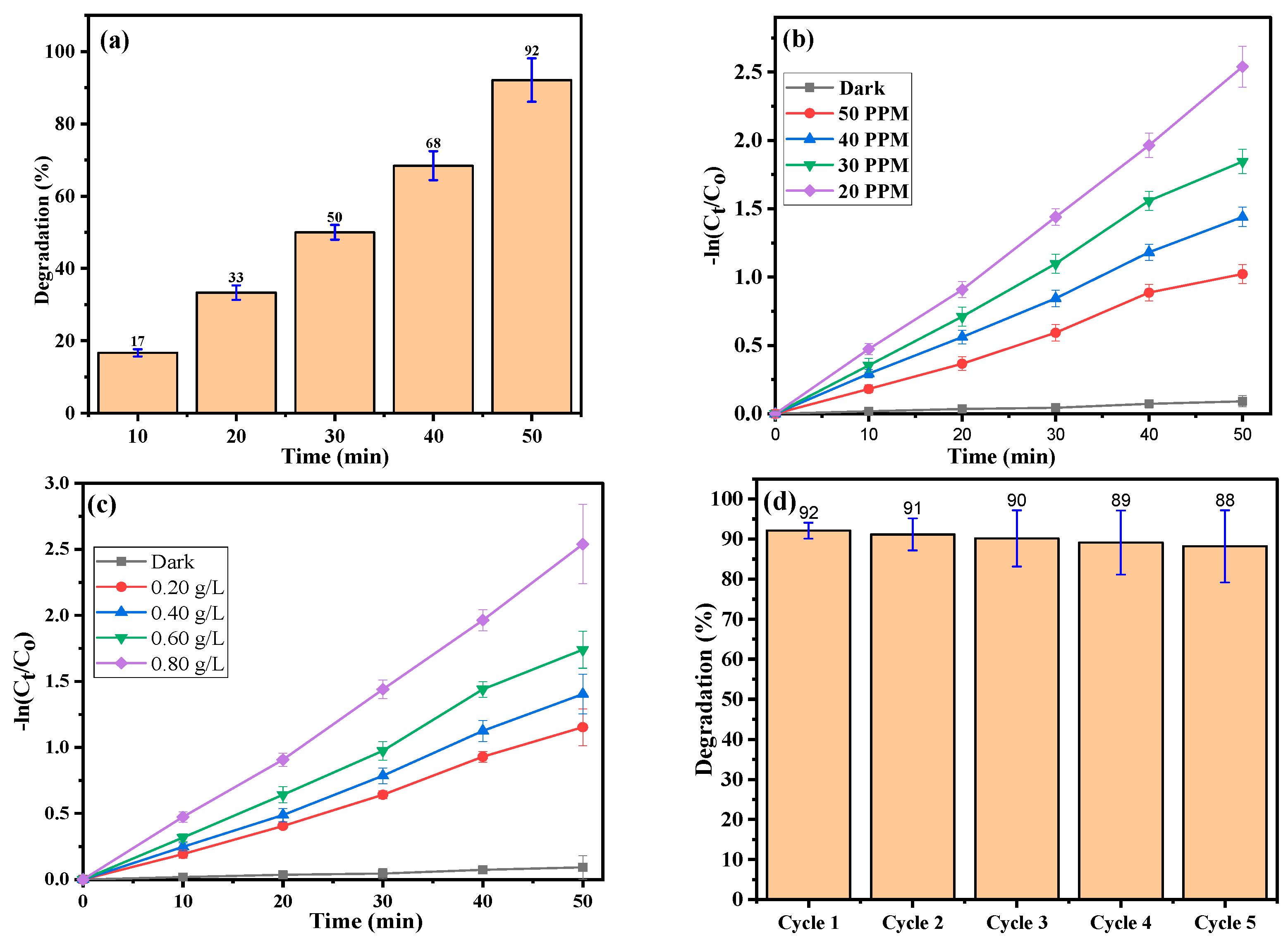
2.6. Photocatalytic Activity
2.7. Dye Concentration
2.8. Photocatalyst Different Doses
2.9. Stability and Reusability
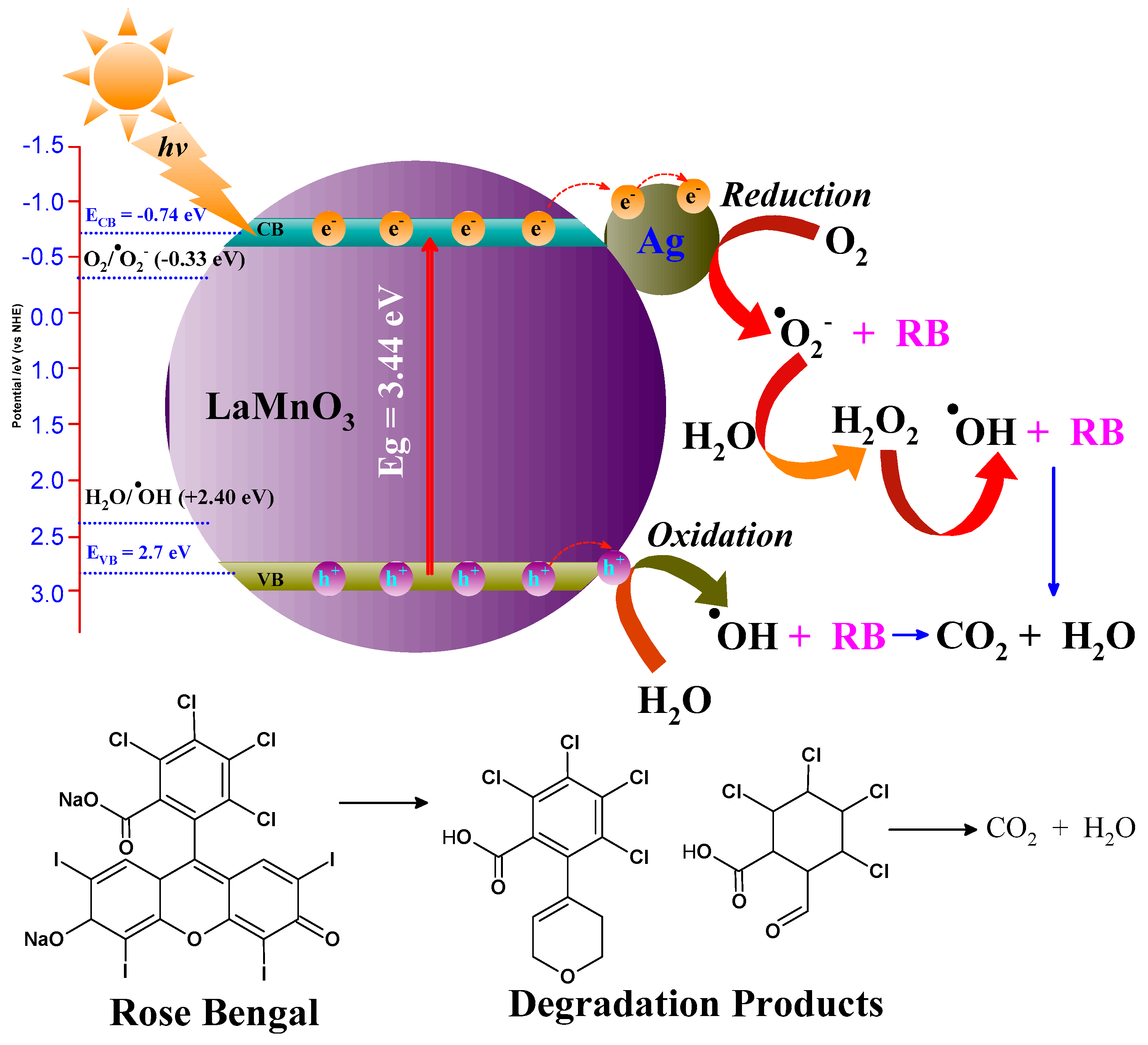
2.10. Mechanism of Dye
2.11. Antibacterial Activities against S. aureus (Gram-Positive) and E. coli (Gram-Negative) Bacteria
3. Methods
3.1. Materials
3.2. Synthesis of LaMnO3 and LaMnO3-Ag Nanocomposite
3.3. Characterization Techniques
3.4. Photocatalytic Application of LaMnO3-Ag Nanocomposites
3.5. Antibacterial Activities of LaMnO3 and LaMnO3-Ag Nanoparticles
3.6. Bacterial Sample Preparation and Antibacterial Study Using Colony Forming Unit (cfu)
3.7. LaMnO3-Ag Nanomaterial and Bacterial Surface Analysis Using Transmission Electron Microscopy (TEM), a Sample Preparation
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomatina, E.; Fukina, D.; Koryagin, A.; Titaev, D.; Suleimanov, E.; Smirnova, L. Preparation and photocatalytic properties of titanium dioxide modified with gold or silver nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106078. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Role of Ag(0) deposited on TiO2 nanoparticles for superior photocatalytic performance induced by calcination. Opt. Mater. 2019, 98, 109407. [Google Scholar] [CrossRef]
- Bak, T.; Li, W.; Nowotny, J.; Atanacio, A.J.; Davis, J. Photocatalytic properties of TiO2: Evidence of the key role of surface active sites in water oxidation. J. Phys. Chem. A 2015, 119, 9465–9473. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef]
- Salas, S.E.; Rosales, B.S.; de Lasa, H. Quantum yield with platinum modified TiO2 photocatalyst for hydrogen production. Appl. Catal. B Environ. 2013, 140, 523–536. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on nonmetal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140, 559–587. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Ebrahim, S.E. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environmental Nanotechnology. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor-based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Chen, P.; Cai, Y.; Wang, J.; Wang, K.; Tao, Y.; Xue, J.; Wang, H. Preparation of protonized titanate nanotubes/Fe3O4/TiO2 ternary composites and dye self-sensitization for visible-light-driven photodegradation of Rhodamine B. Powder Technol. 2018, 326, 272–280. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Wu, Z.; Fang, J.; Westerhoff, P.; Shang, C. Novel visible-light-driven photocatalytic chlorine activation process for carbamazepine degradation in drinking water. Environ. Sci. Technol. 2020, 54, 11584–11593. [Google Scholar] [CrossRef]
- Meneceur, S.; Hemmami, H.; Bouafia, A.; Laouini, S.E.; Tedjani, M.L.; Berra, D.; Mahboub, M.S. Photocatalytic activity of iron oxide nanoparticles synthesized by different plant extracts for the degradation of diazo dyes Evans blue and Congo red. Biomass Convers. Biorefinery 2024, 14, 5357–5372. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Souhaila, M.; Hasan, G.G.; Kir, I.; Abdullah, J.A.A. Green Synthesis of SnO2 Nanoparticles from Laurus nobilis L. Extract for Enhanced Gelatin-Based Films and CEF@SnO2 for Efficient Antibacterial Activity. Food Bioprocess Technol. 2024, 17, 1364–1382. [Google Scholar] [CrossRef]
- Salmi, C.; Souhaila, M.; Eddine, L.S.; Mohammed, H.A.M.; Hasan, G.G.; Mahboub, M.S. Biosynthesis of Mn3O4/PVP Nanocomposite for Enhanced Photocatalytic Degradation of Organic Dyes Under Sunlight Irradiation. J. Clust. Sci. 2024, 35, 201–215. [Google Scholar] [CrossRef]
- Rao, C.N.R. Perovskites. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 707–714. [Google Scholar]
- Irshad, M.; Ain, Q.T.; Zaman, M.; Aslam, M.Z.; Kousar, N.; Asim, M.; Rafique, M.; Siraj, K.; Tabish, A.N.; Usman, M. Photocatalysis and perovskite oxide-based materials: A remedy for a clean and sustainable future. RSC Adv. 2022, 12, 7009–7039. [Google Scholar] [CrossRef]
- Khalil, K.M.; Mahmoud, A.H.; Khairy, M. Formation and textural characterization of size-controlled LaFeO3 perovskite nanoparticles for efficient photocatalytic degradation of organic pollutants. Adv. Powder Technol. 2022, 33, 103429. [Google Scholar] [CrossRef]
- Das, N.; Kandimalla, S. Application of perovskites towards remediation of environmental pollutants: An overview: A review on remediation of environmental pollutants using perovskites. Int. J. Environ. Sci. Technol. 2017, 14, 1559–1572. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Vulchev, V.; Vassilev, L.; Harizanova, S.; Khristov, M.; Zhecheva, E.; Stoyanova, R. Improving the thermoelectric efficiency of LaCoO3 by double substitution with nickel and iron. J. Phys. Chem. C 2012, 116, 13507–13515. [Google Scholar] [CrossRef]
- Han, X.; Ni, H.; Zhao, K.; Xiang, W. Highly sensitive reversible light-driven switches based on LaSrAlO4 single crystals. Opt. Commun. 2011, 284, 5702–5704. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Choudhury, P.; Luo, H. Perovskite oxides as bifunctional oxygen electrocatalysts for oxygen evolution/reduction reactions–A mini-review. Appl. Mater. Today 2019, 16, 56–71. [Google Scholar] [CrossRef]
- Choi, J.-J.; Park, G.-T.; Kim, H.-E.; Kim, D.-Y. Transverse and longitudinal electrooptic properties of highly (100) oriented Pb(Zr, Ti)O3 films grown on glass substrates. Thin Solid Film. 2006, 515, 2437–2441. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, K.-C.; Tsai, D.-Y. A hybrid dielectric resonator antenna based upon novel complex perovskite microwave ceramic. Ceram. Int. 2013, 39, 8043–8048. [Google Scholar] [CrossRef]
- Hou, J.; Liu, H.; Zhao, L. Optimization of radiation absorption model in solar photocatalytic reactors: Coupling photocatalyst concentration distribution. Appl. Therm. Eng. 2019, 160, 114090. [Google Scholar] [CrossRef]
- Acosta-Herazo, R.; Mueses, M.A.; Puma, G.L.; Machuca-Martinez, F. Impact of photocatalyst optical properties on the efficiency of solar photocatalytic reactors rationalized by the concepts of initial rate of photon absorption (IRPA) dimensionless boundary layer of photon absorption and apparent optical thickness. Chem. Eng. J. 2019, 356, 839–849. [Google Scholar] [CrossRef]
- Jong, U.-G.; Yu, C.-J.; Kye, Y.-H. Computational prediction of structural, electronic, and optical properties and phase stability of double perovskites K2SnX6 (X = I, Br, Cl). RSC Adv. 2020, 10, 201–209. [Google Scholar] [CrossRef]
- Thinley, T.; Prabagar, J.S.; Yadav, S.; Anusha, H.S.; Anilkumar, K.M.; Kitirote, W.; Shahmoradi, B.; Shivaraju, H.P. LaNiO3-rGO perovskite interface for sustainable decontaminants of emerging concerns under visible light photocatalysis. J. Mol. Struct. 2023, 1285, 135413. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Men, J.; Zhang, L.; Huang, H. Ag modified LaMnO3 nanorods-reduced graphene oxide composite applied in the photocatalytic discoloration of direct green. Solid State Sci. 2016, 61, 239–245. [Google Scholar] [CrossRef]
- Kumar, R.D.; Thangappan, R.; Jayavel, R. Enhanced visible light photocatalytic activity of LaMnO3 nanostructures for water purification. Res. Chem. Intermed. 2018, 44, 4323–4337. [Google Scholar] [CrossRef]
- Kumar, S.R.; Abinaya, C.; Amirthapandian, S.; Ponpandian, N. Enhanced visible light photocatalytic activity of porous LaMnO3 sub-micron particles in the degradation of rose bengal. Mater. Res. Bull. 2017, 93, 270–281. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Manganese oxides/LaMnO3 perovskite materials and their application in the oxygen reduction reaction. Energy 2022, 247, 123456. [Google Scholar] [CrossRef]
- Luo, K.; Zheng, Q.; Yu, Y.; Wang, C.; Jiang, S.; Zhang, H.; Liu, Y.; Guo, Y. Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery. Batteries 2023, 9, 90. [Google Scholar] [CrossRef]
- Song, L.; Zhu, Y.; Yang, Z.; Wang, C.; Lu, X. Oxidase-mimicking activity of perovskite LaMnO3+ δ nanofibers and their application for colorimetric sensing. J. Mater. Chem. B 2018, 6, 5931–5939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Li, X.; Zhu, Z.; Wang, Q.; Luo, S.; Xie, A. Honeycomb LaMnO3 perovskite synthesized by a carbon sphere as a self-sacrificing template for supercapacitors. Energy Fuels 2021, 35, 13457–13465. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M= Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Rao, K.N.; Malik, M.A. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials 2022, 12, 2985. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Guo, Y.; Lu, G.; Boreave, A.; Retailleau, L.; Baylet, A.; Giroir-Fendler, A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl. Catal. B Environ. 2014, 148, 490–498. [Google Scholar] [CrossRef]
- Priyatharshni, S.; Kumar, S.R.; Viswanathan, C.; Ponpandian, N. Morphologically tuned LaMnO3 as an efficient nanocatalyst for the removal of organic dye from aqueous solution under sunlight. J. Environ. Chem. Eng. 2020, 8, 104146. [Google Scholar] [CrossRef]
- Karunakaran, G.; Sudha, K.G.; Ali, S.; Cho, E.-B. Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules 2023, 28, 4527. [Google Scholar] [CrossRef]
- Okwu, D.E.; Awurum, A.N.; Okoronkwo, J.I. Phytochemical composition and in vitro antifungal activity screening of extracts from citrus plants against Fusarium oxysporum of okra plant (Hibiscus esculentus). Pest Technol. 2007, 1, 145–148. [Google Scholar]
- John, S.; Monica, S.J.; Priyadarshini, S.; Arumugam, P. Investigation on phytochemical profile of Citrus limonum peel extract. Int. J. Food Sci. Nutr. 2017, 2, 65–67. [Google Scholar]
- Arabi, A.; Fazli, M.; Ehsani, M. Synthesis and characterization of calcium-doped lanthanum manganite nanowires as a photocatalyst for degradation of methylene blue solution under visible light irradiation. Bull. Mater. Sci. 2018, 41, 77. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Alzahrani, E.A.; Nabi, A.; Kamli, M.R.; Albukhari, S.M.; Althabaiti, S.A.; Al-Harbi, S.A.; Khan, I.; Malik, M.A. Facile Green Synthesis of ZnO NPs and Plasmonic Ag-Supported ZnO Nanocomposite for Photocatalytic Degradation of Methylene Blue. Water 2023, 15, 384. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
- Susanti, Y.D.; Afifah, N.; Saleh, R. Ag modified LaMnO3 nanoparticles for methylene blue degradation via photosonocatalytic activities. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012037. [Google Scholar] [CrossRef]
- Saha, P.; Mahiuddin, M.; Islam, A.N.; Ochiai, B. Biogenic synthesis and catalytic efficacy of silver nanoparticles based on peel extracts of citrus macroptera fruit. ACS Omega 2021, 6, 18260–18268. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Men, J.; Liu, Y.; Huang, H.; Jiao, T. One-pot synthesis of Ag-modified LaMnO3–graphene hybrid photocatalysts and application in the photocatalytic discoloration of an azo-dye. RSC Adv. 2015, 5, 54028–54036. [Google Scholar] [CrossRef]
- Farhadi, S.; Mahmoudi, F.; Amini, M.M.; Dusek, M.; Jarosova, M. Synthesis and characterization of a series of novel perovskite-type LaMnO3/Keggin-type polyoxometalate hybrid nanomaterials for fast and selective removal of cationic dyes from aqueous solutions. Dalton Trans. 2017, 46, 3252–3264. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Ivanovici, M.-G.; Poienar, M.; Ianasi, C.; Vlazan, P. Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds. Processes 2022, 10, 2688. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jatawa, S.K.; Tiwari, A. Phytochemical analysis of Citrus limonum pulp and peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 369–371. [Google Scholar]
- Venkatesh, N.; Murugadoss, G.; Mohamed, A.A.A.; Kumar, M.R.; Peera, S.G.; Sakthivel, P. A Novel Nanocomposite Based on Triazine Based Covalent Organic Polymer Blended with Porous g-C3N4 for Photo Catalytic Dye Degradation of Rose Bengal and Fast Green. Molecules 2022, 27, 7168. [Google Scholar] [CrossRef] [PubMed]
- Bamola, P.; Sharma, M.; Dwivedi, C.; Singh, B.; Ramakrishna, S.; Dalapati, G.K.; Sharma, H. Interfacial interaction of plasmonic nanoparticles (Ag, Au) decorated floweret TiO2 nanorod hybrids for enhanced visible light driven photocatalytic activity. Mater. Sci. Eng. B 2021, 273, 115403. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Jiang, X.; Luan, Z.-Y.; Guo, J. Rational Design and Synthesis of Fe-Doped Co-Based Coordination Polymer Composite Photocatalysts for the Degradation of Norfloxacin and Ciprofloxacin. Inorg. Chem. 2024, 63, 6514–6525. [Google Scholar]
- Shaterian, M.; Enhessari, M.; Rabbani, D.; Asghari, M.; Salavati-Niasari, M. Synthesis, characterization and photocatalytic activity of LaMnO3 nanoparticles. Appl. Surf. Sci. 2014, 318, 213–217. [Google Scholar] [CrossRef]
- Tran, T.H.; Phi, T.H.; Nguyen, H.N.; Pham, N.H.; Nguyen, C.V.; Ho, K.H.; Doan, Q.K.; Le, V.Q.; Nguyen, T.T.; Nguyen, V.T. Sr doped LaMnO3 nanoparticles prepared by microwave combustion method: A recyclable visible light photocatalyst. Results Phys. 2020, 19, 103417. [Google Scholar] [CrossRef]
- Nayak, H.; Padhi, B. Degradation of methylene blue using Ca-doped LaMnO3 as a photocatalyst under visible light irradiation. Results Chem. 2023, 6, 101104. [Google Scholar] [CrossRef]
- van Veen, H.W.; Abee, T.; Kortstee, G.J.; Konings, W.N.; Zehnder, A.J. Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 1994, 33, 1766–1770. [Google Scholar] [CrossRef]
- Persy, V.P.; Behets, G.J.; Bervoets, A.R.; De Broe, M.E.; D’Haese, P.C. Lanthanum: A Safe Phosphate Binder. Semin. Dial. 2006, 19, 195–199. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Shi, W.; He, J.; Feng, H.; Xu, Y.; Cui, F.; Wang, C. Carbon nanofiber matrix with embedded LaCO3OH synchronously capture phosphate and organic carbon to starve bacteria. J. Mater. Chem. A 2016, 4, 12799–12806. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Dai, S.; Cui, F. Synchronous, efficient and fast removal of phosphate and organic matter by carbon-coated lanthanum nanorods. RSC Adv. 2018, 8, 11754–11763. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Shi, W.; Cui, F. La2O3 nanoparticle/polyacrylonitrile nanofibers for bacterial inactivation based on phosphate control. RSC Adv. 2016, 6, 99353–99360. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg-Al–La ternary (hydro)oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Alamier, W.M.; Hasan, N.; Syed, I.S.; Bakry, A.M.; Ismail, K.S.; Gedda, G.; Girma, W.M. Silver Nanoparticles’ Biogenic Synthesis Using Caralluma subulata Aqueous Extract and Application for Dye Degradation and Antimicrobials Activities. Catalysts 2023, 13, 1290. [Google Scholar] [CrossRef]
- Alamier, W.M.; Oteef, M.D.Y.; Bakry, A.M.; Hasan, N.; Ismail, K.S.; Awad, F.S. Green Synthesis of Silver Nanoparticles Using Acacia ehrenbergiana Plant Cortex Extract for Efficient Removal of Rhodamine B Cationic Dye from Wastewater and the Evaluation of Antimicrobial Activity. ACS Omega 2023, 8, 18901–18914. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial, Anti-Candida, Acaricides, and Photocatalytic Activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Boutalbi, A.; Meneceur, S.; Laouini, S.E.; Mohammed, H.A.M.; Hasan, G.G.; Bouafia, A. Synthesis of Ag nanoparticles loaded with potassium polyacrylate hydrogel for rose bengal dye removal and antibacterial activity. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed]

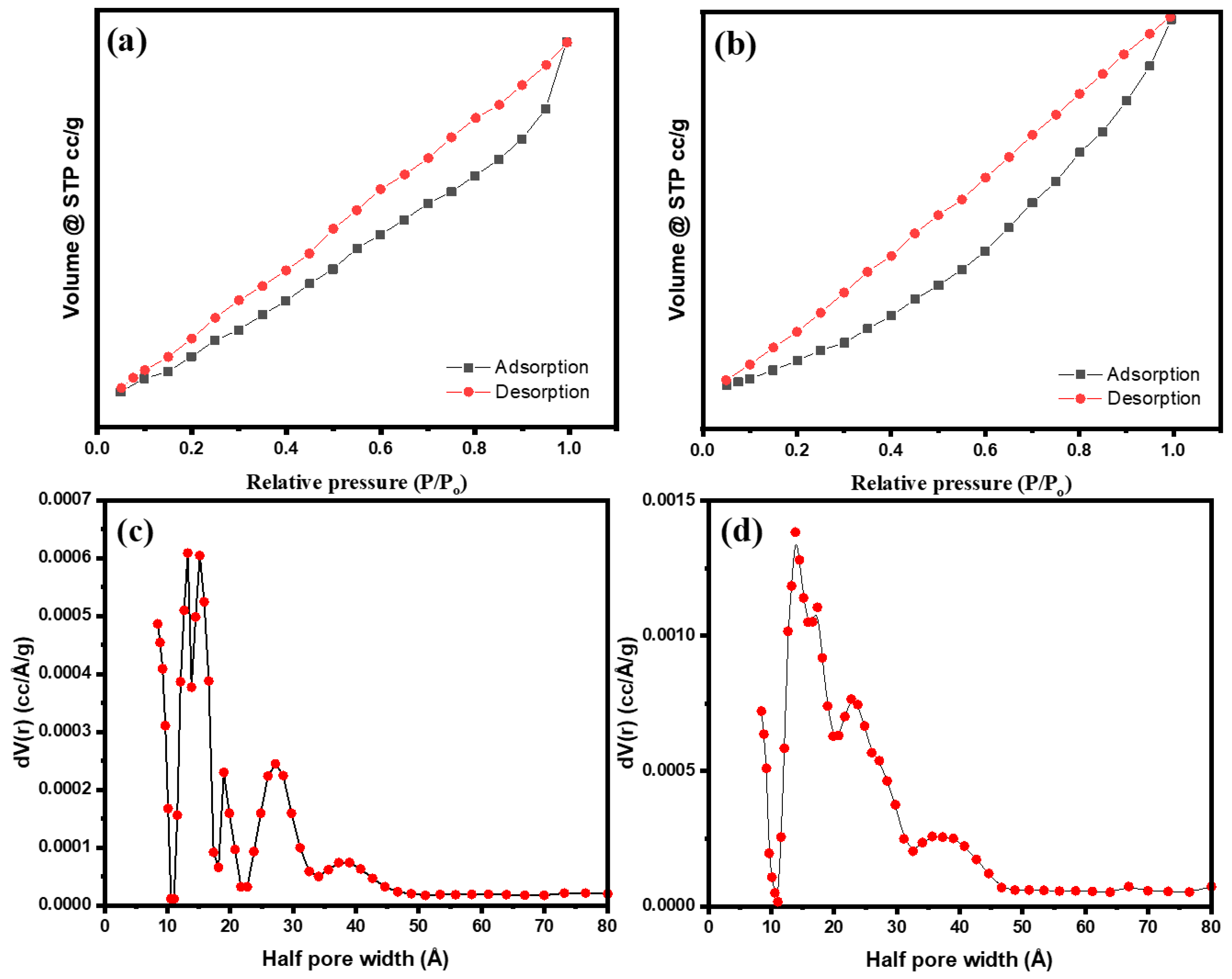



| Sample | BET Surface Area (m2/g) | Pore Volume (cc/g) | Average Pore Size (nm) |
|---|---|---|---|
| LaMnO3 | 12.642 | 0.012 | 1.26 |
| LaMnO3-Ag | 16.209 | 0.023 | 1.38 |
| Initial Dye Concentration (ppm) | Rate Constant k (min−1) | R2 | Catalyst Dosage (mg) | Rate Constant k (min−1) | R2 |
|---|---|---|---|---|---|
| 20 | 0.05057 | 0.997 | 10 | 0.02346 | 0.995 |
| 30 | 0.0378 | 0.996 | 20 | 0.02843 | 0.995 |
| 40 | 0.02899 | 0.998 | 30 | 0.03544 | 0.995 |
| 50 | 0.02129 | 0.990 | 40 | 0.05057 | 0.995 |
| S. No | Perovskite Type and Synthesis Routes | Type of Dye | Efficiency of Method (g/ppm/min) * | Dye Degradation (%) | Dark or Light | References |
|---|---|---|---|---|---|---|
| 1 | LaMnO3–graphene-Ag (Sol-gel) | Direct Green BE | 0.02/20/120 | 97.8 | Light irradiation | [53] |
| 2 | LaMnO3 (sol-gel) | Methyl orange | 0.12/12/80 | 96.0 | Dark | [55] |
| 3 | LaMnO3 (sol-gel) | Methyl orange | 0.06/6/90 | 98.0 | Visible light irradiation | [60] |
| 4 | LaMnO3-Sr (microwave) | Methylene blue | 0.20/10/120 | 96.0 | Light irradiation | [61] |
| 5 | LaMnO3-Ca (sol-gel) | Methylene blue | 0.05/5/100 | 68.5 | Light irradiation | [62] |
| 6 | LaMnO3-Ag (green-synthesis) | Rose Bengal | 0.8/20/50 | 92.0 | Light irradiation | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, N. Green Synthesis of LaMnO3-Ag Nanocomposites Using Citrus limon (L.) Burm Peel Aqueous Extract: Photocatalytic Degradation of Rose Bengal Dye and Antibacterial Applications. Catalysts 2024, 14, 609. https://doi.org/10.3390/catal14090609
Hasan N. Green Synthesis of LaMnO3-Ag Nanocomposites Using Citrus limon (L.) Burm Peel Aqueous Extract: Photocatalytic Degradation of Rose Bengal Dye and Antibacterial Applications. Catalysts. 2024; 14(9):609. https://doi.org/10.3390/catal14090609
Chicago/Turabian StyleHasan, Nazim. 2024. "Green Synthesis of LaMnO3-Ag Nanocomposites Using Citrus limon (L.) Burm Peel Aqueous Extract: Photocatalytic Degradation of Rose Bengal Dye and Antibacterial Applications" Catalysts 14, no. 9: 609. https://doi.org/10.3390/catal14090609








