Zeolitized Clays and Their Use for the Capture and Photo-Fenton Degradation of Methylene Blue
Abstract
1. Introduction
2. Results and Discussion
2.1. Zeolites Characterization
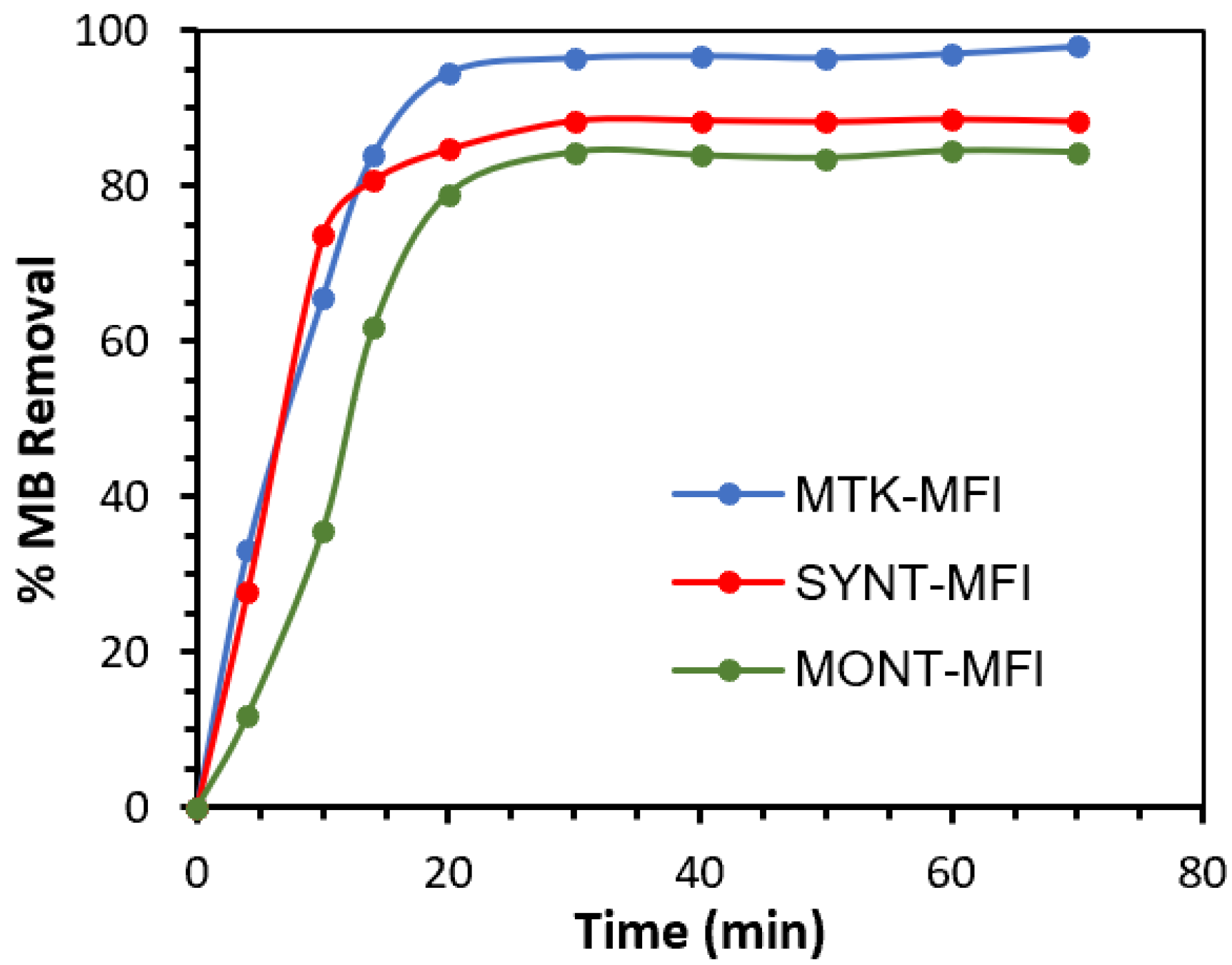
2.2. MB Adsorption Tests
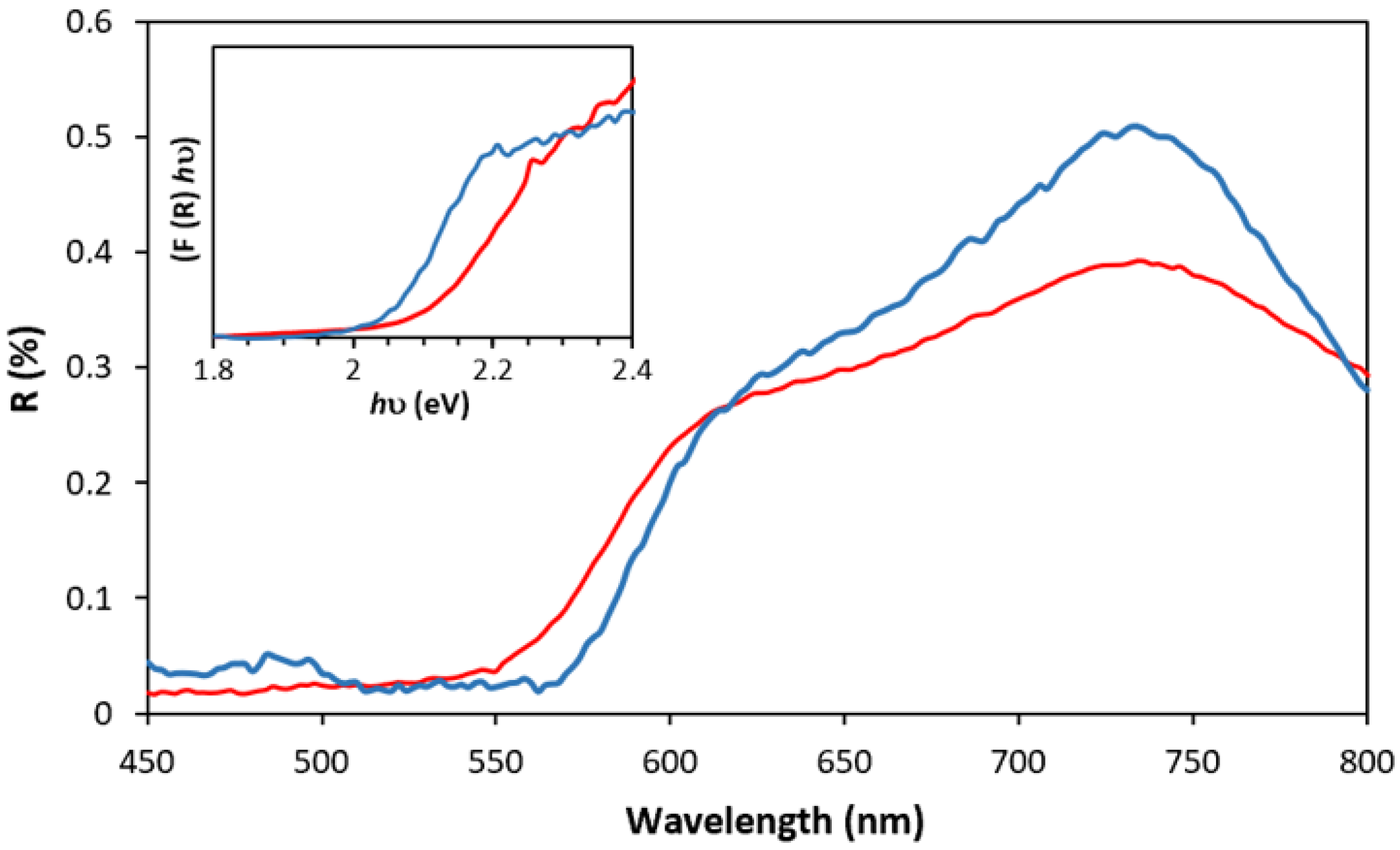
2.3. Degradation of MB by the Heterogeneous Photo-Fenton Process
3. Materials and Methods
3.1. Clay Pre-Treatment
3.2. Zeolitization of Clays
3.3. Photo-Fenton Catalyst Preparation
3.4. Characterization
3.5. Adsorption Tests
3.6. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPCA | Integrated photocatalyst adsorbent |
| MB | Methylene blue |
| AOP | Advanced oxidation process |
| NPs | Nanoparticles |
| MTK | Metakaolin after acid washing |
| MONT | Montmorillonite after acid washing |
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Azha, S.F.; Shamsudin, M.S.; Shahadat, M.; Ismail, S. Low cost zwitterionic adsorbent coating for treatment of anionic and cationic dyes. J. Ind. Eng. Chem. 2018, 67, 187–198. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Sik Ok, Y.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef]
- Lundehøj, L.; Jensen, H.C.; Wybrandt, L.; Nielsen, U.G.; Christensen, M.L.; Quist-Jensen, C.A. Layered double hydroxides for phosphorus recovery from acidified and non-acidified dewatered sludge. Water Res. 2019, 153, 208–216. [Google Scholar] [CrossRef]
- Kumar, N.; Nieminen, V.; Demirkan, K.; Salmi, T.; Murzin, D.Y.; Laine, E. Effect of synthesis time and mode of stirring on physico-chemical and catalytic properties of ZSM-5 zeolite catalysts. Appl. Catal. A Gen. 2002, 235, 113–123. [Google Scholar] [CrossRef]
- Richards, M.D.; Pope, C.G. Adsorption of Methylene Blue from aqueous solutions by amorphous aluminosilicate gels and zeolite X. J. Chem. Soc.—Faraday Trans. 1996, 92, 317–323. [Google Scholar] [CrossRef]
- Hadda Aya, H.; Djamel, N.; Samira, A.; Otero, M.; Ali Khan, M. Optimizing methylene blue adsorption conditions on hydrothermally synthesized NaX zeolite through a full two-level factorial design. RSC Adv. 2024, 14, 23816–23827. [Google Scholar] [CrossRef]
- Melgoza, D.; Hernández-Ramírez, A.; Peralta-Hernández, J.M. Comparative efficiencies of the decolourisation of Methylene Blue using Fenton’s and photo-Fenton’s reactions. Photochem. Photobiol. Sci. 2009, 8, 596–599. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Shangguan, Z.; Yuan, X.; Jiang, L.; Zhao, Y.; Qin, L.; Zhou, X.; Wu, Y.; Chew, J.W.; Wang, H. Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater. Chin. Chem. Lett. 2022, 33, 4719–4731. [Google Scholar] [CrossRef]
- Quynh, H.G.; Thanh, H.V.; Phuong, N.T.T.; Duy, N.P.T.; Hung, L.H.; Dung, N.V.; Duong, N.T.H.; Long, N.Q. Rapid removal of methylene blue by a heterogeneous photo-Fenton process using economical and simple-synthesized magnetite–zeolite composite. Environ. Technol. Innov. 2023, 31, 103155. [Google Scholar] [CrossRef]
- Ning, M.; Li, L.; Xu, X. Degradation of Methylene Blue Using Fe-ZSM-5 Zeolite as a Heterogeneous Fenton Catalyst. J. Phys. Conf. Ser. 2023, 2463, 012055. [Google Scholar] [CrossRef]
- Schumacher de Oliveira, J.; Mazutti, M.A.; Urquieta-González, E.A.; Foletto, E.L. Preparation of Mesoporous Fe2O3-Supported ZSM-5 Zeolites by Carbon-Templating and their Evaluation as Photo-Fenton Catalysts to Degrade Organic Pollutant. Mater. Res. 2016, 19, 1399–1406. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Park, K.H.; Seo, D.C. Heterogeneous fenton oxidation of methylene blue with Fe-impregnated biochar catalyst. Biochar 2020, 2, 165–176. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, W.; Zhang, M.; Lin, Y.; Xu, N.; Chen, F.; Wang, D.; Chen, Z. Adsorption and Synergetic Fenton-like Degradation of Methylene Blue by a Novel Mesoporous α-Fe2O3/SiO2 at Neutral pH. Ind. Eng. Chem. Res. 2018, 57, 5539–5549. [Google Scholar] [CrossRef]
- Šuligoj, A.; Ristić, A.; Dražić, G.; Pintar, A.; Logar, N.Z.; Tušar, N.N. Bimetal Cu-Mn porous silica-supported catalyst for Fenton-like degradation of organic dyes in wastewater at neutral pH. Catal. Today 2020, 358, 270–277. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B Environ. 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Geng, T.; Yan, J.; Li, B.; Yan, H.; Guo, L.; Sun, Q.; Guan, Z.; Zhao, C.; Xu, J.; Wang, W. Efficient degradation of methylene blue at near neutral pH based on heterogeneous Fenton-like system catalyzed by Fe2O3/MnO2. Results Chem. 2024, 11, 101795. [Google Scholar] [CrossRef]
- Aguilar-Mamani, W.; García, G.; Hedlund, J.; Mouzon, J. Comparison between leached metakaolin and leached diatomaceous earth as raw materials for the synthesis of ZSM-5. SpringerPlus 2014, 3, 292. [Google Scholar] [CrossRef]
- Schmidt, W.; Wilczok, U.; Weidenthaler, C.; Medenbach, O.; Goddard, R.; Buth, G.; Cepak, A. Preparation and morphology of pyramidal MFI single-crystal segments. J. Phys. Chem. B 2007, 111, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Taufiqurrahmi, N.; Mohamed, A.R.; Bhatia, S. Nanocrystalline zeolite beta and zeolite y as catalysts in used palm oil cracking for the production of biofuel. J. Nanopart. Res. 2011, 13, 3177–3189. [Google Scholar] [CrossRef]
- Iryani, A.; Nur, H.; Santoso, M.; Hartanto, D. Adsorption study of rhodamine B and methylene blue dyes with ZSM-5 directly synthesized from Bangka Kaolin without organic template. Indones. J. Chem. 2020, 20, 130–140. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 20160164. [Google Scholar] [CrossRef]
- Piccinin, S. The band structure and optical absorption of hematite (α-Fe2O3): A first-principles GW-BSE study. Phys. Chem. Chem. Phys. 2019, 21, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Deotale, A.J.; Nandedkar, R.V. Correlation between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Kyriakou, G.; Theocharis, C.R. The Anomalous Sorptive Behaviour of Zsm-5 And Silicalite-I: Observation of Low-Pressure Hysteresis in Nitrogen Adsorption. Stud. Surf. Sci. Catal. 2002, 144, 709–716. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schüβler, F.; D’Amico, A.; Danilina, N.; van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of Zeolites in Hot Liquid Water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, P.; Yan, W.; Boronat, M.; Li, X.; Su, J.-H.; Wang, J.; Li, Y.; Corma, A.; Xu, R.; et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 2016, 351, 1188–1191. [Google Scholar] [CrossRef]
- Aguilar-Mamani, W.; Akhtar, F.; Hedlund, J.; Mouzon, J. Solution-mediated growth of NBA-ZSM-5 crystals retarded by gel entrapment. J. Cryst. Growth 2018, 487, 57–64. [Google Scholar] [CrossRef]
- Comboni, D.; Pagliaro, F.; Lotti, P.; Gatta, G.D.; Merlini, M.; Milani, S.; Migliori, M.; Giordano, G.; Catizzone, E.; Collings, I.E.; et al. The elastic behavior of zeolitic frameworks: The case of MFI type zeolite under high pressure methanol intrusion. Catal. Today 2020, 345, 88–96. [Google Scholar] [CrossRef]
- Deplazes, R.; Teles, C.A.; Ciotonea, C.; Sfeir, A.; Canilho, N.; Richard, F.; Royer, S. Melt infiltration for the preparation of finely dispersed Ni-FeOx nanoparticles on SBA-15 for the hydrodeoxygenation of m-cresol. Catal. Today 2024, 430, 114514. [Google Scholar] [CrossRef]
- Jiang, X.; Li, L.; Cui, Y.; Cui, F. New branch on old tree: Green-synthesized RGO/Fe3O4 composite as a photo-Fenton catalyst for rapid decomposition of methylene blue. Ceram. Int. 2017, 43, 14361–14368. [Google Scholar] [CrossRef]
- Almojil, S.F.; Ning, J.; Almohana, A.I. Photo-Fenton process for degradation of methylene blue using copper ferrite@sepiolite clay. Inorg. Chem. Commun. 2024, 166, 112623. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, F.T.; Liu, Q.Q.; Zhao, X.; Liu, S.Q. Application of heterogenous catalyst of tris(1,10)-phenanthroline iron(II) loaded on zeolite for the photo-Fenton degradation of methylene blue. React. Kinet. Mech. Catal. 2011, 103, 299–310. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Rasool, R.T.; Hassan, M.; Zhang, L.; Guo, H. Heterogeneous catalytic activation of BaCu-based M-hexaferrite nanoparticles for methylene blue degradation under photo-Fenton-like system. Mol. Catal. 2021, 505, 111501. [Google Scholar] [CrossRef]
- Quynh, H.G.; Kiet, N.A.; Van Thanh, H.; Tue, T.M.; Phuong, N.T.T.; Long, N.Q. Removal of aqueous organic pollutant by photo-Fenton process using low-cost Fe3O4/zeolite A. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012013. [Google Scholar] [CrossRef]
- Finger, L.W.; Hazen, R.M. Crystal Structure and Isothermal Compression of Fe2O3, Cr2O3, and V2O3 to 50 Kbars. J. Appl. Phys. 1980, 51, 5362–5367. [Google Scholar] [CrossRef]

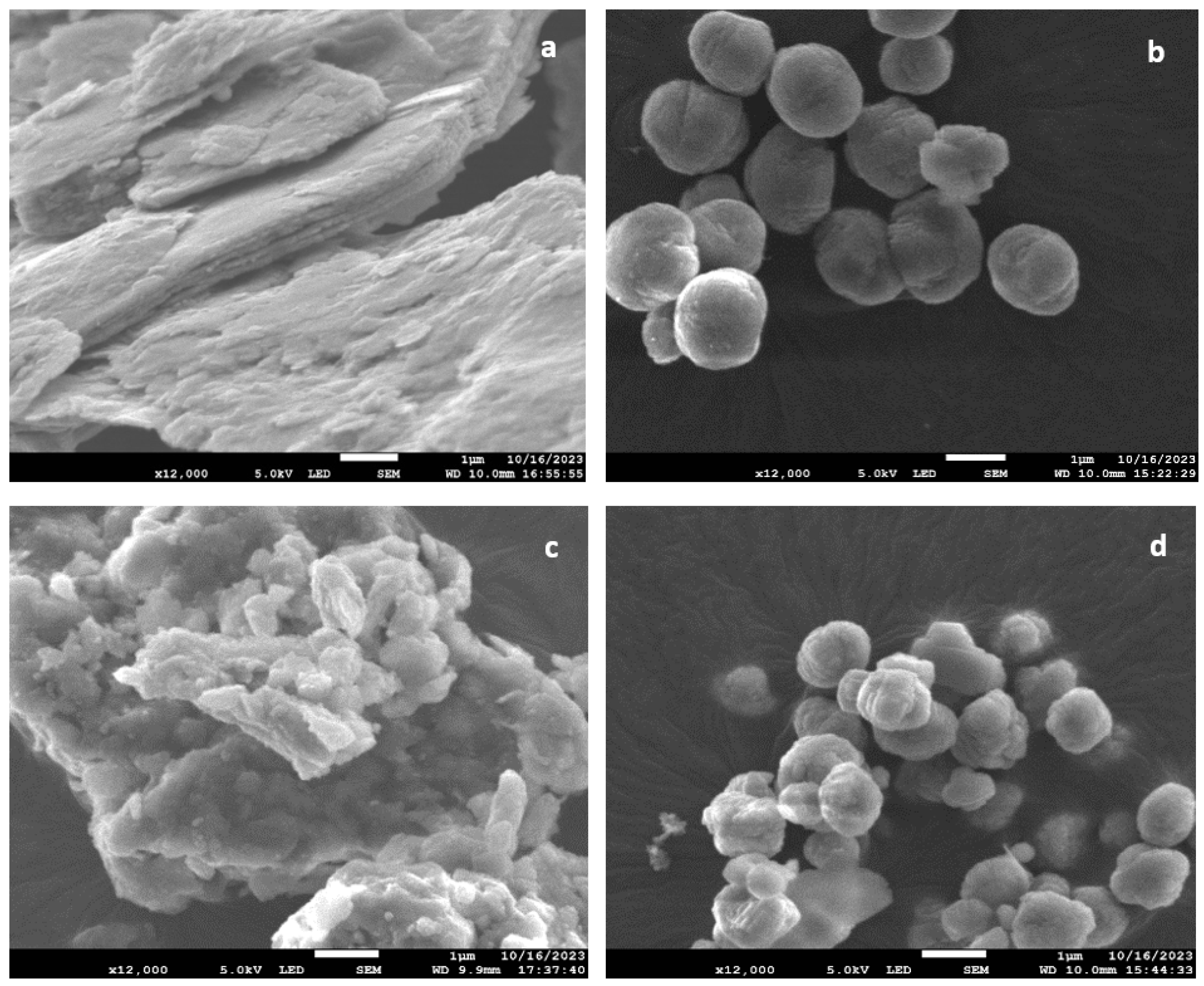
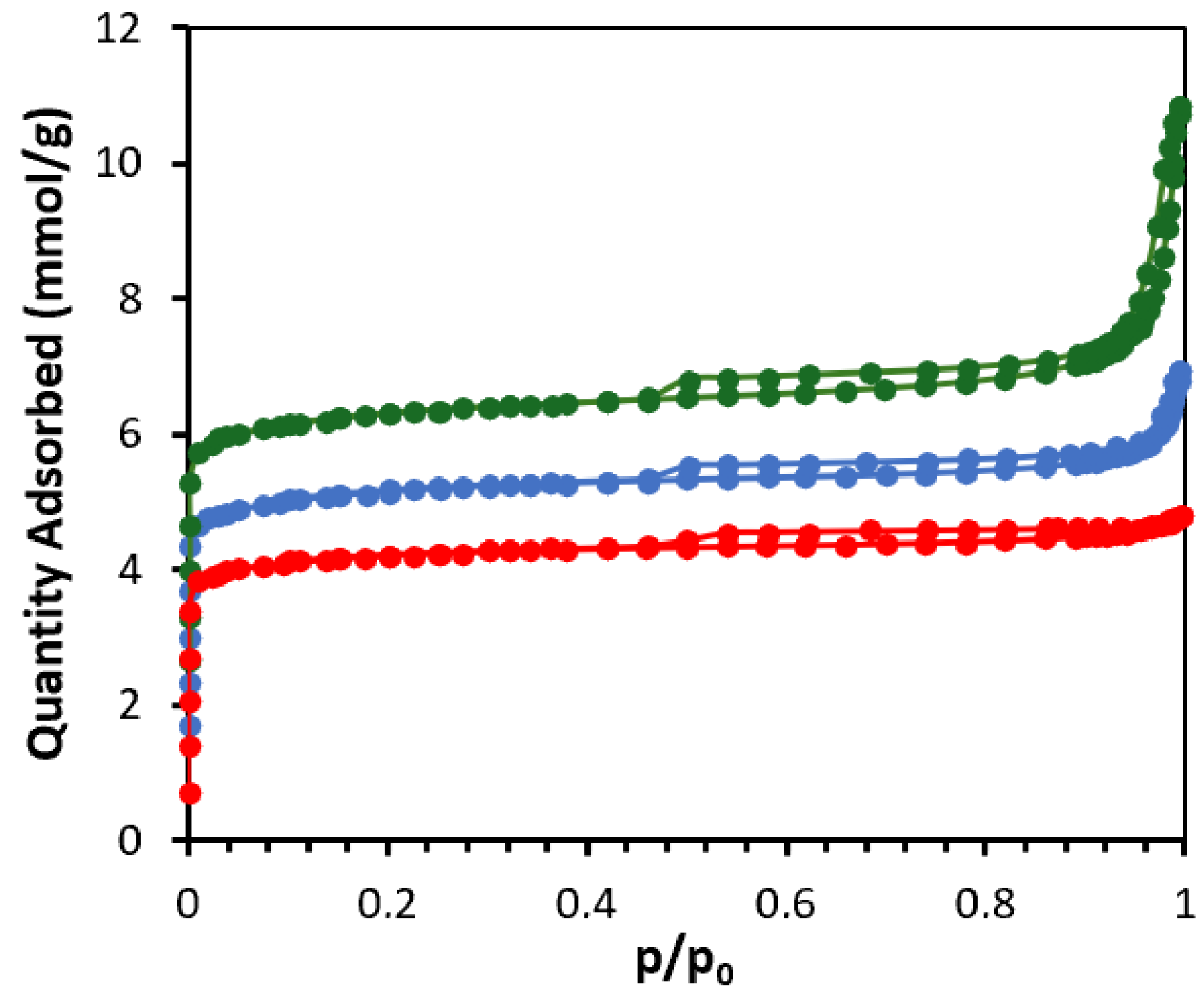


| Materials | SBET (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Acidity (mmol NH3/g) |
|---|---|---|---|---|---|
| Metakaolin | 12.68 | 0.04 | 0.00 | 0.04 | - |
| MTK | 188.3 | 0.12 | 0.07 | 0.05 | - |
| MONT | 224.6 | 0.36 | 0.09 | 0.27 | - |
| MTK-MFI | 324.0 | 0.18 | 0.12 | 0.06 | 0.63 |
| MONT-MFI | 330.4 | 0.26 | 0.13 | 0.13 | 0.88 |
| SYNT-MFI | 321.4 | 0.15 | 0.13 | 0.02 | 0.92 |
| Materials | Experimental Data | Adsorption Kinetic Model | |||
|---|---|---|---|---|---|
| % Removal | qe Exp. (mg/g) | k1 | qe Theor. (mg/g) | R2 | |
| MTK-MFI | 98.0 | 4.98 | 0.16 | 4.94 | 0.96 |
| SYNT-MFI | 88.6 | 4.50 | 0.21 | 5.95 | 0.99 |
| MONT-MFI | 84.5 | 4.28 | 0.11 | 4.23 | 0.91 |
| Catalysts | Fe2O3 NPs (nm) a | Fe (wt.%) b | Fe (wt.%) c | MB Removal (%) d |
|---|---|---|---|---|
| Fe/MTK-MFI | 29 | 9.6 | 1.5 | 97.6 |
| Fe/SYNT-MFI | 32 | 8.4 | 2.2 | 90.5 |
| Fe/MONT-MFI | 31 | 2.0 * | 2.1 | 80.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouadio, K.S.; Tchirioua, E.; Dhainaut, J. Zeolitized Clays and Their Use for the Capture and Photo-Fenton Degradation of Methylene Blue. Catalysts 2025, 15, 188. https://doi.org/10.3390/catal15020188
Kouadio KS, Tchirioua E, Dhainaut J. Zeolitized Clays and Their Use for the Capture and Photo-Fenton Degradation of Methylene Blue. Catalysts. 2025; 15(2):188. https://doi.org/10.3390/catal15020188
Chicago/Turabian StyleKouadio, Koffi Simeon, Ekou Tchirioua, and Jérémy Dhainaut. 2025. "Zeolitized Clays and Their Use for the Capture and Photo-Fenton Degradation of Methylene Blue" Catalysts 15, no. 2: 188. https://doi.org/10.3390/catal15020188
APA StyleKouadio, K. S., Tchirioua, E., & Dhainaut, J. (2025). Zeolitized Clays and Their Use for the Capture and Photo-Fenton Degradation of Methylene Blue. Catalysts, 15(2), 188. https://doi.org/10.3390/catal15020188






