Abstract
The versatility and significance of imines (Schiff bases) make them highly attractive for many industrial applications. This study investigates photocatalytic routes for the one-pot synthesis of Schiff bases using alcohol and an aromatic nitro compound as reagents, rather than the more conventional amine and aldehyde or ketone. Utilizing photoirradiation at 370 nm with TiO2 loaded with various metals, we demonstrate the exceptional efficiency of the one-pot synthesis of Schiff bases under an inert atmosphere. Notably, the Fe3O4@TiO2 magnetic catalyst offers an excellent option for synthesizing the corresponding imine, achieving a remarkable production rate of 6.8 mmol h−1 during the first 6 h of irradiation with UVA light and reaching over 99% yield after 20 h. This success is attributed to a series of reactions involving the photocatalytic oxidation of benzyl alcohol to benzaldehyde and the simultaneous in situ reduction of nitrobenzene to aniline. The subsequent catalytic condensation of these products, facilitated by the active sites at the TiO2-metal interface, ultimately yields the desired imine. Additionally, while irradiation in the UVA region alone can photocatalyze the process, incorporating blue light (450 nm) accelerates it significantly. Dual-wavelength irradiation increased the production of the benzaldehyde to 77.9 mmol and more than doubled the Schiff base yield, from 7.5 mmol (with UVA light) to 17 mmol in 3 h of irradiation. Additionally, the reusability of the catalyst under simultaneous 450 nm and 370 nm light exposure significantly enhanced Schiff base production, which rose from 16.9 mmol to 48.9 mmol after adding fresh 0.1 M nitrobenzene for the second use. This highlights the effectiveness of color-coordinated catalysis in advancing sustainable chemistry through two-color photochemistry. The magnetic catalytic system not only demonstrates remarkable performance but also shows excellent reusability, representing a promising alternative for sustainable and efficient chemical transformations.
1. Introduction
Schiff bases are condensation products of primary amines with carbonyl compounds such as aldehydes or ketones [1]. Their common structural feature is the azomethine (imine) group with a general formula of RN = CR′ (R′ ≠ H), where R and R′ can be alkyl, aryl, or heterocyclic groups, which can be substituted in various ways. The term Schiff base originates from Hugo Schiff, a German chemist, who in 1864 reported the first synthesis of imines and introduced a new class of organic compounds [2,3].
These organic compounds are extensively used in different fields, serving multiple purposes such as dyes, catalysts, and intermediate species in organic synthesis. They can also work as polymer photo stabilizers through UV absorption or screening, peroxide decomposition, and radical-scavenging mechanisms. They also have diverse biological activities, including antibacterial and antifungal properties [4,5]. Schiff bases are also adaptable ligands capable of coordinating a range of metal ions [6,7].
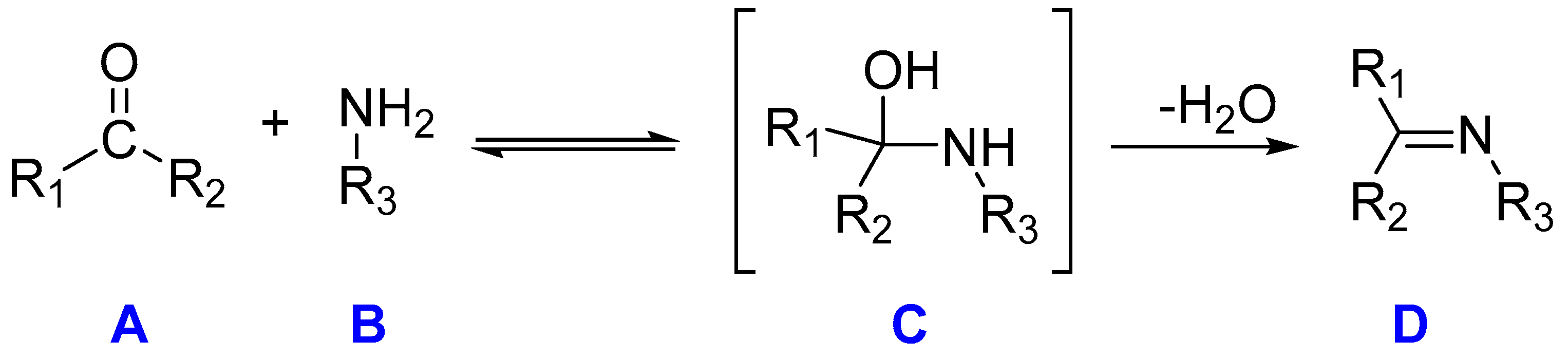
Schiff bases can be synthesized through simple and versatile methods, most frequently through the condensation reaction between primary amines and carbonyl compounds, wherein, specifically, there is a nucleophilic addition of the NH2 group from the amine (B) compound to the C=O of the aldehyde or ketone (A), resulting in the creation of a hemiaminal [8] compound (C) under reflux conditions while simultaneously eliminating water. Subsequently, dehydration occurs, leading to the production of an imine (compound D), as shown in Scheme 1:
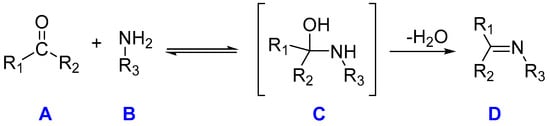
Scheme 1.
Thermal Schiff base synthesis. Molecule A corresponds to a ketone, molecule B corresponds to an amine compound, molecule C is the hemiaminal (an intermediate compound), and molecule D is the Schiff base, characterized by the distinctive double bond C=N.
The photocatalytic synthesis of imines has been explored using homogenous catalysts [9]. More recently, the use of heterogeneous catalysts has gained attention for its potential advantages. Using heterogenous Pt@TiO2, Shiraishi et al. [10] observed yields of up to 84% for N-benzylideneaniline using Pt@TiO2 and using benzyl alcohol and aniline as initial substances.
An alternative approach involves employing nitroarenes as reactants, culminating in a one-pot synthesis of imines via the oxidative coupling of an alcohol and a nitroarene. This method uses stable and cost-effective reactants while reducing the required synthetic steps. This approach was previously explored by Selvam et al. [11] who observed the production of Schiff bases from the photoirradiation (λ 300 nm) of Pd-loaded TiO2 in an aqueous solution of benzyl alcohol and nitrobenzene. However, due to the strong hydrogenation ability of palladium, the products had higher selectivity towards secondary amines [12].
In this report, we have studied several catalysts, but quickly center on Fe3O4@TiO2, a magnetic photocatalyst that has proven extremely useful, allowing us to start the synthesis of Schiff bases from readily available alcohols and nitro compounds, rather than the conventional carbonyls and amines of Scheme 1. We aimed at producing a hybrid material capable of catalyzing both oxidations and reductions. In fact, over a century ago [13,14], Sabatier recognized that hydrogenation catalysts that could work with sacrificial hydrogen donors necessarily had to also be good oxidation (or dehydrogenation) catalysts. In our case, Fe3O4@TiO2 can perform both functions—reduction for the nitro compound and oxidation for the alcohol, thus generating both reaction partners in situ. While irradiation in the UVA region can photocatalyze the complete process, we have discovered that two-color irradiation can dramatically accelerate the process, as blue light, selectively absorbed by magnetite, can convert alcohols to aldehydes or ketones. While no Schiff bases are formed with just blue light, when combined with UVA, the result is a dramatic enhancement of the catalyst’s performance, which is the central piece of this work. As such, the color-coordinated catalysis of Schiff base formation provides another example where two-color photochemistry [15] contributes to the goals of sustainable chemistry [16].
The group of heterogeneous catalysts explored here includes TiO2-P25 and Pd@TiO2, and we compared their activity with new materials involving first-row transition metals such as Fe and Cu on TiO2 (Fe3O4@TiO2 and Cu@TiO2). Aiming at practical industrial applications and greener alternatives, the catalysts were tested in a one-pot approach for the synthesis of imines, starting from nitrobenzene and benzyl alcohol. Our initial efforts showed that Fe3O4@TiO2 exhibits similar activity to Pd@TiO2 and TiO2 catalysts. Considering the experimental results, along with concerns about element depletion and the environmental impact of certain chemicals, iron presents itself as an attractive option. Further, Fe3O4 nanoparticles exhibit superparamagnetism, enabling magnetic separation in the presence of a magnetic field. The reactions were initially driven using UVA light, the spectral region largely absorbed by TiO2; however, the discovery that two-color irradiation could enhance catalyst performance clearly gives added value to Fe3O4@TiO2 as a high-performance Schiff photocatalyst. Under UVA light, this catalyst shows production rates of up to 6.8 mmol/h over a 3 h period, which is more than ten times higher than previously reported values, and is enhanced up to 16.9 mmol/h under two-color irradiation.
2. Results and Discussion
2.1. Catalyst Characterization
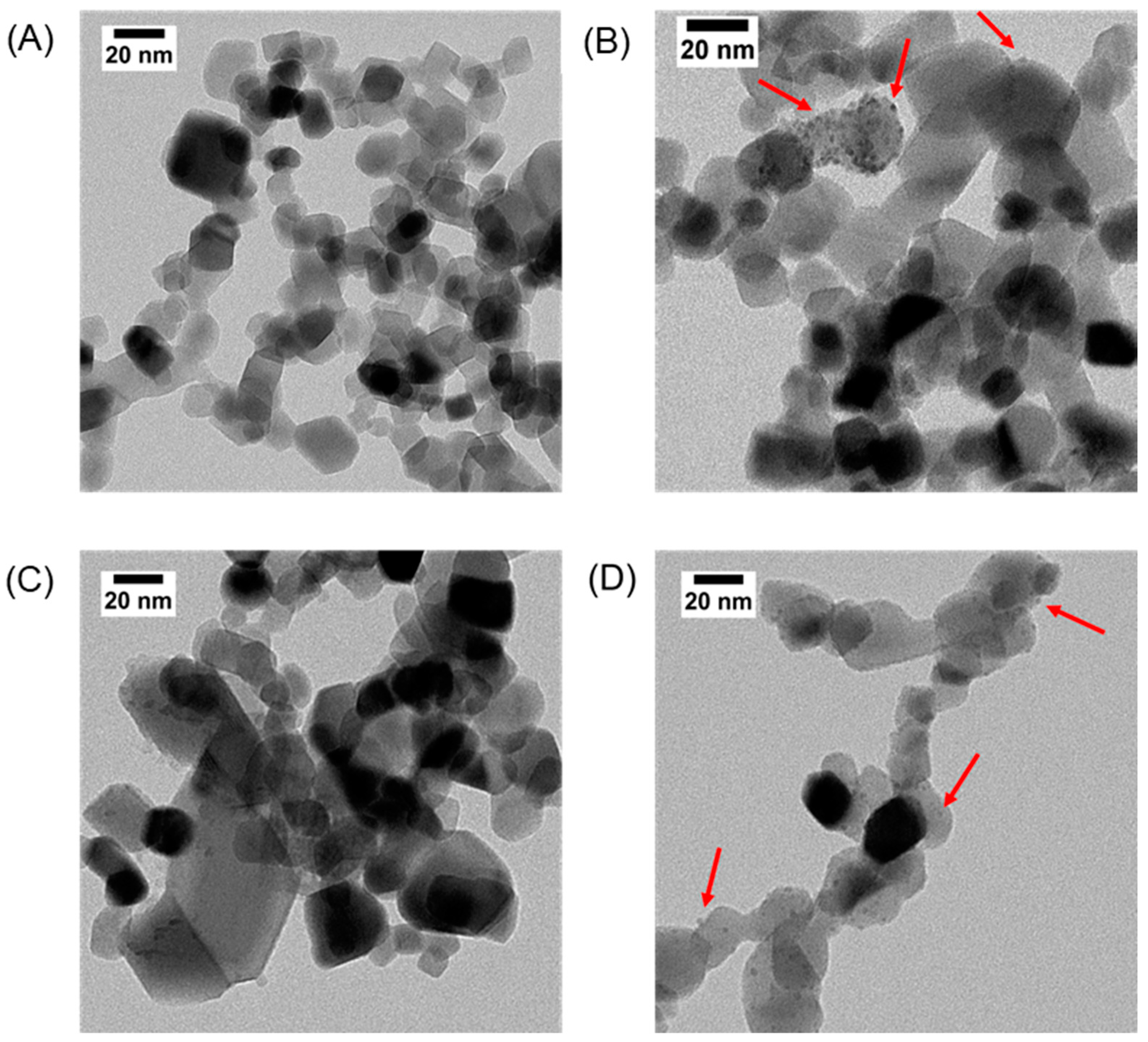
In this work, four TiO2-based catalysts were selected to evaluate possible photocatalytic routes for one-pot imine synthesis. Transmission electron microscopy (TEM) was used as a powerful tool to learn more about the nanoparticles, their morphology, and polydispersity. Figure 1 shows the TEM images of the catalysts, showing the distinctive spherical morphology of the nanoparticles (NPs). Upon closer examination of Figure 1, it becomes evident that the NPs were deposited onto the catalyst surface. This deposition is particularly visible in panel B, for Pd@TiO2, where the TEM image distinctly shows the presence of diminutive particles decorating the TiO2 surface. The same scenario is visible for panels C and D. Previous publications in our group [17] have shown that the mean particle size of the metallic NPs is around 3.0 ± 0.2 nm. Since the distinction between two metal-oxide in TEM is challenging due to the similar electron densities, the diameter of the Fe3O4 over P25 could not be measured. However, previous publications from our group, using the same synthetic route, produced NPs with diameters around 12.5 ± 3 nm [18].
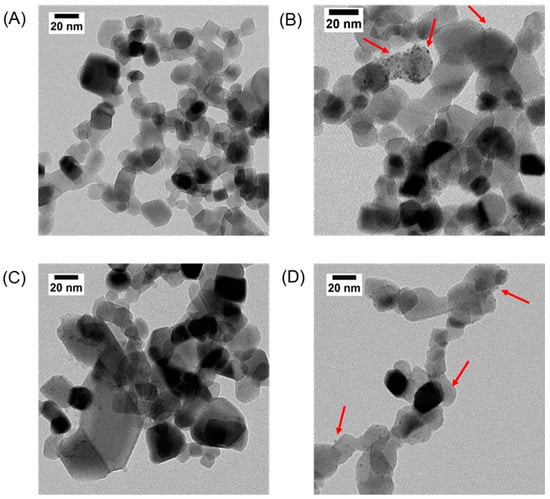
Figure 1.
Representative TEM images of TiO2 (A), Pd@TiO2 (B), Fe3O4@TiO2 (C) and Cu@TiO2 (D). Red arrows indicate representative Pd and Cu nanoparticles on P25. Scale bar: 20 nm.
For the bimetallic nanoparticles (NPs), metal loadings were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis, as shown in Table 1. Synthesis via photo-deposition resulted in lower metal loadings of 2% and 3% for Pd and Cu, respectively. Iron oxide on TiO2 was synthesized by co-precipitation on the semiconductor surface, yielding a 12% loading. The higher loading of iron oxide was intentional, aiming to explore magnetite’s catalytic properties and its magnetism, which can facilitate catalyst removal. Indeed, after synthesis, Fe3O4@TiO2 demonstrated a strong response to an external magnetic field, as shown in Figure S3.

Table 1.
Metal loading of the three bimetallic catalysts evaluated in this work. Metal quantification was carried out using ICP-OES following Fe, Pd, and Cu emission lines.
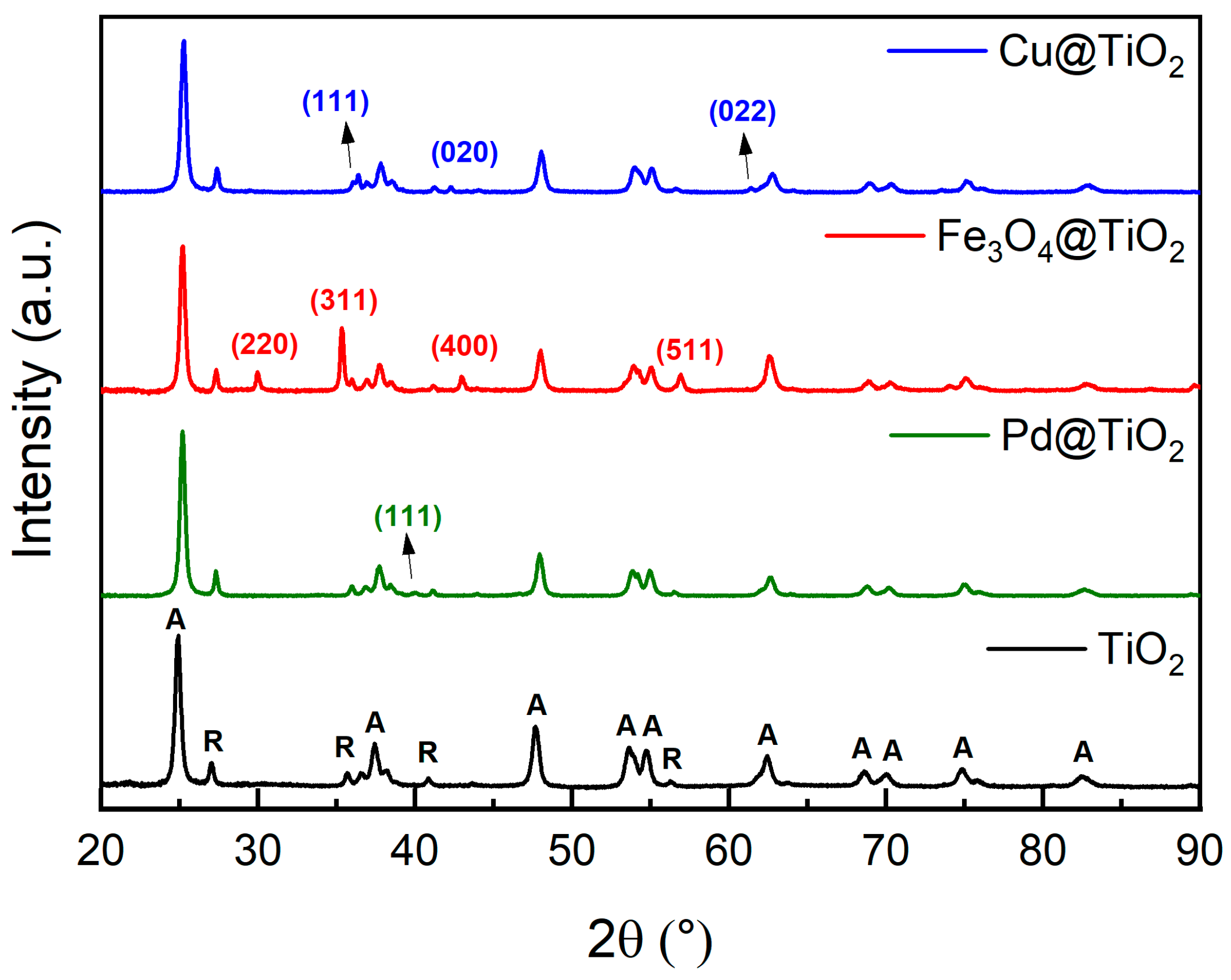
X-ray diffraction (XRD) helped us to acquire insights into the crystalline structures of the synthesized materials. For our tests, P25 was the source of titanium dioxide. P25 is formed from TiO2 nanoparticles, with a diameter between 10 and 50 nm (Figure 1A), composed of 80% anatase and 20% rutile phase [19]. The diffractogram of P25 (Figure 2) confirms its crystalline structure. Peaks assigned for anatase and rutile are identified as A and R, respectively, according to ICCD cards n° 01-083-5916 and 01-076-1940. In the diffractogram, for all the metal-loaded TiO2 catalysts the same peaks assigned to TiO2 (P25) can be identified (Figure 2). From the diffractogram of Cu@TiO2, we identified the structure of Cuprite (Cu2O) (ICCD: 96-900-7498), a cubic-shaped crystal formed from the oxidation of Cu0. The diffractogram peaks assigned to Cu2O can be found at 2θ values 36.4°, 42.3°, and 61.4°, relative to the crystal planes (111), (020), and (022), respectively.
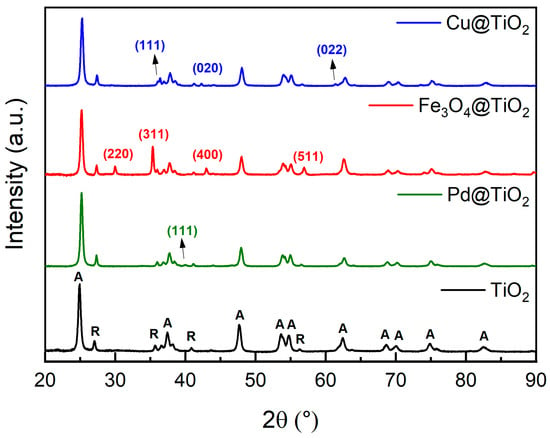
Figure 2.
XRD patterns of the catalysts used: TiO2 (P25) (black), Pd@TiO2 (green), Fe@TiO2 (red), and Cu@TiO2 (blue). TiO2 peaks are identified with A for anatase and R for rutile TiO2; those peaks are present in all samples. The remaining peaks were assigned to the decorating metals and were labeled with their corresponding (h k l) interplanar planes for each crystalline phase, with their colors matching the decorating metal in each sample.
On the other hand, XRD of Fe3O4@TiO2 reveals the formation of cubic magnetite (Fe3O4), according to ICCD card n° 04-009-8439, using the peaks corresponding to the planes (220), (311), (400) and (511) at 2θ values of, 29.9°, 35.3°, 43.0°, and 56.9° to detect the crystal form. As for Pd@TiO2, we can observe the dominance of TiO2 peaks, with the only identifiable peak for Pd being at 2θ of 40.01°, which corresponds to the (111) plane of cubic Pd (ICCD: 96-153-4922).
Table S1 presents the d-spacing measurements for all materials, calculated using Bragg’s law. For most peaks, the d-spacing values are consistent with those obtained from the ICDD reference cards. However, slight discrepancies are observed in the d-spacing values for Cu and Pd when compared to the reference patterns. These differences are attributed to the small particle sizes, which are below 10 nm [20]. At this scale, significant peak broadening occurs, resulting in reduced signal intensity, making accurate analysis of these peaks challenging (Figure 2).
Diffuse reflectance measurements were conducted on all catalysts to examine their optical properties. These measurements, recorded between 200 nm and 800 nm in diffuse reflectance mode, were used to calculate the band gaps via the Tauc plot method. For the Pd- and Cu-decorated P25 catalysts, a distinct absorbance peak appeared at 375–400 nm, corresponding to the band gap of TiO2 (Figure S4). Additionally, visible light absorbance was detected in these materials. Specifically, for Cu@TiO2, two distinct absorbance bands were identified: one at ~3.1 eV, attributed to TiO2, and another at ~2.05 eV, corresponding to Cu2O (Figure S5B). For Pd@TiO2, it was not possible to obtain the band gap; as can be seen in Figure S4, the material has a continuous absorbance in the entire measured region. This suggests that the band edges of both materials interact strongly, resulting in a continuum of intraband states, known as band-tail states, rather than isolated band transitions [21,22]. This phenomenon is also observed for Fe3O4@TiO2, but in this case, a single band gap calculated at 1.72 eV can be detected for this composite material (Figure S5).
2.2. Selecting the Optimal Catalyst: Exploring Variations for Enhanced Reaction Performance
At the beginning of our studies, the aim was to evaluate whether the system chosen yields imines using benzyl alcohol and nitrobenzene as reagents. Benzyl alcohol was chosen as an alkylation surrogate for aldehydes due to its cost-effectiveness. Previous research has demonstrated that an excess of benzyl alcohol enhances the reaction; thus, we employed it as a solvent, facilitating potential solution reusability.
Across all reactions, the primary products observed included benzaldehyde (BenzA) and N-Benzylideneaniline (Schiff base). A representative chromatogram using Fe3O4@TiO2 as the catalyst is depicted in Figure S6. The mass spectra of the products shown in Figure S6 can be seen in Figure S7. Similarly, the pure Schiff base compound was analyzed using GC:MS (Figure S8, panel C) to compare its retention time with that observed after the reaction, as depicted in Figure S6. The mass spectrometry data, shown in Figure S8, Panel D, confirmed the presence of the molecular ion peak for N-Benzylideneaniline. Specifically, a tandem process occurred where the nitroarene underwent reduction to form an aromatic amine through interaction with the alcohol, concurrently oxidizing the alcohol to yield the aldehyde (BenzA). Following this process, the aldehyde (BenzA) and the amine (aniline) coupled to produce the corresponding imine (Schiff base, N-benzylideneaniline). The presence of BenzA demonstrates the successful oxidation of the benzyl alcohol for all catalysts (Figures S9–S11) and its high abundance is attributed to the chosen stoichiometry.
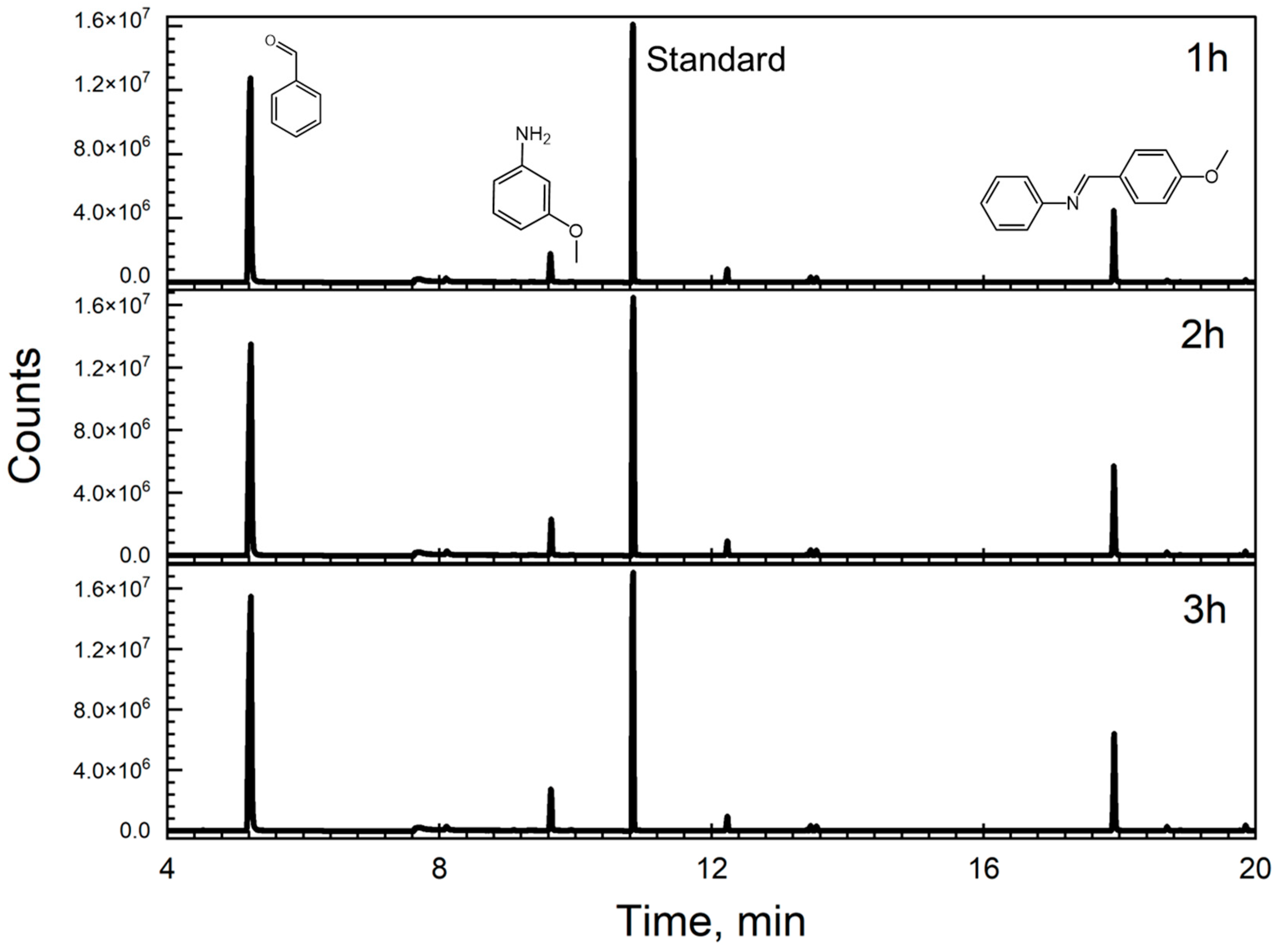
Aniline is also one of the products, although it was present at low concentrations, indicating our catalysts’ dual ability to successfully oxidize benzyl alcohol while reducing the nitrobenzene to aniline. In our study, gas chromatography revealed a challenge in detecting the aniline peak due to its proximity to the retention time of the solvent (benzyl alcohol) when nitrobenzene was employed as the substrate. However, subsequent experiments using 3-nitroanisole (3-NA) keeping benzyl alcohol as the solvent and Fe3O4@TiO2 as the catalyst, yielded more distinct results, as depicted in Figure 3. In this particular case, although m-anisidine was present in relatively low concentrations, it was discernible in the chromatogram (retention time of 9.63 min), which is indicative of our catalysts’ proficiency in facilitating the oxidation of benzyl alcohol while concurrently reducing nitro compounds to amines. This observation underscores the importance of substrate selection and catalyst design in optimizing reaction outcomes, providing valuable insights for the development of efficient catalytic systems. Additionally, the smaller peak observed for m-anisidine (9.63 min) compared to benzaldehyde indicates that m-anisidine is the limiting reagent in the formation of the Schiff base. This is because the condensation reaction between an aniline derivative, such as m-anisidine, and benzaldehyde is essential for Schiff base synthesis; without sufficient aniline, the reaction cannot proceed effectively. To potentially enhance the yield of the Schiff base, incorporating blue light into the process could be beneficial. By increasing the energy input with blue light, we thought that the reaction could be driven more effectively, promoting better interaction between the compounds and thereby increasing the production of the Schiff base.
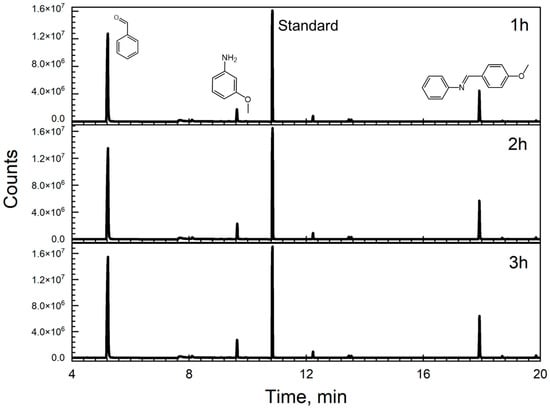
Figure 3.
Chromatograms showing the peaks obtained after performing the reaction with 3-nitroanisole 0.1 M in benzyl alcohol and 10 mg of Fe3O4@TiO2. We irradiated 5 mL of the solution for 3 h under UVA irradiation and continuous stirring. Samples were taken every hour and filtered using a PTFE syringe filter. 3,5-Di-tert-butyltoluene was used as an external standard (peak at 10.84 min) The products had the following retention times: BenzA—5.17 min, m-anisidine—9.63 min, Schiff base N-(4-Methoxybenzylidene)aniline—17.94 min. The timed events feature of the local user interface was used (turned off at 6.20 min and activated at 8.70 min) to prevent the risk of a high concentration of benzyl alcohol reaching the detector.
Figure 3 shows the success of this approach for the synthesis of the Schiff base. To further confirm this, the pure Schiff base compound was analyzed by GC:MS (Figure S8, panel A), and its retention time was compared to that observed in Figure 3. The mass spectrometry data (Figure S8, Panel B) verified the presence of the molecular ion peak and fragmentation pattern for N-(4-Methoxybenzylidene)aniline. However, a comparison of the results depicted in Figure 3 and Figure S6 reveals a notable difference: following a 3 h reaction period, the product peak observed when using nitrobenzene as the starting substrate is higher than that of 3-nitroanisole. This difference is attributed to electronic effects. In fact, when comparing the electron density contributions of the nitro group in molecules such as nitrobenzene to that of the methoxy group on the aromatic ring, the electron-donating effect of the methoxy group is stronger [23]. This leads to a lower electron density around the nitro group in 3-anisole, which indicates that the electronic effect of substituents on the aromatic ring is also an important factor in the reaction. Additionally, as stated by Hirao et al. [24], analyzing the electronic structure and the distribution of electron density within the aromatic ring is important to determine the reactivity of the ortho, meta, and para positions when exposed to an electrophile. In this case, the presence of a methoxy group enhances electron density within the ring.
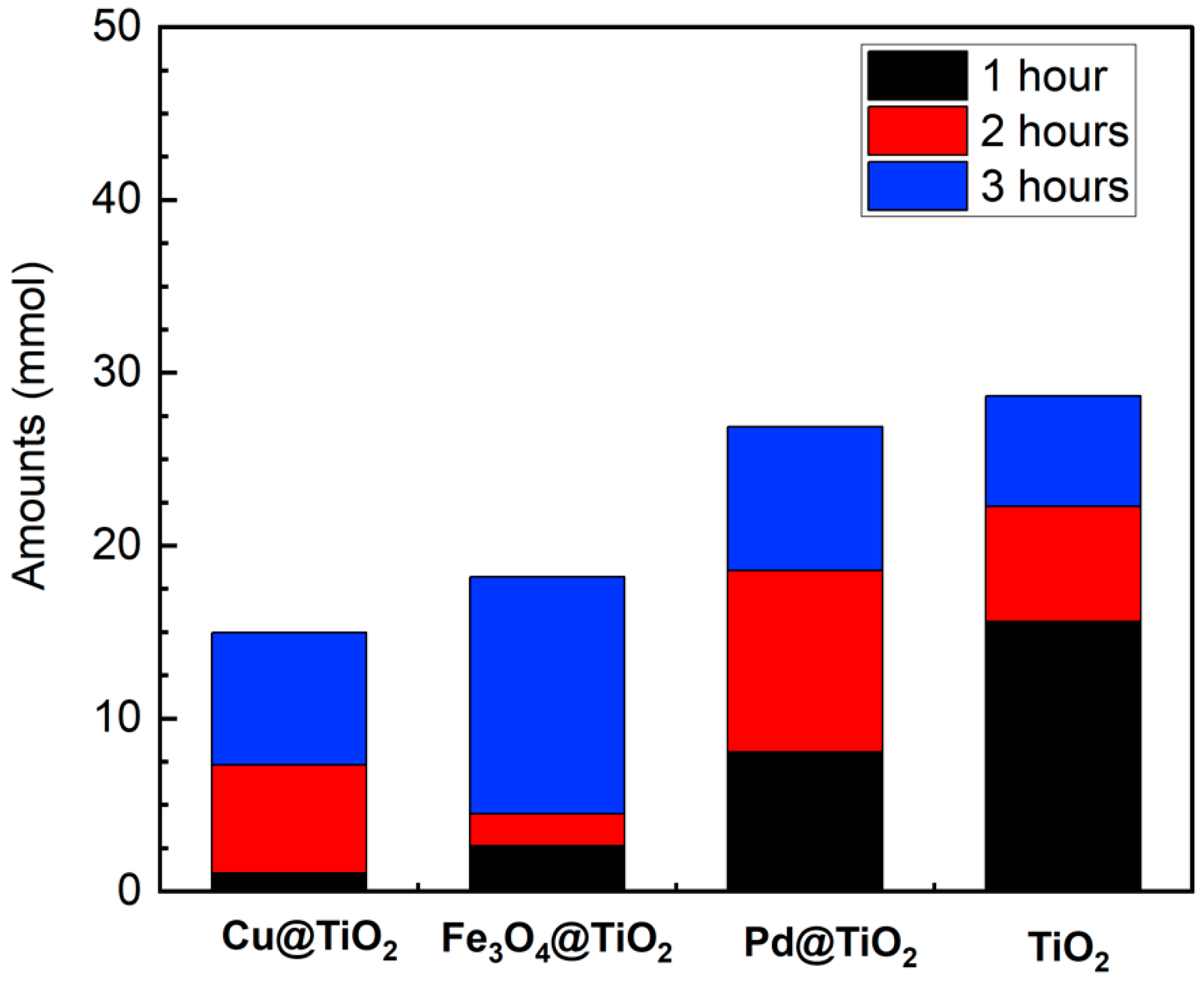
The photocatalytic activity of four TiO2-based catalysts was compared. In the comparative analysis, the samples were subjected to irradiation for up to 3 h, revealing varying imine yields dependent on the catalysts employed. As shown in Figure 4, Pd@TiO2 exhibited a maximum production of 26.9 mmol (27%) after 3 h of irradiation. Remarkably, Pd@TiO2 was the only catalyst capable of yielding the fully reduced product, N-Benzylaniline. This observation is highlighted in Figure S10, where a distinct peak begins to appear around 15.65 min at the 2 h mark. This is a result of the strong hydrogenation ability of noble metals, which decreases the selectivity towards the desired imine [11,12,13]. TiO2 produced a similar Schiff base, producing 28.7 mmol (Figure 4). Prior publications had showcased the potential of TiO2 for the tandem imine formation from alcohols and nitroarenes [25,26]. In particular, P25 takes advantage of rutile’s high photocatalytic reduction of nitrobenzene coupled with the higher concentration of Lewis acid sites on anatase which facilitates the condensation of BenzA and aniline [26].
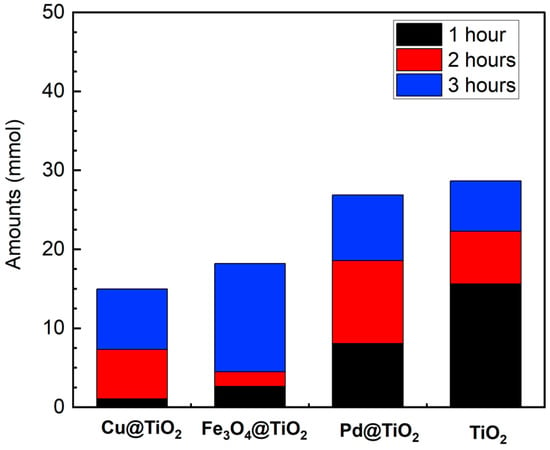
Figure 4.
Photocatalytic performance for the selectively generated Schiff base N-Benzylideneaniline in the coupled system of benzyl alcohol and nitrobenzene using different catalysts under UVA light irradiation for 1–3 h. Reaction conditions: benzyl alcohol (as the solvent, 5 mL), nitrobenzene (0.1 mmol/L), M@TiO2 (10 mg), under argon, UVA light irradiation (λ 370 nm). Schiff base production was quantified using GC-MS.
Interestingly, the Fe- and Cu-loaded catalysts exhibited a delayed onset in catalyzing conversion, evident from their low yields within the initial 2 h, followed by a pronounced increase in yield from 2 to 3 h. In our previous studies with Cu@TiO2, a similar slower catalytic rate compared to Pd-loaded TiO2 in the hydrogenation of alkynes [17] was observed. Just as with the alkynes, Cu exhibited superior control over the selectivity of the reaction, while Pd excelled in enhancing its rate. This difference highlights how various catalysts play a crucial role in controlling both the speed and specificity of chemical reactions.
Despite their slower kinetics relative to other catalysts, Cu@TiO2 and Fe3O4@TiO2 demonstrated substantial Schiff base formation within 3 h, at 15.3 and 18 mmol, respectively (Figure 4). This contrasts with previous reports utilizing Fe- and Co-based catalysts, where reaction completion often required days [27,28], showing that the association with TiO2 is beneficial to improve the yields for those catalysts. It is also noteworthy that we did not employ bases, in contrast with prior reports [27,28,29,30], and yet achieved satisfactory yields in a short time.
Based on the previous results, P25 exhibited higher yields in the synthesis of Schiff bases; however, its powdered form hinders separation processes, often requiring an extra centrifugation step. In contrast, Fe3O4@TiO2 offers the distinct advantage of magnetic removal, facilitating the separation process while keeping good reaction yields. Therefore, recognizing this advantage, we directed our efforts towards enhancing the yield of this magnetic catalyst, as its facile removal makes it an attractive candidate for scaling up applications in organic synthesis.
2.3. Optimizing Reaction Conditions Using Fe3O4@TiO2 as a Catalyst
Our initial observation was that the reactions using Fe3O4@TiO2 had a delayed start compared to pure TiO2. Consequently, the Schiff base yield was first evaluated at longer irradiation times and compared it with TiO2 alone. After running the reactions with both catalysts for 6 h, P25 produced 36.6 mmol (Table 2, entry 2) of the Schiff base, while Fe3O4@TiO2 produced 41.7 mmol (Table 2, entry 1). These results indicate that the Schiff base rate achieved with the magnetic catalysts is 6.8 mmol h−1, which is over ten times higher than the values previously published, which has rates ranging from 4.6 µmol h−1 to 0.34 mmol h−1 reported (Table S2) [25,26,31,32,33,34]. In fact, Table S2 details the production rates (in mmol h−1) achieved with various catalytic materials under different conditions. Notably, among the catalysts reviewed, only the magnetic catalyst from this work achieved the highest production rate. Given that our magnetic catalysts demonstrated superior performance, we extended the reaction time to further evaluate its efficiency. With 20 h of irradiation, we achieved over 99% of the desired product. Thus, even with the longer irradiation time, a significantly high average production rate was obtained. This further underscores the exceptional catalytic activity and stability of the catalyst, highlighting its potential for efficient synthesis processes.

Table 2.
Optimizing Schiff base yield produced by Fe3O4@TiO2 as a catalyst.
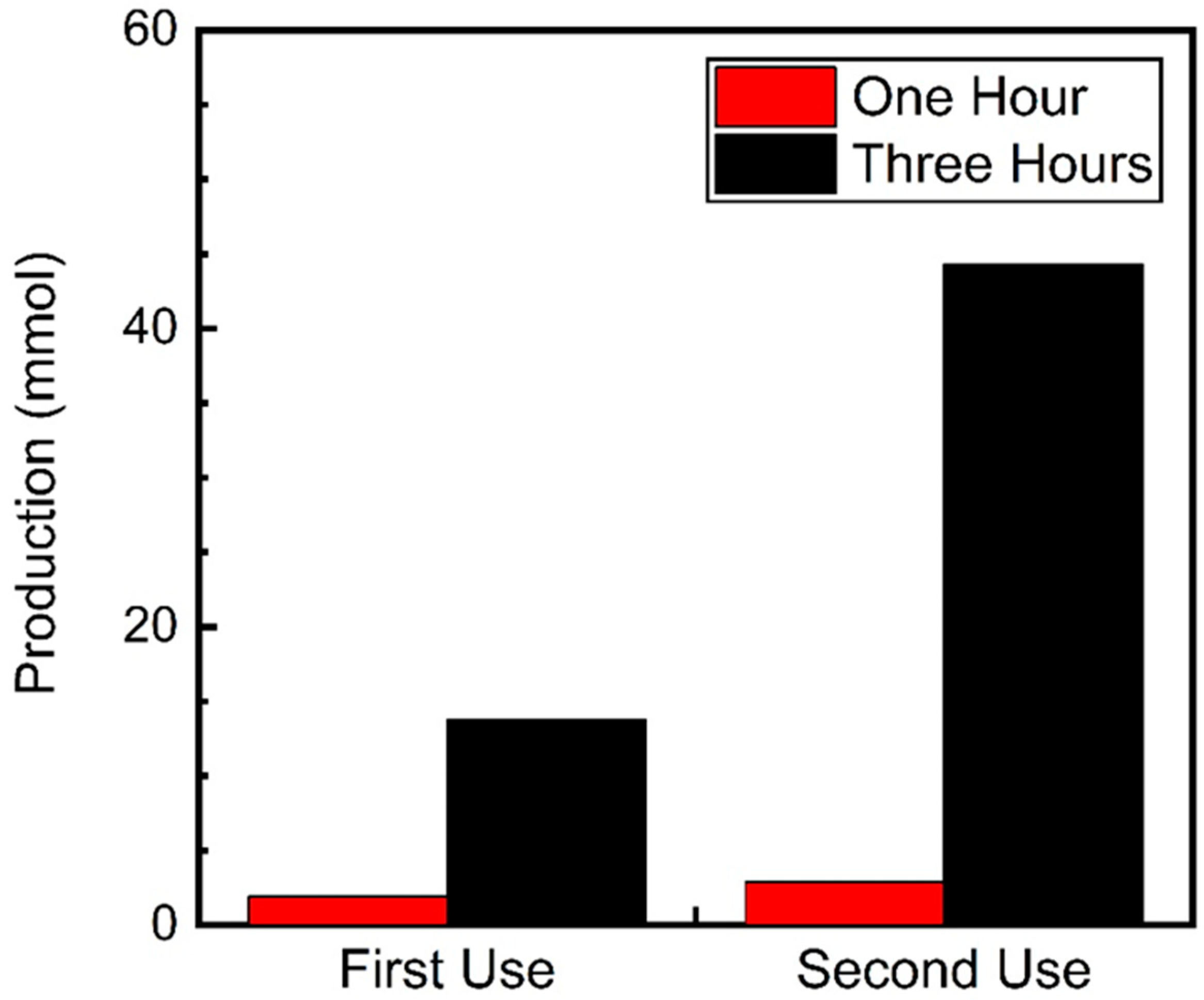
We also investigated whether the catalytic delay persisted during a second use of the catalyst. To test this, a reaction for 3 h under UVA irradiation was conducted. After this period, 0.1 M of fresh nitrobenzene was added to the solution, which would avoid wasting the excess benzyl alcohol and the produced BenzA. The reaction was then irradiated for another three hours. Figure 5 compares the results from the first and second uses of the catalyst difference between the first use of the catalyst. During the first use, Fe3O4@TiO2 produced 13.8 mmol of N-benzylamine after three hours. However, during the second use, the catalyst yielded over 44.3 mmol of the desired Schiff base in the same time frame.
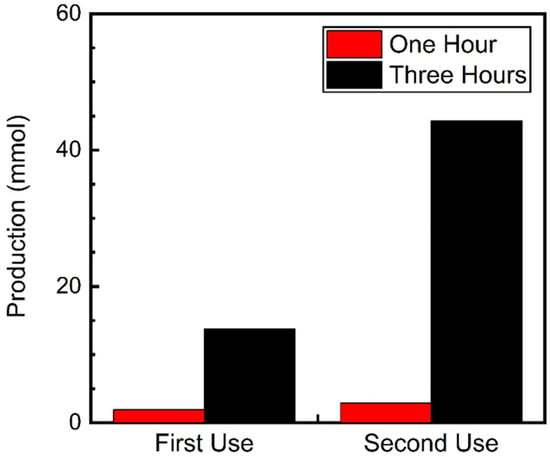
Figure 5.
N-benzylamine production using Fe3O4@TiO2 as catalyst (10 mg) irradiated for up to 3 h with UVA (λ 370 nm) under a N2 atmosphere. After the first round of irradiation (first use), 0.1 M of nitrobenzene was added to the solution and irradiated for another 3 h (second use). Schiff base production was quantified using GC-MS.
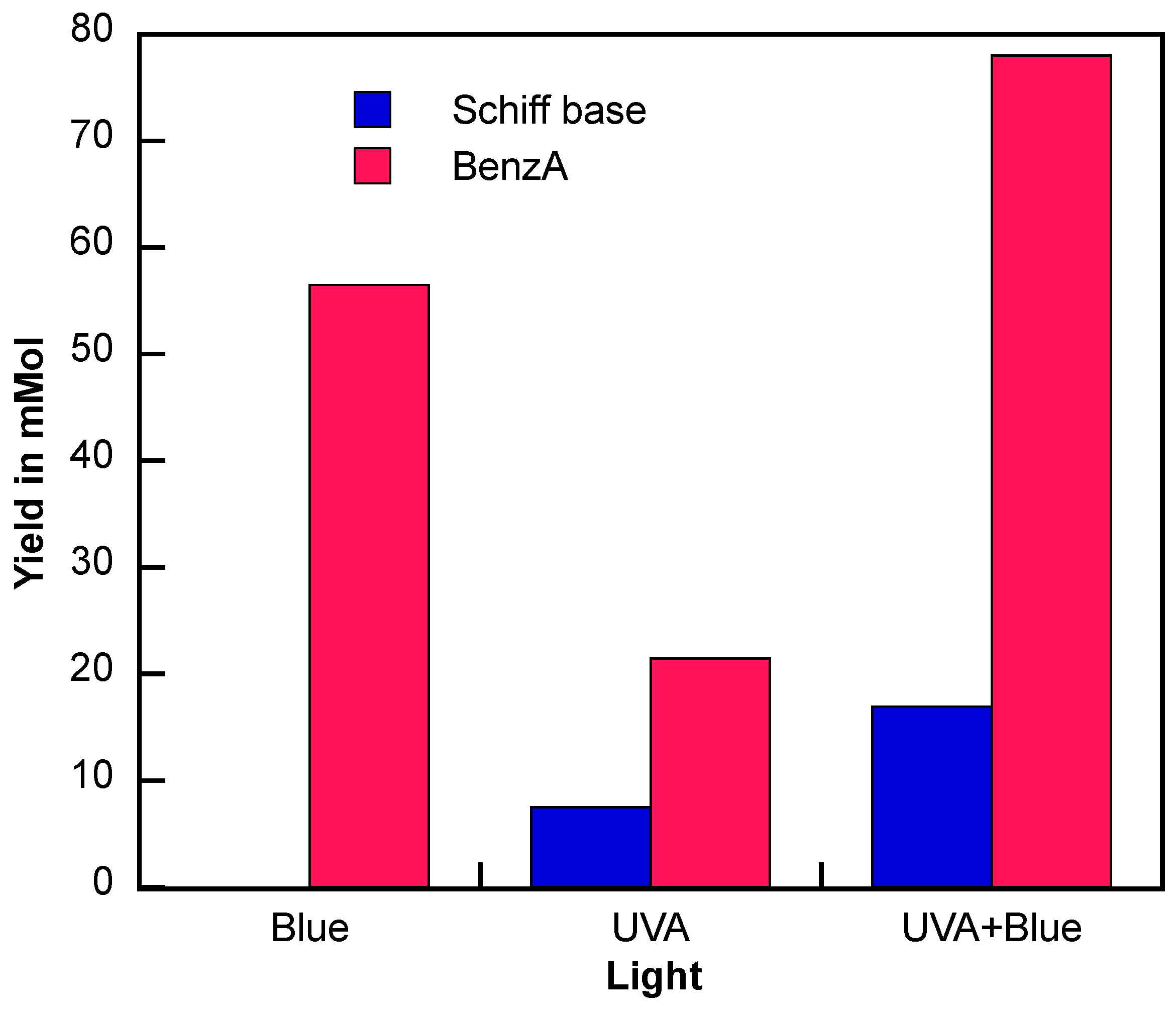
As a follow-up, changes in lighting conditions were evaluated to enhance the Schiff base production yield. We compared the product yield at the 3 h mark for all the optimization reactions. Since irradiance can alter the distribution of products [35,36], the resulting imine yields were evaluated at lower irradiance. For our system, reducing the irradiance resulted in a lower Schiff base yield (Table 2, entry 4). Next, the influence of wavelength was evaluated by irradiating the reaction with a blue LED (450 nm), which selectively excites the iron oxide but not TiO2. This led to a substantial production of BenzA, while neither a Schiff base nor an amine was detected (Table 2, entry 3 and Figure S12). Likewise, the absence of irradiation resulted in no Schiff base formation (Table 2, entry 6 and Figure S13) and a significantly lower BenzA (5.17 min) production. These results suggest that TiO2 excitation is essential for the reaction to occur.
Since aniline was identified as the limiting reagent (analysis from Figure 3) and BenzA production continues with UVA and blue light (Figures S6 and S11), this suggests that using both wavelengths together could further enhance the reaction. Therefore, we used simultaneous irradiation using both blue light and UVA LEDs. The results of this combined approach are shown in Figure 6 and compared to those obtained with UVA light alone. Figure S14 shows the experimental setup for the dual irradiation experiments.
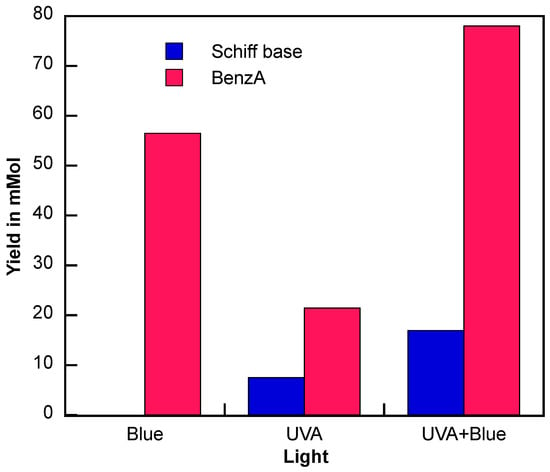
Figure 6.
N-benzylamine (Schiff base, blue) and benzaldehyde (BenzA, red) production using Fe3O4@TiO2 as catalyst (10 mg) irradiated for up to 3 h with blue LED (λ = 450 nm), UVA (λ = 370 nm), and UVA and blue light simultaneously (UVA + Blue) under N2 atmosphere. Schiff base production was quantified using GC-MS.
When both wavelengths of irradiation are used, the BenzA production increases significantly. Given that BenzA is a crucial intermediate for synthesizing the desired Schiff base, combining both irradiation methods leverages the benefits of blue light for higher BenzA production and the nitro reduction and condensation steps facilitated by UVA irradiation. The results of this experiment are presented in Figure 6, showing the Schiff base production (blue) and BenzA concentration (red) after 3 h of irradiation. Notably, the production of the carbonyl intermediate increased with dual-wavelength irradiation, reaching 77.9 mmol. Even more interestingly, the dual irradiation more than doubled the production of the Schiff base, increasing from 7.5 mmol to 17 mmol, clearly demonstrating that, the dual excitation effect leads to a more effective activation of the hybrid catalyst.
From the results of the reusability tests (Figure 5), in an environment with higher BenzA concentration and the dual light experiments, we can observe that excess BenzA is favorable for Schiff base production. This could be attributed to the enhanced oxidation rates of benzyl alcohol, shown by the increased BenzA concentration (Figure 6). This supports the notion that benzyl alcohol oxidation and nitrobenzene reduction are coupled processes, where the protons released during alcohol dehydrogenation are employed in the hydrogenation of nitrobenzene. A similar result was observed by Ling and colleagues [37], who found that a higher alcohol concentration led to an increased aniline yield and the absence of the initial alcohol resulted in no aniline production. Other studies have also reported consistent findings [31,38,39,40], demonstrating that increased alcohol dehydrogenation enhances nitrobenzene hydrogenation, thereby boosting N-benzylamine yield.
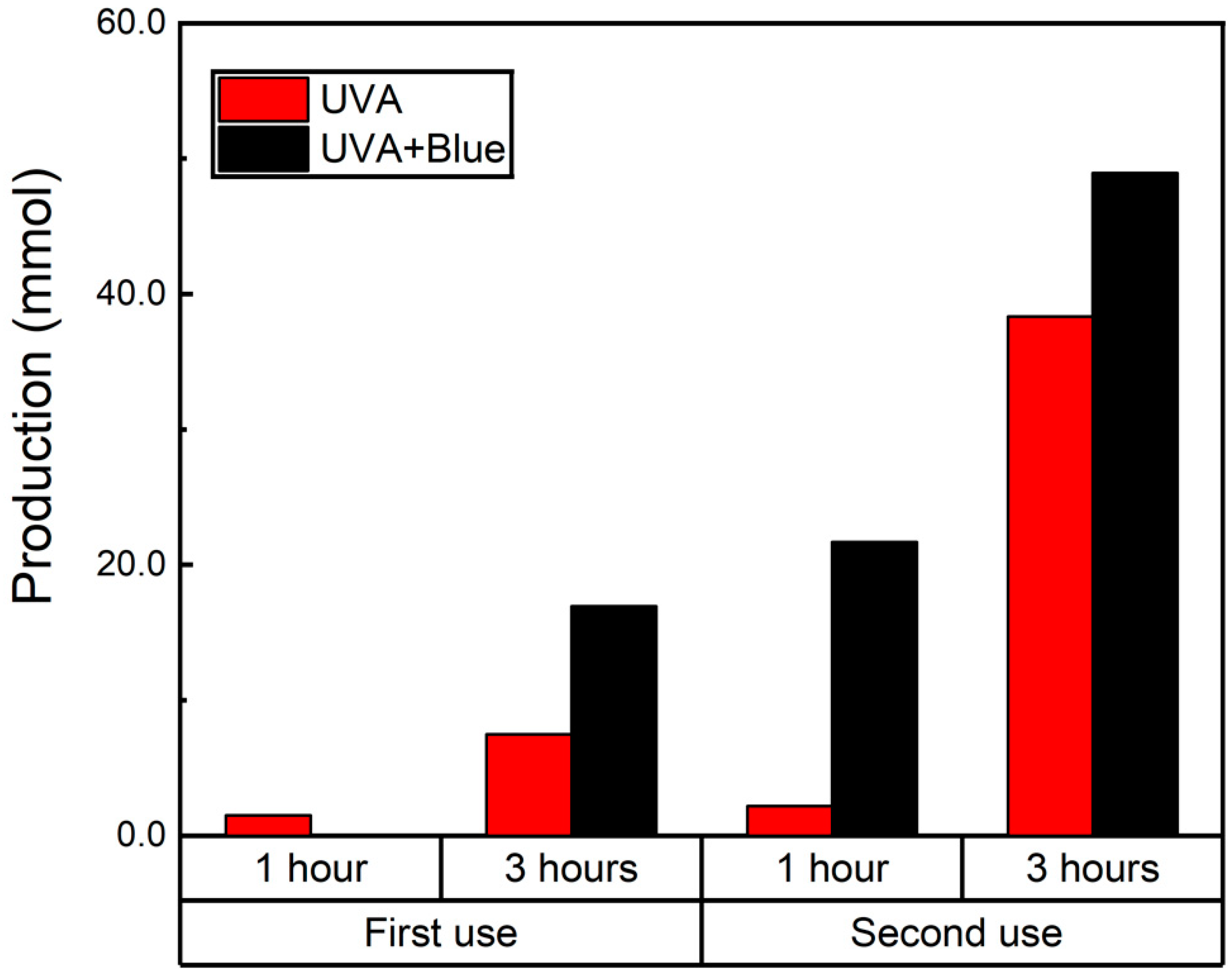
Similarly to the reusability experiments utilizing UVA irradiation, we decided to compare the reusability of the catalysts when exposed simultaneously to 450 and 370 nm light. The results for these reusability tests are presented in Figure 7 for the Schiff base and in Figure S15 for the BenzA production. As previously observed, the higher BenzA yield from the first use is a key factor in the significant increase in Schiff base production, which went from 16.9 mmol to 48.9 mmol in the second use after adding fresh 0.1 M nitrobenzene (Figure 7). This substantial increase highlights the improved efficiency of the catalyst in subsequent reactions, demonstrating its enhanced performance with continued use. Additionally, this approach improves the catalytic efficiency of the magnetic catalyst, particularly when it has been reused. Therefore, the dual-light system not only enhances the catalyst’s performance but also extends its reusability. Additionally, the crystalline structure of the catalyst was evaluated after use using XRD and compared to the diffractograms of the fresh samples. Both diffractograms are presented in Figure S16, revealing no alterations in the crystalline structure of the materials. The primary difference between the samples is the intensity of the magnetite peaks, identified by their Miller indices, which are less intense after use. This reduction in peak intensity may be attributed to either the leaching of a portion of the iron catalyst or the coverage of iron catalyst sites with organic material.
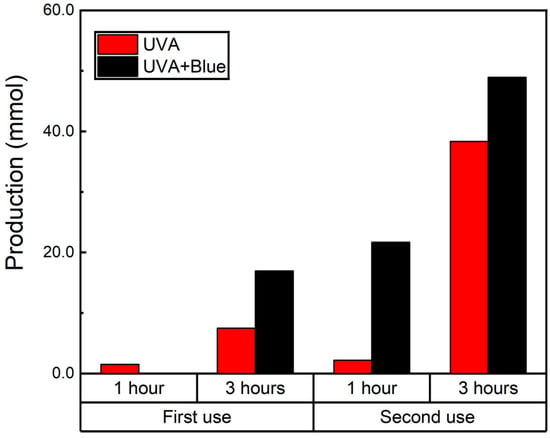
Figure 7.
N-benzylamine production using Fe3O4@TiO2 as catalyst (10 mg) irradiated for up to 3 h with UVA (λ 370 nm) and UVA and blue light (λ 450 nm) simultaneously under a N2 atmosphere. After the first round of irradiation (first use), 0.1 M of nitrobenzene was added to the solution and irradiated for another 3 h (second use). Schiff base production was quantified using GC-MS.
2.4. Mechanistic Insights into the Synthesis of Schiff Bases from Nitrobenzene and Benzyl Alcohol
Based on our previous findings, a plausible mechanism is proposed to elucidate the synthesis of Schiff bases from nitrobenzene and benzyl alcohol catalyzed by M@TiO2. Initially, the photocatalytic reaction on M@TiO2 is initiated by the photoexcitation of TiO2, leading to the generation of electron (e−) and hole (h+) pairs. The moderate oxidation potential of the photogenerated holes allows the h+ to oxidize benzyl alcohol, yielding BenzA and hydrogen ions (H+) [39]. BenzA was identified as one of the main products in our experiments, which is crucial in this process. The H+ ions formed are subsequently reduced on the surface of the metal to generate H-metal species. This is the case of Pd@TiO2, in which it has been proposed that Pd-H species will likely form in materials like Pd-decorated TiO2 when exposed to light [41]. In fact, Paul Sabatier, who won the Nobel Prize in 1912 [13] and worked with nickel (Ni) and different molecules such as hydrocarbons, alcohols, and acids, mentioned that the hydrogen coming in contact with the metal of a catalyst forms very quickly on the surface of each grain. These species can serve as active sites for reducing agents on the surface of Pd, facilitating specific reactions like converting nitrobenzene into aniline. This is supported by our experiment using 3-nitroanisole, in which m-anisidine, an aniline derivative, was identified. The catalytic condensation of BenzA with aniline on the Lewis acid sites of the TiO2 surface leads to the formation of the Schiff base. Our experimental results, particularly, highlighted in Figure 3, demonstrate the efficiency of this reaction on the M@TiO2 catalysts.
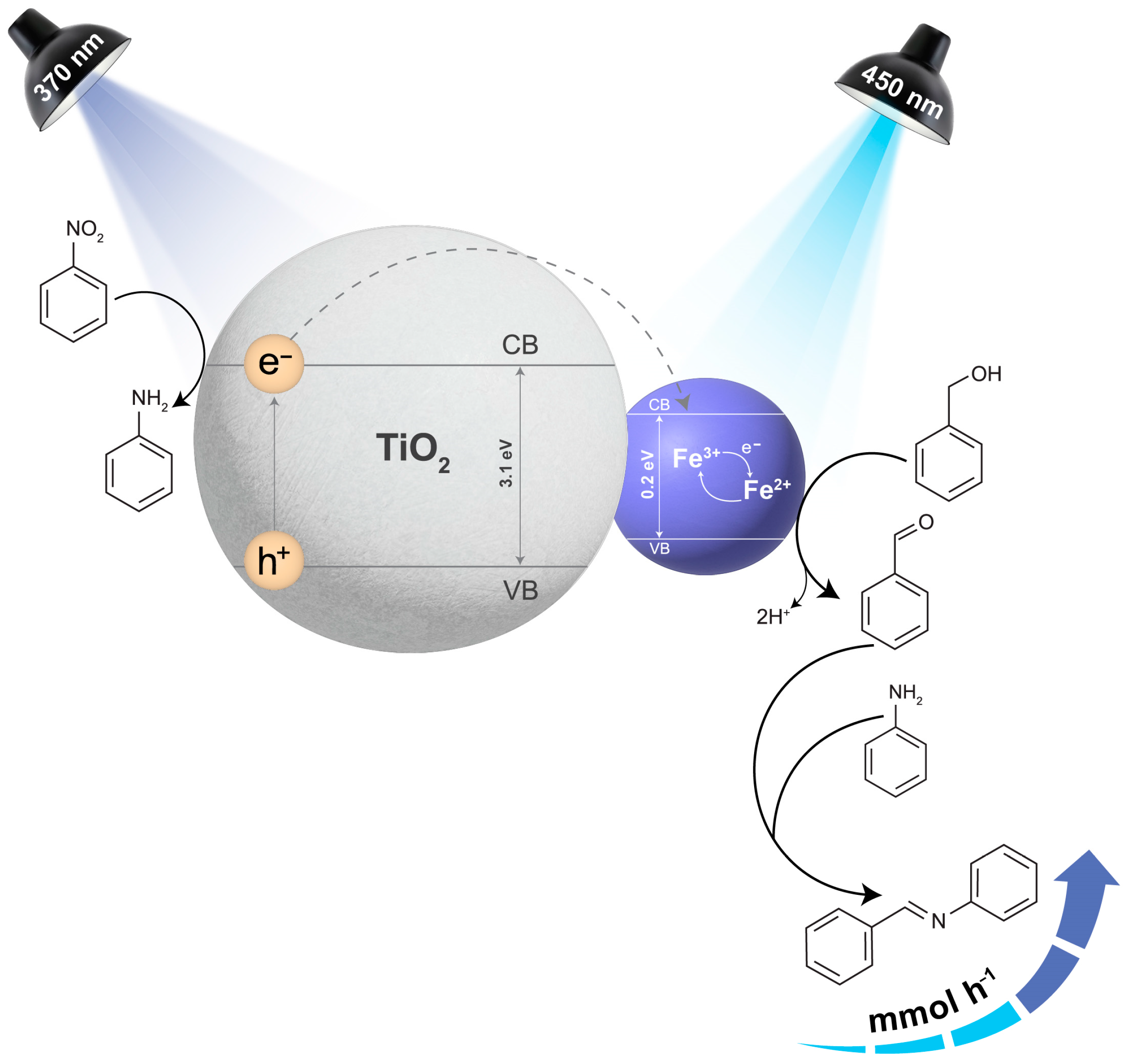
It should be noted that the reaction could vary depending on the metal used to decorate TiO2. For Fe3O4@TiO2, we propose that upon UVA irradiation, the h+ generated on the TiO2 surface may oxidize benzyl alcohol and the e− transferred to Fe3O4 (Figure S17). Xu et al. [42] found out that when TiO2 interacts with Fe3+, it facilitates the Fe3+/Fe2+ cycling [41,42]. Particularly, in the presence of P25, large amounts of Fe2+ were detected at the beginning of the reaction. This is likely due to the positive energy difference between the conduction band (CB) of TiO2, and the CB of Fe3O4 [43]. This difference allows for easy transfer of the photogenerated e− to the magnetic catalyst, decreasing the rate of charge recombination. Moreover, during the regeneration of Fe3+, the electrons could potentially reduce nitrobenzene to aniline. Another possible route involves photogenerated e− helping in the reduction of nitrobenzene in the presence of H+ ions. H-Fe species may also be generated, assisting in the reduction of the nitro moiety, yielding the amine or intermediate conversion, such as those involving phenylhydroxylamine, eventually leading to the condensation between benzaldehyde and aniline [29]. A plausible scenario of this is depicted in Figure S17, which shows the results obtained with UVA irradiation alone. Another possible route involves photogenerated electrons helping in the reduction of nitrobenzene to form aniline in the presence of H+ ions. H-Fe species may also be generated, assisting in the reduction of the nitro moiety, yielding the amine or intermediate conversion, such as those involving phenylhydroxylamine, eventually leading to the condensation between benzaldehyde and aniline [29]. On the other hand, under irradiation with simultaneous UVA and blue light, we observed several key enhancements. The addition of blue light provided an alternative way to directly photoactivate the Fe3O4 component, whose h+ is also trapped by benzyl alcohol (Figure 8). Thus, BenzA formation can occur either on the magnetite or TiO2 sites. The high dehydrogenation rates culminate in higher available hydrogen for nitrobenzene or phenylhydroxylamine hydrogenation. As demonstrated in our experiments, BenzA then reacts with aniline to produce the Schiff base. This synergistic effect of UVA and blue light improves overall reaction efficiency by enhancing both oxidation and reduction processes. Thus, Fe3O4 serves three primary functions in this catalyst: (1) trapping electrons and reducing charge recombination, (2) transferring trapped electrons to nitrobenzene, facilitating its reduction, and (3) under blue light, generating additional holes for benzyl alcohol oxidation, thereby increasing the availability of hydrogen for nitro reduction.
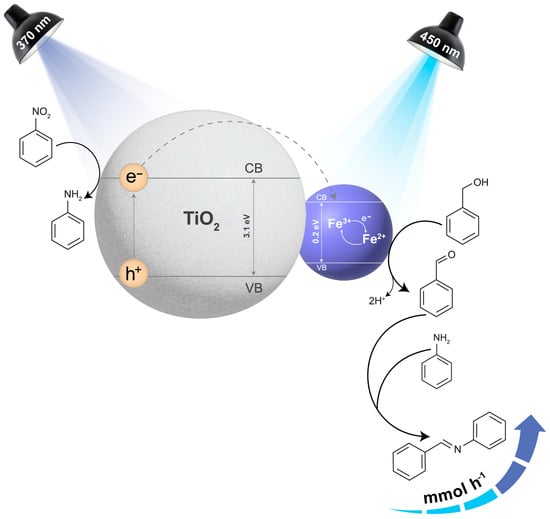
Figure 8.
A plausible scenario to understand the activity shown by Fe3O4@TiO2 when irradiating with UVA light (370 nm) and blue light (450 nm). Note that the hole in TiO2 can be trapped by benzyl alcohol, being an alternative route for BenzA production. The band gap values have been added based on references or the TiO2 [44] and or magnetite [45].
It is important to highlight that, as mentioned by Moniz et al. [40], who also worked with a nanocomposite of iron, the role of this metal in catalytic processes, particularly when it interacts with TiO2, remains a subject of ongoing research, presenting intriguing possibilities. While some studies propose that its presence facilitates hole transfer, others suggest its involvement in electron transfer mechanisms [46,47]. Recent investigations have highlighted the electron transfer phenomena occurring between iron and TiO2 [48].
Light plays an essential role in the formation of Schiff bases from nitro compounds and alcohols. Specifically, in the two-LED system, the dual color exposure leads to a higher conversion to the desired Schiff base. The drastic enhancement shown in Figure 7 suggests that blue irradiation of the magnetite component in the catalyst does more than just make BenzA, likely assisting in the nitro-to-amine process, for example, in its intermediate stages, such as phenyl hydroxylamine reduction.
3. Experimental Details
All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) and used as received unless otherwise stated. They were of analytical pure grade (99%) and used without further purification. This was confirmed as per the manufacturer’s specifications. TiO2 P25, technical-grade powder, was purchased from Univar Canada (Richmond, BC, Canada).
3.1. Catalyst Synthesis
Fe3O4@TiO2: Magnetic TiO2 NPs were synthesized by in situ precipitation of Fe3O4 on TiO2 NPs. In brief, a 0.32 mg/mL aqueous solution of commercial P25 was added to a 3-neck round bottom flask. To this solution, 0.056 g of Fe(NO)3 and 0.32 g of Fe(SO)4 were added to obtain a proportion of 2:1 of Fe (III) to Fe(II). The solution was purged for 10 min with nitrogen and then heated at 85 °C and kept at this temperature for 10 min under N2 flow, to equilibrate the temperature. To this solution, 360 µL of NH4OH was added dropwise and the resulting dark solution was kept under N2 for another 10 min. The resulting material was separated using an external magnet (0.5 T) washed five times with Milli-Q water (Thermo Scietific™ Barnstead™ GenPure™ water purification system—Waltham, MA, USA) and dried under vacuum. A representative illustration of the synthesis is shown in Figure S1.
Pd@TiO2: Based on a reported synthesis [17], approximately 15 mg of metal precursor salt (PdCl2) was mixed with TiO2 (~500 mg) in 150 mL of Milli-Q water and sonicated for 20 min. The slurry was irradiated in a photoreactor (Luzchem Research Inc.—Ottawa, ON, Canada) equipped with 14 UVA bulbs for 24 h with vigorous stirring in a crystallizing Pyrex dish (Sigma Aldrich—St. Louis, MO, USA) with a Pyrex cover. The slurry was centrifuged and washed with Milli-Q water four times to remove unreacted metal precursor salt and dried overnight in a desiccator under vacuum. The powder obtained had a dark gray color.
Cu@TiO2: Based on a reported synthesis [17], a total of 55 mg of CuCl2·2H2O were mixed with TiO2 (~300 mg) and 95 mg of the benzoin-type photoinitiator Irgacure-2959 (Gifted from BASF) in 130 mL of Milli-Q water and purged with argon for 15 min. After this, the slurry was irradiated in a Luzchem photoreactor equipped with 14 UVA bulbs for 24 h with vigorous stirring. The slurry was filtered and washed with Milli-Q water at least seven times to remove unreacted metal precursor salt and dried overnight in a desiccator under vacuum. The powder had an intense beige color with green tones.
3.2. Catalyst Characterization
Transmission electron microscopy (TEM) images were collected on a JEM2100F FETEM (JEOL, Akishima City, Japan) operating at 200 kV. Diffuse reflectance measurements were carried out using an Agilent Cary 7000 UV-Vis-NIR Universal Measurement Spectrophotometer (Agilent, Santa Clara, CA, USA) coupled with an Agilent praying Mantis accessory. The metal composition of the materials was determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), using an Agilent Vista Pro ICP Emission Spectrometer. Approximately 10 mg portions were accurately weighed in triplicate and digested with aqua regia. Solutions were further diluted and measured by ICP-OES. The following emission lines were used for quantification when applicable: Fe 238.20 nm, Pd 340.45 nm, and Cu 327.39 nm. Powder X-ray diffraction analysis was carried out at room temperature utilizing a Bruker D8 Endeavor Polycrystalline X-Ray Diffractometer (PXRD), equipped with LynxEye XE-T 1-D silicon strip detector with 192 channels (Bruker, Billerica, MA, USA) in Bregg Brentano geometry, using Cu Kα radiation (λ = 1.5418 Å), powered at 40 kV and 25 mA (1 kW). Diffractograms were collected in a 2θ range from 20° to 90°.
3.3. Photocatalytic Activity
The photocatalytic performance of the synthetized catalysts was performed by adding 5 mL of fresh solution of nitrobenzene (0.1 M) in benzyl alcohol in a Pyrex glass tube, loaded with 10 mg of the catalyst. The solution was sonicated and purged for 10 min with N2. Irradiation was conducted using a UVA LED (λ 370 nm) with an irradiance of 13.5 mW.cm−2 (Figure S2). In the two-color experiments, the Pyrex test tube was positioned 3 cm away from the center between the UVA and blue (450 nm) LEDs. Samples were irradiated and stirred for up to 6 h. The catalysts were removed using a hydrophilic PTFE syringe filter, with 0.2 µm pore size. Quantification of products was performed by mass spectrometry in an Agilent 6890-N Gas Chromatograph with an Agilent 5973 mass selective detector, 3,5-Di-tert-butyltoluene (4 mM in dichloromethane as the solvent) as an external standard. The timed events feature was used within the setpoint control of the local user interface (OFF at 6.20 min/ON at 8.70 min) to prevent an excessive concentration of benzyl alcohol from reaching the detector. In addition to mass spectrometry, we characterized the resultant Schiff base compound using Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) spectroscopy with a Thermo Scientific Nicolet 6700 FTIR/ATR instrument (Thermo Scientific, Waltham, MA, USA). Although the FTIR spectrum was crowded in the region of the C=N stretching, the results were consistent with those obtained from the pure Schiff base compound. Please refer to Figure S18.
4. Conclusions
To the best of our knowledge, Fe3O4@TiO2 is not frequently employed in organic synthesis, including the formation of Schiff bases. However, our study demonstrates its significant potential for broader applications. Our results indicate that Fe3O4@TiO2 is highly effective in synthesizing Schiff bases, achieving approximately 99% yield after 20 h of UVA light irradiation. Notably, the reaction efficiency improved with subsequent use, showing increased production rates and coupled with solvent and catalyst reusability. Further, the introduction of dual-color irradiation enhanced the catalyst’s performance, more than doubling the yield of the desired product. In the second use, a 50% yield was achieved after just three hours, highlighting the catalyst’s improved efficiency. Our findings underscore the promising catalytic role of Fe3O4@TiO2 in the field of photocatalysis research, particularly due to its ease of separation from reaction mixtures. This catalyst offers an environmentally friendly alternative and helps mitigate the use of scarce resources such as palladium. Expanding its application to other organic transformations and exploring its potential in diverse chemical reactions could significantly enhance its utility. Additionally, studying the effects of different reaction conditions and substrates on the catalyst’s performance may uncover new optimization opportunities. These future research directions could contribute to advancing the field of photocatalysis, promoting more sustainable and efficient chemical processes while addressing emerging challenges in synthetic chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14090612/s1, Figure S1: Representative illustration of the synthesis of Fe3O4@TiO2; Figure S2: Emission spectra of light sources used in this work; Figure S3: Picture of Fe3O4@TiO2 after its synthesis; Table S1: Calculated interplanar spacing for the materials used in this study; Figure S4: Diffuse Reflectance of the catalysts used in this study; Figure S5: Tauc plots of the used catalysts; Figure S6: Chromatograms showing the peaks obtained after performing the reaction with Fe3O4@TiO2; Figure S7: Mass spectra of the products; Figure S8: Chromatograms displaying the peaks for the pure Schiff bases; Figure S9: Chromatograms showing the peaks obtained after performing the reaction with of TiO2; Figure S10: Chromatograms showing the peaks obtained after performing the reaction with Pd@TiO2; Figure S11: Chromatograms showing the peaks obtained after performing the reaction with Cu@TiO2; Table S2: Comparison table illustrating the findings of this study alongside previous research; Figure S12: Chromatogram showing the peaks obtained after 3 hours at 450 nm light irradiation; Figure S13: Chromatogram showing the results for the experiment without light; Figure S14: Image of the experimental setup for dual irradiation; Figure S15: Benzaldehyde production using Fe3O4@TiO2 as catalyst and using dual light illumination; Figure S16: XRD diffractograms of Fe3O4@TiO2 after synthesis and after use in the reaction; Figure S17: Plausible scenario to understand the activity shown by Fe3O4@TiO2 when irradiating with UVA light only. Figure S18: FTIR spectra of the initial and final solutions. References [49,50] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.C.-P. and J.C.S.; Investigation, D.R.C.d.S., M.C.-P. and J.C.S.; Resources, J.C.S.; Writing—original draft, D.R.C.d.S. and M.C.-P.; Writing—review & editing, D.R.C.d.S., M.C.-P. and J.C.S.; Supervision, J.C.S.; Funding acquisition, J.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant), the Canada Foundation for Innovation, and the Canada Research Chairs Program (CRC1).
Data Availability Statement
Original data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fieser, L.F.; Fieser, M. Advanced Organic Chemistry; Reinhold Publishing Corp: New York, NY, USA, 1961. [Google Scholar]
- Schiff, H. A new series of organic diamines. Ann. Chem. Pharm. 1866, 3, 343–370. [Google Scholar]
- Schiff, H. Sopra una nuova serie di basi organiche. Sci. Nat. Econ. Palermo 1866, 2, 201–257. [Google Scholar]
- de Souza, A.O.; Galetti, F.C.S.; Silva, C.L.; Bicalho, B.; Parma, M.M.; Fonseca, S.F.; Marsaioli, A.J.; Trindade, A.C.L.B.; Gil, R.P.F.; Bezerra, F.S.; et al. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quim. Nova 2007, 30, 1563–1566. [Google Scholar] [CrossRef]
- Jarrahpour, A.A.; Motamedifar, M.; Pakshir, K.; Hadi, N.; Zarei, M. Synthesis of Novel Azo Schiff Bases and Their Antibacterial and Antifungal Activities. Molecules 2004, 9, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Majeed, A.S.; Al-Sammarrae, K.W.; Salih, N.A.; Salimon, J.; Abdullah, B.M. Metal complexes of Schiff base: Preparation, characterization and antibacterial activity. Arab. J. Chem. 2017, 10, S1639–S1644. [Google Scholar] [CrossRef]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff bases and their metal Complexes: A review on the history, synthesis, and applications. Inorg. Chem. Commun. 2023, 150, 110451. [Google Scholar] [CrossRef]
- Urbansky, E.T. Carbinolamines and Geminal Diols in Aqueous Environmental Organic Chemistry. J. Chem. Educ. 2000, 77, 1644. [Google Scholar] [CrossRef]
- Saranya, S.; Ramesh, R.; Grzegorz Małecki, J. One-Pot Catalytic Approach for the Selective Aerobic Synthesis of Imines from Alcohols and Amines Using Efficient Arene Diruthenium(II) Catalysts under Mild Conditions. Eur. J. Org. Chem. 2017, 2017, 6726–6733. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Ikeda, M.; Tsukamoto, D.; Tanaka, S.; Hirai, T. One-pot synthesis of imines from alcohols and amines with TiO2 loading Pt nanoparticles under UV irradiation. Chem. Commun. 2011, 47, 4811–4813. [Google Scholar] [CrossRef]
- Selvam, K.; Sakamoto, H.; Shiraishi, Y.; Hirai, T. One-pot synthesis of secondary amines from alcohols and nitroarenes on TiO2 loaded with Pd nanoparticles under UV irradiation. New J. Chem. 2015, 39, 2467–2473. [Google Scholar] [CrossRef]
- Geng, L.; Jian, W.; Jing, P.; Zhang, W.; Yan, W.; Bai, F.-Q.; Liu, G. Crystal phase effect of iron oxides on the aerobic oxidative coupling of alcohols and amines under mild conditions: A combined experimental and theoretical study. J. Catal. 2019, 377, 145–152. [Google Scholar] [CrossRef]
- Sabatier, P. How I Have Been Led to the Direct Hydrogenation Method by Metallic Catalysts. Ind. Eng. Chem. Res. 1926, 18, 1005–1008. [Google Scholar] [CrossRef]
- Sabatier, P. Nobel Lecture: The Method of Direct Hydrogenation by Catalysis; Nobel Lectures, Chemistry 1901–1921; Elsevier Publishing Company: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Marina, N.; Lanterna, A.E.; Scaiano, J.C. Expanding the Color Space in the Two-Color Heterogeneous Photocatalysis of Ullmann C–C Coupling Reactions. ACS Catal. 2018, 8, 7593–7597. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Cely-Pinto, M.; Wang, B.; Scaiano, J.C. Photocatalytic Semi-Hydrogenation of Alkynes: A Game of Kinetics, Selectivity and Critical Timing. Nanomaterials 2023, 13, 2390. [Google Scholar] [CrossRef]
- da Silva, D.R.C.; Scaiano, J.C. Exploring the Antibacterial Properties of Lignin-coated Magnetic Nanoparticles Synthesized in a One-pot Process. Photochem. Photobiol. 2023, 99, 706–715. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc plot method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Zhang, Y.; Fulajtárová, K.; Kubů, M.; Mazur, M.; Hronec, M.; Čejka, J. Electronic/steric effects in hydrogenation of nitroarenes over the heterogeneous Pd@BEA and Pd@MWW catalysts. Catal. Today 2020, 345, 39–47. [Google Scholar] [CrossRef]
- Hirao, H.; Ohwada, T. Theoretical study of reactivities in electrophilic aromatic substitution reactions: Reactive hybrid orbital analysis. J. Phys. Chem. A 2003, 107, 2875–2881. [Google Scholar] [CrossRef]
- Tong, J.; Wang, J.; Shen, X.; Zhang, H.; Wang, Y.; Fang, Q.; Chen, L. One-Pot Synthesis of Schiff Bases by Defect-Induced TiO2–x-Catalyzed Tandem Transformation from Alcohols and Nitro Compounds. Inorg. Chem. 2021, 60, 10715–10721. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Katayama, M.; Shiraishi, Y.; Sakamoto, H.; Wang, K.; Ohtani, B.; Ichikawa, S.; Tanaka, S.; Hirai, T. One-pot synthesis of imines from nitroaromatics and alcohols by tandem photocatalytic and catalytic reactions on degussa (Evonik) P25 titanium dioxide. ACS Appl. Mater. Interfaces 2015, 7, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.; Ramón, D.J.; Yus, M. Impregnated ruthenium on magnetite as a recyclable catalyst for the N-alkylation of amines, sulfonamides, sulfinamides, and nitroarenes using alcohols as electrophiles by a hydrogen autotransfer process. J. Org. Chem. 2011, 76, 5547–5557. [Google Scholar] [CrossRef]
- Zanardi, A.; Mata, J.A.; Peris, E. One-Pot Preparation of Imines from Nitroarenes by a Tandem Process with an Ir–Pd Heterodimetallic Catalyst. Chem. Eur. J. 2010, 16, 10502–10506. [Google Scholar] [CrossRef]
- Wu, J.; Darcel, C. Iron-catalyzed hydrogen transfer reduction of nitroarenes with alcohols: Synthesis of imines and AZA heterocycles. J. Org. Chem. 2020, 86, 1023–1036. [Google Scholar] [CrossRef]
- Pérez, J.M.; Cano, R.; Yus, M.; Ramón, D.J. Straightforward Synthesis of Aromatic Imines from Alcohols and Amines or Nitroarenes Using an Impregnated Copper Catalyst. Eur. J. Org. Chem. 2012, 24, 4548–4554. [Google Scholar] [CrossRef]
- Zou, G.; Cao, R.; Cui, C.; Luo, Y.; Huang, C.; Cui, X.; Wang, Z.; Song, Y. Photocatalytic one-pot alkylation of nitrobenzene with benzyl alcohol for the precise synthesis of N-benzylideneaniline over F-doped Bi2MoO6 nanosheets. Catal. Sci. Technol. 2023, 13, 3916–3926. [Google Scholar] [CrossRef]
- Higashimoto, S.; Nakai, Y.; Azuma, M.; Takahashi, M.; Sakata, Y. One-pot synthesis of imine from benzyl alcohol and nitrobenzene on visible-light responsive CdS–TiO2 photocatalysts. RSC Adv. 2014, 4, 37662–37668. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Zhang, H.; Liu, M.; Zhang, W.; Xu, D.; Gao, J. Direct reductive coupling of nitroarenes and alcohols catalysed by Co-N-C/CNT@AC. Green Chem. 2019, 21, 2129–2137. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Y.; Wu, Y.; Zhang, X.; Wang, X.; Chen, S. Constructing a system for effective utilization of photogenerated electrons and holes: Photocatalytic selective transformation of aromatic alcohols to aromatic aldehydes and hydrogen evolution over Zn3In2S6 photocatalysts. Appl. Catal. B Environ. 2019, 242, 302–311. [Google Scholar] [CrossRef]
- Gawargy, T.A.; Costa, P.; Lanterna, A.E.; Scaiano, J.C. Photochemical benzylic radical arylation promoted by supported Pd nanostructures. Org. Biomol. Chem. 2020, 18, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Wang, Q.-X.; Yi, J.; Chen, L.; Wu, D.-Y.; Wang, Y.; Xie, Z.-X.; Moskovits, M.; Tian, Z.-Q. Plasmonic nanoreactors regulating selective oxidation by energetic electrons and nanoconfined thermal fields. Sci. Adv. 2021, 7, eabf0962. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Ye, X.; Zhang, J.; Zhang, J.; Zhang, S.; Meng, S.; Fu, X.; Chen, S. Solvothermal synthesis of CdIn2S4 photocatalyst for selective photosynthesis of organic aromatic compounds under visible light. Sci. Rep. 2017, 7, 27. [Google Scholar] [CrossRef]
- Dai, X.; Xie, M.; Meng, S.; Fu, X.; Chen, S. Coupled systems for selective oxidation of aromatic alcohols to aldehydes and reduction of nitrobenzene into aniline using CdS/g-C3N4 photocatalyst under visible light irradiation. Appl. Catal. B Environ. 2014, 158–159, 382–390. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, M.J.; Park, M.; Kim, K.-j.; Lee, H.; Kim, H.S. Selective photocatalytic conversion of benzyl alcohol to benzaldehyde or deoxybenzoin over ion-exchanged CdS. Appl. Catal. B Environ. 2022, 304, 120967. [Google Scholar] [CrossRef]
- Ning, X.; Meng, S.; Fu, X.; Ye, X.; Chen, S. Efficient utilization of photogenerated electrons and holes for photocatalytic selective organic syntheses in one reaction system using a narrow band gap CdS photocatalyst. Green Chem. 2016, 18, 3628–3639. [Google Scholar] [CrossRef]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Xu, L.; Meng, L.; Zhang, X.; Mei, X.; Guo, X.; Li, W.; Wang, P.; Gan, L. Promoting Fe3+/Fe2+ cycling under visible light by synergistic interactions between P25 and small amount of Fenton reagents. J. Hazard. Mater. 2019, 379, 120795. [Google Scholar] [CrossRef]
- Aguinaco, A.; Mánuel, J.M.; Blanco, E.; Domínguez, M.; Litrán, R.; Delgado, J.J.; Ramírez-del-Solar, M. Fe3O4-TiO2 Thin Films in Solar Photocatalytic Processes. Materials 2022, 15, 6718. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Yaghmaei, M.; da Silva, D.R.C.; Rutajoga, N.; Currie, S.; Li, Y.; Vallieres, M.; Joshi, N.; Wang, B. Visible light photocatalytic water remediation strategies using a novel black TiO2 based material optimized for in-flow applications. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Liu, H.; Di Valentin, C. Band Gap in Magnetite above Verwey Temperature Induced by Symmetry Breaking. J. Phys. Chem. C 2017, 121, 25736–25742. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xie, T.; Lu, Y.; Fan, H.; Wang, D. Synthesis, photoelectric properties and photocatalytic activity of the Fe2O3/TiO2 heterogeneous photocatalysts. Phys. Chem. Chem. Phys. 2010, 12, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge Carrier Dynamics at TiO2 Particles: Reactivity of Free and Trapped Holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Nolan, M. Electronic coupling in iron oxide-modified TiO2 leads to a reduced band gap and charge separation for visible light active photocatalysis. Phys. Chem. Chem. Phys. 2011, 13, 18194–18199. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, Y.; Ling, C.; Ding, R.; Wang, X.; Zhang, X.; Chen, S. One-Pot Synthesis of Schiff Base Compounds via Photocatalytic Reaction in the Coupled System of Aromatic Alcohols and Nitrobenzene Using CdIn2S4 Photocatalyst. Dalton Trans. 2018, 47, 10915–10924. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Zhang, S.; Meng, S.; Fu, X.; Wang, X.; Zhang, X.; Chen, S. Photocatalytic Synthesis of Schiff Base Compounds in the Coupled System of Aromatic Alcohols and Nitrobenzene Using CdXZn1−XS Photocatalysts. J. Catal. 2018, 359, 151–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).