Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future
Abstract
1. Introduction
| Reaction | Electrolyte | Half-Reaction (Equation) | Reaction Pathways | Ref. |
|---|---|---|---|---|
| ORR | Acidic Alkaline | O2 + 4H+ + 4e− → 2H2O O2 + 2H2O + 4e− → 4OH− | Direct 4-electron pathway or 2-electron pathway (H2O2 intermediate) Same as acidic, but OH− formation is dominant | [12] |
| OER | Acidic Alkaline | 2H2O → O2 + 4H+ + 4e− 4OH− → O2 + 2H2O + 4e− | Oxygen evolution via water oxidation Oxygen evolution via hydroxide oxidation | [13] |
| HER | Acidic Alkaline | 2H+ + 2e− → H2 2H2O + 2e− → H2 + 2OH− | Proton reduction Water reduction | [14] |
| CO2RR | Acidic Alkaline | CO2 + 2H+ + 2e− → CO + H2O CO2 + H2O + 2e− → CO + 2OH− | Multiple pathways: CO, CH4, HCOOH, etc., based on the catalyst Similar to hydroxide products | [15] |
| NRR | Acidic Alkaline | N2 + 6H+ + 6e− → 2NH3 N2 + 6H2O + 6e− → 2NH3 + 6OH− | Ammonia production via stepwise hydrogenation A similar pathway with water-splitting | [16] |
| NO3RR | Acidic Alkaline | NO3− + 10H+ + 8e− → NH4+ + 3H2O NO3− + 6H2O + 8e− → NH4++ 10OH− | Stepwise reduction to NH4+ or N2 Similar reduction with hydroxide products | [17] |
2. Scope of This Review
3. Synthesis of SACs
3.1. Bottom-Up Strategies
3.2. Top-Down Strategies
4. Substrate’s Engineering of SACs
4.1. Carbonaceous Substrate
4.1.1. Morphology Engineering
4.1.2. Vacancy Engineering
4.1.3. Heteroatom Doping
4.2. Metal-Oxide-Based Substrate
4.2.1. Morphology Engineering
4.2.2. Vacancy Engineering
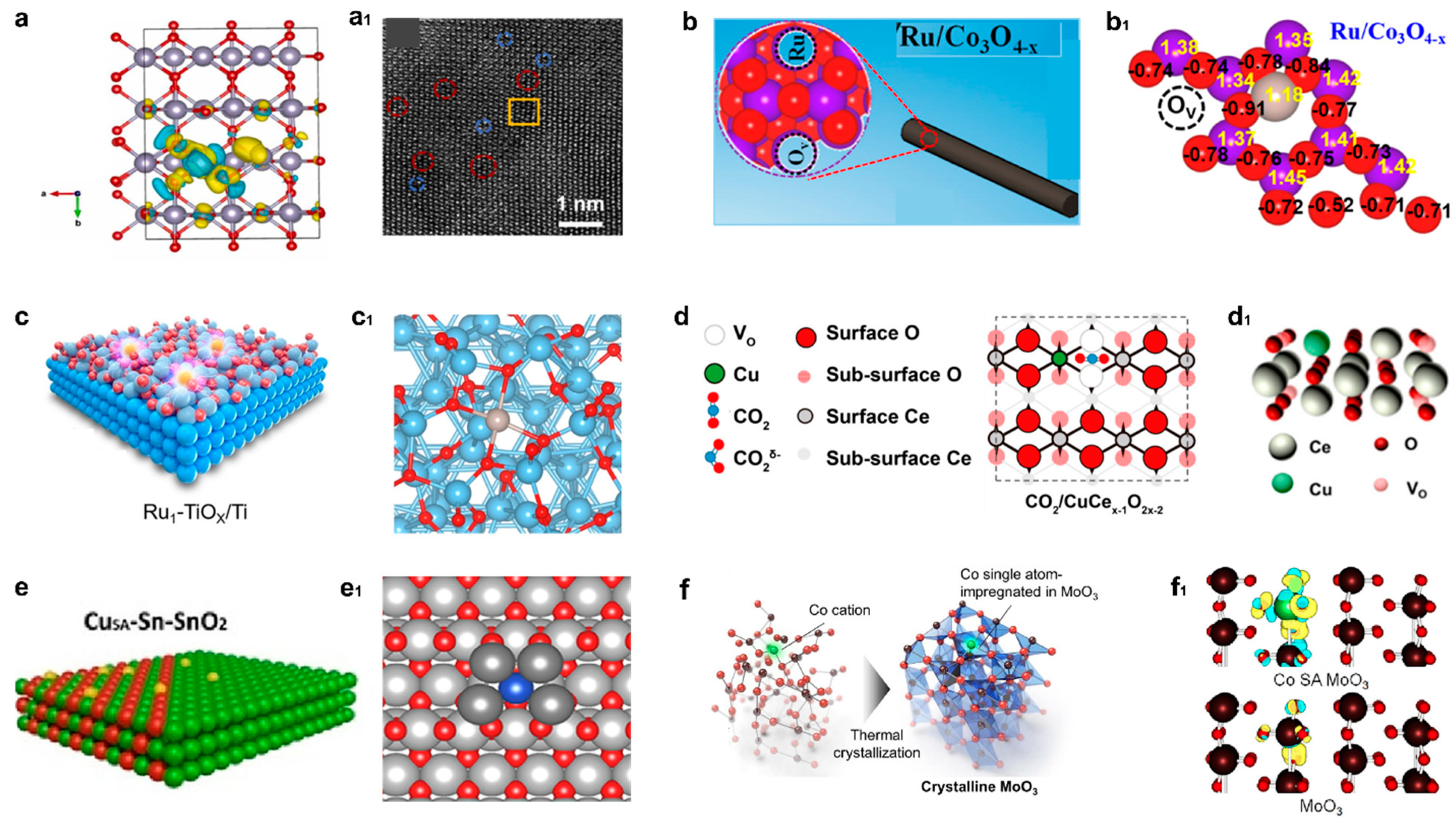
4.2.3. Heteroatom Doping
4.2.4. Facet Engineering
4.2.5. Crystallinity Control
4.3. Alloy-Based Substrate
4.3.1. Morphology Engineering
4.3.2. Vacancy Engineering
4.3.3. Heteroatom Doping
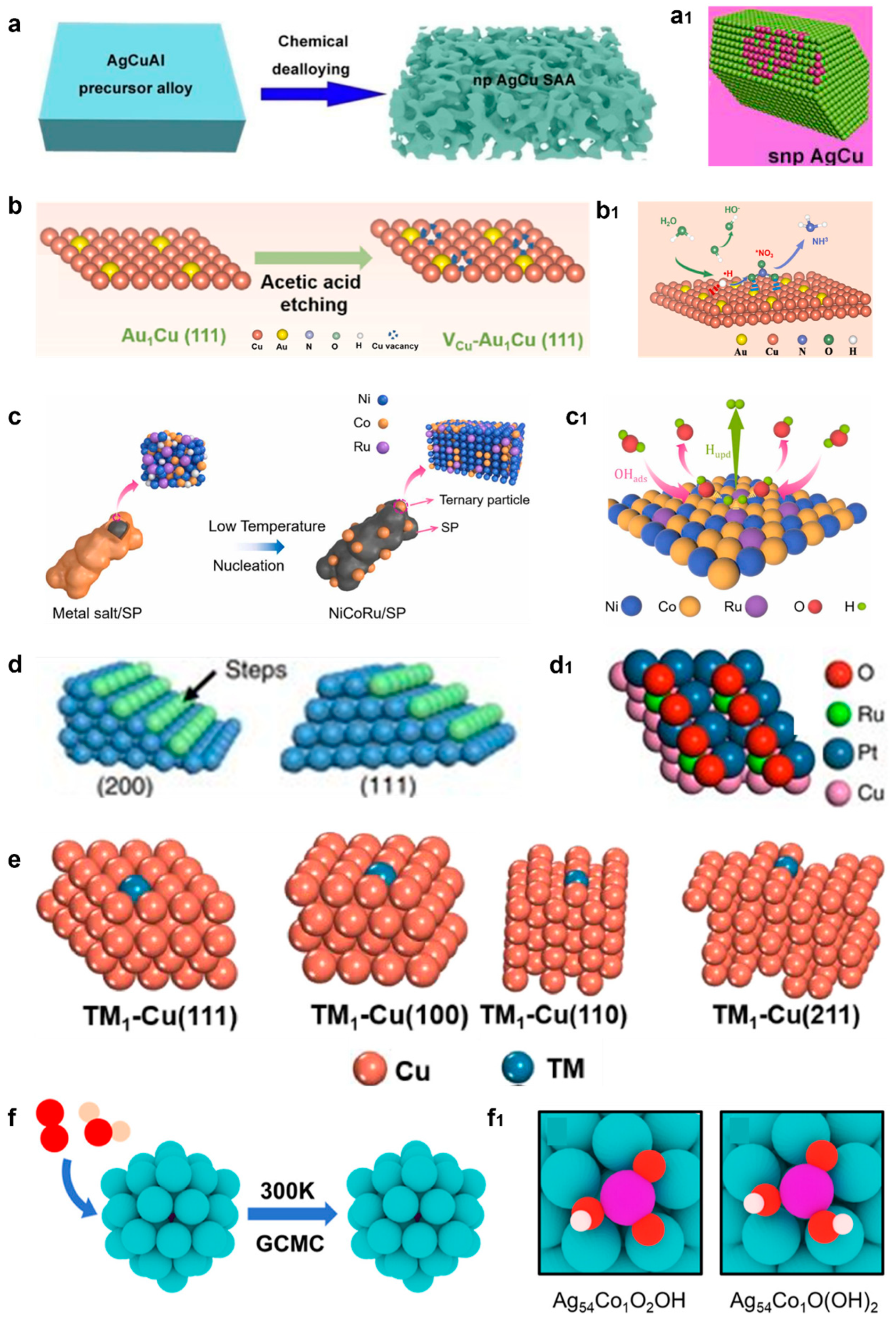
4.3.4. Facet Engineering
4.3.5. Crystallinity Control
4.4. TMD-Based Substrate
4.4.1. Morphology Engineering
4.4.2. Vacancy Engineering
4.4.3. Heteroatom Doping
4.4.4. Facet Engineering
4.4.5. Crystallinity Control

4.5. MXene-Based Substrate
4.5.1. Morphology Engineering
4.5.2. Vacancy Engineering
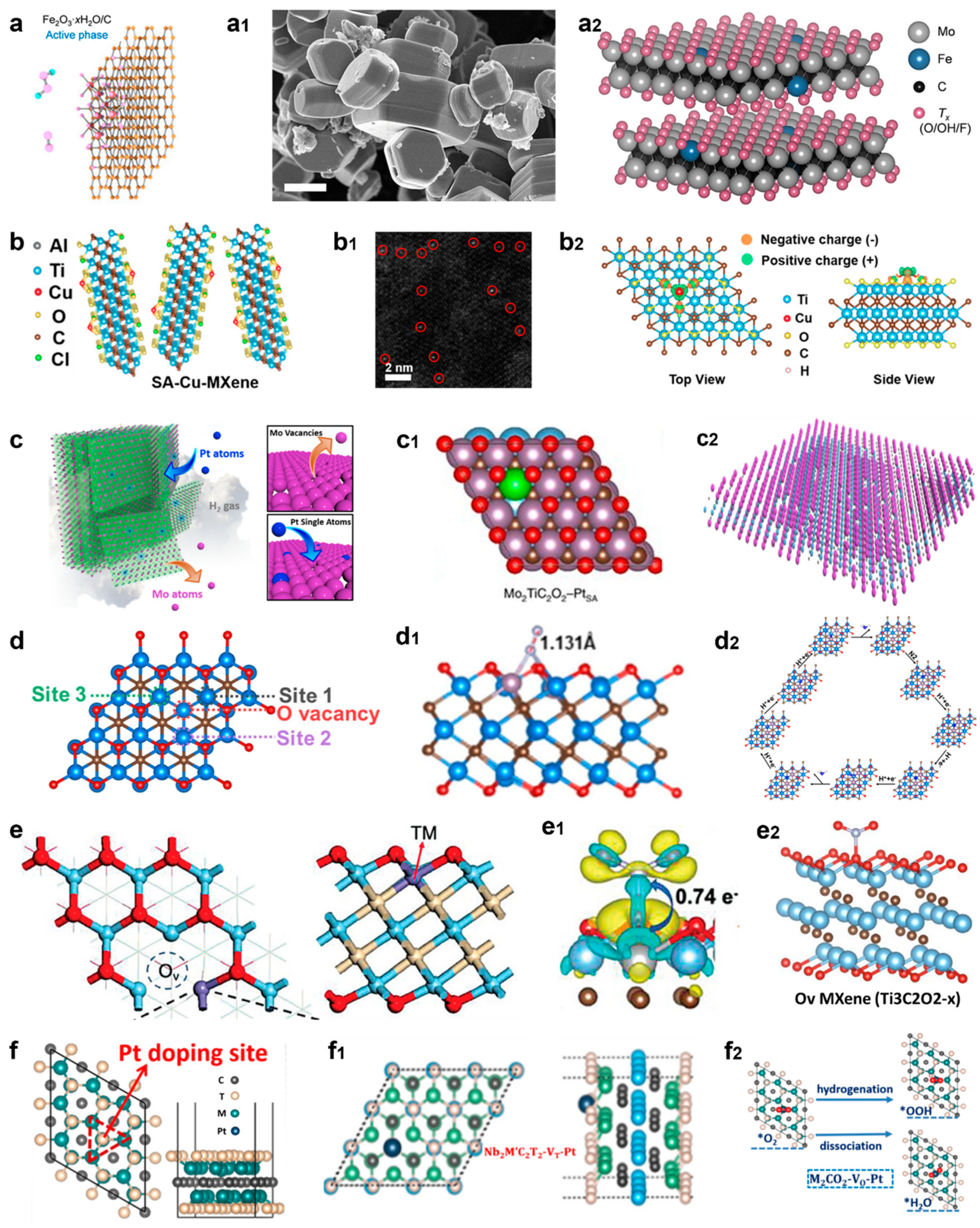
4.5.3. Heteroatom Doping
4.6. MOF-Based Substrate
4.6.1. Morphology Engineering
4.6.2. Vacancy Engineering

4.6.3. Heteroatom Doping
4.6.4. Crystallinity Control
5. Conclusions
6. Challenges
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chen, C.; Xu, L.; Zhang, Y.; Wei, W.; Zhao, E.; Wu, Y.; Chen, C. Challenges and Perspectives of Single-Atom-Based Catalysts for Electrochemical Reactions. JACS Au 2023, 3, 736–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, H.; He, Y.; Li, L.; Zhu, S.; Shen, H.; Xie, P.; Fu, X.; Zhou, G.; Feng, C.; et al. Advanced Electrocatalysts with Single-Metal-Atom Active Sites. Chem. Rev. 2020, 120, 12217–12314. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Y.; Wang, Q.; He, G.; Chen, H. Supports promote single-atom catalysts toward advanced electrocatalysis. Coord. Chem. Rev. 2022, 451, 214261. [Google Scholar] [CrossRef]
- Matthews, T.; Mashola, T.A.; Adegoke, K.A.; Mugadza, K.; Fakude, C.T.; Adegoke, O.R.; Adekunle, A.S.; Ndungu, P.; Maxakato, N.W. Electrocatalytic activity on single atoms catalysts: Synthesis strategies, characterization, classification, and energy conversion applications. Coord. Chem. Rev. 2022, 467, 214600. [Google Scholar] [CrossRef]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Ling, C.; Niu, X.; Li, Q.; Du, A.; Wang, J. Metal-Free Single Atom Catalyst for N2 Fixation Driven by Visible Light. J. Am. Chem. Soc. 2018, 140, 14161–14168. [Google Scholar] [CrossRef]
- Jiang, K.; Luo, M.; Peng, M.; Yu, Y.; Lu, Y.-R.; Chan, T.-S.; Liu, P.; de Groot, F.M.F.; Tan, Y. Dynamic active-site generation of atomic iridium stabilized on nanoporous metal phosphides for water oxidation. Nat. Commun. 2020, 11, 2701. [Google Scholar] [CrossRef]
- Liang, L.; Jin, H.; Zhou, H.; Liu, B.; Hu, C.; Chen, D.; Wang, Z.; Hu, Z.; Zhao, Y.; Li, H.-W.; et al. Cobalt single atom site isolated Pt nanoparticles for efficient ORR and HER in acid media. Nano Energy 2021, 88, 106221. [Google Scholar] [CrossRef]
- Liu, D.; Barbar, A.; Najam, T.; Javed, M.S.; Shen, J.; Tsiakaras, P.; Cai, X. Single noble metal atoms doped 2D materials for catalysis. Appl. Catal. B Environ. 2021, 297, 120389. [Google Scholar] [CrossRef]
- Roth-Zawadzki, A.M.; Nielsen, A.J.; Tankard, R.E.; Kibsgaard, J. Dual and Triple Atom Electrocatalysts for Energy Conversion (CO2RR, NRR, ORR, OER, and HER): Synthesis, Characterization, and Activity Evaluation. ACS Catal. 2024, 14, 1121–1145. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Huang, W.; Huang, T.; Wu, B. Coordination environment manipulation of single atom catalysts: Regulation strategies, characterization techniques and applications. Coord. Chem. Rev. 2024, 515, 215952. [Google Scholar] [CrossRef]
- Monini, V.; Bonechi, M.; Bazzicalupi, C.; Bianchi, A.; Gentilesca, P.; Giurlani, W.; Innocenti, M.; Meoli, A.; Romano, G.M.; Savastano, M. Oxygen reduction reaction (ORR) in alkaline solution catalysed by an atomically precise catalyst based on a Pd(ii) complex supported on multi-walled carbon nanotubes (MWCNTs). Electrochemical and structural considerations. Dalton Trans. 2024, 53, 2487–2500. [Google Scholar] [CrossRef]
- Hao, Y.; Cao, X.; Lei, C.; Chen, Z.; Yang, X.; Gong, M. Chemical oxygen species on electrocatalytic materials during oxygen evolution reaction. Mater. Today Catal. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Wu, J.; Liu, Y.; Geng, D.; Meng, X. Understanding the high-electrocatalytic performance of two-dimensional MoS2 nanosheets and their composite materials. J. Chem. A 2017, 5, 24540–24563. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Wen, J.; Zuo, L.; Sun, H.; Wu, X.; Huang, T.; Liu, Z.; Wang, J.; Liu, L.; Wu, Y.; Liu, X.; et al. Nanomaterials for the electrochemical nitrogen reduction reaction under ambient conditions. Nanoscale Adv. 2021, 3, 5525–5541. [Google Scholar] [CrossRef]
- Zhang, R.; Li, C.; Cui, H.; Wang, Y.; Zhang, S.; Li, P.; Hou, Y.; Guo, Y.; Liang, G.; Huang, Z.; et al. Electrochemical nitrate reduction in acid enables high-efficiency ammonia synthesis and high-voltage pollutes-based fuel cells. Nat. Commun. 2023, 14, 8036. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, C.; Wang, D.; Zhou, S.; Wang, R.; Hu, S.; Li, H.; Zuo, M.; Kong, Y.; Bao, J.; et al. Selectively anchoring single atoms on specific sites of supports for improved oxygen evolution. Nat. Commun. 2022, 13, 2473. [Google Scholar] [CrossRef]
- Ajmal, S.; Kumar, A.; Mushtaq, M.A.; Tabish, M.; Zhao, Y.; Zhang, W.; Khan, A.S.; Saad, A.; Yasin, G.; Zhao, W. Uniting Synergistic Effect of Single-Ni Site and Electric Field of B- Bridged-N for Boosted Electrocatalytic Nitrate Reduction to Ammonia. Small 2024, 20, 2310082. [Google Scholar] [CrossRef]
- Wang, C.; Wang, K.; Feng, Y.; Li, C.; Zhou, X.; Gan, L.; Feng, Y.; Zhou, H.; Zhang, B.; Qu, X.; et al. Co and Pt Dual-Single-Atoms with Oxygen-Coordinated Co–O–Pt Dimer Sites for Ultrahigh Photocatalytic Hydrogen Evolution Efficiency. Adv. Mater. 2021, 33, 2003327. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, G.; Feng, H.; Chen, L.; Wang, H.; Wang, B.; Zhang, X.; Zheng, L.; Hong, S.; Wei, M. Platinum–copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 2019, 10, 5812. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Qi, M.-Y.; Li, Y.-H.; Tang, Z.-R.; Wang, T.; Gong, J.; Xu, Y.-J. Activating two-dimensional Ti3C2Tx-MXene with single-atom cobalt for efficient CO2 photoreduction. Cell Rep. Phys. Sci. 2021, 2, 100371. [Google Scholar] [CrossRef]
- Jiang, K.; Luo, M.; Liu, Z.; Peng, M.; Chen, D.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.F.; Tan, Y. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat. Commun. 2021, 12, 1687. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Ro, I.; Qi, J.; Lee, S.; Xu, M.; Yan, X.; Xie, Z.; Zakem, G.; Morales, A.; Chen, J.G.; Pan, X.; et al. Bifunctional hydroformylation on heterogeneous Rh-WOx pair site catalysts. Nature 2022, 609, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Zhang, S.; Huang, H.; Liu, X.; Li, B.; Lee, Y.; Wang, X.; Bai, Y.; Sun, M.; Wu, Y.; et al. General Synthesis of a Diatomic Catalyst Library via a Macrocyclic Precursor-Mediated Approach. J. Am. Chem. Soc. 2023, 145, 4819–4827. [Google Scholar] [CrossRef]
- Shan, J.; Ye, C.; Chen, S.; Sun, T.; Jiao, Y.; Liu, L.; Zhu, C.; Song, L.; Han, Y.; Jaroniec, M.; et al. Short-Range Ordered Iridium Single Atoms Integrated into Cobalt Oxide Spinel Structure for Highly Efficient Electrocatalytic Water Oxidation. J. Am. Chem. Soc. 2021, 143, 5201–5211. [Google Scholar] [CrossRef]
- Zhang, L.; Si, R.; Liu, H.; Chen, N.; Wang, Q.; Adair, K.; Wang, Z.; Chen, J.; Song, Z.; Li, J.; et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 2019, 10, 4936. [Google Scholar] [CrossRef]
- DeRita, L.; Dai, S.; Lopez-Zepeda, K.; Pham, N.; Graham, G.W.; Pan, X.; Christopher, P. Catalyst Architecture for Stable Single Atom Dispersion Enables Site-Specific Spectroscopic and Reactivity Measurements of CO Adsorbed to Pt Atoms, Oxidized Pt Clusters, and Metallic Pt Clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Liu, H.; Liu, X.; Luo, J. Potential-Cycling Synthesis of Single Platinum Atoms for Efficient Hydrogen Evolution in Neutral Media. Angew. Chem. Int. Ed. 2017, 56, 13694–13698. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-Coordinated Dual-Metal Sites: A Stable and Active Pt-Free Catalyst for Acidic Oxygen Reduction Reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Xu, J.; Sun, H.; Li, Z.; Liu, W.; Yuan, T.; Liu, W.; Wang, X.; Cheong, W.-C.; et al. Recover the activity of sintered supported catalysts by nitrogen-doped carbon atomization. Nat. Commun. 2020, 11, 335. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Chen, H.; Cheng, Y.; Sun, Q.; Zhang, H.; He, Q.; Zhang, Y.; Guo, G.; He, X.; et al. Synthesis of Nitrogen-doped Carbon Supported Cerium Single Atom Catalyst by Ball Milling for Selective Oxidation of Ethylbenzene. Chem. Res. Chin. Univ. 2022, 38, 1258–1262. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Yang, X.; Shi, Q.; Qiao, Z.; Lucero, M.; Ma, Q.; More, K.L.; Cullen, D.A.; Feng, Z.; et al. Chemical Vapor Deposition for Atomically Dispersed and Nitrogen Coordinated Single Metal Site Catalysts. Angew. Chem. Int. Ed. 2020, 59, 21698–21705. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Rykov, A.I.; Im, Y.-K.; Yu, S.-Y.; Jeon, J.-P.; Kim, S.-J.; Zhou, W.; Ge, R.; Ao, Z.; et al. Abrading bulk metal into single atoms. Nat. Nanotechnol. 2022, 17, 403–407. [Google Scholar] [CrossRef]
- Hai, X.; Zhao, X.; Guo, N.; Yao, C.; Chen, C.; Liu, W.; Du, Y.; Yan, H.; Li, J.; Chen, Z.; et al. Engineering Local and Global Structures of Single Co Atoms for a Superior Oxygen Reduction Reaction. ACS Catal. 2020, 10, 5862–5870. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, X.; Zhang, Y.; Li, J.; Xu, L.; Li, S.; Fu, H.; An, X. Constructing asymmetric-electron-density Pd-Zn dual-atoms on N-doped defective mesoporous carbon for electrochemical reduction of CO2. Chem. Eng. J. 2024, 500, 156603. [Google Scholar] [CrossRef]
- Yu, P.-W.; Elmas, S.; Roman, T.; Pan, X.; Yin, Y.; Gibson, C.T.; Andersson, G.G.; Andersson, M.R. Highly active platinum single-atom catalyst grafted onto 3D carbon cloth support for the electrocatalytic hydrogen evolution reaction. Appl. Surf. Sci. 2022, 595, 153480. [Google Scholar] [CrossRef]
- Han, J.; Bao, H.; Wang, J.-Q.; Zheng, L.; Sun, S.; Wang, Z.L.; Sun, C. 3D N-doped ordered mesoporous carbon supported single-atom Fe-N-C catalysts with superior performance for oxygen reduction reaction and zinc-air battery. Appl. Catal. B Environ. 2021, 280, 119411. [Google Scholar] [CrossRef]
- Lü, F.; Zhao, S.; Guo, R.; He, J.; Peng, X.; Bao, H.; Fu, J.; Han, L.; Qi, G.; Luo, J.; et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy 2019, 61, 420–427. [Google Scholar] [CrossRef]
- Li, T.; Ren, S.; Zhang, C.; Qiao, L.; Wu, J.; He, P.; Lin, J.; Liu, Y.; Fu, Z.; Zhu, Q.; et al. Cobalt single atom anchored on N-doped carbon nanoboxes as typical single-atom catalysts (SACs) for boosting the overall water splitting. Chem. Eng. J. 2023, 458, 141435. [Google Scholar] [CrossRef]
- Lv, C.; Li, B.; Ren, Y.; Zhang, G.; Lu, Z.; Li, L.; Zhang, X.; Yang, X.; Yu, X. A “MOF-plus-MOF” strategy to synthesize Co-N3C1 single-atom catalyst for rechargeable Zn-air battery. Chem. Eng. J. 2024, 495, 153670. [Google Scholar] [CrossRef]
- Musa, S.; Pirzada, B.M.; Talib, S.H.; Anjum, D.H.; Haija, M.A.; Mohamed, S.; Qurashi, A. Growth of copper-nickel (Cu-Ni) dual atom catalysts over graphene variants as active anodes for clean oxygen generation: Integrative experimental and computational validation. Nano Energy 2024, 125, 109479. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Tian, S.; Yang, J.; Li, J.; Guan, Q.; Yin, F.; Xiang, X.; Wang, Y.; Meng, X.; et al. Structurally optimized rosette-like microspheres carbon with Fe-Ni single atom sites for bifunctional oxygen electrocatalysis in Zinc-Air batteries. Chem. Eng. J. 2024, 497, 154963. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, F.; Huang, R.; Liu, X.; Zhang, Z.; Yao, T.; Zhang, Y.; Wu, Y. Symmetry Evolution Induced 2D Pt Single Atom Catalyst with High Density for Alkaline Hydrogen Oxidation. Adv. Mater. 2024, 36, 2404672. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, S.; Chen, Z.; Lu, C.; Han, S.; Ke, C.; Zhu, J.; Zhang, J.; Tranca, D.; Zhuang, X. Carbon nanosheets supporting Ni–N3S single-atom sites for efficient electrocatalytic CO2 reduction. Carbon 2021, 178, 488–496. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, J.; Zhang, L.; Wan, W.; Ding, Y.; Sun, X. Synergy of dual-atom catalysts deviated from the scaling relationship for oxygen evolution reaction. Nat. Commun. 2023, 14, 4449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, Y.; Ma, L.; Wang, Y.; Huang, L.; Wei, B.; Xue, Y.; Zhu, H.; Jiang, R. Single transition metal atom anchored on g-C3N4 as an electrocatalyst for nitrogen fixation: A computational study. Int. J. Hydrogen Energy 2023, 48, 7621–7631. [Google Scholar] [CrossRef]
- Pang, Y.; Ding, Z.; Ma, A.; Fan, G.; Xu, H. Electroreduction of nitrate to ammonia on graphyne-based single-atom catalysts by combined density functional theory and machine learning study. Sep. Purif. Technol. 2025, 354, 129422. [Google Scholar] [CrossRef]
- Mehmood, R.; Long, G.; Fan, W.; Li, M.; Liu, L.; Zhang, F. One dimensional nickel phosphide polymorphic heterostructure as carbon-free functional support loading single-atom iridium for promoted electrocatalytic water oxidation. J. Energy Chem. 2023, 79, 410–417. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, M.; He, D.; Yin, H.; Huang, Y.; Cao, L.; Fang, Y.; Chu, D.; Liu, Y.; Chen, H.; et al. Co-Zn single atoms anchored carbon nanotubes derived from anti-perovskite carbides for boosted hydrogen evolution and oxygen reduction reactions. Chem. Eng. J. 2024, 496, 154255. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Chen, M.; Zhou, S.; Wu, L. An isolated bimetallic Fe–Ru single-atom catalyst for efficient electrochemical nitrogen reduction. J. Mater. Chem. A 2023, 11, 14900–14910. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, Z.; Liu, Y.; Liu, N.; Li, Y.; Cheng, Y.; Yu, J.; Yu, R.; Wang, D.; Li, H. Shear-Strained Pd Single-Atom Electrocatalysts for Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202411396. [Google Scholar]
- Chen, W.; Luo, X.; Ling, S.; Zhou, Y.; Shen, B.; Slater, T.J.A.; Fernandes, J.A.; Lin, T.; Wang, J.; Shen, Y. Hemoglobin-derived Fe-Nx-S species supported by bamboo-shaped carbon nanotubes as efficient electrocatalysts for the oxygen evolution reaction. Carbon 2020, 168, 588–596. [Google Scholar] [CrossRef]
- Narendra Kumar, A.V.; Muthu Prabhu, S.; Shin, W.S.; Yadav, K.K.; Ahn, Y.; Abdellattif, M.H.; Jeon, B.-H. Prospects of non-noble metal single atoms embedded in two-dimensional (2D) carbon and non-carbon-based structures in electrocatalytic applications. Coord. Chem. Rev. 2022, 467, 214613. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Song, D.; Wang, C.; Yuan, Y.; Liu, Y.; Dong, K. From one-dimensional to three-dimensional, the criss-crossed fiber materials forge high-performance lithium-sulfur batteries. Chem. Eng. J. 2024, 495, 153126. [Google Scholar]
- Tian, H.; Song, A.; Zhang, P.; Sun, K.; Wang, J.; Sun, B.; Fan, Q.; Shao, G.; Chen, C.; Liu, H.; et al. High Durability of Fe–N–C Single-Atom Catalysts with Carbon Vacancies toward the Oxygen Reduction Reaction in Alkaline Media. Adv. Mater. 2023, 35, 2210714. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhang, H.; Song, Y.; Liu, P.; Hou, Y.; Xu, B.; Liao, T.; Guo, J.; Sun, Z. Electronic Asymmetry Engineering of Fe–N–C Electrocatalyst via Adjacent Carbon Vacancy for Boosting Oxygen Reduction Reaction. Adv. Sci. 2023, 10, 2305194. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Hu, X.; Lee, S.; Fan, W.; Kim, G.; Zhang, J.; Zhou, Z.; Kang, Y.-M. Oxygen Reduction Kinetics of Fe–N–C Single Atom Catalysts Boosted by Pyridinic N Vacancy for Temperature-Adaptive Zn–Air Batteries. J. Am. Chem. Soc. 2024, 146, 4803–4813. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, B.; Xu, X.; Peng, L.; Liu, B.; He, J.; Zhang, J. Oxygen vacancy rich δ-MnO2 nanosheets encapsulating single cobalt atoms-anchored carbon nanotubes for efficient oxygen evolution. Mater. Today Energy 2024, 40, 101515. [Google Scholar] [CrossRef]
- Yi, M.; Lv, S.; Yang, Q.; Lei, S.; Wang, H.; Huang, J.; Zhang, J. Ionic liquid meets ZnIn2S4: Synergistically tuning coordination environment of ZnIn2S4 grown on porous carbon by N, F doping and S-vacancies to load high concentration of single-atom Sb for efficient flexible Zn-Air batteries. Appl. Catal. B Environ. Energy 2025, 361, 124697. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Gao, G.; Yan, X.; Chen, N.; Chen, J.; Soo, M.T.; Wood, B.; Yang, D.; Du, A.; et al. Graphene Defects Trap Atomic Ni Species for Hydrogen and Oxygen Evolution Reactions. Chem 2018, 4, 285–297. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Yuan, P.; Jia, Y.; Zhuang, L.; Zhang, H.; Yan, X.; Liu, G.; Zhao, Y.; Liu, J.; et al. Single Carbon Vacancy Traps Atomic Platinum for Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2022, 144, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.; Choi, H.; Yadav, S.; Jiménez, J.D.; Kim, C.; Van Nguyen, T.; Chen, K.; Uthirakumar, P.; Van Le, Q.; Senanayake, S.D.; et al. LaCeOx coupled N-doped graphene/Ru single-atoms as a binary-site catalyst for efficient hydrogen evolution based on hydrogen spillover. Appl. Catal. B Environ. 2024, 343, 123452. [Google Scholar] [CrossRef]
- An, B.; Zhou, J.; Zhu, Z.; Li, Y.; Wang, L.; Zhang, J. Uncovering the coordination effect on the Ni single-atom catalysts for CO2 reduction including vacancy defect and non-vacancy defect structures. Fuel 2022, 310, 122472. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.-J.; Lu, X.-L.; Si, R.; Lu, T.-B. Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 1961–1965. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Pei, J.; Li, H.; Ji, S.; Shi, L.; Zhang, Y.; Li, C.; Tang, C.; Liao, J.; et al. Continuous electroproduction of formate via CO2 reduction on local symmetry-broken single-atom catalysts. Nat. Commun. 2023, 14, 6849. [Google Scholar] [CrossRef]
- Choi, C.; Back, S.; Kim, N.-Y.; Lim, J.; Kim, Y.-H.; Jung, Y. Suppression of Hydrogen Evolution Reaction in Electrochemical N2 Reduction Using Single-Atom Catalysts: A Computational Guideline. ACS Catal. 2018, 8, 7517–7525. [Google Scholar] [CrossRef]
- Guo, X.; Gu, J.; Lin, S.; Zhang, S.; Chen, Z.; Huang, S. Tackling the Activity and Selectivity Challenges of Electrocatalysts toward the Nitrogen Reduction Reaction via Atomically Dispersed Biatom Catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. [Google Scholar] [CrossRef]
- Lee, C.H.; Pahari, S.; Barteau, M.A.; Kwon, J.S.-I. Exploring dynamics in single atom catalyst research: A comprehensive DFT-kMC study of nitrogen reduction reaction with focus on TM aggregation. Appl. Catal. B Environ. Energy 2024, 358, 124434. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lim, D.-H. DFT investigation into efficient transition metal single-atom catalysts supported on N-doped graphene for nitrate reduction reactions. Chem. Eng. J. 2023, 468, 143466. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Liu, H.; Liu, S.; Yuan, Y.; Meng, Y.; Wang, M.; Shen, C.; Peng, Q.; Chen, J.; et al. Breaking Local Charge Symmetry of Iron Single Atoms for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2023, 62, e202308044. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Bu, Z.; Yang, F.; Luo, J.; An, Q.; Zeng, Z.; Wang, J.; Deng, S. Boosting CO2-to-CO conversion on a robust single-atom copper decorated carbon catalyst by enhancing intermediate binding strength. J. Mater. Chem. A 2021, 9, 1705–1712. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, S.; Xue, Y.; Hu, X.; Cui, H.; Zhou, Z. Nickel single-atom catalysts intrinsically promoted by fast pyrolysis for selective electroreduction of CO2 into CO. Appl. Catal. B Environ. 2022, 304, 120997. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Zachman, M.J.; Zeng, Y.; Yu, H.; Li, B.; Wang, M.; Braaten, J.; Liu, J.; Meyer, H.M.; et al. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 2022, 7, 652–663. [Google Scholar] [CrossRef]
- Rebarchik, M.; Bhandari, S.; Kropp, T.; Mavrikakis, M. Insights into the Oxygen Evolution Reaction on Graphene-Based Single-Atom Catalysts from First-Principles-Informed Microkinetic Modeling. ACS Catal. 2023, 13, 5225–5235. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, J.; Zhang, J.; He, W.; Li, Y.; Liang, L.; Liu, C.; Liu, H.; Hao, Q. Engineering Co and Ru dual-metal atoms on nitrogen-doped carbon as highly efficient bifunctional oxygen electrocatalysts. Catal. Sci. Technol. 2022, 12, 5435–5441. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Flahaut, E.; Peljo, P.; Sainio, J.; Davodi, F.; Lobiak, E.V.; Mustonen, K.; Kauppinen, E.I. Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions. ACS Catal. 2020, 10, 4647–4658. [Google Scholar] [CrossRef]
- Li, T.; Lu, T.; Li, X.; Xu, L.; Zhang, Y.; Tian, Z.; Yang, J.; Pang, H.; Tang, Y.; Xue, J. Atomically Dispersed Mo Sites Anchored on Multichannel Carbon Nanofibers toward Superior Electrocatalytic Hydrogen Evolution. ACS Nano 2021, 15, 20032–20041. [Google Scholar] [CrossRef]
- Kuang, P.; Wang, Y.; Zhu, B.; Xia, F.; Tung, C.-W.; Wu, J.; Chen, H.M.; Yu, J. Pt Single Atoms Supported on N-Doped Mesoporous Hollow Carbon Spheres with Enhanced Electrocatalytic H2-Evolution Activity. Adv. Mater. 2021, 33, 2008599. [Google Scholar] [CrossRef]
- Bala Musa, A.; Tabish, M.; Kumar, A.; Selvaraj, M.; Abubaker Khan, M.; Al-Shehri, B.M.; Arif, M.; Asim Mushtaq, M.; Ibraheem, S.; Slimani, Y.; et al. Microenvironment engineering of Fe-single-atomic-site with nitrogen coordination anchored on carbon nanotubes for boosting oxygen electrocatalysis in alkaline and acidic media. Chem. Eng. J. 2023, 451, 138684. [Google Scholar] [CrossRef]
- Zheng, T.; Han, X.; Wang, J.; Xia, Z. Role of heteroatom-doping in enhancing catalytic activities and the stability of single-atom catalysts for oxygen reduction and oxygen evolution reactions. Nanoscale 2022, 14, 16286–16294. [Google Scholar] [CrossRef]
- Yasin, G.; Ali, S.; Ibraheem, S.; Kumar, A.; Tabish, M.; Mushtaq, M.A.; Ajmal, S.; Arif, M.; Khan, M.A.; Saad, A.; et al. Simultaneously Engineering the Synergistic-Effects and Coordination-Environment of Dual-Single-Atomic Iron/Cobalt-sites as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. ACS Catal. 2023, 13, 2313–2325. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, C.; Xia, J.; Li, L.; Qian, X.; Yin, F.-X.; He, G.; Chen, Q.; Chen, H. FeN4S1 Single-Atom Sites Anchored on Three-Dimensional Porous Carbon for Highly Efficient and Durable Oxygen Electrocatalysis. ACS Nano 2024, 18, 32995–33004. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, Z.; Qin, G.; Wang, B.; Liu, M.; Liang, Q.; Li, X.; Pang, R.; Guo, Y.; Li, Y.; et al. Asymmetrically Coordinated Cu Dual-Atom-Sites Enables Selective CO2 Electroreduction to Ethanol. Adv. Mater. 2024, 36, 2409797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, J.; Zhou, H.; Xing, L.; Qiao, S.; Yuan, J.; Mei, B.; Wei, Z.; Zhao, S.; Tang, Y.; et al. Coordination Shell Dependent Activity of CuCo Diatomic Catalysts for Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reaction. Adv. Funct. Mater. 2024, 34, 2311664. [Google Scholar] [CrossRef]
- Tao, X.; Liu, Y.; Lu, R.; Liu, J.; Wang, H.I.; Yang, J.; Bonn, M.; Müllen, K.; Zhou, Y. Phosphorus-Enhanced Bimetallic Single-Atom Catalysts for Hydrogen Evolution. Adv. Energy Mater. 2024, 2404167. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.-Y.; Li, Q.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Modulating the d-band centers by coordination environment regulation of single-atom Ni on porous carbon fibers for overall water splitting. Nano Energy 2022, 98, 107266. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, R.; Tao, X.; Qiu, Z.; Chen, G.; Yang, J.; Zhao, Y.; Feng, X.; Müllen, K. Boosting Oxygen Electrocatalytic Activity of Fe–N–C Catalysts by Phosphorus Incorporation. J. Am. Chem. Soc. 2023, 145, 3647–3655. [Google Scholar] [CrossRef]
- Li, S.-L.; Peng, M.; Song, Y.; Chen, Y.; Qiao, L.; Feng, Y.; Zhao, Y.; Gan, L.-Y. Screening transition metal and nonmetal atoms co-doped graphyne as efficient single-atom catalysts for nitrogen reduction. Chem. Eng. J. 2024, 495, 153275. [Google Scholar] [CrossRef]
- Jiao, D.; Liu, Y.; Cai, Q.; Zhao, J. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: A computational study. J. Mater. Chem. A 2021, 9, 1240–1251. [Google Scholar] [CrossRef]
- Han, L.; Hou, M.; Ou, P.; Cheng, H.; Ren, Z.; Liang, Z.; Boscoboinik, J.A.; Hunt, A.; Waluyo, I.; Zhang, S.; et al. Local Modulation of Single-Atomic Mn Sites for Enhanced Ambient Ammonia Electrosynthesis. ACS Catal. 2021, 11, 509–516. [Google Scholar] [CrossRef]
- Shi, L.; Bi, S.; Qi, Y.; He, R.; Ren, K.; Zheng, L.; Wang, J.; Ning, G.; Ye, J. Anchoring Mo Single-Atom Sites on B/N Codoped Porous Carbon Nanotubes for Electrochemical Reduction of N2 to NH3. ACS Catal. 2022, 12, 7655–7663. [Google Scholar] [CrossRef]
- Lu, X.; Wei, J.; Lin, H.; Li, Y.; Li, Y.-y. Boron Regulated Fe Single-Atom Structures for Electrocatalytic Nitrate Reduction to Ammonia. ACS Appl. Nano Mater. 2024, 7, 14654–14664. [Google Scholar] [CrossRef]
- Lu, S.; Lou, F.; Zhao, Y.; Yu, Z. Regulating the coordination environment of single-atom catalysts for electrocatalytic CO2 reduction. J. Colloid Interface Sci. 2023, 646, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, R.; Xiao, S.; Yang, Y.; Lai, L.; Chen, J.S.; Chen, Y. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction. Chem. Eng. J. 2022, 430, 132882. [Google Scholar] [CrossRef]
- Ma, M.; Tang, Q. Axial coordination modification of M–N4 single-atom catalysts to regulate the electrocatalytic CO2 reduction reaction. J. Mater. Chem. C 2022, 10, 15948–15956. [Google Scholar] [CrossRef]
- Han, S.-G.; Ma, D.-D.; Zhou, S.-H.; Zhang, K.; Wei, W.-B.; Du, Y.; Wu, X.-T.; Xu, Q.; Zou, R.; Zhu, Q.-L. Fluorine-tuned single-atom catalysts with dense surface Ni-N4 sites on ultrathin carbon nanosheets for efficient CO2 electroreduction. Appl. Catal. B Environ. 2021, 283, 119591. [Google Scholar] [CrossRef]
- Sui, R.; Zhang, X.; Wang, X.; Wang, X.; Pei, J.; Zhang, Y.; Liu, X.; Chen, W.; Zhu, W.; Zhuang, Z. Silver based single atom catalyst with heteroatom coordination environment as high performance oxygen reduction reaction catalyst. Nano Res. 2022, 15, 7968–7975. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, Z.; Li, Y.C.; Li, J.; Li, F.; Lum, Y.; Nam, D.-H.; Chen, B.; Wicks, J.; Xu, A.; et al. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen. Nat. Commun. 2019, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, E.; Sun, C.; Shi, C.; Yu, J.; Yang, X.; Xu, M. Isolated single-atom Fe-N4O1 catalytic site from a pre-oxidation strategy for efficient oxygen reduction reaction. Chem. Eng. J. 2023, 463, 142468. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, K.; Yuan, S.; Xiang, L.; Wang, K.; Jing, S.; Li, N. Coupling Ru single-atom and oxygen vacancy in Ru/SnO2−x for efficient nitrogen reduction to ammonia via electronic synergistic effect. J. Alloys Compd. 2023, 962, 171028. [Google Scholar] [CrossRef]
- Lu, F.; Yi, D.; Liu, S.; Zhan, F.; Zhou, B.; Gu, L.; Golberg, D.; Wang, X.; Yao, J. Engineering Platinum–Oxygen Dual Catalytic Sites via Charge Transfer towards Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 17712–17718. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Lian, S.; Li, J.; Sun, K.; Zhao, S.; Kim, Y.D.; Ren, Y.; Zhang, M.; Liu, Q.; et al. Engineering the oxygen vacancies enables Ni single-atom catalyst for stable and efficient C-H activation. Appl. Catal. B Environ. 2022, 314, 121516. [Google Scholar] [CrossRef]
- Yuan, C.-Z.; Wang, S.; San Hui, K.; Wang, K.; Li, J.; Gao, H.; Zha, C.; Zhang, X.; Dinh, D.A.; Wu, X.-L.; et al. In Situ Immobilizing Atomically Dispersed Ru on Oxygen-Defective Co3O4 for Efficient Oxygen Evolution. ACS Catal. 2023, 13, 2462–2471. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, L.; Dai, J.; Wang, J.; Fang, C.; Zhan, G.; Zheng, Q.; Hou, W.; Zhang, L. Single Atom Ru Monolithic Electrode for Efficient Chlorine Evolution and Nitrate Reduction. Angew. Chem. Int. Ed. 2022, 61, e202208215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, N.; Wang, Y.; Jiao, X.; Xia, Y.; Chen, D. Boosting Electrochemical Reduction of CO2 to Formate over Oxygen Vacancy Stabilized Copper–Tin Dual Single Atoms Catalysts. Adv. Funct. Mater. 2023, 33, 2303473. [Google Scholar] [CrossRef]
- Sial, M.A.Z.G.; Guo, N.; Jalil, A.; Abbas, M.; Mateen, M.; Ullah, S.; Alam, U.; Bhat, Z.M.; Hussain, A.; Cai, X.; et al. Electrochemical C-C coupling mediated by novel Sn-SnO2 supported Cu single atoms: The case of CO2 conversion to ethanol. Chem. Eng. J. 2024, 489, 151099. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.; Bak, S.-M.; Nam, C.-Y.; Moon, J.H. Amorphous-crystalline transition-driven synthesis of Co single-atom catalysts on MoO3 for enhanced hydrogen evolution in acidic and alkaline media. Chem. Eng. J. 2024, 488, 150976. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Zhong, X.; Hu, Z.; Huang, W.-H.; Pao, C.-W.; Cheng, H.; Alonso-Vante, N.; Ma, J. Universal synthesis strategy for preparation of transition metal oxide electrocatalysts doped with noble metal single atoms for oxygen evolution reaction. Energy Adv. 2024, 3, 2002–2012. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Ting, Y.-C.; Yen, F.-Y.; Li, G.-R.; Lee, C.-H.; Lee, K.-A.; Chang, S.-I.; Chen, H.-Y.T.; Lu, S.-Y. Synergistic Mo and W single atoms co-doped surface hydroxylated NiFe oxide as bifunctional electrocatalysts for overall water splitting. Appl. Catal. B Environ. Energy 2024, 358, 124356. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Han, P.; Du, Y.; Gu, Z.; Xu, X.; Zheng, G. Single-Atomic Cu with Multiple Oxygen Vacancies on Ceria for Electrocatalytic CO2 Reduction to CH4. ACS Catal. 2018, 8, 7113–7119. [Google Scholar] [CrossRef]
- Zang, W.; Lee, J.; Tieu, P.; Yan, X.; Graham, G.W.; Tran, I.C.; Wang, P.; Christopher, P.; Pan, X. Distribution of Pt single atom coordination environments on anatase TiO2 supports controls reactivity. Nat. Commun. 2024, 15, 998. [Google Scholar] [CrossRef]
- Hu, B.; Sun, K.; Zhuang, Z.; Chen, Z.; Liu, S.; Cheong, W.-C.; Chen, C.; Hu, M.; Cao, X.; Ma, J.; et al. Distinct Crystal-Facet-Dependent Behaviors for Single-Atom Palladium-On-Ceria Catalysts: Enhanced Stabilization and Catalytic Properties. Adv. Mater. 2022, 34, 2107721. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, Q.; Cao, L.; Gao, Z.; Lin, J.; Li, L.; Pan, X.; Lin, S.; Wang, X.; Zhang, T. Facet-dependent electronic state of Pt single atoms anchoring on CeO2 nanocrystal for CO (preferential) oxidation. J. Catal. 2022, 415, 174–185. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.-G.; Li, J. Theoretical Investigations of Pt1@CeO2 Single-Atom Catalyst for CO Oxidation. J. Phys. Chem. C 2017, 121, 11281–11289. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Liu, H.; Sun, J.; Xie, R.; Qiu, Y.; Lü, F.; Liu, Y.; Zhuo, L.; Liu, X.; et al. Amorphous MoOX-Stabilized single platinum atoms with ultrahigh mass activity for acidic hydrogen evolution. Nano Energy 2020, 70, 104529. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Lu, Y.; Zheng, L.; Sun, S.; Li, H.; Chen, G.; Zhang, J. Single-atom alloy with Pt-Co dual sites as an efficient electrocatalyst for oxygen reduction reaction. Appl. Catal. B Environ. 2022, 306, 121112. [Google Scholar] [CrossRef]
- Cai, J.; Wei, Y.; Cao, A.; Huang, J.; Jiang, Z.; Lu, S.; Zang, S.-Q. Electrocatalytic nitrate-to-ammonia conversion with ~100% Faradaic efficiency via single-atom alloying. Appl. Catal. B Environ. 2022, 316, 121683. [Google Scholar] [CrossRef]
- Li, X.; Shen, P.; Luo, Y.; Li, Y.; Guo, Y.; Zhang, H.; Chu, K. PdFe Single-Atom Alloy Metallene for N2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202205923. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; He, C.-T.; Pei, J.; Chen, W.; He, D.; He, Y.; Zhuang, Z.; Chen, C.; Peng, Q.; Wang, D.; et al. Accelerating water dissociation kinetics by isolating cobalt atoms into ruthenium lattice. Nat. Commun. 2018, 9, 4958. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, X.; Yu, T.; Lu, X.; Qian, L.; Liu, P.; Lei, P. Surface restructuring in AgCu single-atom alloy catalyst and self-enhanced selectivity toward CO2 reduction. Electrochim. Acta 2022, 426, 140774. [Google Scholar] [CrossRef]
- Gao, Q.; Yao, B.; Pillai, H.S.; Zang, W.; Han, X.; Liu, Y.; Yu, S.-W.; Yan, Z.; Min, B.; Zhang, S.; et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat. Synth. 2023, 2, 624–634. [Google Scholar] [CrossRef]
- Yao, Y.; Sulei, H.; Chen, W.; Huang, Z.-Q.; Wei, W.; Yao, T.; Liu, R.; Zang, K.; Wang, X.; Wu, G.; et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. In Controllable Synthesis and Atomic Scale Regulation of Noble Metal Catalysts; Springer Nature: London, UK, 2019; Volume 2, pp. 55–92. [Google Scholar]
- Zhao, Y.; Liu, X.; Chen, D.; Liu, Z.; Yang, Q.; Lin, X.; Peng, M.; Liu, P.; Tan, Y. Atomic-level-designed copper atoms on hierarchically porous gold architectures for high-efficiency electrochemical CO2 reduction. Sci. China Mater. 2021, 64, 1900–1909. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wang, W.; Yin, L.; Crittenden, J.C. Electrocatalytic nitrate reduction to ammonia on defective Au1Cu (111) single-atom alloys. Appl. Catal. B Environ. 2022, 310, 121346. [Google Scholar] [CrossRef]
- Darby, M.T.; Stamatakis, M. Single-Atom Alloys for the Electrochemical Oxygen Reduction Reaction. ChemPhysChem 2021, 22, 499–508. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Clark, E.L.; Pham, H.H.; Bell, A.T.; Head-Gordon, M. Quantum Mechanical Screening of Single-Atom Bimetallic Alloys for the Selective Reduction of CO2 to C1 Hydrocarbons. ACS Catal. 2016, 6, 7769–7777. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, C.; Guo, C.; Zhang, M.; Xu, L.; Jiang, Q.; Xue, W.; Li, H.; Li, A.; Pao, C.-W.; et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 2021, 16, 1386–1393. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Wang, L.; Su, B.-J.; Wu, K.-H.; Juang, J.-Y.; Hou, F.; Yin, L.; Dou, S.X.; Liu, J.; et al. Carbon-Shielded Single-Atom Alloy Material Family for Multi-Functional Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2205654. [Google Scholar] [CrossRef]
- Zheng, G.; Li, Y.; Qian, X.; Yao, G.; Tian, Z.; Zhang, X.; Chen, L. High-Throughput Screening of a Single-Atom Alloy for Electroreduction of Dinitrogen to Ammonia. ACS Appl. Mater. Interfaces 2021, 13, 16336–16344. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Zhu, Y.; Chen, G.; She, S.; Guo, X.; Li, H.; Liu, T.; Lin, Z.; Zhou, H.; et al. Atomic ruthenium modification of nickel-cobalt alloy for enhanced alkaline hydrogen evolution. Appl. Catal. B Environ. 2023, 331, 122710. [Google Scholar] [CrossRef]
- Gao, D.; Yi, D.; Xia, J.; Yang, Y.; Wang, X. First-principles screening of Cu-based single-atom alloys for highly efficient electrocatalytic nitrogen reduction. Mol. Catal. 2024, 555, 113879. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, J.-L.; Zhao, J.-W.; Feng, L.; Zhu, J.; Jiang, X.; Li, W.-X.; Liu, J.-X. Nature of the Active Center for the Oxygen Reduction on Ag-Based Single-Atom Alloy Clusters. JACS Au 2024, 4, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lu, S.; Wang, J.-a.; Wang, X.; Wang, M.; Fruehwald, H.M.; Wang, L.; Zhang, B.; Guo, T.; Mills, J.P.; et al. Selectively Reducing Nitrate into NH3 in Neutral Media by PdCu Single-Atom Alloy Electrocatalysis. ACS Catal. 2023, 13, 10560–10569. [Google Scholar] [CrossRef]
- Feng, Y.; An, W.; Wang, Z.; Wang, Y.; Men, Y.; Du, Y. Electrochemical CO2 Reduction Reaction on M@Cu(211) Bimetallic Single-Atom Surface Alloys: Mechanism, Kinetics, and Catalyst Screening. ACS Sustain. Chem. Eng. 2020, 8, 210–222. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Liu, S.; Norouzi Banis, M.; Song, Z.; Li, J.; Yang, L.; Markiewicz, M.; Zhao, Y.; Li, R.; et al. Pt/Pd Single-Atom Alloys as Highly Active Electrochemical Catalysts and the Origin of Enhanced Activity. ACS Catal. 2019, 9, 9350–9358. [Google Scholar] [CrossRef]
- Xi, S.; Zhao, P.; He, C.; Zhang, W. High-throughput screening of single-atom catalysts on 1 T-TMD for highly active and selective CO2 reduction reaction: Computational and machine learning insights. J. Catal. 2024, 436, 115610. [Google Scholar] [CrossRef]
- Ling, F.; Xia, W.; Li, L.; Zhou, X.; Luo, X.; Bu, Q.; Huang, J.; Liu, X.; Kang, W.; Zhou, M. Single Transition Metal Atom Bound to the Unconventional Phase of the MoS2 Monolayer for Catalytic Oxygen Reduction Reaction: A First-Principles Study. ACS Appl. Mater. Interfaces 2021, 13, 17412–17419. [Google Scholar] [CrossRef]
- Ran, N.; Song, E.; Wang, Y.; Zhou, Y.; Liu, J. Dynamic coordination transformation of active sites in single-atom MoS2 catalysts for boosted oxygen evolution catalysis. Energy Environ. Sci. 2022, 15, 2071–2083. [Google Scholar] [CrossRef]
- Pattengale, B.; Huang, Y.; Yan, X.; Yang, S.; Younan, S.; Hu, W.; Li, Z.; Lee, S.; Pan, X.; Gu, J.; et al. Dynamic evolution and reversibility of single-atom Ni(II) active site in 1T-MoS2 electrocatalysts for hydrogen evolution. Nat. Commun. 2020, 11, 4114. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, H.; Cheng, D. Design of high-performance MoS2 edge supported single-metal atom bifunctional catalysts for overall water splitting via a simple equation. Nanoscale 2019, 11, 20228–20237. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Qi, K.; Zhao, Z. Single atom supported on MoS2 as efficient electrocatalysts for the CO2 reduction reaction: A DFT study. Appl. Surf. Sci. 2022, 602, 154211. [Google Scholar] [CrossRef]
- Yang, T.; Song, T.T.; Zhou, J.; Wang, S.; Chi, D.; Shen, L.; Yang, M.; Feng, Y.P. High-throughput screening of transition metal single atom catalysts anchored on molybdenum disulfide for nitrogen fixation. Nano Energy 2020, 68, 104304. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, C.; Zheng, L.; Petit, E.; Qi, K.; Zhang, Y.; Wu, H.; Wang, W.; Tiberj, A.; et al. 3.4% Solar-to-Ammonia Efficiency from Nitrate Using Fe Single Atomic Catalyst Supported on MoS2 Nanosheets. Adv. Funct. Mater. 2022, 32, 2108316. [Google Scholar] [CrossRef]
- Tursun, M.; Abdukayum, A.; Wu, C.; Wang, C. Screening WS2−based single−atom catalysts for electrocatalytic nitrate reduction to ammonia. Int. J. Hydrogen Energy 2024, 73, 183–190. [Google Scholar] [CrossRef]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single atom Ru doping 2H-MoS2 as highly efficient hydrogen evolution reaction electrocatalyst in a wide pH range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Li, H.; Zeng, Z. Boosting Electrocatalytic Reduction of CO2 to HCOOH on Ni Single Atom Anchored WTe2 Monolayer. Small 2022, 18, 2203759. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Tang, S.; Liu, T.; Li, X.; Wang, X.; Zhong, W.; Luo, Y.; Jiang, J. Regulating Electronic Spin Moments of Single-Atom Catalyst Sites via Single-Atom Promoter Tuning on S-Vacancy MoS2 for Efficient Nitrogen Fixation. J. Phys. Chem. Lett. 2021, 12, 8355–8362. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, W. Accelerating electrocatalytic reduction of nitrate to ammonia by utilizing steric hindrance in single boron-decorated 2H/1T-MoS2: A theoretical insight. Appl. Surf. Sci. 2022, 596, 153624. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Zhu, T.; Gan, X.; Xiao, Z.; Dai, S.; Xiao, H.; Zhang, S.; Dong, S.; Zhao, H.; Wang, P. Single-atom dispersed Cu or Co on 2H-MoS2 monolayer for improving electrocatalytic activity of overall water splitting. Surf. Interfaces 2021, 27, 101538. [Google Scholar] [CrossRef]
- Hoa, V.H.; Tran, D.T.; Prabhakaran, S.; Kim, D.H.; Hameed, N.; Wang, H.; Kim, N.H.; Lee, J.H. Ruthenium single atoms implanted continuous MoS2-Mo2C heterostructure for high-performance and stable water splitting. Nano Energy 2021, 88, 106277. [Google Scholar] [CrossRef]
- Nie, Q.-J.; Ding, C.-C.; Liu, T.; Jin, W.; Hu, J.-S. Transition metal ions doped into Haeckelite-MoS2 as highly efficient and selective electrocatalyst for nitrogen reduction reaction. Mater. Today Commun. 2024, 39, 109423. [Google Scholar] [CrossRef]
- Sun, T.; Tang, Z.; Zang, W.; Li, Z.; Li, J.; Li, Z.; Cao, L.; Dominic Rodriguez, J.S.; Mariano, C.O.M.; Xu, H.; et al. Ferromagnetic single-atom spin catalyst for boosting water splitting. Nat. Nanotechnol. 2023, 18, 763–771. [Google Scholar] [CrossRef]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q.; et al. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; Zhang, W.; Tang, L.; Mu, Z.; Liu, C.; Tian, B.; Fei, M.; Sun, Y.; Su, H.; et al. Boosting the performance of single-atom catalysts via external electric field polarization. Nat. Commun. 2022, 13, 3063. [Google Scholar] [CrossRef]
- Aggarwal, P.; Sarkar, D.; Awasthi, K.; Menezes, P.W. Functional role of single-atom catalysts in electrocatalytic hydrogen evolution: Current developments and future challenges. Coord. Chem. Rev. 2022, 452, 214289. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Chen, Z.; Kumar, P.V.; Tsoukalou, A.; Kierzkowska, A.; Abdala, P.M.; Safonova, O.V.; Fedorov, A.; Müller, C.R. Single Site Cobalt Substitution in 2D Molybdenum Carbide (MXene) Enhances Catalytic Activity in the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 17809–17816. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Chen, Z.; Abdala, P.M.; Safonova, O.V.; Fedorov, A.; Müller, C.R. Single-Atom-Substituted Mo2CTx:Fe-Layered Carbide for Selective Oxygen Reduction to Hydrogen Peroxide: Tracking the Evolution of the MXene Phase. J. Am. Chem. Soc. 2021, 143, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, N.; Ong, W.-J.; Zhou, N. Single atom-supported MXene: How single-atomic-site catalysts tune the high activity and selectivity of electrochemical nitrogen fixation. J. Mater. Chem. A 2019, 7, 27620–27631. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S.; et al. Selective Etching Quaternary MAX Phase toward Single Atom Copper Immobilized MXene (Ti3C2Clx) for Efficient CO2 Electroreduction to Methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Yang, W.; Liu, S.; Zhang, X.; Yu, Y.; Cheong, W.-C.; Zheng, L.; Ren, F.; Ying, G.; et al. MXene (Ti3C2) Vacancy-Confined Single-Atom Catalyst for Efficient Functionalization of CO2. J. Am. Chem. Soc. 2019, 141, 4086–4093. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Y.; Tian, W.; Zhang, H.; Wang, Y. Single, double, and triple transition metal atoms embedded in defective V3C2O2 for nitrogen reduction reaction: A DFT study. Appl. Surf. Sci. 2021, 569, 151020. [Google Scholar] [CrossRef]
- Gao, X.; Tse, E.C.M. Unraveling the Performance Descriptors for Designing Single-Atom Catalysts on Defective MXenes for Exclusive Nitrate-To-Ammonia Electrocatalytic Upcycling. Small 2024, 20, 2306311. [Google Scholar] [CrossRef]
- Kan, D.; Wang, D.; Cheng, Y.; Lian, R.; Sun, B.; Chen, K.; Huo, W.; Wang, Y.; Chen, G.; Wei, Y. Designing of Efficient Bifunctional ORR/OER Pt Single-Atom Catalysts Based on O-Terminated MXenes by First-Principles Calculations. ACS Appl. Mater. Interfaces 2021, 13, 52508–52518. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varadhan, P.; Fu, H.-C.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1903841. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. From Metal–Organic Frameworks to Single-Atom Fe Implanted N-doped Porous Carbons: Efficient Oxygen Reduction in Both Alkaline and Acidic Media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Sun, L.; Wang, M.; Hong, J.; Zou, L.; Liu, H.; Wang, Y.; Zhang, M.; Liu, Z.; Li, Y.; et al. Fabricating Dual-Atom Iron Catalysts for Efficient Oxygen Evolution Reaction: A Heteroatom Modulator Approach. Angew. Chem. Int. Ed. 2020, 59, 16013–16022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Pu, Z.; Zhou, H.; Yu, J.; Amiinu, I.S.; Zhu, J.; Liang, Q.; Yang, J.; He, D.; Hu, Z.; et al. A universal synthesis strategy for single atom dispersed cobalt/metal clusters heterostructure boosting hydrogen evolution catalysis at all pH values. Nano Energy 2019, 59, 472–480. [Google Scholar] [CrossRef]
- Jiao, L.; Zhu, J.; Zhang, Y.; Yang, W.; Zhou, S.; Li, A.; Xie, C.; Zheng, X.; Zhou, W.; Yu, S.-H.; et al. Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO2 Electroreduction. J. Am. Chem. Soc. 2021, 143, 19417–19424. [Google Scholar] [CrossRef]
- Tao, H.; Choi, C.; Ding, L.-X.; Jiang, Z.; Han, Z.; Jia, M.; Fan, Q.; Gao, Y.; Wang, H.; Robertson, A.W.; et al. Nitrogen Fixation by Ru Single-Atom Electrocatalytic Reduction. Chem 2019, 5, 204–214. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Dong, H.; Zhou, L.; Xu, H.; Wang, H.-R.; Yan, X.; Jiang, Y.; Zhang, J.; Gu, Z.-G. Fe single-atom catalysts with pre-organized coordination structure for efficient electrochemical nitrate reduction to ammonia. Appl. Catal. B Environ. 2022, 317, 121750. [Google Scholar] [CrossRef]
- Shankar, A.; Marimuthu, S.; Maduraiveeran, G. High-valent iron single-atom catalysts for improved overall water splitting via a reduced energy barrier and stabilization of the active center. J. Mater. Chem. A 2024, 12, 121–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, L.; Yang, W.; Xie, C.; Jiang, H.-L. Rational Fabrication of Low-Coordinate Single-Atom Ni Electrocatalysts by MOFs for Highly Selective CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7607–7611. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pei, J.; He, C.-T.; Wan, J.; Ren, H.; Wang, Y.; Dong, J.; Wu, K.; Cheong, W.-C.; Mao, J.; et al. Single Tungsten Atoms Supported on MOF-Derived N-Doped Carbon for Robust Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1800396. [Google Scholar] [CrossRef]
- Han, X.; Ling, X.; Yu, D.; Xie, D.; Li, L.; Peng, S.; Zhong, C.; Zhao, N.; Deng, Y.; Hu, W. Atomically Dispersed Binary Co-Ni Sites in Nitrogen-Doped Hollow Carbon Nanocubes for Reversible Oxygen Reduction and Evolution. Adv. Mater. 2019, 31, 1905622. [Google Scholar] [CrossRef]
- Yan, C.; Li, H.; Ye, Y.; Wu, H.; Cai, F.; Si, R.; Xiao, J.; Miao, S.; Xie, S.; Yang, F.; et al. Coordinatively unsaturated nickel–nitrogen sites towards selective and high-rate CO2 electroreduction. Energy Environ. Sci. 2018, 11, 1204–1210. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, Y.-N.; Liu, H.; Banis, M.N.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Sham, T.-K.; Yang, L.; et al. Engineering the Low Coordinated Pt Single Atom to Achieve the Superior Electrocatalytic Performance toward Oxygen Reduction. Small 2020, 16, 2003096. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Zhang, H.; Liu, K.; Cullen, D.; More, K.; Wang, M.; Feng, Z.; Wang, G.; Wu, G.; Li, Y. Unveiling Active Sites of CO2 Reduction on Nitrogen-Coordinated and Atomically Dispersed Iron and Cobalt Catalysts. ACS Catal. 2018, 8, 3116–3122. [Google Scholar] [CrossRef]
- Zhang, R.; Jiao, L.; Yang, W.; Wan, G.; Jiang, H.-L. Single-atom catalysts templated by metal–organic frameworks for electrochemical nitrogen reduction. J. Mater. Chem. A 2019, 7, 26371–26377. [Google Scholar] [CrossRef]




| Supports | |||||||
|---|---|---|---|---|---|---|---|
| Approach | Advantages and Disadvantages | Carbon Support | Metal Oxide Support | Alloy Support | Transition Metal Dichalcogenides (TMDs) Support | MXenes Support | Metal–Organic Framework (MOF) Support |
| Morphology Engineering | Advantages | High surface area, tunable porosity, lightweight, enhanced mass transport | Diverse morphologies, robustness, wide bandgap tunability | Tunable surface, improved electron transport, alloy synergy effects | Layered structures, abundant active edges, high catalytic activity | High conductivity, layered structure, enhanced active site exposure | Tailorable structure, ultrahigh porosity, and versatility in design |
| Disadvantages | Limited chemical functionality; weak interaction with metal atoms | Brittle nature, low conductivity | Complexity in precise morphology tuning, potential phase segregation | Instability under certain conditions, higher synthesis cost | Susceptibility to oxidation, challenging defect creation | Structural fragility in liquid-phase reactions, scalability issues | |
| Vacancy Engineering | Advantages | Improves catalytic activity and conductivity; lightweight | Enhances active site density and binding energies | Provides electronic tunability; synergetic effects enhance catalytic performance | Boosts catalytic sites at edges and vacancies; enables fine-tuning of electronic states | Promotes metallic conductivity and excellent adsorption properties | Facilitates enhanced adsorption properties and active site creation |
| Disadvantages | Limited vacancy stability under reaction conditions | Difficulty in maintaining vacancy stability over time | Formation of vacancies can weaken overall structural integrity | Complex fabrication methods, moderate thermal stability | High sensitivity to oxidative and acidic conditions | Hard to control vacancies precisely | |
| Heteroatom Doping | Advantages | Enhances electronic properties, provides functional groups for improved binding | Tailors catalytic activity via bandgap engineering | Strengthens catalytic performance by introducing new alloy phases and interactions | Modifies electronic structure to improve catalytic selectivity and activity | Facilitates synergistic effects with intrinsic conductivity | Allows diverse doping combinations, enhancing versatility |
| Disadvantages | Limited thermal stability; uneven dopant dispersion | High temperature is often required for dopant diffusion | Dopant clustering may reduce uniformity and effectiveness | Reduced mechanical stability due to strain effects | Possible degradation of intrinsic conductivity upon heavy doping | May alter intrinsic stability, reducing long-term durability | |
| Facet Engineering | Advantages | Enhances site-specific catalytic activity; tunable electronic interactions | Enables site-selective reactions and improved stability | Offers higher selectivity and activity by controlling atomic arrangements | Improves edge-site activity with facet exposure | Specific facet control for electronic property optimization | Facilitates selective catalysis with exposed active facets |
| Disadvantages | Facet exposure control can be challenging in porous carbon | Limited options for controlling facets in certain oxides | Surface diffusion may compromise facet selectivity | Facet engineering may reduce stability in some catalytic environments | Limited reproducibility in precise facet exposure | Achieving stable facet configurations can be difficult | |
| Crystallinity Control | Advantages | Enhances electronic conductivity and stability | Improves charge transport and catalytic stability | Reduces grain boundaries, improving structural integrity and activity | Increases intrinsic activity by reducing defects while maintaining conductivity | Enables fine-tuning of electronic and catalytic properties | Allows structural and catalytic property optimization |
| Disadvantages | Difficult to achieve uniform crystallinity in disordered carbons | Requires high temperatures, challenging scalability | Balancing crystallinity with alloy composition can be challenging | Limited options for achieving precise control | Susceptible to oxidation and degradation | Trade-off between crystallinity and porosity | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajmal, S.; Huang, J.; Guo, J.; Tabish, M.; Mushtaq, M.A.; Alam, M.M.; Yasin, G. Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future. Catalysts 2025, 15, 137. https://doi.org/10.3390/catal15020137
Ajmal S, Huang J, Guo J, Tabish M, Mushtaq MA, Alam MM, Yasin G. Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future. Catalysts. 2025; 15(2):137. https://doi.org/10.3390/catal15020137
Chicago/Turabian StyleAjmal, Saira, Junfeng Huang, Jianwen Guo, Mohammad Tabish, Muhammad Asim Mushtaq, Mohammed Mujahid Alam, and Ghulam Yasin. 2025. "Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future" Catalysts 15, no. 2: 137. https://doi.org/10.3390/catal15020137
APA StyleAjmal, S., Huang, J., Guo, J., Tabish, M., Mushtaq, M. A., Alam, M. M., & Yasin, G. (2025). Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future. Catalysts, 15(2), 137. https://doi.org/10.3390/catal15020137







