Abstract
Cleavage of C-C bonds is crucial for hydrogen production via aqueous phase reforming of biomass-derived oxygenates. In this study, the hydrogen production performance and C-C bond cleavage capacity of Ni-W/AC catalysts with varying W/Ni ratios are evaluated using ethylene glycol as a model compound. A series of APR experiments conducted suggests that Ni-0.2W/AC catalyst exhibits the highest C1/C2+ ratio of 15.87 and achieves a hydrogen yield of 47.76%. The enhanced Ni-W bimetallic interactions, which significantly improve the efficiency of C-C bond cleavage and increase catalyst activity by promoting active site dispersion, are confirmed by detailed characterization techniques. Further analysis of product distribution provides insights into the reaction pathways of ethylene glycol and the reaction mechanism for ethanol during aqueous phase reforming. All the results indicate that this catalytic reforming method effectively facilitates C-C bond cleavage and hydrogen production, contributing to a better understanding of APR mechanisms for biomass-derived oxygenates.
1. Introduction
Biomass, an abundant and renewable resource on earth, can be converted into valuable chemicals and clean fuels through various processes such as physical, biological, chemical, or thermochemical treatments, though challenges in the efficient use of biomass during biorefinery processes remain unsolved [1,2,3]. In 2002, Dumesic et al. [2] proposed a highly promising pathway for efficient conversion of biomass or its oxygenated derivatives into hydrogen, carbon monoxide, carbon dioxide, and methane through a catalytic reforming process under hydrothermal conditions, termed aqueous phase reforming (APR). Compared to those complex and costly technologies such as enzymatic hydrolysis, gasification, and steam reforming, APR offers the advantages of milder reaction conditions, lower energy consumption, fewer gaseous by-products, and minimal carbon deposition [4,5,6,7,8]. Moreover, Recent studies have demonstrated the potential of APR technology in real-world applications, such as converting dairy wastewater and plastic waste into hydrogen fuel [9,10]. These advancements emphasize the need for development in APR processes. Hydrogen production via APR involves the adsorption of biomass-derived oxygenates onto the catalyst, followed by decomposition, activation through dehydrogenation, and C-C bond cleavage to produce CO* intermediates which then react with water to further generate more hydrogen [11]. The primary difficulty in converting biomass and its oxygenated derivatives into hydrogen energy through a one-pot APR process stems from the complex structure of biomass, which largely consists of multi-carbon organic compounds rich in C-C bonds, thereby complicating the reaction process [12].
Due to the favorable characteristics for C-C bond cleavage and efficient hydrogen production, biomass-derived oxygenates with a C/O atomic ratio of 1:1 are considered ideal model compounds for investigating hydrogen production via the APR process [3]. Among these, ethylene glycol stands out as a particularly promising model compound due to its simple structure, containing a single C-C bond and two hydroxyl groups, making it ideal for studying C-C bond cleavage during APR. In addition, ethylene glycol is one of the most readily available products, as well as a waste material in industrial aqueous residues [13,14]. Widely recognized mechanisms for hydrogen production in APR of ethylene glycol are the reforming reaction (Equation (1)) and the subsequent WGS reaction (Equation (2)) [11]. Theoretically, one mol of ethylene glycol can generate five mol of H2 by APR. However, certain side reactions, such as C-O bond cleavage, consume hydrogen and cause rearrangement and tautomerization of derived molecules, forming short- and long-chain alkanes or carboxylic acids, which reduce the hydrogen yield [15]. Kandoi et al. [16] demonstrated through DFT calculations and microkinetic models that the cleavage mechanism of the C-C bond in ethylene glycol dehydrogenation intermediates is a competitive reaction pathway worthy of in-depth study, but it has received relatively little attention [17,18]. Enhancing the adsorption and C-C bond cleavage performance of ethylene glycol dehydrogenation intermediates in APR will not only strengthen the competitiveness of the C-C bond cleavage pathway but also reduce side reactions, significantly increasing hydrogen yield. However, the complexity of hydrothermal conditions in APR has led to an incomplete understanding of the reaction mechanisms, compounded by the presence of stubborn byproducts.
C2H6O2 (l) + 2H2O (l) → 2CO2 (g) + 5H2 (g)
C2H6O2 (l) → 2CO (g) +3H2 (g)
CO (g) + H2O (l) → CO2 (g) + H2 (g)
Both non-precious Ni-based and noble Pt-based catalysts exhibit comparable C-C bond cleavage activities for the APR of oxygenated compounds, particularly ethylene glycol [19]. However, Ni-based catalysts encounter challenges in large-scale industrial applications due to their pronounced methanation activity, which can reduce the selectivity and yield of desired products [20]. Selective C-C bond cleavage is crucial for optimizing hydrogen production in the APR process, while the cleavage of C-O bonds inevitably leads to methane formation. Further insights are provided by Davda et al. [21], who reported that the TOFH2 on Ni metal active sites in ethylene glycol APR is ten times lower than that of Pt. Given the lower cost of nickel-based catalysts compared to platinum-based catalysts, the rational design of nickel-based catalysts and the addition of promoters contribute to extending their application in catalytic APR reactions.
Ni-W bimetallic catalysts have shown high activity for hydrogen production through reactions such as cellulose conversion [22], auto-thermal reforming [23], and hydrothermal gasification [24]. However, their application in APR, particularly for C-C bond cleavage, remains largely underexplored. Furthermore, tungsten-based catalysts have shown a facilitating effect on the cleavage of long-chain C-C bonds and in promoting water-splitting processes [25,26,27]. However, the role of Ni-W bimetallic catalysts in improving C-C bond cleavage and boosting hydrogen yields from ethylene glycol remains unclear, particularly regarding the underlying mechanisms. On the other hand, the adsorption properties of key intermediates on Ni4W have been well established. For example, the adsorption energy of *CHOHCHOH on Ni4W is calculated to be −4.759 eV. Other intermediates, including *CHOCHO, *CHCH2OH, and *CH2CH2OH, exhibit negative adsorption energies, demonstrating the strong affinity of NiW bimetallic catalysts for alcohol dehydrogenation intermediates [28,29]. Nevertheless, a fundamental investigation of Ni-W interaction and the influence on the reaction mechanisms driving hydrogen production from ethylene glycol and similar substrates is essential. Addressing these gaps will enhance both the efficiency and applicability of Ni-W catalytic systems in APR of multi-carbon biomass oxygenated derivatives [24].
To achieve efficient hydrogen production from the APR of biomass-derived oxygenates, this study synthesized Ni-W/AC catalysts using the impregnation method, and ethylene glycol was used as a model compound to evaluate the catalytic APR performance, as well as the C-C bond cleavage ability. The physicochemical properties of the catalysts were investigated through a series of characterizations to reveal their effect on C-C bond cleavage in the APR process of ethylene glycol. The reaction pathways for catalytic APR of ethylene glycol on the Ni-W/AC catalyst were deduced based on product distribution and catalyst characterizations. A detailed investigation into the ethylene glycol APR reaction mechanism was also conducted by using ethanol as a representative byproduct.
2. Catalytic Tests
All APR tests of ethylene glycol and ethanol (as a typical byproduct) were conducted in a stainless-steel batch reactor. All catalysts were transferred to the reactor under inert gas protection. Initially, 20 mL of a 10 wt% solution of either ethylene glycol or ethanol, along with 0.2 g of catalyst powder, was introduced into the reactor. The reactor was then hermetically sealed and purged three times with pure argon gas to eliminate any residual air. The reaction was carried out at 250 °C with continuous magnetic stirring at 400 rpm. After reacting for 6 h, the reactor was naturally cooled to room temperature, and the gaseous products were collected in a gas bag. The total gas volume was measured using the drainage method, and its composition was analyzed by gas chromatography (GC-2014C, Shimadzu, Kyoto, Japan) with a TDX-01 column and a thermal conductivity detector (TCD), using argon as the carrier gas. The liquid products were filtered and weighed. Their concentrations were determined and verified by high-performance liquid chromatography (HPLC) using a Waters e2695 Alliance system (Milford, MA, USA). This detailed procedure ensured precise analysis and quantification of both gas and liquid products resulting from the APR of ethylene glycol. All catalytic tests were conducted in triplicate to ensure reproducibility, with average values reported and standard deviations below 5%. Table 1 shows the parameters and equations used to evaluate the performance of APR. To evaluate the efficiency of C-C bond cleavage in the reaction condition of APR of ethylene glycol, the ratio of single-carbon products to multi-carbon products (C1/C2+) was introduced as a bond cleavage performance metric of APR. Carbon balance should be in the range of 90–100% to ensure data accuracy.

Table 1.
Parameters and equations for evaluating APR performance.
3. Results and Discussion
3.1. Catalyst Characterizations
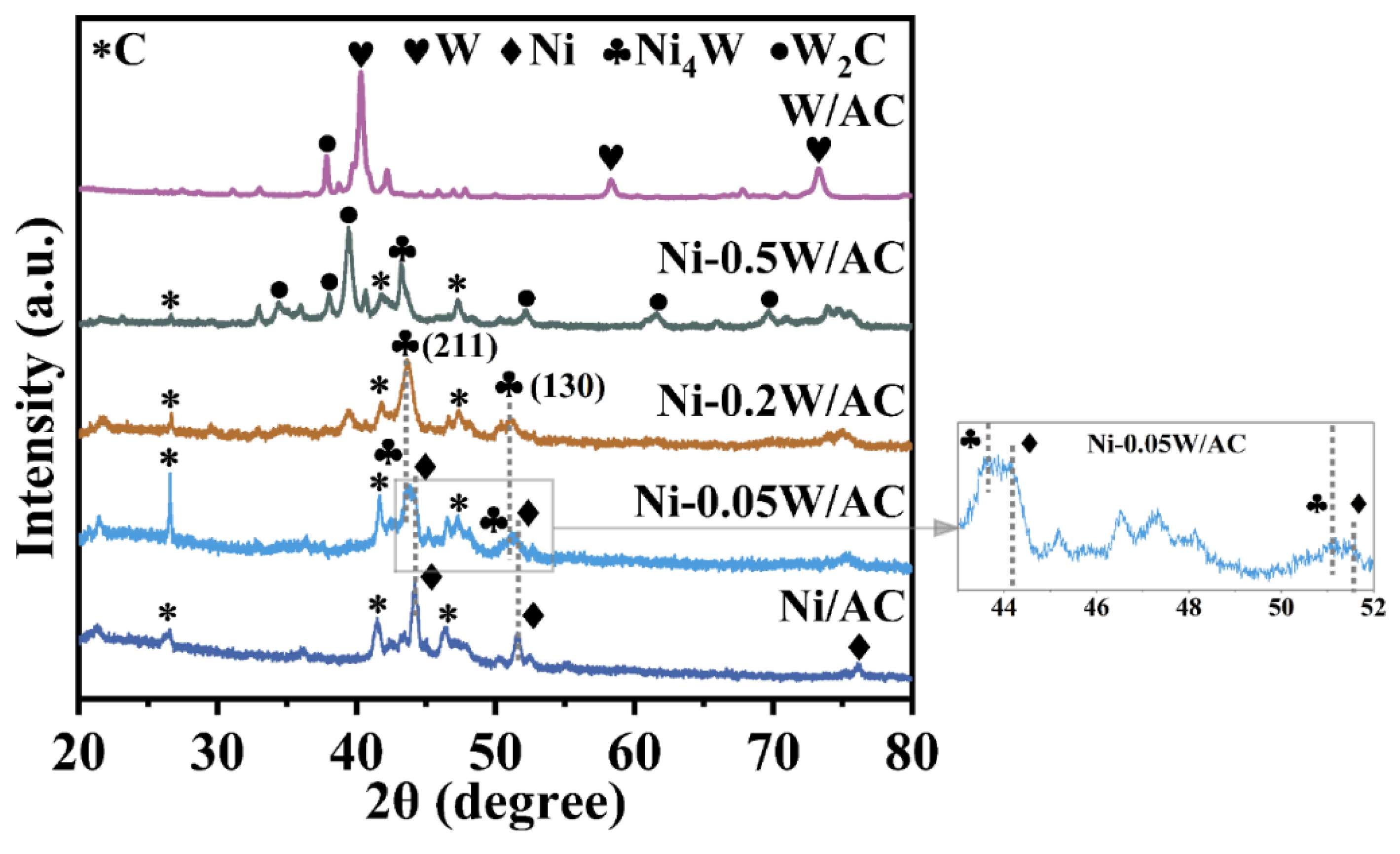
To explore the crystal structure and morphology of the catalysts, a series of characterizations were performed. As shown in Figure 1, for the Ni/AC catalyst without W loading, sharp diffraction peaks at 2θ = 44.4°, 51.8°, and 76.3° correspond to the (111), (200), and (220) crystal planes of cubic Ni (PDF 04-010-6148) [30]. For the W/AC catalyst without Ni loading, diffraction peaks at 2θ = 40.2°, 58.3°, and 73.4° correspond to the metal W (PDF 01-075-6145), the low-intensity diffraction peaks at 2θ = 26.6° and between about 45° and 50° are mainly from the amorphous carbon [31]. With the increase in W atomic content, the Ni peaks progressively shift towards lower angles and split into two distinct diffraction components. Diffraction peaks at 2θ = 43.5° and 50.3°, corresponding to the (211) and (130) crystal planes of tetragonal Ni4W (PDF 04-003-5612), emerge [32]. In the case of the Ni-0.2W/AC catalyst, the strongest Ni4W characteristic diffraction peak becomes prominent, while the Ni peak vanishes, indicating that the composition of active Ni species on the catalyst surface is significantly influenced by W loading. Moreover, the XRD patterns of the Ni/AC, Ni-0.05W/AC, and Ni-0.2W/AC catalysts show that as the W atomic ratio increases, the Ni diffraction peaks shift gradually to lower angles, aligning with the Ni4W peak, as highlighted by the gray dashed line in Figure 1. This observation indicates that within the W/Ni atomic ratio range of 0 to 0.2, an increase in W content favors the formation of Ni species in the form of Ni4W. Simultaneously, comprehensive analysis for the diffraction peaks for the Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts reveals that the intensity of the Ni4W peaks decreases with increasing W loading, indicating at higher W ratios, W species preferentially interact with the carbon support, forming large hexagonal W2C crystals (PDF 00-035-0776, 20.83 nm, see Table 2) corresponding to the diffraction peaks around 2θ = 39.6° and 43.5° in the Ni-0.5W/AC catalyst of Figure 1 [33,34,35]. The average crystallite sizes of the active metallic species were quantitatively calculated from the three strongest diffraction peaks using the Scherrer equation, with the results summarized in Table 2. The W2C crystal formed at high W loading measures 20.83 nm. However, the Ni-0.2W/AC catalyst showed the smallest average crystallite size, measuring only 8.23 nm. Changes in phase composition may affect the catalytic activity.
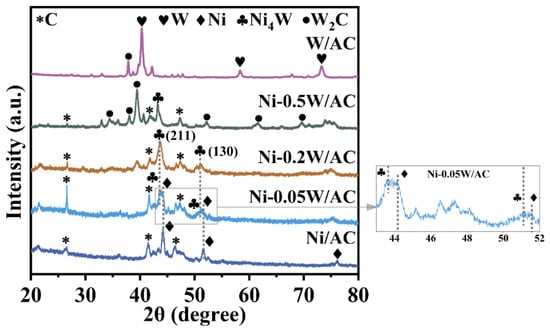
Figure 1.
XRD patterns of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts. The * symbol denotes the diffraction peaks corresponding to the carbon support.

Table 2.
The physical–chemical properties of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts.
The ICP results of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts are listed in Table 2. The actual W/Ni loading ratios closely match the theoretical values calculated based on the W/Ni atomic ratio, with the corresponding actual atomic ratios being 0, 0.05, 0.22, and 0.52, respectively. W/AC represents the catalyst that contains no nickel.
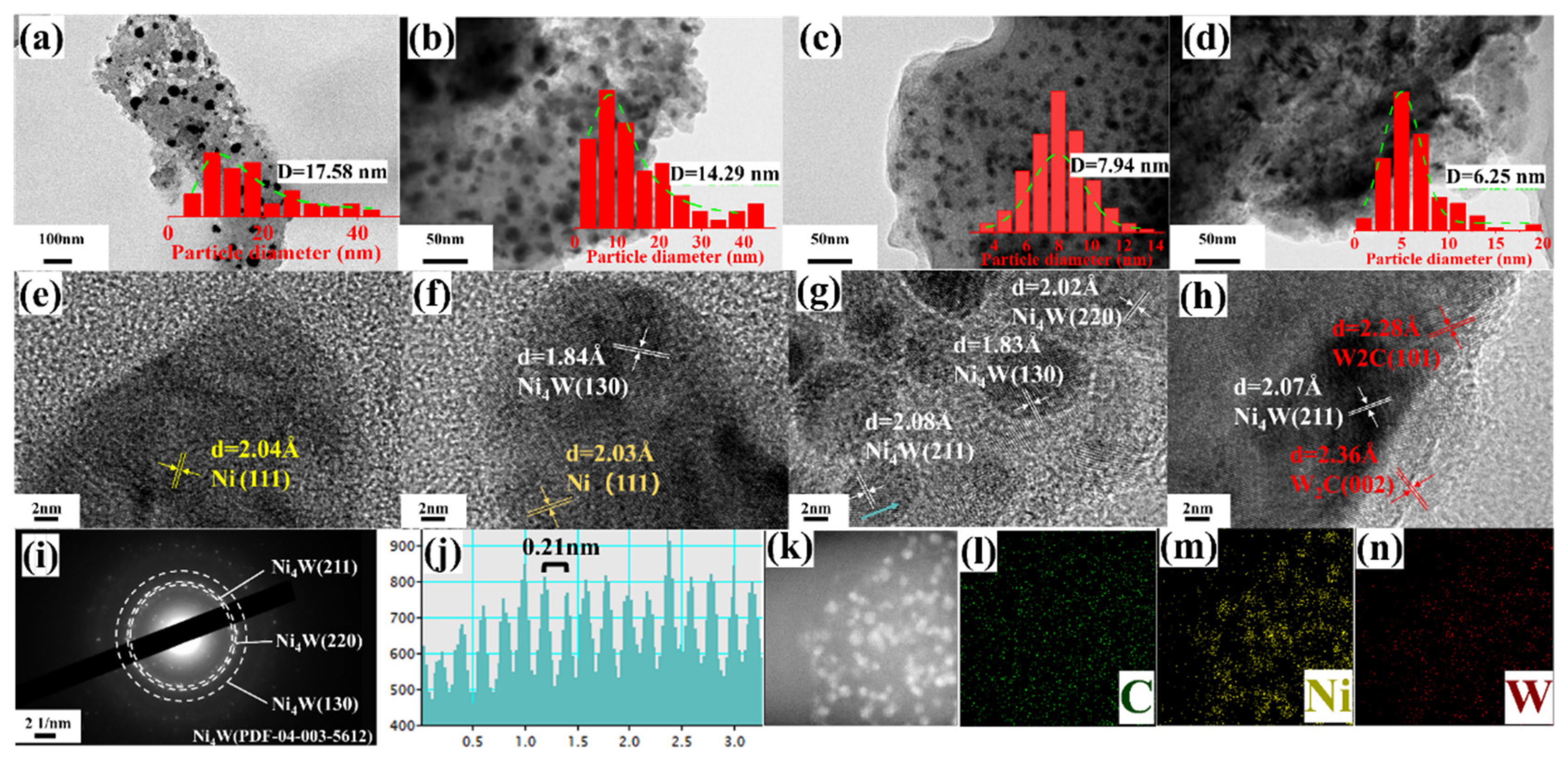
To investigate the influence of the W/Ni atomic ratio on the morphology and elemental composition of the selected samples, TEM, HRTEM, and EDS characterizations were performed. TEM images (Figure 2a–d) reveal quasi-spherical particles with nanoscale sizes in the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, and Ni-0.5W/AC catalysts, respectively. Among them, Ni-0.2W/AC shows the most uniform particle size distribution (Figure 2c), and the EDS mapping (Figure 2k–n) also illustrates the homogeneous dispersion of Ni and W species on the AC support. As the W/Ni atomic ratio increased, particle size decreased, and the size distribution became more concentrated, as seen in the spectra for Ni/AC, Ni-0.05W/AC, and Ni-0.2W/AC (Figure 2a–c). This trend in mean particle size aligns with the crystallite size calculated using the Scherrer equation (Table 1). The corresponding lattice fringes observed in HRTEM (Figure 2e–g) show the interplanar distances for Ni about 2.04 Å in the Ni/AC and Ni-0.05W/AC catalysts, and about 1.84 Å for Ni4W in the Ni-0.05W/AC and Ni-0.2W/AC catalysts, confirming the changes in species on the support. For the Ni-0.2W/AC catalyst, the (211), (130), and (220) crystal planes identified in the SAED pattern (Figure 2i), along with the lattice spacing of approximately 0.21 nm observed in the FFT pattern (Figure 2j, fitted from the area indicated by the blue arrow in Figure 2g), confirm the presence of Ni4W, further supporting the XRD results. However, the (220) crystal plane is difficult to detect in the XRD patterns due to its inherently low peak intensity. In contrast, excessive W loading results in thicker and amorphous W2C layer deposits that envelop Ni4W particles on the Ni-0.5W/AC catalyst, which serves as weaker active sites for the reaction. These observations are visible in HRTEM images (lattice spacings of 0.24 nm and 0.23 nm for W2C, Figure 2h) and Figure 2d. In summary, the Ni/AC and Ni-0.05W/AC catalysts exhibit some agglomerated Ni4W particles, while excessive W loading, as in Ni-0.5W/AC, results in agglomerated W2C blocks covering the Ni4W particles. The TEM and XRD results conclude that the Ni-0.2W/AC catalyst shows the most uniform particle distribution and the smallest Ni4W particle size.
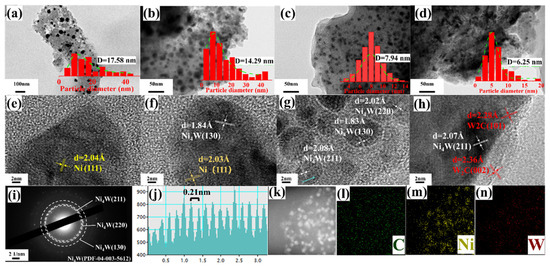
Figure 2.
TEM and HRTEM images of the sample Ni/AC catalyst (a,e), Ni-0.05W/AC catalyst (b,f), Ni-0.2W/AC catalyst (c,g), Ni-0.5W/AC catalyst (d,h), and SAED (i), FFT image (j), and EDS mapping images (k–n) of the Ni-0.2W/AC catalyst.
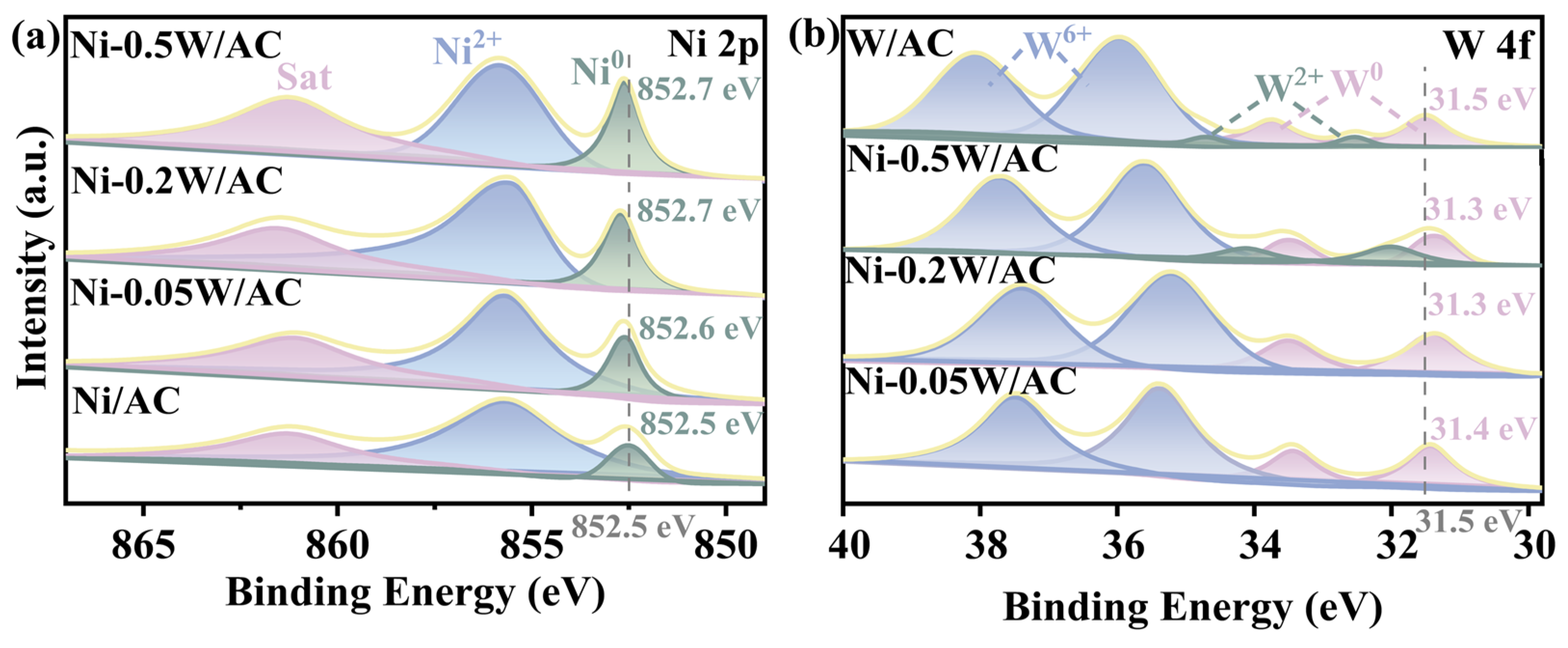
The electronic interaction between Ni and W species was examined using XPS. Deconvolutions were performed using a Gaussian-Lorentzian curve-fitting procedure to identify Ni and W species in different chemical states based on the positions of the Ni 2p (Figure 3a) and W 4f (Figure 3b) levels. Binding energy values at approximately 855.6 eV and 852.5 eV were attributed to Ni2+ and Ni0 species, with a satellite peak observed at around 861.2 eV [36,37,38]. Compared to the binding energy for Ni0 in the Ni/AC catalyst, the binding energies for Ni0 in the Ni-0.05W/AC, Ni-0.2W/AC, and Ni-0.5W/AC catalysts exhibited distinct blue shifts of 0.1, 0.2, and 0.2 eV, respectively. The shift indicates that the electronic interaction between Ni and W species modifies the electronic structure, reducing the d-band density of Ni, which aligns with the findings reported by Yang et al. [39]. For W 4f (Figure 3b), each chemical state appears as doublets due to spin-orbital coupling [40]. The peaks at approximately 35.9 and 38.0 eV correspond to W6+, while the double peaks of W0 in the W/AC catalyst are observed at 33.7 and 31.5 eV. The presence of Ni2+ and W6+ on the catalyst surface is likely due to the unavoidable oxidation of active metals or incomplete reduction in the precursor [41,42]. An approximately 0.1–0.2 eV redshift in the XPS peak of W0 is observed in samples with W/Ni atomic ratios compared to the W/AC catalyst. Figure 3a,b show shifts in peak positions toward lower and higher binding energy values for Ni0 and W0, respectively, suggesting the formation of an alloy, which corroborates the XRD and TEM results. Notably, the Ni-0.2W/AC and Ni-0.5W/AC catalysts exhibit the largest binding energy shift of 0.2 eV, indicating a strong interaction between the bimetallic Ni and W species. The doublet peaks of W2+ observed at 32.4 and 34.5 eV correspond to the W-C bond in W2C, suggesting that W species preferentially transform from the precursor state to W2C rather than Ni4W at higher temperatures and W/Ni atomic ratios [43]. This observation was strongly consistent with the XRD analysis results. Additionally, alloying Ni with W may significantly improve catalytic efficiency due to electron transfer between the two metals, which causes the Ni surface to attract negatively charged intermediates, such as OH*. The ratios of Ni0/∑Nix shown in Table 2 reveal that the Ni-0.2W/AC and Ni-0.5W/AC catalysts have a higher proportion of Ni0 than the other catalysts, indicating enhanced reoxidation of the bimetallic Ni-W precursor. However, the Ni-0.2W/AC catalyst exhibits a high surface Ni concentration (W/Ni = 0.16, Table 2), which is attributed to the uniform dispersion of Ni4W particles. By contrast, the deposition of W2C layers, as shown by TEM images (Figure 2), resulted in low Ni species concentration for the Ni-0.5W/AC catalyst.
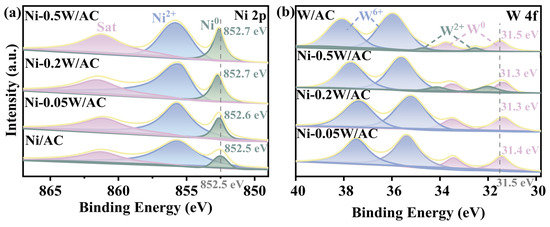
Figure 3.
High-resolution XPS spectra of (a) Ni 2p3/2 for the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, and Ni-0.5W/AC catalysts. (b) W 4f5/2 and W 4f7/2 for the Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC and W/AC catalysts.
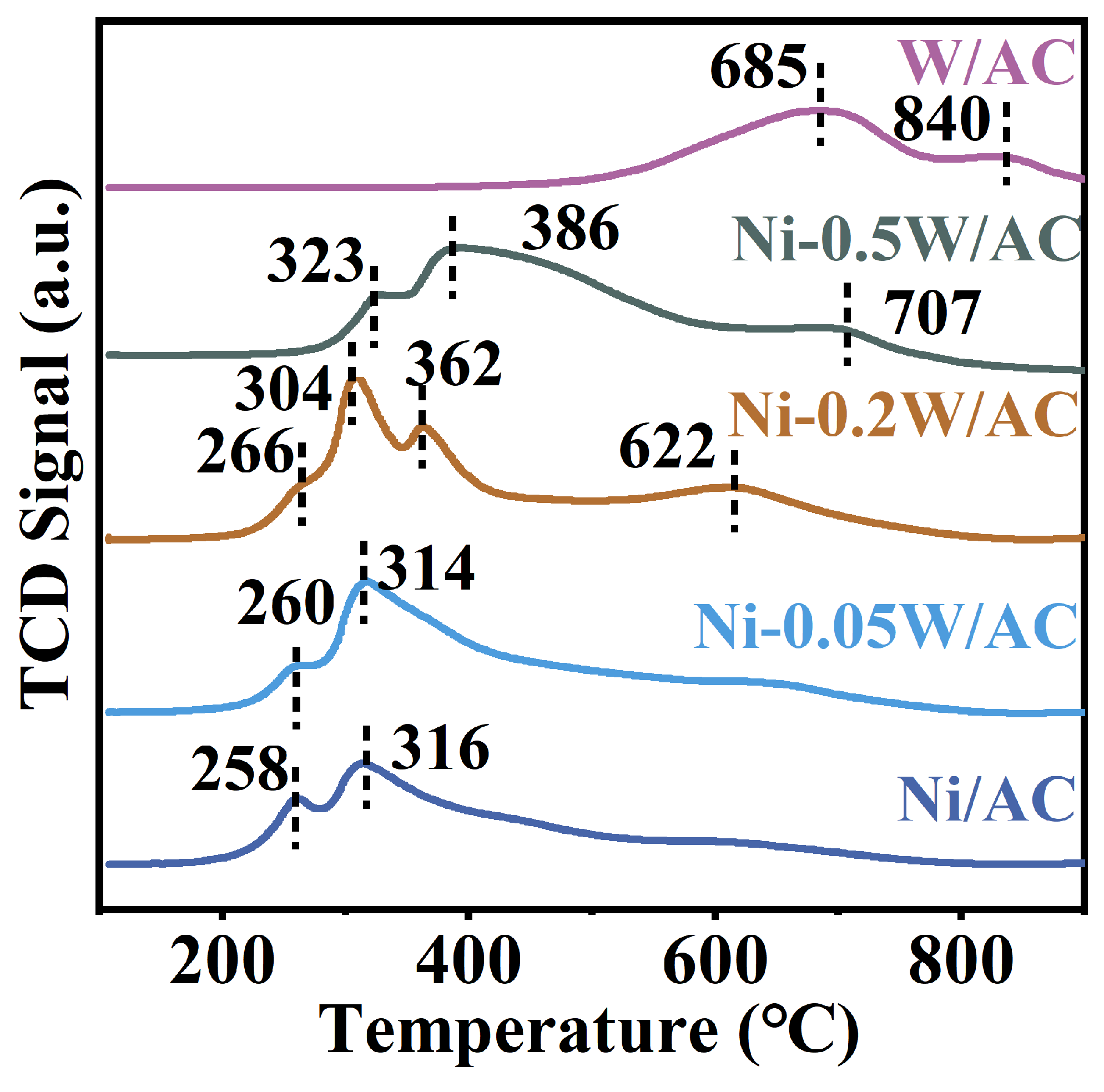
The reduction behaviors and interactions of bimetallic Ni-W within the temperature range of 100–900 °C for the above catalysts were investigated using H2-TPR characterization. Typically, the low-temperature reduction peaks centered at 258 °C and 316 °C can be attributed to NiO reduction (Figure 4) [44]. The high-temperature reduction peak emerges at 685 °C and reaches a maximum at approximately 840 °C, which can be attributed to the reduction in the tungsten oxide in the W/AC catalyst [45]. The observed reduction peaks of Ni-0.05W/AC, Ni-0.2W/AC, and Ni-0.5W/AC catalysts within the same temperature range varied significantly, likely due to different interaction strengths between Ni and W species on each catalyst. These variations led to inconsistent C-C bond cleavage performance. Additionally, the peak shape of the Ni-0.05W/AC catalyst resembles that of the Ni/AC catalyst, indicating that a low W/Ni atomic ratio has no significant impact on the reduction characteristic peak of NiO species. Broad peaks are observed around 386 °C and 707 °C in the Ni-0.5W/AC catalyst, corresponding to the transformation of NiWOx into Ni4W and W2C (Figure 4), respectively [39]. Combined with TEM results, it suggests that under strong reduction conditions, a higher W/Ni atomic ratio promotes the formation of a W2C layer, covering the metal nanoparticles on the surface [46]. The strong peaks at 304 °C, 362 °C, and 622 °C for the Ni-0.2W/AC catalyst (Figure 4), along with its highest hydrogen consumption of 186 μmol (Table 2), clearly demonstrate the enhanced reduction and dispersion of the active metal species [47]. Therefore, an optimal W/Ni atomic ratio facilitates the reduction and further dispersion of active metal species on AC at lower temperatures. Herein, from the characterization results, it can be concluded that a lower W loading leads to a weaker bimetallic interaction, while a higher W loading results in the coverage of active sites. However, when the W/Ni ratio reaches 0.2, the bimetallic interaction is maximized, significantly enhancing the uniform dispersion of active species on the catalyst surface.
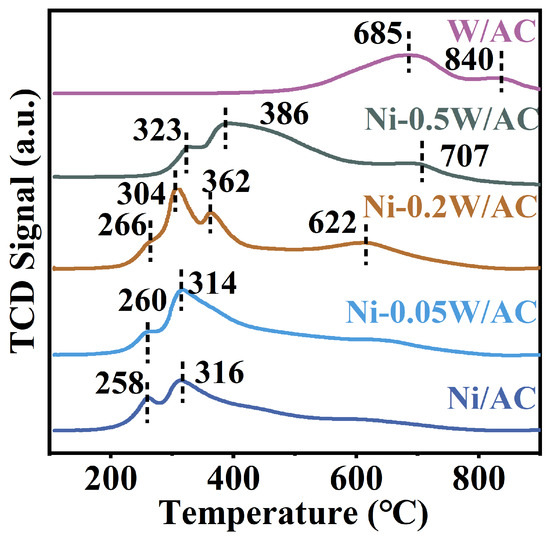
Figure 4.
H2-TPR profiles of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts.
3.2. Catalytic APR Performances of Ethylene Glycol
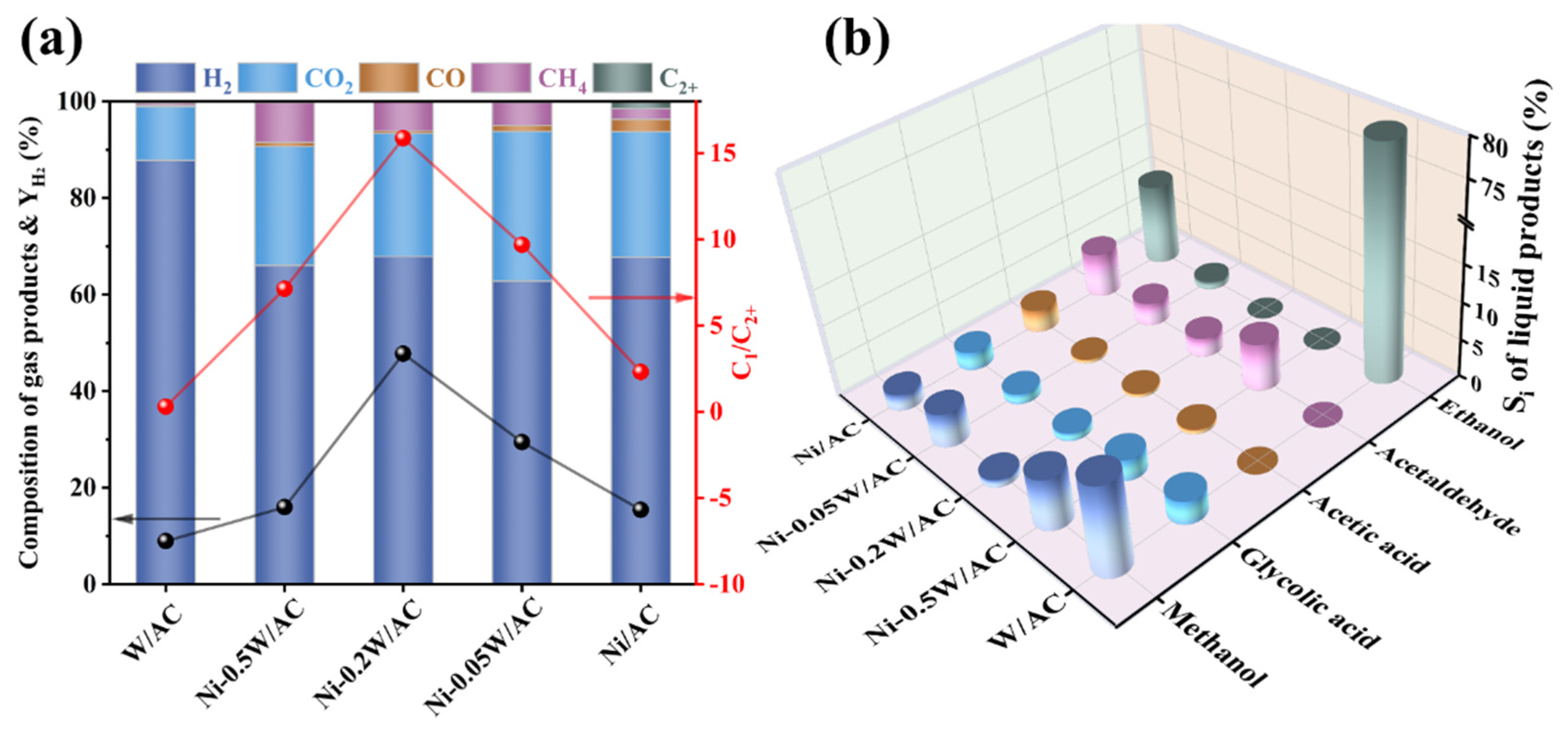
In this study, to systematically evaluate the effect and interaction between Ni and W species on the APR of ethylene glycol, the reaction performances of ethylene glycol were investigated in a stainless-steel batch reactor. Figure 5 illustrates the catalytic performance of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts, highlighting contrasts in gaseous product composition, hydrogen yield, C1/C2+ product ratio, and carbon-based selectivity of liquid products. The primary gaseous products examined in the reaction include H2, CO2, CO, CH4, and other multi-carbon compounds, while the liquid products consist of methanol, glycolic acid, acetic acid, ethanol, and acetaldehyde. Trace amounts of formic acid and 1,2-propanediol were detected, but these were disregarded due to their low concentration and minimal impact on the reaction.
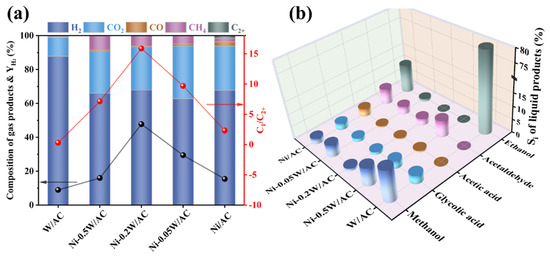
Figure 5.
(a) Gas phase product composition, , and C1/C2+ of ethylene glycol APR on the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts. (b) Selectivity for liquid product distribution of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts. Reaction conditions: 0.2 g catalyst, 20 mL of 10 wt% ethylene glycol, temperature at 250 °C.
Figure 5a illustrated that the bimetallic Ni-W catalysts significantly enhanced the yield of hydrogen ( = 16–48%) and the cleavage performance of the C-C bond (C1/C2+ = 7–16) for the ethylene glycol feedstock, far surpassing the Ni/AC and W/AC catalysts. The C1/C2+ ratio is used to reflect the strength of C-C bond cleavage ability in ethylene glycol. Interestingly, based on the product distributions reported for Sn-Ni-based catalysts in reference [11] and Pt-Mn-based catalysts in reference [48] for the aqueous phase reforming of ethylene glycol, we calculated their C1/C2+ ratios to be approximately 14 and 10, respectively. In contrast, the Ni-0.2W/AC catalyst developed in this study exhibits a higher C1/C2+ ratio of 15.87, indicating superior C-C bond cleavage activity. This difference may be attributed to the synergistic interaction between Ni and W, which promotes the adsorption and dehydrogenation of intermediates, as detailed in Section 3.1. The aforementioned ratios were calculated using the molar yields of single-carbon products and multi-carbon products as reported in the literature. Moreover, the Ni-0.2W/AC catalyst exhibited the highest XEG of 80.08% (Table 3) and a relatively low CO concentration, highlighting the synergistic advantage of bimetallic Ni-W in improving C-C bond cleavage and hydrogen production from ethylene glycol.

Table 3.
Catalytic performance of the Ni/AC, Ni-0.05W/AC, Ni-0.2W/AC, Ni-0.5W/AC, and W/AC catalysts.
The red and black lines in Figure 5a correspond to C1/C2+ and , respectively, both following a trend of initially increasing and then decreasing with rising W loading, peaking at those on Ni-0.2W/AC catalyst. The CCgas and XEG data in Table 3 indicated a strong correlation between hydrogen production and the conversion mechanism of ethylene glycol. The characterization results also revealed a correlation between the particle size and dispersion of active metallic particles in the APR of ethylene glycol and the proportion of single-carbon products. The strong interaction between bimetallic Ni and W species synergistically promoted the dispersion and the reduction in active Ni4W nanoparticles on the Ni-0.2W/AC catalyst. These enhanced catalytic properties resulted in small and uniformly distributed active sites on the surface of the catalyst, which significantly increased the contact between reaction stock, dehydrogenation intermediates, and the active sites, thereby promoting feedstock conversion and achieving a high carbon conversion (XEG = 80.08%). This interaction also promoted both the decomposition of ethylene glycol and the forward WGS reaction, enhancing the efficiency of conversion to gas products. This process depended on the C-C bond cleavage ability, reflecting the improved performance of the Ni-0.2W/AC catalyst. Furthermore, the increased hydrogen yield demonstrated that C-C bond cleavage contributed directly to hydrogen production.
In comparison, the Ni-0.5W/AC catalyst exhibited a similar low conversion to the Ni/AC catalyst but with a higher proportion of CH4. This reveals that the large W2C layer covers some of the metal particles, leading to insufficient contact area between the reactants and the catalytic active sites and resulting in an insufficient feedstock conversion of 30.41% (Figure 2). Additionally, a small amount of CO formed by the cleavage of the C-C bond could not contact the active site in time for the WGS reaction to produce hydrogen, indirectly increasing the probability of alkylation or methanation reactions and resulting in a CH4 concentration close to 10%, as shown in Figure 5a. Interestingly, the W/AC catalyst exhibited an unusually high hydrogen proportion of 87.79% and an H2/CO2 ratio of 7.87 (see Table 3), far exceeding the other catalysts. Such performance was attributed to the significantly lower activation energy required for C-H bond cleavage compared to C-C bond cleavage in the APR system, particularly for catalysts with low C-C bond cleavage activity [16]. In the absence of electron-deficient Ni, monometallic W served as an active site but could not effectively facilitate C-C bond cleavage and the conversion of ethylene glycol, leading to the lowest C1/C2+ product ratio and the lowest CCgas. It plays a role in dehydrogenation by facilitating partial C-H bond cleavage. Thus, the total hydrogen yield remained limited at only 14.58 mmol, indicating that the W/AC catalyst could not be efficiently employed in complex APR systems.
For gas phase products (Figure 5a), hydrogen typically predominates in APR products, and variations in CO concentration reflect the intensity of the WGS reaction. The production of CH4 and CO2 can also provide indirect insights into the efficiency of C-C bond cleavage [3]. In comparison to the Ni/AC, Ni-0.05W/AC, and Ni-0.5W/AC catalysts, XRD and XPS results revealed that Ni metal alloyed in Ni4W remained electron deficient, giving the Ni-0.2W/AC catalyst a high affinity for OH* from the dissociation of H2O. This catalytic property was favorable for the subsequent WGS reaction, promoting hydrogen production and CO conversion [12]. The conversion of CO by WGS reaction re-exposed the active sites on the catalyst surface, further promoting the reforming reaction. As a result, CO accumulation in the reaction system was effectively prevented, avoiding the formation of multi-carbon species. Indirect evidence of this conclusion was observed from the relatively low methanol selectivity (1.1%) of the Ni-0.2W/AC catalyst in Figure 5b. The conversion of methanol is widely accepted to be primarily driven by its dehydrogenation activity [49]. The rapid consumption of CO encouraged the further conversion of methanol in the liquid phase through the reforming reaction pathway to hydrogen, although inevitably some methanol was hydrogenated to form methane. Thus, the H2/CO2 ratio of the Ni-0.2W/AC catalyst in Table 3 reached 2.66, exceeding the theoretical stoichiometric ratio of 2.5. This phenomenon can be explained by the dehydrogenation reactions of intermediates, which produce additional H2. Furthermore, a small amount of carbon is retained in liquid-phase products or converted into other carbon-containing gasses, such as methane, contributing to this increase. In contrast, the Ni-0.5W/AC and Ni-0.05W/AC catalysts exposed fewer active sites, resulting in the delayed conversion of CO, which led to the accumulation of intermediates. Accumulated methanol tended to undergo alkylation reactions, producing alkanes.
Figure 5a demonstrated that the Ni-0.2W/AC catalyst generated only a small amount of CH4. Correspondingly, the trends in Figure 5b revealed a decline in acetaldehyde selectivity, suggesting that a portion of the methane may have resulted from the conversion of acetaldehyde. Moreover, as shown in Figure 5b, methanol and acetaldehyde were the primary liquid products for the bimetallic catalysts. Conversely, ethanol was undetectable, in stark contrast to the ethanol-dominated liquid products observed with the monometallic Ni/AC and W/AC catalysts. This suggests that the Ni-W bimetallic synergistic effect either inhibited the formation of ethanol as an intermediate product or converted the produced ethanol thoroughly, indirectly indicating possible interconversion between ethanol, methanol, and acetaldehyde. Additionally, the accumulation of multi-carbon liquid products may have increased the probability of forming multi-carbon alkanes or olefins. Given the high ethanol selectivity of 80.46%, and acetic acid and acetaldehyde selectivity of nearly zero for the W/AC catalyst in Figure 5b, compared to the Ni-W catalysts with clearly detectable acetic acid and acetaldehyde, it could be inferred that metallic W in tungsten-based catalysts, as a weakly metallic site for C-C bond cleavage, possessed dehydrogenation activity, resulting in ethanol accumulation. Moreover, the Ni/AC catalyst exhibited the highest proportion of C2+ alkanes, which was related to its poor adsorption performance for intermediates and weak C-C bond cleavage ability.
In summary, the Ni-0.2W/AC catalyst exhibited the strongest bimetallic Ni-W synergistic effect and the most uniformly dispersed active sites under APR conditions, promoting intermediate adsorption and C-C bond cleavage of ethylene glycol. Moreover, the electron-deficient Ni in Ni4W alloy nanoparticles on the Ni-0.2W/AC catalyst further facilitated the conversion of CO into more favorable hydrogen-producing pathways. Ultimately, the Ni-0.2W/AC catalyst demonstrated superior performance in ethylene glycol conversion, hydrogen yield, and C-C bond cleavage efficiency.
3.3. Potential Reaction Mechanism
Since the Ni-0.2W/AC catalyst exhibited the best catalytic performance, this discussion focused on the origin of the products observed with the Ni-0.2W/AC catalyst. The reaction pathways in Scheme 1 are designed to provide a plausible explanatory framework for the primary product origins in the aqueous-phase reforming process over the Ni-0.2W/AC catalyst.
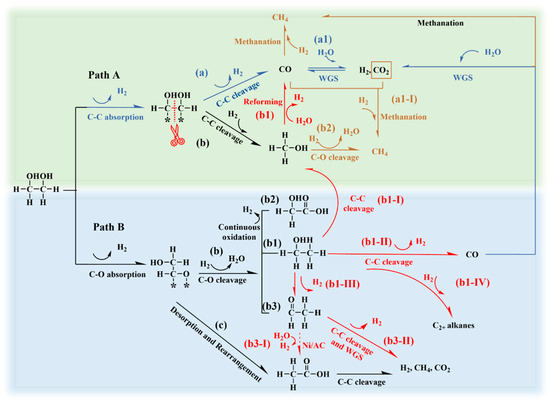
Scheme 1.
Proposed mechanism of the APR over the Ni-0.2W/AC catalyst based on experimental observations and literature-derived evidence. Red, brown, and blue arrows represent newly identified potential pathways, methanation, and the optimum hydrogen production mechanism, respectively [11,19,50,51].
Scheme 1.
Proposed mechanism of the APR over the Ni-0.2W/AC catalyst based on experimental observations and literature-derived evidence. Red, brown, and blue arrows represent newly identified potential pathways, methanation, and the optimum hydrogen production mechanism, respectively [11,19,50,51].
It is widely recognized that the primary chemisorption behaviors of ethylene glycol at the active Ni sites involve both C-C (Path A) and C-O (Path B) bond cleavage, as depicted in Scheme 1. Path A: a, a1 involves the C-C bond cleavage of ethylene glycol adsorbed on active sites, leading to the formation of CO intermediates that subsequently generate a large amount of hydrogen through the WGS reaction [12]. This pathway is crucial for maximizing hydrogen yield, as one molecule of ethylene glycol can produce up to five molecules of hydrogen [2,12]. During the process, the Ni-0.2W/AC catalyst, characterized by its uniformly dispersed active sites and strong electron-transfer properties (See XPS results), may exhibit a greater attraction for the dehydrogenation intermediates (e.g., *HOCHCHOH and *CHOCHO) compared to the Ni/AC and W/AC catalysts [11,52]. Simultaneously, the electron-deficient Ni in Ni4W nanoparticles could show an affinity for the electron-rich OH*, which facilitated the dissociation of OH* from water, producing CO2 and H2 [53]. This rapid consumption of CO* in turn stimulated further C-C bond cleavage reactions to compensate for the consumed CO*, promoting the absorption of additional dehydrogenation intermediates [2]. Therefore, hydrogen production was enhanced by Path A: a, a1. The observed high hydrogen yield and low CO concentration suggest that C-C bond cleavage may be the predominant pathway, followed by an efficient WGS reaction, which aligns with the product distribution characteristics. Furthermore, the absence of ethanol in the liquid-phase products indicates that the Ni-W bimetallic interaction, as confirmed by XPS and TEM data, suppresses the C-O bond cleavage pathway. Comparisons with monometallic Ni and W catalysts further support this interpretation. However, we acknowledge that the current explanation relies on indirect evidence and literature inference due to the lack of direct intermediate validation data. Future investigations could employ in situ characterization techniques to further elucidate the details of these pathways.
However, the inherent hydrogenation activity of metallic Ni also promotes methanation (Path A: a2, a1-I) and hydrogenation (Path A: b) reactions to produce alkanes and methanol. Methanol is typically considered a derivative of Path A; yet, the methanol production mechanism on the Ni-0.2W/AC catalyst could further contribute to hydrogen production [54]. Previous studies confirmed that the decomposition of methanol into hydrogen at 250 °C was thermodynamically favorable [44]. Additionally, the conversion mechanism of methanol to produce hydrogen, Path A: b1, along with the accompanying methanation reaction (Path A: b2), was discussed in Section 3.2. However, when the energy barrier for C-C bond cleavage was higher than that for C-O bond cleavage, multi-carbon liquid products such as ethanol (Path B: b1), acetic acid, acetaldehyde (Path B: b3), and glycolic acid (Path B: b2) were formed. However, Path B is widely recognized as a hydrogen-consuming pathway [11].
Ethanol is commonly recognized as an important byproduct in the catalytic APR of ethylene glycol, and its accumulation can impede the release of hydrogen from the liquid phase [50,54]. Suppressing its generation during APR is essential for highly efficient hydrogen production. Further investigation and understanding of its reaction pathway are of great necessity to optimize hydrogen yield. The concentration of ethanol in products indicates the intensity of competitive reactions. A high ethanol concentration in the product can influence the hydrogen release from the reaction mixture. Nevertheless, the conversion mechanism of ethanol over the Ni-W catalysts remains unclear. Understanding this mechanism could provide insights into the formation of acetaldehyde, acetic acid, and methanol in ethylene glycol APR, thus advancing research on feedstock conversion and hydrogen production. As indicated in Figure 5b, the Ni-0.2W/AC catalyst showed no selectivity for ethanol, in stark contrast to the Ni/AC and W/AC catalysts. Given that ethanol was detected in the products of the Ni/AC and W/AC catalysts and all reactions maintained a high carbon balance, equipment failure can be ruled out. This suggested two possibilities for ethanol conversion: (I) ethanol was effectively adsorbed and converted into other products during APR; or (II) the conversion of ethylene glycol did not proceed through the ethanol formation pathway (Path B: b1). The nearly 0.2% proportion of multi-carbon alkanes shown in Figure 5a supports the ethanol pathway, partially ruling out hypothesis (II).
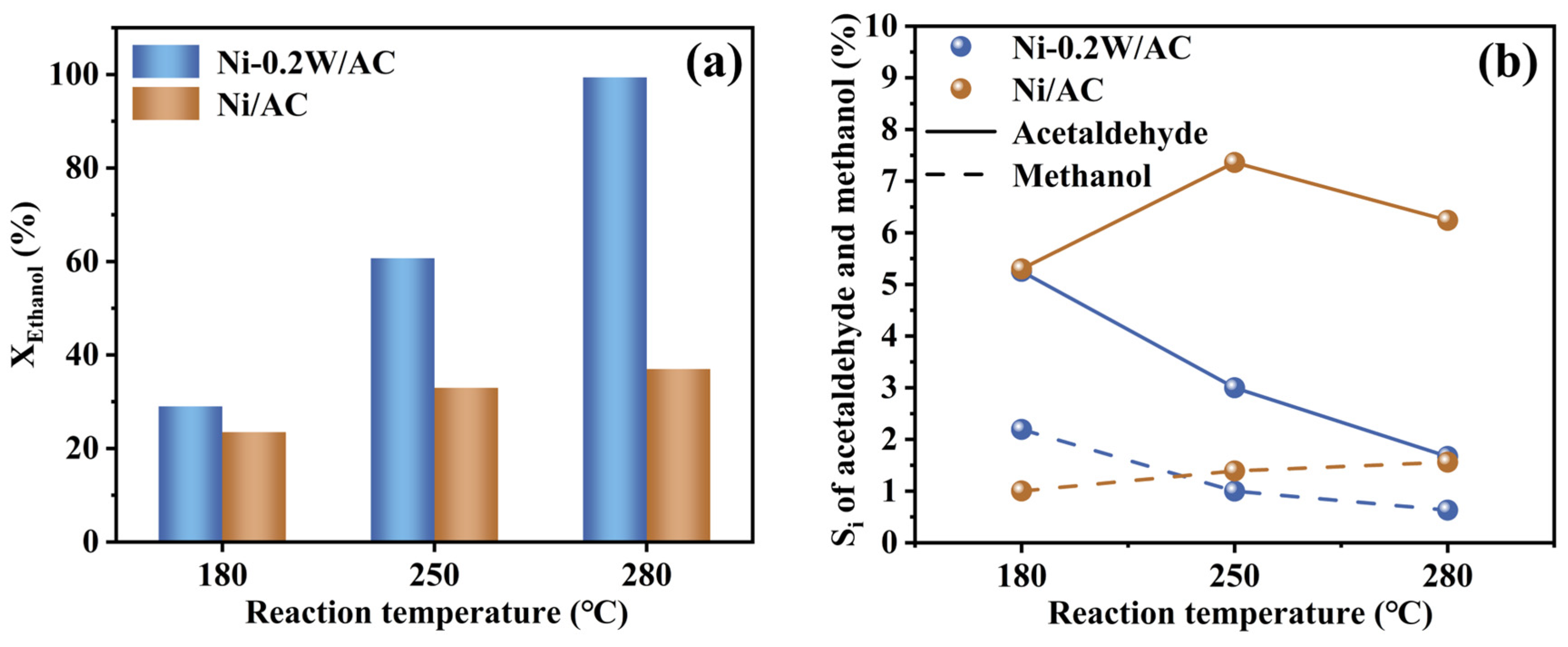
To explore ethanol conversion trends with temperature under hydrothermal conditions of APR and further verify the hypothesis (I), APR simulations of low-concentration ethanol solutions were conducted at sequentially increasing temperatures: 180 °C, 250 °C, and 280 °C, with the Ni/AC catalyst serving as the control. Extending the reaction time to 10 h ensured equilibrium and other experimental conditions were identical to those of ethylene glycol APR. Results revealed that ethanol APR primarily yielded acetaldehyde and methanol, indicating that ethanol conversion under similar conditions follows the conversion mechanisms of acetaldehyde and methanol. Performance evaluation (Figure 6a,b) showed that the Ni-0.2W/AC catalyst exhibited higher conversion, especially at elevated temperatures, compared to the Ni/AC catalyst. Previous studies reported that ethanol can be completely converted at 500 °C by the Ni-W catalysts during a steam reforming reaction [55]. Similarly, in this study, the Ni-0.2W/AC catalyst achieved efficient ethanol conversion at 280 °C, suggesting that the Ni-W bimetallic synergy enhanced the conversion of low-carbon alcohols (e.g., ethanol, ethylene glycol) under APR conditions. Thus, ethanol appears more readily convertible on the Ni-0.2W/AC catalyst at higher temperatures.
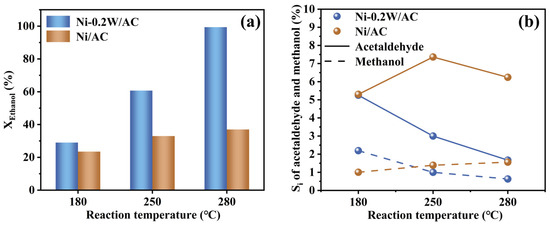
Figure 6.
(a) Conversion of ethanol (XEthanol) and Si of acetaldehyde and methanol (b) in APR of 10mL of 10% ethanol solution at 180 °C, 250 °C and 280 °C on the Ni-0.2W/AC and Ni/AC catalysts.
For the Ni-0.2W/AC catalyst, the conversion of low-concentration ethanol aqueous solution was significantly higher than that of the Ni/AC catalyst within the temperature range of 180 °C to 280 °C, indicating higher reactivity of Ni-0.2W/AC catalyst. Simultaneously, ethanol likely underwent C-C bond cleavage to form methanol and dehydrogenation to produce acetaldehyde on Ni-based catalysts. The selectivity (Si) of acetaldehyde and methanol, the main liquid products of ethanol APR, gradually decreased with increasing temperature, exhibiting a trend opposite to that of the Ni/AC catalyst. The strong bimetallic interaction likely facilitated the conversion of acetaldehyde and methanol into other products, such as H2, as thermodynamic energy increased. This is evident from the trend of increasing XEthanol and decreasing Si in Figure 6a,b. In contrast, under the same temperature conditions, ethanol catalyzed by the Ni/AC catalyst tended to convert into continuously accumulating acetaldehyde and methanol. Therefore, it can be concluded that the ethanol conversion pathway includes its transformation into acetaldehyde and methanol. The 0% ethanol selectivity in aqueous phase reforming catalyzed by the Ni-0.2W/AC catalyst may result from the cleavage of acetaldehyde and methanol into gas products at high temperatures. The consumption of acetaldehyde and methanol further promoted the conversion of ethanol into other intermediates, leading to a decrease in the selectivity for acetaldehyde and methanol, two key liquid products, thereby confirming the hypothesis (I). In summary, the APR process of ethylene glycol for ethanol conversion primarily involves four mechanisms (Scheme 1): dehydrogenation (Path B: b1-III) to acetaldehyde, followed by further reactions (Path B: b3-II), C-C bond cleavage (Path B: b1-I) to form methanol, reforming (Path B: b1-II) to produce hydrogen and other gas products, and hydrogenation of intermediates to form CH4 and C2+ alkanes (Path B: b1-IV).
Additionally, active metals on the catalyst can be leached by the acetic acid existing in the liquid phase during the APR process, thereby diminishing catalytic performance and stability [11]. On bimetallic Ni-W/AC catalysts, the selectivity for glycolic acid and acetic acid shows little variation and follows a similar trend, suggesting that their formation may not depend on active metal dispersion or Ni-W interaction. The formation of glycolic acid could be influenced by the pH of the reaction solution, which is highly related to the concentration of acetic acid. Typically, acetic acid forms through aldehyde oxidation (Path b3-I) [56]. Unlike W species, Ni catalyzes the conversion of ethanol to acetaldehyde by promoting dehydrogenation and facilitating acetic acid formation. Specifically, this process occurs through desorption and rearrangement of oxygenated adsorbates from ethylene glycol, followed by acetaldehyde oxidation (Path B: b3-I). The trends of Si for ethanol, acetaldehyde, and acetic acid in Figure 5b suggested that bimetallic Ni-W interaction promoted the conversion of ethanol to acetaldehyde. However, this interaction did not appear to facilitate the subsequent oxidation of acetaldehyde to acetic acid, explaining the lower acetic acid selectivity for Ni-W catalysts compared to the Ni/AC catalyst.
For longer carbon chain organic compounds, the industrialization of APR faces significant challenges due to limitations in hydrogen yield. Taking glycerol as an example, conventional Pt/Al2O3 catalysts achieve of only about 17% [54]. In contrast, in the APR of ethylene glycol, other catalysts such as the commonly used Pt/CMK-3 [57] and NiSnAl [58] hydrotalcite catalysts achieve approximately 27% and 35%, respectively, while the Ni-0.2W/AC bimetallic catalyst described in this study realizes a of 47.76%. Given its bimetallic interactions and confinement effects, we hypothesize that the Ni-W catalyst may offer advantages in the APR of glycerol or longer carbon organic compounds.
3.4. Catalyst Stability
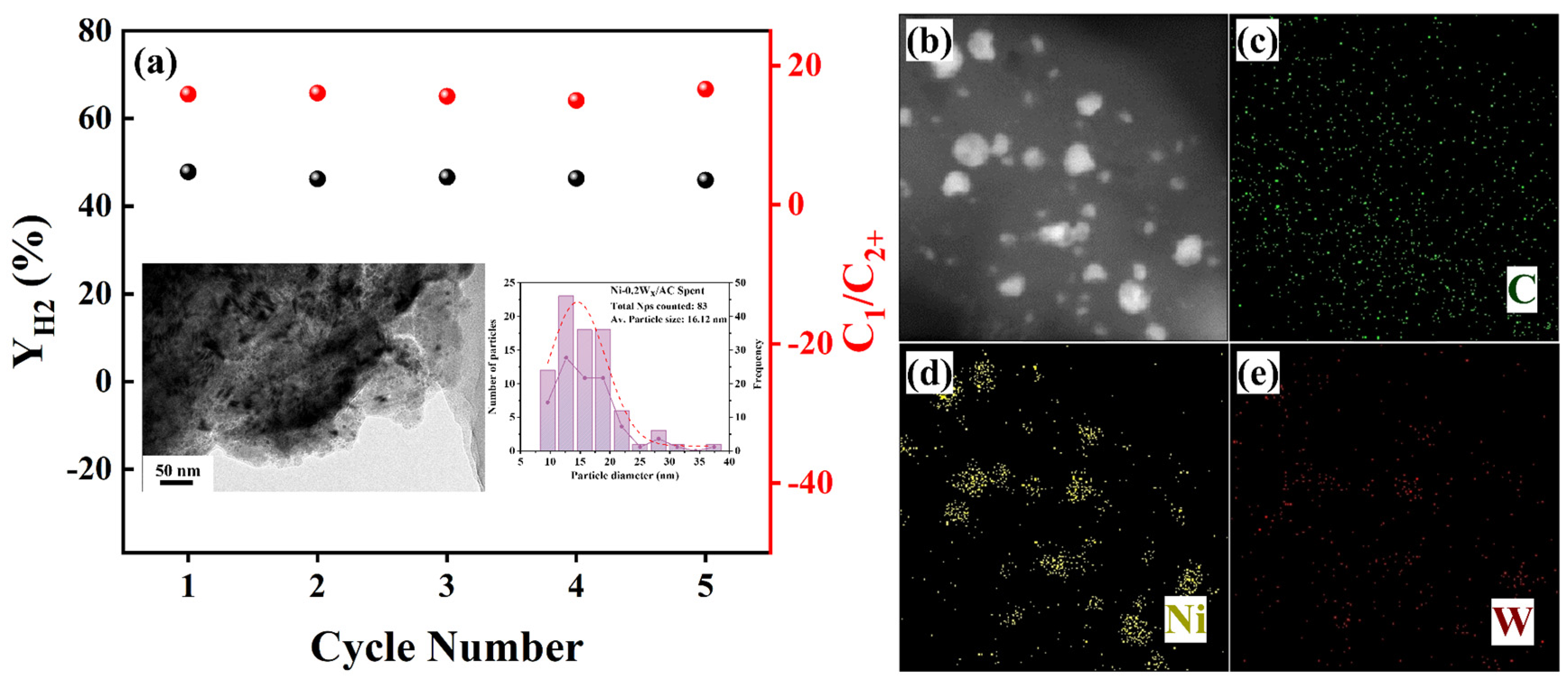
The catalyst stability of Ni-0.2W/AC was tested for five cycles. As shown in Figure 7a, the and C1/C2+ of the Ni-0.2W/AC catalyst showed a small amount of variation in the five cycling reactions, demonstrating good catalyst stability. TEM and EDS characterization tests (Figure 7b) were performed on the spent catalyst after five cycles. It showed that the spent Ni-0.2W/AC catalyst exhibited slight agglomeration of metal nanoparticles, but the metal distribution shown in the EDS plots in Figure 7b–e indicated that there was no significant loss of metals. The generation of acetic acid during the APR reaction of ethylene glycol leads to metal leaching [44,51]. For the Ni-0.2W/AC catalyst in this work, a slight decrease in catalytic performance can be attributed to the strong Ni-W interaction and the inhibition of the acetic acid intermediate. As confirmed by XPS and XRD characterizations, the formation of the Ni4W alloy enhances the bimetallic interaction through the confinement effect, which stabilizes the Ni metal and improves its resistance to leaching by acidic liquid phase products, such as acetic acid, under APR conditions. This stability is evidenced by only minimal agglomeration and abundant Ni metal distributed on the support, as observed in TEM images after five cycles (Figure 7a).
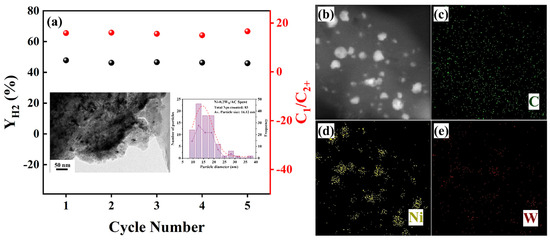
Figure 7.
(a) The cycle stability of the Ni-0.2W/AC catalyst for and C1/C2+. The inset illustrations show TEM of the spent catalyst recovered after 5 cycles and the corresponding particle size analysis. (b–e) EDS mapping images of the spent Ni-0.2W/AC catalyst.
4. Materials and Methods
4.1. Chemicals and Materials
Activated carbon (AC) was obtained from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Ni(NO3)2·6H2O, (NH4)6H2W12O40·xH2O, and methanol were obtained from Aladdin Biochemical Company (Shanghai, China). Ethylene glycol (99.5% purity) and ethanol (99.8% purity) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Hydrogen was supplied by Foshan Kedi Gas Chemical Co., Ltd. (Foshan, China).
4.2. Synthesis of Catalysts
All catalysts were synthesized via the wet impregnation method. Initially, 10 g of AC was treated by placing it in a 2.2 M HNO3 solution and refluxing at 80 °C for 5 h. Following reflux, the AC was thoroughly washed with deionized water until the wash water reached a neutral pH, and subsequently dried at 60 °C [59].
To prepare the Ni/AC catalyst, 0.5 g of Ni(NO3)2·6H2O was dissolved in aqueous methanol solutions (50% v/v methanol), thoroughly mixed with nitric acid-treated AC, dried at 110 °C, and then reduced at 800 °C under a hydrogen atmosphere for 180 min. For the W/AC catalyst, (NH4)6H2W12O40·xH2O was dissolved in aqueous methanol solutions (50% v/v methanol), mixed with the treated AC, dried, and reduced at 350 °C in the air to form WOx/AC. The WOx/AC was further reduced at 800 °C under a hydrogen atmosphere at 5 °C/min for 180 min. The W precursor loading was controlled to match the metal loading of Ni in the Ni/AC catalyst. Finally, Ni-0.05W/AC, Ni-0.2W/AC, and Ni-0.5W/AC catalysts were prepared through a two-step impregnation of W and Ni precursors. The W precursor was loaded following the same procedure as for W/AC, after which 0.5 g of Ni(NO3)2·6H2O was dissolved in aqueous methanol solutions (50% v/v methanol), mixed thoroughly with the treated AC, dried at 110 °C, and then reduced at 800 °C at a heating rate of 5 °C/min under a hydrogen atmosphere for 180 min. Ni loading remained constant, while W loadings were adjusted to achieve W/Ni molar ratios of 0.05, 0.2, and 0.5.
4.3. Characterization
To ensure a comprehensive understanding of the catalyst properties, advanced characterization techniques were employed. Powder X-ray Diffraction (XRD) was utilized to determine the crystallographic structure and phase composition, using an X’Pert Pro MPD system from PANalytical (Malvern, Worcestershire, UK). Diffraction patterns were recorded across a 2θ range of 20 to 80 degrees, with a scanning speed of 10 degrees/min. The elemental composition of Ni and W was measured using a Perkin Elmer Optima 8000 ICP-OES spectrometer (PerkinElmer, Inc. Waltham, MA, USA). Prior to ICP-OES analysis, the catalyst samples were digested using a mixture of nitric acid and hydrochloric acid (3:1 v/v) to ensure complete dissolution of the metal species. Prior to ICP-OES analysis, the catalyst samples were digested using a mixture of nitric acid and hydrochloric acid (3:1 v/v) to ensure complete dissolution of the metal species. X-ray Photoelectron Spectroscopy (XPS) was performed on an ESCALAB 250Xi with an AlKα X-ray source (Thermo Scientific, USA) to analyze the surface chemical composition and valence states. Transmission Electron Microscopy (TEM) and Energy-Dispersive X-ray Spectroscopy (EDS) were employed to analyze nanoscale morphology and elemental distribution. H2-Temperature Programmed Reduction (H2-TPR) tests were conducted to investigate the reduction behavior and metal-metal/metal-support interactions of the samples. The samples were first heated from ambient temperature to 300 °C at 10 °C/min, then purged with helium (50 mL/min) for 2 h. After cooling to 50 °C, a hydrogen/argon mixture (10% H2) was introduced for 30 min until stabilization. The samples were then heated to 900 °C in a hydrogen/argon atmosphere at 5 °C/min.
5. Conclusions
Briefly, bimetallic Ni-W catalysts with various W/Ni atomic ratios were employed for the APR of ethylene glycol. The results showed that the C1/C2+ product ratio for the Ni-0.2W/AC catalyst reached 15.87 and a hydrogen yield of 47.76%. Characterization results attributed enhanced C-C bond cleavage efficiency to the strong bimetallic Ni-W interaction, which promoted the uniform dispersion of active sites and facilitated electron transfer. In contrast, the Ni-0.5W/AC catalyst showed fewer exposed Ni4W nanoparticles, while the Ni-0.05W/AC catalyst exhibited weak bimetallic interaction due to lower W loading. Experimental results revealed that improved C-C bond cleavage efficiency promoted the conversion of ethylene glycol into gaseous products, significantly boosting hydrogen production. Moreover, the study elaborates on the hydrogen production mechanism and achieves complete conversion of the by-product ethanol during APR of ethylene glycol over the Ni-0.2W/AC catalyst. Further in-depth study of the reaction mechanism suggested that ethanol conversion proceeded through four possible pathways, resulting in the absence of ethanol in the liquid products of the ethylene glycol APR. This study underscores the importance of C-C bond cleavage performance for catalysts in the aqueous phase reforming of biomass and biomass-derived oxygenates, providing valuable insights for future research in this field.
Author Contributions
Conceptualization, L.X. and Z.H.; methodology, Y.Z.; software, J.H.; validation, C.W., R.S. and Z.H.; formal analysis, L.X.; investigation, L.L.; resources, J.L.; data curation, Z.T.; writing—original draft preparation, L.X.; writing—review and editing, Z.H.; visualization, J.W.; supervision, C.W.; project administration, Y.C. and Z.T.; funding acquisition, Z.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2024YFE0208200), National Natural Science Foundation of China (Grant No. 52476189), and the Guangzhou Basic and Applied Basic Research Foundation (Grant No. 2024A04J3829). The APC was funded by the National Natural Science Foundation of China through the open access funding program.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
We thank the Analysis and Testing Center of Guangdong University of Technology for the help in catalyst characterizations.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xiong, K.; Yu, W.; Vlachos, D.G.; Chen, J.G. Reaction pathways of biomass-derived oxygenates over metals and carbides: From model surfaces to supported catalysts. ChemCatChem 2015, 7, 1402–1421. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Dumesic, J.A. Kinetics of Aqueous-Phase Reforming of Oxygenated Hydrocarbons: Pt/Al2O3 and Sn-Modified Ni Catalysts. Ind. Eng. Chem. Res. 2004, 43, 3105–3112. [Google Scholar] [CrossRef]
- Coronado, I.; Stekrova, M.; Reinikainen, M.; Simell, P.; Lefferts, L.; Lehtonen, J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions. Int. J. Hydrogen Energy 2016, 41, 11003–11032. [Google Scholar] [CrossRef]
- Lin, L.; Yan, R.; Liu, Y.; Jiang, W. In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose and lignin. Bioresour. Technol. 2010, 101, 8217–8223. [Google Scholar] [CrossRef] [PubMed]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Wang, C.; Liao, M.; Bu, E.; Jiang, Z.; Chen, Y.; Cheng, Z.; Luo, X.; Liang, B.; Shu, R.; Song, Q. Effective hydrogen production from partial oxidation of propane over composite Ni/Al2O3–SiC catalyst. Int. J. Hydrogen Energy 2019, 44, 680–693. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic Reforming of Oxygenates: State of the Art and Future Prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef]
- Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous Phase Reforming of Dairy Wastewater for Hydrogen Production: An Experimental and Energetic Assessment. Sustainability 2024, 16, 1743. [Google Scholar] [CrossRef]
- Ruiz-Garcia, C.; Baeza, J.A.; Oliveira, A.S.; Roldán, S.; Calvo, L.; Gilarranz, M.A. Exploration of operating conditions in the direct aqueous-phase reforming of plastics. Fuel 2024, 374, 132446. [Google Scholar] [CrossRef]
- Rosmini, C.; Urrea, M.P.; Tusini, E.; Indris, S.; Kovacheva, D.; Karashanova, D.; Kolev, H.; Zimina, A.; Grunwaldt, J.D.; Rønning, M.; et al. Unveiling the synergistic effects of pH and Sn content for tuning the catalytic performance of Ni0/NixSny intermetallic compounds dispersed on Ce-Zr mixed oxides in the aqueous phase reforming of ethylene glycol. Appl. Catal. B 2024, 350, 123904. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Zou, Y.Q.; von Wolff, N.; Anaby, A.; Xie, Y.; Milstein, D. Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier. Nat. Catal. 2019, 2, 415–422. [Google Scholar] [CrossRef]
- Qi, K.; Li, Z.; Zhang, C.; Tan, X.; Wan, C.; Liu, X.; Wang, L.; Lee, D.J. Biodegradation of real industrial wastewater containing ethylene glycol by using aerobic granular sludge in a continuous-flow reactor: Performance and resistance mechanism. Biochem. Eng. J. 2020, 161, 107711. [Google Scholar] [CrossRef]
- Van Haasterecht, T.; Ludding, C.C.I.; De Jong, K.P.; Bitter, J.H. Stability and activity of carbon nanofiber-supported catalysts in the aqueous phase reforming of ethylene glycol. J. Energy Chem. 2013, 22, 257–269. [Google Scholar] [CrossRef]
- Kandoi, S.; Greeley, J.; Simonetti, D.; Shabaker, J.; Dumesic, J.A.; Mavrikakis, M. Reaction kinetics of ethylene glycol reforming over platinum in the vapor versus aqueous phases. J. Phys. Chem. C 2011, 115, 961–971. [Google Scholar] [CrossRef]
- Zhang, W.J.; Tian, Z.P.; Huang, J.H.; Wang, J.Y.; Luo, X.L.; Wang, C.; Shu, R.Y.; Liu, J.P.; Chen, Y. Investigation of the promotion effect of metal oxides on the water-gas shift reaction activity over Pt-MOx/CeO2 catalysts for aqueous phase reforming. J. Fuel Chem. Technol. 2023, 51, 1791–1804. [Google Scholar] [CrossRef]
- Dongil, A.B.; Pastor-Pérez, L.; Escalona, N.; Sepúlveda-Escribano, A. Carbon nanotube-supported Ni-CeO2 catalysts. Effect of the support on the catalytic performance in the low-temperature WGS reaction. Carbon 2016, 101, 296–304. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrogen Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Dal Santo, V.; Gallo, A.; Naldoni, A.; Guidotti, M.; Psaro, R. Bimetallic heterogeneous catalysts for hydrogen production. Catal. Today 2012, 197, 190–205. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Zhao, B.; Zhao, R.; Yu, S.; Huang, L. Nickel-tungsten co-doped biochar catalyst boosting ethylene glycol production from cellulose hydrogenolysis. Ind. Crop. Prod. 2024, 207, 117752. [Google Scholar] [CrossRef]
- Chen, H.; Sun, W.; Hu, X.; Wang, Q.; Wu, T.; An, S.; Ding, C.; Chen, C.; Huang, L.; Wang, N. Additive WO2 promotes Ni-based catalyst for hydrogen production from auto-thermal reforming of acetic acid. Fuel 2023, 339, 126914. [Google Scholar] [CrossRef]
- Saquic, B.E.B.; Irmak, S.; Wilkins, M. Enhancement of catalytic performance of graphene supported Pt catalysts by Ni and W for hydrogen gas production by hydrothermal gasification of biomass-derived compounds. Fuel 2022, 308, 122079. [Google Scholar] [CrossRef]
- Qiao, Y.; Xia, G.-J.; Cao, W.; Zeng, K.-H.; Guo, Q.-L.; Yang, X.-F.; Wang, A.-Q.; Wang, Y.-G. Breaking the C-C bond of glucose on tungsten oxide-based catalysts in aqueous phase. J. Catal. 2023, 427, 115114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Hao, C.; Wang, S.; Liu, H. Unveiling the mechanism for selective cleavage of C-C bonds in sugar reactions on tungsten trioxide–based catalysts. Proc. Natl. Acad. Sci. USA 2022, 119, e2206399119. [Google Scholar] [CrossRef]
- Lercher, J.A. Novel mechanism for selective cracking of sugars on WO3 and its significance for biomass utilization. Chin. J. Catal. 2023, 46, 1–3. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Chanussot, L.; Das, A.; Goyal, S.; Lavril, T.; Shuaibi, M.; Riviere, M.; Tran, K.; Heras-Domingo, J.; Ho, C.; Hu, W.; et al. The Open Catalyst 2020 (OC20) Dataset and Community Challenges. ACS Catal. 2021, 11, 6059–6072, Erratum in ACS Catal. 2021, 11, 13062–13065. https://doi.org/10.1021/acscatal.1c04408. [Google Scholar] [CrossRef]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef]
- Hon, K.; Couet, S.; Kumar Vudya Sethu, K.; Swerts, J.; Kar, G.S. Effect of nitrogen doping on the structure of metastable β-W on SiO2. Thin Solid Films 2021, 732, 138795. [Google Scholar] [CrossRef]
- Cury, R.; Joubert, J.M.; Tusseau-Nenez, S.; Leroy, E.; Allavena-Valette, A. On the existence and the crystal structure of Ni4W, NiW and NiW2 compounds. Intermetallics 2009, 17, 174–178. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, T.; Zheng, M.; Wang, A.; Wang, H.; Wang, X.; Chen, J.G. Direct catalytic conversion of cellulose into ethylene glycol using nickel-promoted tungsten carbide catalysts. Angew. Chem. Int. Ed. 2008, 47, 8510–8513. [Google Scholar] [CrossRef]
- Sauerwein, J.C.; Dalton, G.R. Standard Reference Data Publications, 1964–1984; NBS Special Publication 708; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1985. [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef]
- Du, H.; Zhou, C.; Xie, X.; Li, H.; Qi, W.; Wu, Y.; Liu, T. Pseudocapacitance of nanoporous Ni@NiO nanoparticles on Ni foam substrate: Influence of the annealing temperature. Int. J. Hydrogen Energy 2017, 42, 15236–15245. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, P.; Wang, J.; Wang, H.; Yang, L.; Zhao, X. In situ synthesis of Ni nanofibers via vacuum thermal reduction and their efficient catalytic properties for hydrogen generation. J. Mater. Chem. A 2018, 6, 11370–11376. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, J.; Zeng, R.; Xing, F.; Huang, C. Schottky barrier tuning via surface plasmon and vacancies for enhanced photocatalytic H2 evolution in seawater. Appl. Catal. B 2022, 310, 121321. [Google Scholar] [CrossRef]
- Yang, F.; Komarneni, M.R.; Libretto, N.J.; Li, L.; Zhou, W.; Miller, J.T.; Ge, Q.; Zhu, X.; Resasco, D.E. Elucidating the Structure of Bimetallic NiW/SiO2 Catalysts and Its Consequences on Selective Deoxygenation of m-Cresol to Toluene. ACS Catal. 2021, 11, 2935–2948. [Google Scholar] [CrossRef]
- Tran, C.C.; Akmach, D.; Kaliaguine, S. Hydrodeoxygenation of vegetable oils over biochar supported bimetallic carbides for producing renewable diesel under mild conditions. Green Chem. 2020, 22, 6424–6436. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, L.H.; Ma, C.Q.; Yuan, Z.T.; Song, J. Enhancing the sensing performance of WO2.72 toward n-butanol via loading CeO2 nanoparticles. J. Mater. Chem. A 2024, 12, 21156–21164. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Shan, J.; Xu, Z.; Fang, Z.; Wang, L.; Liu, S. Strong hetero-interface interaction in 2D/2D WSe2/ZnIn2S4 heterostructures for highly-efficient photocatalytic hydrogen generation. J. Energy Chem. 2024, 97, 379–387. [Google Scholar] [CrossRef]
- Vinay, G.; Singh, N.K.; Khan, S.W.; Kant, R.; Singh, H. XPS insights for optimization of laser-remelting as a post-processing technique for coatings. Surf. Interfaces 2024, 47, 104212. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Luo, X.; Wang, C.; Shu, R.; Song, Q.; Liu, J.; Tian, Z.; Chen, Y. Hydrogen production by aqueous phase reforming over stable La-promoted Ni-based hydrotalcite catalysts. Int. J. Hydrogen Energy 2024, 50, 681–689. [Google Scholar] [CrossRef]
- Martínez, A.; Prieto, G.; Arribas, M.A.; Concepción, P.; Sánchez-Royo, J.F. Influence of the preparative route on the properties of WOx-ZrO2 catalysts: A detailed structural, spectroscopic, and catalytic study. J. Catal. 2007, 248, 288–302. [Google Scholar] [CrossRef]
- Zhou, K.; Du, X.; Zhou, L.; Yang, H.; Lei, X.; Zeng, Y.; Li, D.; Hu, C. The deoxygenation of jatropha oil to high quality fuel via the synergistic catalytic effect of Ni, W2C and WC species. Catalysts 2021, 11, 469. [Google Scholar] [CrossRef]
- He, K.; Liu, S.; Zhao, G.; Qin, Y.; Bi, Y.; Song, L. Ni-W Catalysts Supported on Mesoporous SBA-15: Trace W Steering CO2 Methanation. Chem. Res. Chin. Univ. 2022, 38, 1504–1511. [Google Scholar] [CrossRef]
- Urrea, M.P.; Herold, F.; Chen, D.; Rønning, M. Nitrogen-containing carbon nanofibers as supports for bimetallic Pt-Mn catalysts in aqueous phase reforming of ethylene glycol. Catal. Today 2023, 418, 114066. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, Y.; Zhang, W.; Shu, R.; Luo, X.; Song, Q.; Lei, L.; Wang, C.; Chen, Y.; Ma, L. Investigation on the hydrogen production by methanol aqueous phase reforming over Pt/CexMg1-xO2 catalyst: Synergistic effect of support basicity and oxygen vacancies. Renew. Energy 2024, 230, 120807. [Google Scholar] [CrossRef]
- De Vlieger, D.J.M.; Mojet, B.L.; Lefferts, L.; Seshan, K. Aqueous Phase Reforming of ethylene glycol—Role of intermediates in catalyst performance. J. Catal. 2012, 292, 239–245. [Google Scholar] [CrossRef]
- Pazos Urrea, M.; Meilinger, S.; Herold, F.; Gopakumar, J.; Tusini, E.; De Giacinto, A.; Zimina, A.; Grunwaldt, J.-D.; Chen, D.; Rønning, M. Aqueous Phase Reforming over Platinum Catalysts on Doped Carbon Supports: Exploring Platinum–Heteroatom Interactions. ACS Catal. 2024, 14, 4139–4154. [Google Scholar] [CrossRef]
- Syuhada, A.; Ameen, M.; Azizan, M.T.; Aqsha, A.; Yusoff, M.H.M.; Ramli, A.; Alnarabiji, M.S.; Sher, F. In-situ hydrogenolysis of glycerol using hydrogen produced via aqueous phase reforming of glycerol over sonochemically synthesized nickel-based nano-catalyst. Mol. Catal. 2021, 514, 111860. [Google Scholar] [CrossRef]
- So, J.; Chung, Y.; Sholl, D.S.; Sievers, C. In-situ ATR-IR study of surface reaction during aqueous phase reforming of glycerol, sorbitol and glucose over Pt/γ-Al2O3. Mol. Catal. 2019, 475, 110423. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Aqueous phase reforming (APR) of glycerol over platinum supported on Al2O3 catalyst. Renewable Energy 2016, 85, 1116–1126. [Google Scholar] [CrossRef]
- Hernández, I.P.; Gochi-Ponce, Y.; Contreras Larios, J.L.; Fernández, A.M. Steam reforming of ethanol over nickel-tungsten catalyst. Int. J. Hydrogen Energy 2010, 35, 12098–12104. [Google Scholar] [CrossRef]
- Han, S.; Shin, K.; Henkelman, G.; Buddie Mullins, C. Selective Oxidation of Acetaldehyde to Acetic Acid on Pd-Au Bimetallic Model Catalysts. ACS Catal. 2019, 9, 4360–4368. [Google Scholar] [CrossRef]
- Kim, H.-D.; Park, H.J.; Kim, T.-W.; Jeong, K.-E.; Chae, H.-J.; Jeong, S.-Y.; Lee, C.-H.; Kim, C.-U. Hydrogen production through the aqueous phase reforming of ethylene glycol over supported Pt-based bimetallic catalysts. Int. J. Hydrogen Energy 2012, 37, 8310–8317. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, N. Hydrogen Production from Ethylene Glycol Aqueous Phase Reforming over Ni–Al Layered Hydrotalcite-Derived Catalysts. Catalysts 2020, 10, 54. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Y.; Shu, R.; Wang, J.; Liu, J.; Wang, C.; Tian, Z.; Chen, Y. Efficient hydrogen production by methanol aqueous phase reforming over KMnO4 modified PtMnK/AC catalyst: Regulating the hydrophilicity of carbon support. Mol. Catal. 2024, 559, 114105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).