Abstract
Biodiesel is a renewable and sustainable alternative fuel to petrol-derived diesel. Decreasing the operating costs by improving the catalyst’s characteristics is an effective way to increase the competitiveness of biodiesel in the fuel market. An aqueous solution of sodium methoxide (CH3ONa), which is a traditional alkaline catalyst, was immersed in nanometer-sized particles of titanium dioxide (TiO2) powder to prepare the strong alkaline catalyst TiO2/CH3ONa. The immersion method was used to enhance the transesterification reaction. The mixture of TiO2 and CH3ONa was calcined in a high-temperature furnace in a range between 150 and 450 °C continuously for 4 h. The heterogeneous alkaline catalyst TiO2/CH3ONa was then used to catalyze the strong alkaline transesterification reaction of palm oil with methanol. The highest content of fatty acid methyl esters (FAMEs), which amounted to 95.9%, was produced when the molar ratio of methanol to palm oil was equal to 6, and 3 wt.% TiO2/CH3ONa was used, based on the weight of the palm oil. The FAMEs produced from the above conditions were also found to have the lowest kinematic viscosity of 4.17 mm2/s, an acid value of 0.32 mg KOH/g oil, and a water content of 0.031 wt.%, as well as the highest heating value of 40.02 MJ/kg and cetane index of 50.05. The lower catalyst amount of 1 wt.%, in contrast, resulted in the lowest cetane index of 49.31. The highest distillation temperature of 355 °C was found when 3 wt.% of the catalyst was added to the reactant mixture with a methanol/palm oil molar ratio of 6. The prepared catalyst is considered effective for improving the fuel characteristics of biodiesel.
1. Introduction
Biodiesel has been considered a promising alternative to diesel fuel for diesel engines or boilers due to its relatively lower elemental carbon content, superior combustion efficiency, low toxic gas emissions, and almost no sulfur oxide pollutants [1]. Biodiesel also has the excellent characteristics of biodegradability and lubricity for the moving parts of combustion engines [2]. Available sources of feedstock oils, including animal fats, vegetable oils, and microalgae lipids, are pivotal for determining the price of biodiesel products and application extent in the energy industry. To strengthen its competitiveness in the fuel market with traditional fossil fuel, degraded feedstock oils, inedible plant oils, and even inferior lignocellulose biomass are used to produce biodiesel to reduce its cost [3]. However, these raw materials, with poor properties, often result in low fuel quality, which leads to the abnormal operation of combustion engines or burners, and in turn, they frequently fail or even shut down. Adopting an effective catalyst could facilitate the transesterification reaction of these degraded raw oils with short-chained alcohol [4] and improve the fuel characteristics of biodiesel products. Three major types of catalysts have been developed for the transesterification process in biodiesel production, including enzymatic, homogeneous, and heterogeneous catalysts [5]. The former two catalysts are more effective in biodiesel production but have a few drawbacks, including high water and oil consumption and costly purification processes [6]. In contrast, heterogeneous catalysts bear the advantages of highly reduced production costs and are much more environmentally friendly [7]. The representative catalysts of the first type are potassium hydroxide (KOH), sodium hydroxide (NaOH), and sodium methoxide (CH3ONa), which are most frequently applied in industrial biodiesel production [8]. The conversion rate from feedstock oil to biodiesel might reach as high as 98% depending on the feedstock oil used, reaction time and temperature, and catalyst material [9].
Biodiesel is produced from various types of edible and non-edible recycled waste oils, including straight vegetable oil (SVO), hydrogenated vegetable oil (HVO), animal fats, or microalgae lipid [10] primarily composed of triglycerides, through a transesterification reaction under the catalytic effects of a heterogeneous or homogeneous reaction [11]. Strong alkaline catalysts are mostly used in the biodiesel production industry primarily due to their high tolerance for both the contents of free fatty acids and moisture [12]. The transesterification of strong alkaline catalysts also curtails the occurrence of saponification, increasing the formation of fatty acid methyl esters and reducing the post-treatment processes after biodiesel production [13]. Biodiesel is regarded as a crucial sustainable and renewable energy resource. The fuel characteristics of biodiesel are required to meet the specifications, such as ASTM D6751 or EN 14214 [14].
Both the contents of water and free fatty acids (FFAs) in the feedstocks change the transesterification process. The molar ratio of methanol to vegetable oil is another fundamental factor that alters ester formation [15]. Chuah et al. [16] found that the maximum ester conversion occurred at a molar ratio of 6:1. The primary pros of enzymatic catalysts are that they react at low temperatures, have a high yield, and are eco-friendly, but they have high costs, are sensitive to high temperatures, and have a low reaction rate, inhibiting their wide applicability in industrial practices [17].
Titania has three phases, including nature anatase, rutile, and brookite, which are used for various applications [18]. The activity and selectivity of titanium catalysts are expanding in industrial practices. The titania-based catalysts used for biodiesel production include sulfated titanium dioxide [19], such as SO42−/TiO2, SO42−/TiO2-ZrO2, SO42−/TiO2-SiO2, SO42−/TiO2/La3+, etc. The production methods for these TiO2-based catalyst are sol-gel, precipitation, wet impregnation, solid-state, etc. [20]. Titanium dioxide (TiO2) is currently the most widely used catalyst material for wastewater treatment, the degradation of organic chemicals, and other industrial applications. Titanium dioxide is an n-type semiconductor. Its chemical properties are highly stable, and it will not react with most chemical reagents under normal circumstances [21]. Titanium dioxide has two crystalline forms, including rutile and anatase phases. Titanium dioxide of the anatase phase has superior photocatalytic activity and is used in most photocatalytic applications [22]. In addition, sodium methoxide (CH3ONa) is frequently used as a catalyst to enhance the transesterification reaction for the biodiesel production industry. Udayakumar [23] used a bentonite-supported sodium methoxide catalyst to accelerate biodiesel production from used cooking sunflower oil at three different temperatures, which were 50, 55, and 60 °C. The optimum transesterification conditions were a reaction time of 70 min and a methanol/oil molar ratio of 12. Shiferaw et al. [24] prepared a supported catalyst based on zeolite Y doped with sodium methoxide (CH3ONa) for manufacturing biodiesel from waste cooking oil. The CH3ONa/zeolite was revealed to be a prominent catalyst through a solvent-free and ball-milling process. The optimum reaction conditions for the zeolite-supported CH3ONa catalyst were 20% catalyst loading, a reaction temperature of 60 °C, and a molar methanol/oil ratio of 16. In addition, the CH3ONa/zeolite was considered to have a rather high catalytic activity and a high oil treatment rate, which reached 250 g of oil per gram of catalyst. Carbonaceous catalysts, including biochar and activated carbon from agricultural residues, have been applied for biodiesel production [25] by adopting their texture characteristics. Deep eutectic solvents (DESs) belong to the class of ionic liquids (ILs), which are non-toxic and environmentally friendly. Some DESs, which are composed of halide salts and a hydrogen-bonding organic material, were found to be a promising catalyst, co-solvent, and extracting agent in biodiesel production processes [26]. Homogeneous, heterogeneous, and enzyme catalysts are available for biodiesel production via transesterification and esterification [27].
The yield and fuel characteristics of fatty acid methyl esters (FAMEs) were compared between biodiesel prepared from waste cooking oil using a homogeneous catalyst of sodium methoxide (CH3ONa) and a heterogeneous catalyst of calcium oxide (CaO). The biodiesel yields using CH3ONa and CaO were 80.6% and 76.1%, while the heating values at 40 °C were 34.02 and 30.84 MJ/kg, respectively [28]. However, a homogeneous catalyst might result in higher FAME production and superior fuel characteristics than a heterogeneous catalyst [29]. The latter catalyst generally requires fewer treatment procedures and lower operating costs for the downstream process of a biodiesel product and is considered more competitive for industrial biodiesel production. Based on their proton strength, heterogeneous catalysts can be divided into non-metal oxides, metal phosphates and acids, cation-exchange resins, metal oxides, alkaline earth oxides, alkaline oxides, etc. [30]. Heterogeneous catalysts can be recycled and reused until their deactivation, and thus, they are more cost-effective in biodiesel production [31]. The strong alkaline catalyst sodium methoxide (CH3ONa) has been widely used to catalyze the transesterification reaction for biodiesel production. For example, Lin et al. [32] used 1 wt.% strong alkaline catalyst CH3ONa, based on the weight of soybean oil, to manufacture soybean-oil biodiesel under a reacting temperature of 60 °C for 1 h using the transesterification process. Chen et al. [33] found that the use of a pure CH3ONa catalyst resulted in a higher biodiesel yield than a NaOH catalyst. Using a heterogeneous catalyst, MgO, 5.6 tons was required to produce 100,000 tons of biodiesel, while 88 tons of homogeneous NaOH catalyst was consumed to produce 8000 tons of biodiesel [34]. This is partly because heterogeneous catalysts can be recycled and reused. In addition, homogeneous catalysts produce waste oil due to their mixing with the product. The separation and purification processes, such as washing and distillation, require much higher input energy and operating costs [35].
Pure TiO2 nanoparticles have no effect on the catalyzing transesterification reaction. However, a catalyst that unites TiO2 with some suitable metallic or non-metallic oxides can result in highly efficient biodiesel production through transesterification [36]. In particular, TiO2 nanoparticles have promising catalytic and distinctive semiconducting properties; they are non-toxic, biocompatible, and chemically stable, and they have a large surface area and high photocatalytic activity [37]. TiO2 catalysts have been applied in water splitting for H2 generation, the photo degradation of organic molecules, the disinfection of waste water, self-cleaning coatings for buildings, etc. In addition, TiO2 is widely used as a white pigment due to its high refractive index and small particle size of around 250 nm. When TiO2 is excited by light irradiation with an energy equal or higher than the band gap of 3.2 ev, electron/hole pairs of TiO2 can be generated. This leads to the occurrence of photocatalytic properties [38]. TiO2 characteristics can be further modified by doping other adequate materials. For example, Azer et al. [39] investigated the effects of doping Cl and F in TiO2 on the particle structure and properties. They found that the F- and Cl-doped TiO2 powders were suppressed by their transformation from amorphous to anatase and from anatase to rutile. The doped anatase powders were more toxic and hemolytic than the undoped anatase powders. A study on biodiesel production using a heterogeneous catalyst of TiO2 impregnated with CH3ONa has not been found in the literature, although the catalyst is considered promising for the efficient transesterification of raw oil with methanol [40]. Moreover, the fuel characteristics of biodiesel produced through transesterification by adding the heterogeneous catalyst TiO2/CH3ONa to the reactant mixture have not been investigated yet [41]. Palm oil has become the primary source of edible oil for biodiesel production, accounting for 45 wt.% of all edible oils used for the production of biodiesel [42]. Palm oil also has the highest oil content (5000 kg oil/ha) among all available edible oils for biodiesel production [43]. Hence, a heterogeneous catalyst of TiO2 sintered and calcined with CH3ONa was used to enhance the biodiesel production rate of palm oil and improve the biodiesel properties. The biodiesel properties of heating value, kinematic viscosity, and acid value produced in this study were analyzed to explore the optimum reaction conditions, including the methanol/palm oil molar ratio and the amount of catalyst added.
2. Results and Discussion
Biodiesel was produced through the transesterification reaction of palm oil with methanol under various molar ratios of methanol to palm oil ranging from 5 to 9 and different amounts of TiO2/CH3ONa catalyst. The techniques and preparation methods used in this study can be used in large-scale industrial applications according to the analysis of Sun et al. [44]. After they reviewed the application of a TiO2-based catalyst in biomass utilization and chemical synthesis, they found that the preparation method for the catalyst and biodiesel production can be developed for use in industrial-scale production [45]. The effects of the production parameters on the fuel properties of the biodiesel are analyzed and discussed in the following sections.
2.1. Effects of TiO2/CH3ONa Catalyst and Molar Ratio on Methyl Ester Formation
Fatty acid methyl esters (FAMEs) are produced through the transesterification of triglycerides contained in animal fats, vegetable oils, and algae lipids. The complete degree of transesterification can be judged by the FAME formation content.
The highest methyl ester content, 95.95%, was produced under the reaction conditions of an alcohol/oil molar ratio of 6 and 3 wt.% of a solid-state TiO2/CH3ONa catalyst based on the weight of palm oil. Decreasing the catalyst weight ratio from 3 wt.% to 1 wt. % of the palm oil weight reduced the formation of FAME to 94.0%. Chuah et al. [46] found that too much or too little methanol added to the reactant mixture resulted in various degrees of adverse effects on the transesterification reaction. Based on the results of other molar ratios of alcohol/oil, it can be seen that the methyl ester content increased significantly with the increase in the amount of the catalyst. When the alcohol/oil molar ratio was 5, there was a relatively lower amount of methanol causing the formation of saponification products, and therefore, lower methyl esters in turn [47]. The FAME formation increased with the increase in the alcohol/oil molar ratio until it reached 6. The continual increase in the alcohol/oil molar ratio to a value higher than 6 reduced the formation of methyl esters. This was due to too much methanol mixed with an insufficient amount of the catalyst added to the palm oil at a higher molar ratio than 8. The increase in the catalyst addition from 1 wt.% to 3 wt.% caused higher FAME formation, as shown in Figure 1.
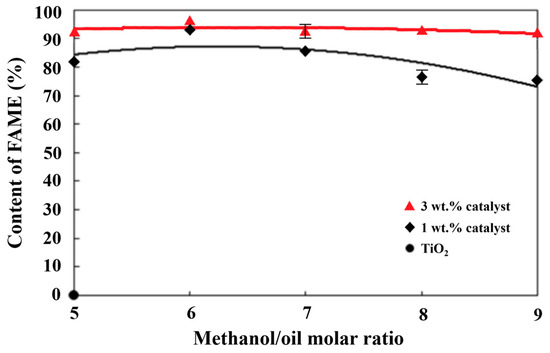
Figure 1.
Effects of different methanol/palm oil molar ratios and catalyst amounts on methyl ester content.
Kumar et al. [48] used a sulfonated carbon catalyst (SCG) derived from glycerol to catalyze waste cooking oil (WCO) to prepare biodiesel. After two consecutive cycles, they found that the yield of fatty acid methyl esters (FAMEs) remained higher than 90% for reusability. Guo et al. [49] used a calcined CH3ONa with a gel-type resin monosphere catalyst for the transesterification of soybean oil. They observed that the FAME yield of the regenerated catalyst lost 5% in comparison to the fresh one. Our FAME yield also revealed a 5% reduction for each use of a regenerated catalyst. Each fresh catalyst might be used for transesterification for four consecutive cycles to maintain a FAME yield greater than the acceptable 80%.
2.2. Effects of TiO2/CH3ONa Catalyst and Molar Ratio on the Kinematic Viscosity
The kinematic viscosity of a liquid fuel is related to the lubrication and atomization characteristics of the mechanical components of burners or combustion engines. Fuel with a low kinematic viscosity will result in insufficient lubrication on the surfaces of the mechanical components and cause abnormal wear. For the atomization characteristics of a combustor, the higher the viscosity, the longer the spray penetration distance and the larger the spray particle size, which leads to longer ignition delay times [50]. The EN 14214 specification of the kinematic viscosity of biodiesel fuel lies within the range of 3.5–5.0 mm2/s [51]. The kinematic viscosity of palm oil at 40 °C is 47.7 mm2/s [52].
In Figure 2, it can be seen that the kinematic viscosities of the liquid fuel ranged from 4 to 8 mm2/s. The highest kinematic viscosity occurred under a methanol/palm oil molar ratio of 5 and a catalyst amount of 1 wt.% of the weight of palm oil. The increase in the catalyst weight percentage from 1 wt.% to 3 wt.% decreased the kinematic viscosity. Lin and Ma [45] found that the structure of fatty acids and the content of methyl esters affected the kinematic viscosity of biodiesel. A low content of methyl esters would cause the viscosity to increase. Table 1 shows the variations in the fatty acid methyl ester content with the weight concentrations of the solid alkaline TiO2/CH3ONa catalyst from 1 wt.% to 3 wt.% with a methanol/palm oil molar ratio of 6. It is shown that the fatty acid was mostly composed of C16 and C18, which accounted for 91.58 wt.% and 91.31 wt.% in the cases of the alkaline TiO2/CH3ONa catalyst additions of 1 wt.% and 3 wt.%, respectively. In addition, the saturated fatty acids increased from 36.20 wt.% to 39.70 wt.% when the catalyst addition increased from 1 wt.% to 3 wt.%. This indicates that the increase in the catalyst addition enhanced the complete extent of the transesterification toward biodiesel production. The effects of the molar ratio on the kinematic viscosity in the case of the 3 wt.% catalyst addition were insignificant, primarily due to the relatively lower kinematic viscosity from 4.17 to 4.36 mm2/s, which is difficult to distinguish, resulting in experimental errors. The higher contents of either a carbon chain longer than C16 or saturated fatty acids increased the kinematic viscosity and cetane index [53]. The reacting conditions of a molar ratio of 6 and a catalyst amount of 3 wt.% resulted in more complete transesterification. A higher viscous triglyceride content was converted into lower amounts of viscous biodiesel products, leading to a reduction in the kinematic viscosity, as shown in Figure 2.
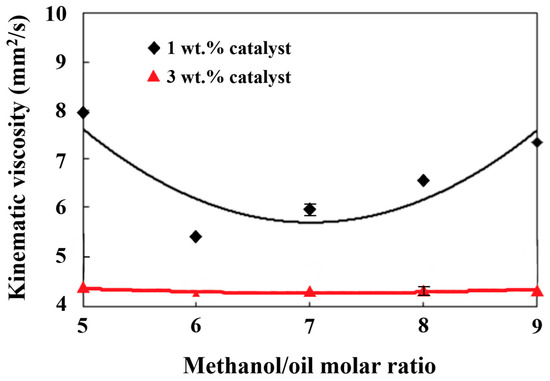
Figure 2.
Effects of different methanol/palm oil molar ratios and catalyst amounts on kinematic viscosity.

Table 1.
Weight percentages (wt.%) of fatty acid methyl esters prepared with different catalyst concentrations of 1 wt.% and 3 wt.% TiO2/CH3ONa at a methanol/palm oil molar ratio of 6.
2.3. Effects of TiO2/CH3ONa Catalyst and Molar Ratio on the Acid Value and Moisture Content
High water content in fats or oils frequently leads to their hydrolytic degradation and the formation of free fatty acids, resulting in higher acidity [54]. Waste cooking oil generally has a high acid value primarily due to the formation of high amounts of free fatty acids during high-temperature frying processes for a long time. A high water content is prone to forming in the lipid as well. Hasan and Ratnam [55] found that more than a 1 wt.% water content in the feedstock oil resulted in an increase in the acid value of the biodiesel product by 3 mg KOH/g. Hence, the acid value of biodiesel is influenced by the amount of water in the feedstock oil. As shown in Figure 3, there was about a 0.035 wt.% water content in the biodiesel product after removing the moisture content through distillation under the production conditions of various molar ratios of methanol/palm oil from 5 to 9 and catalyst additions from 1 wt.% to 3 wt.%. The increases in the molar ratio together with the decreases in the catalyst addition tended to increase the water content slightly. This might be ascribed to the hydrolysis of more methanol at larger methanol/palm oil molar ratios and less transesterification under lower catalyst additions, leading to higher water formation, as shown in Figure 3. Lin and Ma [56] found that water formed during the mixing process of an alkaline catalyst and methanol, which may lead to the saponification of raw oil. Hence, the dissociation of methanol under the catalyst effect produced OH− radicals, which resulted in water production in the biodiesel. Various water contents may have even been left behind after the water removal process through distillation during biodiesel production. The acid value of the biodiesel increased with the water’s existence in turn. The effects of different alcohol/oil molar ratios and catalyst amounts on the acid value of the biodiesel are shown in Figure 4. The trends of the acid value with the molar ratios of methanol/palm oil in Figure 4 agree well with those of the water content in Figure 4 due to the significant influence of water content on the acid value of biodiesel [57]. The acid value was also affected by the methyl ester content. In comparison with Figure 1, which presents the lowest methyl ester content under the methanol/palm oil molar ratio of 5 and a catalyst addition of 1 wt.%, the highest acid value occurred under the same production conditions in Figure 4. Consequently, the variation in the methyl ester content with the molar ratio of methanol/palm oil was the opposite to that of the acid value, in the comparison of Figure 1 and Figure 4.
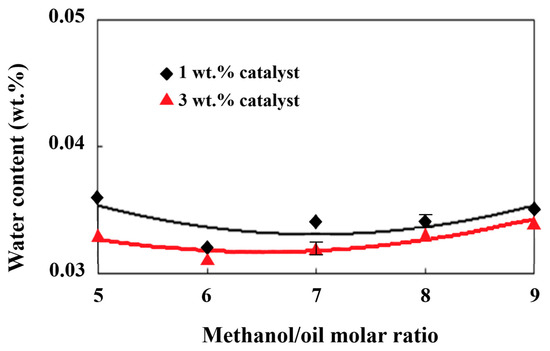
Figure 3.
Effects of different methanol/palm oil molar ratios and catalyst amounts on water content.
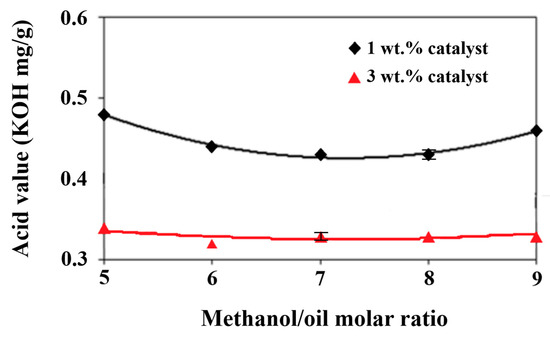
Figure 4.
Effects of different methanol/palm oil molar ratios and catalyst amounts on acid value.
2.4. Effects of TiO2/CH3ONa Catalyst and Molar Ratio on the Distillation Temperature and Cetane Index
Distillation temperature can be applied to indicate the volatilization extent and combustion characteristics of a liquid fuel. The distillation temperature distribution curve, which is termed the ASTM D-86 curve, displays the range of boiling points corresponding to the distilled and condensed volumes of the various compounds in the fuel sample. The distillation temperature curves of the biodiesel made using the methanol/palm oil ratio of 6 and the catalyst additions ranging from 1 to 3 wt.% are shown in Figure 5. The distillation temperatures were distributed between 320 °C and 360 °C. The increase in the catalyst addition from 1 wt.% to 3 wt.% enhanced the extent of transesterification and in turn formed heavier hydrocarbon compounds, leading to a slight increase in the distillation temperatures. Yeong et al. [58] found that the increase in the contents of long carbon-chain fatty acids and saturated fatty acids of biodiesel increased the distillation temperature. The saturated fatty acids increased from 36.2 wt.% to 39.7 wt.%, and the methyl esters from C14 to C22 increased from 94.0 wt.% to 95.95 wt.% when the catalyst addition increased from 1 wt.% to 3 wt.% and the methanol/palm oil molar ratio was 6, as shown in Table 1. Hence, slightly higher distillation temperatures were observed under the same biodiesel production conditions where the molar ratio and catalyst addition were 6 and 3 wt.%, respectively, achieving more complete transesterification.
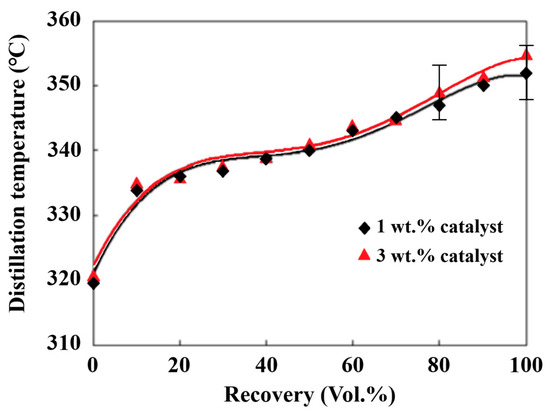
Figure 5.
Distillation temperature curve for the biodiesel prepared at a methanol/palm oil molar ratio of 6.
The cetane number of diesel fuel can represent its compression ignition quality in a diesel engine. Liquid fuel with a higher cetane number can shorten the ignition delay after the fuel is injected into the engine, reducing the burning time and residence period of the peak flame temperature of the fuel in the combustion chamber, detonation occurrence, and emissions of nitrogen oxides. Khethiwe et al. [59] indicated that the cetane number of a biodiesel fuel varies with its composition of fatty acids. Biodiesel composed of larger amounts of longer carbon chains tends to have a higher cetane number. The cetane indices of the biodiesel prepared by adding from 1 wt.% to 3 wt.% of a TiO2/CH3ONa alkaline catalyst based on the weight of the palm oil were calculated, according to Equation (3), to be 49.29 and 50.05, respectively. This indicates that the increase in the catalyst amount increased the content of the saturated fatty acids, and the cetane index in turn.
2.5. Effects of TiO2/CH3ONa Catalyst and Molar Ratio on the Heating Value
Fuel with a higher heating value indicates a higher heat release per unit mass, which is more fuel efficient and requires a lower fuel consumption rate to obtain the same engine power output [43]. The heating values of the biodiesel produced under various methanol/palm oil molar ratios ranging between 5 and 9 and catalyst additions from 1 wt.% to 3 wt.% ranged between 38 and 40 MJ/kg, as shown in Figure 6. The highest heating value, which was 40.02 MJ/kg, occurred for the biodiesel produced when the methanol/palm oil molar ratio was 6 and the amount of catalytic addition was 3 wt.%. Compared to the results of the methyl ester content in Figure 3, it was found that the lower heating value appeared with the biodiesel with a lower content of methyl ester. The biodiesel composed of long-chained methyl esters or higher weight percentages of elemental carbon appeared to have a higher heating value [60]. Comparing the variations in the water content in the biodiesel in Figure 3, the biodiesel that contained a lower water content appeared to have a higher heating value, as shown in Figure 6.
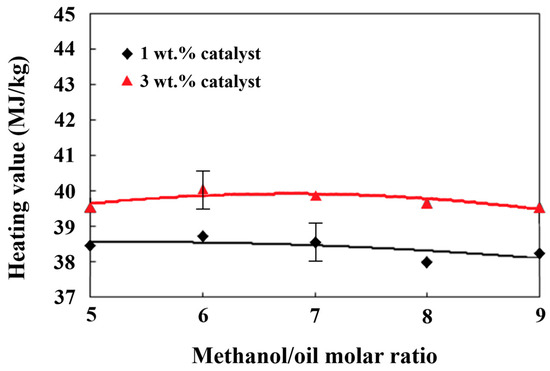
Figure 6.
Effects of different methanol/palm oil molar ratios and catalyst amounts on the heating value.
3. Experimental Details
A TiO2/CH3ONa catalyst was utilized to catalyze the transesterification of palm oil to produce biodiesel. The experimental equipment, procedures, and materials are described below.
3.1. Materials Used for the Catalyst Preparation
A catalyst of titanium dioxide (TiO2) impregnated with the strong alkaline catalyst sodium methoxide (CH3ONa) was used to catalyze the transesterification reaction of palm oil with methanol. The anatase type of TiO2 is considered the most promising catalyst and bears the most active photocatalytic effects [61]. TiO2 powder acts as a catalyst carrier due to its physical structure of full pores with large specific surface areas that can accommodate the aqueous solution of a strong acid or alkaline catalyst. The specific surface area (BET), purity, average primary particle size, and pH value in a 4% dispersion of the TiO2 nanoparticles were 50.5 m2/g, 99.5%, 21 nm, and 3.5~4.5, respectively [62].
The sodium methoxide (CH3ONa) solution, with a 5 M concentration, was provided by Merck Taiwan Ltd. (Taipei City, Taiwan). CH3ONa has been widely applied to produce biodiesel through transesterification as a strong alkaline catalyst. The amount of catalyst used is generally in the range between 1 and 5 wt.%.
3.2. Synthesized Catalyst Method for Biodiesel Production
In the first step of catalyst preparation, 1 g of TiO2 powder nanoparticles with a 0.1 M concentration was mixed in an aqueous sodium methoxide solution, allowing 10 mL of CH3ONa solution to flow into the empty holes of the TiO2 powder, which lasted for 12 h. The aqueous TiO2 and CH3ONa mixture was then filtered through a ceramic funnel and a glass fiber using a vacuum pump to remove impurities. The catalyst TiO2 was then impregnated with CH3ONa solution. A high-temperature furnace (model DF404, Deng Yng Ltd., New Taipei City, Taiwan) was then used to heat, dry, and forge the mixture at 6 various temperatures between 150 °C and 450 °C. The furnace was first heated from room temperature to 100 °C in 0.5 h. and remained at 100 °C for 3 h to distill away the volatile compound and water. It was then heated to 200 °C in 2 h where it remained for 3 h. The two calcined catalysts were thus physically ligated together to produce an effective catalyst for further manufacturing biodiesel through the transesterification reaction [63]. The preparation procedures for the TiO2/CH3ONa nanocatalyst are illustrated in Figure 7, where the experimental conditions of the high temperature and strong alkaline catalyst can be observed [64].
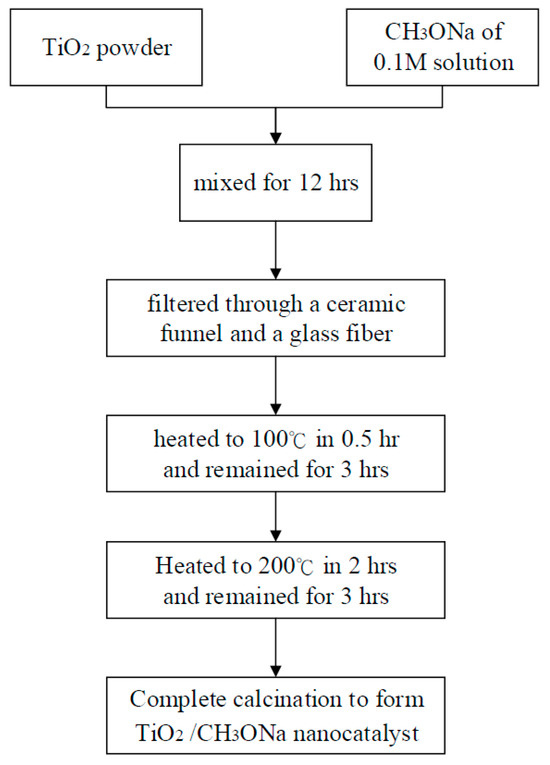
Figure 7.
Preparation procedures for the TiO2/CH3ONa nanocatalyst.
3.3. Transesterification of Palm Oil Using Solid Alkaline Catalysts
For transesterification with methanol (purchased from First Chemical Materials Ltd., Taipei City, Taiwan), 200 mL of palm oil (provided by Formosa Oilseed Processing Co., Ltd., Taichung City, Taiwan), whose oil properties are shown in Table 2 [65], was used as the feedstock oil with the assistance of a TiO2/CH3ONa solid catalyst to produce fatty acid methyl esters (FAMEs), which are frequently called biodiesel. The transesterification conditions were a 1~3 wt.% of the TiO2/CH3ONa solid alkali catalyst, based on the palm oil weight, molar ratios of methanol/palm oil from 5 to 9, a reaction time of 1 h, and a reaction temperature of 62 °C. After completing the conversion processes of the feedstock oil, the corresponding amount of strong alkaline CH3ONa was added to the glacial acetic acid and mixed and stirred to neutralize the pH value of the crude product. The crude product was then centrifuged to separate the biodiesel from the glycerol at the upper and lower layers due to the obvious density differences between these two compounds. After excluding the glycerol, the crude biodiesel was heated to 70 °C for 20 min to vaporize the methanol and other volatile materials away from the crude biodiesel. Then, 10 vol.% deionized water, based on the weight of the crude biodiesel, was used to wash the biodiesel three times, and then centrifuged away from the water content. The biodiesel was finally heated at 110 °C to remove the water, methanol, and other volatiles to obtain a biodiesel sample. The procedures for biodiesel production and analysis of the fuel properties are illustrated in Figure 8.

Table 2.
Physical properties of palm oil [65].
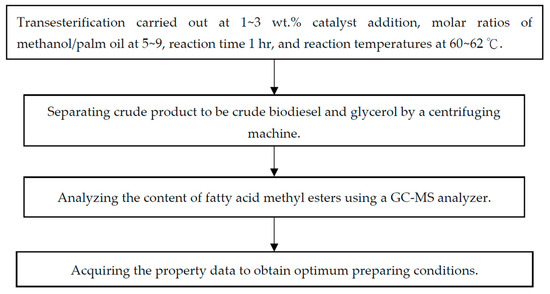
Figure 8.
Experimental procedures for analyzing biodiesel properties.
3.4. Analysis of Fuel Properties of Palm-Oil Biodiesel
The fatty acids of the palm oil and fatty acid methyl esters after the transesterification reaction were analyzed using a gas chromatograph (Model GC-14a, Shimadzu Ltd., Chiyoda-ku, Tokyo, Japan) associated with a flame ionization detector (FID). The separation column model used for the GC analysis was the Zebron ZB-5HT Inferno Column (Phenomenex Ltd., Torrance, CA, USA). The length, inside diameter, and film thickness of the column were 30 m, 0.32 mm, and 0.25 μm, respectively. The reagent in the injector of the GC was maintained at a temperature of 250 °C, and nitrogen was used as the carrier gas at a flow rate of 1 mL/min. The tests were carried out using the ASTM E1387-01 standard method [66]. The total fatty acid methyl ester content was determined by substituting the value calculated by the software chromatography data system (CDS) of Chromeleon 7.3.2 [67]. High-performance liquid chromatography (HPLC) or gas chromatography (GC) can be used to analyze FAME contents. In this study, gas chromatography (GC) was used for the quantitative determination of fatty acid methyl esters.
The heating value of a fuel is defined as the amount of heat energy released per unit mass or volume of fuel when it is completely burned at a standard temperature (25 °C). In this experiment, the heating value of biodiesel in units of cal/g or MJ/kg was determined using an oxygen bomb calorimeter (model 1261, Parr Inc., Moline, IL, USA). The water contents in biodiesel samples were determined using a volumetric Karl Fischer-type moisture titrator (DL-31 model, Mettler-Toledo Inc., Greifensee Schweiz, Switzerland), following the Coulomb electricity method of EN 12973 [68]. Excessive water in the feedstock oil hinders the transesterification reaction for biodiesel production [69], so the water needs to be removed by an adequate method before the reaction process. Water’s existence in a biodiesel product will also affect the combustion performance of the engine and the emissions from burning the fuel [70].
The property of kinematic viscosity is one of the major indicators of the fluidity of a liquid fuel. A U-tube viscometer (model K698, Cannon Instrument Ltd., State College, PA, USA) immersed in a constant-temperature water bath (model D-606, Deng Yng Ltd., New Taipei City, Taiwan) was used to measure the kinematic viscosity of the fuel samples according to the ASTM D445 standard test method [71]. The liquid fuel in the test tube remained at 40 °C. The time (t in a unit of sec) required for the fuel sample to flow through two indicated points in the tube was recorded.
The kinematic viscosity can be calculated using the following formula:
where k = 0.001045 mm2/s2 is the kinematic viscosity coefficient for the designated viscometer, and ν is the kinematic viscosity in units of mm2/s.
ν = k × t
The specific gravity (SG) of biodiesel can be converted to API gravity (G) using the equation below:
G = 141.5/SG (at 15 °C) − 131.5
API gravity can be used to calculate the cetane index of a liquid fuel [72]. A liquid-phase hydrometer placed in a cylinder filled with 100 mL of a liquid fuel sample was used to measure the specific gravity of the fuel based on the ASTM S1298-99e2 standard method [73]. Liquid fuel is composed of many compounds with various boiling points. The lighter substances will be the first to vaporize and be distilled away from the fuel. The distillation temperatures corresponding to the first drop, 10 vol.%, 20 vol.%, 50 vol.%, 90 vol.%, and the last drop of the fuel heated, distilled, and collected through a distillation thermometer were recorded and denoted as TIBP, T10, T20, T50, T90, and TEP. The subscripts IBP and EP are the abbreviations of the initial boiling point and the end point, respectively.
The cetane index (CI) is calculated from the data of API gravity (G) and the 50 vol.% distillation temperature (T50) in units of °F, as shown in the following formula:
CI = −420.34 + 0.016G2 + 1.192G (logT50) + 65.01 (logT50)2 − 0.0001809T502
The CI (cetane index) is the alternative property to the cetane number, which indicates the delay time of compression ignition after the sample fuel is injected into the combustion chamber of a diesel engine [74].
The acid value of a lipid represents its degree of rancidity and contents of free fatty acids. A higher acid value implies that the lipid is prone to deteriorate, and therefore, has a shorter preservation period. An automatic titrator (model 785 DMP Titrino, Metrohm AG, Herisau, Switzerland) was used to measure the acid value of the biodiesel fuel samples according to the consumed titrant based on the ASTM D664-18e2 standard procedure [75]. Each experiment was repeated 3 to 5 times to obtain their mean values. The experimental values of the FAME content, kinematic viscosity, water content, acid value, distillation temperature, and heating value were 2.26%, 2.15%, 1.57%, 1.46%, 1.21%, and 1.37%, respectively.
4. Conclusions
The biodiesel made from palm oil was catalyzed using a synthesized catalyst of TiO2/CH3ONa under various preparation conditions of the methanol/feedstock oil molar ratios and the amount of catalyst added to the reactant mixture. TiO2 powder consists of a porosity structure that can be used to tolerate strong alkaline catalysts of CH3ONa, leading to combined catalytic effects. The fuel properties of the produced biodiesel, such as the formation of fatty acid methyl esters (FAMEs), kinematic viscosity, moisture content, distillation temperature, and cetane index, were analyzed to pursue the optimum conditions for preparing the catalyst and the biodiesel product. The major experimental results are summarized below.
- (1)
- The highest content of fatty acid methyl esters, 95.9 wt.%, was achieved under the reaction conditions of a reaction temperature of 60 °C, a reaction time of 1 h, a methanol/palm oil molar ratio of 6, and a catalyst amount of 3 wt.% of the weight of the feedstock palm oil.
- (2)
- The biodiesel under the above optimum transesterification conditions obtained a minimum kinematic viscosity of 4.17 mm2/s, a minimum water content of 0.031 wt.%, the lowest acid value of 0.32 mg KOH/g oil, the highest heating value of 40.02 MJ/kg, and the highest cetane index of 50.05. In contrast, the lowest cetane index of 49.31 for the biodiesel occurred when using 1 wt.% of the strong alkaline catalyst and the same methanol/palm oil molar ratio of 6.
- (3)
- There was no significant difference in the distillation temperature distribution of the biodiesel obtained from the transesterification reaction with 1 to 3 wt.% of the strong alkaline TiO2/CH3ONa catalyst added and a methanol/palm oil molar ratio of 6. However, the highest distillation temperature reached 355 °C when the reaction was carried out with a 3 wt.% catalyst addition and a methanol/palm oil molar ratio of 6.
Author Contributions
Conceptualization, C.-Y.L.; methodology, C.-Y.L.; formal analysis, S.-L.T.; investigation, C.-Y.L. and S.-L.T.; data curation, S.-L.T.; writing—original draft preparation, C.-Y.L. and S.-L.T.; writing—review and editing, C.-Y.L.; supervision, C.-Y.L.; project administration, C.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Science and Technology, Taiwan, under the contract number NCST 110-2221-E-019-055-MY2.
Data Availability Statement
The data presented in this study are contained within this article.
Acknowledgments
The authors would like to gratefully acknowledge the financial support from the National Council of Science and Technology, Taiwan, under the contract number NCST 110-2221-E-019-055-MY2.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, C.Y. The influences of promising feedstock variability on advanced biofuel production: A review. J. Mar. Sci. Technol. 2022, 29, 714–730. [Google Scholar] [CrossRef]
- Narayana Sarma, R.; Vinu, R. Current status and future prospects of biolubricants: Properties and applications. Lubricants 2022, 10, 70. [Google Scholar] [CrossRef]
- Zambare, V.; Patankar, R.; Bhusare, B.; Christopher, L. Recent Advances in Feedstock and Lipase Research and Development towards Commercialization of Enzymatic Biodiesel. Processes 2021, 9, 1743. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. BioEnergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Maleki, B.; Talesh, S.A.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Mater. Today Sustain. 2022, 18, 100157. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling catalysts for biodiesel production via transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Brahma, S.; Hoque, M.; Das, B.K.; Selvaraj, M.; Brahma, S.; Basumatary, S. Advances in CaO-based catalysts for sustainable biodiesel synthesis. Green Energy Resour. 2023, 1, 100032. [Google Scholar] [CrossRef]
- Arachchige, U.S.P.R.; Miyuranga, K.V.; Thilakarathne, D.; Jayasinghe, R.A.; Weerasekara, N.A. Biodiesel-alkaline transesterification process for methyl ester production. Nat. Environ. Pollut. Technol. 2021, 20, 1973–1980. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Sadik, W.; Sadek, O.M.; Kasaby, M.A. A review on biodiesel feedstocks and production technologies. J. Chil. Chem. Soc. 2021, 66, 5098–5109. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tseng, Y.M. Effects of LED irradiation and sea water culture on the lipid characteristics of Nannochloropsis oculata. J. Renew. Sustain. Energy 2018, 10, 023102. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, K.H. Comparison of the engine performance of soybean oil biodiesel emulsions prepared by phase inversion temperature and mechanical homogenization methods. Processes 2023, 11, 907. [Google Scholar] [CrossRef]
- Najjar, A.; Hassan, E.A.; Zabermawi, N.; Saber, S.H.; Bajrai, L.H.; Almuhayawi, M.S.; Harakeh, S. Optimizing the catalytic activities of methanol and thermotolerant Kocuria flava lipases for biodiesel production from cooking oil wastes. Sci. Rep. 2021, 11, 13659. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Imran, S.; Kalam, M.A.; Zulkifli, N.W.; Masjuki, H.H. Future needs of the biodiesel industry. In Sustainable Biodiesel; Academic Press: Cambridge, MA, USA, 2023; pp. 373–383. [Google Scholar]
- Lapisa, R.; Yuvenda, D.; Putra, R.P. Experimental study on fuel consumption and smoke opacity of defective coffee-bean-based biodiesel fuel engines. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2023; Volume 2582, p. 012007. [Google Scholar]
- Chuah, L.F.; Klemeš, J.J.; Bokhari, A.; Asif, S. A review of biodiesel production from renewable resources: Chemical reactions. Chem. Eng. Trans. 2021, 88, 943–948. [Google Scholar]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Hidayat, S.; Rahayu, I. Heterophase polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for efficient photocatalyst: Fabrication and activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Venkatesh, Y.K.; Ravikumar, M.P.; Ramu, S.; Ravikumar, C.H.; Mohan, S.; Geetha Balakrishna, R. Developments in Titanium-Based Alkali and Alkaline Earth Metal Oxide Catalysts for Sustainable Biodiesel Production: A Review. Chem. Rec. 2023, 23, e202300277. [Google Scholar] [CrossRef]
- Din, I.U.; Nasir, Q.; Garba, M.D.; Alharthi, A.I.; Alotaibi, M.A.; Usman, M. A review of preparation methods for heterogeneous catalysts. Mini-Rev. Org. Chem. 2022, 19, 92–110. [Google Scholar]
- Stephen, L. Titanium dioxide versatile solid crystalline: An overview. Assorted Dimens. Reconfig. Mater. 2020, 1, 92056. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Udayakumar, M. Kinetics and thermodynamic analysis of transesterification of waste cooking sunflower oil using bentonite-supported sodium methoxide catalyst. Biomass Convers. Biorefin. 2023, 13, 9701–9714. [Google Scholar] [CrossRef]
- Argaw Shiferaw, K.; Mathews, J.M.; Yu, E.; Choi, E.Y.; Tarte, N.H. Sodium methoxide/zeolite-supported catalyst for transesterification of soybean waste cooking oil for biodiesel production. Inorganics 2023, 11, 163. [Google Scholar] [CrossRef]
- Soodesh, C.Y.; Seriyala, A.K.; Chattopadhyay, P.; Rozhkova, N.; Michalkiewicz, B.; Chatterjee, S.; Roy, B. Carbonaceous catalysts (biochar and activated carbon) from agricultural residues and their application in produc tion of biodiesel: A review. Chem. Eng. Res. Des. 2024, 203, 759–788. [Google Scholar] [CrossRef]
- Najaf-Abadi, M.K.; Ghobadian, B.; Dehghani-Soufi, M. A review on application of deep eutectic solvents as green catalysts and co-solvents in biodiesel production and purification processes. Biomass Convers. Biorefin. 2024, 14, 3117–3134. [Google Scholar] [CrossRef]
- Martínez, A.; Mijangos, G.E.; Romero-Ibarra, I.C.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y. In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel 2019, 235, 277–287. [Google Scholar] [CrossRef]
- Patel, A.; Vachhani, H.; Patel, Y. Thermal and fluid property analysis of biodiesel produced from waste cooking oil using sodium methoxide and calcium oxide. In Recent Advances in Fluid Dynamics: Select Proceedings of ICAFFTS 2021; Springer Nature: Singapore, 2022; pp. 83–92. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Synthesis of biodiesel from tall oil fatty acids by homogeneous and heterogeneous catalysis. Sustain. Chem. 2021, 2, 206–221. [Google Scholar] [CrossRef]
- AlMohamadi, H.; Awad, S.A.; Sharma, A.K.; Fayzullaev, N.; Távara-Aponte, A.; Chiguala-Contreras, L.; Esmaeili, H. Photo catalytic Activity of Metal-and Non-Metal-Anchored ZnO and TiO2 Nanocatalysts for Advanced Photocatalysis: Comparative Study. Catalysts 2024, 14, 420. [Google Scholar] [CrossRef]
- Sheng, Y.; Tian, F.; Wang, X.; Jiang, N.; Zhang, X.; Chen, X.; Liang, C.; Wang, A. Carbon-encapsulated Ni catalysts derived from citrate complexes for highly efficient hydrogenation of furfural to tetrahydrofurfuryl alcohol. Energy 2024, 292, 130360. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, K.H.; Yang, H. Effects of surfactant characteristics on fuel properties of emulsions of alternative engine fuel through the phase inversion method. Processes 2023, 11, 1864. [Google Scholar]
- Chen, K.S.; Lin, Y.C.; Hsu, K.H.; Wang, H.K. Improving biodiesel yields from waste cooking oil by using sodium methoxide and a microwave heating system. Energy 2012, 38, 151–156. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, K.P. The impact of heterogeneous catalyst on biodiesel production; a review. Mater. Today Proc. 2023, 78, 364–371. [Google Scholar] [CrossRef]
- Kokkinos, N.C.; Emmanouilidou, E.; Paschou, V.; Mitkidou, S. Applicatiof membrane reactors in homogeneous catalytic processes. In Homogeneous Catalysis Concepts and Basics; Elsevier: Amsterdam, The Netherlands, 2024; pp. 279–298. [Google Scholar]
- Lin, C.Y.; Ma, L. Influences of water content in feedstock oil on burning characteristics of fatty acid methyl esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Veza, I.; Roslan, M.F.; Said, M.F.M.; Latiff, Z.A.; Abas, M.A. Physico-chemical properties of Acetone-Butanol-Ethanol (ABE)-diesel blends: Blending strategies and mathematical correlations. Fuel 2021, 286, 119467. [Google Scholar] [CrossRef]
- Effiom, S.O. Effect of process parameters on biodiesel yield produced from palm kernel shell oil (PKSO) using eggshell as catalyst. Int. J. Front. Eng. Technol. Res. 2023, 4, 001–017. [Google Scholar] [CrossRef]
- Azer, B.B.; Gulsaran, A.; Pennings, J.R.; Saritas, R.; Kocer, S.; Bennett, J.L.; Yavuz, M. A Review: TiO2 based photoele trocatalytic chemical oxygen demand sensors and their usage in industrial applications. J. Electroanal. Chem. 2022, 918, 116466. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. Valorization of de-oiled microalgal biomass as a carbon-based heterogeneous catalyst for a sustainable biodiesel production. Bioresour. Technol. 2021, 337, 125424. [Google Scholar] [CrossRef]
- Atgur, V.; Manavendra, G.; Banapurmath, N.R.; Rao, B.N.; Rajhi, A.A.; Khan, T.M.Y.; Venkatesh, R. Essence of thermal analysis to assess biodiesel combustion performance. Energies 2022, 15, 6622. [Google Scholar] [CrossRef]
- Guan, T.; Liu, B.; Wang, R.; Huang, Y.; Luo, J.; Li, Y. The enhanced fatty acids flavor release for low-fat cheeses by carrier immobilized lipases on O/W Pickering emulsions. Food Hydrocoll. 2021, 116, 106651. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, C.; Ge, Y.; Hao, L.; Tan, J.; Wang, X.; Li, J. Fuel consumption and emission performance from light-duty conventional/hybrid-electric vehicles over different cycles and real driving tests. Fuel 2020, 278, 118340. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, X.; Zhang, G. TiO2-based catalysts for photothermal catalysis: Mechanisms, materials and applications. J. Clean Prod. 2022, 381, 135156. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ma, L. Fluid characteristics of biodiesel produced from palm oil with various initial water contents. Processes 2021, 9, 309. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Bokhari, A.; Asif, S.; Cheng, Y.W.; Chong, C.C.; Show, P.L. A review of intensification technologies for biodiesel production. Biofuels Biorefin. 2022, 2, 87–116. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Griffiths, G.; Duraisamy, G.; Thomas, J.J. Investigation on yield, fuel properties, ageing and low temperature flow of fish oil esters. Energy Convers. Manag. X 2022, 14, 100217. [Google Scholar] [CrossRef]
- Kumar, S.; Shamsuddin, M.R.; Farabi, M.A.; Saiman, M.I.; Zainal, Z.; Taufiq-Yap, Y.H. Production of methyl esters from waste cooking oil and chicken fat oil via simultaneous esterification and transesterification using acid catalyst. Energy Convers. Manag. 2020, 226, 113366. [Google Scholar] [CrossRef]
- Guo, Y.; Delbari, S.A.; Namini, A.S.; Van Le, Q.; Park, J.Y.; Kim, D.; Li, C. Recent developments in solid acid catalysts for biodiesel production. Mol. Catal. 2023, 547, 113362. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.W. Engine Performance of High-Acid Oil-Biodiesel through Supercritical Transesterification. ACS Omega 2024, 9, 3445–3453. [Google Scholar] [CrossRef]
- Yaşar, F. Comparison of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- El-Araby, R.; Amin, A.; El Morsi, A.K.; El-Ibiari, N.N.; El-Diwani, G.I. Study on the characteristics of palm oil–biodiesel–diesel fuel blend. Egypt. J. Pet. 2018, 27, 187–194. [Google Scholar] [CrossRef]
- He, X.; Xu, K.; Xu, Y.; Zhang, Z.; Wei, W. Effects of nozzle diameter on the characteristic time scales of diesel spray two-stage ignition under cold-start conditions. Fuel 2023, 335, 126700. [Google Scholar] [CrossRef]
- Deepanraj, B.; Senthilkumar, N.; Mala, D.; Sathiamourthy, A. Cashew nut shell liquid as alternate fuel for CI engine—Optimization approach for performance improvement. Biomass Convers. Biorefin. 2022, 12, 1715–1728. [Google Scholar] [CrossRef]
- Hasan, N.; Ratnam, M.V. Biodiesel production from waste animal fat by transesterification using H2SO4 and KOH catalysts: A study of physiochemical properties. Int. J. Chem. Eng. 2022, 2022, 6932320. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ma, L. Comparison of water-removal efficiency of molecular sieves vibrating by rotary shaking and electromagnetic stirring from feedstock oil for biofuel production. Fermentation 2021, 7, 132. [Google Scholar] [CrossRef]
- Anantapinitwatna, A.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Anantpinijwatna, A.; Quitain, A.T.; Assabumrungrat, S. Water influence on the kinetics of transesterification using CaO catalyst to produce biodiesel. Fuel 2021, 296, 120653. [Google Scholar] [CrossRef]
- Yeong, S.P.; San Chan, Y.; Law, M.C.; Ling, J.K.U. Improving cold flow properties of palm fatty acid distillate biodiesel through vacuum distillation. J. Bioresour. Bioprod. 2022, 7, 43–51. [Google Scholar] [CrossRef]
- Khethiwe, E.; Clever, K.; Jerekias, G. Effects of fatty acids composition on fuel properties of Jatropha curcas biodiesel. Smart Grid Renew. Energy 2020, 11, 165–180. [Google Scholar] [CrossRef]
- Ahmad, R.K.; Sulaiman, S.A.; Yusup, S.; Dol, S.S.; Inayat, M.; Umar, H.A. Exploring the potential of coconut shell biomass for charcoal production. Ain Shams Eng. J. 2022, 13, 101499. [Google Scholar] [CrossRef]
- Kuss, V.V.; Kuss, A.V.; da Rosa, R.G.; Aranda, D.A.G.; Cruz, Y.R. Potential of biodiesel production from palm oil at Brazilian Amazon. Renew. Sustain. Energy Rev. 2015, 50, 1013–1020. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Kim, M.; Salley, S.O.; Ng, K.Y.S. Transesterification of glycerides using a heterogeneous resin catalyst combined with a homogeneous catalyst. Energy Fuels 2008, 22, 3594–3599. [Google Scholar] [CrossRef]
- Formosa Oilseed Processing Ltd. Test Report of Palm Oil. 2021; Taichung City, Taiwan. [Google Scholar]
- Kassem, Y.; Çamur, H.; Alassi, E. Biodiesel production from four residential waste frying oils: Proposing blends for improving the physicochemical properties of methyl biodiesel. Energies 2020, 13, 4111. [Google Scholar] [CrossRef]
- Pathmasiri, T.K.K.S.; Perera, G.I.P. Potential of using polyethylene as viscosity enhancer of palm oil to use as a lubricating oil. Adv. Mech. Eng. 2020, 12, 1687814020970745. [Google Scholar] [CrossRef]
- EN 12973:2000; Technical Committee of European Standard, Value Management, Value Analysis, Function Analysis. CEN-CENELEC Management Centre: Brussel, Belgium, 2000.
- Lin, C.Y. Effects of the degree of unsaturation of fatty acid esters on engine performance and emission characteristics. Processes 2022, 10, 2161. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, X.E. Determination of cetane number from fatty acid compositions and structures of biodiesel. Processes 2022, 10, 1502. [Google Scholar] [CrossRef]
- ASTM D445; ASTM International. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2024. [Google Scholar]
- Vicentini-Polette, C.M.; Ramos, P.R.; Gonçalves, C.B.; De Oliveira, A.L. Determination of free fatty acids in crude vegetable oil samples obtained by high-pressure processes. Food Chem. X 2021, 12, 100166. [Google Scholar] [CrossRef]
- ASTM S1298-99e2; ASTM International. Standard Test Method for Density, Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Cheng, C.; Cordtz, R.F.; Førby, N.L.; Schramm, J. Experimental and simulation investigation of n-heptane/ammonia dual fuel on a light-duty compression ignition engine. Int. J. Hydrogen Energy 2024, 57, 1339–1353. [Google Scholar] [CrossRef]
- ASTM D664-18e2; ASTM International. Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).