One-Pot In Situ Synthesis of Porous Vanadium-Doped g-C3N4 with Improved Photocatalytic Removal of Pharmaceutical Pollutants
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication Process of V/CN Catalysts
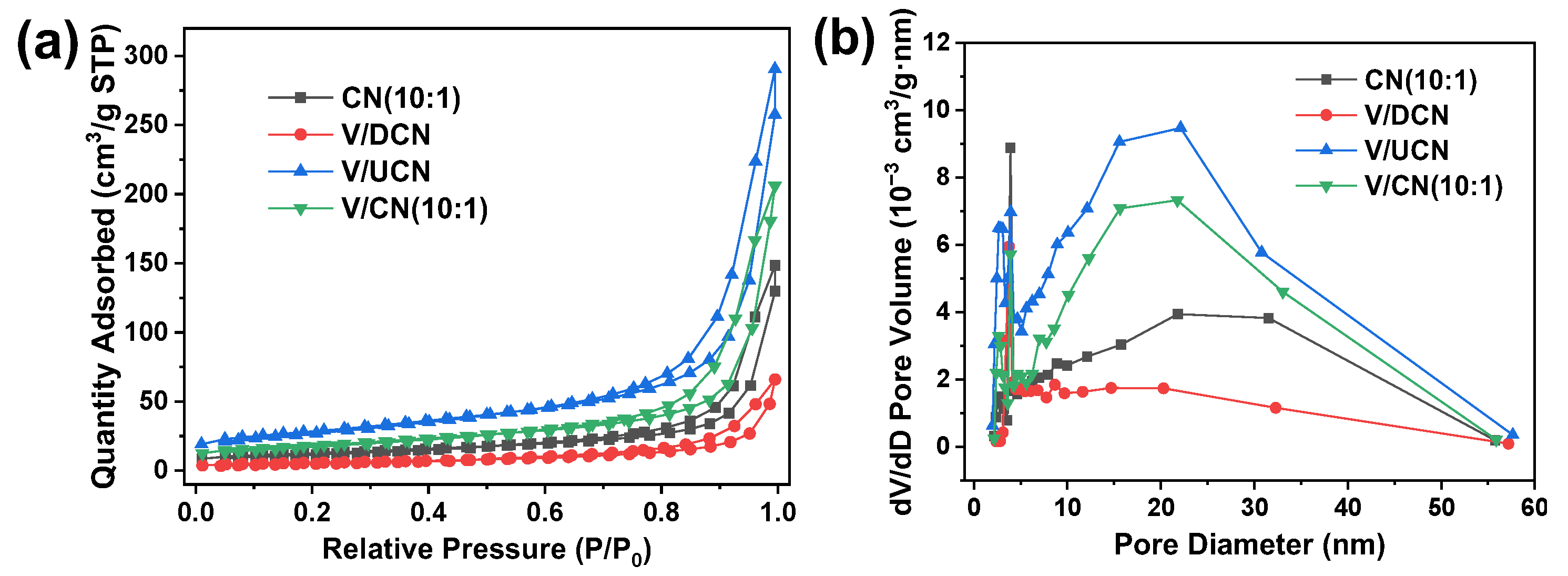
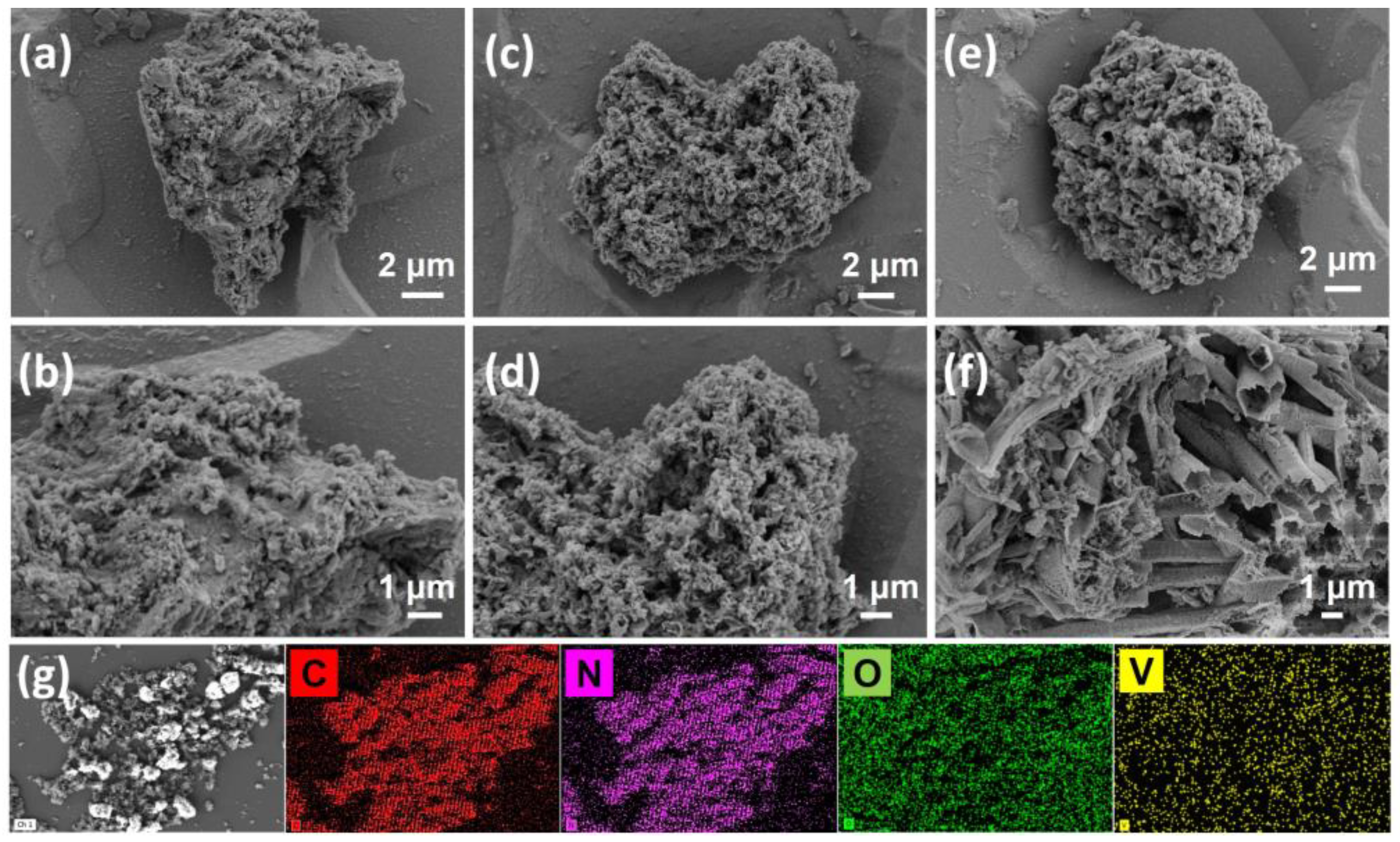
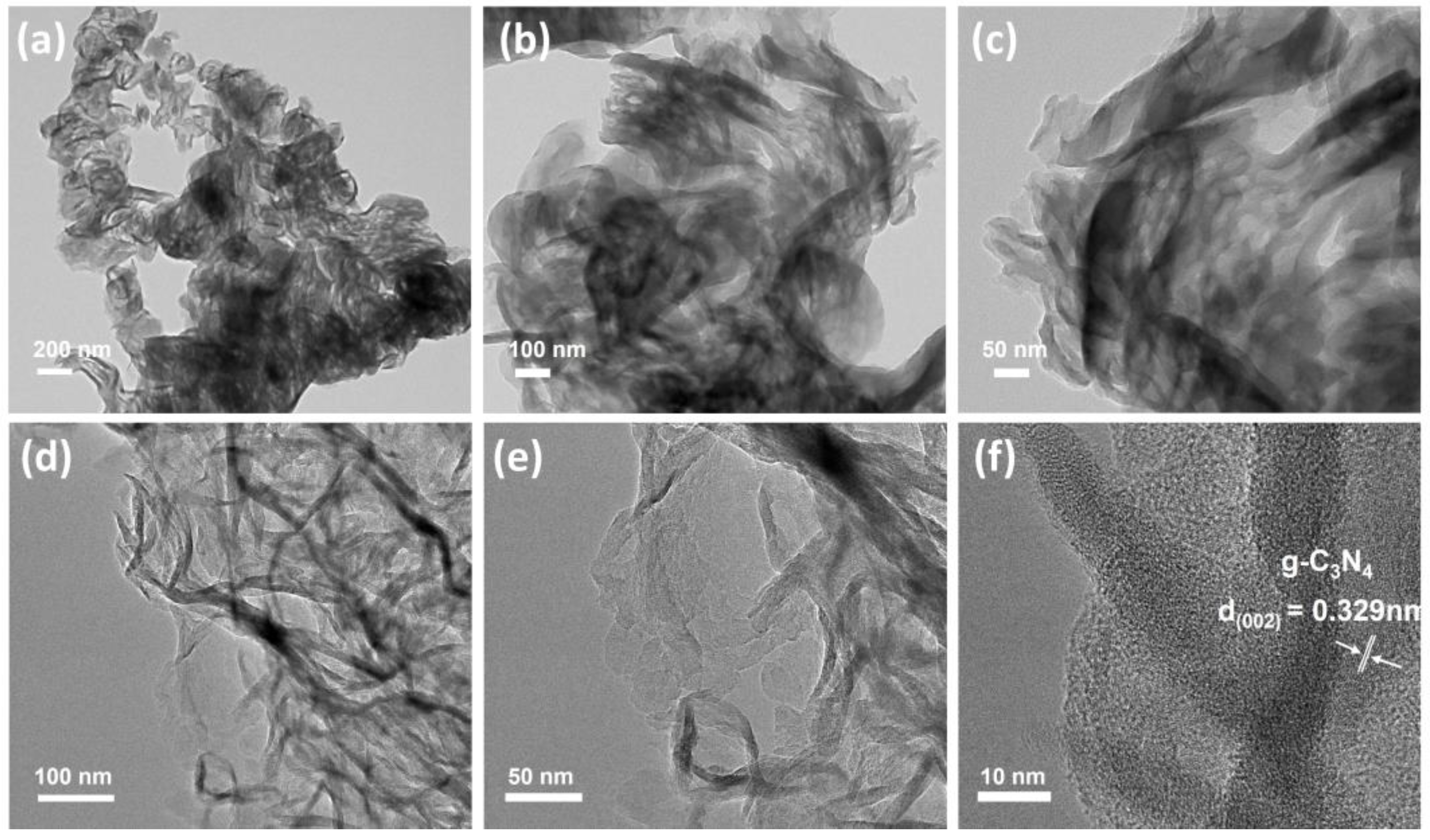
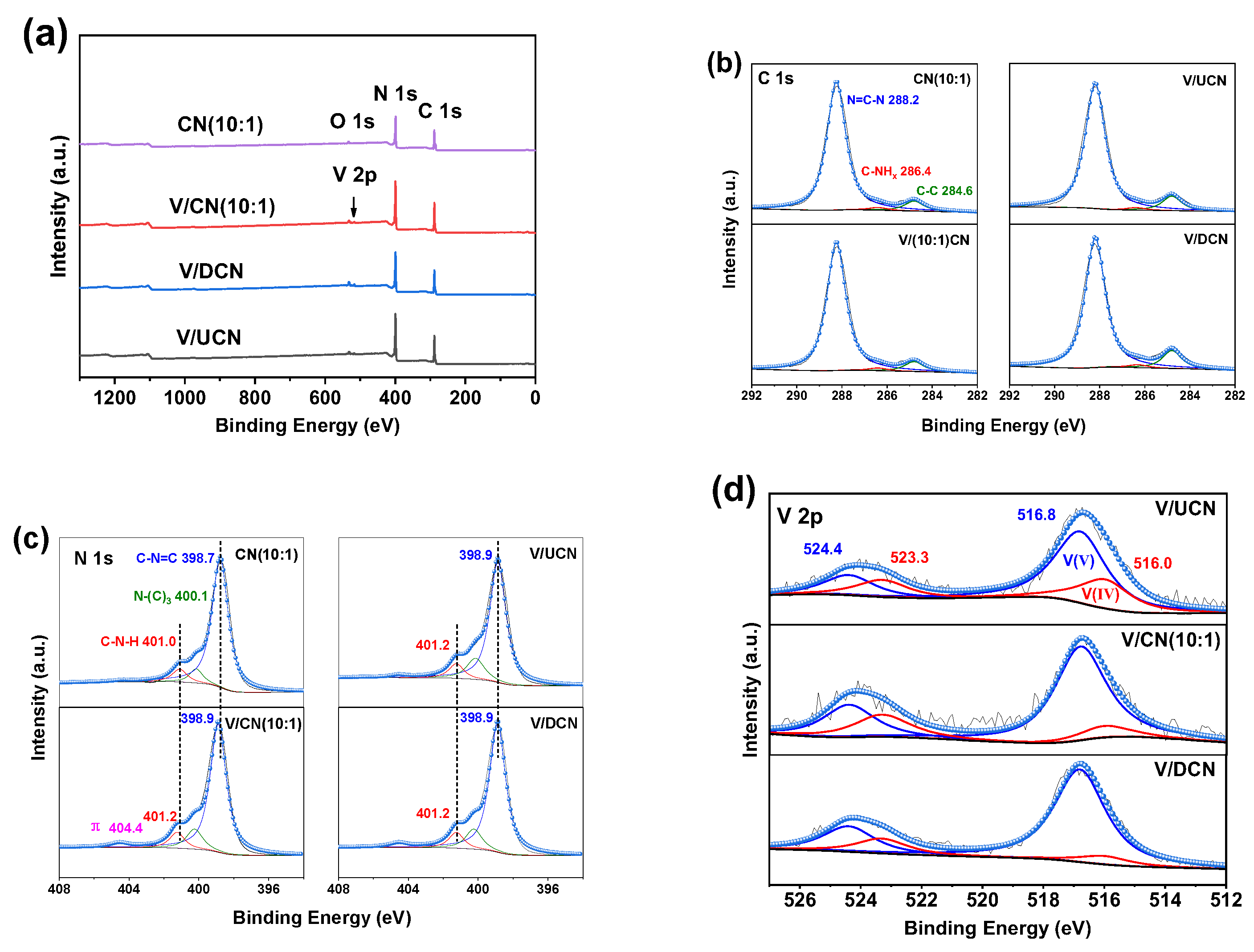
2.2. Characterizations of V/CN Catalysts
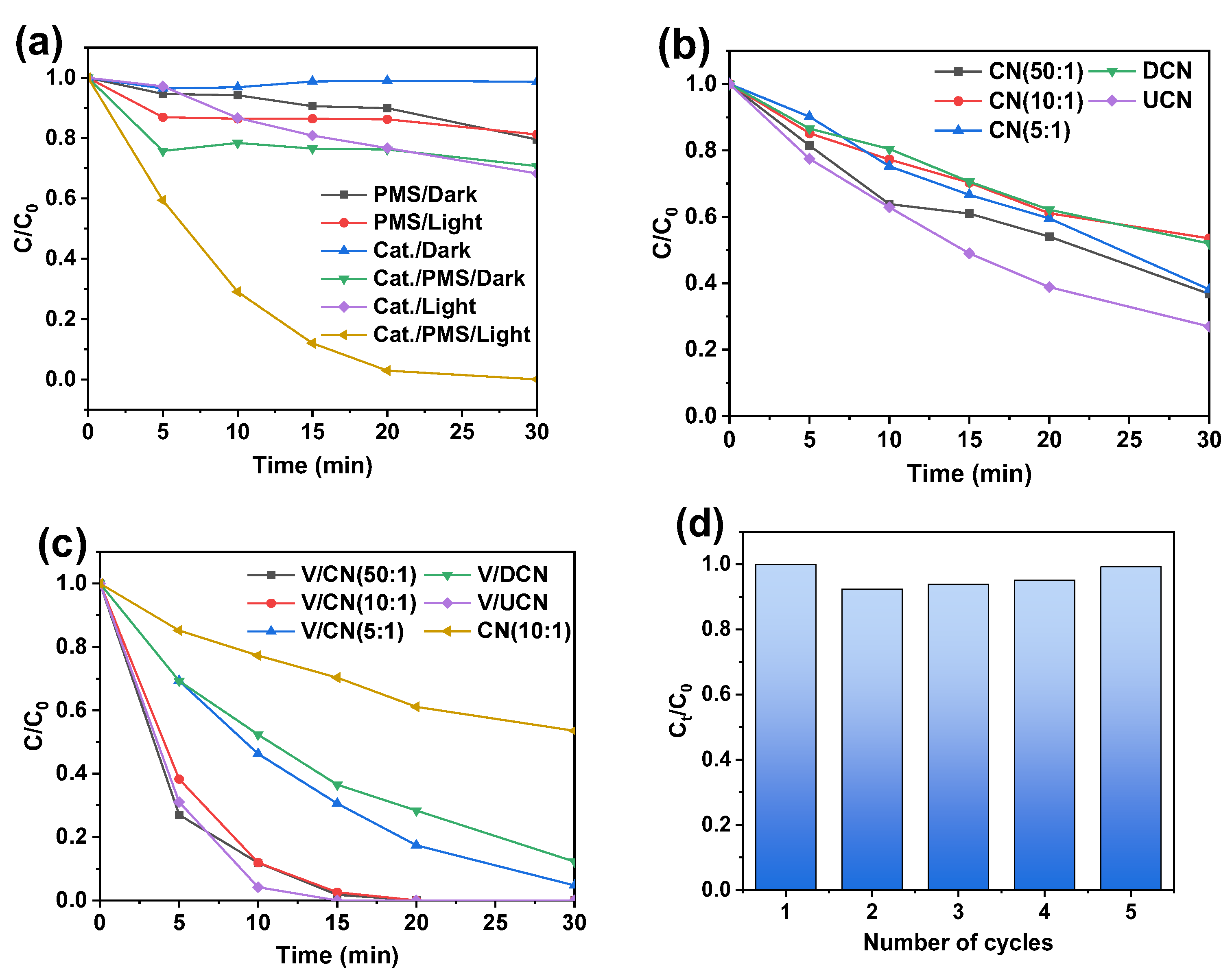
2.3. Photocatalytic Performance Evaluation
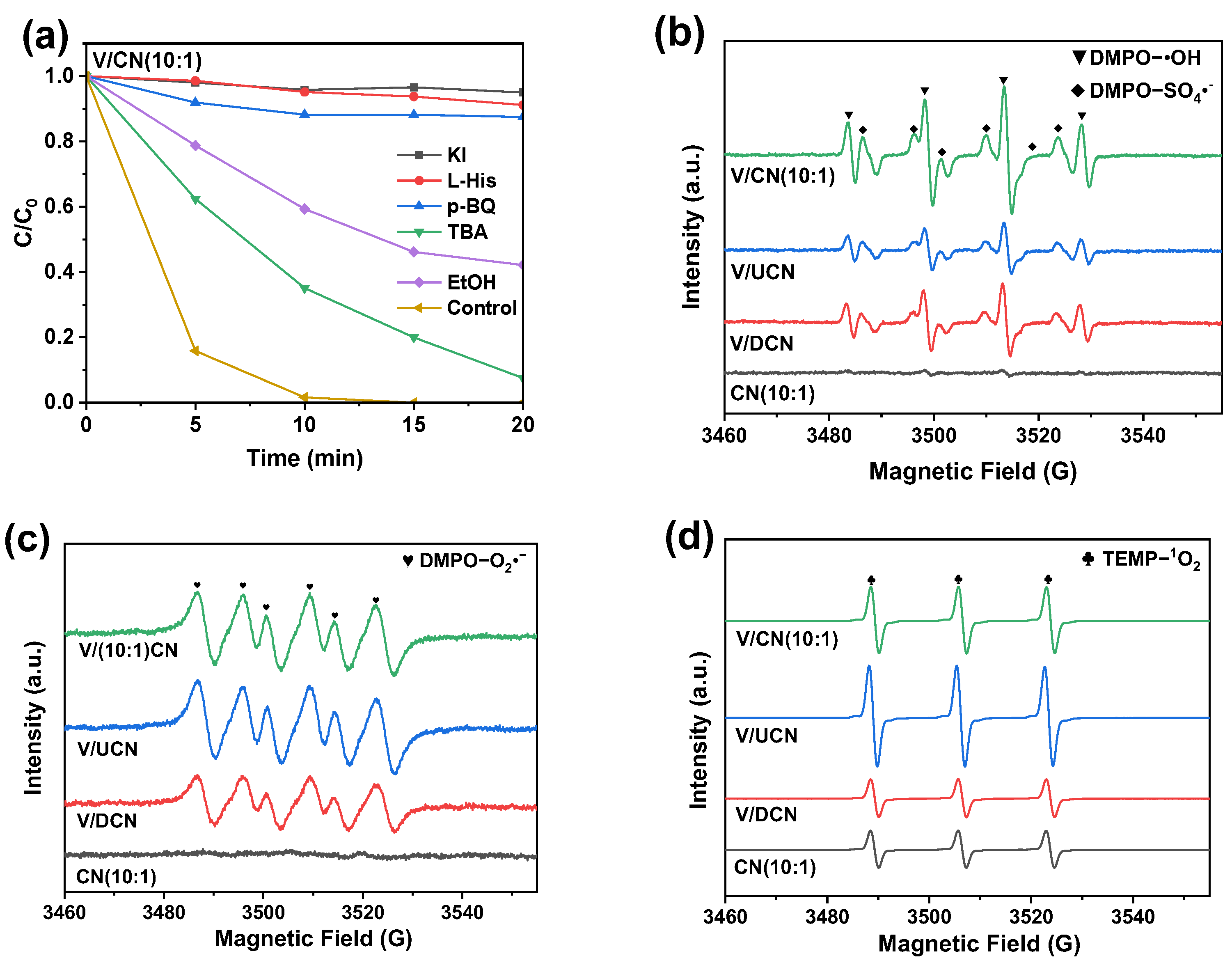
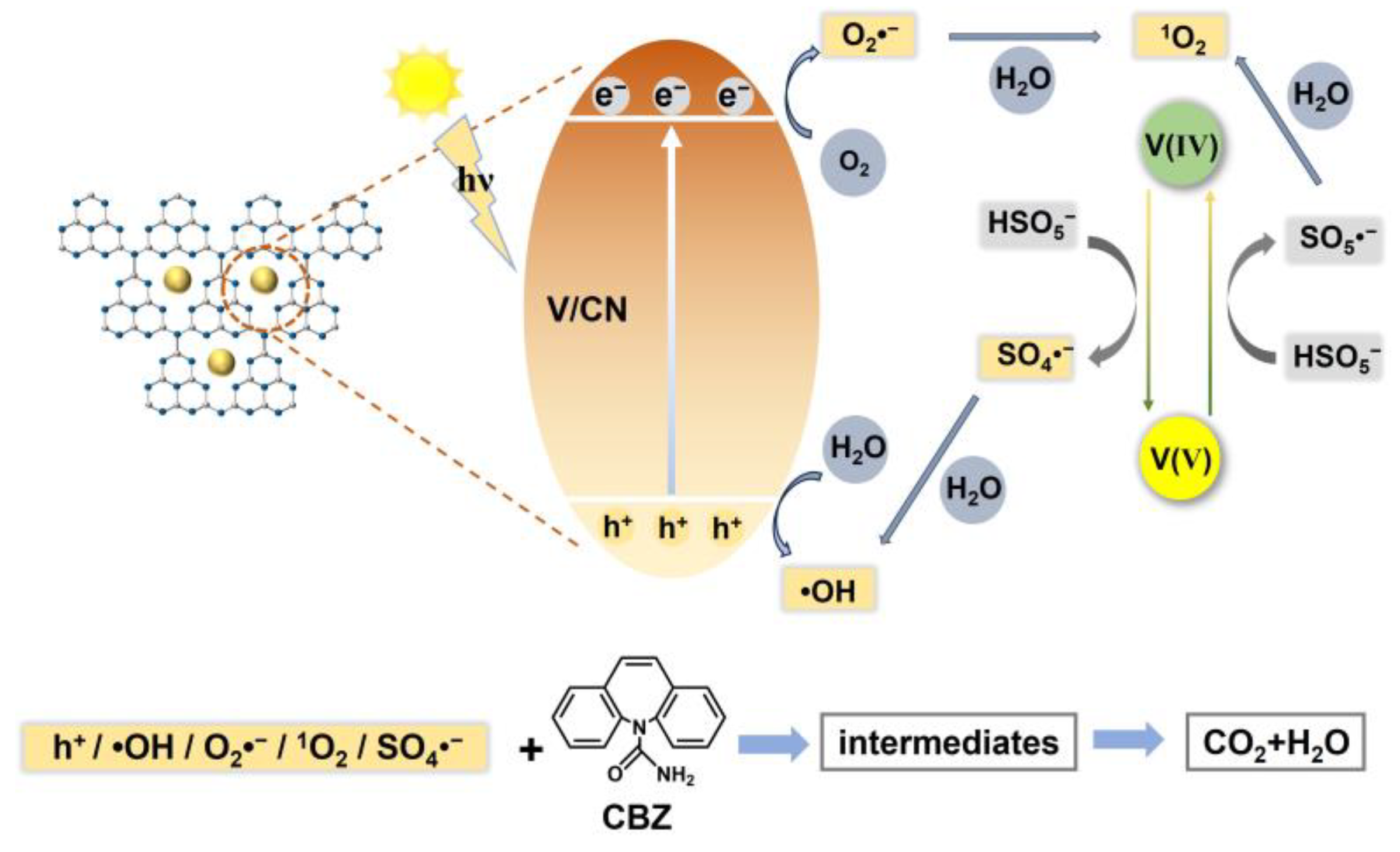
2.4. Reaction Mechanism
2.5. CBZ Degradation Pathway
3. Experimental Section
3.1. Materials
3.2. Synthesis of V-Doped g-C3N4 Catalyst
3.3. Characterization
3.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, K.; Shen, C.; Yan, R.; Liu, Y.; Zhuang, C.; Li, S. Integration of Plasmonic Effect and S-Scheme Heterojunction into Ag/Ag3PO4/C3N5 Photocatalyst for Boosted Photocatalytic Levofloxacin Degradation. Acta Phys.-Chim. Sin. 2024, 40, 2310013. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, R.; Gong, J.; Luo, Y.; Zhuang, Y.; Zhang, P. S-Scheme WO3/SnIn4S8 Heterojunction for Water Purification: Enhanced Photocatalytic Performance and Mechanism. Catalysts 2023, 13, 1450. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, L.; Zhang, S.; Tang, J. Nitrogen-Doped Biochar (N-Doped BC) and Iron/Nitrogen Co-Doped Biochar (Fe/N Co-Doped BC) for Removal of Refractory Organic Pollutants. J. Hazard. Mater. 2023, 446, 130727. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Tian, K.; Cao, M.; Li, L.; Shi, F.; Qu, J.; Zheng, Q.; Zhang, G. Application of Nickel Foam Supported Cu–MnO2 in Microwave Enhanced Fenton-like Process for p-Nitrophenol Removal: Degradation, Synergy and Mechanism Insight. J. Clean. Prod. 2023, 397, 136442. [Google Scholar] [CrossRef]
- Stella, R.; Sreevani, I.; Gurugubelli, T.; Ravikumar, R.; Koutavarapu, R. Enhanced Solar Light-Driven Photocatalytic Degradation of Tetracycline Using Fe3+-Doped CdO/ZnS Nanocomposite: Mechanistic Insights and Performance Evaluation. Catalysts 2023, 13, 1312. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Wang, Y.; Wu, T.; Shao, B.; He, Q.; Zhou, L.; Li, T.; Liu, S.; Huang, X.; et al. MOF Derived MnFeOx Supported on Carbon Cloth as Electrochemical Anode for Peroxymonosulfate Electro-Activation and Persistent Organic Pollutants Degradation. Chem. Eng. J. 2024, 481, 148646. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Chen, Y.; Dou, L.; Ren, Y.; Li, N.; Lai, B.; Tan, B. Cobalt Doped g-C3N4 Activated Peroxymonosulfate for Organic Pollutant Degradation: Alterations in Cobalt Species and Reactive Oxygen Species. Chemosphere 2024, 369, 143763. [Google Scholar] [CrossRef]
- Lai, B.; Wu, P.; Li, B.; Liu, L.; Wang, T.; Zhu, N.; Dang, Z. Activation of Peroxomonosulfate by Manganese-Containing Non-Homogeneous Catalysts Prepared via a Soft Template Calcination Strategy for Efficient Degradation of Carbamazepine. Chem. Eng. J. 2024, 500, 157199. [Google Scholar] [CrossRef]
- Ying, Y.; Liang, S.; Zhang, F.; Xu, X.; Qian, C.; Jiang, L.; Zhou, J.; Wan, Y.; Wang, L.; Yao, Y. Accelerated Fe3+/Fe2+ Cycle in Mo2C-Based Fe Catalyst to Promote Peroxymonosulfate Activation. Chemosphere 2024, 367, 143380. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, L.; Yang, S.; Yao, Y.; Chen, H.; Wang, W. Fe-Based Carbonitride as Fenton-like Catalyst for the Elimination of Organic Contaminants. Environ. Res. 2021, 198, 110486. [Google Scholar] [CrossRef]
- Zhen, J.; Sun, J.; Xu, X.; Wu, Z.; Song, W.; Ying, Y.; Liang, S.; Miao, L.; Cao, J.; Lv, W.; et al. M−N3 Configuration on Boron Nitride Boosts Singlet Oxygen Generation via Peroxymonosulfate Activation for Selective Oxidation. Angew. Chem. Int. Ed. 2024, 63, e202402669. [Google Scholar] [CrossRef]
- Yan, H.; Zhen, J.; Yao, Y. Fe3+ and H2O2 Assisted Dopamine Rapid Polymerization on Melamine Foam to Activate PMS for Organic Pollutant Degradation. Environ. Sci.-Water Res. Technol. 2024, 10, 2698–2708. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Sun, J.; Zhen, J.; Lv, W. Layered Double Hydroxide/Carbonitride Heterostructure with Potent Combination for Highly Efficient Peroxymonosulfate Activation. Chemosphere 2023, 313, 137394. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Sun, X.; Wang, Z.; Naushad, M.; Boczkaj, G. Activated Persulfate and Peroxymonosulfate Based Advanced Oxidation Processes (AOPs) for Antibiotics Degradation—A Review. Water Resour. Ind. 2023, 29, 100194. [Google Scholar] [CrossRef]
- Hassani, A.; Scaria, J.; Ghanbari, F.; Nidheesh, P.V. Sulfate Radicals-Based Advanced Oxidation Processes for the Degradation of Pharmaceuticals and Personal Care Products: A Review on Relevant Activation Mechanisms, Performance, and Perspectives. Environ. Res. 2023, 217, 114789. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, Y.-X.; He, Y.; Xu, J.; Ke, Z.; Zhang, X.; Ouyang, G.; Yang, X. Highly Effective Activation of Peroxymonosulfate via Oxygen-Coordinated Single-Atom Iron for Water Decontamination. Chem. Eng. J. 2024, 485, 149782. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, G.; Han, Z.; Li, H.; Chi, S.; Hu, G.; Zhao, X. Interfacial Anchoring Cobalt Species Mediated Advanced Oxidation: Degradation Performance and Mechanism of Organic Pollutants. J. Colloid Interface Sci. 2025, 679, 67–78. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Q.; Shen, W.; Chen, Z.; Hong, X.; Fu, Y.; Si, Y. Synthesis of Highly Crystallized g-C3N4 by Regulating Staged Gaseous Intermediates for Hydrogen Production. J. Mater. Res. 2023, 38, 3214–3226. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C.; Shen, W.; Fu, Y.; Si, Y. Regulating Intermediate Concentration to Synthesize Highly Crystalline g-C3N4 under Spontaneous Ultrahigh Pressure. ChemNanoMat 2023, 9, e202300102. [Google Scholar] [CrossRef]
- Zhuang, C.; Chang, Y.; Li, W.; Li, S.; Xu, P.; Zhang, H.; Zhang, Y.; Zhang, C.; Gao, J.; Chen, G.; et al. Light-Induced Variation of Lithium Coordination Environment in g-C3N4 Nanosheet for Highly Efficient Oxygen Reduction Reactions. ACS Nano 2024, 18, 5206–5217. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and Modification Strategies of g-C3N4 Nanosheets for Photocatalytic Applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Gao, L.-J.; Ren, J.-T.; Yuan, Z.-Y. Engineering g-C3N4 Based Materials for Advanced Photocatalysis: Recent Advances. Green Energy Environ. 2024, 9, 166–197. [Google Scholar] [CrossRef]
- Li, Z.; Lv, H.; Tong, K.; He, Y.; Zhai, C.; Yun, Y.; Zhu, M. Modulating the Precursors of Carbon Nitride to Boost Local Electron Delocalization for H2O2 Photosynthesis to Remove Oxytetracycline and Its Antibiotic Resistant Genes. Appl. Catal. B Environ. 2024, 345, 123690. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Zhang, H.; Song, P.; Yang, P. Effect of Precursors on Cu Particle Distribution in g-C3N4 Nanosheets towards Efficient Photocatalytic Degradation and H2 Generation. Int. J. Hydrogen Energy 2024, 68, 463–471. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, S.; Chen, X.; Chen, F.; Chen, X.; Lu, W. Convenient Fabrication of Ultrafine VOx Decorated on Porous g-C3N4 for Boosting Photocatalytic Degradation of Pharmaceuticals with Peroxymonosulfate. Surf. Interfaces 2023, 42, 103300. [Google Scholar] [CrossRef]
- Peng, X.; Chen, X.; Pang, R.; Cheng, L.; Chen, F.; Lu, W. The Impact of Polymerization Atmosphere on the Microstructure and Photocatalytic Properties of Fe-Doped g-C3N4 Nanosheets. Catalysts 2024, 14, 520. [Google Scholar] [CrossRef]
- Wu, X.; Tan, L.; Chen, G.; Kang, J.; Wang, G. g-C3N4-Based S-Scheme Heterojunction Photocatalysts. Sci. China-Mater. 2024, 67, 444–472. [Google Scholar] [CrossRef]
- Hou, S.; Gao, X.; Lv, X.; Zhao, Y.; Yin, X.; Liu, Y.; Fang, J.; Yu, X.; Ma, X.; Ma, T.; et al. Decade Milestone Advancement of Defect-Engineered g-C3N4 for Solar Catalytic Applications. Nano-Micro Lett. 2024, 16, 70. [Google Scholar] [CrossRef]
- Cong, X.; Li, A.; Guo, F.; Qin, H.; Zhang, X.; Wang, W.; Xu, W. Construction of CdS@g-C3N4 Heterojunction Photocatalyst for Highly Efficient Degradation of Gaseous Toluene. Sci. Total Environ. 2024, 913, 169777. [Google Scholar] [CrossRef]
- Deng, J.; Zeng, Y.; Almatrafi, E.; Liang, Y.; Wang, Z.; Wang, Z.; Song, B.; Shang, Y.; Wang, W.; Zhou, C.; et al. Advances of Carbon Nitride Based Atomically Dispersed Catalysts from Single-Atom to Dual-Atom in Advanced Oxidation Process Applications. Coord. Chem. Rev. 2024, 505, 215693. [Google Scholar] [CrossRef]
- Wei, F. The Future of Carbon Catalysis, Energy, Material and Engineering. Carbon Future 2024, 1, 9200006. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, W. Rational Design of Multifunctional Framework Materials for Sustainable Photocatalysis. Carbon Future 2024, 1, 9200018. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Yang, Y.; Chen, X.; Chen, F.; Lu, W. Construction of High-Density Fe Clusters Embedded in a Porous Carbon Nitride Catalyst with Effectively Selective Transformation of Benzene. ACS Sustain. Chem. Eng. 2023, 11, 1518–1526. [Google Scholar] [CrossRef]
- Kalidasan, K.; Mallapur, S.; Munirathnam, K.; Nagarajaiah, H.; Reddy, M.B.M.; Kakarla, R.R.; Raghu, A.V. Transition Metals-Doped g-C3N4 Nanostructures as Advanced Photocatalysts for Energy and Environmental Applications. Chemosphere 2024, 352, 141354. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Zhang, Y.; Lin, L.; Wang, Y.; Zhou, M.; Li, Z.; Xu, S. Reasonably Design of Hollow Spherical g-C3N4/Mn0.25Cd0.75S Heterojunction for Efficient Photocatalytic Hydrogen Production and Tetracycline Degradation. J. Environ. Chem. Eng. 2024, 12, 111956. [Google Scholar] [CrossRef]
- Cao, G.; Shen, Z.; Cui, J.; Yu, M.; Li, W. Bifunctional Activation of Peroxymonosulfate over CuS/g-C3N4 Composite for Efficient Degradation of Tetracycline Antibiotics. Chem. Eng. J. 2024, 483, 149082. [Google Scholar] [CrossRef]
- Shentu, Q.; Wu, Z.; Song, W.; Pan, S.; Zhou, Z.; Lv, W.; Song, C.; Yao, Y. Carbon Doped Boron Nitride Nanosheet as Efficient Metal-Free Catalyst for Peroxymonosulfate Activation: Important Role of B-N-C Moieties. Chem. Eng. J. 2022, 446, 137274. [Google Scholar] [CrossRef]
- Wang, L.; Rao, L.; Ran, M.; Shentu, Q.; Wu, Z.; Song, W.; Zhang, Z.; Li, H.; Yao, Y.; Lv, W.; et al. A Polymer Tethering Strategy to Achieve High Metal Loading on Catalysts for Fenton Reactions. Nat. Commun. 2023, 14, 7841. [Google Scholar] [CrossRef]
- Sun, S.; Peng, X.; Guo, X.; Chen, X.; Liu, D. Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon. Catalysts 2024, 14, 158. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Xiao, Q.; Guo, X.; Chen, F.; Liu, X.; Lu, W. Controlling Transformation of Sorbitol into Glycols over Ru-WOx Modifed Biomass-derived N-doped Carbon. J. Mater. Sci. 2024, 59, 8186. [Google Scholar] [CrossRef]
- Sun, L.; Yang, M.; Huang, J.; Yu, D.; Hong, W.; Chen, X. Freestanding Graphitic Carbon Nitride Photonic Crystals for Enhanced Photocatalysis. Adv. Funct. Mater. 2016, 26, 4943–4950. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Xin, T.; Li, Y.; Wu, X.; Zhang, G. Single-Atom V-N Charge-Transfer Bridge on Ultrathin Carbon Nitride for Efficient Photocatalytic H2 Production and Formaldehyde Oxidation under Visible Light. Chem. Eng. J. 2022, 429, 132229. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Shi, Y.; Cao, L.; Cheng, X.; Shi, W.; Chen, L.; Lin, X. A Ragged Porous Hollow Tubular Carbon Nitride towards Boosting Visible-Light Photocatalytic Hydrogen Production in Water and Seawater. Renew. Energy 2022, 188, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Fu, X.; Antonietti, M.; Wang, X. Fe-g-C3N4-Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light. J. Am. Chem. Soc 2009, 131, 11658–11659. [Google Scholar] [CrossRef]
- Wan, S.; Xu, J.; Cao, S.; Yu, J. Promoting Intramolecular Charge Transfer of Graphitic Carbon Nitride by Donor–Acceptor Modulation for Visible-light Photocatalytic H2 Evolution. Interdiscip. Mater. 2022, 1, 294–308. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jeong, Y.J.; Cho, I.S.; Park, S.-J.; Lee, C.-G.; Alvarez, P.J.J. Facile Synthesis of N Vacancy g-C3N4 Using Mg-Induced Defect on the Amine Groups for Enhanced Photocatalytic •OH Generation. J. Hazard. Mater. 2023, 449, 131046. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Guan, J.; Chen, X.; Guo, X.; Zhao, F.; Hou, T.; Mu, X. Metal-free g-C3N4 Photocatalyst by Sulfuric Acid Activation for Selective Aerobic Oxidation of Benzyl Alcohol under Visible Light. Mater. Res. Bull. 2014, 59, 84–92. [Google Scholar] [CrossRef]
- Sun, H.; Guo, F.; Pan, J.; Huang, W.; Wang, K.; Shi, W. One-Pot Thermal Polymerization Route to Prepare N-Deficient Modified g-C3N4 for the Degradation of Tetracycline by the Synergistic Effect of Photocatalysis and Persulfate-Based Advanced Oxidation Process. Chem. Eng. J. 2021, 406, 126844. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhou, Y.; Zhang, X.; Cui, H.; Cheng, G.; Tian, J. Co Doped MoS2 as Cocatalyst Considerably Improved Photocatalytic Hydrogen Evolution of g-C3N4 in an Alkalescent Environment. Chem. Eng. J. 2021, 421, 130016. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, Q.; Yang, Y.; Dong, B.; Zhao, Z. Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efffcient Hydrogenation of Quinolines under Mild Conditions. Catalysts 2024, 14, 345. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.; Hu, Y.; Xiao, Q.; Chen, X.; Lu, W. Robust Co3O4 Nanocatalysts Supported on Biomass-derived Porous N-doped Carbon Toward Low-Pressure Hydrogenation of Furfural. Front. Mater. Sci. 2023, 17, 230645. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Shi, X.; Chen, X.; Yang, Y. Breaking the activity-selectivity trade-off in photocatalytic semihydrogenation of alkynes over palladium nanoparticles with phosphate modiffcation. Appl. Catal. B Environ. Energy 2025, 365, 124948. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, H.; Wang, W.; Li, W.; Ren, Y.; Li, X. Synthesis of Fe0/Fe3O4@porous Carbon through a Facile Heat Treatment of Iron-Containing Candle Soots for Peroxymonosulfate Activation and Efficient Degradation of Sulfamethoxazole. J. Hazard. Mater. 2021, 411, 124952. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bo, S.; Qin, Y.; An, Q.; Xiao, Z.; Zhai, S. Transforming Goat Manure into Surface-Loaded Cobalt/Biochar as PMS Activator for Highly Efficient Ciprofloxacin Degradation. Chem. Eng. J. 2020, 395, 125063. [Google Scholar] [CrossRef]
- Yang, T.; Fan, S.; Li, Y.; Zhou, Q. Fe-N/C Single-Atom Catalysts with High Density of Fe-Nx Sites toward Peroxymonosulfate Activation for High-Efficient Oxidation of Bisphenol A: Electron-Transfer Mechanism. Chem. Eng. J. 2021, 419, 129590. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, Z.; Hu, Y.; Li, Y.; Ye, J.; Tong, M.; Liang, J. Insight into the Role of Fe in the Synergetic Effect of Persulfate/Sulfite and Fe2O3@g-C3N4 for Carbamazepine Degradation. Sci. Total Environ. 2022, 819, 152787. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Pang, R.; Sun, S.; Chen, X.; Chen, F.; Lu, W. One-Pot In Situ Synthesis of Porous Vanadium-Doped g-C3N4 with Improved Photocatalytic Removal of Pharmaceutical Pollutants. Catalysts 2025, 15, 206. https://doi.org/10.3390/catal15030206
Huang Y, Pang R, Sun S, Chen X, Chen F, Lu W. One-Pot In Situ Synthesis of Porous Vanadium-Doped g-C3N4 with Improved Photocatalytic Removal of Pharmaceutical Pollutants. Catalysts. 2025; 15(3):206. https://doi.org/10.3390/catal15030206
Chicago/Turabian StyleHuang, Yafeng, Rui Pang, Shanshan Sun, Xiufang Chen, Fengtao Chen, and Wangyang Lu. 2025. "One-Pot In Situ Synthesis of Porous Vanadium-Doped g-C3N4 with Improved Photocatalytic Removal of Pharmaceutical Pollutants" Catalysts 15, no. 3: 206. https://doi.org/10.3390/catal15030206
APA StyleHuang, Y., Pang, R., Sun, S., Chen, X., Chen, F., & Lu, W. (2025). One-Pot In Situ Synthesis of Porous Vanadium-Doped g-C3N4 with Improved Photocatalytic Removal of Pharmaceutical Pollutants. Catalysts, 15(3), 206. https://doi.org/10.3390/catal15030206






