Recent Advances in Biochar Production, Characterization, and Environmental Applications
Abstract
1. Introduction
2. History of Biochar
3. Synthetic Protocols of Biochar
3.1. Pyrolysis
| S. No. | Biomass | Pyrolysis Condition | Biochar Yield, % | Application | References | |||
|---|---|---|---|---|---|---|---|---|
| Heating Source | Temperature, °C | Heating Rate, °C/min | Residence Time, Minute | |||||
| 1 | Rice husk | Thermal | 300 | 20 | 90 | 37.71 | - | [24] |
| 2 | Rape straw | Thermal | 500 | 10 | 120 | - | Pb removal | [16] |
| 3 | Litchi seeds | Thermal | 700 | 10 | 120 | - | Fabrication of supercapacitor | [22] |
| 4 | Cashew nutshell | Thermal | 500 | Fast | Minimal | 26–28 | CO2 adsorbent | [25] |
| 5 | Sewage sludge and bamboo waste (4:1) | Thermal | 700 | 10 | 30 | - | Ciprofloxacin adsorption | [26] |
| 6 | Straw | Thermal | 500 | 10 | - | - | Co-combustion with coal | [19] |
| 7 | Pinewood | Thermal | 500 | Fast | Minimal | - | A catalyst for green needle coke production | [27] |
| 8 | Wood biomass + Coal | Thermal | 350 | 10 | 20 | - | Fuel cell | [21] |
| 9 | Corn straw + seaweed | Thermal | 400 | - | 120 | 31.6 | Soil amendment | [15] |
| 10 | Rice husk | Thermal | 500 | 10 | 60 | - | Pb2+ and Cu2+ adsorption | [17] |
| 11 | Cotton textile waste | Thermal | 450 | 5 | 120 | - | Activated carbon | [28] |
| 12 | Citrus peel fruit waste | Thermal | 300–700 | 5 | 60 | 53.62–22.01 | Solid biofuel | [20] |
| 13 | Wheat straw | Microwave (100 to 600 W) | - | - | - | 24.25–74.66 | Adsorption of heavy metals (Cu2+, Cd2+, Pb2+) | [18] |
| 14 | Sugarcane bagasse | Thermal | 600 | 10 | 60 | - | Phenol adsorption | [29] |
| 15 | Lignin | Thermal | 750 | 10 | 120 | - | Methyl orange adsorption | [6] |
| 16 | Canola straw | Microwave | 300–500 | - | 30 | 41.9–29.8 | - | [14] |
| 17 | Wheat straw | Microwave | 300–500 | - | 30 | 43.3–31 | - | [14] |
| 18 | Corn stalk | Microwave | 400–600 | - | 45 | 36.4–24.8 | - | [30] |
| 19 | Pinewood | Microwave | 400–600 | - | 45 | 33.1–19.3 | - | [30] |
| 20 | Algae | Microwave | 400–600 | - | 45 | 13.4–10.8 | - | [30] |
3.2. Hydrothermal Liquefaction
| S. No. | Biomass | HTL Condition | Biochar Yield, % | Application | References | |||
|---|---|---|---|---|---|---|---|---|
| Heating Source | Temperature, °C | Pressure | Residence Time, Minute | |||||
| 1 | Rice straw | Thermal | 350 | 18 MPa | 30 | 36.4 | Fertilizer | [33] |
| 2 | Corn stalk | Thermal | 190–240 | - | 30 | 56.96–42.31 | Solid fuel | [34] |
| 3 | Green waste | Microwave | 190 | - | 60 | - | Solid fuel and adsorbent | [42] |
| 4 | Sunflower stalk | Thermal | 230 | - | 1440 | - | Supercapacitor | [37] |
| 5 | Corncob residue | Thermal | 230 | - | 60 | 48 | Solid fuel | [43] |
| 6 | Olive tree | Thermal | 200 | - | 60 | 58.2 | Solid fuel | [44] |
| 7 | Beech wood | Thermal | 220 | 45 bars | 300 | 56 | Activated carbon | [41] |
| 8 | Cotton | Thermal | 250 | - | 180 | - | Heavy metal Adsorption (Pb2+, Cd2+) | [38] |
| 9 | Wood dust | Thermal | 180 | - | 600 | - | Metal Adsorption (Cr, Sb) | [39] |
| 10 | Pinecone | Thermal | 200 | - | 300 | - | Adsorption (Pb, Cd) | [40] |
3.3. Gasification
3.4. Torrefaction
3.5. Solvothermal Liquefaction
4. Characterization of Biochar
4.1. Fourier Transform Infrared (FTIR) Spectroscopy
4.2. Raman Spectroscopy
4.3. Scanning Electron Microscopy (SEM)
4.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.5. X-Ray Diffraction (XRD)
4.6. Brunauer–Emmett–Teller Analysis
4.7. X-Ray Photoelectron Spectroscopy (XPS)
4.8. Thermogravimetric Analysis (TGA)
5. Applications of Biochar
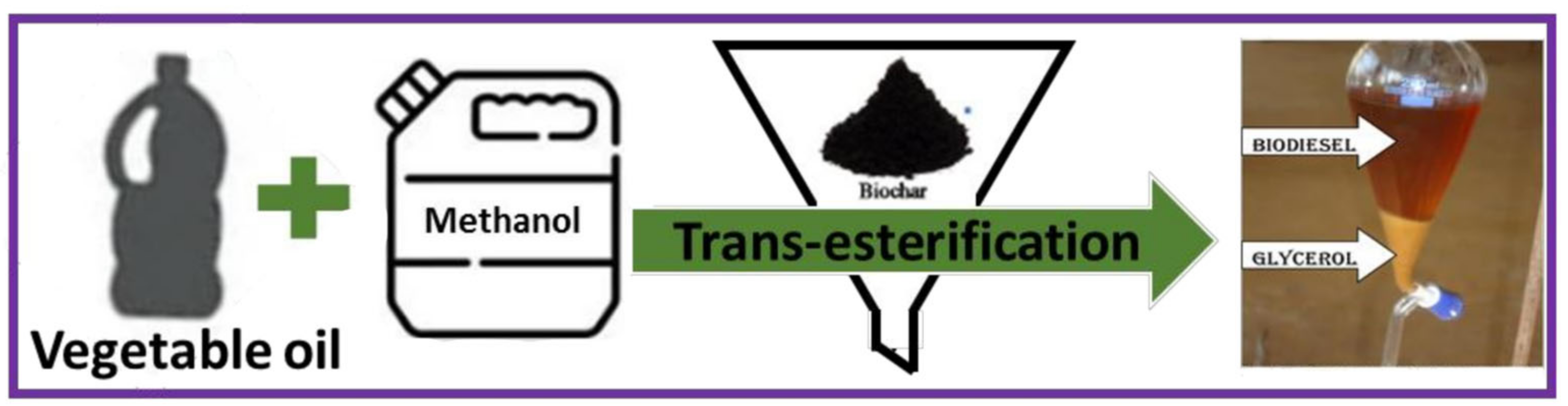
5.1. Biodiesel Synthesis
5.2. Catalyst Support
5.3. Soil Remediation
5.3.1. Adsorption and Ion Exchange Mechanism
5.3.2. Microbial Activity Enhancement
5.3.3. Improvement in Soil Structure
5.3.4. Long-Term Soil Stability
5.3.5. Soil Carbon Sequestration
5.4. Wastewater Treatment
5.4.1. Odor Control
5.4.2. Biochar Filters
5.4.3. Adsorption of Heavy Metals
5.4.4. Removal of Dye and Phosphorous Compounds
5.5. Storage Devices and Supercapacitors
5.5.1. Thermal Energy Storage
5.5.2. Biochar-Based Batteries
5.5.3. Biochar-Based Supercapacitors
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- International Biochar Initiative. International Biochar Initiative Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Int. Biochar Initiat. 2012, 1–47. [Google Scholar]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A.; Al-Wabel, M.I.; Oleszczuk, P.; Lee, S.S. The effects of biochar amendment on soil fertility. Agric. Environ. Appl. Biochar Adv. barriers 2016, 63, 123–144. [Google Scholar]
- Zhang, Y.; Maierdan, Y.; Guo, T.; Chen, B.; Fang, S.; Zhao, L. Biochar as carbon sequestration material combines with sewage sludge incineration ash to prepare lightweight concrete. Constr. Build. Mater. 2022, 343, 128116. [Google Scholar] [CrossRef]
- Saini, K.; Sahoo, A.; Biswas, B.; Kumar, A.; Bhaskar, T. Preparation and characterization of lignin-derived hard templated carbon (s): Statistical optimization and methyl orange adsorption isotherm studies. Bioresour. Technol. 2021, 342, 125924. [Google Scholar] [CrossRef]
- Hunt, J.; Duponte, M.; Sato, D.; Kawabata, A. The Basics of Biochar: A Natural Soil Amendment. Soil Crop Manag. 2010, 30, 1–6. [Google Scholar]
- Biochar for Environmental Management. 2012. Available online: https://www.css.cornell.edu/faculty/lehmann/publ/First%20proof%2013-01-09.pdf (accessed on 23 February 2025).
- Lehmann, J. Terra Preta Nova—Where to from Here. In BT—Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A., Rebellato, L., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 473–486. ISBN 978-1-4020-9031-8. [Google Scholar]
- Fryda, L.; Visser, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Safarian, S. Performance analysis of sustainable technologies for biochar production: A comprehensive review. Energy Rep. 2023, 9, 4574–4593. [Google Scholar] [CrossRef]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- Uddin, M.N.; Daud, W.M.A.W.; Abbas, H.F. Effects of pyrolysis parameters on hydrogen formations from biomass: A review. Rsc Adv. 2014, 4, 10467–10490. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Arshad, M.; Ulah, A.; Naeth, M.A.; Chang, S.X. Fuel, thermal and surface properties of microwave-pyrolyzed biochars depend on feedstock type and pyrolysis temperature. Bioresour. Technol. 2021, 320, 124282. [Google Scholar] [CrossRef] [PubMed]
- Suo, F.; You, X.; Yin, S.; Wu, H.; Zhang, C.; Yu, X.; Sun, R.; Li, Y. Preparation and characterization of biochar derived from co-pyrolysis of Enteromorpha prolifera and corn straw and its potential as a soil amendment. Sci. Total Environ. 2021, 798, 149167. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Fu, Q.; Hu, H.; Wang, Q.; Liu, Y.; Zhu, J. Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J. Hazard. Mater. 2019, 371, 191–197. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of different biomass species and pyrolysis temperatures on heavy metal adsorption, stability and economy of biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Chang, S.; Wang, H.; Wang, M.; Xiang, W.; Gao, B. Microwave biochar produced with activated carbon catalyst: Characterization and adsorption of heavy metals. Environ. Res. 2023, 216, 114732. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.; Wang, W.; Liu, T.; Liu, E.; Liu, H.; Li, Z. Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renew. Energy 2020, 161, 867–877. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Eom, S.Y.; Rhie, Y.H.; Sung, Y.M.; Moon, C.E.; Choi, G.M.; Kim, D.J. Utilization of wood biomass char in a direct carbon fuel cell (DCFC) system. Appl. Energy 2013, 105, 207–216. [Google Scholar] [CrossRef]
- Rawat, S.; Boobalan, T.; Sathish, M.; Hotha, S.; Thallada, B. Utilization of CO2 activated litchi seed biochar for the fabrication of supercapacitor electrodes. Biomass Bioenergy 2023, 171, 106747. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Garg, S.; Das, P. Microporous carbon from cashew nutshell pyrolytic biochar and its potential application as CO2 adsorbent. Biomass Convers. Biorefinery 2020, 10, 1043–1061. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Wang, Y. Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ. Sci. Pollut. Res. 2020, 27, 22806–22817. [Google Scholar] [CrossRef]
- Reyes Molina, E.A.; Vook, T.; Sagues, W.J.; Kim, K.; Labbé, N.; Park, S.; Kelley, S.S. Green Needle Coke Production from Pyrolysis Biocrude toward Bio-based Anode Material Manufacture: Biochar Fines Addition Effect as “Physical Template” on the Crystalline Order. ACS Sustain. Chem. Eng. 2023, 11, 6944–6955. [Google Scholar] [CrossRef]
- Rubi, R.V.C.; Allayban, J.P.O.; Deduque, D.A.B.; Mena, J.A.B.; Robles, N.M.D.; Rubio, B.D.; Roque, E.C.; Joy Janaban, P.; Olay, J.G. Production and characterization of activated carbon from pyrolysis biochar of cellulosic cotton-based textile wastes. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Saini, K.; Biswas, B.; Kumar, A.; Sahoo, A.; Kumar, J.; Bhaskar, T. Screening of sugarcane bagasse-derived biochar for phenol adsorption: Optimization study using response surface methodology. Int. J. Environ. Sci. Technol. 2022, 19, 8797–8810. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Jiang, P.; Wu, T. Microwave-assisted biomass pyrolysis polygeneration process using a scaled-up reactor: Product characterization, thermodynamic assessment and bio-hydrogen production. Biomass Bioenergy 2020, 139, 105651. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Harisankar, S.; Vishnu Mohan, R.; Choudhary, V.; Vinu, R. Effect of water quality on the yield and quality of the products from hydrothermal liquefaction and carbonization of rice straw. Bioresour. Technol. 2022, 351, 127031. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Shen, X.; Yi, W.; Li, Z.; Li, Y.; Tian, C. Comparison study on fuel properties of hydrochars produced from corn stalk and corn stalk digestate. Energy 2018, 165, 527–536. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and biochar: Production, physicochemical properties and techno-economic analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Wang, X.; Yun, S.; Fang, W.; Zhang, C.; Liang, X.; Lei, Z.; Liu, Z. Layer-Stacking Activated Carbon Derived from Sunflower Stalk as Electrode Materials for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11397–11407. [Google Scholar] [CrossRef]
- Li, Y.; Shao, M.; Huang, M.; Sang, W.; Zheng, S.; Jiang, N.; Gao, Y. Enhanced remediation of heavy metals contaminated soils with EK-PRB using β-CD/hydrothermal biochar by waste cotton as reactive barrier. Chemosphere 2022, 286, 131470. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Shao, Y.; Huang, W.; Cheng, X. Different adsorption behaviors and mechanisms of a novel amino-functionalized hydrothermal biochar for hexavalent chromium and pentavalent antimony. Bioresour. Technol. 2020, 310, 123438. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, J.; Yuan, Y.; Song, H.; Liu, Y.; Wang, S.; Tao, Y.; Zhao, Y.; Li, Z. Simultaneous scavenging of Cd (II) and Pb (II) from water by sulfide-modified magnetic pinecone-derived hydrochar. J. Clean. Prod. 2022, 341, 130758. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the Carbonization Process on Activated Carbon Properties from Lignin and Lignin-Rich Biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Shao, Y.; Tan, H.; Shen, D.; Zhou, Y.; Jin, Z.; Zhou, D.; Lu, W.; Long, Y. Synthesis of improved hydrochar by microwave hydrothermal carbonization of green waste. Fuel 2020, 266, 117146. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal carbonization of corncob residues for hydrochar production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Duman, G.; Balmuk, G.; Cay, H.; Kantarli, I.C.; Yanik, J. Comparative evaluation of torrefaction and hydrothermal carbonization: Effect on fuel properties and combustion behavior of agricultural wastes. Energy Fuels 2020, 34, 11175–11185. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef]
- Allesina, G.; Pedrazzi, S.; Allegretti, F.; Morselli, N.; Puglia, M.; Santunione, G.; Tartarini, P. Gasification of cotton crop residues for combined power and biochar production in Mozambique. Appl. Therm. Eng. 2018, 139, 387–394. [Google Scholar] [CrossRef]
- Cheah, S.; Malone, S.C.; Feik, C.J. Speciation of sulfur in biochar produced from pyrolysis and gasification of oak and corn stover. Environ. Sci. Technol. 2014, 48, 8474–8480. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Zhang, S.; Asadullah, M.; Dong, L.; Tay, H.L.; Li, C.Z. An advanced biomass gasification technology with integrated catalytic hot gas cleaning. Part II: Tar reforming using char as a catalyst or as a catalyst support. Fuel 2013, 112, 646–653. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Sirico, A.; Bernardi, P.; Belletti, B.; Malcevschi, A.; Dalcanale, E.; Domenichelli, I.; Fornoni, P.; Moretti, E. Mechanical characterization of cement-based materials containing biochar from gasification. Constr. Build. Mater. 2020, 246, 118490. [Google Scholar] [CrossRef]
- Xiao, N.; Luo, H.; Wei, W.; Tang, Z.; Hu, B.; Kong, L.; Sun, Y. Microwave-assisted gasification of rice straw pyrolytic biochar promoted by alkali and alkaline earth metals. J. Anal. Appl. Pyrolysis 2015, 112, 173–179. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Labaki, M. Energy Applications of Coffee Processing by-Products; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128112915. [Google Scholar]
- Wang, M.J.; Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave-induced torrefaction of rice husk and sugarcane residues. Energy 2012, 37, 177–184. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Lu, Q. Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Patel, B.; Gami, B.; Bhimani, H. Improved fuel characteristics of cotton stalk, prosopis and sugarcane bagasse through torrefaction. Energy Sustain. Dev. 2011, 15, 372–375. [Google Scholar] [CrossRef]
- Roy, M.; Kundu, K. Production of biochar briquettes from torrefaction of pine needles and its quality analysis. Bioresour. Technol. Rep. 2023, 22, 101467. [Google Scholar] [CrossRef]
- Satpathy, S.K.; Tabil, L.G.; Meda, V.; Naik, S.N.; Prasad, R. Torrefaction of wheat and barley straw after microwave heating. Fuel 2014, 124, 269–278. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Gan, Z.; Zhuang, X.; Ba, Y. Comparative study of electric-heating torrefaction and solar-driven torrefaction of biomass: Characterization of property variation and energy usage with torrefaction severity. Appl. Energy Combust. Sci. 2022, 9, 100051. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Riaz, S.; Siddiqui, M.T.H.; Takkalkar, P.; Tunio, M.M.; Mazari, S.; Bhutto, A.W. Solvothermal Liquefaction of Corn Stalk: Physico-Chemical Properties of Bio-oil and Biochar. Waste Biomass Valorization 2019, 10, 1957–1968. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, Z.; Huang, J.; Wu, X.; Kong, L.; Liang, Z.; Yang, L.; Lin, Z. Preparation and Characterization of Magnetic Biochar Nanocomposites via a Modified Solvothermal Method and Their Use as Efficient Heterogeneous Fenton-like Catalysts. Ind. Eng. Chem. Res. 2020, 59, 1809–1821. [Google Scholar] [CrossRef]
- Kariim, I.; Park, J.Y.; Kazmi, W.W.; Swai, H.; Lee, I.G.; Kivevele, T. Solvothermal liquefaction of orange peels into biocrude: An experimental investigation of biocrude yield and energy compositional dependency on process variables. Bioresour. Technol. 2024, 391, 129928. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.H.; Chan, F.L.; Nizamuddin, S.; Baloch, H.A.; Kundu, S.; Czajka, M.; Griffin, G.J.; Tanksale, A.; Shah, K.; Srinivasan, M. Comparative study of microwave and conventional solvothermal synthesis for magnetic carbon nanocomposites and bio-oil from rice husk. J. Environ. Chem. Eng. 2019, 7, 103266. [Google Scholar] [CrossRef]
- Li, F.; Shen, K.; Long, X.; Wen, J.; Xie, X.; Zeng, X.; Liang, Y.; Wei, Y.; Lin, Z.; Huang, W.; et al. Preparation and characterization of biochars from eichornia crassipes for cadmium removal in aqueous solutions. PLoS ONE 2016, 11, e0148132. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Correa-Navarro, Y.M.; Giraldo, L.; Moreno-Piraján, J.C. Dataset for effect of pH on caffeine and diclofenac adsorption from aqueous solution onto fique bagasse biochars. Data Br. 2019, 25, 104111. [Google Scholar] [CrossRef]
- Da Silva Veiga, P.A.; Cerqueira, M.H.; Gonçalves, M.G.; da Silva Matos, T.T.; Pantano, G.; Schultz, J.; de Andrade, J.B.; Mangrich, A.S. Upgrading from batch to continuous flow process for the pyrolysis of sugarcane bagasse: Structural characterization of the biochars produced. J. Environ. Manag. 2021, 285, 112145. [Google Scholar] [CrossRef]
- De Almeida, S.G.C.; Tarelho, L.A.C.; Hauschild, T.; Costa, M.A.M.; Dussán, K.J. Biochar production from sugarcane biomass using slow pyrolysis: Characterization of the solid fraction. Chem. Eng. Process.-Process Intensif. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Al-Saad, K. Application of Infrared Spectroscopy in the Characterization of Lignocellulosic Biomasses Utilized in Wastewater Treatment. In Infrared Spectroscopy—Perspectives and Applications; El-Azazy, M., Al-Saad, K., El-Shafie, A.S., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 8; ISBN 978-1-80356-282-7. [Google Scholar]
- Rehman, R.; Farooq, S.; Mahmud, T. Use of Agro-waste Musa acuminata and Solanum tuberosum peels for economical sorptive removal of Emerald green dye in ecofriendly way. J. Clean. Prod. 2019, 206, 819–826. [Google Scholar] [CrossRef]
- Alfattani, R.; Shah, M.A.; Siddiqui, M.I.H.; Ali, M.A.; Alnaser, I.A. Biochar characterization produced from walnut shell biomass through slow pyrolysis: Sustainable for soil amendment and an alternate bio-fuel. Energies 2022, 15, 1. [Google Scholar] [CrossRef]
- Adekanye, T.; Dada, O.; Kolapo, J. Pyrolysis of maize cob at different temperatures for biochar production: Proximate, ultimate and spectroscopic characterisation. Res. Agric. Eng. 2022, 68, 27–34. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, J.; Yu, G. Understanding the Effect of Different Biomass Ash Additions on Pyrolysis Product Distribution, Char Physicochemical Characteristics, and Char Gasification Reactivity of Bituminous Coal. Energy Fuels 2019, 33, 3068–3076. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Chang, W.; Li, R.; Li, Y.; Wang, C. Structural features and gasification reactivity of biomass chars pyrolyzed in different atmospheres at high temperature. Energy 2018, 147, 25–35. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Ling, P.; Zhang, X.; Xu, K.; He, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Tsaneva, V.N.; Kwapinski, W.; Teng, X.; Glowacki, B.A. Assessment of the structural evolution of carbons from microwave plasma natural gas reforming and biomass pyrolysis using Raman spectroscopy. Carbon N. Y. 2014, 80, 617–628. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Berrueco, C.; Fidalgo, B.; Paterson, N.; Millan, M. Influence of temperature and particle size on structural characteristics of chars from Beechwood pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 127–134. [Google Scholar] [CrossRef]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.-S.; Espinal, J.F.; Garcia-Perez, M. Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles. Carbon N. Y. 2016, 100, 678–692. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon N. Y. 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, Y.; Li, A. Self-activation of biochar from furfural residues by recycled pyrolysis gas. Waste Manag. 2018, 77, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, X.; He, M.; Xu, Z.; Hou, D.; Zhang, W.; Ok, Y.S.; Rinklebe, J.; Wang, L.; Tsang, D.C.W. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Jawad, A.H.; Razuan, R.; Appaturi, J.N.; Wilson, L.D. Adsorption and mechanism study for methylene blue dye removal with carbonized watermelon (Citrullus lanatus) rind prepared via one-step liquid phase H2SO4 activation. Surf. Interfaces 2019, 16, 76–84. [Google Scholar] [CrossRef]
- Jayakumar, M.; Hamda, A.S.; Abo, L.D.; Daba, B.J.; Venkatesa Prabhu, S.; Rangaraju, M.; Jabesa, A.; Periyasamy, S.; Suresh, S.; Baskar, G. Comprehensive review on lignocellulosic biomass derived biochar production, characterization, utilization and applications. Chemosphere 2023, 345, 140515. [Google Scholar] [CrossRef]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen Structure–Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Article biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eicchornia crassipes) biomass hydrolysis. Sustainability 2021, 13, 245. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi Damuchali, A.; Soltan, J.; McPhedran, K.N. Treatment of aqueous arsenic—A review of biochar modification methods. Sci. Total Environ. 2020, 739, 139750. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Hajialigol, S.; Masoum, S. Optimization of biosorption potential of nano biomass derived from walnut shell for the removal of Malachite Green from liquids solution: Experimental design approaches. J. Mol. Liq. 2019, 286, 110904. [Google Scholar] [CrossRef]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. CO2 adsorption of bagasse waste feedstock using thermogravimetric analyses. J. Therm. Anal. Calorim. 2022, 147, 5973–5984. [Google Scholar] [CrossRef]
- Xiao, B.; Dai, Q.; Yu, X.; Yu, P.; Zhai, S.; Liu, R.; Guo, X.; Liu, J.; Chen, H. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge. J. Hazard. Mater. 2018, 343, 347–355. [Google Scholar] [CrossRef]
- Khan, E.A.; Shahjahan; Khan, T.A. Adsorption of methyl red on activated carbon derived from custard apple (Annona squamosa) fruit shell: Equilibrium isotherm and kinetic studies. J. Mol. Liq. 2018, 249, 1195–1211. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Elemental composition of biochars is affected by methods used for its determination. J. Anal. Appl. Pyrolysis 2021, 156, 105174. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Ilić, M.; Haegel, F.-H.; Lolić, A.; Nedić, Z.; Tosti, T.; Ignjatović, I.S.; Linden, A.; Jablonowski, N.D.; Hartmann, H. Surface functional groups and degree of carbonization of selected chars from different processes and feedstock. PLoS ONE 2022, 17, e0277365. [Google Scholar] [CrossRef]
- De Castro, A.E.; Penido, E.S.; Souza, T.F.; Camargos, J.B.; Lobato, R.L.M.; Ribeiro-Soares, J.; Ferreira, G.M.D.; Ferreira, G.M.D. Biochars from modified sugarcane bagasse for manganese removal from mining effluents. J. Environ. Chem. Eng. 2023, 11, 110761. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Physicochemical Characterization and Pyrolysis Kinetic Study of Sugarcane Bagasse Using Thermogravimetric Analysis. J. Energy Resour. Technol. 2016, 138, 052205. [Google Scholar] [CrossRef]
- Athira, G.; Bahurudeen, A.; Appari, S. Thermochemical Conversion of Sugarcane Bagasse: Composition, Reaction Kinetics, and Characterisation of By-Products. Sugar Tech. 2021, 23, 433–452. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gokalp, I.; Petrus, S.; Belandria, V.; Bostyn, S. Energy recovery analysis from sugar cane bagasse pyrolysis and gasification using thermogravimetry, mass spectrometry and kinetic models. J. Anal. Appl. Pyrolysis 2018, 132, 225–236. [Google Scholar] [CrossRef]
- Jagnade, P.; Panwar, N.L.; Agarwal, C. Experimental Investigation of Kinetic Parameters of Bamboo and Bamboo Biochar Using Thermogravimetric Analysis Under Non-isothermal Conditions. BioEnergy Res. 2023, 16, 1143–1155. [Google Scholar] [CrossRef]
- Mathur, R.; Srivastava, V.K. Crop Residue Burning: Effects on Environment; Springer: Singapore, 2019; ISBN 9789811332722. [Google Scholar]
- Jain, N.; Bhatia, A.; Pathak, H. Emission of air pollutants from crop residue burning in India. Aerosol Air Qual. Res. 2014, 14, 422–430. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop residue burning in India: Policy challenges and potential solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef]
- US Energy Information Administration Annual Energy Outlook. 2023. Available online: https://www.eia.gov/outlooks/aeo/ (accessed on 23 February 2025).
- Kumar, P.; Varkolu, M.; Mailaram, S.; Kunamalla, A.; Maity, S.K. Chapter 12—Biorefinery Polyutilization Systems: Production of Green Transportation Fuels From Biomass. In Polygeneration with Polystorage: For Chemical and Energy Hubs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 373–407. ISBN 9780128133064. [Google Scholar]
- IEA Key World Energy Statistics. 2021. Available online: https://www.iea.org/reports/key-world-energy-statistics-2021 (accessed on 1 September 2021).
- Di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biodiesel antioxidants and their impact on the behavior of diesel engines: A comprehensive review. Fuel Process. Technol. 2022, 232, 107264. [Google Scholar] [CrossRef]
- Kedia, A.G.; Dutta, A.; Kumar, P. Dimethyl Carbonate as a Cost-Effective Substitute of Methanol for Biodiesel Production via Transesterification of Nonedible Oil. Bioenergy Res. 2023, 16, 1134–1142. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Maheshwari, P.; Belal, M.; Yusuf, M.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Dipartimento Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Reyes, D.; Romero-Hermoso, L.; Hidalgo, P.; Meier, S.; Benito, N.; Navia, R. Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production. Energy Convers. Manag. 2017, 137, 165–173. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Yang, S.Y.; Song, H.S.; Park, J.Y.; Park, Y.L.; Han, Y.H.; Choi, Y.K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Hidayat, A.; Rochmadi; Wijaya, K.; Nurdiawati, A.; Kurniawan, W.; Hinode, H.; Yoshikawa, K.; Budiman, A. Esterification of Palm Fatty Acid Distillate with High Amount of Free Fatty Acids Using Coconut Shell Char Based Catalyst. Energy Procedia 2015, 75, 969–974. [Google Scholar] [CrossRef]
- Dong, T.; Gao, D.; Miao, C.; Yu, X.; Degan, C.; Garcia-Pérez, M.; Rasco, B.; Sablani, S.S.; Chen, S. Two-step microalgal biodiesel production using acidic catalyst generated from pyrolysis-derived bio-char. Energy Convers. Manag. 2015, 105, 1389–1396. [Google Scholar] [CrossRef]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Bazargan, A.; Kostić, M.D.; Stamenković, O.S.; Veljković, V.B.; McKay, G. A calcium oxide-based catalyst derived from palm kernel shell gasification residues for biodiesel production. Fuel 2015, 150, 519–525. [Google Scholar] [CrossRef]
- Kostić, M.D.; Bazargan, A.; Stamenković, O.S.; Veljković, V.B.; McKay, G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016, 163, 304–313. [Google Scholar] [CrossRef]
- Nguyen, H.K.D.; Pham, V.V.; Do, H.T. Preparation of Ni/biochar Catalyst for Hydrotreating of Bio-Oil from Microalgae Biomass. Catal. Letters 2016, 146, 2381–2391. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Wu, K.C.W.; Chen, S.S.; Cao, Y.; Tessonnier, J.P.; Shang, J.; Hou, D.; Shen, Z.; Zhang, S.; Ok, Y.S. Effective dispersion of MgO nanostructure on biochar support as a basic catalyst for glucose isomerization. ACS Sustain. Chem. Eng. 2020, 8, 6990–7001. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Yin, Q.; Zhu, L.; Yin, S. Methanation of bio-syngas over a biochar supported catalyst. New J. Chem. 2014, 38, 4471–4477. [Google Scholar] [CrossRef]
- Visser, E.D.; Seroka, N.S.; Khotseng, L. Catalytic Properties of Biochar as Support Material Potential for Direct Methanol Fuel Cell: A Review. ACS Omega 2023, 8, 40972–40981. [Google Scholar] [CrossRef]
- Dong, F.; Liu, X.; Irfan, M.; Yang, L.; Li, S.; Ding, J.; Li, Y.; Khan, I.U.; Zhang, P. Macaroon-like FeCo2O4 modified activated carbon anode for enhancing power generation in direct glucose fuel cell. Int. J. Hydrogren Energy 2019, 44, 8178–8187. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ling, L.L.; Jiang, H. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni-Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, M.; Liang, D.; Lu, Z.; Qin, B.; Li, C.; Yuan, G.; Wang, J.; Yuan, L. Selective oxidation of methane to methanol over lignin-derived biochar supported transition metals catalysts. J. Anal. Appl. Pyrolysis 2024, 177, 106359. [Google Scholar] [CrossRef]
- Ramirez, L.A.; Dennehy, M.; Alvarez, M. Metal oxide-biochar supported recyclable catalysts: A feasible solution for the reduction of 4-nitrophenol in water. Catal. Commun. 2023, 181, 106723. [Google Scholar] [CrossRef]
- Frainetti, A.J.; Klinghoffer, N.B. Biomass and Bioenergy Engineering biochar-supported nickel catalysts for efficient CO2 methanation. Biomass Bioenergy 2024, 184, 107179. [Google Scholar] [CrossRef]
- Yousefian, F.; Babatabar, M.A.; Eshaghi, M.; Poor, S.M.; Tavasoli, A. Pyrolysis of Rice husk, Coconut shell, and Cladophora glomerata algae and application of the produced biochars as support for cobalt catalyst in Fischer–Tropsch synthesis. Fuel Process. Technol. 2023, 247, 107818. [Google Scholar] [CrossRef]
- Gandomi, S.; Rahimpour, M.R. Direct Methane to Methanol Conversion Technologies Methods, Applications, and Future Prospects. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Lahori, A.H.; Guo, Z.; Zhang, Z.; Li, R.; Mahar, A.; Awasthi, M.K.; Shen, F.; Sial, T.A.; Kumbhar, F.; Wang, P.; et al. Use of Biochar as an Amendment for Remediation of Heavy Metal-Contaminated Soils: Prospects and Challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Shabir, S.; Akhtar, N.; Shahzad, A.; Sayyed, R.Z.; Bano, A. Advances in Biochar and PGPR engineering system for hydrocarbon degradation: A promising strategy for environmental remediation. Environ. Pollut. 2022, 305, 119282. [Google Scholar] [CrossRef]
- He, N.; Hu, L.; Jiang, C.; Li, M. Remediation of chromium, zinc, arsenic, lead and antimony contaminated acidic mine soil based on Phanerochaete chrysosporium induced phosphate precipitation. Sci. Total Environ. 2022, 850, 157995. [Google Scholar] [CrossRef]
- Liang, X.; Su, Y.; Wang, X.; Liang, C.; Tang, C.; Wei, J.; Liu, K.; Ma, J.; Yu, F.; Li, Y. Insights into the heavy metal adsorption and immobilization mechanisms of CaFe-layered double hydroxide corn straw biochar: Synthesis and application in a combined heavy metal-contaminated environment. Chemosphere 2023, 313, 137467. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, A.; Wang, P.; Huang, Z.; Fu, Z.; Huang, Z. Two recyclable and complementary adsorbents of coal-based and bio-based humic acids: High efficient adsorption and immobilization remediation for Pb(II) contaminated water and soil. Chemosphere 2023, 318, 137963. [Google Scholar] [CrossRef]
- Ma, M.; Xu, X.; Ha, Z.; Su, Q.; Lv, C.; Li, J.; Du, D.; Chi, R. Deep insight on mechanism and contribution of arsenic removal and heavy metals remediation by mechanical activation phosphogypsum. Environ. Pollut. 2023, 336, 122258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef] [PubMed]
- Annisa, W.; Mukhlis, M.; Hairani, A. Biochar-Materials for Remediation on Swamplands: Mechanisms and Effectiveness. J. Sumberd. Lahan 2021, 15, 13. [Google Scholar] [CrossRef]
- Cen, R.; Feng, W.; Yang, F.; Wu, W.; Liao, H.; Qu, Z. Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas. J. Environ. Manag. 2021, 286, 112198. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ. Pollut. 2021, 291, 118129. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, C.; Chen, Y.; Zheng, L.; Ding, D.; Shan, S. Synergistic mechanism of iron manganese supported biochar for arsenic remediation and enzyme activity in contaminated soil. J. Environ. Manag. 2023, 347, 119127. [Google Scholar] [CrossRef]
- Li, K.; Yang, B.; Wang, H.; Xu, X.; Gao, Y.; Zhu, Y. Dual effects of biochar and hyperaccumulator Solanum nigrum L. on the remediation of Cd-contaminated soil. PeerJ 2019, 7, e6631. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, H.; Tie, B.; Li, D.; Liu, S.; Lei, M.; Du, H. The long-term effectiveness of ferromanganese biochar in soil Cd stabilization and reduction of Cd bioaccumulation in rice. Biochar 2021, 3, 499–509. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, C.; Nie, M.; Ding, M. Bisphenol A adsorption behavior on soil and biochar: Impact of dissolved organic matter. Environ. Sci. Pollut. Res. 2021, 28, 32434–32445. [Google Scholar] [CrossRef]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Wang, X.; Zulfiqar, U.; Farooq, M.; Hussain, S.; Mehmood, T.; Naveed, M.; Li, Y.; Liqun, C.; Saeed, Q.; et al. Biochar application for remediation of organic toxic pollutants in contaminated soils; An update. Ecotoxicol. Environ. Saf. 2022, 248, 114322. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, I.; Usman, M.; Iqbal, R.; Rizwan, M.; Abdel-Hameed, U.K.; Haider, A.A.; Tariq, A. Biochar as a Green Sorbent for Remediation of Polluted Soils and Associated Toxicity Risks: A Critical Review. Separations 2023, 10, 197. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci. Rep. 2017, 7, 12114. [Google Scholar] [CrossRef]
- Gregory, S.J.; Anderson, C.W.N.; Camps-Arbestain, M.; Biggs, P.J.; Ganley, A.R.D.; O’Sullivan, J.M.; McManus, M.T. Biochar in Co-Contaminated Soil Manipulates Arsenic Solubility and Microbiological Community Structure, and Promotes Organochlorine Degradation. PLoS ONE 2015, 10, e0125393. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Gaffar, S.; Arshad, M.; Sheeraz Ahmad, M.; Bibi, F.; Jeddi, K.; Hessini, K. Biostimulation potential of biochar for remediating the crude oil contaminated soil and plant growth. Saudi J. Biol. Sci. 2021, 28, 2667–2676. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Suo, F.; You, X.; Yuan, Y.; Cheng, Y.; Zhang, C.; Li, Y. Effect of biochar and hydrochar from cow manure and reed straw on lettuce growth in an acidified soil. Chemosphere 2022, 298, 134191. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Ling, Q.; Li, S.; Wei, J.; Xin, M.; Xie, D.; Chen, X.; Liu, K.; Yu, F. Bacterial extracellular polymeric substances: Impact on soil microbial community composition and their potential role in heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 2022, 240, 113701. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Formicola, F.; Sbaffoni, S.; Franzetti, A.; Vaccari, M. Insights into rhamnolipid amendment towards enhancing microbial electrochemical treatment of petroleum hydrocarbon contaminated soil. Chemosphere 2022, 307, 136126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Sun, R.; Cao, Y. Neutralization of red mud using bio-acid generated by hydrothermal carbonization of waste biomass for potential soil application. J. Clean. Prod. 2020, 271, 122525. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Ábrego, J.; Gea, G.; Marías, F. Pyrolysis of dairy cattle manure: Evolution of char characteristics. J. Anal. Appl. Pyrolysis 2020, 145, 104724. [Google Scholar] [CrossRef]
- Fan, R.; Ma, W.; Zhang, H. Microbial community responses to soil parameters and their effects on petroleum degradation during bio-electrokinetic remediation. Sci. Total Environ. 2020, 748, 142463. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Bian, Y.; Jiang, X.; Song, Y. The combination of biochar and plant roots improves soil bacterial adaptation to PAH stress: Insights from soil enzymes, microbiome, and metabolome. J. Hazard. Mater. 2020, 400, 123227. [Google Scholar] [CrossRef]
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W.; Shen, Z.; Hou, D. Biochar Aging: Mechanisms, Physicochemical Changes, Assessment, And Implications for Field Applications. Environ. Sci. Technol. 2020, 54, 14797–14814. [Google Scholar] [CrossRef]
- Huang, M.; Yin, X.; Chen, J.; Cao, F. Biochar Application Mitigates the Effect of Heat Stress on Rice (Oryza sativa L.) by Regulating the Root-Zone Environment. Front. Plant Sci. 2021, 12, 711725. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Biochar enhances the retention capacity of nitrogen fertilizer and affects the diversity of nitrifying functional microbial communities in karst soil of southwest China. Ecotoxicol. Environ. Saf. 2021, 226, 112819. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhang, L.; Liu, Y.; Shen, S.; Li, N.; Wu, Z.; Yang, J.; Han, W.; Han, X. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 2022, 298, 134304. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Iqbal, B.; Khan, A.A.; Inamullah; Rehman, A.; Fayyaz, A.; Shakoor, A.; Farooq, T.H.; Wang, L.X. The Interactive Impact of Straw Mulch and Biochar Application Positively Enhanced the Growth Indexes of Maize (Zea mays L.) Crop. Agronomy 2022, 12, 2584. [Google Scholar] [CrossRef]
- Wu, M.; Han, X.; Zhong, T.; Yuan, M.; Wu, W. Soil organic carbon content affects the stability of biochar in paddy soil. Agric. Ecosyst. Environ. 2016, 223, 59–66. [Google Scholar] [CrossRef]
- Sun, Q.; Meng, J.; Lan, Y.; Shi, G.; Yang, X.; Cao, D.; Chen, W.; Han, X. Long-term effects of biochar amendment on soil aggregate stability and biological binding agents in brown earth. CATENA 2021, 205, 105460. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar Carbon Stability in a Clayey Soil As a Function of Feedstock and Pyrolysis Temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef]
- Tian, G.; Franzluebbers, A.J.; Granato, T.C.; Cox, A.E.; O’Connor, C. Stability of soil organic matter under long-term biosolids application. Appl. Soil Ecol. 2013, 64, 223–227. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for Soil Carbon Sequestration: Current Knowledge, Mechanisms, and Future Perspectives. C-J. Carbon Res. 2023, 9, 67. [Google Scholar] [CrossRef]
- Azad, M.; Javeed, I.A.; Bhat, S.A.S. Potential of Biochar to Sequester Carbon and Mitigate Greenhouse Gas Emissions. Curr. J. Appl. Sci. Technol. 2023, 42, 24–31. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, K.; Hu, X.; He, Q.; Yan, J.; Xue, Y. Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration. J. Hazard. Mater. 2021, 408, 124860. [Google Scholar] [CrossRef]
- Chu, J.-H.; Kang, J.-K.; Park, S.-J.; Lee, C.-G. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, S.; Zhang, C.; Zeng, G.; Tan, X.; Song, B.; Zhang, P.; Yang, H.; Li, M.; Chen, Q. Application of biochar for the remediation of polluted sediments. J. Hazard. Mater. 2021, 404, 124052. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef]
- Sashidhar, P.; Kochar, M.; Singh, B.; Gupta, M.; Cahill, D.; Adholeya, A.; Dubey, M. Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ. 2020, 703, 134892. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Margulis, E.; Lang, T.; Tromelin, A.; Ziaikin, E.; Behrens, M.; Niv, M.Y. Bitter Odorants and Odorous Bitters: Toxicity and Human TAS2R Targets. J. Agric. Food Chem. 2023, 71, 9051–9061. [Google Scholar] [CrossRef]
- Giri, B.S.; Mudliar, S.N.; Deshmukh, S.C.; Banerjee, S.; Pandey, R.A. Treatment of waste gas containing low concentration of dimethyl sulphide (DMS) in a bench-scale biofilter. Bioresour. Technol. 2010, 101, 2185–2190. [Google Scholar] [CrossRef]
- Khotsena, C.; Potivichayanon, S. Determination of Appropriate Conditions for Volatile Fatty Acids from Rubber Industrial Wastewater by GC-FID: Headspace Technique. In Proceedings of the 2020 International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE), Pattaya, Thailand, 20–22 October 2020; pp. 1–7. [Google Scholar]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Hasdemir, İ.M.; Yılmazoğlu, E.; Güngör, S.; Hasdemir, B. Adsorption of acetic acid onto activated carbons produced from hazelnut shell, orange peel, and melon seeds. Appl. Water Sci. 2022, 12, 271. [Google Scholar] [CrossRef]
- Perez-Mercado, L.F.; Lalander, C.; Joel, A.; Ottoson, J.; Dalahmeh, S.; Vinnerås, B. Biochar filters as an on-farm treatment to reduce pathogens when irrigating with wastewater-polluted sources. J. Environ. Manag. 2019, 248, 109295. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Duarte, S.; Glaser, B.; Pano, B.L.P.; Cerri, C.E.P. Biochar and sugar cane filter cake interaction on physical and hydrological soil properties under tropical field conditions. Biochar 2020, 2, 195–210. [Google Scholar] [CrossRef]
- Guan, P.; Prasher, S.O.; Afzal, M.T.; George, S.; Ronholm, J.; Dhiman, J.; Patel, R.M. Removal of Escherichia coli from lake water in a biochar-amended biosand filtering system. Ecol. Eng. 2020, 150, 105819. [Google Scholar] [CrossRef]
- Kumkum, P.; Kumar, S. Evaluation of Lead (Pb(II)) Removal Potential of Biochar in a Fixed-bed Continuous Flow Adsorption System. J. Health Pollut. 2020, 10, 201210. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Wurzer, C.; Soldatou, S.; Edwards, C.; Lawton, L.A.; Mašek, O. New directions and challenges in engineering biologically-enhanced biochar for biological water treatment. Sci. Total Environ. 2021, 796, 148977. [Google Scholar] [CrossRef]
- Pritchard, J.C.; Hawkins, K.M.; Cho, Y.-M.; Spahr, S.; Struck, S.D.; Higgins, C.P.; Luthy, R.G. Black Carbon-Amended Engineered Media Filters for Improved Treatment of Stormwater Runoff. ACS Environ. Au 2023, 3, 34–46. [Google Scholar] [CrossRef]
- Pahlavan, F.; Ghasemi, H.; Yazdani, H.; Fini, E.H. Soil amended with Algal Biochar Reduces Mobility of deicing salt contaminants in the environment: An atomistic insight. Chemosphere 2023, 323, 138172. [Google Scholar] [CrossRef]
- Patra, J.M.; Panda, S.S.; Dhal, N.K. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. Biosci. 2020, 6, 1–7. [Google Scholar]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal from Wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Shrestha, R.M.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Double-layer magnetized/functionalized biochar composite: Role of microporous structure for heavy metal removals. J. Water Process Eng. 2021, 39, 101677. [Google Scholar] [CrossRef]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Chen, Y.; Yin, J.; Li, J. An application of waste algae biochar in aquaculture water to remove co-existed cadmium and PAHs and the corresponding mechanism. Environ. Technol. 2023, 44, 1392–1404. [Google Scholar] [CrossRef]
- Elbehiry, F.; Darweesh, M.; Al-Anany, F.S.; Khalifa, A.M.; Almashad, A.A.; El-Ramady, H.; El-Banna, A.; Rajput, V.D.; Jatav, H.S.; Elbasiouny, H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability 2022, 14, 10118. [Google Scholar] [CrossRef]
- Chen, J.; Deng, S.; Jia, W.; Li, X.; Chang, J. Removal of multiple heavy metals from mining-impacted water by biochar-filled constructed wetlands: Adsorption and biotic removal routes. Bioresour. Technol. 2021, 331, 125061. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, L.; Tao, S.; Ma, Y. Removal of Pb(II) and phosphorus in water by γ-Al2O3/biochar. Environ. Sci. Pollut. Res. 2023, 30, 72354–72367. [Google Scholar] [CrossRef]
- Nobaharan, K.; Novair, S.B.; Lajayer, B.A.; Van Hullebusch, E.D. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Barman, S.R.; Das, P.; Mukhopadhayay, A. Biochar from waste Sterculia foetida and its application as adsorbent for the treatment of PAH compounds: Batch and optimization. Fuel 2021, 306, 121623. [Google Scholar] [CrossRef]
- Song, H.J.; Gurav, R.; Bhatia, S.K.; Lee, E.B.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, H.H.; Lee, S.H.; Choi, Y.-K. Treatment of microcystin-LR cyanotoxin contaminated water using Kentucky bluegrass-derived biochar. J. Water Process Eng. 2021, 41, 102054. [Google Scholar] [CrossRef]
- Pap, S.; Boyd, K.G.; Taggart, M.A.; Turk Sekulic, M. Circular economy based landfill leachate treatment with sulphur-doped microporous biochar. Waste Manag. 2021, 124, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Vo, T.-D.-H.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Le, T.-N.-C.; Duong, T.-G.-H.; Bui, M.-H.; et al. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, K.; Li, X.; Wu, L.; Yu, J.; Cao, S.; Zhang, D.; Xu, L.; Parikh, S.J.; Ok, Y.S. Removal of phosphate from water by paper mill sludge biochar. Environ. Pollut. 2022, 293, 118521. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, R.; Yin, Y.; Tu, S.; Ye, L. Two-step pyrolysis biochar derived from agro-waste for antibiotics removal: Mechanisms and stability. Chemosphere 2022, 292, 133454. [Google Scholar] [CrossRef]
- Qian, L.; Yan, S.; Yong, X.; Selvaraj, M.; Ghramh, H.A.; Assiri, M.A.; Zhang, X.; Awasthi, M.K.; Zhou, J. Effective degradation of chloramphenicol in wastewater by activated peroxymonosulfate with Fe-rich porous biochar derived from petrochemical sludge. Chemosphere 2023, 310, 136839. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Maleki Shahraki, Z.; Mao, X. Biochar application in biofiltration systems to remove nutrients, pathogens, and pharmaceutical and personal care products from wastewater. J. Environ. Qual. 2022, 51, 129–151. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.-K.; Zheng, Y.-M.; Zhao, Q.-B.; Yu, H.-Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Ehsani, A.; Parsimehr, H. Electrochemical energy storage electrodes from fruit biochar. Adv. Colloid Interface Sci. 2020, 284, 102263. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bordoloi, U.; Muigai, H.H.; Kalita, P. A novel form stable PCM based bio composite material for solar thermal energy storage applications. J. Energy Storage 2020, 30, 101403. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Wi, S.; Yun, B.Y.; Kim, S. Engineering biochar with multiwalled carbon nanotube for efficient phase change material encapsulation and thermal energy storage. Energy 2021, 216, 119294. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yeol Yun, B.; Uk Kim, Y.; Wi, S.; Kim, S. Introduction of eicosane into biochar derived from softwood and wheat straw: Influence of porous structure and surface chemistry. Chem. Eng. J. 2021, 415, 128887. [Google Scholar] [CrossRef]
- Rajan, A.B.K.; Anandan, S.S. Performance analysis of cold storage system with nanofiller phase change material. Biomass Convers. Biorefinery 2023, 13, 6777–6786. [Google Scholar] [CrossRef]
- Liu, S.; Peng, S.; Zhang, B.; Xue, B.; Yang, Z.; Wang, S.; Xu, G. Effects of biochar pyrolysis temperature on thermal properties of polyethylene glycol/biochar composites as shape-stable biocomposite phase change materials. RSC Adv. 2022, 12, 9587–9598. [Google Scholar] [CrossRef]
- Zhou, J.; Fei, H.; He, Q.; Li, P.; Pan, Y.; Liang, X. Structural characteristics and thermal performances of lauric-myristic-palmitic acid introduced into modified water hyacinth porous biochar for thermal energy storage. Sci. Total Environ. 2023, 882, 163670. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Choi, J.Y.; Yun, B.Y.; Nam, J.; Kim, H.B.; Kim, S. Energy storage and key derives of octadecane thermal stability during phase change assembly with animal manure-derived biochar. Environ. Res. 2024, 240, 117405. [Google Scholar] [CrossRef]
- Salimi, P.; Javadian, S.; Norouzi, O.; Gharibi, H. Turning an environmental problem into an opportunity: Potential use of biochar derived from a harmful marine biomass named Cladophora glomerata as anode electrode for Li-ion batteries. Environ. Sci. Pollut. Res. 2017, 24, 27974–27984. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Lai, C.; Qiu, J.; Li, S.; Hou, Y.; Martens, W.; Mahmood, N.; Zhang, S. Microporous bamboo biochar for lithium-sulfur batteries. Nano Res. 2015, 8, 129–139. [Google Scholar] [CrossRef]
- Liang, H.; Gong, X.; Jia, L.; Chen, F.; Rao, Z.; Jing, S.; Tsiakaras, P. Highly efficient Li-O2 batteries based on self-standing NiFeP@NC/BC cathode derived from biochar supported Prussian blue analogues. J. Electroanal. Chem. 2020, 867, 114124. [Google Scholar] [CrossRef]
- Niu, Z.; Feng, W.; Huang, H.; Wang, B.; Chen, L.; Miao, Y.; Su, S. Green synthesis of a novel Mn–Zn ferrite/biochar composite from waste batteries and pine sawdust for Pb2+ removal. Chemosphere 2020, 252, 126529. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Wang, W.; Shang, Q.; Han, J.; Liu, C.; Tian, Z.; Chen, J. Eco-friendly utilization of sawdust: Ionic liquid-modified biochar for enhanced Li+ storage of TiO2. Sci. Total Environ. 2021, 794, 148688. [Google Scholar] [CrossRef]
- Ding, M.; Ma, Z.; Su, H.; Li, Y.; Yang, K.; Dang, L.; Li, F.; Xue, B. Preparation of porous biochar and its application in supercapacitors. New J. Chem. 2022, 46, 21788–21797. [Google Scholar] [CrossRef]
- Xiaorui, L.; Haiping, Y. A state-of-the-art review of N self-doped biochar development in supercapacitor applications. Front. Energy Res. 2023, 11, 1135093. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. In-situ polymerization of magnetic biochar—polypyrrole composite: A novel application in supercapacitor. Biomass Bioenergy 2017, 98, 95–111. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F.; Dutta, A. Biochar-based composites as electrode active materials in hybrid supercapacitors with particular focus on surface topography and morphology. J. Energy Storage 2020, 29, 101291. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, J.H.; Lee, S.K.; Chun, Y.; Park, C.; Jin, J.-H.; Lee, H.U.; Kim, S.W. Fabricating a modified biochar-based all-solid-state flexible microsupercapacitor using pen lithography. J. Clean. Prod. 2021, 284, 125449. [Google Scholar] [CrossRef]
- Li, X.; Su, Z.; Liang, P.; Zhang, J. Construction of fungus waste-derived porous carbon as electrode materials for electrochemical supercapacitor. Biomass Convers. Biorefinery 2023, 13, 6237–6248. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Chen, X.; Wang, Q.; Zhang, S.; Tian, Y.; Liu, C.; Wang, L.; Wei, Z.; Cao, L.; et al. In Situ N, O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors. Nanomaterials 2023, 13, 2431. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from spent malt rootlets and its application to an energy conversion and storage device. Chemosensors 2021, 9, 57. [Google Scholar] [CrossRef]
- Akgül, G.; Iglesias, D.; Ocon, P.; Moreno Jiménez, E. Valorization of Tea-Waste Biochar for Energy Storage. BioEnergy Res. 2019, 12, 1012–1020. [Google Scholar] [CrossRef]






| S. No. | Biomass | Gasification Condition | Application | Reference | |
|---|---|---|---|---|---|
| Heating Source | Temperature, °C | ||||
| 1 | Mallee wood Gasification | Thermal | 880 | As catalyst for tar reforming | [50] |
| 2 | Pinewood | Thermal | 1200 | Soil amendment | [45] |
| 3 | White oak | Thermal | 600–710 | Additive in anaerobic digestion | [51] |
| 4 | Woodchips | Thermal | 900 | Additives in cement | [52] |
| 5 | Rice straw | Microwave | 550 | [53] | |
| 6 | Beech wood | Thermal | 670–750 | Soil amendment | [10] |
| 7 | Greenhouse waste | Thermal | 670–750 | Soil amendment | [10] |
| 8 | Pinewood chips | Thermal | 700–750 | Carbon sequestration and soil amendment | [46] |
| 9 | Cereal straw | Thermal | 700–750 | Carbon sequestration and soil amendment | [46] |
| 10 | Cotton crops | Thermal | 695–834 | Soil amendment | [47] |
| 11 | Wood chips | Thermal | 850 | CO2 capture | [49] |
| 12 | Oak | Thermal | 850 | Soil mineralization | [48] |
| 13 | Corn stover | Thermal | 850 | Soil mineralization | [48] |
| S. No. | Biomass | Torrefaction Condition | Biochar Yield, % | Application | References | |
|---|---|---|---|---|---|---|
| Heating Source | Temperature, °C | |||||
| 1 | Rice husk | Thermal | 290 | 67.0 | Solid fuel | [56] |
| 2 | Rice husk | Microwave | 220 | 39.71 | Energy | [55] |
| 3 | Sugarcane residues | Microwave | 320 | 32.90 | Energy | [55] |
| 4 | Cotton stalk | Thermal | 300 | 61.0 | Solid fuel | [57] |
| 5 | Sugarcane bagasse | Thermal | 300 | 54.0 | Solid fuel | [57] |
| 6 | Prosopis | Thermal | 300 | 73.0 | Solid fuel | [57] |
| 7 | Pine needles | Thermal | 350 | 44.19 | Energy | [58] |
| 8 | Wheat straw | Microwave | 392 | 66.3 | Energy | [59] |
| 9 | Barley straw | Microwave | 282 | 80.9 | Energy | [59] |
| 10 | Sweet sorghum bagasse | Thermal | 250–300 | 43–65 | Energy | [60] |
| 11 | Peanut shell | Solar thermal | 200–300 | 61.9–96.2 | Solid fuel | [61] |
| 12 | Soybean straw | Solar thermal | 200–300 | 43.2–92 | Solid fuel | [61] |
| 13 | Pine wood | Solar thermal | 200–300 | 53.4–97.8 | Solid fuel | [61] |
| S. No. | Biomass | STL Condition | Biochar Yield, % | Application | References | |||
|---|---|---|---|---|---|---|---|---|
| Heating Source | Temperature, °C | Solvent | Residence Time, Minute | |||||
| 1 | Corn stalk | Thermal | 250 | Ethanol | 75 | 39 | Energy | [62] |
| 2 | Corn stalk | Thermal | 200 | Ethylene glycol | 600 | Magnetic biochar | [63] | |
| 3 | Orange peels | Thermal | 230 | Ethanol/ acetone | 15 | 40.71 | - | [64] |
| 4 | Rice husk with iron precursors | Thermal | 180 | Ethanol | 120 | 41.61 | Magnetic biochar composite | [65] |
| 5 | Rice husk with iron precursors | Microwave | 180 | Ethanol | 120 | 33.59 | Magnetic biochar composite | [65] |
| Biochar | Feed | T, °C | A/C | CX, % | X, % | Key Findings | Ref. |
|---|---|---|---|---|---|---|---|
| PHC | FFA blend with VO and AF | 50–60 | 6:1 | 97 | SAPHC: 1–4 m2/g SAPHC-SO4: 242 m2/g PVPHC: ND PVPHC-SO4: 0.13 cm3g -SO3H Density of PHC: 0 -SO3H Density of PHC-SO4: 0.62 mmol/g | [122] | |
| OH | WCO | 100 and 140 | 10:1 | 10 | 90 Y | SAOH: 49.3 m2 g−1 SAOH: 30.6 m2 g−1 @100 SAOH: 5.4 m2 g−1 @140 | [123] |
| Cork | WCO | 65 | 25:1 | 1.5 | 98 | Pore size of cork char at 600 °C = 2.3 cm decreased to 2.10 at 800 °C. | [124] |
| CS | PFAD | 40–60 | 6:1 to 12:1 | 7 | 87 | [125] | |
| Fir wood | Microalgal oil | 80–120 | 5:1–30:1 | 3–7 | 99Y | CSFFA content: <0.5 Amberlyst-15FFA content = 2.8 | [126] |
| WM | Canola oil | 65 | 15:1 | 5 | 24.5Y, 44.2 Y | (TAD and SA)450: 2.6 mmol/g and 1.88 m2/g (TAD and SA)675: 1.2 mmol/g and 640 m2/g (TAD and SA)875: 0.43 mmol/g and 1411 m2/g | [127] |
| PKS | SO | 60 | 9:1 | 5 | - | PKS is good source for CaO based catalyst to produce biodiesel. | [129] |
| PKS | SO | 65 | 9:1 | 3 | 99 Y | [130] |
| Metal | Biochar | Feed | Reaction Condition Detail | Yield, % | Ref. | ||
|---|---|---|---|---|---|---|---|
| Reaction | Reactor | T, °C; P, MPa | |||||
| Ni | Microalgae | MABO | HDO | Batch | 300 | 80 n-heptadecane | [131] |
| MgO | Wood waste | Glucose | Isomerization | 100 mL Microwave | 100 | 80 S | [132] |
| Ru | Lauan | Bio-syngas | Methanation | Fixed bed | 360–420 | 92 S | [133] |
| FeCo2O4 | Glucose | Oxidation | Fuel cell | 250; 2 | PD: 35.91 W/m2 | [135] | |
| NiMo2 | Saw dust | Lignin | Hydrogenation | 61.3 X | [136] | ||
| Cu, Ni, Zn, Fe, Co | Lignin-derived | Methane | Oxidation | Fixed bed | 240–400 | 686.92 Y | [141] |
| Cu, Co, Mn, and Ni oxide | Pruning waste | 4-nitrophenol | Reduction | - | - | - | [138] |
| Ni | Western red cedar | CO2 | Methanation | Fixed-bed reactor | 400–600 | 58 in 1 h | [139] |
| Co | Rice husk, Coconut shell, algae | Syn gas | FTS | Fixed-bed micro reactor | 220 | 67 XCO | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. https://doi.org/10.3390/catal15030243
Varkolu M, Gundekari S, Omvesh, Palla VCS, Kumar P, Bhattacharjee S, Vinodkumar T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts. 2025; 15(3):243. https://doi.org/10.3390/catal15030243
Chicago/Turabian StyleVarkolu, Mohan, Sreedhar Gundekari, Omvesh, Venkata Chandra Sekhar Palla, Pankaj Kumar, Satyajit Bhattacharjee, and Thallada Vinodkumar. 2025. "Recent Advances in Biochar Production, Characterization, and Environmental Applications" Catalysts 15, no. 3: 243. https://doi.org/10.3390/catal15030243
APA StyleVarkolu, M., Gundekari, S., Omvesh, Palla, V. C. S., Kumar, P., Bhattacharjee, S., & Vinodkumar, T. (2025). Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts, 15(3), 243. https://doi.org/10.3390/catal15030243







