Green Synthesis of Photocatalytically Active ZnO Nanoparticles Using Chia Seed Extract and Mechanistic Elucidation of the Photodegradation of Diclofenac and p-Nitrophenol
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of ZnO Nanoparticles
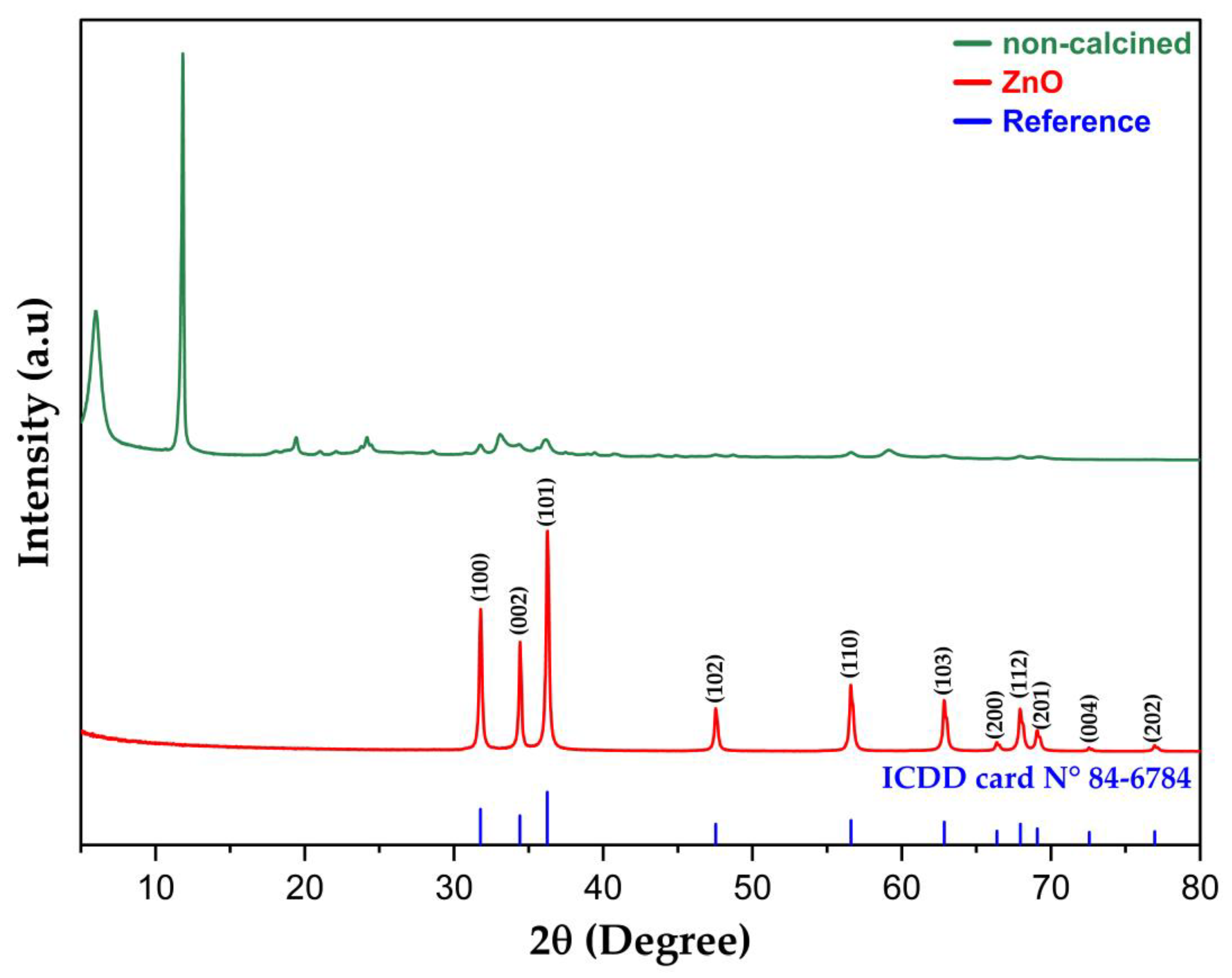
2.1.1. XRD Analysis
2.1.2. Surface Area Determination (BET Analysis)
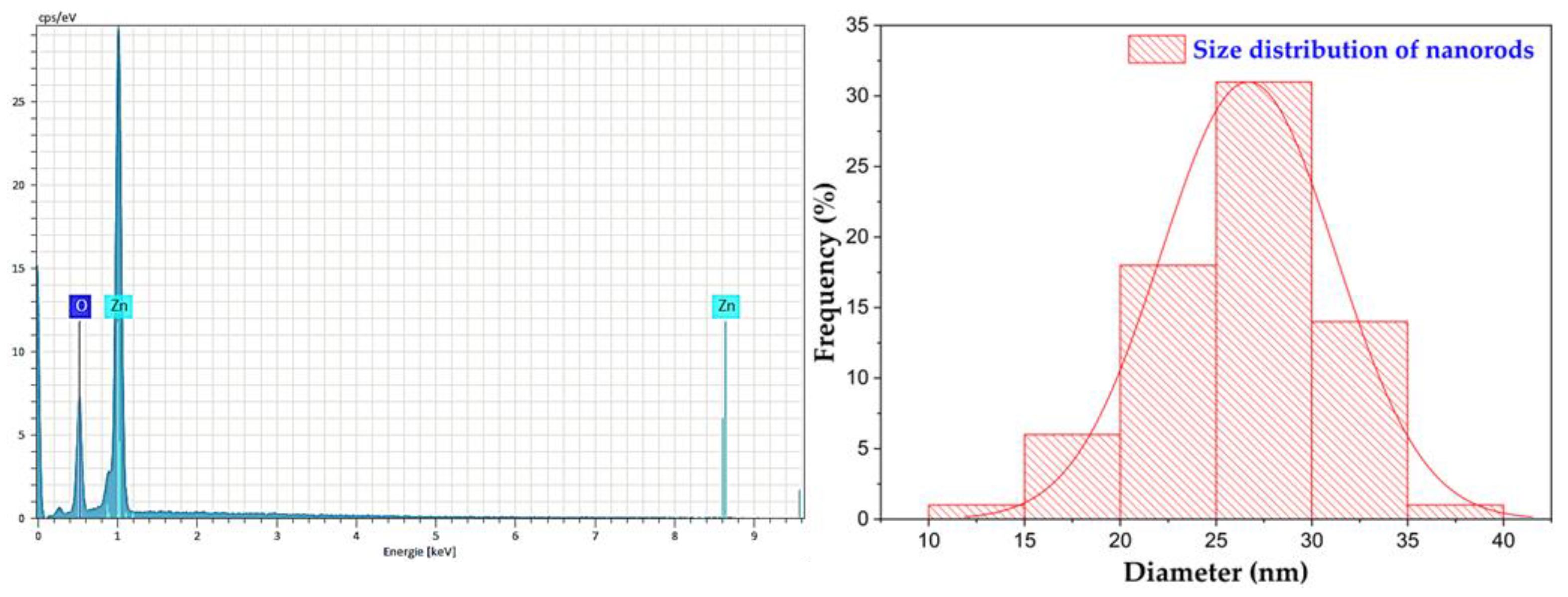
2.1.3. Elemental Analysis (EA)
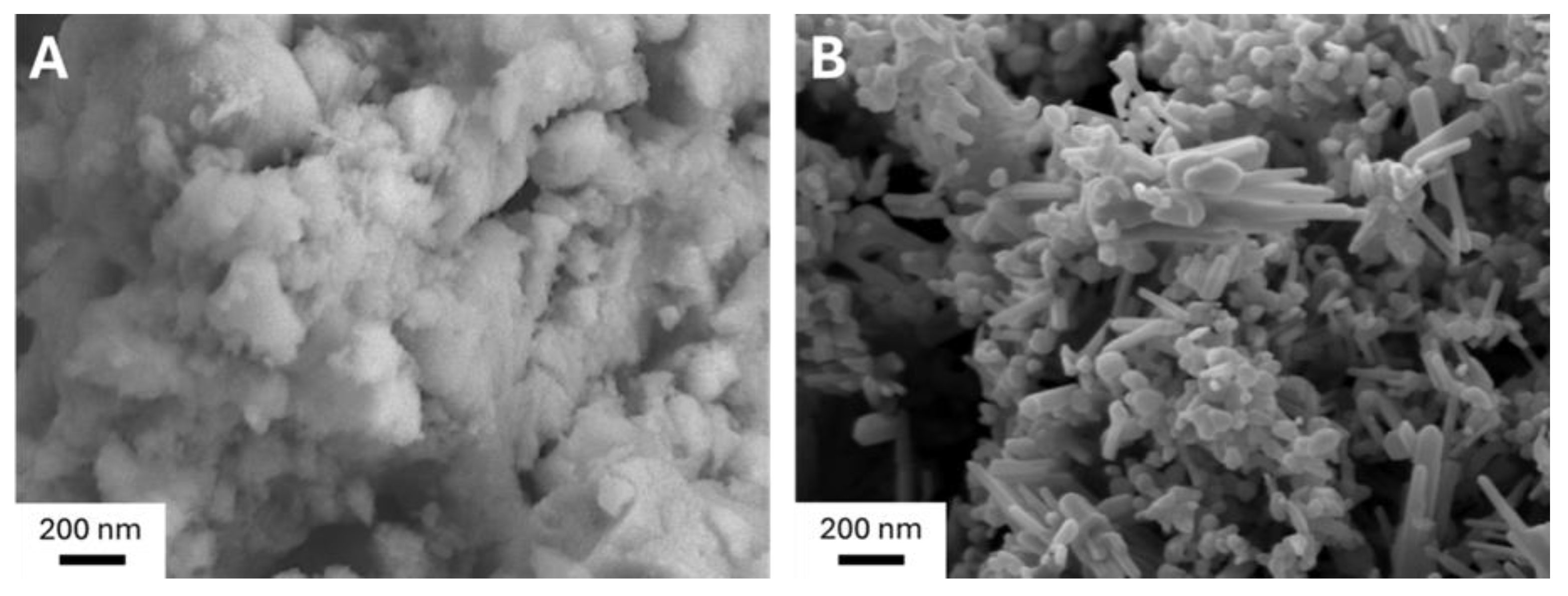
2.1.4. Morphological Insights
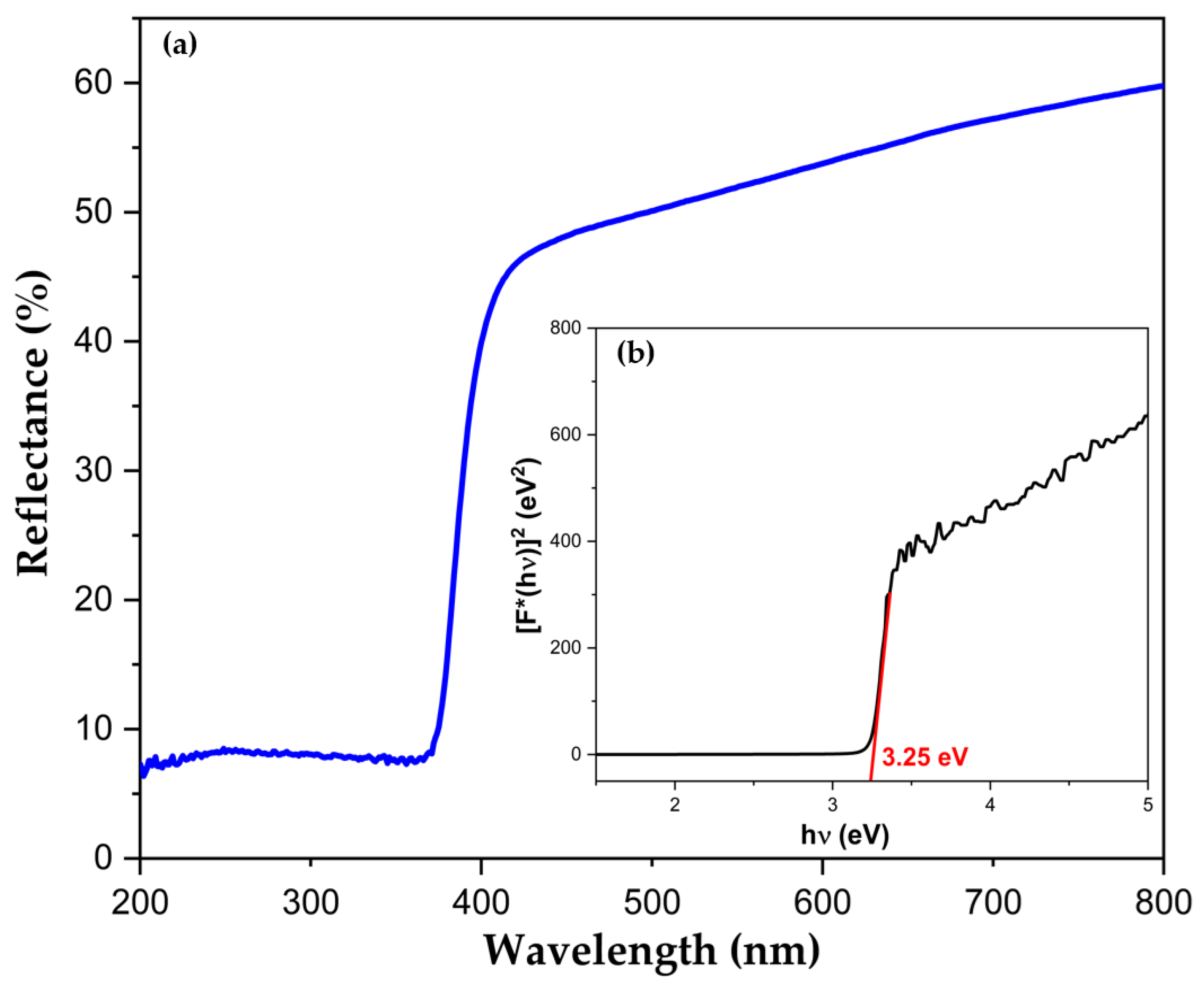
2.1.5. Optical Properties
2.2. Photocatalytic Performance
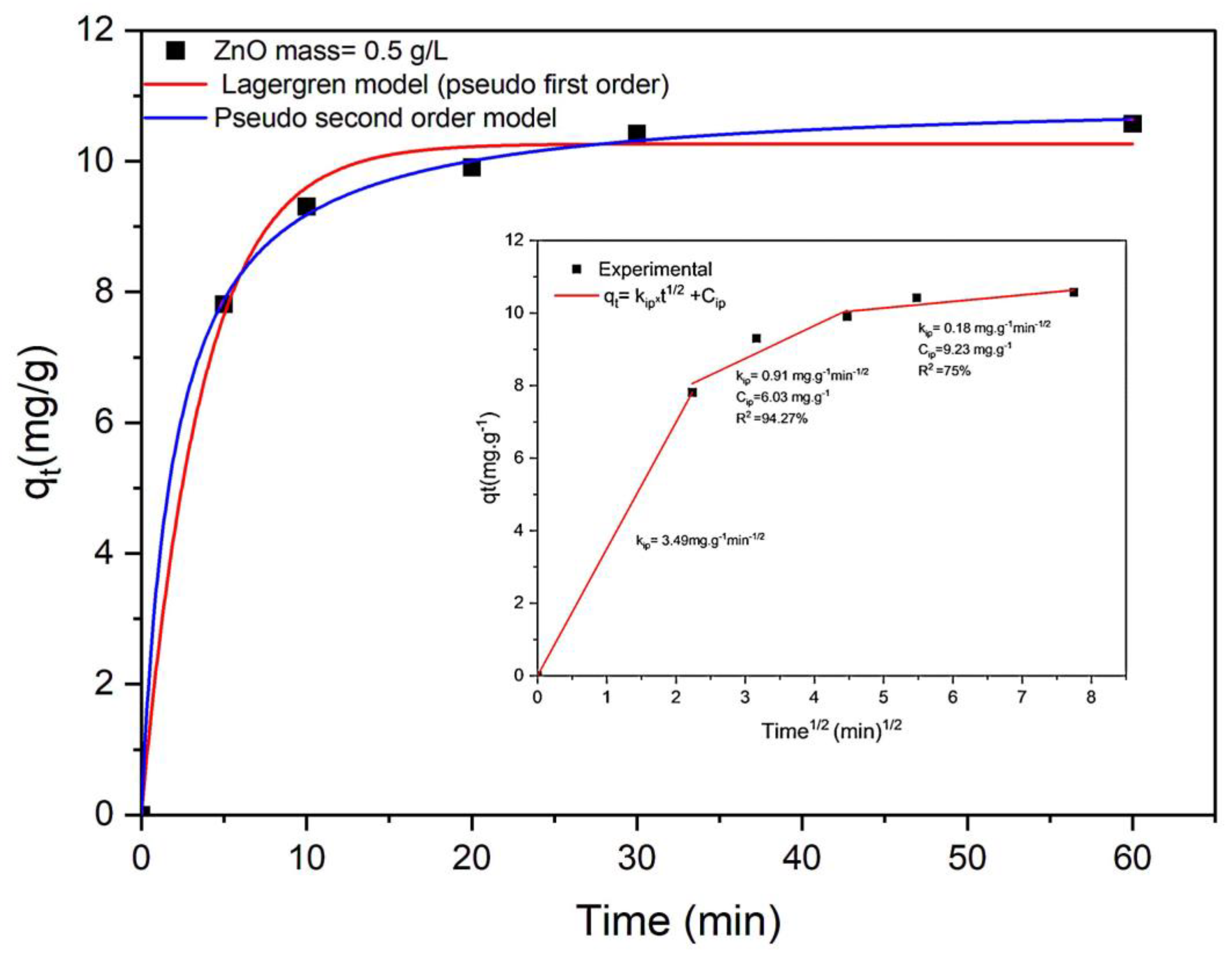
2.2.1. Adsorption Kinetics of DCF
- Surface Adsorption: In this initial stage, DCF molecules occupy the active sites on the exterior surface of ZnO through mechanisms such as hydrophobic partitioning, covalent bonding, and van der Waals forces;
- Intraparticle Diffusion: The second stage involves DCF experiencing mass transfer posed by the outer liquid barrier and diffusing toward the inner surface of the ZnO particles;
- Quasi-Equilibrium Stage: The final stage represents a state where the adsorption rate stabilizes.
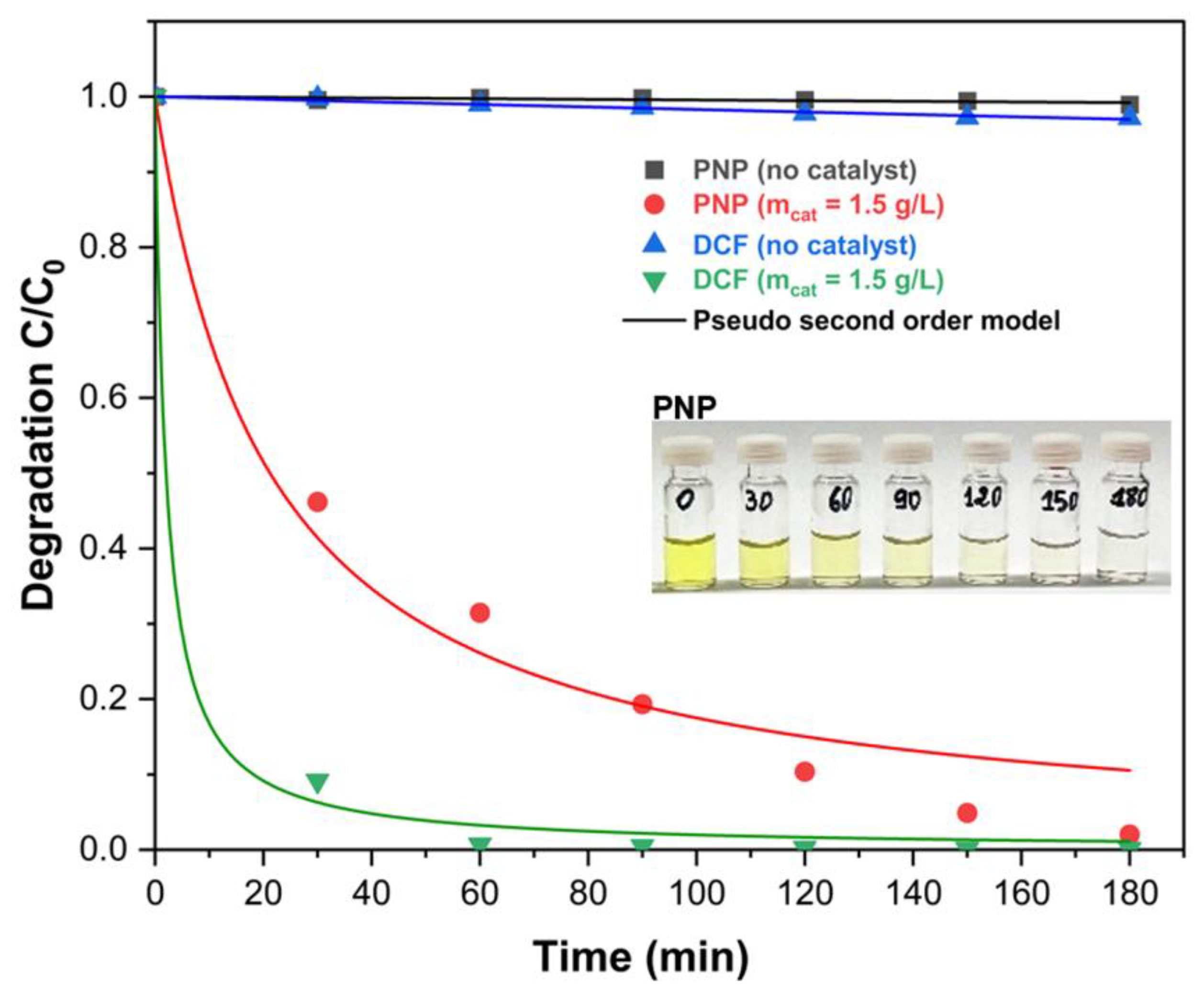
2.2.2. Kinetics of Degradation of PNP and DCF
2.2.3. Photodegradation Process Influencing Factors
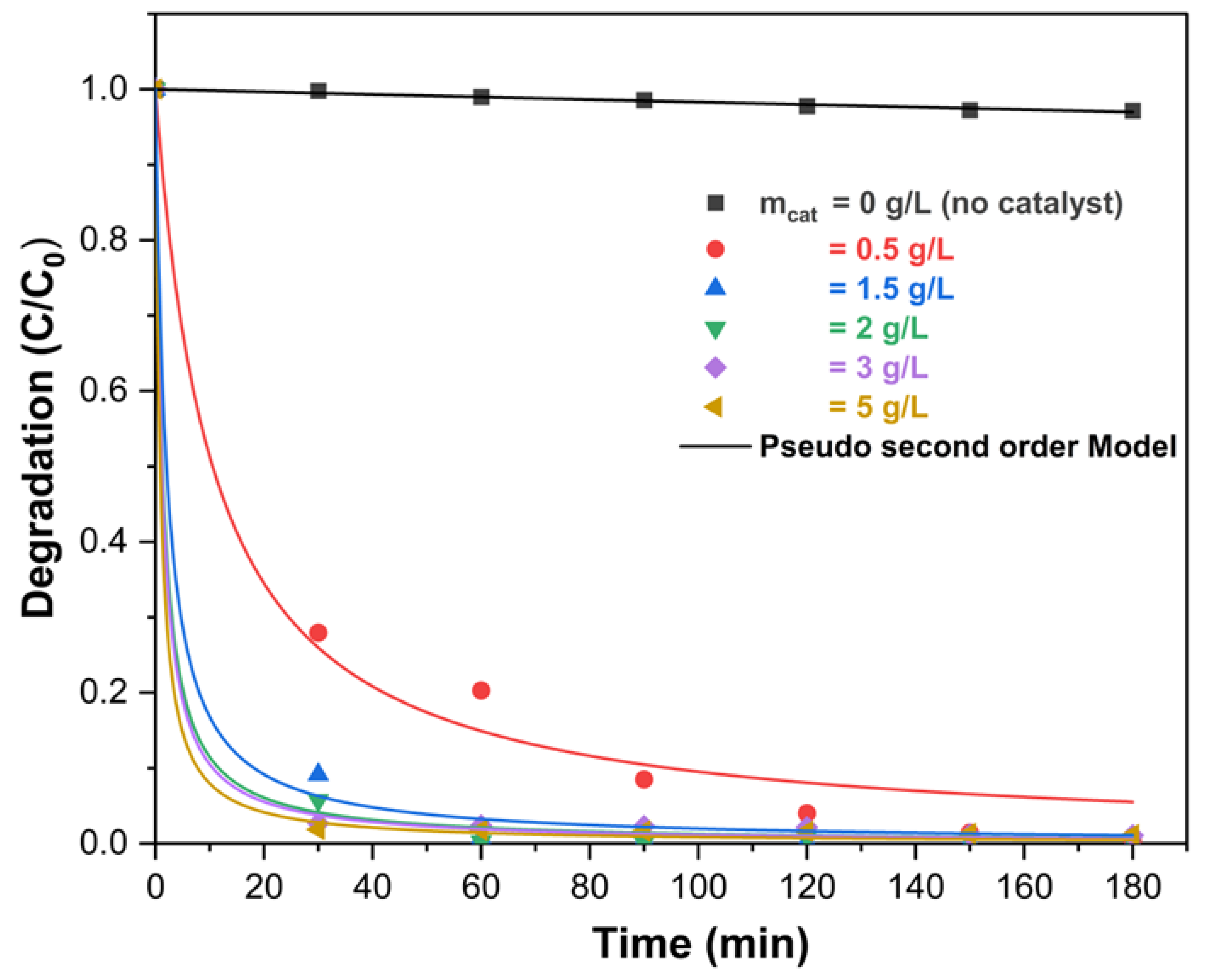
2.2.4. Effect of Catalyst Loading on DCF Degradation
- k (L·mol−1·min−1) is the kinetic constant of the reaction,
- m (grams) is the mass of the catalyst,
- a (L·mol−1·min−1·g−1) is the maximum kinetic constant achievable at low catalyst mass, and
- b (g−1) is a parameter that controls the rate of saturation.
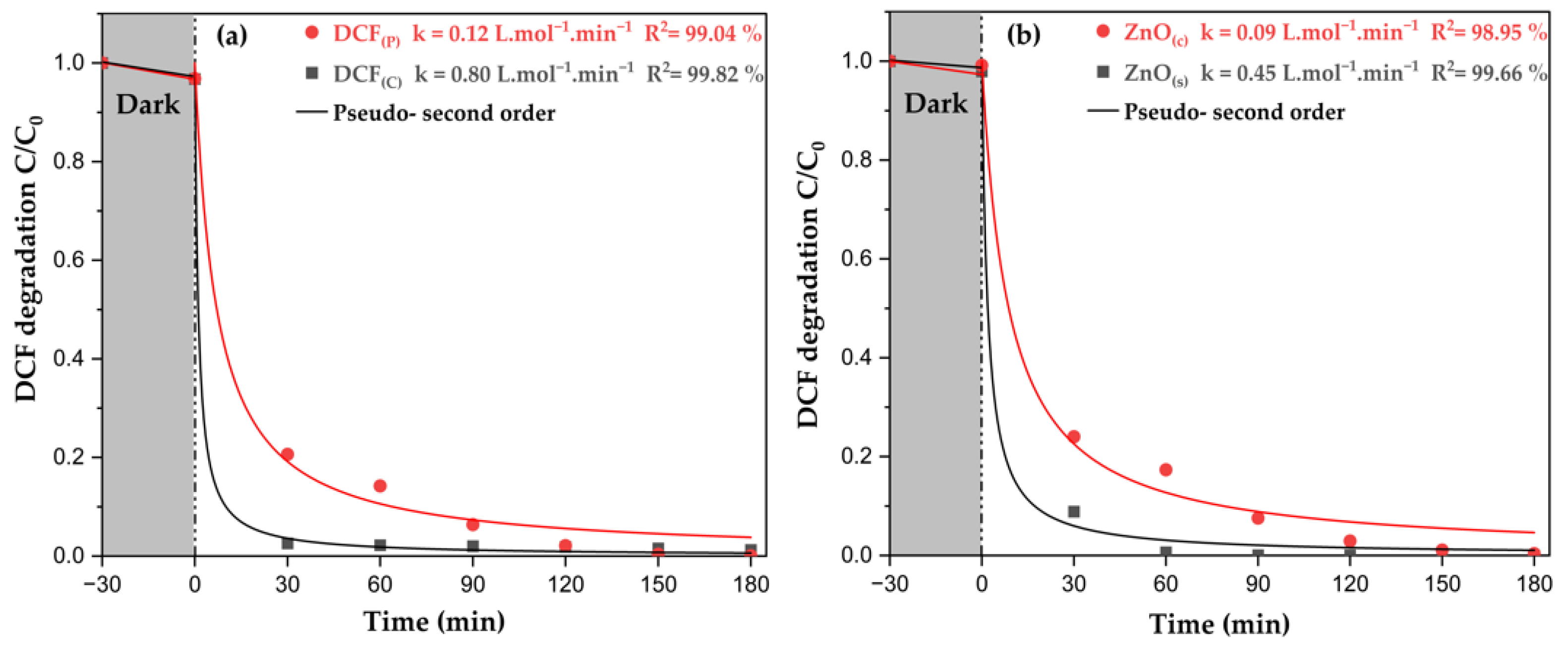
2.2.5. Photocatalytic Performance: Synthesized vs. Commercial ZnO on Diclofenac Degradation
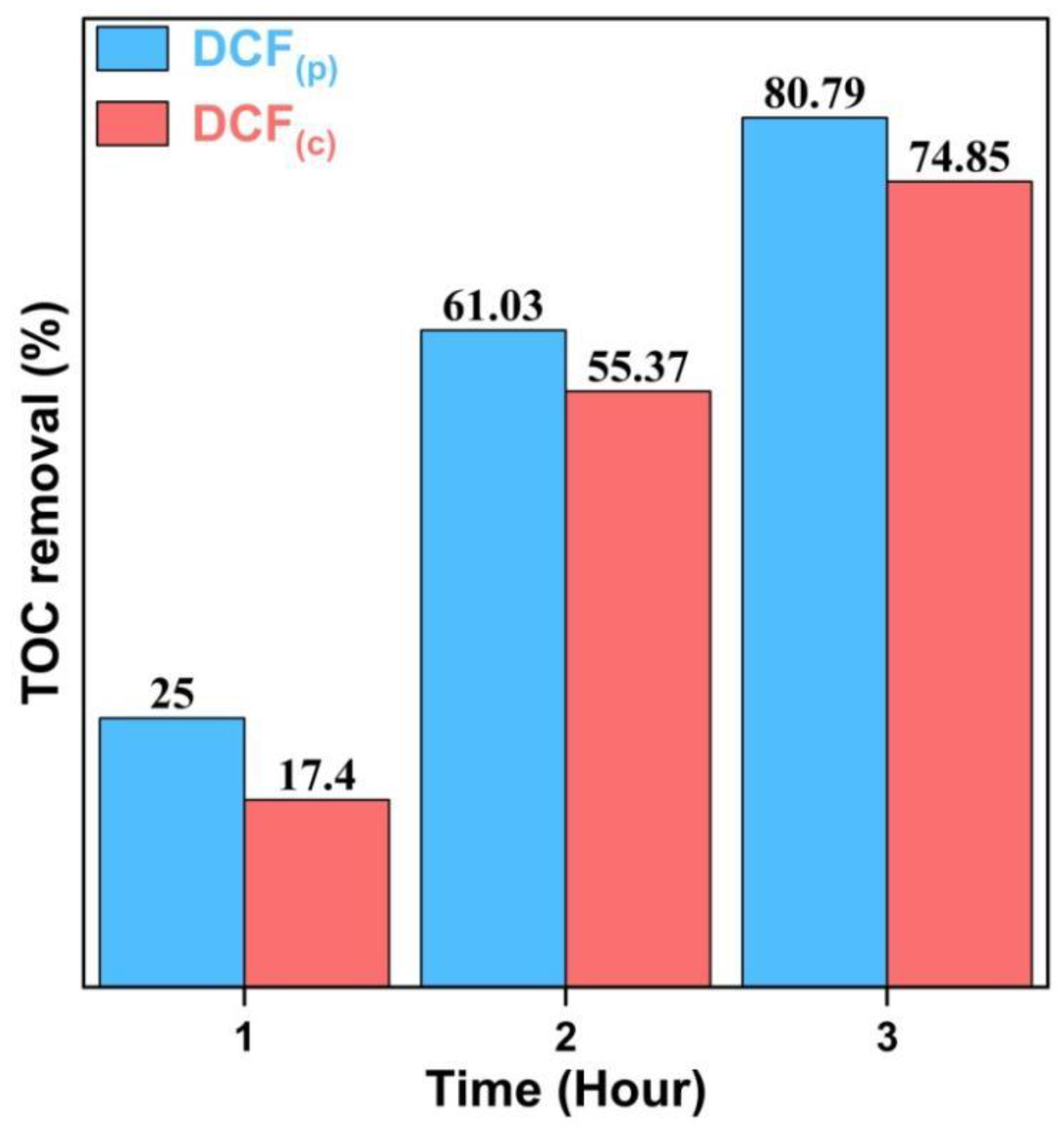
2.2.6. Determination of Total Organic Carbon (TOC)
2.3. Mechanistic Insights into Diclofenac Photodegradation: LC-MS Analysis and Proposed Degradation Pathways
2.4. Assessment of Photocatalyst Stability and Reusability Through Multiple Cycles
2.5. Photocatalytic Reduction of p-Nitrophenol: Spectral Changes and Degradation Insights
2.6. Influence of Trapping Agents on p-Nitrophenol Degradation
3. Experimental
3.1. Materials and Reagents
3.2. Preparation of the Natural Surfactant Extract
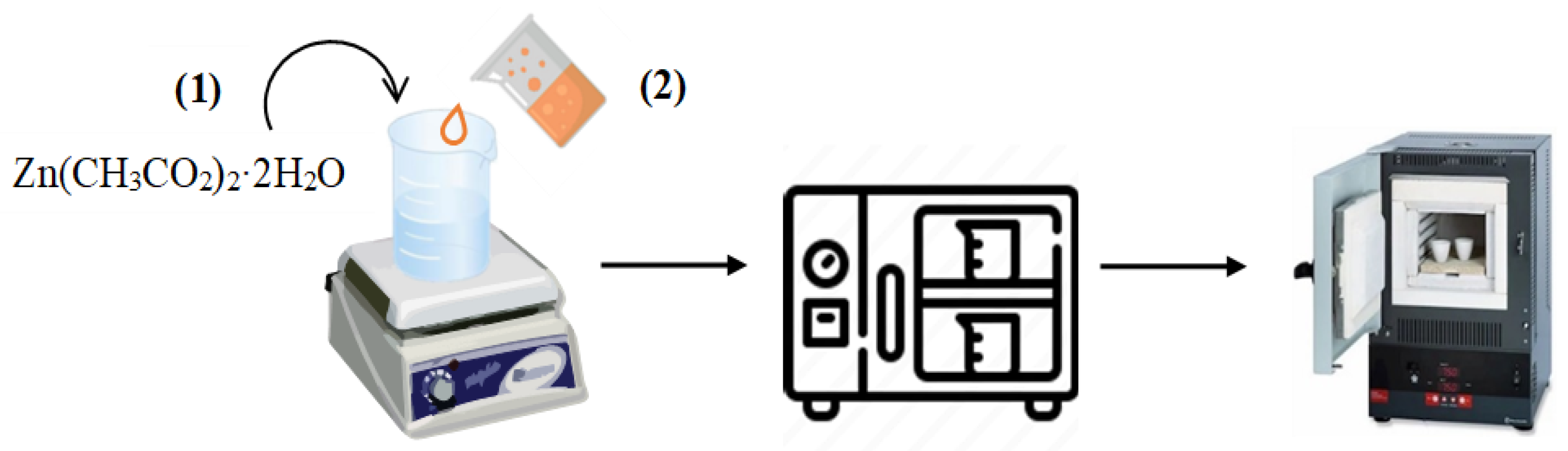
3.3. Synthesis of ZnO Nanoparticles
3.4. Characterization Techniques
3.5. Photocatalytic Activity Assessment
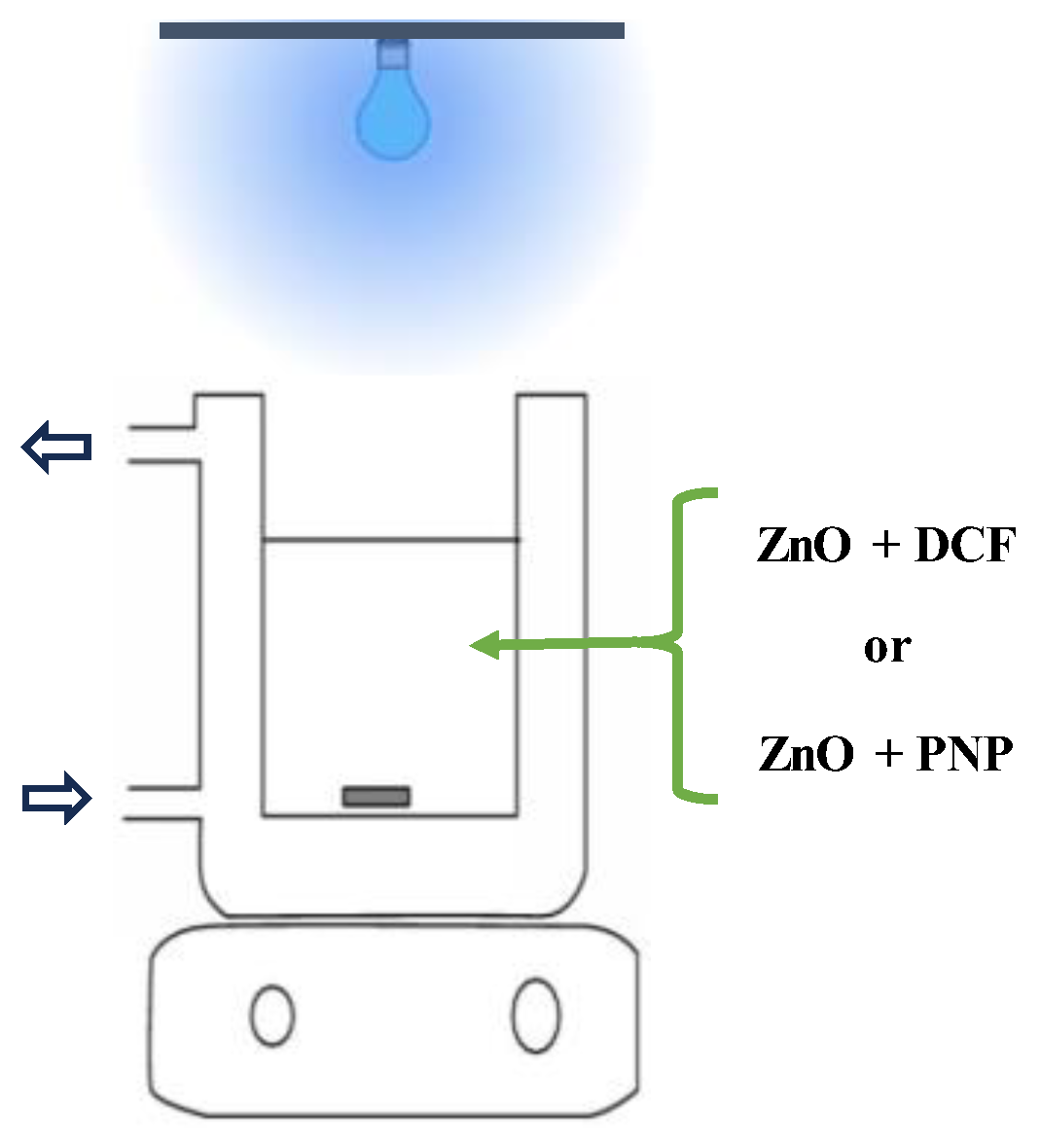
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of nanoparticles: Sources, synthesis, and biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mat. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Campos, J.M.; Montenegro Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotech. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Sundrarajan, M. Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: Spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J. Photochem. Photobio. B Bio. 2015, 149, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium flavum leaf extract. Appl. Nanosci. 2023, 13, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef] [PubMed]
- Abed, M.A.; Jassim, S.M.; Hameed, I.A.; Mohammed, S.B. Physical properties and antibacterial activity of green-iron oxide nanoparticles synthesize with chia seeds. Chem. Data Col. 2023, 44, 101013. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S. A Review on Green Synthesis of Zinc Oxide Nanoparticles Using Plant Extracts and Its Biomedical Applications. BioNanoScience 2020, 10, 848–863. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J.L.; Moreira, J.; Torres-Arellano, S.; Longoria, A.; Okoye, P.U.; Sebastian, P.J. Preparation of a heterogeneous catalyst from moringa leaves as a sustainable precursor for biodiesel production. Fuel 2021, 284, 118983. [Google Scholar] [CrossRef]
- Becerra-Paniagua, D.K.; Torres-Arellano, S.; Martinez-Alonso, C.; Luévano-Hipólito, E.; Sebastian, P.J. Facile and green synthesis of Cu/Cu2O composite for photocatalytic H2 generation. Mat. Sci. Semicond. Proc. 2023, 162, 107485. [Google Scholar] [CrossRef]
- Ramesh, P.; Rajendran, A. Photocatalytic dye degradation activities of green synthesis of cuprous oxide nanoparticles from Sargassum wightii extract. Chem. Phys. Impact 2023, 6, 100208. [Google Scholar] [CrossRef]
- Kovacic, M.; Juretic Perisic, D.; Biosic, M.; Kusic, H.; Babic, S.; Loncaric Bozic, A. UV photolysis of diclofenac in water; kinetics, degradation pathway and environmental aspects. Environ. Sci. Pollut. Res. 2016, 23, 14908–14917. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Tanveer, M.; Tezcanli, G.; Sadiq, M.T.; Kazmi, S.M.; Noshad, N.; Abbas, G.; Ali, A. Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst. Eng. Proc. 2021, 12, 76. [Google Scholar]
- Wojcieszyńska, D.; Łagoda, K.; Guzik, U. Diclofenac Biodegradation by Microorganisms and with Immobilised Systems—A Review. Catalysts 2023, 13, 412. [Google Scholar] [CrossRef]
- Sacher, F.; Lange, F.T.; Brauch, H.-J.; Blankenhorn, I. Pharmaceuticals in groundwaters: Analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the environment: Assessing risks of pharmaceuticals to wildlife and ecosystems. Phil. Trans. Roy. Soc. B Bio. Sci. 2014, 369, 20130569. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Heyd, A.; Eikemper, R.; Köhler, H.R.; Schwaiger, J. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2004, 68, 151–166. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abdelhamid, H.N.; Fouad, D.M.; Ibrahim, S.A. Catalytic reduction of 4-nitrophenol using copper terephthalate frameworks and CuO@C composite. J. Environ. Chem. Eng. 2021, 9, 104401. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mat. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Rasool, A.; Kiran, S.; Gulzar, T.; Abrar, S.; Ghaffar, A.; Shahid, M.; Nosheen, S.; Naz, S. Biogenic synthesis and characterization of ZnO nanoparticles for degradation of synthetic dyes: A sustainable environmental cleaner approach. J. Clean. Prod. 2023, 398, 136616. [Google Scholar] [CrossRef]
- Sanjeev, N.O.; Valsan, A.E. Photocatalytic and antibacterial activity of green synthesized and immobilized zinc oxide nanoparticles for the removal of sulfadiazine and acetaminophen: Effect of operational parameters and degradation pathway. J. Environ. Chem. Eng. 2024, 12, 112649. [Google Scholar] [CrossRef]
- Marcì, G.; Augugliaro, V.; López-Muñoz, M.J.; Martín, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.D.; Venezia, A.M. Preparation Characterization and Photocatalytic Activity of Polycrystalline ZnO/TiO2 Systems. 2. Surface, Bulk Characterization, and 4-Nitrophenol Photodegradation in Liquid−Solid Regime. J. Phys. Chem. B 2001, 105, 1033–1040. [Google Scholar] [CrossRef]
- Lizama, C.; Freer, J.; Baeza, J.; Mansilla, H.D. Optimized photodegradation of Reactive Blue 19 on TiO2 and ZnO suspensions. Catal. Today 2002, 76, 235–246. [Google Scholar] [CrossRef]
- Lathasree, S.; Rao, A.N.; SivaSankar, B.; Sadasivam, V.; Rengaraj, K. Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J. Mol. Catal. A Chem. 2004, 223, 101–105. [Google Scholar] [CrossRef]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Ben Seghir, B.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A review on biogenic green synthesis of ZnO nanoparticles by plant biomass and their applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Sabouri, Z.; Rangrazi, A.; Amiri, M.S.; Khatami, M.; Darroudi, M. Green synthesis of nickel oxide nanoparticles using Salvia hispanica L. (chia) seeds extract and studies of their photocatalytic activity and cytotoxicity effects. Bioprocess Biosyst. Eng. 2021, 44, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Anita; Thakur, V.; Narula, D.; Sharma, A.; Kaur, M.; Sharma, P. Investigation on green synthesized nanocomposites of magnetite using Salvia hispanica for antibacterial activity. Mat. Tod. Proc. 2023. [Google Scholar] [CrossRef]
- Chérif, I.; Dkhil, Y.O.; Smaoui, S.; Elhadef, K.; Ferhi, M.; Ammar, S. X-Ray Diffraction Analysis by Modified Scherrer, Williamson–Hall and Size–Strain Plot Methods of ZnO Nanocrystals Synthesized by Oxalate Route: A Potential Antimicrobial Candidate Against Foodborne Pathogens. J. Clust. Sci. 2023, 34, 623–638. [Google Scholar] [CrossRef]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol-Gel Synthesis of Zinc Oxide (ZnO) Nanoparticles: Study of Structural and Optical Properties. J. Sci. Islam. Repub. Iran 2015, 26, 281–285. [Google Scholar]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solids 2021, 155, 110125. [Google Scholar] [CrossRef]
- Hernández-Morales, L.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Espinoza, K.A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci. 2019, 489, 952–961. [Google Scholar] [CrossRef]
- Aydın, C.; Abd El-sadek, M.S.; Zheng, K.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol–gel calcination technique. Opt. Las. Technol. 2013, 48, 447–452. [Google Scholar] [CrossRef]
- Estrada-Urbina, J.; Cruz-Alonso, A.; Santander-González, M.; Méndez-Albores, A.; Vázquez-Durán, A. Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize. Nanomaterials 2018, 8, 247. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Ojedokun, A.T.; Bello, O.S. Kinetic modeling of liquid-phase adsorption of Congo red dye using guava leaf-based activated carbon. Appl. Water Sci. 2017, 7, 1965–1977. [Google Scholar] [CrossRef]
- Liang, S.; Wang, K.; Wang, K.; Wang, T.; Guo, C.; Wang, W.; Wang, J. Adsorption Behavior of Diclofenac on Polystyrene and Poly(butylene adipate-co-terephthalate) Microplastics: Influencing Factors and Adsorption Mechanism. Langmuir 2023, 39, 12216–12225. [Google Scholar] [CrossRef]
- Awang, H.; Peppel, T.; Strunk, J. Photocatalytic Degradation of Diclofenac by Nitrogen-Doped Carbon Quantum Dot-Graphitic Carbon Nitride (CNQD). Catalysts 2023, 13, 735. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Kadam, V.V.; Shanmugam, S.D.; Ettiyappan, J.P.; Balakrishnan, R.M. Photocatalytic degradation of p-nitrophenol using biologically synthesized ZnO nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 12119–12130. [Google Scholar] [CrossRef] [PubMed]
- Tewari, B.B.; Boodhoo, M. Removal of p-aminophenol and p-nitrophenol from aqueous solution through adsorption on antimony, cadmium, and zirconium ferrocyanides. J. Colloid Interface Sci. 2005, 289, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Marsalek, R. Particle Size and Zeta Potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Badawy, M.I.; Souaya, E.M.R.; Gad-Allah, T.A.; Abdel-Wahed, M.S.; Ulbricht, M. Fabrication of Ag/TiO2 photocatalyst for the treatment of simulated hospital wastewater under sunlight. Environ. Prog. Sus. Energy 2014, 33, 886–894. [Google Scholar] [CrossRef]
- Qutob, M.; Alshehri, S.; Shakeel, F.; Alam, P.; Rafatullah, M. Insight into Photodegradation of Diclofenac: Mechanism, Efficiency, Role of Parameters, Toxicity Assessment and Catalyst Stability. Rev. Environ. Contam. Toxicol. 2023, 261, 27. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic activity of ZnO-WO3 for diclofenac degradation under visible light irradiation. J. Photochem. Photobio. A Chem. 2019, 383, 111993. [Google Scholar] [CrossRef]
- Bhalkaran, S.; Tewari, B.B. Interacción of 2-aminophenol, 4-aminophenol, 2-nitrophenol and 4-nitrophenol with manganese and nickel hexacyanoferrate (II) complexes. Rev. Boliv. Química 2016, 33, 164–173. [Google Scholar]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Cordero-García, A.; Turnes Palomino, G.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Maya-Teviño, L.; Hernández-Ramírez, A. Photocatalytic behaviour of WO3/TiO2-N for diclofenac degradation using simulated solar radiation as an activation source. Environ. Sci. Pollut. Res. 2017, 24, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Aurora-Prado, M.; Steppe, M.; Tavares, M.; Kedor-Hackmann, E.; Santoro, M. Comparison between capillary electrophoresis and liquid chromatography for the determination of diclofenac sodium in a pharmaceutical tablet. J. AOAC Int. 2002, 85, 333–340. [Google Scholar] [PubMed]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef]
- Agüera, A.; Pérez Estrada, L.A.; Ferrer, I.; Thurman, E.M.; Malato, S.; Fernández-Alba, A.R. Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J. Mass Spect. 2005, 40, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous activation of peroxymonosulfate by LaFeO3 for diclofenac degradation: DFT-assisted mechanistic study and degradation pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Drozdek, E.; Boruta, T.; Foszpańczyk, M.; Olak-Kucharczyk, M.; Żyłła, R.; Gmurek, M. Impact of Hydrogen Peroxide on the UVC Photolysis of Diclofenac and Toxicity of the Phototransformation Products. Int. J. Photo. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Iovino, P.; Lavorgna, M.; Orlo, E.; Russo, C.; De Felice, B.; Campolattano, N.; Muscariello, L.; Fenti, A.; Chianese, S.; Isidori, M.; et al. An integrated approach for the assessment of the electrochemical oxidation of diclofenac: By-product identification, microbiological and eco-genotoxicological evaluation. Sci. Total Environ. 2024, 909, 168511. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Cat. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Homlok, R.; Mile, V.; Takács, E.; Járvás, G.; Góger, S.; Wojnárovits, L. Comparison of hydrogen atom and hydroxyl radical reactions with simple aromatic molecules in aqueous solution. Chem. Phys. 2020, 534, 110754. [Google Scholar] [CrossRef]
- Dey, S.; Manogaran, D.; Manogaran, S.; Schaefer, H.F., III. Substituent effects on the aromaticity of benzene—An approach based on interaction coordinates. J. Chem. Phys. 2019, 150, 214108. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Dobrowolski, M.A.; Zborowski, K.; Cyrański, M.K. Relation between the substituent effect and aromaticity. Part II. The case of meta- and para-homodisubstituted benzene derivatives. J. Phys. Org. Chem. 2006, 19, 889–895. [Google Scholar] [CrossRef]
- Anbar, M.; Meyerstein, D.; Neta, P. The Reactivity of Aromatic Compounds toward Hydroxyl Radicals. J. Phys. Chem. 1966, 70, 2660–2662. [Google Scholar] [CrossRef]
- Moore, D.E.; Roberts-Thomson, S.; Zhen, D.; Duke, C.C. Photochemical studies on the antiinflammatory drug diclofenac. Photochem. Photobio. 1990, 52, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Thurman, E.M.; Ferrer, I.; Dotson, A.D.; Linden, K.G. Dimer formation during UV photolysis of diclofenac. Chemosphere 2013, 93, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Svanfelt, J.; Kronberg, L. A Photochemical Study of Diclofenac and Its Major Transformation Products. Photochem. Photobio. 2010, 86, 528–532. [Google Scholar] [CrossRef]
- Ziylan, A.; Dogan, S.; Agopcan, S.; Kidak, R.; Aviyente, V.; Ince, N.H. Sonochemical degradation of diclofenac: Byproduct assessment, reaction mechanisms and environmental considerations. Environ. Sci. Pollut. Res. 2014, 21, 5929–5939. [Google Scholar] [CrossRef] [PubMed]
- Galmier, M.-J.; Bouchon, B.; Madelmont, J.-C.; Mercier, F.; Pilotaz, F.; Lartigue, C. Identification of degradation products of diclofenac by electrospray ion trap mass spectrometry. J. Pharm. Biomed. Anal. 2005, 38, 790–796. [Google Scholar] [CrossRef]
- Elangovan, M.; Bharathaiyengar, S.M.; PonnanEttiyappan, J. Photocatalytic degradation of diclofenac using TiO2-CdS heterojunction catalysts under visible light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 18186–18200. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, M.C.; Valvo, L.; Bertocchi, P.; Manna, L. RP-HPLC study of the degradation of diclofenac and piroxicam in the presence of hydroxyl radicals. J. Pharm. Biomed. Anal. 2003, 32, 151–158. [Google Scholar] [CrossRef]
- Martínez, C.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Cat. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Poirier-Larabie, S.; Segura, P.A.; Gagnon, C. Degradation of the pharmaceuticals diclofenac and sulfamethoxazole and their transformation products under controlled environmental conditions. Sci. Total Environ. 2016, 557–558, 257–267. [Google Scholar] [CrossRef]
- Wang, K.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Chen, G.; Liu, M. Simple Method for Directly Synthesizing Ag Nanoparticles with Silver Ammonia and Polydopamine in a Microreactor toward the Conversion of 4-NP to 4-AP. Ind. Eng. Chem. Res. 2020, 59, 16205–16216. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Fundamentals and misconceptions in photocatalysis. J. Photochem. Photobio. A Chem. 2010, 216, 85–93. [Google Scholar] [CrossRef]
- Azam, A.; Babkair, S.S. Low-temperature growth of well-aligned zinc oxide nanorod arrays on silicon substrate and their photocatalytic application. Int. J. Nanomed. 2014, 9, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Aadnan, I.; Zegaoui, O.; Daou, I.; Esteves da Silva, J.C.G. Synthesis and physicochemical characterization of a ZnO-Chitosan hybrid-biocomposite used as an environmentally friendly photocatalyst under UV-A and visible light irradiations. J. Environ. Chem. Eng. 2020, 8, 104260. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Huang, J.; Tong, J.; Liu, X.; Wang, Y.; Qiao, W.; Han, J. Theoretical and experimental insight into plasma-catalytic degradation of aqueous p-nitrophenol with graphene-ZnO nanoparticles. Sep. Purif. Technol. 2022, 295, 121362. [Google Scholar] [CrossRef]
- Hasan, I.; Shekhar, C.; Bin Sharfan, I.I.; Khan, R.A.; Alsalme, A. Ecofriendly Green Synthesis of the ZnO-Doped CuO@Alg Bionanocomposite for Efficient Oxidative Degradation of p-Nitrophenol. ACS Omega 2020, 5, 32011–32022. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Silveira, J.E.; Carbajo, J.; Zazo, J.A.; Casas, J.A.; Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Diclofenac photodegradation with the Perovskites BaFeyTi1-yO3 as catalysts. Environ. Sci. Poll. Res. 2021, 28, 23822–23832. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ji, Y.; Tian, J.; Du, Z. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015, 262, 1108–1115. [Google Scholar] [CrossRef]
- Smýkalová, A.; Sokolová, B.; Foniok, K.; Matějka, V.; Praus, P. Photocatalytic Degradation of Selected Pharmaceuticals Using g-C3N4 and TiO2 Nanomaterials. Nanomaterials 2019, 9, 1194. [Google Scholar] [CrossRef]
- Suhag, M.H.; Khatun, A.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Visible Light Induced Photocatalytic Degradation of Diclofenac in Aqueous Solution Using Fabricated ZnO/g-C3N4 by Facile Calcination Technique. ACS Omega 2024, 9, 45090–45103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, S.; Li, Y.; Xiao, Y.; Kang, Q.; Cai, Q. High Efficient Photocatalytic Degradation of p-Nitrophenol on a Unique Cu2O/TiO2 p-n Heterojunction Network Catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.; Wu, Q.; Yu, H.; Zhao, Y.; Qu, J.; Huo, M.; Yuan, X. Synthesis of Cu2O nanocrystals/TiO2 photonic crystal composite for efficient p-nitrophenol removal. Col. Surf. A Physicochem. Eng. Asp. 2018, 539, 291–300. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Y.; Yuan, H.; Chen, H.; Chen, G.; Shen, J.; Li, L. The enhanced catalytic degradation of SiO2/Fe3O4/C@TiO2 photo-Fenton system on p-nitrophenol. J. Nano. Res. 2016, 18, 343. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, F.; Huang, Z.; Chen, H.; Zhuang, Z.; Pan, Z.; Long, J.; Gu, F. Photochemical fabrication of SnO2 dense layers on reduced graphene oxide sheets for application in photocatalytic degradation of p-Nitrophenol. Appl. Cat. B Environ. 2017, 215, 8–17. [Google Scholar] [CrossRef]







| Material | SSA (m2/g) | Total Pore Volume (cc/g) | Average Pore Radius (nm) |
|---|---|---|---|
| Non-calcined | 37.7 | 0.2 | 10 |
| Calcined ZnO | 13.4 | 0.1 | 17 |
| Adsorption and Diffusion of DCF (mcat = 0.5 g/L) | |||||
| Pseudo-first-order | k (10−2 min−1) | 27.29 | |||
| R2 (%) | 99.60 | ||||
| Pseudo-second-order | k (10−2 L·mol−1·min−1) | 4.60 | |||
| R2 (%) | 99.94 | ||||
| Diffusion | kip1; kip2; kip3 (mg.g−1min−1/2) | 3.49 | 0.91 | 0.18 | |
| R2 (%) | 100 | 94.27 | 75 | ||
| Degradation of PNP and DCF | |||||
| PNP (no catalyst) | PNP (mcat = 1.5 g/L) | DCF (no catalyst) | DCF (mcat = 1.5 g/L) | ||
| k (10−2 L·mol−1·min−1) | 0.004 | 4.03 | 0.015 | 46.03 | |
| R2 (%) | 66.74 | 97.14 | 96.93 | 99.73 | |
| Compound | Predicted Formula | Measured m/z | Theoretical m/z | Error (ppm) |
|---|---|---|---|---|
| DCF | [C14H11Cl2NO2]− | 294.0094 | 294.0094 | 0 |
| DP1 | [C14H11Cl2NO3]− | 310.0047 | 310.0043 | 1.3 |
| DP2 | [C14H9Cl2NO3]+ | 310.0038 | 310.0032 | 1.9 |
| DP3 | [C13H11Cl2NO]− | 266.0149 | 266.0145 | 1.5 |
| DP4 | [C13H10ClNO]− | 230.0375 | 230.0378 | −1.3 |
| DP5 | [C14H11Cl2NO]+ | 278.0144 | 278.0134 | −3.5 |
| DP6 | [C13H11Cl2N]+ | 250.0194 | 250.0185 | 3.5 |
| DP7 | [C13H10ClN]+ | 214.0424 | 214.0418 | 2.8 |
| DP8 | [C14H11NO3]+ | 242.0820 | 242.0812 | 3.3 |
| Pollutant | Photocatalyst | Type of Irradiation | Removal Efficiency (%) | References |
|---|---|---|---|---|
| DCF | TiO2 (P25) | Xe lamp, 750 W/m2 | 90.4 | [68] |
| BaTiO3 | LED, 1100 W/m2 | 61 | [88] | |
| P25/TEOS | Visible, 79 W/m2 | 65 | [89] | |
| C3N4 | Visible, 85 W/m2 | 77 | [90] | |
| ZnO/g-C3N4 | Visible, 100 W | 97 | [91] | |
| PNP | TiO2 NTs | Xe lamp, 500 W | 36.5 | [92] |
| Cu2O NCs/TiO2 PC | Xe lamp, 300 W | 60 | [93] | |
| SiO2/Fe3O4/C@TiO2 | Xe lamp, 500 W | 93 | [94] | |
| SnO2-rGO | UV, 8 W | 95.6 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ounis Dkhil, Y.; Peppel, T.; Sebek, M.; Strunk, J.; Houas, A. Green Synthesis of Photocatalytically Active ZnO Nanoparticles Using Chia Seed Extract and Mechanistic Elucidation of the Photodegradation of Diclofenac and p-Nitrophenol. Catalysts 2025, 15, 4. https://doi.org/10.3390/catal15010004
Ounis Dkhil Y, Peppel T, Sebek M, Strunk J, Houas A. Green Synthesis of Photocatalytically Active ZnO Nanoparticles Using Chia Seed Extract and Mechanistic Elucidation of the Photodegradation of Diclofenac and p-Nitrophenol. Catalysts. 2025; 15(1):4. https://doi.org/10.3390/catal15010004
Chicago/Turabian StyleOunis Dkhil, Yossra, Tim Peppel, Michael Sebek, Jennifer Strunk, and Ammar Houas. 2025. "Green Synthesis of Photocatalytically Active ZnO Nanoparticles Using Chia Seed Extract and Mechanistic Elucidation of the Photodegradation of Diclofenac and p-Nitrophenol" Catalysts 15, no. 1: 4. https://doi.org/10.3390/catal15010004
APA StyleOunis Dkhil, Y., Peppel, T., Sebek, M., Strunk, J., & Houas, A. (2025). Green Synthesis of Photocatalytically Active ZnO Nanoparticles Using Chia Seed Extract and Mechanistic Elucidation of the Photodegradation of Diclofenac and p-Nitrophenol. Catalysts, 15(1), 4. https://doi.org/10.3390/catal15010004








