Catalytic Decontamination of Carbon Monoxide Using Strong Metal–Support Interactions on TiO2 Microparticles
Abstract
:1. Introduction
2. Results
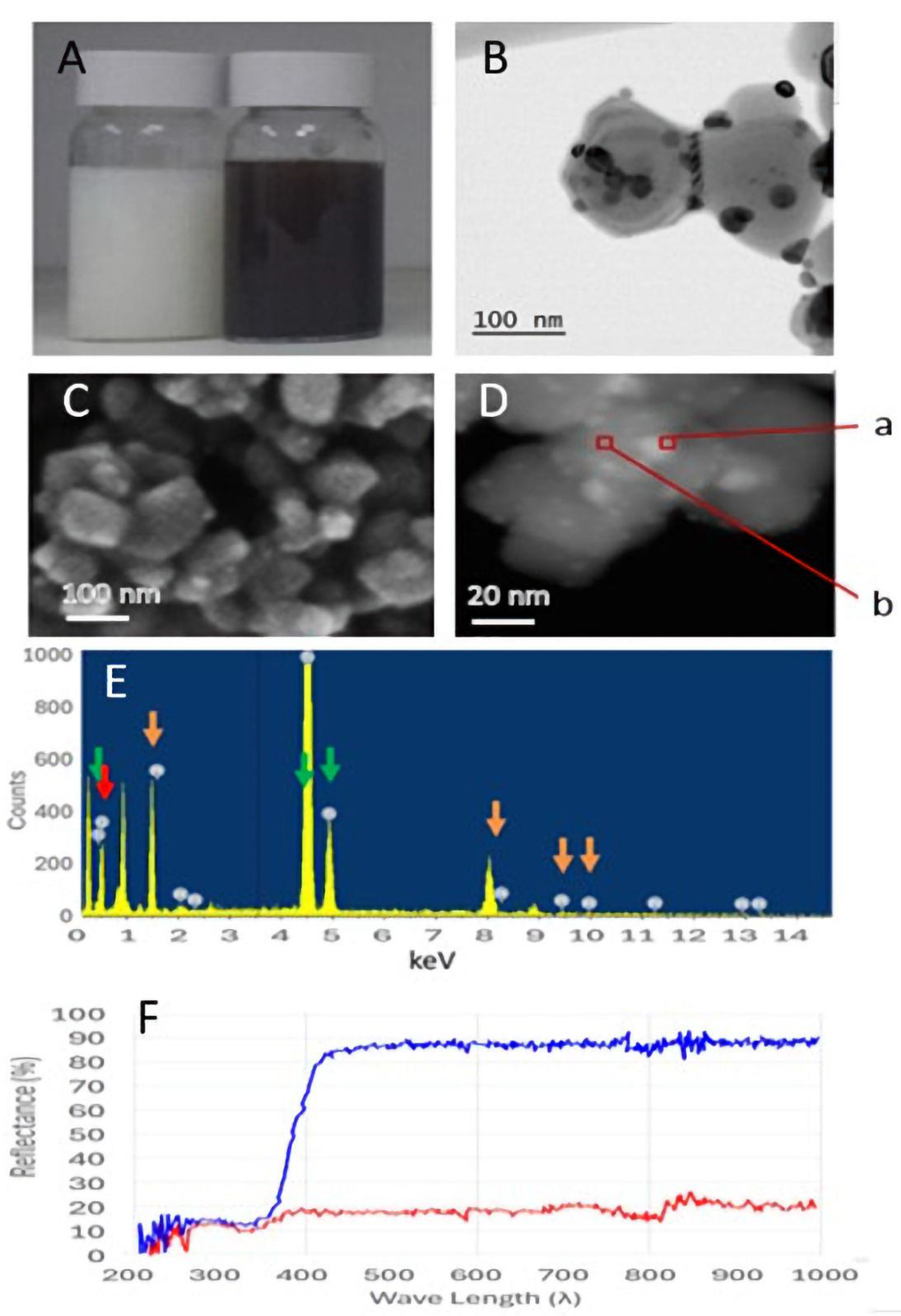
2.1. Structural Properties of TiO2-Pt Microparticles
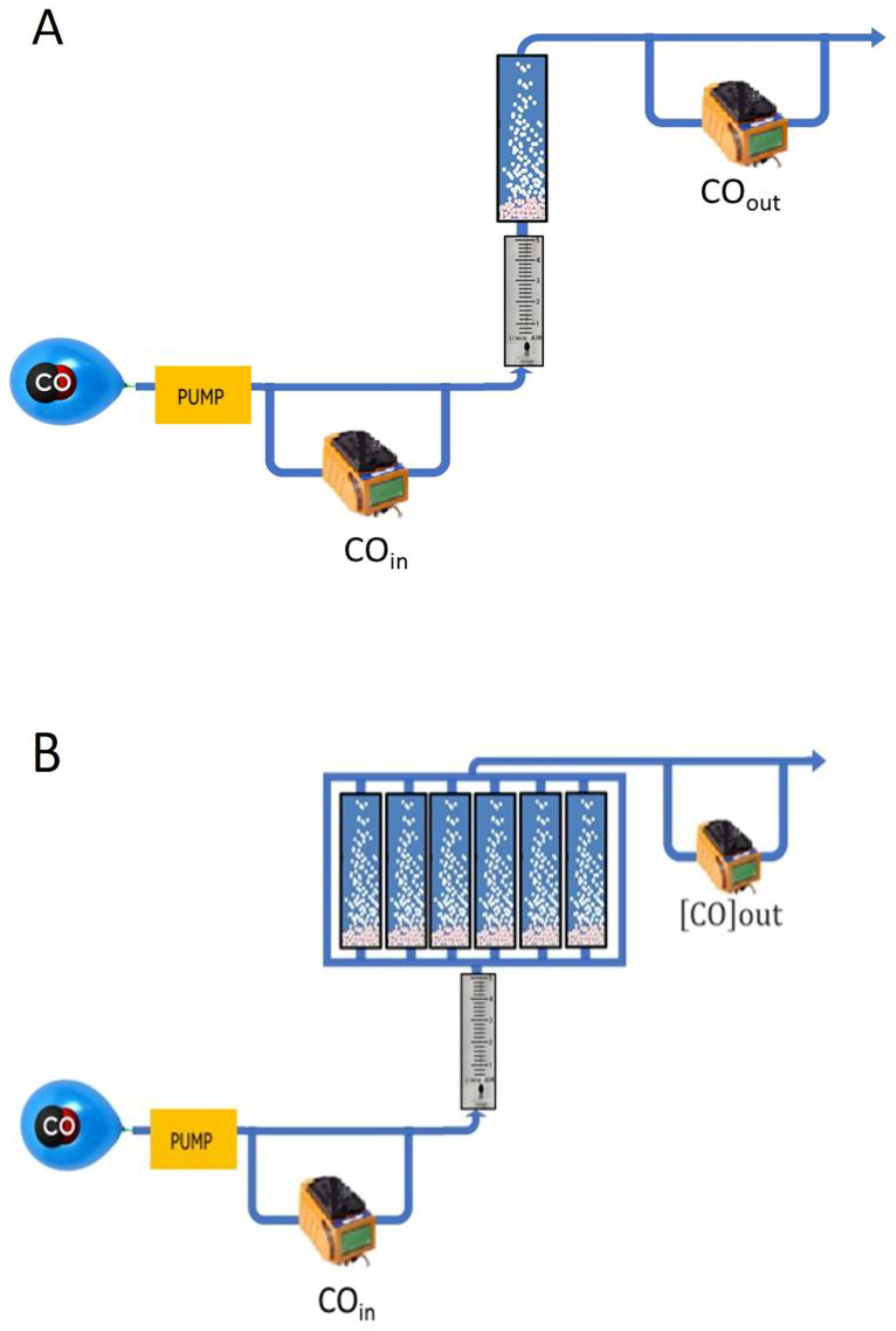
2.2. Modes of Operation
2.2.1. The Environmental Model
| k (1/min) | α | t1/2 (min) | |
|---|---|---|---|
| TiO2 | 0.000 | 0.000 | |
| Max * | 0.150 | 1.000 | 4.6 |
| TiO2-Ag | 0.001 | 0.007 | 693.1 |
| TiO2-Au | 0.001 | 0.007 | 693.1 |
| TiO2-Pd | 0.027 | 0.180 | 25.7 |
| TiO2-Pt | 0.085 | 0.567 | 8.2 |
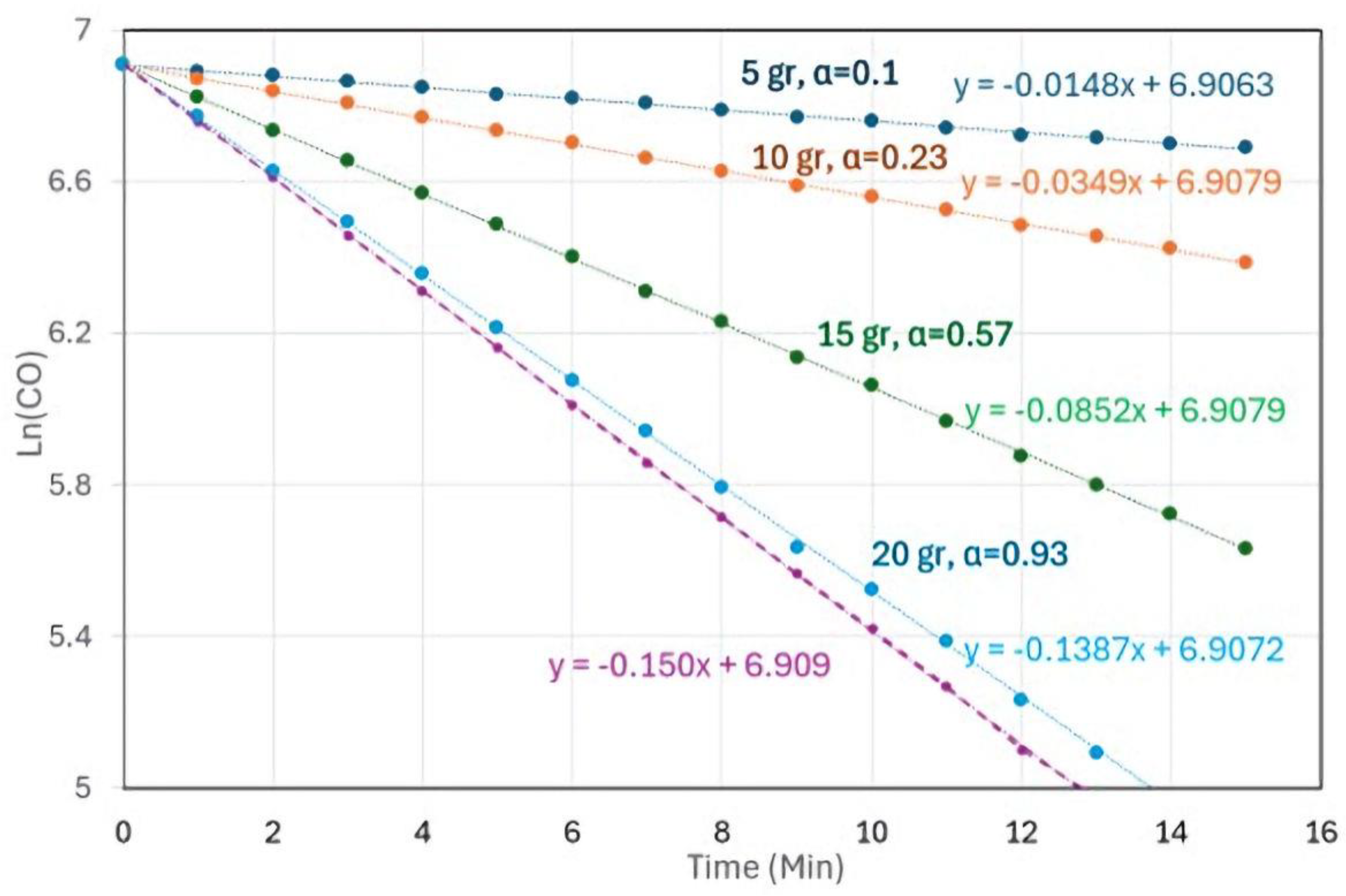
2.2.2. The Linear (Personal) Model
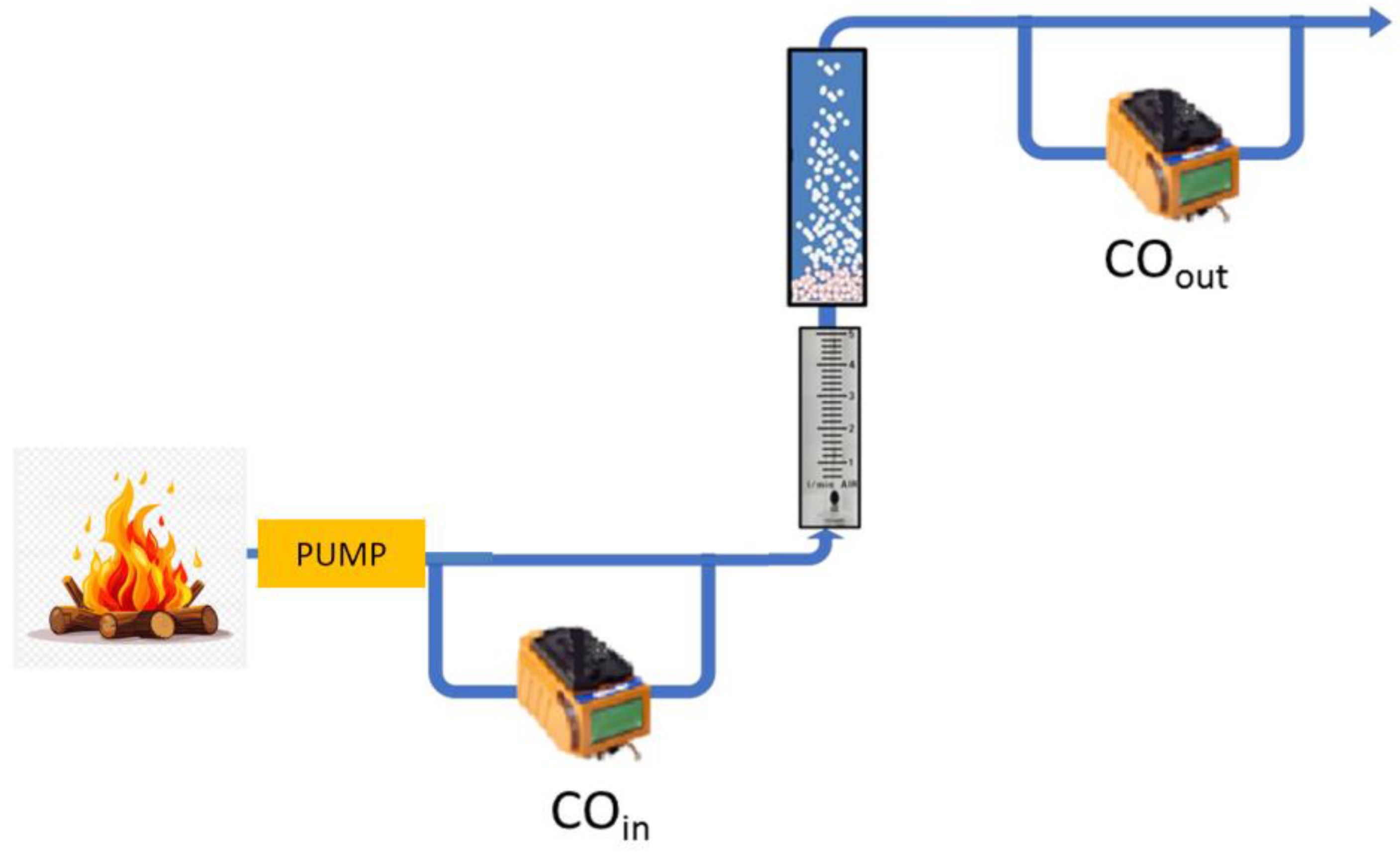
2.2.3. Case Study of Neutralizing CO Produced by a Fire
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Catalyst Preparation
TiO2-Pt
TiO2-Ag, TiO2-Au, and TiO2-Pd
4.2.2. TiO2-Pt Characterization
HRSEM and EDS Analyses
TEM Analysis
ICP Analysis
Reflectance Spectrum
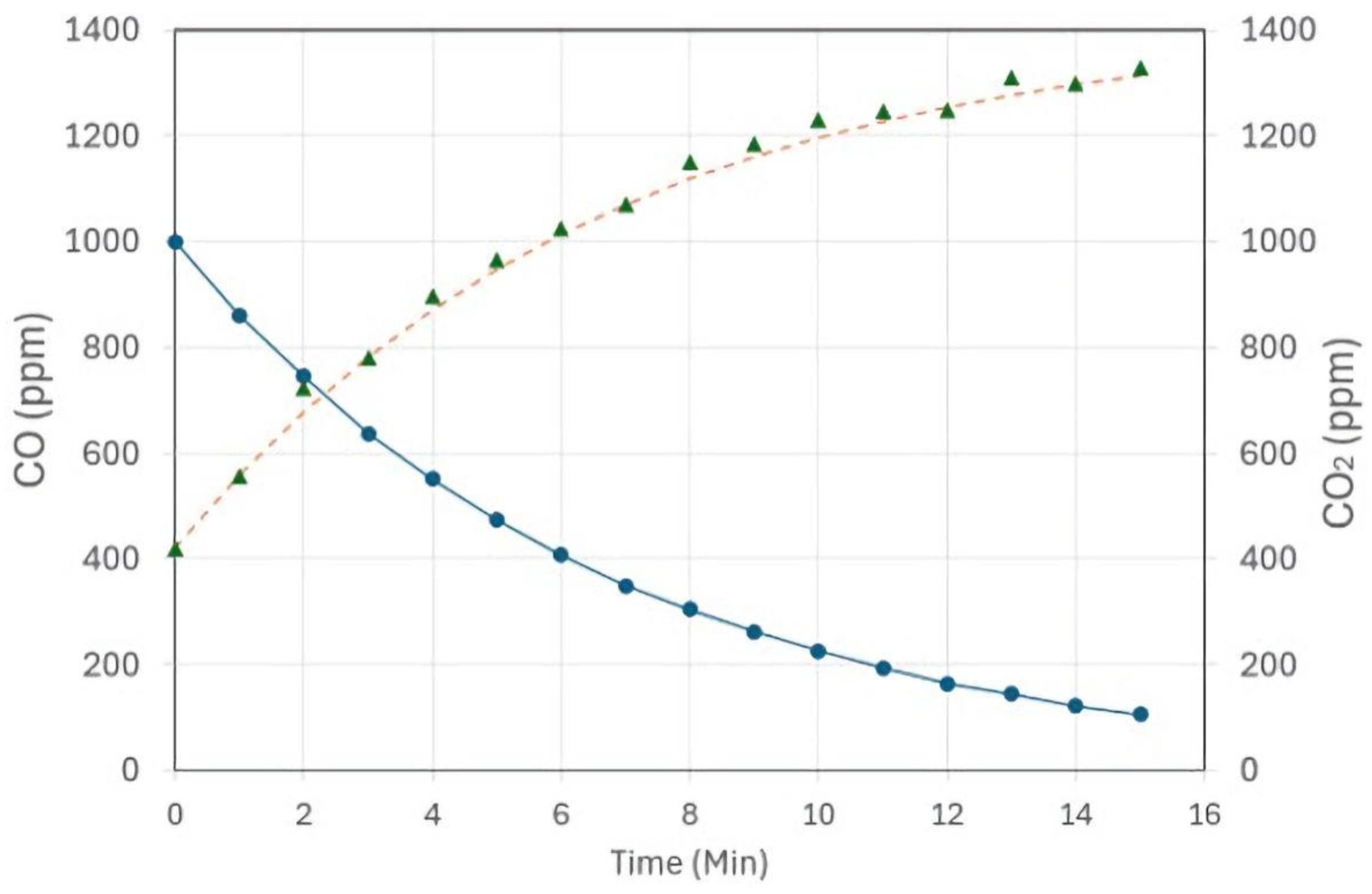
4.2.3. CO and CO2 Measurements
4.2.4. Calculation of Catalytic Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryter, S.W.; Otterbein, L.E. Carbon monoxide in biology and medicine. Bioessays 2004, 26, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Roughton, F.; Darling, R. The effect of carbon monoxide on the oxyhemoglobin dissociation curve. Am. J. Physiol.-Leg. Content 1944, 141, 17–31. [Google Scholar] [CrossRef]
- Sangalli, B.; Bidanset, J. A review of carboxymyoglobin formation: A major mechanism of carbon monoxide toxicity. Vet. Hum. Toxicol. 1990, 32, 449–453. [Google Scholar] [PubMed]
- Piantadosi, C.A. Biological chemistry of carbon monoxide. Antioxid. Redox Signal. 2002, 4, 259–270. [Google Scholar] [CrossRef]
- Coburn, R.F. Mechanisms of carbon monoxide toxicity. Prev. Med. 1979, 8, 310–322. [Google Scholar] [CrossRef]
- Burgess, J.L.; Brodkin, C.A.; Daniell, W.E.; Pappas, G.P.; Keifer, M.C.; Stover, B.D.; Edland, S.D.; Barnhart, S. Longitudinal decline in measured firefighter single-breath diffusing capacity of carbon monoxide values: A respiratory surveillance dilemma. Am. J. Respir. Crit. Care Med. 1999, 159, 119–124. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; Kontos, A.G.; De la Cruz, A.A.; O’shea, K.; Dionysiou, D.D. Synthesis, structural characterization and evaluation of sol–gel-based NF-TiO2 films with visible light-photoactivation for the removal of microcystin-LR. Appl. Catal. B Environ. 2010, 99, 378–387. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Dayan, A.; Mor Yosef, R.; Risphon, J.; Tuval, E.; Fleminger, G. In Situ Detoxification of Venomous Agent X Surrogate Profenofos by Doped Titanium Dioxide Nanoparticles under Illumination at the UV and Visible Ranges. J. Phys. Chem. A 2019, 123, 9456–9461. [Google Scholar] [CrossRef] [PubMed]
- Dayan, A.; Fleminger, G.; Ashur-Fabian, O. RGD-modified dihydrolipoamide dehydrogenase conjugated to titanium dioxide nanoparticles–switchable integrin-targeted photodynamic treatment of melanoma cells. RSC Adv. 2018, 8, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Mukherjee, A.; Roy, K.; Bagchi, J.; Mondal, K. Catalytic converter in automobile exhaust emission. J. Res 2016, 2, 29–33. [Google Scholar]
- Khoiron, A.M.; Anis, S.; Masugino, S.S.M.; Fajri, S.N. Catalytic converter based on titanium oxide (TiO2) to reduce the emission of carbon monoxide and hydrocarbon in exhaust gas of motor vehicles. EIC 2020, 2018, 15–20. [Google Scholar]
- Tauster, S.; Fung, S. Strong metal-support interactions: Occurrence among the binary oxides of groups IIA–VB. J. Catal. 1978, 55, 29–35. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Baker, R.; Horsley, J. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, J.; Zhang, Y.; Pan, C.; Dong, Y.; Zhu, Y. Metal-support interaction for heterogeneous catalysis: From nanoparticles to single atoms. Mater. Today Nano 2020, 12, 100093. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, M.G.; Shin, D.; Han, J.W. Design of ceria catalysts for low-temperature CO oxidation. ChemCatChem 2020, 12, 11–26. [Google Scholar] [CrossRef]
- Pu, T.; Zhang, W.; Zhu, M. Engineering heterogeneous catalysis with strong metal–support interactions: Characterization, theory and manipulation. Angew. Chem. Int. Ed. 2023, 62, e202212278. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhao, G.; Pan, H.; Sun, W. Strong metal–support interaction in heterogeneous catalysts. Adv. Energy Mater. 2022, 12, 2201395. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Zhang, G.; Qiu, W.; He, H.; Chen, G. High active platinum clusters on titanium dioxide supports toward carbon monoxide oxidation. Appl. Catal. B Environ. 2020, 266, 118629. [Google Scholar] [CrossRef]
- Li, N.; Chen, Q.-Y.; Luo, L.-F.; Huang, W.-X.; Luo, M.-F.; Hu, G.-S.; Lu, J.-Q. Kinetic study and the effect of particle size on low temperature CO oxidation over Pt/TiO2 catalysts. Appl. Catal. B Environ. 2013, 142, 523–532. [Google Scholar] [CrossRef]
- Bond, G.C. The origins of particle size effects in heterogeneous catalysis. Surf. Sci. 1985, 156, 966–981. [Google Scholar] [CrossRef]
- Green, R.; Morrall, P.; Bowker, M. CO Spillover and Oxidation on Pt/TiO2. Catal. Lett. 2004, 98, 129–133. [Google Scholar] [CrossRef]
- Song, J.; Yang, Y.; Liu, S.; Li, L.; Yu, N.; Fan, Y.; Chen, Z.; Kuai, L.; Geng, B. Dispersion and support dictated properties and activities of Pt/metal oxide catalysts in heterogeneous CO oxidation. Nano Res. 2021, 14, 4841–4847. [Google Scholar] [CrossRef]
- Li, M.; Noriega-Trevino, M.E.; Nino-Martinez, N.; Marambio-Jones, C.; Wang, J.; Damoiseaux, R.; Ruiz, F.; Hoek, E.M. Synergistic bactericidal activity of Ag-TiO2 nanoparticles in both light and dark conditions. Environ. Sci. Technol. 2011, 45, 8989–8995. [Google Scholar] [CrossRef]
- Shelly, S.; Zaltsman, S.L.; Ben-Gal, O.; Dayan, A.; Ganmore, I.; Shemesh, C.; Atrakchi, D.; Garra, S.; Ravid, O.; Rand, D. Potential neurotoxicity of titanium implants: Prospective, in-vivo and in-vitro study. Biomaterials 2021, 276, 121039. [Google Scholar] [CrossRef]

| Flow Rate (L/min) | COin (ppm) | COout (ppm) | α |
|---|---|---|---|
| 1.0 | 1370 | <10 | 0.99 |
| 1.2 | 1804 | <10 | 0.99 |
| 1.5 | 1610 | <10 | 0.99 |
| 1.8 | 1624 | 10 | 0.99 |
| 2.0 | 1634 | 91 | 0.94 |
| 3.0 | 1720 | 856 | 0.55 |
| Mass (g) | FRmax (L/min) | Residence Time (s) | Activity (µmol/min) | Sp. Activity (µmol/g/min) |
|---|---|---|---|---|
| 12.8 | 1.8 | 1.33 | 73.8 | 5.76 |
| 20.0 | 3.0 | 0.80 | 123.0 | 6.15 |
| 30.0 | 3.7 | 0.65 | 151.6 | 5.05 |
| 42.8 | 6.0 | 0.40 | 245.9 | 5.75 |
| 50.0 | 7.0 | 0.34 | 286.9 | 5.74 |
| T (min) | COin (ppm) | COout (ppm) | α |
|---|---|---|---|
| 1 | 96 | 3 | 0.97 |
| 2 | 24 | 5 | 0.79 |
| 3 | 201 | 5 | 0.98 |
| 4 | 214 | 5 | 0.98 |
| 5 | 474 | 5 | 0.99 |
| 6 | 464 | 5 | 0.99 |
| 7 | 363 | 9 | 0.98 |
| 8 | 1007 | 7 | 0.99 |
| 9 | 770 | 9 | 0.99 |
| 10 | 989 | 8 | 0.99 |
| 11 | 79 | 5 | 0.94 |
| 12 | 20 | 3 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayan, A.; Alter, J.; Fleminger, G. Catalytic Decontamination of Carbon Monoxide Using Strong Metal–Support Interactions on TiO2 Microparticles. Catalysts 2024, 14, 622. https://doi.org/10.3390/catal14090622
Dayan A, Alter J, Fleminger G. Catalytic Decontamination of Carbon Monoxide Using Strong Metal–Support Interactions on TiO2 Microparticles. Catalysts. 2024; 14(9):622. https://doi.org/10.3390/catal14090622
Chicago/Turabian StyleDayan, Avraham, Jacob Alter, and Gideon Fleminger. 2024. "Catalytic Decontamination of Carbon Monoxide Using Strong Metal–Support Interactions on TiO2 Microparticles" Catalysts 14, no. 9: 622. https://doi.org/10.3390/catal14090622








