Iron-Based Alumina-Supported Catalysts for Clean Hydrogen Production from Ammonia
Abstract
1. Introduction
2. Results and Discussion
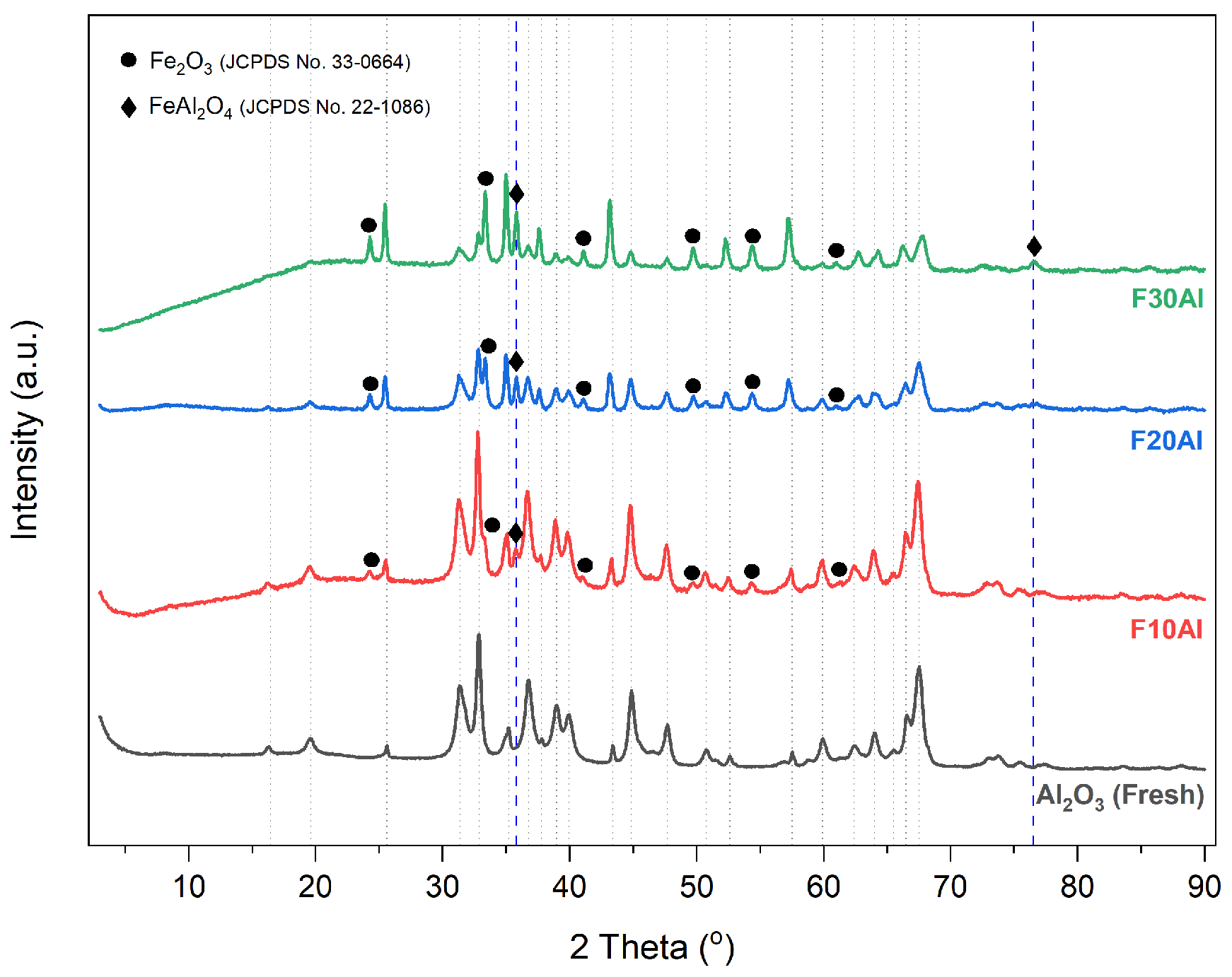
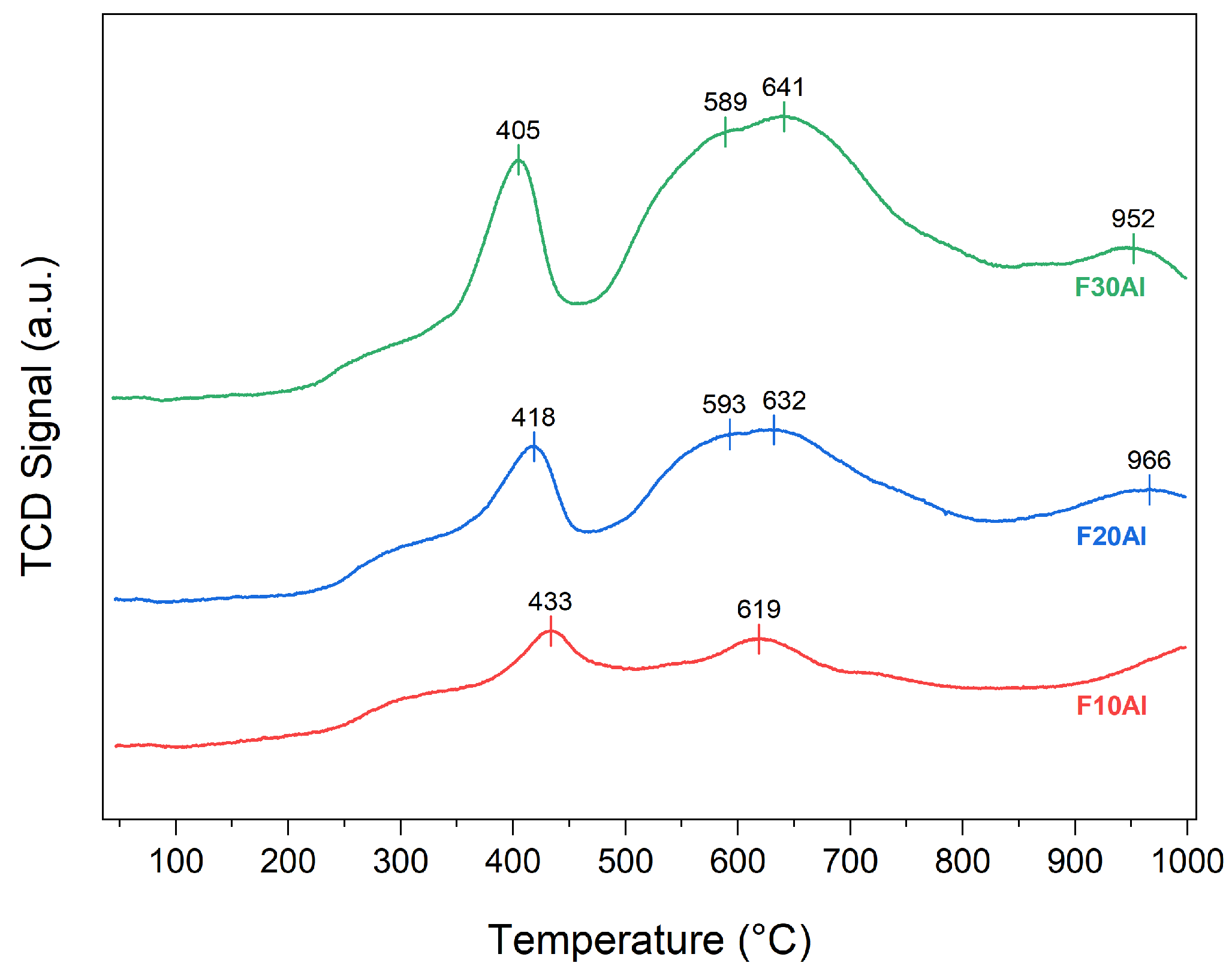
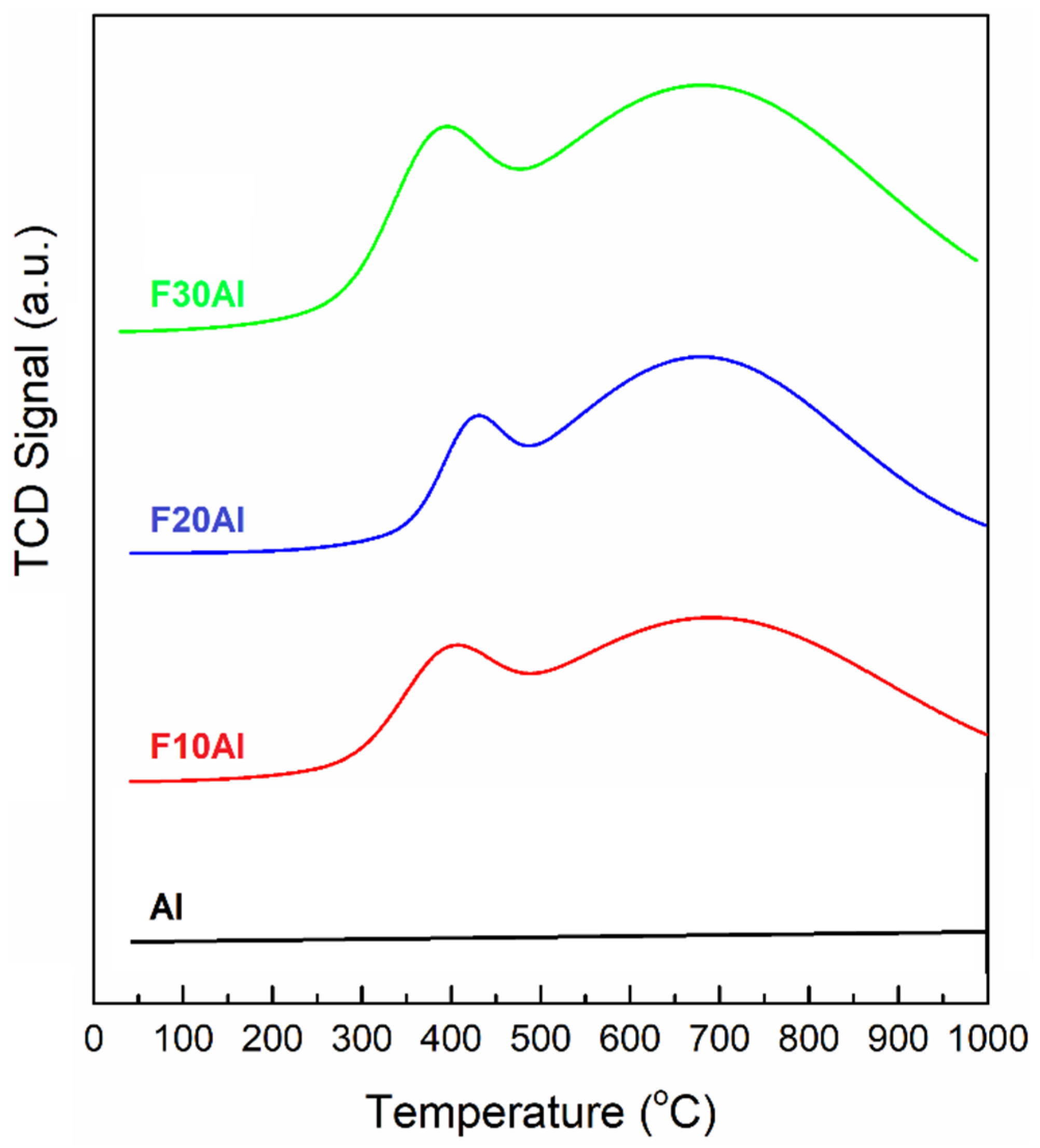
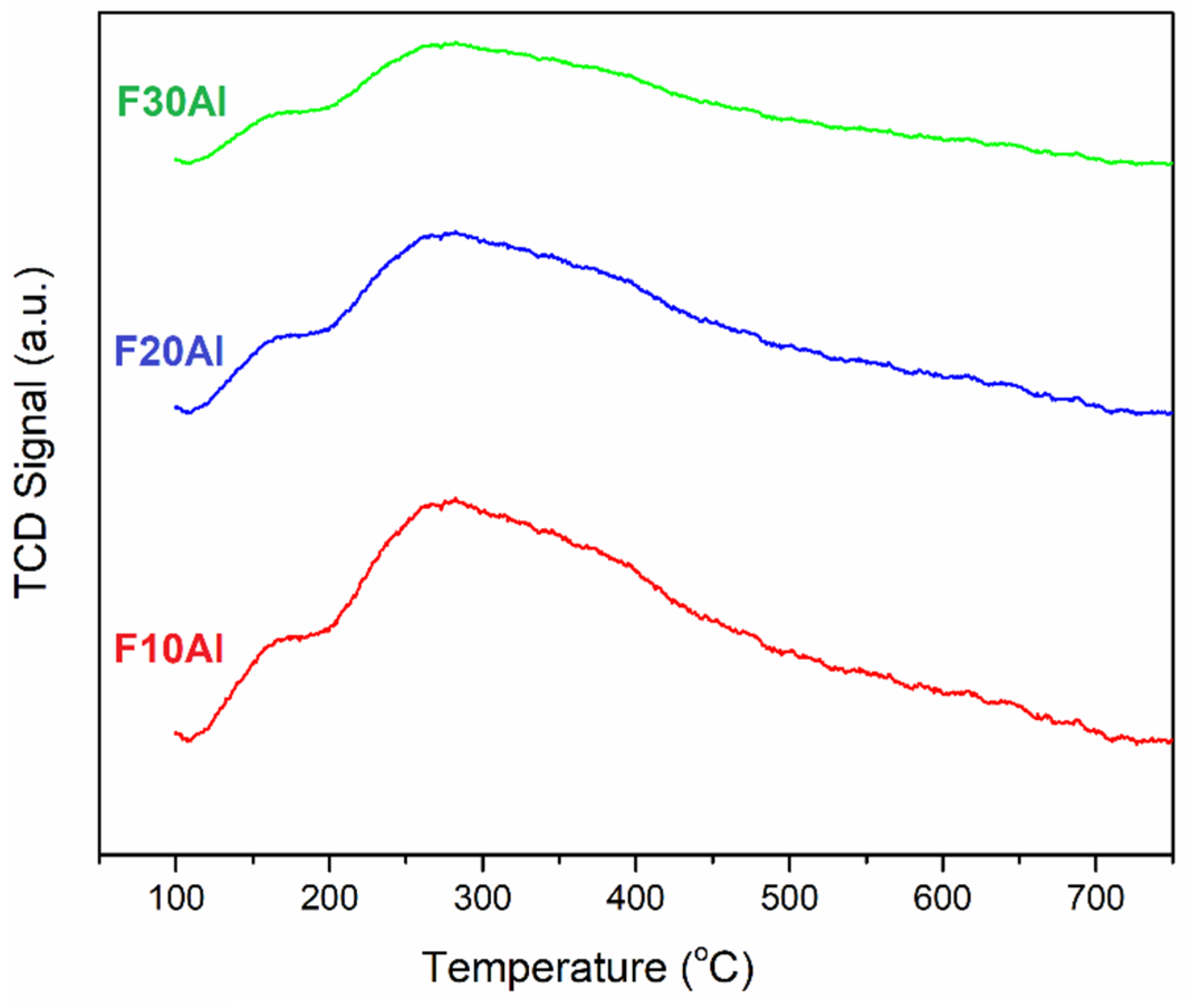
2.1. Characterization of Fresh Catalysts
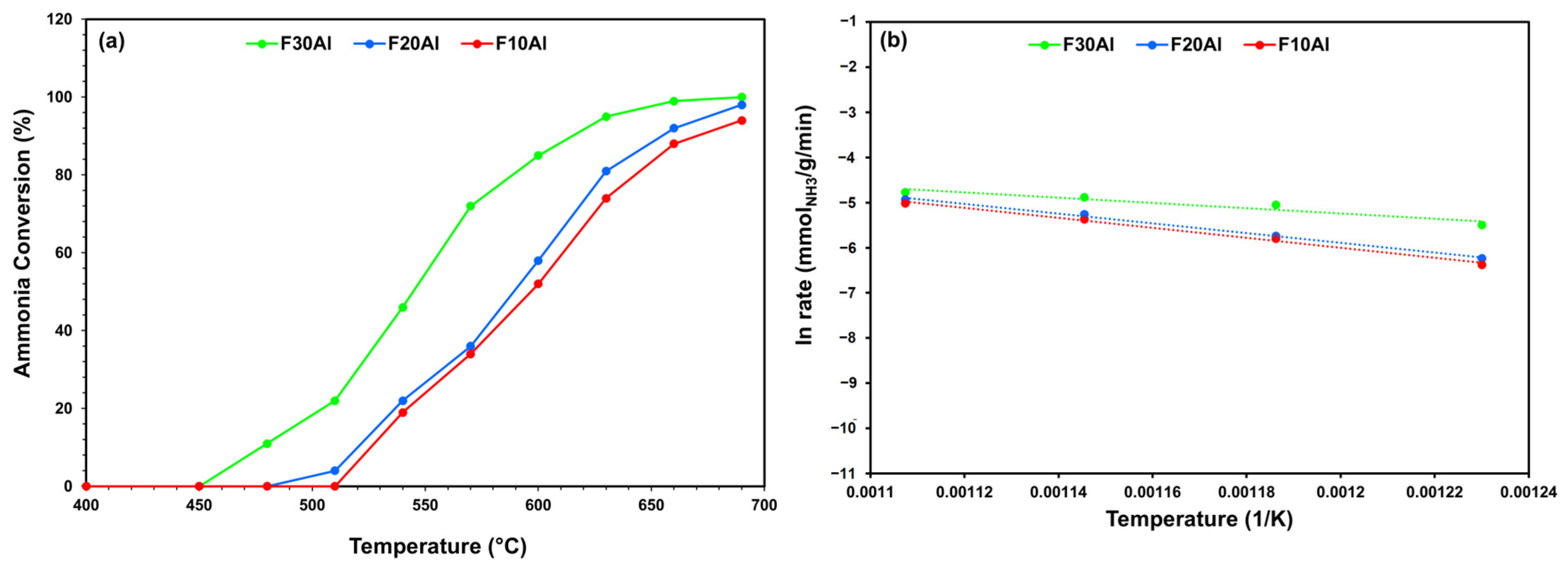
2.2. Activity Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Evaluation
4. Conclusions
- ➢
- XRD profiles indicated that iron oxide, i.e., Fe2O3, was found to be the major active component that contributes to catalytic activity performance during ammonia decomposition reaction.
- ➢
- The amount of hydrogen adsorption sites increased with an increase in iron loading, as depicted by H2-TPD results.
- ➢
- The strong affinity of iron for nitrogen leading to iron nitride formation was observed by N2-TPD, where the amount of N2 desorbed decreased with an increase in iron amount.
- ➢
- The increase in iron facilitated an easier reduction in oxides of iron, as demonstrated by H2-TPR.
- ➢
- The specific surface area decreased with iron loading due to the fact that iron blocked pores of the support.
- ➢
- Activity results showed that with the increase in iron from 10 to 30 wt%, ammonia conversion increased, with F30Al outperforming the rest of the catalysts.
- ➢
- The ammonia conversions of iron-based catalysts of this work were both comparable and, in some cases, higher than previously reported catalysts.
- ➢
- The easily reducible iron species, higher hydrogen desorption, and formation of iron nitride remained as the major factors behind the remarkable catalytic performance of iron-based catalysts of this work.
- ➢
- The scientific findings and insights of this work could provide a guideline for designing an efficient iron-based catalyst in future research and development advancing the field of hydrogen production and transport.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for Hydrogen Storage; A Review of Catalytic Ammonia Decomposition and Hydrogen Separation and Purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, Y.; Kim, H.W.; Lee, S.-U.; Kim, J.-R.; Kim, T.-W.; Lee, Y.-J.; Chae, H.-J. Ru-Supported Lanthania-Ceria Composite as an Efficient Catalyst for COx-Free H2 Production from Ammonia Decomposition. Appl. Catal. B Environ. 2021, 285, 119831. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-Temperature Ammonia Decomposition Catalysts for Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Arabczyk, W.; Zamlynny, J. Study of the Ammonia Decomposition over Iron Catalysts. Catal. Lett. 1999, 60, 167–171. [Google Scholar] [CrossRef]
- Akarçay, Ö.; Kurtoğlu, S.F.; Uzun, A. Ammonia Decomposition on a Highly-Dispersed Carbon-Embedded Iron Catalyst Derived from Fe-BTC: Stable and High Performance at Relatively Low Temperatures. Int. J. Hydrogen Energy 2020, 45, 28664–28681. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Ma, D.; Yang, Z. Adsorption and Dissociation of Ammonia on Small Iron Clusters. Int. J. Hydrogen Energy 2015, 40, 346–352. [Google Scholar] [CrossRef]
- Lanzani, G.; Laasonen, K. NH3 Adsorption and Dissociation on a Nanosized Iron Cluster. Int. J. Hydrogen Energy 2010, 35, 6571–6577. [Google Scholar] [CrossRef]
- Ganley, J.C.; Thomas, F.S.; Seebauer, E.G.; Masel, R.I. A Priori Catalytic Activity Correlations: The Difficult Case of Hydrogen Production from Ammonia. Catal. Lett. 2004, 96, 117–122. [Google Scholar] [CrossRef]
- Hu, X.-C.; Fu, X.-P.; Wang, W.-W.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.-J.; Yan, C.-H. Ceria-Supported Ruthenium Clusters Transforming from Isolated Single Atoms for Hydrogen Production via Decomposition of Ammonia. Appl. Catal. B Environ. 2020, 268, 118424. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO Prepared by a Deposition-Precipitation Method as Highly Active Catalyst for Producing COx-Free Hydrogen from Ammonia Decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Yang, J.; Yan, Y.; Wang, D.; Hu, F.; Wang, X.; Zhang, R.; Feng, G. Ru/La2O3 Catalyst for Ammonia Decomposition to Hydrogen. Appl. Surf. Sci. 2019, 476, 928–936. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, A.; Li, Y.; Zhang, Y.; Cheng, X.; Shi, C. An Investigation of the Thermal Stability, Crystal Structure and Catalytic Properties of Bulk and Alumina-Supported Transition Metal Nitrides. J. Alloys Compd. 2008, 464, 488–496. [Google Scholar] [CrossRef]
- Arabczyk, W.; Zamłynny, J.; Moszyński, D. Kinetics of Nanocrystalline Iron Nitriding. Pol. J. Chem. Technol. 2010, 12, 38–43. [Google Scholar] [CrossRef]
- Wood, T.J.; Makepeace, J.W.; David, W.I.F. Neutron Diffraction and Gravimetric Study of the Iron Nitriding Reaction under Ammonia Decomposition Conditions. Phys. Chem. Chem. Phys. 2017, 19, 27859–27865. [Google Scholar] [CrossRef]
- Pelka, R.; Moszyńska, I.; Arabczyk, W. Catalytic Ammonia Decomposition Over Fe/Fe4N. Catal. Lett. 2008, 128, 72–76. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. Studies of the Kinetics of Reaction Between Iron Catalysts and Ammonia—Nitriding of Nanocrystalline Iron with Parallel Catalytic Ammonia Decomposition. Top. Catal. 2009, 52, 1506–1516. [Google Scholar] [CrossRef]
- Pelka, R.; Kiełbasa, K.; Arabczyk, W. Catalytic Ammonia Decomposition during Nanocrystalline Iron Nitriding at 475 °C with NH3/H2 Mixtures of Different Nitriding Potentials. J. Phys. Chem. C 2014, 118, 6178–6185. [Google Scholar] [CrossRef]
- Yeo, S.C.; Han, S.S.; Lee, H.M. Mechanistic Investigation of the Catalytic Decomposition of Ammonia (NH3) on an Fe(100) Surface: A DFT Study. J. Phys. Chem. C 2014, 118, 5309–5316. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, C.; Gong, W.; Guo, H. Plasma Driven Ammonia Decomposition on a Fe-Catalyst: Eliminating Surface Nitrogen Poisoning. Chem. Commun. 2013, 49, 3787. [Google Scholar] [CrossRef]
- Lei, B.; Wen, J.; Ren, S.; Zhang, L.; Zhang, H. Highly Efficient COx-Free Hydrogen Evolution Activity on Rod Fe2N Catalysts for Ammonia Decomposition. New J. Chem. 2019, 43, 18277–18284. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Zhao, Y.; Zhang, R.; Zhang, J.; Guo, H. NH3 Decomposition for H2 Generation: Effects of Cheap Metals and Supports on Plasma–Catalyst Synergy. ACS Catal. 2015, 5, 4167–4174. [Google Scholar] [CrossRef]
- Bell, T.E.; Torrente-Murciano, L. H2 Production via Ammonia Decomposition Using Non-Noble Metal Catalysts: A Review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Gu, D.; Pistidda, C.; Horstmann, C.; Dornheim, M.; Ternieden, J.; Weidenthaler, C. Tracking the Active Catalyst for Iron-Based Ammonia Decomposition by In Situ Synchrotron Diffraction Studies. ChemCatChem 2018, 10, 4465–4472. [Google Scholar] [CrossRef]
- AlAmoudi, O.M.; Ullah Khan, W.; Hantoko, D.; Bakare, I.A.; Ali, S.A.; Hossain, M.M. Catalytic Activity of Co/γ-Al2O3 Catalysts for Decomposition of Ammonia to Produce Hydrogen. Fuel 2024, 372, 132230. [Google Scholar] [CrossRef]
- Pirola, C.; Bianchi, C.L.; Di Michele, A.; Vitali, S.; Ragaini, V. Fischer Tropsch and Water Gas Shift Chemical Regimes on Supported Iron-Based Catalysts at High Metal Loading. Catal. Commun. 2009, 10, 823–827. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane Decomposition over Iron Catalyst for Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Brunauer, S.; Love, K.S.; Keenan, R.G. Adsorption of Nitrogen and the Mechanism of Ammonia Decomposition Over Iron Catalysts. J. Am. Chem. Soc. 1942, 64, 751–758. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Q.; Ren, S.; Arshid, M.A.; Chu, W.; Chen, C. Implication of Iron Nitride Species to Enhance the Catalytic Activity and Stability of Carbon Nanotubes Supported Fe Catalysts for Carbon-Free Hydrogen Production via Low-Temperature Ammonia Decomposition. Catal. Sci. Technol. 2018, 8, 907–915. [Google Scholar] [CrossRef]
- Lu, B.; Li, L.; Ren, M.; Liu, Y.; Zhang, Y.; Xu, X.; Wang, X.; Qiu, H. Ammonia Decomposition over Iron-Based Catalyst: Exploring the Hidden Active Phase. Appl. Catal. B Environ. 2022, 314, 121475. [Google Scholar] [CrossRef]
- Purcel, M.; Berendts, S.; Bonati, L.; Perego, S.; Müller, A.; Lerch, M.; Parrinello, M.; Muhler, M. Iron Nitride Formation and Decomposition during Ammonia Decomposition over a Wustite-Based Bulk Iron Catalyst. ACS Catal. 2024, 14, 13947–13957. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia Decomposition over Ni/La2O3 Catalyst for on-Site Generation of Hydrogen. Appl. Catal. A Gen. 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- Lorenzut, B.; Montini, T.; Bevilacqua, M.; Fornasiero, P. FeMo-Based Catalysts for H2 Production by NH3 Decomposition. Appl. Catal. B Environ. 2012, 125, 409–417. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.H.; Yan, Z.F.; Lu, G.Q.; Rintoul, L. Catalytic Ammonia Decomposition over Ru/Carbon Catalysts: The Importance of the Structure of Carbon Support. Appl. Catal. A Gen. 2007, 320, 166–172. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Chen, W.; Gong, Q.; Luo, J.; Lin, R.; Xin, H.; Zhang, H.; Wang, D.; Peng, Q.; et al. Sub-Nm Ruthenium Cluster as an Efficient and Robust Catalyst for Decomposition and Synthesis of Ammonia: Break the “Size Shackles”. Nano Res. 2018, 11, 4774–4785. [Google Scholar] [CrossRef]
- Hu, X.-C.; Wang, W.-W.; Jin, Z.; Wang, X.; Si, R.; Jia, C.-J. Transition Metal Nanoparticles Supported La-Promoted MgO as Catalysts for Hydrogen Production via Catalytic Decomposition of Ammonia. J. Energy Chem. 2019, 38, 41–49. [Google Scholar] [CrossRef]
- Gu, Y.-Q.; Jin, Z.; Zhang, H.; Xu, R.-J.; Zheng, M.-J.; Guo, Y.-M.; Song, Q.-S.; Jia, C.-J. Transition Metal Nanoparticles Dispersed in an Alumina Matrix as Active and Stable Catalysts for COx-Free Hydrogen Production from Ammonia. J. Mater. Chem. A 2015, 3, 17172–17180. [Google Scholar] [CrossRef]
- Henpraserttae, S.; Charojrochkul, S.; Klysubun, W.; Lawtrakul, L.; Toochinda, P. Reduced Temperature Ammonia Decomposition Using Ni/Zr-Doped Al2O3 Catalyst. Catal. Lett. 2018, 148, 1775–1783. [Google Scholar] [CrossRef]



| Catalyst | Physisorption Results | Fe2O3 (wt%) | |||
|---|---|---|---|---|---|
| SBET (m2/g) a | P.V. (cm3/g) b | P.D. (nm) c | Target | XRF | |
| Al | 73.7 | 0.33 | 17.7 | - | - |
| F10Al | 55.7 | 0.29 | 20.8 | 10 | 11.26 |
| F20Al | 42.9 | 0.24 | 22.1 | 20 | 20.73 |
| F30Al | 25.9 | 0.18 | 27.1 | 30 | 27.55 |
| Catalyst | H2 Uptake (mmol/g) | N2 Uptake (mmol/g) |
|---|---|---|
| Al | - | - |
| F10Al | 0.097 | 0.511 |
| F20Al | 0.114 | 0.451 |
| F30Al | 0.295 | 0.367 |
| Catalyst | Metal Loading (wt%) | GHSV (L/h/gcat.) | Temperature (°C) | Ammonia Conversion (%) | Ref. |
|---|---|---|---|---|---|
| F10Al | 10 | 12 | 600 | 52 | This work |
| Ni/ZrO2 | 10 | 6 | 600 | 20 | [32] |
| Fe/YSZ | 10 | 46 | 600 | 19 | [33] |
| MP-Fe | 10 | 15 | 610 | 52 | [23] |
| F10Al | 10 | 12 | 540 | 19 | This work |
| Ru/AC | 5 | 30 | 550 | 14.4 | [34] |
| Mo/YSZ | 10 | 46 | 600 | 28 | [33] |
| F20Al | 20 | 12 | 600 | 58 | This work |
| Ni/γ-Al2O3 | 20 | 7.5 | 600 | 88 | [35] |
| Ru/AC | 5 | 15 | 600 | 58 | [36] |
| 20Fe/La-MgO(5) | 20 | 22 | 550 | 61 | [37] |
| F30Al | 30 | 12 | 600 | 85 | This work |
| BM-Fe | 40 | 15 | 610 | 14 | [23] |
| Fe PS3 | 95 | 7 | 500 | 20 | [4] |
| FeAl | 90 | 36 | 600 | 86 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, W.U.; Putra, A.F.P.; AlMohamadi, H.; Hossain, M.M. Iron-Based Alumina-Supported Catalysts for Clean Hydrogen Production from Ammonia. Catalysts 2025, 15, 242. https://doi.org/10.3390/catal15030242
Khan WU, Putra AFP, AlMohamadi H, Hossain MM. Iron-Based Alumina-Supported Catalysts for Clean Hydrogen Production from Ammonia. Catalysts. 2025; 15(3):242. https://doi.org/10.3390/catal15030242
Chicago/Turabian StyleKhan, Wasim Ullah, Achmad Ferdiansyah Pradana Putra, Hamad AlMohamadi, and Mohammad M. Hossain. 2025. "Iron-Based Alumina-Supported Catalysts for Clean Hydrogen Production from Ammonia" Catalysts 15, no. 3: 242. https://doi.org/10.3390/catal15030242
APA StyleKhan, W. U., Putra, A. F. P., AlMohamadi, H., & Hossain, M. M. (2025). Iron-Based Alumina-Supported Catalysts for Clean Hydrogen Production from Ammonia. Catalysts, 15(3), 242. https://doi.org/10.3390/catal15030242








