Exploring Enhanced Oxygen Reduction Reactions: A Study on Nanocellulose, Dopamine, and Cobalt Complex-Derived Non-Precious Electrocatalyst
Abstract
:1. Introduction
| Anode (HOR): | 2H2 → 2H+ + 4e− | E° = 0.00 V vs. SHE |
| Cathode (ORR): | O2 + 4H+ + 4e− → 2H2O | E° = 1.23 V vs. SHE |
| Overall reaction: | 2H2 + O2 → 2H2O | SHE = Standard Hydrogen Electrode |
2. Results and Discussions
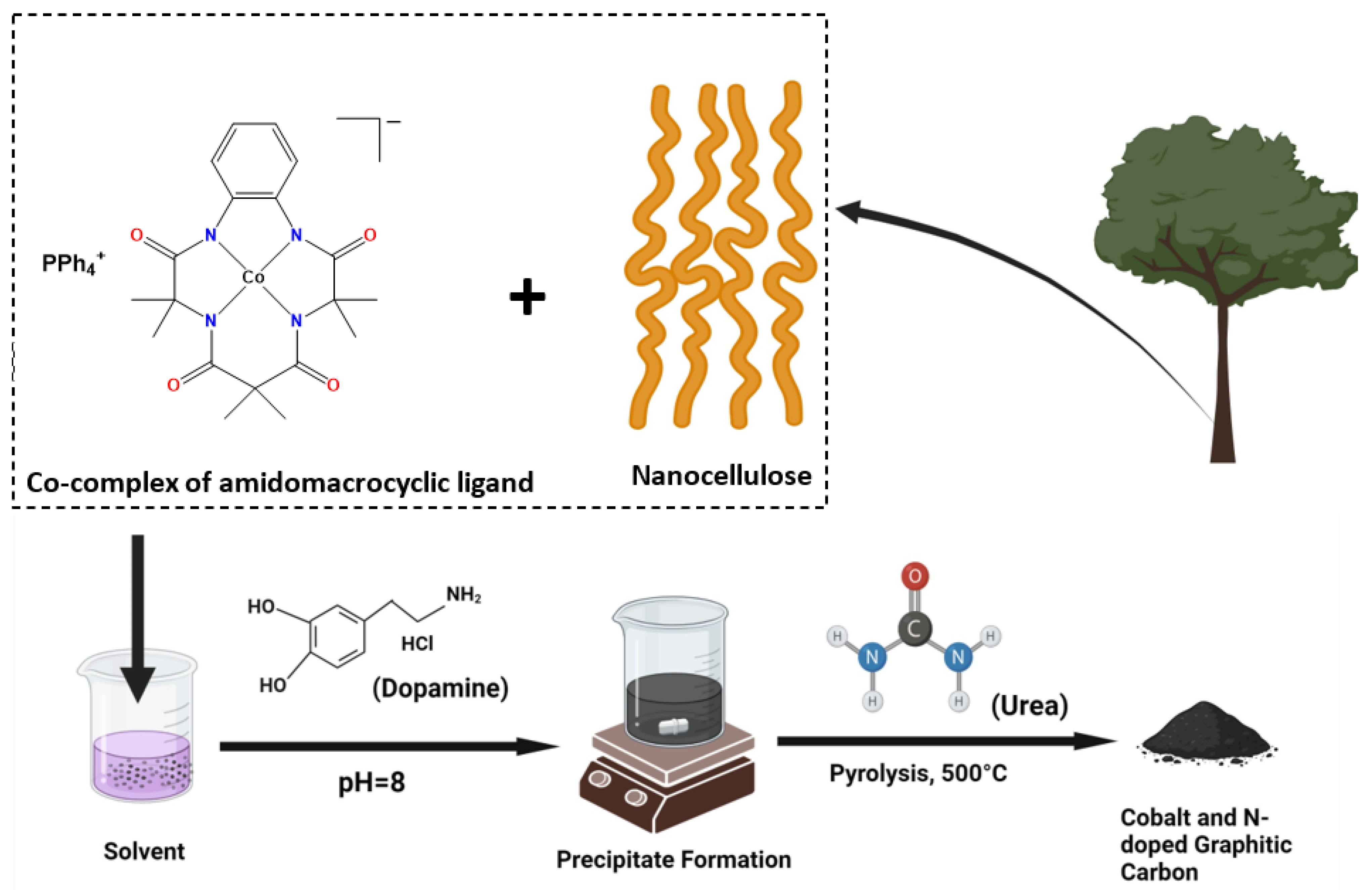
2.1. Synthesis
2.2. Characterizations
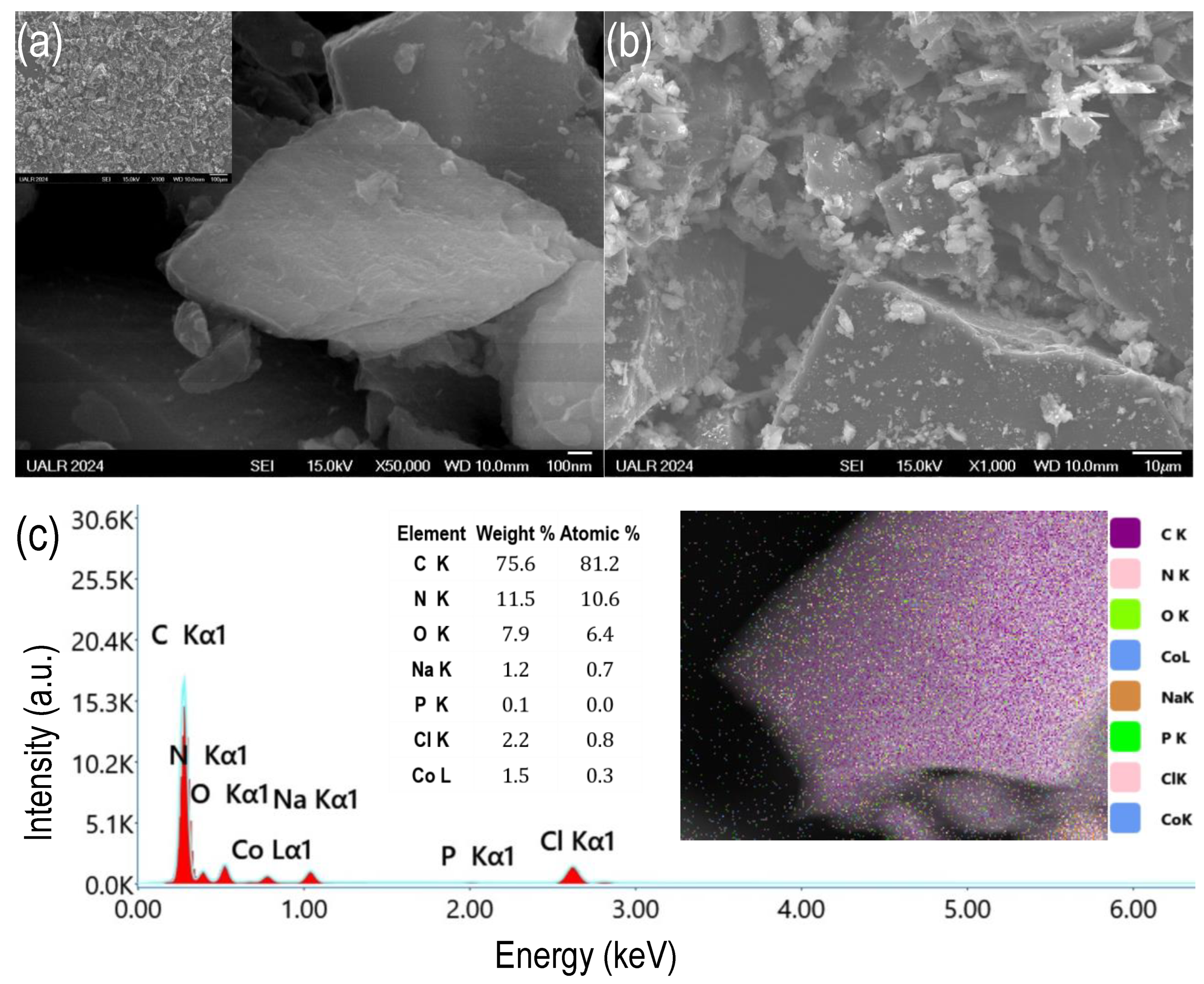
2.2.1. SEM and EDS
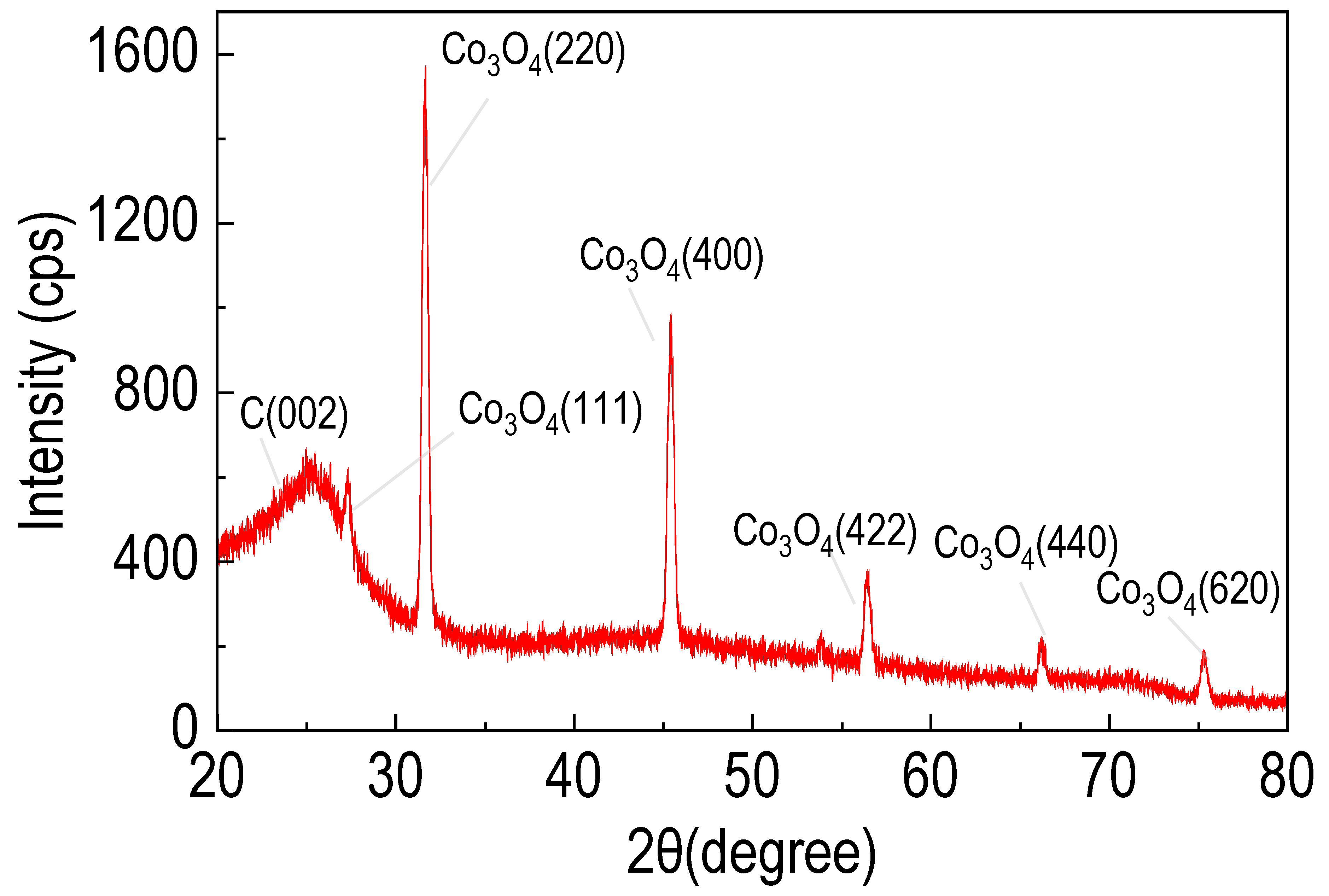
2.2.2. XRD
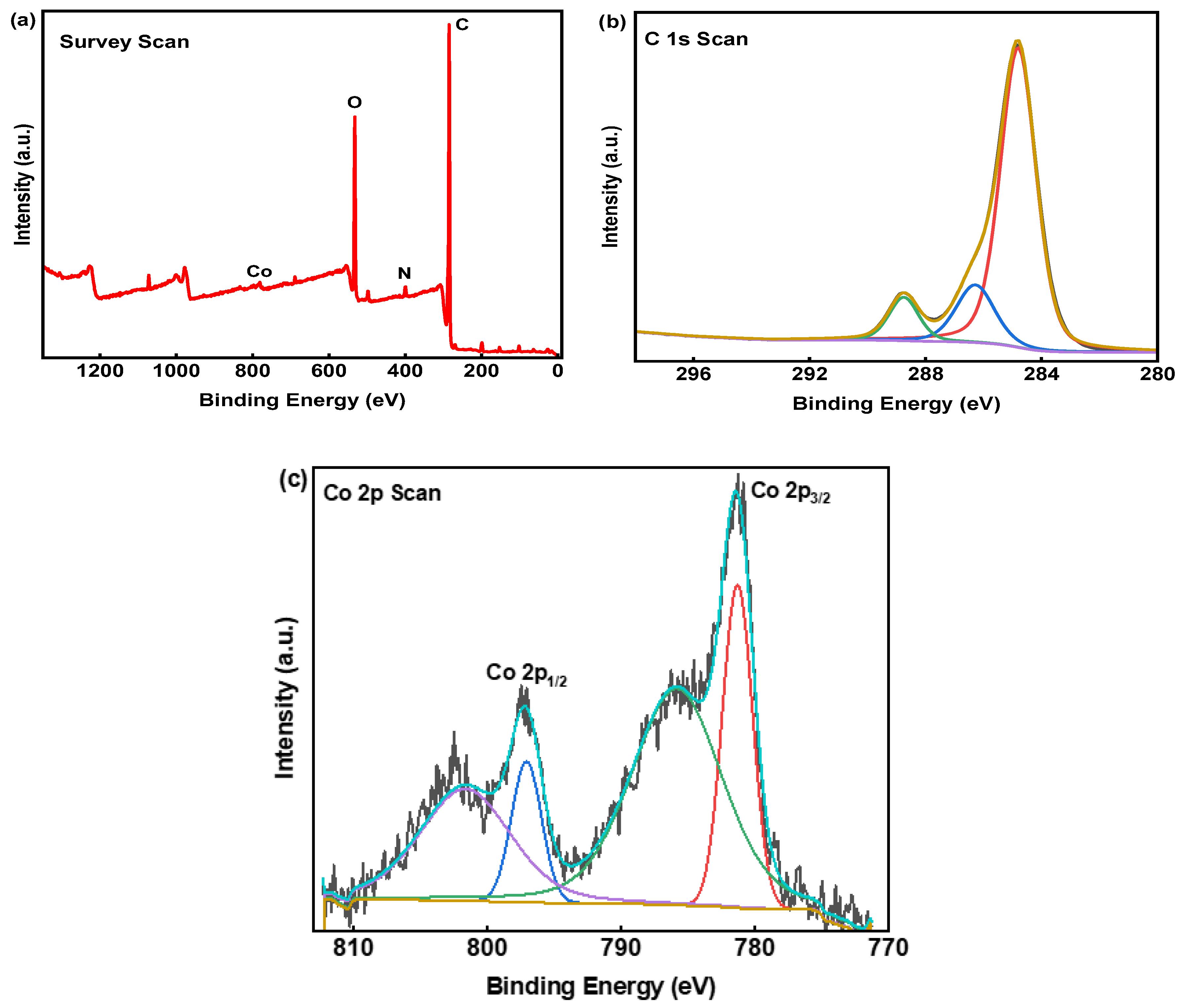
2.2.3. XPS Study
2.3. Cyclic Voltammetry (CV)
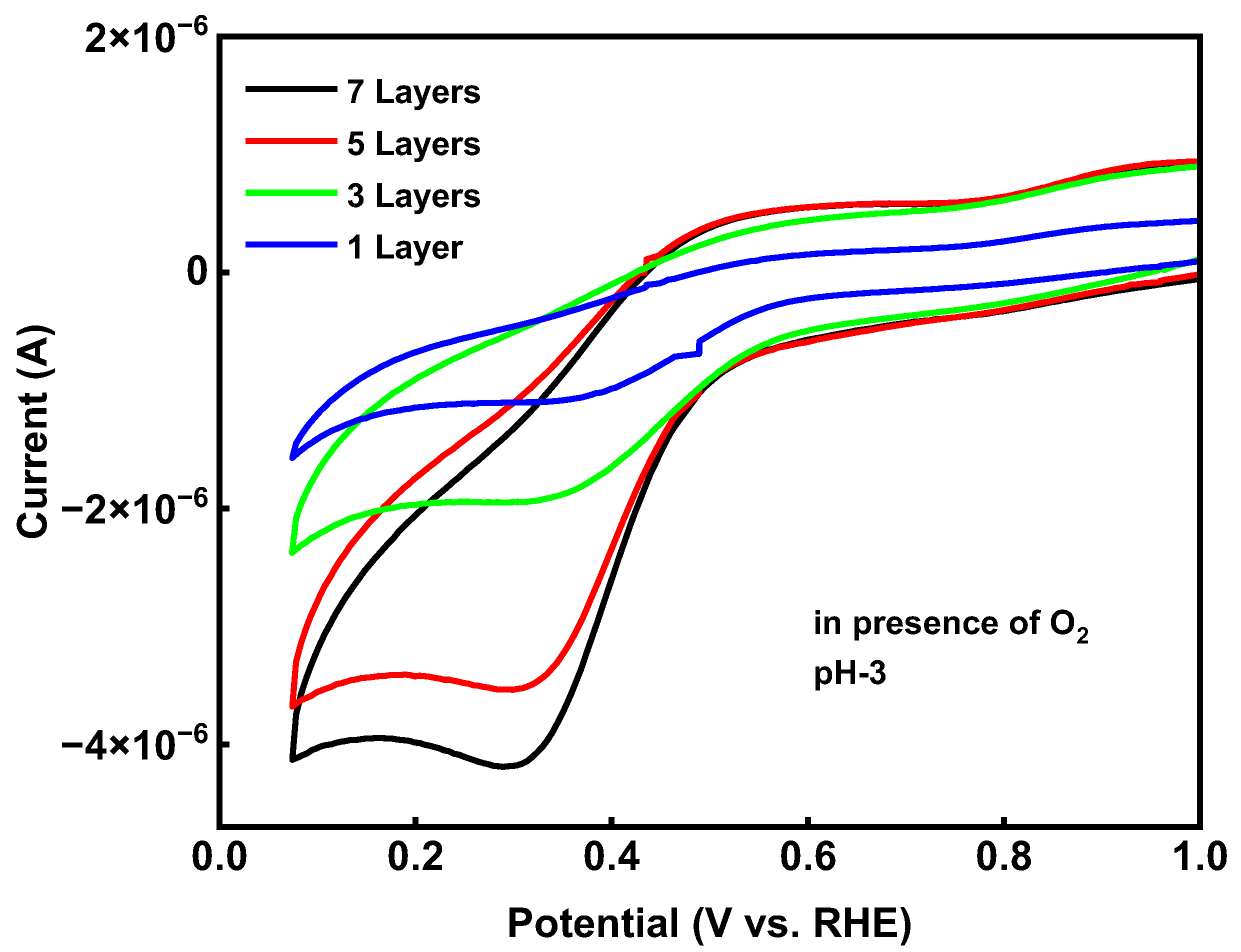
2.3.1. Electrocatalyst Layer Studies
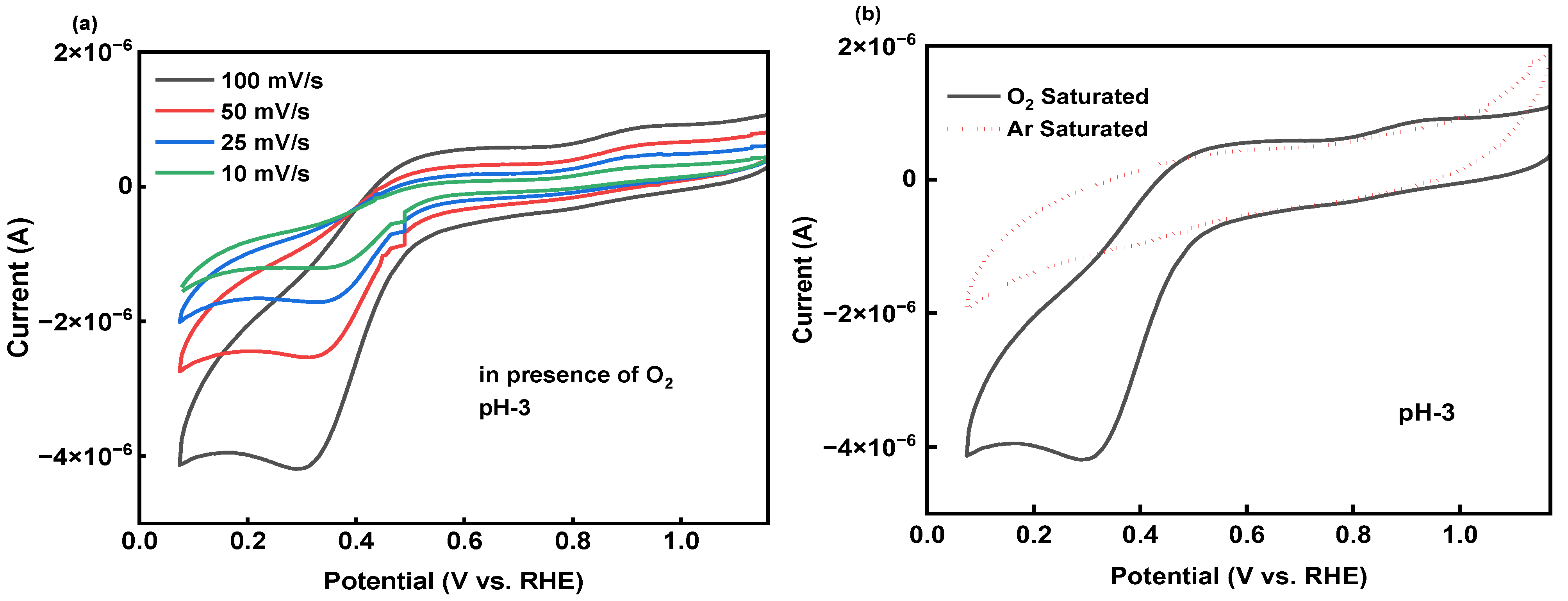
2.3.2. Electrocatalyst pH and Stability Studies
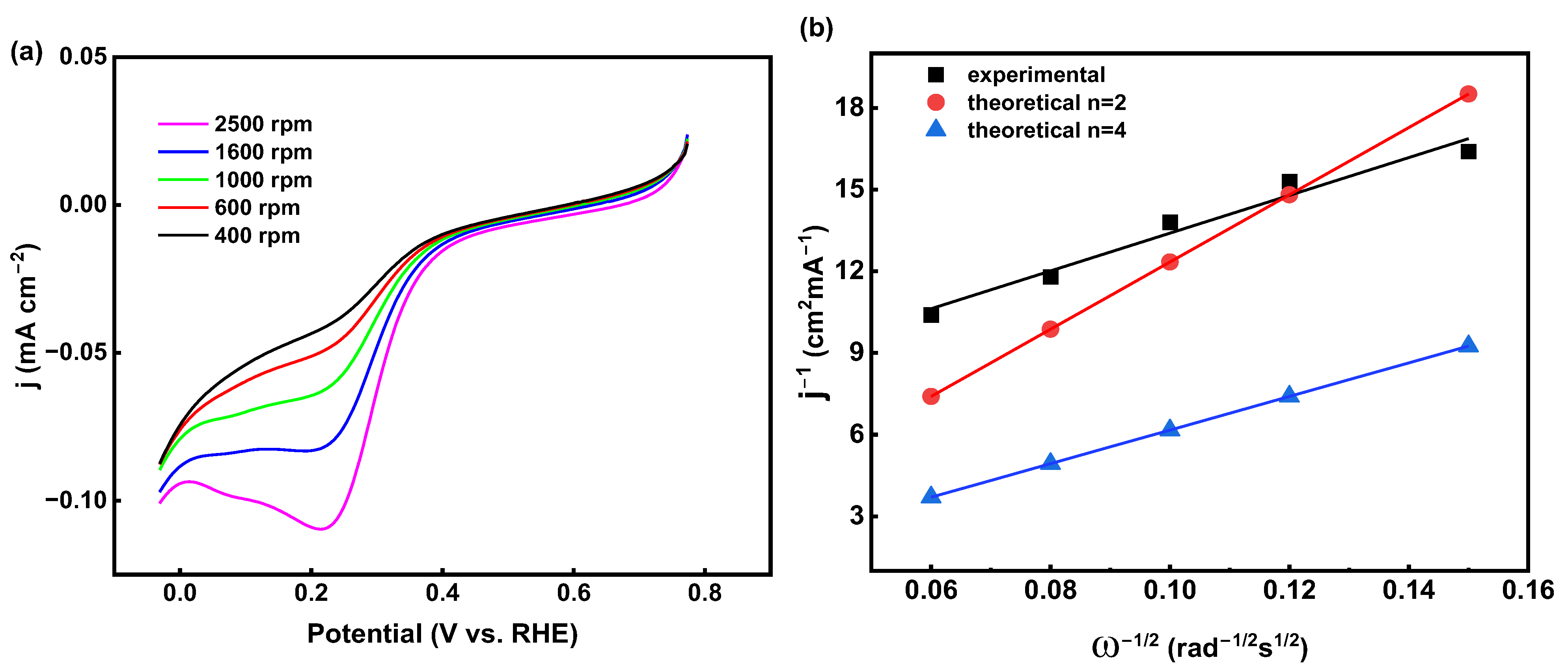
2.3.3. Rotating Disk Electrode (RDE)
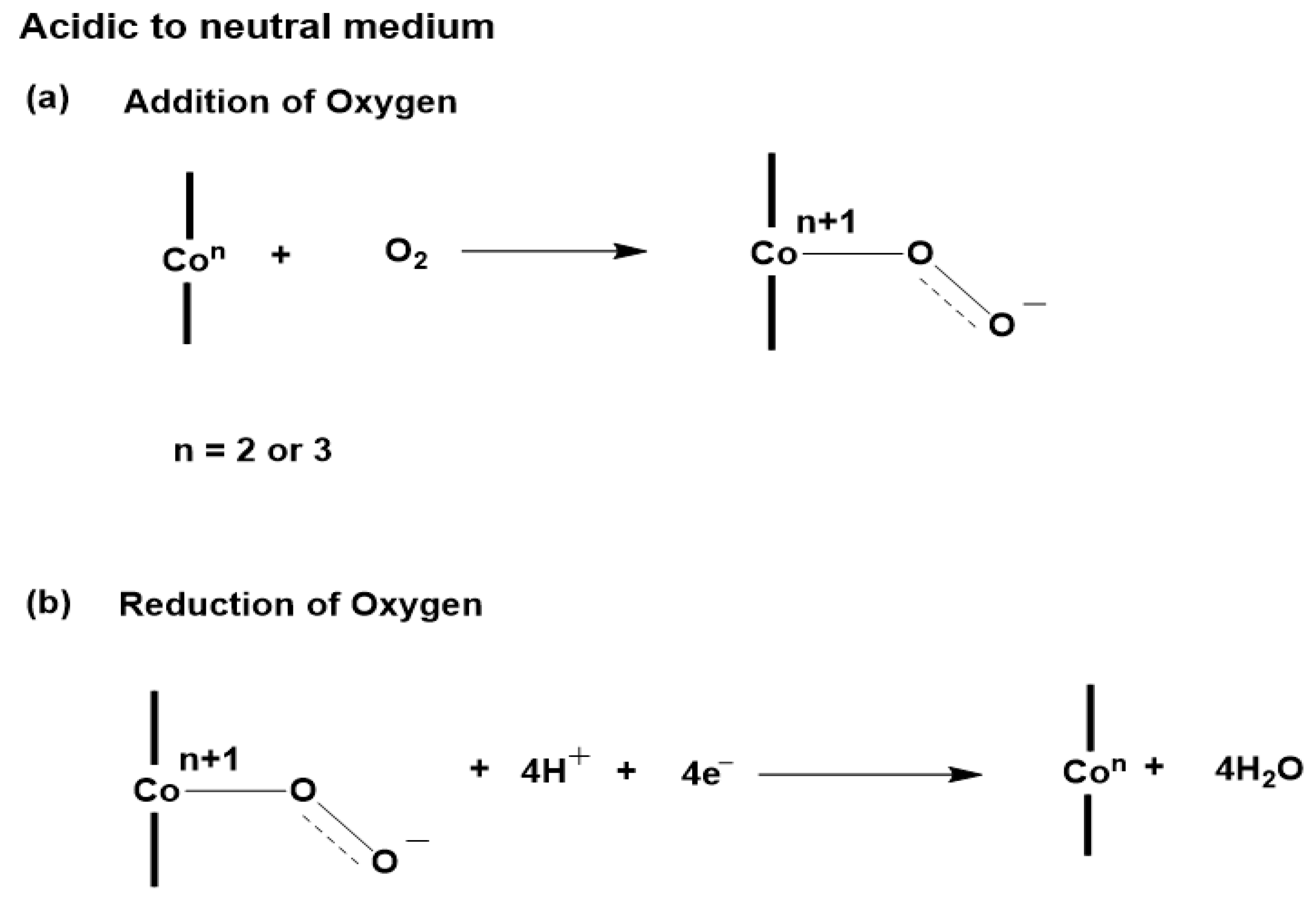
2.4. Proposed Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Cobalt-Doped Carbonaceous Material
3.3. Electrochemical Studies
3.4. Electrode Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhetri, B.; Parnell, C.; Wayland, H.; Rangumagar, A.; Kannarpady, G.; Watanabe, F.; Albkuri, Y.; Biris, A. Chitosan-Derived NiO-Mn2O3/C Nanocarbonaceouss as Non-Precious Catalysts for Enhanced Oxygen Reduction Reaction. Chem. Sel. 2018, 3, 922–932. [Google Scholar] [CrossRef]
- Parnell, C.; Chhetri, B.; Brandt, A.; Watanabe, F.; Alsudani, Z.; Mudalige, T.; Biris, A. Polydopamine-Coated Manganese Complex/Graphene Nanocarbonaceous for Enhanced Electrocatalytic Activity Towards Oxygen Reduction. Sci. Rep. 2016, 6, 31415. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Proietti, E.; Lefevre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuelcells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Parnell, C.M.; Chhetri, B.P.; Mitchell, T.B.; Watanabe, F.; Kannarpady, G.; RanguMagar, A.B.; Zhou, H.; Alghazali, K.M.; Biris, A.S.; Ghosh, A. Simultaneous Electrochemical Deposition of Cobalt Complex and Poly(pyrrole) Thin Films for Supercapacitor Electrodes. Sci. Rep. 2019, 9, 5650. [Google Scholar] [CrossRef]
- Dicks, A.L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141. [Google Scholar] [CrossRef]
- Wang, D.; Haque, S.; Williams, T.; Karim, F.; Hariharalakshmanan, R.K.; Al-Mayalee, K.H.; Karabacak, T. Cyclic voltammetry and specific capacitance studies of copper oxide nanostructures grown by hot water treatment. MRS Adv. 2023, 9, 979–985. [Google Scholar] [CrossRef]
- Haque, S.; Wang, D.; Ergul, B.; Basurrah, A.O.M.; Karabacak, T. Effect of sandblasting and acid surface pretreatment on the specific capacitance of CuO nanostructures grown by hot water treatment for supercapacitor electrode applications. Nanotechnology 2024, 35, 335403. [Google Scholar] [CrossRef]
- Ning, F.; He, X.; Shen, Y.; Jin, H.; Li, Q.; Li, D.; Li, S.; Zhan, Y.; Du, Y.; Jiang, J.; et al. Flexible and Lightweight Fuel Cell with High Specific Power Density. ACS Nano 2017, 11, 5982–5991. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Basile, A.; Ghasemzadeh, K. Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; Available online: https://www.sciencedirect.com/book/9780128241035 (accessed on 5 December 2023).
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Liu, B.; Dong, Q.; Xu, G.; Zhang, L.; Zhang, J. Novel recyclable dual-heterostructured Fe3O4@CeO2/M (M = Pt, Pd and Pt–Pd) catalysts: Synergetic and redox effects for superior catalytic performance. J. Mater. Chem. A 2015, 3, 139–147. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Thorum, M.S. Electrochemical CO2 reduction catalyzed by a cobalt salophen complex. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef]
- Tollefson, J. Climate change: The Earth is warming faster than expected. Nat. News 2010, 464, 1262–1264. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Qiu, C.; Ling, X.; Tian, B.; Chen, W.; Su, C. B, N Codoped and Defect-Rich Nanocarbon Material as a Metal-Free Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Adv. Sci. 2018, 5, 1800036. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Kong, J.; Ismail, S.S.; Lu, X. Integration of inorganic nanostructures with polydopamine-derived carbon: Tunable morphologies and versatile applications. Nanoscale 2016, 8, 1770–1788. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Van Der Westen, R.; Postma, A.; Städler, B. Polydopamine-A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, J.; Liu, M.; Huang, H.; Zhang, X.; Wei, Y. Polydopamine-Based functional materials and their applications in energy, environmental, and catalytic fields: State-of-the-art review. Chem. Eng. J. 2020, 387, 124019. [Google Scholar] [CrossRef]
- Qu, K.; Wang, Y.; Vasileff, A.; Jiao, Y.; Chen, H.; Zheng, Y. Polydopamine-inspired nanomaterials for energy conversion and storage. J. Mater. Chem. A 2018, 6, 21827–21846. [Google Scholar] [CrossRef]
- Martínez-Huerta, M.V.; Lázaro, M.J. Nanocatalysts for low temperature fuel cells. Energy Procedia 2017, 138, 14–19. [Google Scholar] [CrossRef]
- Lim, S.H.; Li, Z.; Poh, C.K.; Lai, L.; Lin, J. Highly active non-precious metal catalyst based on Poly (Vinylpyrrolidone)-wrapped carbon nanotubes complexed with iron-cobalt metal ions for oxygen reduction reaction. J. Power Sources 2012, 214, 15–20. [Google Scholar] [CrossRef]
- Abrham, G.G.; Martínez-Huerta, M.V.; Sebastián, D.; Lázaro, M.J. Transformation of CoFe2O4 spinel structure into active and robust CoFe Alloy/N-doped carbon electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2022, 625, 70–82. [Google Scholar] [CrossRef]
- Rangumagar, A.; Chhetri, B.; Parnell, C.; ParameswaranThankam, A.; Watanabe, F.; Mustafa, T.; Biris, A. Removal of nitrophenols from water using cellulose derived nitrogen doped graphitic carbon material containing titanium dioxide. Part. Sci. Technol. 2019, 37, 444–452. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Rangumagar, A.; Chhetri, B.; ParameswaranThankam, A.; Watanabe, F.; Sinha, A.; Kim, J.-W.; Saini, V.; Biris, A. Nanocrystalline Cellulose-Derived Doped Carbonaceous Material for Rapid Mineralization of Nitrophenols under Visible Light. ACS Omega 2018, 3, 8111–8121. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; He, Y.; Kuhn, A.; Shih, P.-C.; Sun, C.-J.; Wen, X.-D.; Shi, C.; Yang, H. Cobalt-Based Non-Precious Metal Catalysts Derived from Metal–Organic Frameworks for High-Rate Hydrogenation of Carbon Dioxide. ACS Appl. Mater. Interfaces 2019, 11, 27717–27726. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, C.; Pisal, K.B.; Chavan, A.; Skakle, J.; Patil, S.; Patil, P.; Pagare, P. Electrochemical supercapacitive performance study of Spray Pyrolyzed Cobalt Oxide Film. Mater. Today Proc. 2021, 43, 2742–2746. [Google Scholar] [CrossRef]
- Rusi, S.R.M. Electrodeposited Mn3O4-NiO-CO3O4 as a composite electrode material for electrochemical capacitor. Electrochim. Acta 2015, 175, 193–201. [Google Scholar] [CrossRef]
- Alem, A.; Worku, A.; Ayele, D.; Habtu, N.; Ambaw, M.; Yemata, T. Enhancing pseudocapacitive properties of cobalt oxide hierarchical nanostructures via iron doping. Heliyon 2023, 9, e13817. [Google Scholar] [CrossRef]
- Riley, P.; Joshi, P.; Penchev, H.; Narayan, J.; Narayan, R. One-Step Formation of Reduced Graphene Oxide from Insulating Polymers Induced by Laser Writing Method. Crystals 2021, 11, 1308. [Google Scholar] [CrossRef]
- Lopez, G.P.; Castner, D.G.; Ratner, B.D. XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Kruusenberg, I.; Matisen, L.; Tammeveski, K. Oxygen Electroreduction on multi-walled carbon nanotube supported metal phthalocyanines and porphyrins in acid media. Int. J. Electrochem. Sci. 2013, 8, 1057–1066. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interaction of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers—The Scienta ESCA 300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Nemoshkalenko, V.V.; Didyk, V.V.; Krivitskii, V.P.; Senekevich, A.I. Investigation of the atomic charges in iron, cobalt and nickel phosphides. Zh. Neorg. Khimii 1983, 28, 2182–2192. [Google Scholar]
- Bonnelle, J.P.; Grimblot, J.; D’huysser, A. X-ray photoelectron spectroscopy studies of cobalt oxides. J. Electron Spectrosc. Relat. Phenom. 1975, 7, 151. [Google Scholar] [CrossRef]
- Shi, R.; Chen, G.; Ma, W.; Zhang, D.; Qiu, G.; Liu, X. Shape-controlled synthesis, and characterization of cobalt oxides hollow spheres and octahedra. Dalton Trans. 2012, 41, 5981–5987. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Penki, T.R.; Munichandraiah, N.; Shivashankar, S.A. Flower-like porous cobalt(II) monoxide nanostructures as anode material for Li-ion batteries. J. Electroanal. Chem. 2016, 761, 21–27. [Google Scholar] [CrossRef]
- Chuang, T.J.; Bridle, C.R.; Rice, D.W. Interaction of CO2 and CO with clean and oxygen-covered iron surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Yang, H.M.; Ouyang, J.; Tang, A.D. Preparation and characterization of cobalt oxide nanoparticles via a thermal decomposition process. J. Phys. Chem. B 2007, 111, 8006. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Abrutis, A.; Saltyte, Z.; Figueras, A. Chemical vapor deposition of La2NiO4+δ thin films from nickelocene and lanthanum complexes. Chem. Vap. Depos. 2005, 11, 106. [Google Scholar] [CrossRef]
- Dedryvere, R.; Laruelle, S.; Grugeon, S.; Poizot, P.; Gonbeau, D.; Tarascon, J.-M. Characterization of lithium alkyl carbonate species formed on electrochemically cycled graphite electrode in Li-ion batteries. Chem. Mater. 2004, 16, 1056–1061. [Google Scholar]
- Gartia, Y.; Parnell, C.; Watanabe, F.; Szwedo, P.; Biris, A.; Peddi, N.; Alsudani, Z.; Ghosh, A. Graphene-Enhanced Oxygen Reduction by MN4 Type Cobalt(III) Catalyst. ACS Sustain. Chem. Eng. 2015, 3, 97–102. [Google Scholar] [CrossRef]
- Ruan, M.; Liu, J.; Song, P.; Xu, W. Meta-analysis of commercial Pt/C measurements for oxygen reduction reactions via data mining. Chin. J. Catal. 2022, 43, 116–121. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Kim, J.; Park, J.-K. Effect of Nafion dispersion solvent on the interfacial properties between the membrane and the electrode of a polymer electrolyte membrane-based fuel cell. Solid State Ionics 2011, 196, 73–78. [Google Scholar] [CrossRef]
- Nasini, U.B.; Gartia, Y.; Ramidi, P.; Kazi, A.; Shaikh, A.U.; Ghosh, A. Oxygen reduction reaction catalyzed by cobalt(III) complexes of macrocyclic ligands supported on multiwalled carbon nanotubes. Chem. Phys. Lett. 2013, 566, 38. [Google Scholar] [CrossRef]
- Wayland, H.A.; Boury, S.N.; Albkuri, Y.; Watanabe, F.; Biris, A.S.; Parnell, C.M.; Ghosh, A. Graphene-Supported Cobalt(III) Complex of a Tetraamidomacrocyclic Ligand for Oxygen Reduction Reaction. Catal. Lett. 2018, 148, 407–417. [Google Scholar] [CrossRef]
- Ghosh, A.; Ramidi, P.; Pulla, S.; Sullivan, S.Z.; Collom, S.L.; Gartia, Y.; Munshi, P.; Biris, A.S.; Noll, B.C.; Berry, B.C. Cycloaddition of CO2 to Epoxides Using a Highly Active Co(III) Complex of Tetraamidomacrocyclic Ligand. Catal. Lett. 2010, 137, 1–7. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Cho, S.-H.; Jung, J.-W.; Kim, C.; Kim, I.-D. Rational Design of 1-D Co3O4 Nanofibers@Low content Graphene Composite Anode for High Performance Li-Ion Batteries. Sci. Rep. 2017, 7, 45105. [Google Scholar] [CrossRef]
- Leng, X.; Wei, S.; Jiang, Z.; Lian, J.; Wang, G.; Jiang, Q. Carbon-Encapsulated Co3O4 Nanoparticles as Anode Materials with Super Lithium Storage Performance. Sci. Rep. 2015, 5, 16629. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patwary, M.M.; Haque, S.; Szwedo, P.; Hasan, G.; Kondrapolu, R.S.; Watanabe, F.; KC, K.; Wang, D.; Ghosh, A. Exploring Enhanced Oxygen Reduction Reactions: A Study on Nanocellulose, Dopamine, and Cobalt Complex-Derived Non-Precious Electrocatalyst. Catalysts 2024, 14, 613. https://doi.org/10.3390/catal14090613
Patwary MM, Haque S, Szwedo P, Hasan G, Kondrapolu RS, Watanabe F, KC K, Wang D, Ghosh A. Exploring Enhanced Oxygen Reduction Reactions: A Study on Nanocellulose, Dopamine, and Cobalt Complex-Derived Non-Precious Electrocatalyst. Catalysts. 2024; 14(9):613. https://doi.org/10.3390/catal14090613
Chicago/Turabian StylePatwary, Md Mohsin, Shanzida Haque, Peter Szwedo, Ghada Hasan, Raja Shekhar Kondrapolu, Fumiya Watanabe, Krishna KC, Daoyuan Wang, and Anindya Ghosh. 2024. "Exploring Enhanced Oxygen Reduction Reactions: A Study on Nanocellulose, Dopamine, and Cobalt Complex-Derived Non-Precious Electrocatalyst" Catalysts 14, no. 9: 613. https://doi.org/10.3390/catal14090613






