Abstract
The aim of this study was to design model emulsion systems based on enzymatic modification fats for shaping the quality of target products in the food, cosmetic, and pharmaceutical industries. In this study, a catalysis process carried out in the presence of immobilized lipase as a catalyst was used to obtain the fatty mixtures constituting the fat base of the emulsions. It was assumed to produce stable emulsion products containing modified fat with a sufficient amount of emulsifiers and a variable concentration of a viscosity modifier, which was a mixture of xanthan gum and microcrystalline cellulose (XGMCC). The following methods were used in the evaluation of emulsions: evaluation of the stability of systems using the Turbiscan test, evaluation of average particle size, microscopic evaluation of emulsions, and evaluation of texture and viscosity. Based on the results obtained for XGMCC-stabilized emulsion systems containing enzymatically modified fats, it was found that some of the systems had satisfactory stability. No correlation was observed between the applied concentration of a texture modifier and emulsion stability. However, the type of fatty phase used influenced the stability of the analyzed systems. Taking the above relationship into account, emulsion E67, which was characterized by a small degree of destabilization changes, was evaluated as the best system. This emulsion was characterized by the lowest droplet diameter of the dispersed phase at all measuring points during the storage process. This system can be used as a stable model system as a starting point in the development of a new food or cosmetic formulation.
1. Introduction
Interestrification is a catalytic reaction. It can be carried out in the presence of a chemical catalyst but also in the presence of enzymes [1]. The latter type of catalysis is nowadays much more widespread due to issues, such as waste-free technology, but also the use of biological reaction catalysts [2]. The current diversity of enzymes is also a factor that allows enzymatic catalysis to be considered superior to chemical catalysis [3]. Interesterification is a valuable process in fat modification, as it alters the arrangement of fatty acids within triacylglycerols (TAGs) without changing the fatty acids themselves [4,5]. This can improve the physical and functional properties of fats, such as the melting point, texture, and stability, making them more suitable for various food applications. By redistributing fatty acids, interesterification can help achieve specific characteristics that align with consumer preferences, such as reduced trans fats or enhanced creaminess in products like margarine or shortenings [6]. Additionally, this process can improve nutritional profiles by incorporating healthier fatty acid compositions, catering to current dietary trends [6].
Emulsions play a crucial role in the cosmetic industry, serving as the foundation for many formulations [7]. Their two-phase systems, typically consisting of oil and water, allow for a range of textures and performances that cater to various consumer needs [8]. In color cosmetics, emulsions enable smooth application and even distribution of pigments, while in skincare, they help deliver moisturizing agents and active ingredients effectively. Additionally, emulsions can enhance the stability and shelf life of products, making them essential for both product performance and consumer satisfaction [9].
The use of surfactants and stabilizers is key to maintaining the integrity of emulsions, preventing separation, and ensuring a uniform product [10]. Innovations in emulsion technology continue to expand their application, leading to more effective and appealing cosmetic formulations. Innovations in ingredients, such as natural and organic components, are becoming more prominent as consumers seek safer and environmentally friendly options.
Xanthan gum (XG) is prized in various industries, including food, cosmetics, pharmaceuticals, and more, due to its ability to thicken and stabilize solutions [11]. Even at low concentrations, it can significantly increase viscosity, making it effective for products like salad dressings, sauces, and dairy products. Its unique properties also allow it to maintain consistency over a wide range of temperatures and pH levels, which is why it is so widely used [12].
The benefits of using xanthan gum are also found in cosmetic formulations, for instance, in toothpaste, lotions, shampoo, and dermatological products [13]. In creams and lotions, xanthan gum helps maintain the stability of oil and water mixtures, preventing them from separating.
A fairly commonly used thickener and at the same time stabilizer in the food, cosmetic, and medical industries, is microcrystalline cellulose (MCC). It is used as a regulator of flow characteristics in systems applied to final products and as a reinforcing agent for final products, such as medical tablets [14].
Microcrystalline cellulose is a purified form of cellulose that is mainly used as a formulation ingredient to improve texture properties as well as the physical stability of emulsion systems [14]. The emulsion stabilizing and thickening functions of cellulose are due to its affinity to both water and oil [15]. MCC increases the viscosity of the aqueous phase of emulsion systems, thus preventing the approach of the dispersed phase droplets and, consequently, their merging [15].
It has been found that the co-processing of MCC with other excipients can improve the yield of these excipients [16], so in the study presented here, a commercial mixture of xanthan gum and microcrystalline cellulose was used as the viscosity modifier.
The aim of this work was to prepare new model emulsion systems that could be used as the basis for products in various industries. Since each area requires long-term stability and stability over a range of variable temperatures, for example, it was important to carry out a series of physicochemical determinations to confirm the validity of producing emulsions based on modified fats by enzymatic catalysis. In addition to characterizing the emulsions with regard to the type of fat base, the paper also describes the characteristics of the systems, depending on the amount of viscosity modifier used.
2. Results and Discussion
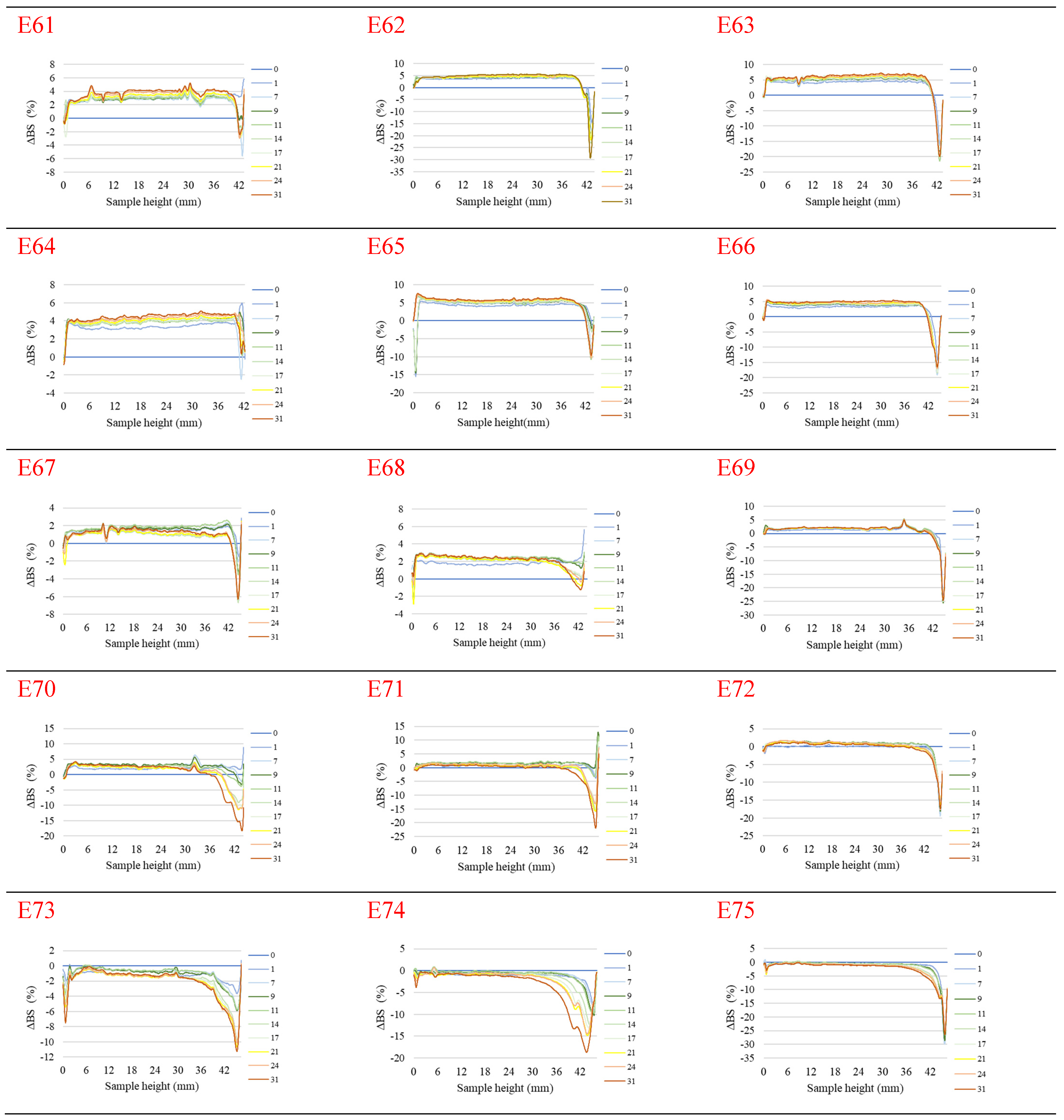
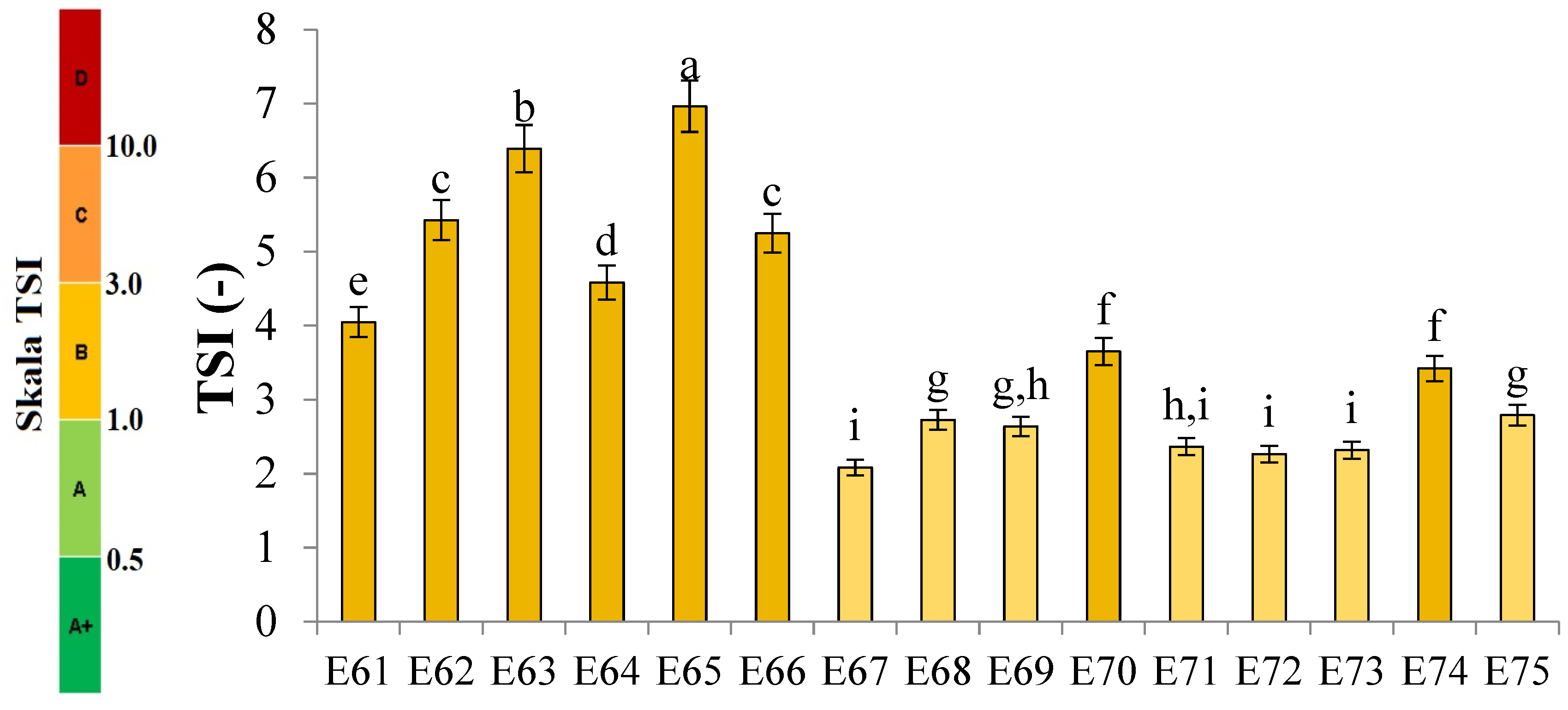
Figure 1 shows the profiles of backscattered light intensity as a function of sample height in the reference mode for emulsions E61–E75, differing from each other by the fatty phase and the amount of the introduced texture modifier containing XG and MCC. Different stages of destabilization processes after the storage period were observed in the systems produced. No correlation between the advancement of these changes and the variable concentration of texture modifiers used in the systems was observed. The lowest stability and the highest degree of destabilization changes were characteristic of systems E61–E66, in which the fatty phase consisted of modified fat blends containing a predominance of mutton tallow. Analyzing the profiles of changes in the backscattered light intensity of these systems, it was found that the mean values of ΔBS in the central part of the graphs, corresponding to the central zone of the measuring vials, exceeded 2.0%. This indicates that processes of aggregation or merging of dispersed phase droplets (flocculation or coalescence) have occurred in these systems. It was found that in systems to which 0.8 and 1.0% of the texture modifier was introduced (E62, E63, E65, and E66), the process of gravitational separation began, which caused the migration of fatty phase droplets to the upper parts of the measuring vials. The above changes were already observed 24 h after emulsion formation. The stability coefficients of emulsions E61–E66 were in the range 4.0–7.0 (category C—poor stability), and the values obtained were the highest among the values determined for the analyzed systems containing XGMCC (Figure 2).
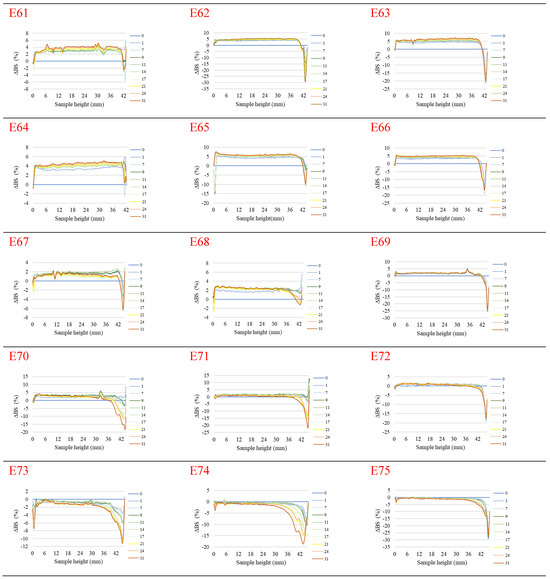
Figure 1.
Profiles of changes in backscattered light intensity as a function of sample height for emulsions E61–E75 when stored for 31 days at 25 °C.
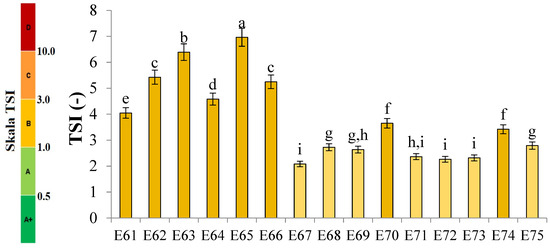
Figure 2.
TSI values for E61–E75 systems stored for 31 days at 25 °C. a, b, c, ...—different letters denote statistically significant differences between the means (p ≤ 0.05).
Analyzing the changes in the backscattered light intensity of systems E70–E75, it was found that they were characterized by similar ΔBS profiles. The dominant destabilizing process that occurred in these systems was the creaming process, which was evidenced by the BS changes in the parts of the graphs corresponding to the upper zone of the measurement vials. However, it can be concluded that this process was not advanced to a significant degree, as no clarification occurred in the lower part of the samples after the storage period in any of the systems. The thickness of the cream produced was greater for systems E70, E71, E73, and E74 containing 0.6 and 0.8% w/w XGMCC than for E72 and E75 containing 1.0% w/w of this component. For systems E71–E75, the mean ΔBS values in the part of the graphs corresponding to the middle zone of the measurement vials did not exceed 2.0%, suggesting that aggregation and droplet merging processes did not occur. Systems E70–E75 were characterized by statistically significant (p ≤ 0.05) lower TSI values. Based on the stability coefficient values obtained (2.4–2.8), systems E71, E72, E73, and E75 were classified in category B (satisfactory stability). On the other hand, the E70 and E74 systems with TSI values above 3.0 (3.7 and 3.4) were classified as category C (poor system stability).
The ΔBS profiles of emulsions E67–E69 indicated the occurrence of destabilizing changes of weak intensity in these systems. The process of migration of fatty phase particles into the upper parts of the measuring vials started in all three emulsions, and its least advance was recorded for system E68. Analyzing the changes in the intensity of backscattered light of the systems in the part of the graphs corresponding to the middle zone of the measuring vials, it was found that only in the case of system E67 did flocculation or coalescence processes not occur. Also, in the case of systems E67–E69, statistically significant (p ≤ 0.05) lower values of the TSI coefficient were recorded in comparison with the values of this parameter determined for systems E61–E66 discussed above. These values determined after the storage period were within the range of 2.2–2.7, which qualified them into category B, indicating their satisfactory stability.
Photographs of the E61–E75 systems after 30 days of storage are shown in Figure 3. In general, no changes were observed to conclude that destabilizing processes had started in the emulsions. The visual assessment of the emulsions was not consistent with the provided results from the Turbiscan test. However, this assessment can be explained by the information provided by Araújo et al. (2009) [17], who report that Turbiscan is a highly sensitive tool that provides reliable and rapid information to assess emulsion changes at a very early stage when visual analysis does not yet capture these changes.

Figure 3.
Photograph of E61–E75 systems after 31 days of storage at 25 °C.
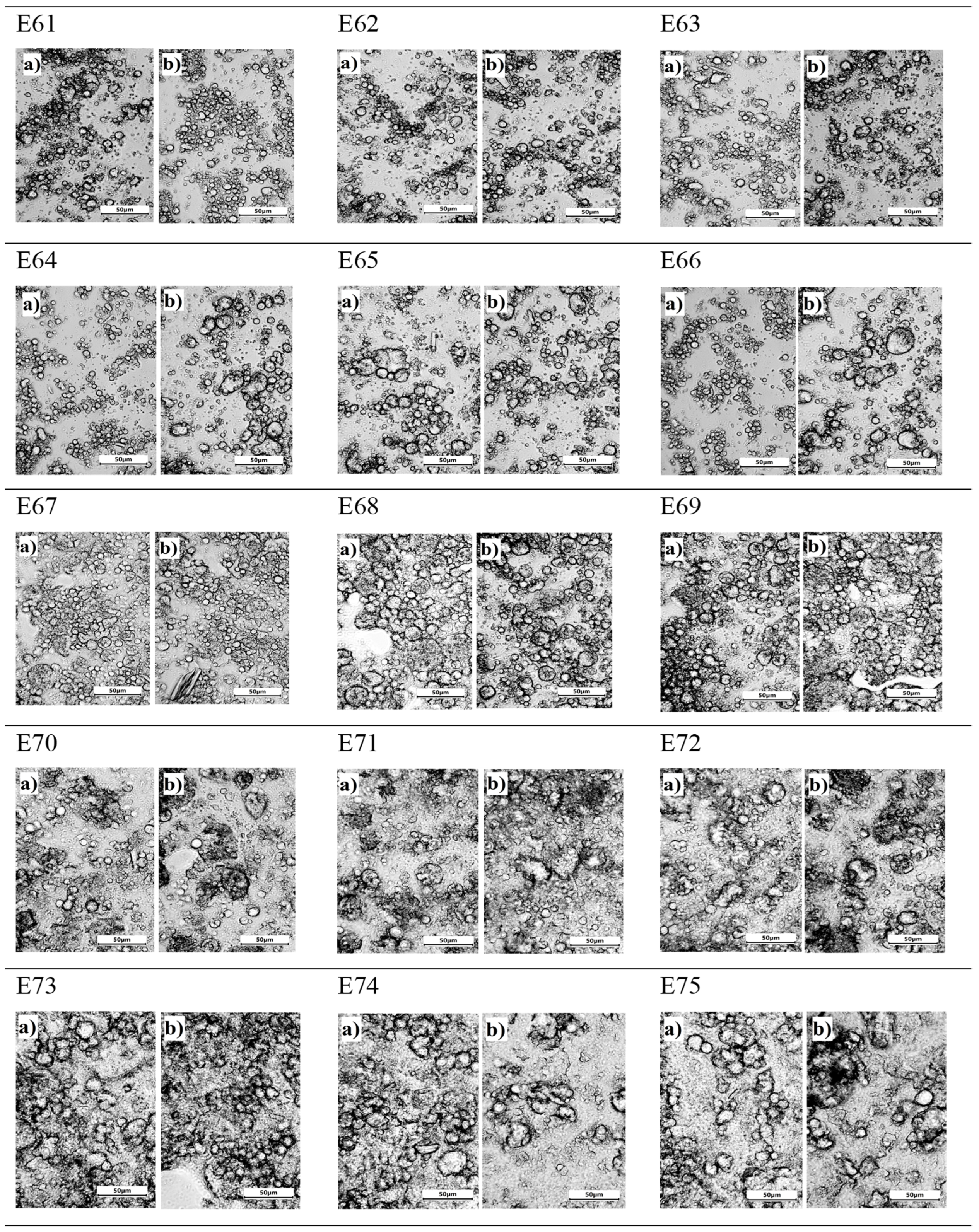
Analyzing the microstructure of all systems, it is clear that the smallest, almost invisible to the naked eye, changes were recorded for emulsion E67. Both the distribution and size of droplets were similar 24 h after manufacturing and after 30 days of emulsion storage (Figure 4). The largest droplets were in emulsions E73, E74, and E75, with no effect on the storage period of these systems, i.e., the systems with the highest hemp oil content. It should also be pointed out that for these systems, varying the concentration of the thickener also had no effect on the image of the obtained emulsion microstructure. For emulsions E64 and E66, it was observed that the droplet size increased after 30 days of storage. For the emulsion systems, i.e., E61, E62, E63, E65, E68, and E69, the presence of droplets of different sizes was observed. Between the small droplets, there were larger droplets, which tended to form larger agglomerates. According to Alizadeh et al. (2019) [18], systems with this structure and arrangement of dispersed phase droplets show instability during storage. In these systems, no clear change in droplet size was observed after a 30-day storage period. Evaluating the microstructure for E70, E71, and E72 systems, it should be noted that these systems are characterized by a similar small particle size, with no effect of the concentration of the thickener or the influence of the storage period on the appearance of the emulsion (Figure 4).
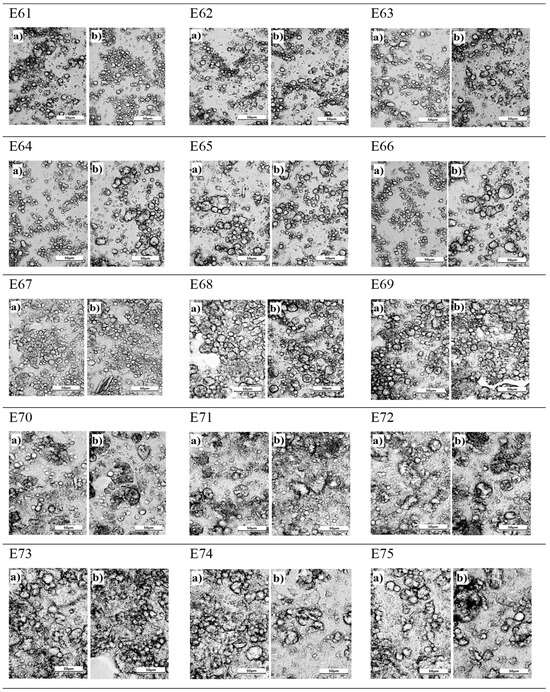
Figure 4.
The microstructure of emulsion systems E61–E75 determined 24 h after their preparation (a) and after 31 days of storage (b).
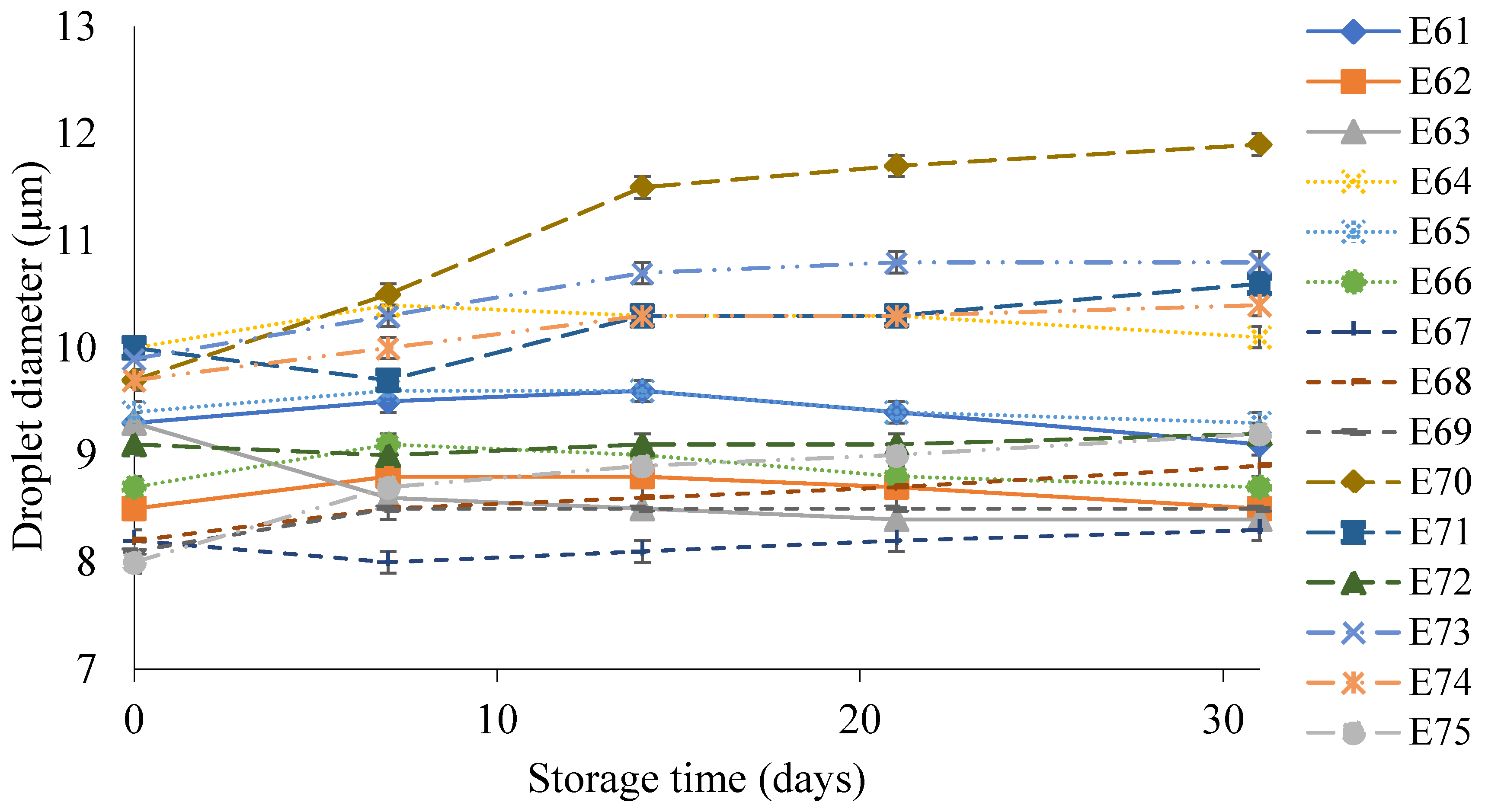
Figure 5 shows the droplet diameter of the dispersed phase of the E61–E75 systems during their storage for 31 days. These emulsions had droplet diameters in the range 7.5–11.2 µm. The largest increase in droplet size during storage of 2.0 µm was characteristic of emulsion E70. On the other hand, after manufacturing, the smallest droplet size of the dispersed phase (7.7 µm) was recorded for systems E67 and E68. After 31 days, the increase in droplet diameter for E67 was only 0.1 µm, while for E68, it was 0.7 µm.
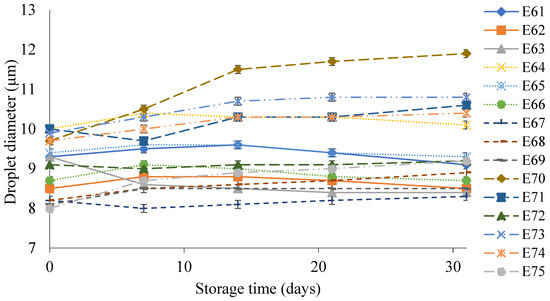
Figure 5.
Droplet diameter of the dispersed phase of E61–E75 systems when stored for 31 days at 25 °C.
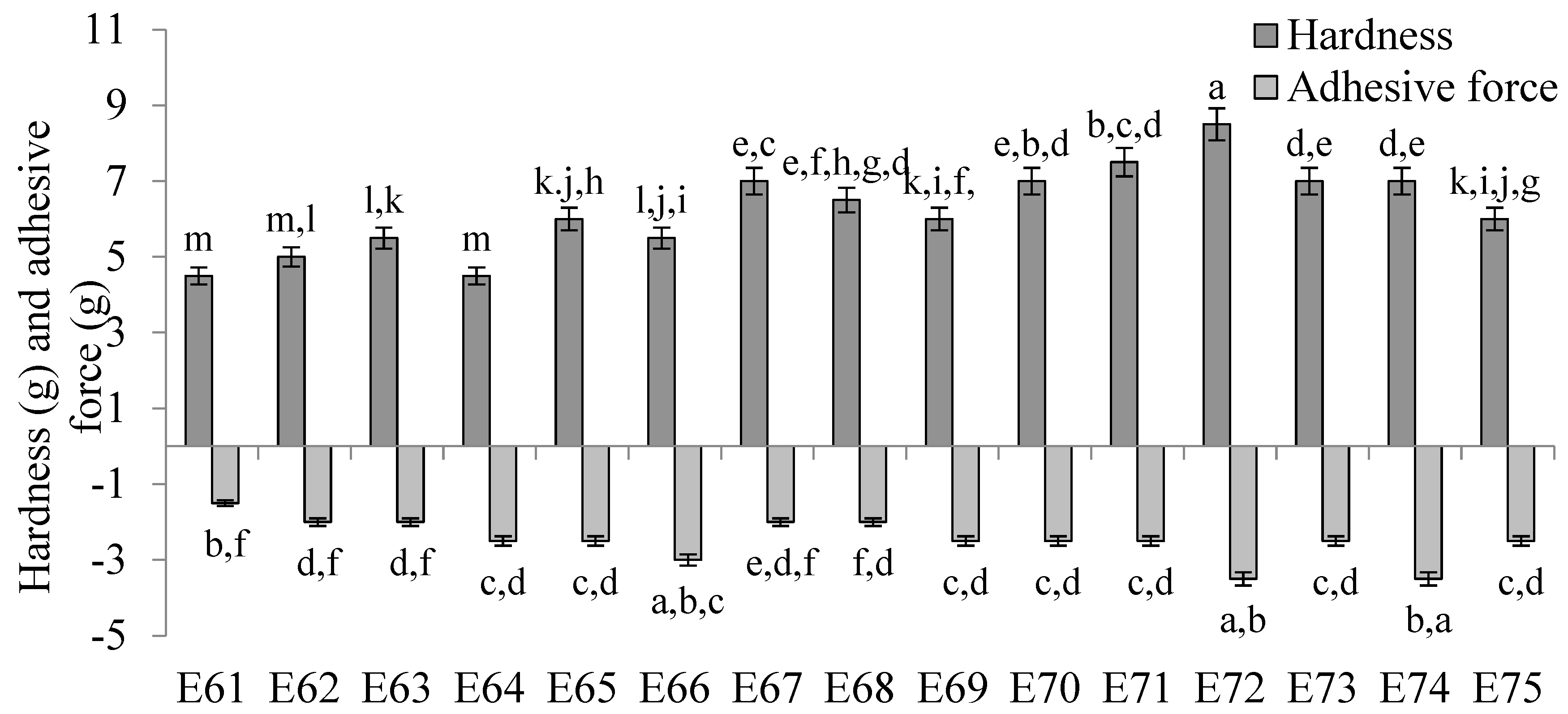
The values of texture parameters characterizing emulsion systems E61–E75 are shown in Figure 6. The hardness of these emulsions was slightly dependent on the type of fatty phase used. The lowest hardness values, in the range of 4.5–6.0 g, were obtained for systems E61–E66, which contained as a fatty phase a blend of mutton tallow and hemp seed oil fats with a predominance of mutton tallow. These values were statistically significantly (p ≤ 0.05) lower than those obtained for emulsions E70–E75 containing hemp seed oil predominance as well as for emulsions E67–E69 containing equal parts of both fats. Systems E70–E72 were characterized by the highest values of this parameter, remaining in the range of 7.0–8.5 g. In contrast, from the hardness results obtained, no clear trend of increase or decrease was observed between the values of this parameter and the ratio of animal fat to vegetable oil used in the fat phase. Rather, the changes in hardness were due to a change in the consistency of the fats resulting from the interestrification process. The concentration of the texture modifier also showed no clear, biased effect on the hardness of the analyzed systems. Considering systems containing the same fatty phase but differing in XGMCC concentration by 0.2% w/w (i.e., 0.6 and 0.8% w/w or 0.8 and 1.0% w/w), it was observed that only systems E64 and E65, E71 and E72, and E74 and E75 were characterized by statistically significant (p ≤ 0.05) different hardness values.
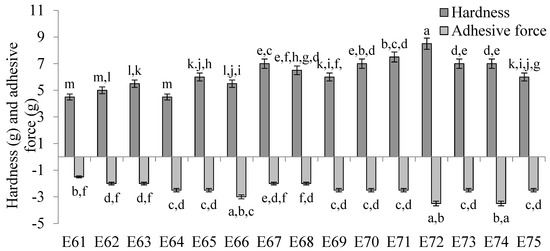
Figure 6.
The hardness and adhesive force of E61–E75 systems determined 48 h after their preparation. a, b, c, ...—different letters denote statistically significant differences between the means for individual texture parameters (i.e., separately for hardness and adhesive force) (p ≤ 0.05).
No correlation was observed between the determined values of adhesive force for systems E61–E75 and the amount of XGMCC introduced into the system, as well as the ratio of fats in the fatty phases of the emulsion. In general, the values of this parameter were very similar for all systems and ranged from 1.5 to 3.5 g.
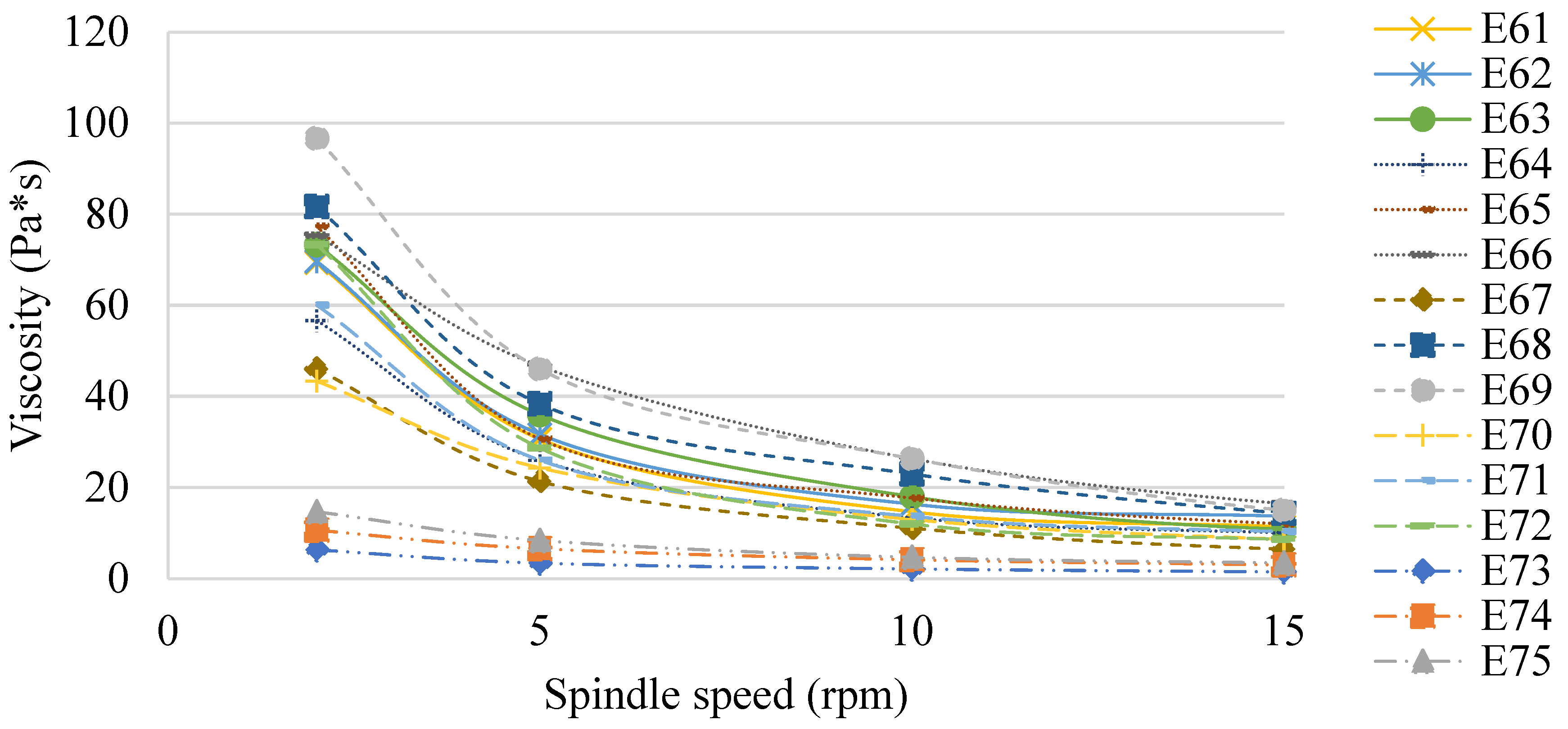
Figure 7 shows the viscosity of the E61–E75 systems determined 48 h after their preparation. Similar to Van Aken et al. (2011) and Maia Filho (2012) [19,20], this study also confirmed that the viscosity of the emulsion products analyzed was dependent on the type of fat phase used, although no correlation was observed between the values obtained and the ratio of fats in these phases. However, the effect of XGMCC concentration on the viscosity of the analyzed systems was observed. The viscosity values increased with increasing viscosity modifier concentration (E61 < E62 < E63, E64 < E65 < E66, etc.). This relationship was observed for spindle speeds of 2, 5, and 10 rpm, while at 15 rpm, no such relationship was observed. For a spindle speed of 2 rpm, the highest viscosity values were recorded for E68 and E69 (82 and 97 Pa×, respectively). In turn, the lowest viscosity values were recorded for systems E73–E75 (in the range of 6–15 Pa×s for 2 rpm). E61–E75 systems stabilized with a mixture of xanthan gum and microcrystalline cellulose and showed characteristics of pseudoplastic liquids [21].
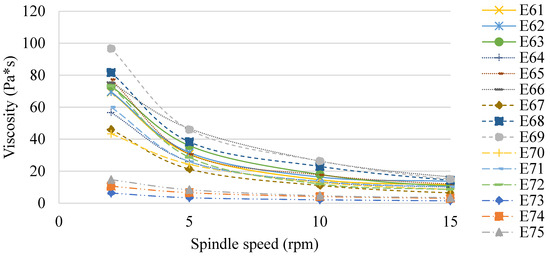
Figure 7.
The viscosity of E61–E75 determined 48 h after their preparation.
3. Material and Methods
3.1. Research Material
Cold-pressed hemp seed oil (Oleofarm, Wrocław, Poland) and lamb tallow (Meat-Farm Radosław Łuczak, Stefanowo, Poland) were used as fatty raw materials in this study.
The catalyst used in the enzymatic interesterification reactions was a lipase from Rhizomucor miehei, immobilized on immobead 150, ≥300 U g−1 (Sigma Aldrich, Saint Louis, MO, USA).
Xanthan gum and microcrystalline cellulose, Vivapur CS 032 XV (XGCM) (producer: J. Rettenmaier & Söhne (Rosenberg, Germany), were used as stabilizers in the emulsion.
As a preservative in emulsions, the commercial preparation Euxyl K712 (Schülke & Mayr GmbH, Norderstedt, Germany) consisting of an aqueous solution of sodium benzoate and potassium sorbate was used.
3.2. Research Methodology
3.2.1. Procedure for the Enzymatic Reaction of the Fatty Mixtures of Mutton Tallow and Hemp Seed Oil
The blends used in the research part of the work were prepared by weighing 150 g of fats into a 250 mL Erlenmeyer flask. The fat blends used in this study contained the following mass ratios of mutton tallow and hemp seed oil: 3:1 (T1), 3:2 (T2), 3:3 (T3), 2:3 (T4), and 1:3 (T5).
The raw materials were thermostated at 60 °C for 15 min in a shaker (SWB 22N, Labo Play, Warsaw, Poland). After this time, an enzyme catalyst (immobilized lipase) was added to the mixtures at 5% by weight of the reaction substrates and 2.50% distilled water (taking into account the water content of the enzyme preparation of about 0.5% by weight). The addition of water in the amount of 2.5% was dictated by obtaining the assumed amount of mono- and diacylglycerols as emulsifiers by weight of the total fat. The indicated amount of emulsifiers was sufficient to form emulsion systems. The determination of the addition of water added to the catalytic reaction was described in detail in [22]. The reaction was carried out for 6 h at 200 rpm at 60 °C. Filtering out the enzyme terminated the process.
3.2.2. Preparation of Emulsion
- The aqueous phase of the emulsion was a mixture of water and XGCM. This phase was prepared by adding the viscosity modifier in small portions to a beaker of water placed on an activated magnetic stirrer. The mixing process lasted 30 min, and then the total was homogenized for 1 min and left for 24 h.
- The next step was to heat both phases (water and fat) to 50–55 °C.
- Both phases were mixed and homogenized using an Ultra-Turrax T18 rotor-stator homogenizer equipped with an S18G–19G dispersing tool (IKA, Guangzhou, China).
- A preservative was added to the finished emulsions.
Each emulsion was prepared as a 100 g sample. Details of the quantities of emulsion ingredients are given in Table 1 and Table 2.

Table 1.
Parameters and components for emulsion preparation.

Table 2.
Definition and composition of tested emulsions.
3.3. Methods to Evaluate the Quality of Emulsion Systems
3.3.1. Determination of Emulsion Stability Using the Turbiscan Test
A Turbiscan Lab Expert (Formulaction, L’Union, France) was used to access the destabilization processes occurring in the formed emulsions. The light source of the device was a radiation-emitting diode (λ = 880 nm). The device has two synchronized detectors: one receiving transmitted light (T) and the other receiving backscattered light (BS) through the sample. Measurements were made by scanning with a moving optical head over the entire height of the vessel in which the emulsion was present (about 40 mm). Measurements were made every few days, although the entire test took days. The temperature at which the measurements were conducted averaged 23 °C. The parameters summarized in Table 3 were used to evaluate the stability of the emulsion systems.

Table 3.
Parameters used in this study to determine the stability of emulsions.
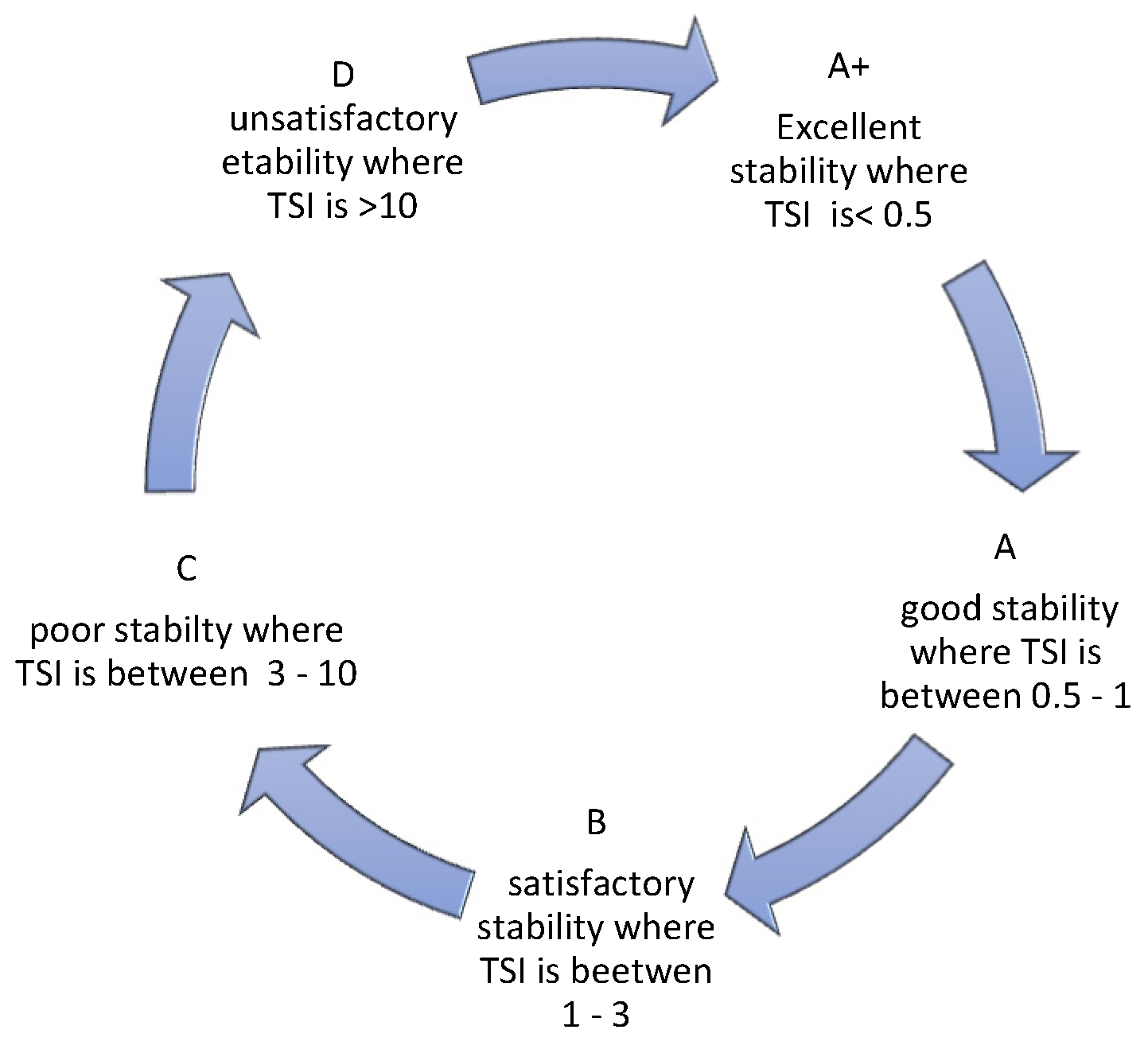
TSI allows for assessing the kinetics of destabilization changes occurring in a sample. As reported by Nastaj et al., 2020 [25], it takes values from 0 to 100, where 0 is taken by systems with no signs of destabilization and 100 characterizes systems in which destabilization is far advanced. The manufacturer of the device Turbiscan, in an application note (2019) [26], indicates that dispersions can be divided into 5 categories, depending on the TSI value obtained. Also, the category description can be found in reference [27] (Figure 8).
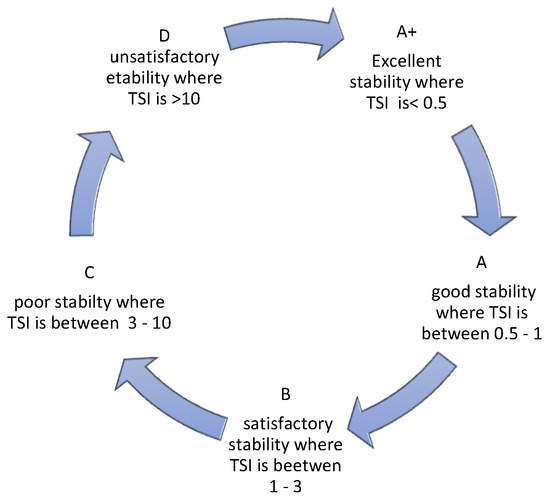
Figure 8.
Characteristics of TSI.
3.3.2. Microstructure Evaluation of Emulsion Systems
The microstructure of all emulsions was observed 24 h after manufacturing and 30 days of storage. This study was carried out using a Genetic Pro Trino optical microscope (Delta Optical, Warsaw, Poland) and DLT Cam Pro cameras (Delta Optical, Warsaw, Poland). The magnification used for the pictures was Gx400.
3.3.3. Emulsion Texture Determination
The texture of the emulsion was determined using a CT3 texture analyzer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA). A 25.4 mm diameter nylon sphere-shaped probe was used. Emulsions were tested in cylindrical dishes measuring 70 × 50 mm. Each system was penetrated once to a depth of 10 mm at a probe speed (in both directions) of 2 mm/s. Tests were carried out at 20 °C 48 h after emulsion formation (in triplicate). The emulsion texture parameters (hardness and adhesion force) were defined as the maximum and minimum peak force (g) for the compression cycle, respectively.
3.3.4. Dynamic Viscosity Determination
The dynamic viscosity of the emulsion was measured using a Brookfield DV-III Ultra, model HA viscometer (Brookfield Engineering laboratories, Middleboro, MA, USA), incorporating the Helipath system. Tests were conducted at 20 °C using a T-C spindle (No. 93) and speeds of 2, 5, 10, and 15 rpm.
3.3.5. Statistical Analysis
The statistical results were processed according to the rules given by Statistica 13 software (Statsoft, Krakow, Poland). A one-way analysis of variance (ANOVA) was used to evaluate the experimental results. The Tukey test was used to indicate the significance of differences between means (p ≤ 0.05).
4. Conclusions
Based on the results obtained for XGMCC-stabilized emulsion systems containing enzymatically modified fats, it was found that some of the systems had satisfactory stability. No correlation was observed between the applied concentration of the texture modifier and emulsion stability. However, the type of fatty phase used influenced the stability of the analyzed systems. Taking the above relationship into account, emulsion E67, which was characterized by a small degree of destabilization changes, was evaluated as the best system. This emulsion was characterized by the lowest droplet diameter of the dispersed phase at all measuring points during the storage process.
The rationale for the research was to create a new model emulsion system that can be used as a basis for further modifications to obtain a target food or cosmetic product. Physicochemical studies have confirmed that the proposed systems with new fats produced during the reaction catalyzed by immobilized lipase can also be an important factor in the development of pharmaceutical products, where the fat base is often the carrier of active substances. In addition, studies show a trend towards the use of animal fat in combination with the nutritionally beneficial hemp oil, thus creating a fat base that does not exist in nature. The biocatalysis reaction used in this study to produce this type of fat is in line with current trends and sustainability, not only because it increases the nutritional value of the fat obtained but also because it is one of the waste-free methods.
Author Contributions
Conceptualization, M.K and M.W.; methodology, M.W. and M.K.; validation, A.Z.; formal analysis, A.M.; investigation, M.W. and J.O.; data curation, M.W.; writing—original draft preparation, M.W. and M.K.; writing—review and editing, A.Z.; visualization, A.M.; supervision, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study is available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dijkstra, A.J. Interesterification, chemical or enzymatic catalysis. Lipid Technol. 2015, 27, 134–136. [Google Scholar]
- Sheldon, R.A.; John, M.W. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah SZ, H.; Bharagava, R.N.; Iqbal, H.M. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransfor. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Rozendaal, A.; Macrae, A.R. Interesterification of oils and fats. In Lipid Technologies and Applications; Routledge: New York, NY, USA, 2018; pp. 223–263. [Google Scholar]
- Singh, P.K.; Chopra, R.; Garg, M.; Dhiman, A.; Dhyani, A. Enzymatic interesterification of vegetable oil: A review on physicochemical and functional properties, and its health effects. J. Oleo Sci. 2022, 71, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Zbikowska, K.; Feszterová, M. Trends in fat modifications enabling alternative partially hydrogenated fat products proposed for advanced application. Gels 2023, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Kapila, S.; Ganesan, N.G.; Rangarajan, V. A review on green nanoemulsions for cosmetic applications with special emphasis on microbial surfactants as impending emulsifying agents. J. Surfactants Deterg. 2022, 25, 303–319. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Angardi, V.; Ettehadi, A.; Yücel, Ö. Critical review of emulsion stability and characterization techniques in oil processing. J. Energy Resour. Technol. 2022, 144, 040801. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Furtado, I.F.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Krawczyk, G.; Venables, A.; Tuason, D. Microcrystalline cellulose. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; CRC Press: Boca Raton, FL, USA, 2009; pp. 740–759. [Google Scholar]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef]
- Araújo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Bahmaei, M. Characterization of sodium caseinate/Hydroxypropyl methylcellulose concentrated emulsions: Effect of mixing ratio, concentration and wax addition. Int. J. Biol. Macromol. 2019, 128, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, G.A.; Vingerhoeds, M.H.; De Wijk, R.A. Textural perception of liquid emulsions: Role of oil content, oil viscosity and emulsion viscosity. Food Hydrocoll. 2011, 25, 789–796. [Google Scholar] [CrossRef]
- Maia Filho, D.C.; Ramalho, J.B.; Spinelli, L.S.; Lucas, E.F. Aging of water-in-crude oil emulsions: Effect on water content, droplet size distribution, dynamic viscosity and stability. Colloids Surf. A 2012, 396, 208–212. [Google Scholar] [CrossRef]
- Wei, W.; Pengyu, W.; Li, K.; Jimiao, D.; Kunyi, W.; Jing, G. Prediction of the apparent viscosity of non-Newtonian water-in-crude oil emulsions. Pet. Explor. Dev. 2013, 40, 130–133. [Google Scholar]
- Kowalska, M.; Woźniak, M.; Krzton-Maziopa, A.; Tavernier, S.; Pazdur, Ł.; Żbikowska, A. Development of the emulsions containing modified fats formed via enzymatic interesterification catalyzed by specific lipase with various amount of water. J. Dispers. Sci. Technol. 2019, 40, 192–205. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. Lwt-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Nastaj, M.; Terpiłowski, K.; Sołowiej, B.G. The effect of native and polymerised whey protein isolate addition on surface and microstructural properties of processed cheeses and their meltability determined by Turbiscan. Int. J. Food Sci. Technol. 2020, 55, 2179–2187. [Google Scholar] [CrossRef]
- Application Note Formulaction Turbiscan Stability Scale. The Stability Criteria and Correlation to Visual Observation; Touluse, France, 2019. Available online: https://www.google.com/search?q=Application+Note+Formulaction+Turbiscan+Stability+Scale.+The+stability+criteria+and+correlation+to+visual+observation.+2019.&sca_esv=09a4cde28335567a&ei=Avx5Z-XWCIWj1e8PnY3wEQ&ved=0ahUKEwjl396s0d2KAxWFUfUHHZ0GPAIQ4dUDCBA&oq=Application+Note+Formulaction+Turbiscan+Stability+Scale.+The+stability+criteria+and+correlation+to+visual+observation.+2019.&gs_lp=Egxnd3Mtd2l6LXNlcnAifEFwcGxpY2F0aW9uIE5vdGUgRm9ybXVsYWN0aW9uIFR1cmJpc2NhbiBTdGFiaWxpdHkgU2NhbGUuIFRoZSBzdGFiaWxpdHkgY3JpdGVyaWEgYW5kIGNvcnJlbGF0aW9uIHRvIHZpc3VhbCBvYnNlcnZhdGlvbi4gMjAxOS5IAFAAWABwAHgAkAEAmAEAoAEAqgEAuAEMyAEA-AEBmAIAoAIAmAMAkgcAoAcA&sclient=gws-wiz-serp (accessed on 28 December 2024).
- Domian, E.; Marzec, A.; Kowalska, H. Assessing the effectiveness of colloidal microcrystalline cellulose as a suspending agent for black and white liquid dyes. Int. J. Food Sci. Technol. 2020, 56, 2504–2515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).