Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluating the Different Biocatalysts in the Hydrolysis of Babassu Oil
2.2. Determining the Best Reaction Conditions
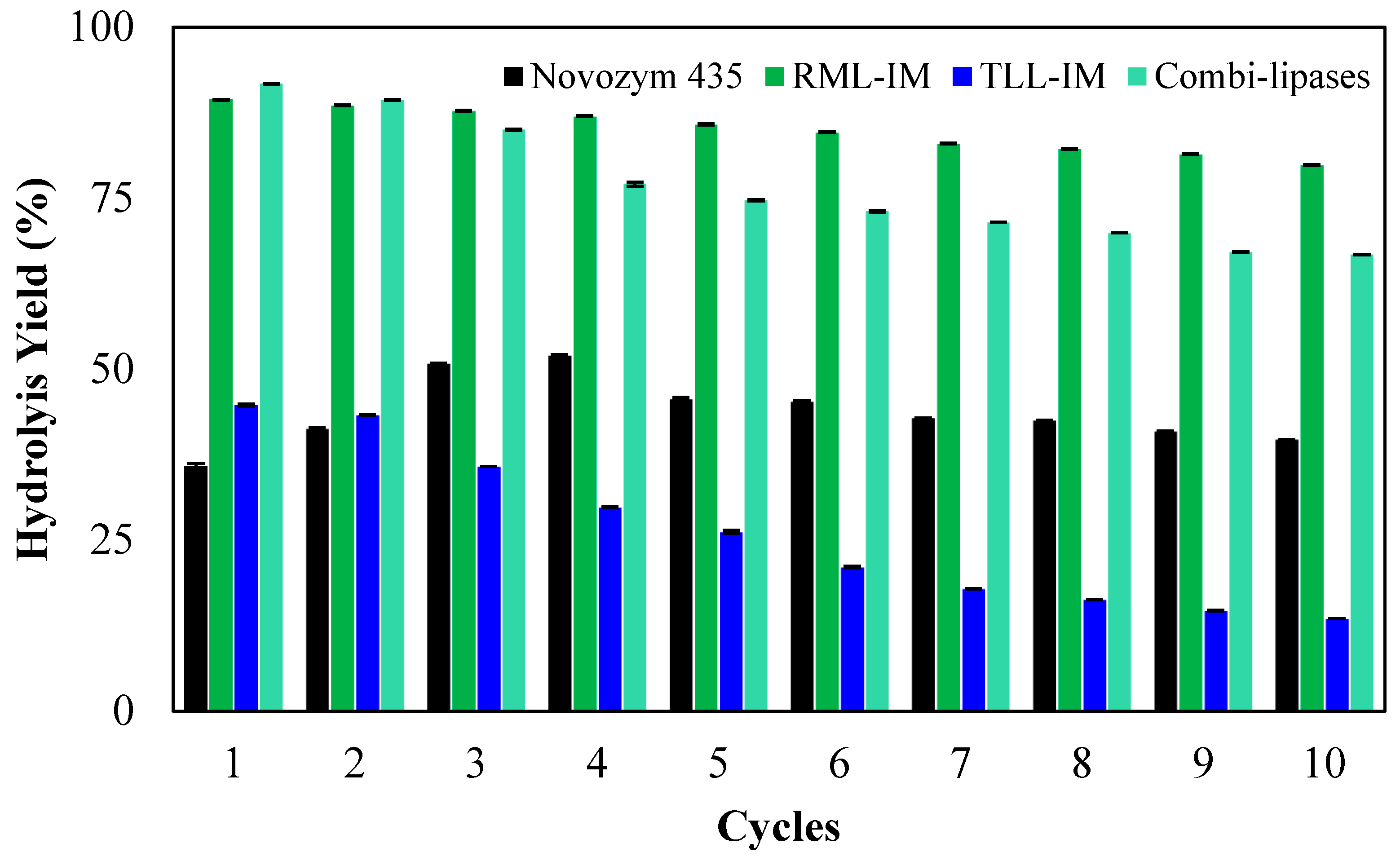
2.3. Operational Stability of the Biocatalysts
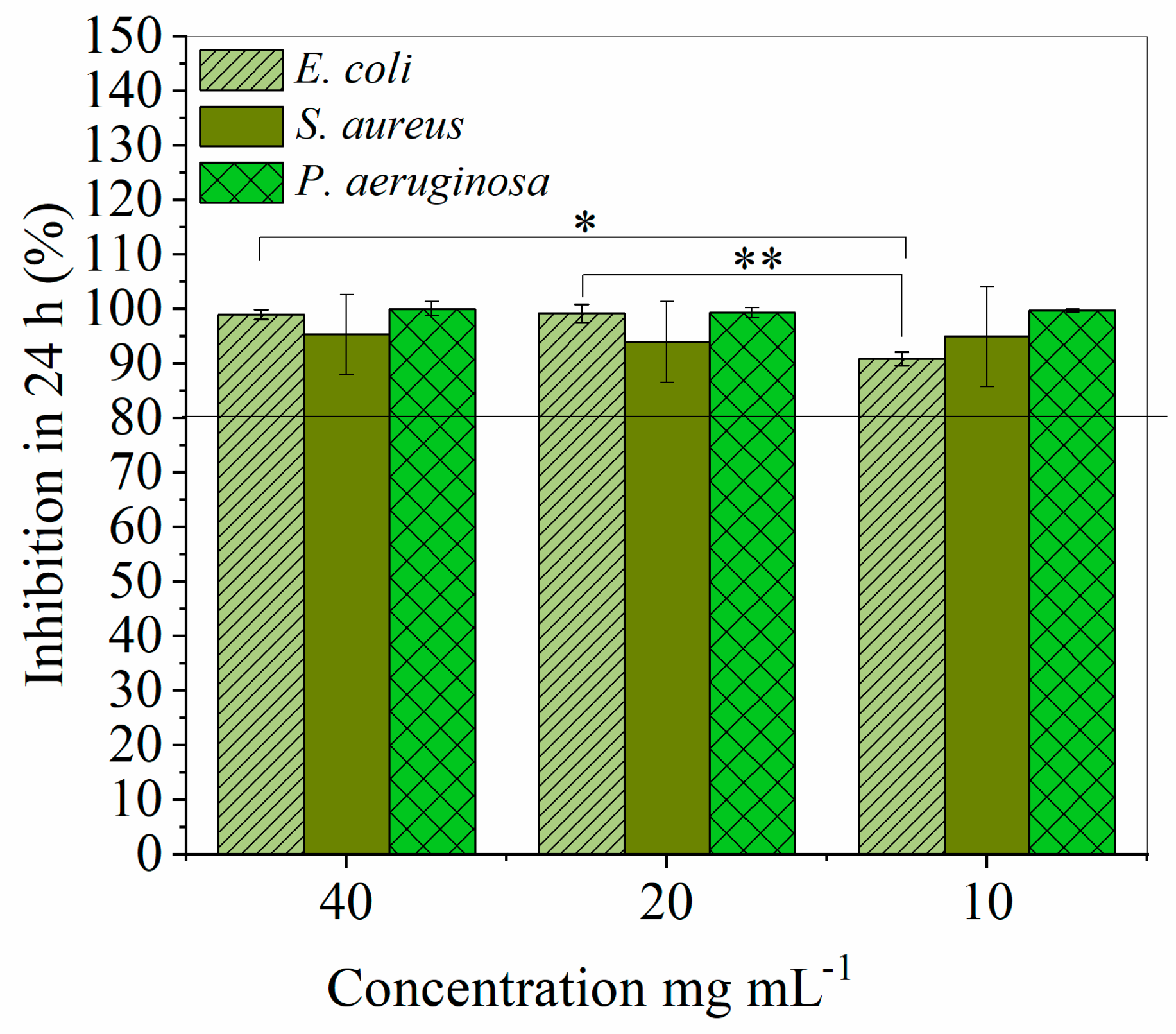
2.4. Antimicrobial Activity of the Produced FFAs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Experimental Design of the Combi-Lipase Biocatalyst
3.2.2. Experimental Design of the Reaction Conditions
3.2.3. Antimicrobial Activity of the Produced FFAs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| dF | SS | MS | F | p-Value | |
|---|---|---|---|---|---|
| Biocatalyst Content | 21.12 | 2 | 10.56 | 21.97 | 0.007 |
| {Substrate Ratio} | 1.14 | {2} | |||
| Temperature | 63.46 | 2 | 31.73 | 66.015 | 0.001 |
| {Stirring} | 0.78 | {2} |
References
- Issariyakul, T.; Dalai, A.K. Biodiesel from Vegetable Oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Samantaray, B.; Mohapatra, S.; Mishra, R.R.; Behera, B.C.; Thatoi, H. Bioethanol Production from Agro-Wastes: A Comprehensive Review with a Focus on Pretreatment, Enzymatic Hydrolysis, and Fermentation. Int. J. Green Energy 2024, 21, 1398–1424. [Google Scholar] [CrossRef]
- Thirunavukarasu, N.; Panda, R.C. Modeling, Identification, and Control for the Production of Glycerol by the Hydrolysis of Tallow. Rev. Chem. Eng. 2015, 31, 345–359. [Google Scholar] [CrossRef]
- Hartman, L. Effect of Temperature on the Twitchell Fat Splitting Process and Its Catalysts. J. Am. Oil Chem. Soc. 1953, 30, 349–350. [Google Scholar] [CrossRef]
- Hartman, L. Kinetics of the Twitchell Hydrolysis. Nature 1951, 167, 199. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From Lignocellulosic Biomass to Levulinic Acid: A Review on Acid-Catalyzed Hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Hydrolysis of Vegetable Oils and Fats to Fatty Acids over Solid Acid Catalysts. Appl. Catal. A Gen. 2011, 391, 427–435. [Google Scholar] [CrossRef]
- Stirton, A.J.; Hammaker, E.M.; Herb, S.F.; Roe, E.T. Comparison of Fat-Splitting Reagents in the Twitchell Process. Oil Soap 1944, 21, 148–151. [Google Scholar] [CrossRef]
- Goswami, D.; Basu, J.K.; De, S. Lipase Applications in Oil Hydrolysis with a Case Study on Castor Oil: A Review. Crit. Rev. Biotechnol. 2013, 33, 81–96. [Google Scholar] [CrossRef]
- Casali, B.; Brenna, E.; Parmeggiani, F.; Tessaro, D.; Tentori, F. Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes. Sustain. Chem. 2021, 2, 74–91. [Google Scholar] [CrossRef]
- Costa, C.Z.; Sousa-Aguiar, E.F.; Couto, M.A.P.G.; Filho, J.F.S. de C. Hydrothermal Treatment of Vegetable Oils and Fats Aiming at Yielding Hydrocarbons: A Review. Catalysts 2020, 10, 843. [Google Scholar] [CrossRef]
- Holliday, R.L.; King, J.W.; List, G.R. Hydrolysis of Vegetable Oils in Sub- And Supercritical Water. Ind. Eng. Chem. Res. 1997, 36, 932–935. [Google Scholar] [CrossRef]
- Peters, M.A.; Alves, C.T.; Wang, J.; Onwudili, J.A. Subcritical Water Hydrolysis of Fresh and Waste Cooking Oils to Fatty Acids Followed by Esterification to Fatty Acid Methyl Esters: Detailed Characterization of Feedstocks and Products. ACS Omega 2022, 7, 46870–46883. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xue, K.; Fan, W.; Li, C.; Nan, G.; Li, Z. Hydrolysis of Vegetable Oils to Fatty Acids Using Brønsted Acidic Ionic Liquids as Catalysts. Ind. Eng. Chem. Res. 2014, 53, 11653–11658. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Han, F.; Kang, X.; Gu, H.; Yang, L. Microwave-Assisted Method for Simultaneous Hydrolysis and Extraction in Obtaining Ellagic Acid, Gallic Acid and Essential Oil from Eucalyptus Globulus Leaves Using Brönsted Acidic Ionic Liquid [HO3S(CH2)4mim]HSO4. Ind. Crop. Prod. 2016, 81, 152–161. [Google Scholar] [CrossRef]
- Khodadadi, M.R.; Malpartida, I.; Tsang, C.-W.; Lin, C.S.K.; Len, C. Recent Advances on the Catalytic Conversion of Waste Cooking Oil. Mol. Catal. 2020, 494, 111128. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Khan, E.; Wang, L.; Ok, Y.S.; Poon, C.S. Valorization of Cellulosic Food Waste into Levulinic Acid Catalyzed by Heterogeneous BrØnsted Acids: Temperature and Solvent Effects. Chem. Eng. J. 2017, 327, 328–335. [Google Scholar] [CrossRef]
- Lu, T.; Guo, Y.; Shi, J.; Li, X.; Wu, K.; Li, X.; Zeng, Z.; Xiong, Y. Identification and Safety Evaluation of Ochratoxin A Transformation Product in Rapeseed Oil Refining Process. J. Agric. Food Chem. 2022, 70, 14931–14939. [Google Scholar] [CrossRef]
- Faillace, E.; Brunini-Bronzini de Caraffa, V.; Mariani, M.; Berti, L.; Maury, J.; Vincenti, S. Optimizing the First Step of the Biocatalytic Process for Green Leaf Volatiles Production: Lipase-Catalyzed Hydrolysis of Three Vegetable Oils. Int. J. Mol. Sci. 2023, 24, 12274. [Google Scholar] [CrossRef]
- Baena, A.; Orjuela, A.; Rakshit, S.K.; Clark, J.H. Enzymatic Hydrolysis of Waste Fats, Oils and Greases (FOGs): Status, Prospective, and Process Intensification Alternatives. Chem. Eng. Process. Process Intensif. 2022, 175, 108930. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in Lipid Modification. Annu. Rev. Food Sci. Technol. 2018, 9, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Da Silva, S.S.O.; Cavalcante, C.L.; De Luna, F.M.T.; Bolivar, J.M.; Vieira, R.S.; Fernandez-Lafuente, R. Biosynthesis of Alkanes/Alkenes from Fatty Acids or Derivatives (Triacylglycerols or Fatty Aldehydes). Biotechnol. Adv. 2022, 61, 108045. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Enzymes in Lipid Modification: From Classical Biocatalysis with Commercial Enzymes to Advanced Protein Engineering Tools. Oléagineux Corp. Gras Lipides 2013, 20, 45–49. [Google Scholar] [CrossRef]
- Murty, V.R.; Bhat, J.; Muniswaran, P.K.A. Hydrolysis of Oils by Using Immobilized Lipase Enzyme: A Review. Biotechnol. Bioprocess Eng. 2002, 7, 57–66. [Google Scholar] [CrossRef]
- Pedro, K.C.N.R.; Da Silva, G.A.R.; Da Silva, M.A.P.; Henriques, C.A.; Langone, M.A.P. Immobilization of Lipase on Zeolite, Silica, and Silica-Aluminas and Its Use in Hydrolysis, Esterification, and Transesterification Reactions. Catal. Today 2025, 447, 115141. [Google Scholar] [CrossRef]
- Syaima, M.T.S.; Ong, K.H.; Mohd Noor, I.; Zamratul, M.I.M.; Brahim, S.A.; Hafizul, M.M. The Synthesis of Bio-Lubricant Based Oil by Hydrolysis and Non-Catalytic of Palm Oil Mill Effluent (POME) Using Lipase. Renew. Sustain. Energy Rev. 2015, 44, 669–675. [Google Scholar] [CrossRef]
- Guerrero-Elias, H.Y.; Camacho-Ruiz, M.A.; Espinosa-Salgado, R.; Mateos-Díaz, J.C.; Camacho-Ruiz, R.M.; Asaff-Torres, A.; Rodríguez, J.A. Spectrophotometric Assay for the Screening of Selective Enzymes towards DHA and EPA Ethyl Esters Hydrolysis. Enzym. Microb. Technol. 2025, 182, 110531. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; De Luna, F.M.T.; Lomonaco, D.; Fernandez-Lafuente, R.; Vieira, R.S. Improving the Performance of Lipases in the Full Hydrolysis of Residual Coconut Oil by Immobilization on Hydrophobic Supports. Ind. Crop. Prod. 2024, 222, 120014. [Google Scholar] [CrossRef]
- Mhadmhan, S.; Yoosuk, B.; Henpraserttae, S. Selective Lipase-Catalyzed Hydrolysis for Removal of Diglyceride in Palm Oil. Sep. Purif. Technol. 2024, 349, 127897. [Google Scholar] [CrossRef]
- Liu, R.; Li, P.; Li, S.; Zhuang, Y.; Chen, S.; Huang, Y.; Zheng, Y.; Xiong, Y.; Wang, D.; He, M.; et al. Immobilized Burkholderia Cepacia Lipase and Its Application in Selective Enrichment of EPA by Hydrolysis of Fish Oil. LWT 2024, 206, 116598. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Lan, M.N.; Lee, S.H.; Hwang, S.M.; Koo, Y.M. Lipase-Catalyzed Biodiesel Production from Soybean Oil in Ionic Liquids. Enzym. Microb. Technol. 2007, 41, 480–483. [Google Scholar] [CrossRef]
- Gupta, S. Comparative Study on Hydrolysis of Oils by Lipase Immobilized Biocatalytic PS Membranes Using Biphasic Enzyme Membrane Reactor. J. Environ. Chem. Eng. 2016, 4, 1797–1809. [Google Scholar] [CrossRef]
- Nguyen, V.D.H.; Huynh, T.N.P.; Nguyen, T.T.T.; Ho, H.H.; Trinh, L.T.P.; Nguyen, A.Q. Expression and Characterization of a Lipase EstA from Bacillus Subtilis KM-BS for Application in Bio-Hydrolysis of Waste Cooking Oil. Protein Expr. Purif. 2024, 215, 106419. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; De Andrade Souza, L.T.; Moreno-Pérez, S.; Pinto, M.C.C.; Manoel, E.A.; De Oliveira, D.; Pessela, B.C. Application of Goat and Lamb Lipases on the Development of New Immobilized Biocatalysts Aiming at Fish Oil Hydrolysis. Appl. Biochem. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. One Pot Use of Combilipases for Full Modification of Oils and Fats: Multifunctional and Heterogeneous Substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, Z.; Wang, Y. Evolution of the Diacylglycerol Lipases. Prog. Lipid Res. 2016, 64, 85–97. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Lipase-Catalyzed Syntheses of Monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Oda, M.; Kaieda, M.; Hama, S.; Yamaji, H.; Kondo, A.; Izumoto, E.; Fukuda, H. Facilitatory Effect of Immobilized Lipase-Producing Rhizopus Oryzae Cells on Acyl Migration in Biodiesel-Fuel Production. Biochem. Eng. J. 2005, 23, 45–51. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Matsumoto, T.; Ban, K.; Kondo, A.; Shimada, Y.; Noda, H.; Nomoto, F.; Ohtsuka, K.; Izumoto, E.; et al. Biodiesel Fuel Production from Plant Oil Catalyzed by Rhizopus Oryzae Lipase in a Water-Containing System without an Organic Solvent. J. Biosci. Bioeng. 1999, 88, 627–631. [Google Scholar] [CrossRef]
- Park, Y.K.; Pastore, G.M.; de Almeida, M.M. Hydrolysis of Soybean Oil by a Combined Lipase System. J. Am. Oil Chem. Soc. 1988, 65, 252–254. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, F.; Guan, W.; Zuo, J.; Feng, D. Optimisation of Combi-Lipases from Aspergillus Niger for the Synergistic and Efficient Hydrolysis of Soybean Oil. Anim. Sci. J. 2017, 88, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, Q.; Bu, W.; Zhang, C.; Yang, Z.; Zhang, X.; Zhang, K. Ultrasound-Assisted Hydrolysis of Lard for Free Fatty Acids Catalyzed by Combined Two Lipases in Aqueous Medium. Bioengineered 2020, 11, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.E.S.; Monteiro, R.R.C.; Rocha, T.G.; Moreira, K.S.; Cavalcante, F.T.T.; de Sousa Braz, A.K.; de Souza, M.C.M.; dos Santos, J.C.S. Sonohydrolysis Using an Enzymatic Cocktail in the Preparation of Free Fatty Acid. 3 Biotech 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-Lipase for Heterogeneous Substrates: A New Approach for Hydrolysis of Soybean Oil Using Mixtures of Biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Alonazi, M.; Al-Diahan, S.K.; Alzahrani, Z.R.A.; Ben Bacha, A. Combined Immobilized Lipases for Effective Biodiesel Production from Spent Coffee Grounds. Saudi J. Biol. Sci. 2023, 30, 103772. [Google Scholar] [CrossRef]
- Zeng, L.; He, Y.; Jiao, L.; Li, K.; Yan, Y. Preparation of Biodiesel with Liquid Synergetic Lipases from Rapeseed Oil Deodorizer Distillate. Appl. Biochem. Biotechnol. 2017, 183, 778–791. [Google Scholar] [CrossRef]
- Karmee, S.K. Preparation of Biodiesel from Nonedible Oils Using a Mixture of Used Lipases. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2727–2733. [Google Scholar] [CrossRef]
- Jang, M.G.; Kim, D.K.; Park, S.C.; Lee, J.S.; Kim, S.W. Biodiesel Production from Crude Canola Oil by Two-Step Enzymatic Processes. Renew. Energy 2012, 42, 99–104. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, Y.; Li, L.; Lin, L.; Li, C.; Ye, Y. Insight into the Medium-long-medium Structured Lipids Made from Camellia Oil: Composition–Structure Relationship. J. Food Sci. 2023, 88, 3384–3397. [Google Scholar] [CrossRef]
- Huang, C.; Lin, Z.; Zhang, Y.; Liu, Z.; Tang, X.; Li, C.; Lin, L.; Huang, W.; Ye, Y. Structure-Guided Preparation of Fuctional Oil Rich in 1,3-Diacylglycerols and Linoleic Acid from Camellia Oil by Combi-Lipase. J. Sci. Food Agric. 2023, 103, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Sabir, J.S.M.; Baeshen, N.A.; Akoh, C.C. Enzymatic Synthesis of Extra Virgin Olive Oil Based Infant Formula Fat Analogues Containing ARA and DHA: One-Stage and Two-Stage Syntheses. J. Agric. Food Chem. 2013, 61, 10590–10598. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.; Eid, M.; Abou-Elmagd, W.; Abou-Elregal, M. Lipase Catalysed Transesterification of Palm Stearin with Ferulic Acid in Solvent-Free Media. Biocatal. Biotransformation 2022, 40, 378–385. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces Lanuginosus: Uses and Prospects as an Industrial Biocatalyst. J. Mol. Catal. B: Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Yousefi, M.; Marciello, M.; Guisan, J.M.; Fernandez-Lorente, G.; Mohammadi, M.; Filice, M. Fine Modulation of the Catalytic Properties of Rhizomucor Miehei Lipase Driven by Different Immobilization Strategies for the Selective Hydrolysis of Fish Oil. Molecules 2020, 25, 545. [Google Scholar] [CrossRef]
- Kotogán, A.; Furka, Z.T.; Kovács, T.; Volford, B.; Papp, D.A.; Varga, M.; Huynh, T.; Szekeres, A.; Papp, T.; Vágvölgyi, C.; et al. Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential. Foods 2022, 11, 1711. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C.; Mesa, M.; Wilson, L. Immobilization of Lipases in Hydrophobic Chitosan for Selective Hydrolysis of Fish Oil: The Impact of Support Functionalization on Lipase Activity, Selectivity and Stability. Int. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, Z.; Xia, Q.; Luo, T.; Mao, Q.; Sun, Q.; Han, Z.; Liu, Y.; Wei, S.; Liu, S. Comparative Lipidomics Profiling of Acylglycerol from Tuna Oil Selectively Hydrolyzed by Thermomyces Lanuginosus Lipase and Candida Antarctica Lipase A. Foods 2022, 11, 3664. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Freire, C.C.C.; Almeida, L.C.; Freitas, L.S.; Souza, R.L.; Pereira, E.B.; Mendes, A.A.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Optimization of the Enzymatic Hydrolysis of Moringa Oleifera Lam Oil Using Molecular Docking Analysis for Fatty Acid Specificity. Biotechnol. Appl. Biochem. 2019, 66, 823–832. [Google Scholar] [CrossRef]
- Bauer, L.C.; Santos, L.S.; Sampaio, K.A.; Ferrão, S.P.B.; Fontan, R.d.C.I.; Minim, L.A.; Veloso, C.M.; Bonomo, R.C.F. Physicochemical and Thermal Characterization of Babassu Oils (Orbignya Phalerata Mart.) Obtained by Different Extraction Methods. Food Res. Int. 2020, 137, 109474. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A. Babassu-A New Approach for an Ancient Brazilian Biomass. Biomass Bioenergy 2008, 32, 857–864. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Mitja, D.; Delaître, E.; Demagistri, L.; de Souza Miranda, I.; Libourel, T.; Petit, M. Estimating Babassu Palm Density Using Automatic Palm Tree Detection with Very High Spatial Resolution Satellite Images. J. Environ. Manag. 2017, 193, 40–51. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Total Production and Sales Related to Babassu Oil in Brazil in 2023. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/babacu/br (accessed on 15 November 2024).
- Paiva, E.J.M.; Da Silva, M.L.C.P.; Barboza, J.C.S.; De Oliveira, P.C.; De Castro, H.F.; Giordani, D.S. Non-Edible Babassu Oil as a New Source for Energy Production-a Feasibility Transesterification Survey Assisted by Ultrasound. Ultrason. Sonochem. 2013, 20, 833–838. [Google Scholar] [CrossRef]
- Meira, M.; Quintella, C.M.; Ribeiro, E.M.O.; Silva, H.R.G.; Guimarães, A.K. Overview of the Challenges in the Production of Biodiesel. Biomass Convers. Biorefinery 2015, 5, 321–329. [Google Scholar] [CrossRef]
- De Medeiros Melo Neto, O.; Minervina Silva, I.; De Figueiredo Lopes Lucena, L.C.; De Figueiredo Lopes Lucena, L.; De Sousa, T.M.; Wan Hu, J.; Youssef, A. Assessment of Rheological Properties of Asphalt Binder Modified with Babassu Oil. Case Stud. Constr. Mater. 2024, 21, e03599. [Google Scholar] [CrossRef]
- Da Silva, M.J.F.; Rodrigues, A.M.; Costa, M.C.P.; Camara, A.L.; Cabral, L.M.; Ricci Junior, E.; Vanzan, D.F.; Matos, A.P.D.S.; Da Silva Honorio, T.; Borges, A.C.R. Solid Lipid Nanoparticles Based on Babassu Oil and Copaiba Oleoresin: A Promising Approach for Prostate Cancer Therapy. Nanomaterials 2024, 14, 1014. [Google Scholar] [CrossRef]
- Lima, R.C.; Carvalho, A.P.A.D.; Almeida, A.E.C.C.D.; Conte-Junior, C.A. Bioactive Compounds and Benefits of By-Products of Amazon Babassu Oil Production: Potential for Dietary Supplement, Biomedical and Food Applications. Food Funct. 2024, 15, 6232–6253. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nitti, F.; Jumina, J.; Detha, A.I.R. Antimicrobial Properties of Lauric Acid and Monolaurin in Virgin Coconut Oil: A Review. ChemBioEng Rev. 2022, 9, 442–461. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Hu, X.; Yao, X.; Han, L.; Wu, X.; Li, R.; Wen, T.; Ming, L. Lauric Acid Induces Apoptosis of Rice Sheath Blight Disease Caused by Rhizoctonia Solani by Affecting Fungal Fatty Acid Metabolism and Destroying the Dynamic Equilibrium of Reactive Oxygen Species. J. Fungi 2022, 8, 153. [Google Scholar] [CrossRef]
- Do Couto, M.V.S.; Da Costa Sousa, N.; Paixão, P.E.G.; Dos Santos Medeiros, E.; Abe, H.A.; Meneses, J.O.; Cunha, F.S.; Filho, R.M.N.; De Sousa, R.C.; Maria, A.N.; et al. Is There Antimicrobial Property of Coconut Oil and Lauric Acid against Fish Pathogen? Aquaculture 2021, 545, 737234. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Park, S.; Kim, S.; Lee, J. Inhibition of Polymicrobial Biofilm Formation by Saw Palmetto Oil, Lauric Acid and Myristic Acid. Microb. Biotechnol. 2022, 15, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Gao, W.; Ge, T.; Tan, X.; Wang, J.; Liu, H.; Wang, Y.; Han, C.; Xu, Q.; Wang, Q. Lauric Acid Is a Potent Biological Control Agent That Damages the Cell Membrane of Phytophthora Sojae. Front. Microbiol. 2021, 12, 666761. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Lee, S.W. The Antibacterial Effect of Fatty Acids on Helicobacter Pylori Infection. Korean J. Intern. Med. 2016, 31, 30–35. [Google Scholar] [CrossRef]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 2017, 5, 170. [Google Scholar] [CrossRef]
- Jankovic, A.; Chaudhary, G.; Goia, F. Energy & Buildings Designing the Design of Experiments (DOE)—An Investigation on the Influence of Different Factorial Designs on the Characterization of Complex Systems. Energy Build. 2021, 250, 111298. [Google Scholar] [CrossRef]
- Durakovic, B. Design of Experiments Application, Concepts, Examples: State of the Art. Period. Eng. Nat. Sci. 2017, 5, 421–439. [Google Scholar] [CrossRef]
- Weissman, S.A.; Anderson, N.G. Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Results in Engineering Response Surface Methodology (RSM) for Optimizing Engine Performance and Emissions Fueled with Biofuel: Review of RSM for Sustainability Energy Transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Buruk Sahin, Y.; Aktar Demirtaş, E.; Burnak, N. Mixture Design: A Review of Recent Applications in the Food Industry. Pamukkale Univ. J. Eng. Sci. 2016, 22, 297–304. [Google Scholar] [CrossRef]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi Methodology as a Statistical Tool for Biotechnological Applications: A Critical Appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Ilzarbe, L.; Álvarez, M.J.; Viles, E.; Tanco, M. Practical Applications of Design of Experiments in the Field of Engineering: A Bibliographical Review. Qual. Reliab. Eng. Int. 2008, 24, 417–428. [Google Scholar] [CrossRef]
- Kapur, K.C.; Chen, G. Signal-to-noise Ratio Development for Quality Engineering. Qual. Reliab. Eng. Int. 1988, 4, 133–141. [Google Scholar] [CrossRef]
- Altekar, M.; Homon, C.A.; Kashem, M.A.; Mason, S.W.; Nelson, R.M.; Patnaude, L.A.; Yingling, J.; Taylor, P.B. Assay Optimization: A Statistical Design of Experiments Approach. Clin. Lab. Med. 2007, 27, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Georgantzinos, S.K.; Kastanos, G.; Tseni, A.D.; Kostopoulos, V. Efficient Optimization of the Multi-Response Problem in the Taguchi Method through Advanced Data Envelopment Analysis Formulations Integration. Comput. Ind. Eng. 2024, 197. [Google Scholar] [CrossRef]
- Karazi, S.M.; Moradi, M.; Benyounis, K.Y. Statistical and Numerical Approaches for Modeling and Optimizing Laser Micromachining Process-Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780128035818. [Google Scholar]
- Chowdhury, A.; Chakraborty, R.; Mitra, D.; Biswas, D. Optimization of the Production Parameters of Octyl Ester Biolubricant Using Taguchi’s Design Method and Physico-Chemical Characterization of the Product. Ind. Crop. Prod. 2014, 52, 783–789. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ayub, M.A.Z. Effects of the Combined Use of Thermomyces Lanuginosus and Rhizomucor Miehei Lipases for the Transesterification and Hydrolysis of Soybean Oil. Process Biochem. 2011, 46, 682–688. [Google Scholar] [CrossRef]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; de Lima Camargo Giordano, R.; Tardioli, P.W. Lipase-Catalyzed Production of Biodiesel by Hydrolysis of Waste Cooking Oil Followed by Esterification of Free Fatty Acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Corradini, F.A.S.; Alves, E.S.; Kopp, W.; Ribeiro, M.P.A.; Mendes, A.A.; Tardioli, P.W.; Giordano, R.C.; Giordano, R.L.C. Kinetic Study of Soybean Oil Hydrolysis Catalyzed by Lipase from Solid Castor Bean Seeds. Chem. Eng. Res. Des. 2019, 144, 115–122. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Hasan, M.; Ramachandran, K.B. Kinetics of the Enzymatic Hydrolysis of Palm Oil by Lipase. Process Biochem. 2003, 38, 1155–1163. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Chang, C. -S Kinetics of Lipase-catalyzed Hydrolysis of Lipids in Biphasic Organic—Aqueous Systems. J. Chem. Technol. Biotechnol. 1993, 57, 147–154. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of High-Pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Santos, K.C.; Cassimiro, D.M.J.; Avelar, M.H.M.; Hirata, D.B.; de Castro, H.F.; Fernández-Lafuente, R.; Mendes, A.A. Characterization of the Catalytic Properties of Lipases from Plant Seeds for the Production of Concentrated Fatty Acids from Different Vegetable Oils. Ind. Crop. Prod. 2013, 49, 462–470. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Li, X. A Novel and Rapid Method for Fatty Acid Preparation by the Lipase-Catalyzed Hydrolysis of Phoenix Tree Seeds. 3 Biotech 2018, 8, 403. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Preparation of a Six-Enzyme Multilayer Combi-Biocatalyst: Reuse of the Most Stable Enzymes after Inactivation of the Least Stable One. ACS Sustain. Chem. Eng. 2022, 10, 3920–3934. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Coimmobilization of Lipases Exhibiting Three Very Different Stability Ranges. Reuse of the Active Enzymes and Selective Discarding of the Inactivated Ones. Int. J. Biol. Macromol. 2022, 206, 580–590. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Cortés Corberan, V.; Fernandez-Lafuente, R. Multi-Combilipases: Co-Immobilizing Lipases with Very Different Stabilities Combining Immobilization via Interfacial Activation and Ion Exchange. The Reuse of the Most Stable Co-Immobilized Enzymes after Inactivation of the Least Stable Ones. Catalysts 2020, 10, 1207. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterling, R.; Rocha-Martin, J.; Fernandez-Lafuente, R. The Combination of Covalent and Ionic Exchange Immobilizations Enables the Coimmobilization on Vinyl Sulfone Activated Supports and the Reuse of the Most Stable Immobilized Enzyme. Int. J. Biol. Macromol. 2022, 199, 51–60. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- De Albuquerque, T.L.; Rueda, N.; Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy Stabilization of Interfacially Activated Lipases Using Heterofunctional Divinyl Sulfone Activated-Octyl Agarose Beads. Modulation of the Immobilized Enzymes by Altering Their Nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Alcántara, A.R.; Tardioli, P.W.; Fernandez-Lafuente, R. Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces Lanuginosus and Covalently Attached to the Support. Catalysts 2023, 13, 108. [Google Scholar] [CrossRef]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Sánchez-Montero, J.M.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R. Advantages of Heterofunctional Octyl Supports: Production of 1,2-Dibutyrin by Specific and Selective Hydrolysis of Tributyrin Catalyzed by Immobilized Lipases. ChemistrySelect 2016, 1, 3259–3270. [Google Scholar] [CrossRef]
- Machado, J.F.; Costa, M.D.S.; Tintino, S.R.; Rodrigues, F.F.G.; Nobre, C.B.; Coutinho, H.D.M.; Da Costa, J.G.M.; De Menezes, I.R.A.; Sousa, E.O. De Antibiotic Activity Potentiation and Physicochemical Characterization of the Fixed Orbignya Speciosa Almond Oil against Mdr Staphylococcus Aureus and Other Bacteria. Antibiotics 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.F.; Parente, E.J.S.; Manrique-Rueda, E.D.; Cavalcante, C.L.; Luna, F.M.T. Fatty Acid Alkyl Esters Obtained from Babassu Oil Using C1–C8 Alcohols and Process Integration into a Typical Biodiesel Plant. Chem. Eng. Res. Des. 2020, 160, 224–232. [Google Scholar] [CrossRef]
- Rooney, D.; Weatherley, L.R. The Effect of Reaction Conditions upon Lipase Catalysed Hydrolysis of High Oleate Sunflower Oil in a Stirred Liquid-Liquid Reactor. Process Biochem. 2001, 36, 947–953. [Google Scholar] [CrossRef]
- Mattos, F.R.; Júnior, J.M.; Sabi, G.J.; Garcia, P.H.D.; Carvalho, P.O.; Luiz, J.H.H.; Mendes, A.A. Design of a New Chemoenzymatic Process for Producing Epoxidized Monoalkyl Esters from Used Soybean Cooking Oil and Fusel Oil. Catalysts 2023, 13, 543. [Google Scholar] [CrossRef]
- MO7-A10; Clinical and Laboratory Standards Institute (CLSI) M07-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—Tenth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; pp. 15–47.

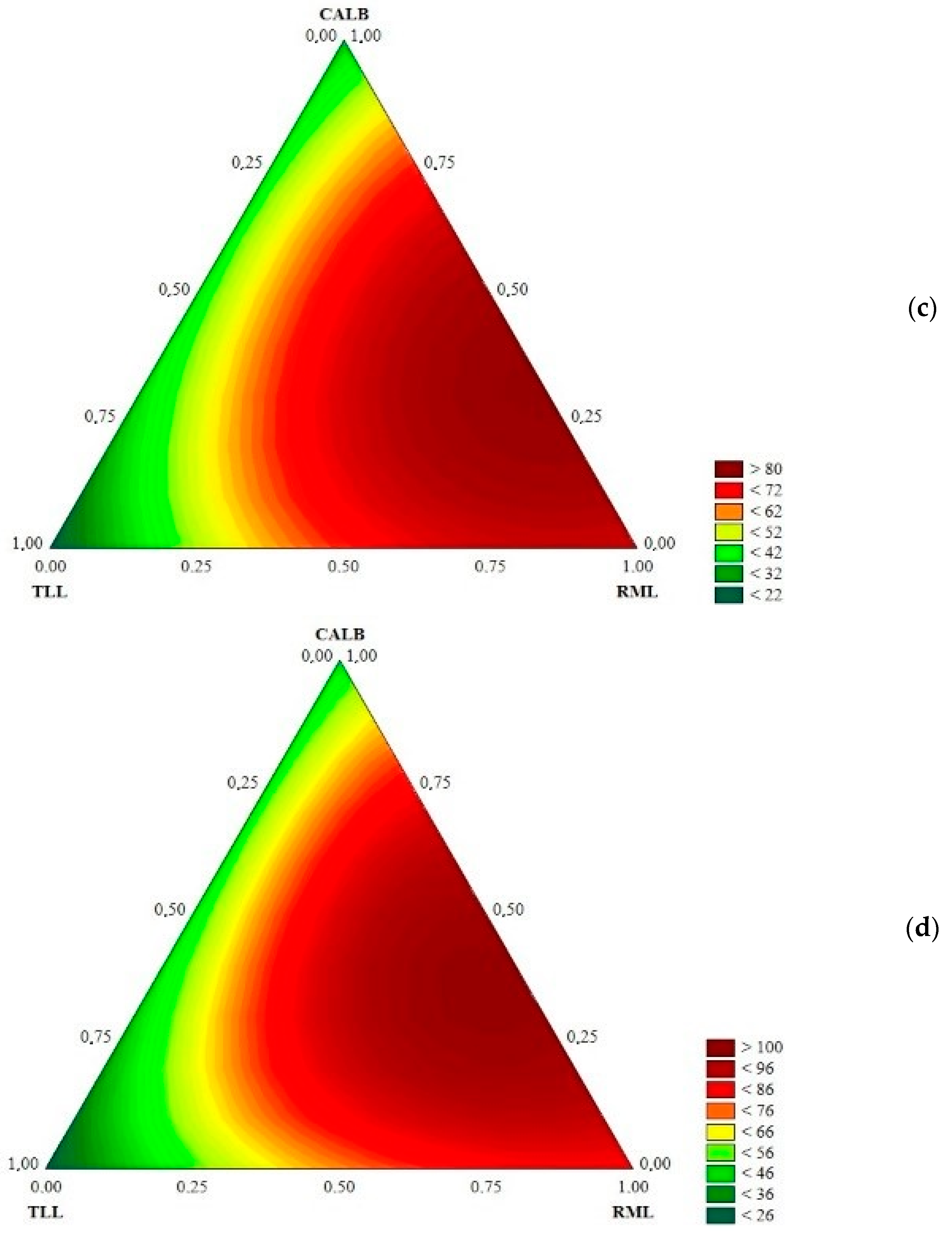
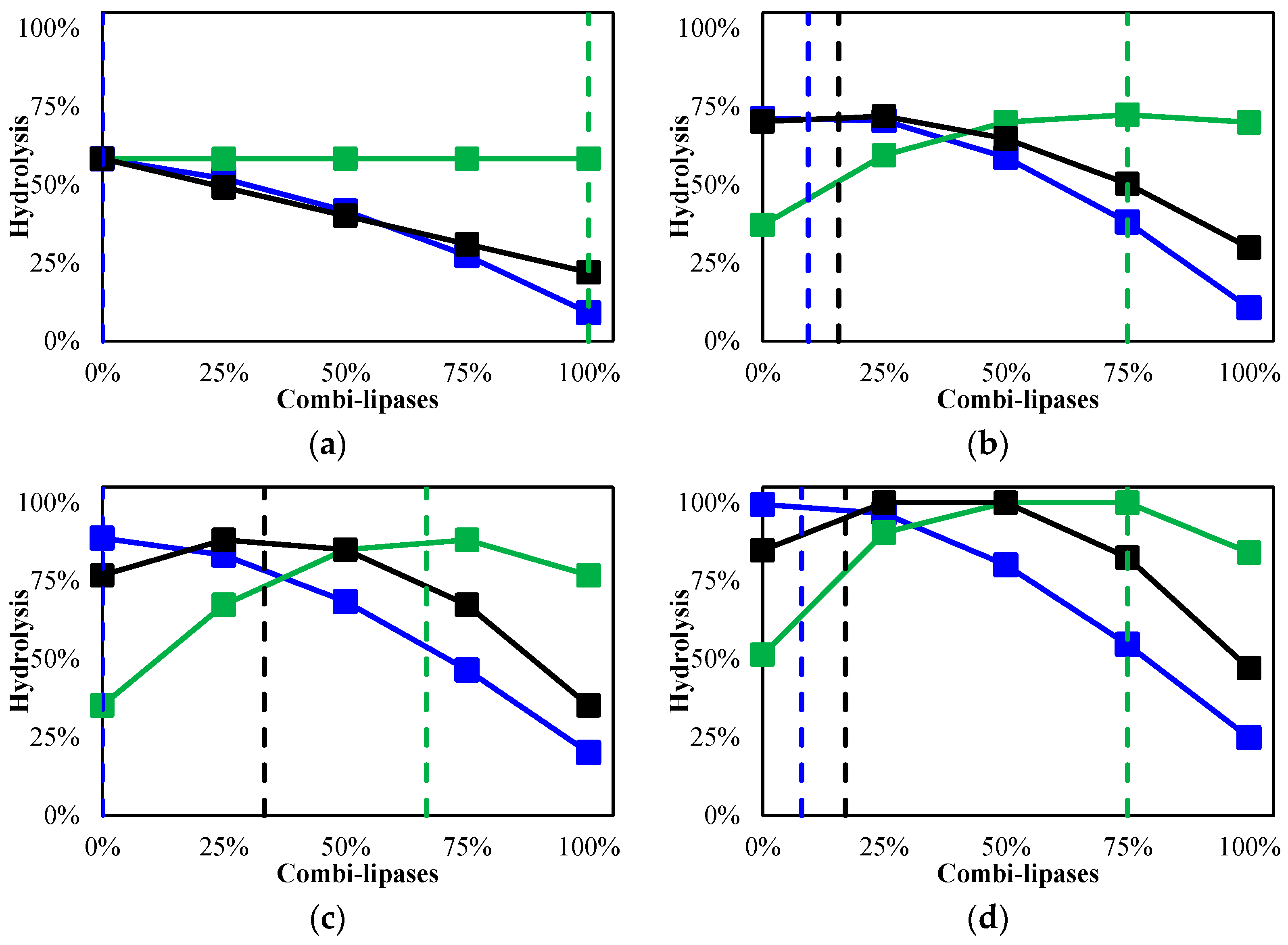

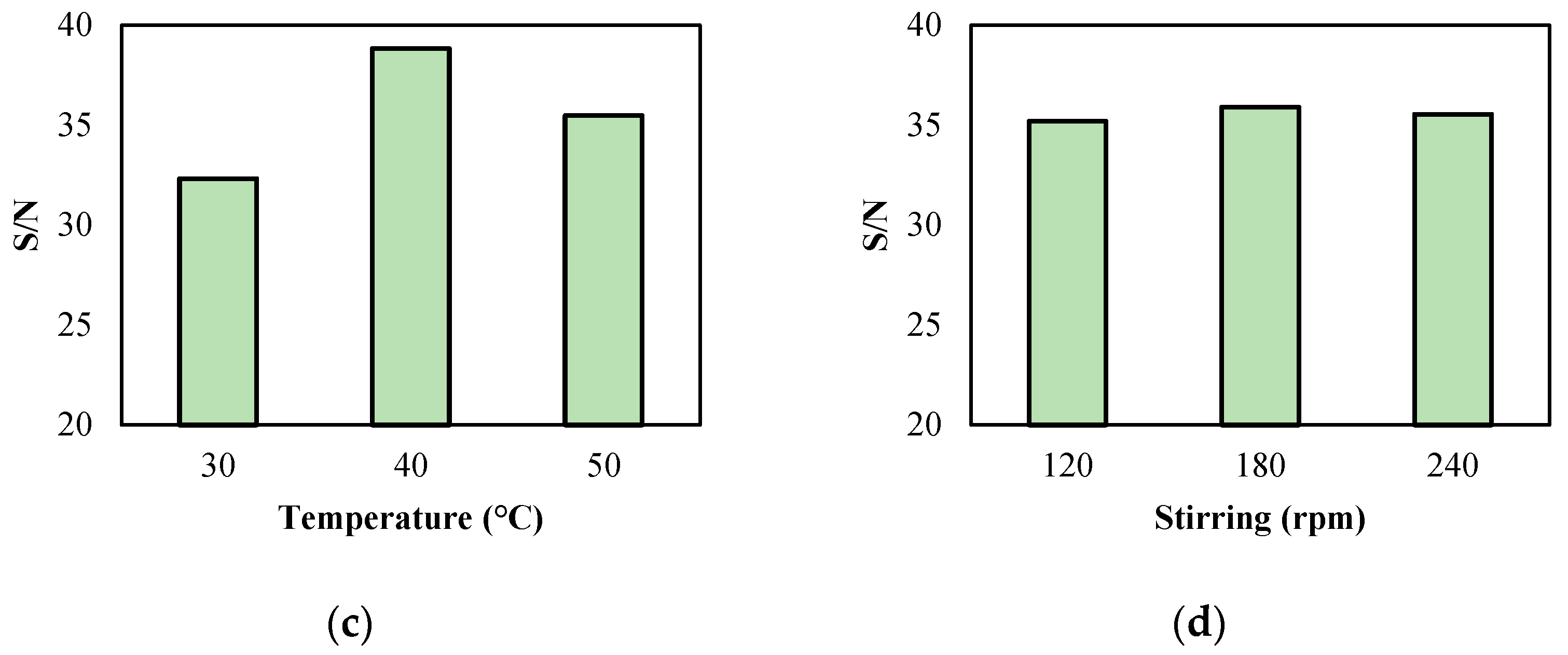
| Run | TLL-IM | RML-IM | Novozym 435 | Hydrolysis (%) | |||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | ||||
| 1 | 100.00 | 0.00 | 0.00 | 10.2 ± 0.2 | 11.1 ± 0.2 | 19.8 ± 0.4 | 24.5 ± 0.5 |
| 2 | 0.00 | 100.00 | 0.00 | 58.1 ± 2.4 | 70.8 ± 3.1 | 78.2 ± 3.5 | 85.0 ± 3.7 |
| 3 | 0.00 | 0.00 | 100.00 | 20.9 ± 0.4 | 27.3 ± 0.5 | 32.8 ± 0.7 | 43.5 ± 1.4 |
| 4 | 50.00 | 50.00 | 0.00 | 42.5 ± 1.2 | 58.9 ± 2.5 | 68.3 ± 2.7 | 73.4 ± 3.4 |
| 5 | 50.00 | 0.00 | 50.00 | 21.1 ± 0.4 | 33.5 ± 0.6 | 39.1 ± 0.9 | 45.1 ± 1.4 |
| 6 | 0.00 | 50.00 | 50.00 | 38.8 ± 0.8 | 62.5 ± 2.6 | 84.1 ± 3.6 | 99.6 ± 4.4 |
| 7 | 33.33 | 33.33 | 33.33 | 43.0 ± 1.2 | 59.5 ± 2.5 | 72.3 ± 3.1 | 85.2 ± 3.7 |
| 8 | 66.67 | 16.67 | 16.67 | 25.9 ± 0.5 | 43.0 ± 1.3 | 54.2 ± 2.3 | 66.6 ± 2.5 |
| 9 | 16.67 | 66.67 | 16.67 | 52.8 ± 2.2 | 71.0 ± 3.1 | 82.1 ± 3.6 | 99.9 ± 4.5 |
| 10 | 16.67 | 16.67 | 66.67 | 36.2 ± 0.8 | 60.6 ± 2.5 | 71.2 ± 3.1 | 93.4 ± 4.1 |
| Run | Biocatalyst Content (wt.%) | Substrates Ratio (Oil/Water) | Temperature (°C) | Stirring (rpm) | Hydrolysis (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1:1 | 30 | 120 | 29.8 ± 0.5 |
| 2 | 1 | 1:2 | 40 | 180 | 75.7 ± 3.3 |
| 3 | 1 | 1:3 | 50 | 240 | 46.3 ± 1.3 |
| 4 | 5 | 1:1 | 40 | 240 | 88.6 ± 3.9 |
| 5 | 5 | 1:2 | 50 | 120 | 63.9 ± 2.4 |
| 6 | 5 | 1:3 | 30 | 180 | 45.3 ± 1.3 |
| 7 | 9 | 1:1 | 50 | 180 | 70.8 ± 3.3 |
| 8 | 9 | 1:2 | 30 | 240 | 52.2 ± 1.6 |
| 9 | 9 | 1:3 | 40 | 120 | 99.5 ± 4.6 |
| Level | Biocatalyst Content | Substrate Ratio | Temperature | Stirring |
|---|---|---|---|---|
| Level 1 | 33.46 | 35.15 | 32.32 | 35.19 |
| Level 2 | 36.06 | 36.01 | 38.83 | 35.91 |
| Level 3 | 37.10 | 35.46 | 35.47 | 35.53 |
| Delta | 3.64 | 0.86 | 6.50 | 0.72 |
| Ranking | 2 | 3 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.P.S.; Araujo, L.L.; Oliveira, A.A., Jr.; da Silva, T.F.; Rocha, T.G.; Fernandez-Lafuente, R.; Monteiro, R.R.C.; Vieira, R.S. Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases. Catalysts 2025, 15, 209. https://doi.org/10.3390/catal15030209
Santos RPS, Araujo LL, Oliveira AA Jr., da Silva TF, Rocha TG, Fernandez-Lafuente R, Monteiro RRC, Vieira RS. Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases. Catalysts. 2025; 15(3):209. https://doi.org/10.3390/catal15030209
Chicago/Turabian StyleSantos, Rayan P. S., Lucas L. Araujo, Airton A. Oliveira, Jr., Thamyres F. da Silva, Thales G. Rocha, Roberto Fernandez-Lafuente, Rodolpho R. C. Monteiro, and Rodrigo S. Vieira. 2025. "Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases" Catalysts 15, no. 3: 209. https://doi.org/10.3390/catal15030209
APA StyleSantos, R. P. S., Araujo, L. L., Oliveira, A. A., Jr., da Silva, T. F., Rocha, T. G., Fernandez-Lafuente, R., Monteiro, R. R. C., & Vieira, R. S. (2025). Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases. Catalysts, 15(3), 209. https://doi.org/10.3390/catal15030209









