Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Biomass-Derived Nanocomposites (Co/MPC)
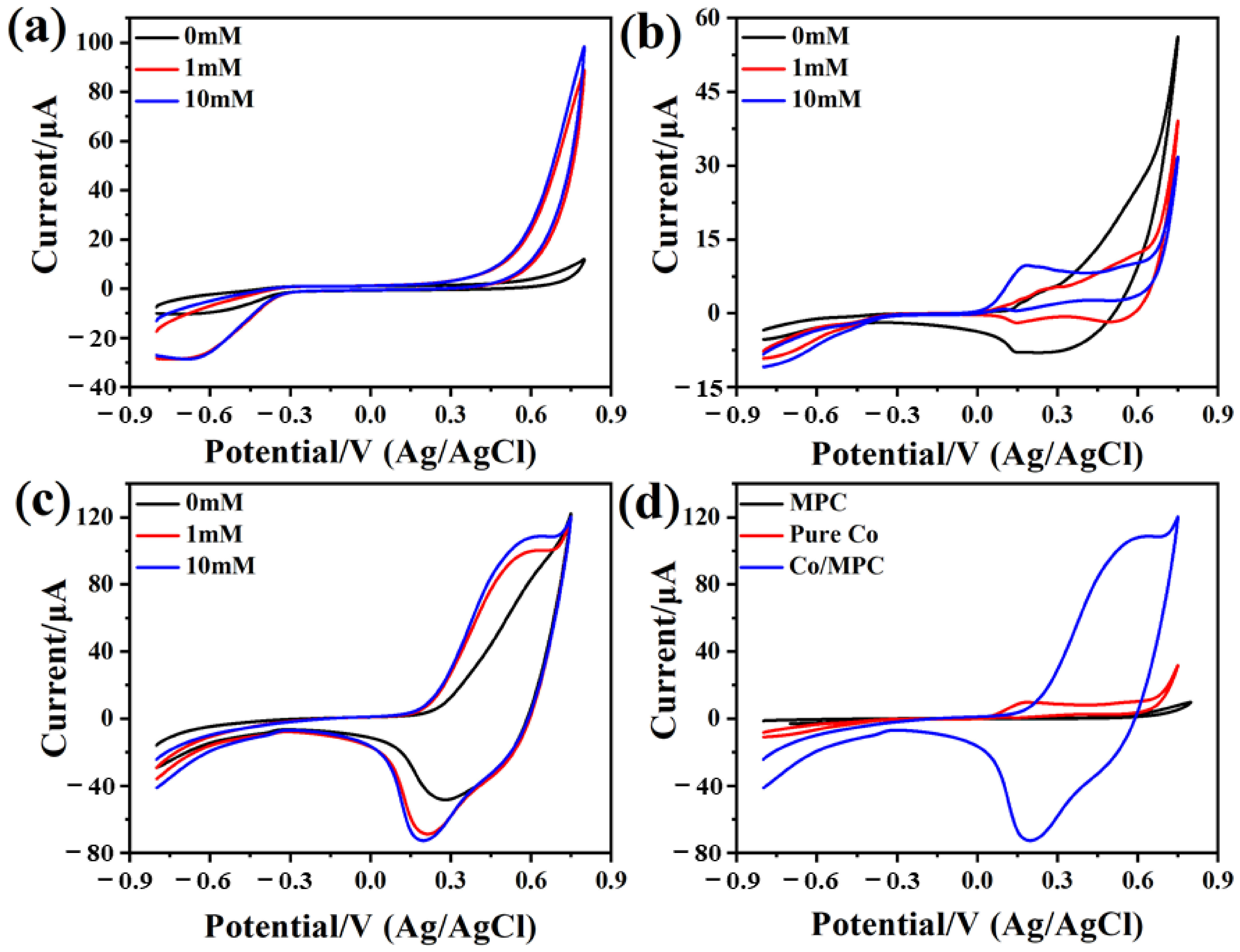
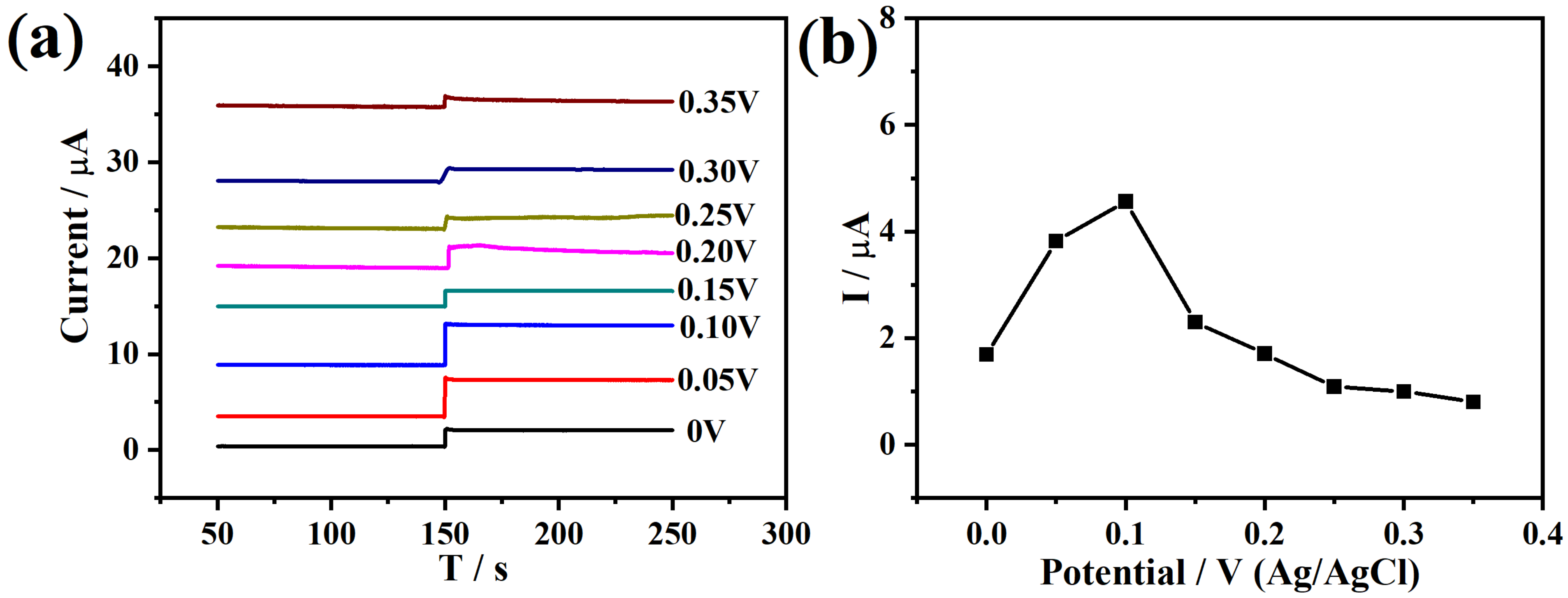
2.2. Electrochemical Properties and Electrocatalysis of the Co/MPC Electrode Toward H2O2
2.3. Reproducibility, Anti-Interference, and Stability Test of Co/MPC/GCE for Hydrogen Peroxide
3. Materials and Methods
3.1. Materials
3.2. Preparation of Biochar (MPC) via Microwave Pyrolysis
3.3. Preparation of Co/MPC Composite
3.4. Characterization of the Synthesized Materials
3.5. Preparation of Co/MPC-Modified Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Yan, D.; Liang, B.; Shi, F.; Yan, Y.; Wang, S.; Xue, F. Boosting electrochemical fenton process Via Cu, S-doped FeOOH sheet for hydrogen peroxide detection. Electrochim. Acta 2024, 486, 144122. [Google Scholar] [CrossRef]
- Ullah, I.; Yaqub, A.; Haq, M.Z.U.; Ajab, H.; Jafry, A.T.; Khan, M.K. Sensitive and cost-effective colorimetric sensor based on enzyme mimic MoS2@CoTiO3 nanocomposite for detection of hydrogen peroxide in milk and tap water. J. Food Compos. Anal. 2023, 124, 105689. [Google Scholar] [CrossRef]
- Buzdar, M.; Yaqub, A.; Hayat, A.; Ul Haq, M.Z.; Khan, A.; Ajab, H. Paper based colorimetric sensor using novel green magnetized nanocomposite of pinus for hydrogen peroxide detection in water and milk. Food Biosci. 2023, 55, 103014. [Google Scholar] [CrossRef]
- Chen, P.-C.; Kirankumar, R.; Lin, P.-Y.; Chuang, Z.-W.; Hsieh, S. Perovskite nanowire-based electrochemical sensing for selective and rapid detection of hydrogen peroxide. J. Alloys Compd. 2024, 1002, 175320. [Google Scholar] [CrossRef]
- da Silva, W.; Guedes, E.A.B.; Faustino, L.C.; Goulart, M.O.F.; Gerôncio, E.T.S. Tailored electrochemical biosensor with poly-diallydimethylammonium chloride-functionalised multiwalled carbon nanotubes/gold nanoparticles/manganese dioxide, and haemoglobin for sensitive hydrogen peroxide detection. Talanta 2024, 276, 126290. [Google Scholar] [CrossRef]
- Zhu, Y.; Kang, K.; Jia, Y.; Guo, W.; Wang, J. General and fast synthesis of graphene frameworks using sugars for high-performance hydrogen peroxide nonenzymatic electrochemical sensor. Microchim. Acta. 2020, 18, 669. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Xie, Y.; Guo, E.; Bai, H.; Jiang, F.; Li, Q.; Yue, H. Design and Fabrication of Island-Like CoNi2S4@NiCo-LDH/Biomass Carbon Heterostructure as Advanced Electrodes for High-Performance Hybrid Supercapacitors. Adv. Energy Mater. 2024, 14, 2400493. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Li, J.-H.; Chen, Y.-C.; Ho, W.H.; Song, Y.-D.; Kung, C.-W. Electrodeposition of pore-confined cobalt in metal–organic framework thin films toward electrochemical H2O2 detection. Electrochim. Acta 2020, 347, 136276. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Zhao, P.; Chen, Y.; Lei, J.; Hou, J.; Hou, C.; Huo, D. An electrochemical sensor based on FeCo bimetallic single-atom nanozyme for sensitive detection of H2O2. Anal. Chim. Acta 2023, 1281, 341867. [Google Scholar] [CrossRef]
- Dai, Y.; Wen, X.; Fan, H.; Hong, J.; Huang, L.; Zhang, Q.; Xu, J.; Zhang, J.; Zhang, H.; Zhu, W.; et al. Carbon Dots Dually Doped with Fe and Co for Visual Detection of H2O2 and Glutathione. ACS Appl. Nano Mater. 2024, 7, 17795–17803. [Google Scholar] [CrossRef]
- Xia, L.; Luan, X.; Qu, F.; Lu, L. Co-MOF/titanium nanosheet array: An excellent electrocatalyst for non-enzymatic detection of H2O2 released from living cells. J. Electroanal. Chem. 2020, 878, 114553. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, Y.; Li, Y.; Li, C.; Wang, L.; Shaojun, G. MOF derived Co,O4/N-doped carbon nanotubes hybrids as efficient catalysts for sensitive detection of HzOz and glucose. Chin. Chem. Lett. 2020, 31, 774–778. [Google Scholar] [CrossRef]
- Long, L.; Liu, H.; Liu, X.; Chen, L.; Wang, S.; Liu, C.; Dong, S.; Jia, J. Co-embedded N-doped hierarchical carbon arrays with boosting electrocatalytic activity for in situ electrochemical detection of H2O2. Sens. Actuators B 2020, 318, 128242. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, K.; Ouyang, T.; Wang, Z.; Chen, Y.; Li, N.; Liu, Z.-Q. Co-Cr mixed spinel oxide nanodots anchored on nitrogen-doped carbon nanotubes as catalytic electrode for hydrogen peroxide sensing. J. Colloid Interface Sci. 2021, 585, 605–613. [Google Scholar] [CrossRef]
- Du, H.; Ai, M.; Lyu, S.-S.; Zhang, P.-P.; Chen, X.-G.; Jin, A.; Ye, Y. Systematic fabrication and electromagnetic performance of porous biomass carbon/ferrite nanocomposites. J. Alloys Compd. 2022, 896, 163048. [Google Scholar] [CrossRef]
- Samanth, A.; Selvaraj, R.; Murugesan, G.; Varadavenkatesan, T.; Vinayagam, R. Efficient adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) using biomass derived magnetic activated carbon nanocomposite in synthetic and simulated agricultural runoff water. Chemosphere 2024, 361, 142513. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Algethami, J.S.; Alkorbi, A.S.; Harraz, F.A. Facile synthesis of CeO2·CuO-decorated biomass-derived carbon nanocomposite for sensitive detection of catechol by electrochemical technique. Mater. Sci. Semicond. Process. 2024, 172, 108098. [Google Scholar] [CrossRef]
- Bi, Y.; Hei, Y.; Wang, N.; Liu, J.; Ma, C.-B. Synthesis of a clustered carbon aerogel interconnected by carbon balls from the biomass of taros for construction of a multi-functional electrochemical sensor. Anal. Chim. Acta 2021, 1164, 338514. [Google Scholar] [CrossRef]
- Wang, N.; Hei, Y.; Liu, J.; Sun, M.; Sha, T.; Hassan, M.; Bo, X.; Guo, Y.; Zhou, M. Low-cost and environment-friendly synthesis of carbon nanorods assembled hierarchical meso-macroporous carbons networks aerogels from natural apples for the electrochemical determination of ascorbic acid and hydrogen peroxide. Anal. Chim. Acta 2019, 1047, 36–44. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and opportunities in microwave-assisted catalytic pyrolysis of biomass: A review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Gianola, G.; Garino, N.; Bartoli, M.; Sacco, A.; Pirri, C.F.; Zeng, J. Microwave-assisted synthesis of N/S-doped CNC/SnO2 nanocomposite as a promising catalyst for oxygen reduction in alkaline media. Mater. Chem. Phys. 2023, 308, 128205. [Google Scholar] [CrossRef]
- Bai, L.; Huang, A.; Feng, J.; Su, X.; Mo, W.; Ma, S.; Lin, H. One-step microwave pyrolysis synthesis of bagasse biochar/ferrites nanocomposite and synergistic effect on As (V) adsorption in water. Mater. Chem. Phys. 2022, 283, 126035. [Google Scholar] [CrossRef]
- Cai, J.; Vasudevan, S.V.; Wang, M.; Mao, H.; Bu, Q. Microwave-assisted synthesized renewable carbon nanofiber/nickel oxide for high-sensitivity detection of H2O2. J. Electroanal. Chem. 2022, 924, 116876. [Google Scholar] [CrossRef]
- Arief, I.; Bhattacharjee, Y.; Prakash, O.; Sahu, M.; Suwas, S.; Bose, S. Tunable CoNi microstructures in flexible multilayered polymer films can shield electromagnetic radiation. Compos. Part B 2019, 177, 107283. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition tailoring via N and S co-doping and structure tuning by constructing hierarchical pores: Metal-free catalysts for high-performance electrochemical reduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 15476–15480. [Google Scholar] [CrossRef]
- Hu, Y.; Hojamberdiev, M.; Geng, D. Recent advances in enzyme-free electrochemical hydrogen peroxide sensors based on carbon hybrid nanocomposites. J. Mater. Chem. C 2021, 9, 6970–6990. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Fasheun, D.O.; de Oliveira, R.A.; Bon, E.P.S.; Silva, A.S.A.d.; Teixeira, R.S.S.; Ferreira-Leitão, V.S. Dry extrusion pretreatment of cassava starch aided by sugarcane bagasse for improved starch saccharification. Carbohydr. Polym. 2022, 285, 119256. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, J. Environmentally friendly synthesis of Co-based zeolitic imidazolate framework and its application as H2O2 sensor. Chem. Eng. J. 2020, 392, 123690. [Google Scholar] [CrossRef]
- Dong, X.-x.; Li, M.-y.; Feng, N.-n.; Sun, Y.-m.; Yang, C.; Xu, Z.-l. A nanoporous MgO based nonenzymatic electrochemical sensor for rapid screening of hydrogen peroxide in milk. RSC Adv. 2015, 5, 86485–86489. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Dai, X.; Wang, Y.; Sun, M.; Zhou, C.; Du, H.; Rao, H. Novel flexible bifunctional amperometric biosensor based on laser engraved porous graphene array electrodes: Highly sensitive electrochemical determination of hydrogen peroxide and glucose. J. Hazard. Mater. 2021, 402, 123774. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, M.V.; Melchionna, M.; Giuliani, A.; Nasi, L.; Tavagnacco, C.; Prato, M.; Fornasiero, P. H2O2 sensing enhancement by mutual integration of single walled carbon nanohorns with metal oxide catalysts: The CeO2 case. Sens. Actuators B 2017, 239, 923–932. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wu, D.; Wei, Q. Preparation of PbS NPs/RGO/NiO nanosheet arrays heterostructure: Function-switchable self-powered photoelectrochemical biosensor for H2O2 and glucose monitoring. Biosens. Bioelectron. 2021, 173, 112803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Z.; Chen, C.; Wu, Y.; Zhu, Z.; Zhao, H.; Lan, M. A Novel Biomimetic Hydrogen Peroxide Biosensor Based on Pt Flowers-decorated Fe3O4/Graphene Nanocomposite. Electroanalysis 2017, 29, 1518–1523. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Lin, S.; Wang, L.; Wang, C. Synthesis of PtAu bimetallic nanoparticles on graphene–carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor. Talanta 2013, 112, 111–116. [Google Scholar] [CrossRef]
- Garate, O.; Veiga, L.S.; Tancredi, P.; Medrano, A.V.; Monsalve, L.N.; Ybarra, G. High-performance non-enzymatic hydrogen peroxide electrochemical sensor prepared with a magnetite-loaded carbon nanotube waterborne ink. J. Electroanal. Chem. 2022, 915, 116372. [Google Scholar] [CrossRef]
- Liu, M.; An, M.; Xu, J.; Liu, T.; Wang, L.; Liu, Y.; Zhang, J. Three-dimensional carbon foam supported NiO nanosheets as non-enzymatic electrochemical H2O2 sensors. Appl. Surf. Sci. 2021, 542, 148699. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Rajkumar, M.; Rajavel, K.; Zhu, P.; Xuan, W.; Xu, Z.-X.; Wang, F. Porous nickel oxide microsphere and Ti3C2Tx hybrid derived from metal-organic framework for battery-type supercapacitor electrode and non-enzymatic H2O2 sensor. Electrochim. Acta 2019, 322, 134771. [Google Scholar] [CrossRef]
- Qiao, R.; Lei, Y.; Liu, Q.; Tang, L.; Xiao, X.; Zhang, G.; He, T.; Zhang, Y.; Liang, C.; Chen, S. Ruthenium, nitrogencodoped carbon aerogels for real-time electrochemical monitoring of cellular hydrogen peroxide release. J. Electroanal. Chem. 2024, 952, 117997. [Google Scholar] [CrossRef]
- Achari, D.S.; Santhosh, C.; Deivasegamani, R.; Nivetha, R.; Bhatnagar, A.; Jeong, S.K.; Grace, A.N. A non-enzymatic sensor for hydrogen peroxide based on the use of α-Fe2O3 nanoparticles deposited on the surface of NiO nanosheets. Microchim. Acta 2017, 184, 3223–3229. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Oyman, G.; Cınar, E.; Odabas, G. A new polyaniline–catalase–glutaraldehyde-modified biosensor for hydrogen peroxide detection. Prep. Biochem. Biotechnol. 2016, 47, 86–93. [Google Scholar] [CrossRef]
- Bu, Q.; Yu, F.; Cai, J.; Bai, J.; Xu, J.; Wang, H.; Lin, H.; Long, H. Preparation of sugarcane bagasse-derived Co/Ni/N/MPC nanocomposites and its application in H2O2 detection. Ind. Crops Prod. 2024, 211, 118218. [Google Scholar] [CrossRef]
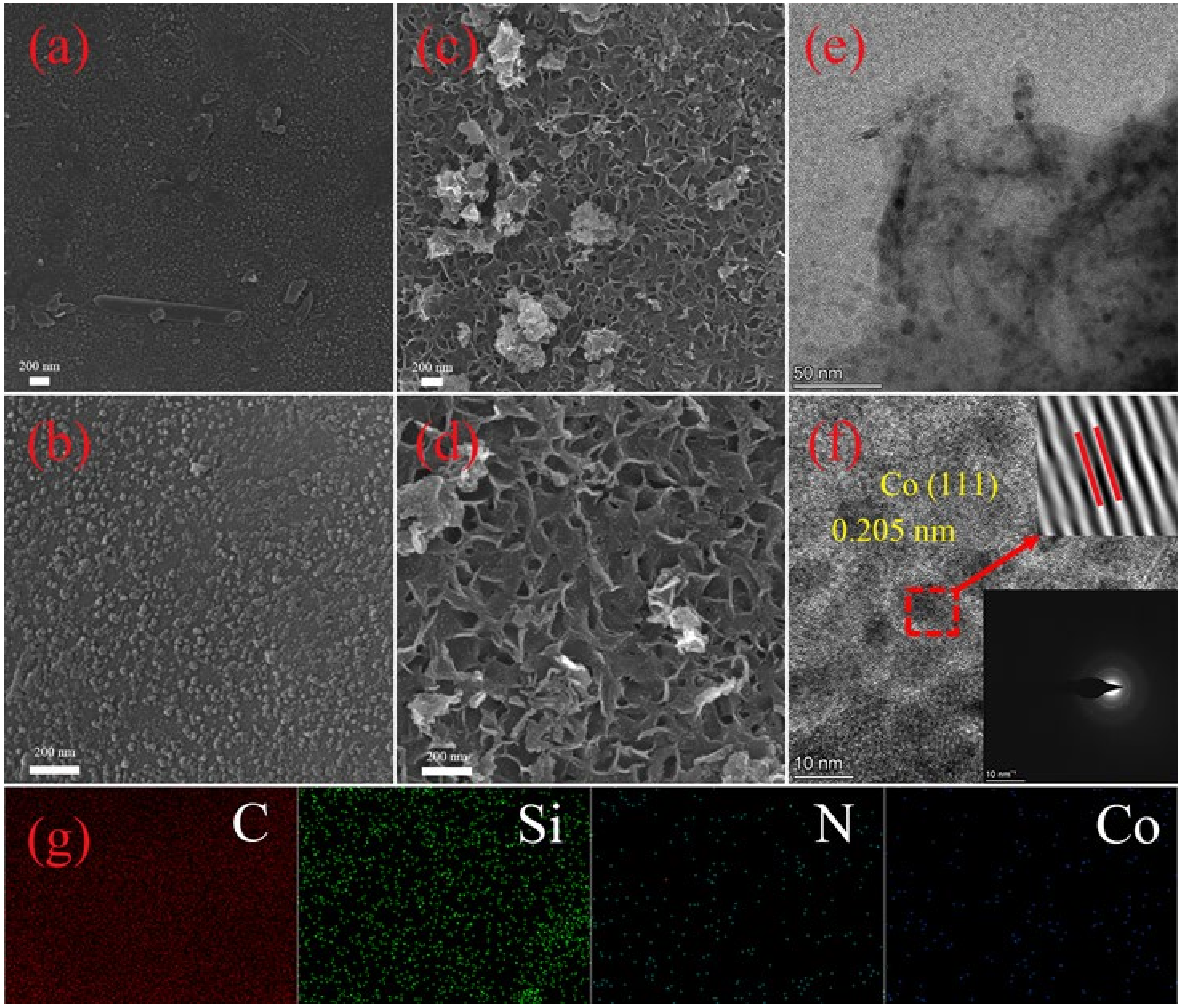
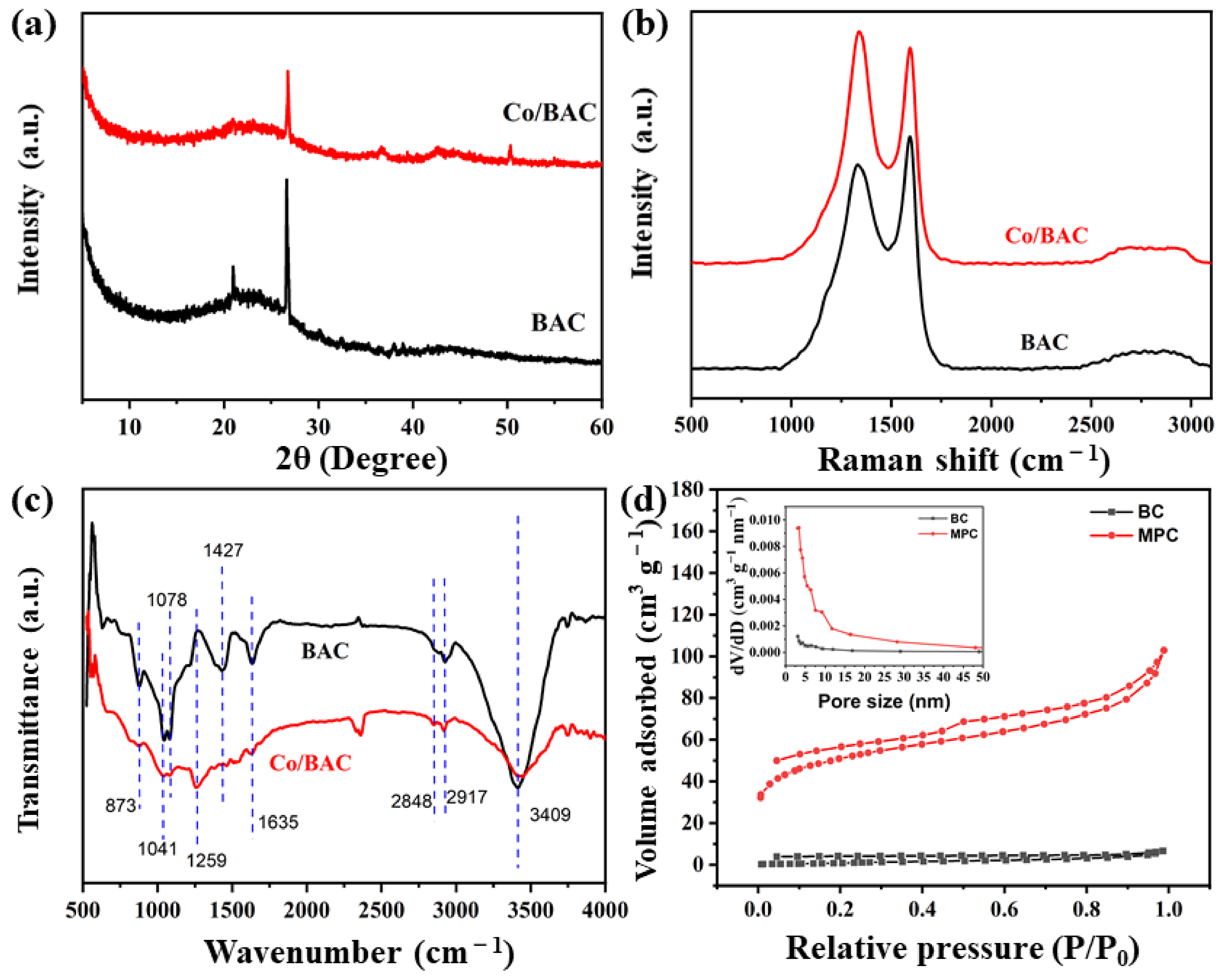


| Materials | SBET (m2 g−1) | Average Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| BC | 10.41 | - | 0.01 |
| MPC | 184.16 | 3.39 | 0.09 |
| Electrode Materials | Sensitivity | Linear Detection Range | Detection Limit | Ref. |
|---|---|---|---|---|
| ox-SWCNH@CeO2 | 160 µAcm−2 mM−1 | 10 μM–1.4 mM | 2.7 μM | [32] |
| PbS NPS/RGO/NiO | - | 0–100 mM | 18 μM | [33] |
| Pt/Fe3O4/rGO | 100–2400 μM | 1.58 μM | [34] | |
| PtAu/G-CNTs/GCE | - | 2 μM–8.56 mM | 0.6 μM | [35] |
| Fe3O4/CNT | 1040 µAcm−2 mM−1 | 0.001–2 mM | 0.5 μM | [36] |
| NiO-NSs/CF-1801/GCE | 23.30 µAcm−2 mM−1 | 0.20–3.75 mM | 0.01 μM | [37] |
| NiO/Ti3C2Tx | - | 0.01–4.54 mM | 0.35 μM | [38] |
| Ru-NCAG | - | 0.1–1000 μM | 0.01 μM | [39] |
| NiO/α-Fe203 | 146.98 µAcm−2 mM−1 | 500–3000 μM | 50 μM | [40] |
| Co/MPC | 103.45 µAcm−2 mM−1 | 0.55–100.05 mM | 1.38 μM | This work |
| Sample | Dosage (μmol/L) | Average Measured Value (μmol/L) | Recovery Rate (%) | Standard Derivation (%) |
|---|---|---|---|---|
| Tap water | 30 | 28.2 | 94.0 | 6.5 |
| 50 | 48.6 | 97.2 | 5.4 | |
| 100 | 97.6 | 97.6 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Cai, J.; Jiao, L.; Bu, Q. Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction. Catalysts 2024, 14, 624. https://doi.org/10.3390/catal14090624
Wang M, Cai J, Jiao L, Bu Q. Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction. Catalysts. 2024; 14(9):624. https://doi.org/10.3390/catal14090624
Chicago/Turabian StyleWang, Mei, Jin Cai, Lihua Jiao, and Quan Bu. 2024. "Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction" Catalysts 14, no. 9: 624. https://doi.org/10.3390/catal14090624
APA StyleWang, M., Cai, J., Jiao, L., & Bu, Q. (2024). Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction. Catalysts, 14(9), 624. https://doi.org/10.3390/catal14090624







