Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs
Abstract
:1. Background
1.1. Nucleotides in Metabolism and Industrial Applications
1.2. Enzymes Involved in Nucleotide Metabolism
1.2.1. Enzymes Involved in the Synthesis of Natural and Modified 5′-NMPs
1.2.2. Enzymes Synthesizing 5′-NDPs and Their Analogs
1.2.3. Enzymatic 5´-NTP Synthesis
1.2.4. Conversion of 5′-NMPs and 5′-NDPs to 5′-NTPs by Polyphosphate Kinases
1.3. Chemical Synthesis of NTPs and Their Analogs
2. Enzymatic Cascade Reactions to Produce Natural and Modified 5′-NTPs
2.1. Cascades Starting from Nucleosides and Using Nucleoside and NMP Kinases
2.2. Biocatalytic Synthesis of 5′-NTPs Starting from Nucleobases and Using NMP Kinases
2.3. Biocatalytic Synthesis of 5′-NTPs Using Polyphosphate Kinases
2.4. Other Interesting Approaches to Produce 5′-NTPs
3. Importance of ATP-Regeneration Systems in the Synthesis of 5′-NTPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcK | acetate kinase |
| AD | adenine deaminase |
| ADA | adenosine deaminase |
| ADP | adenosine-5′-diphosphate |
| AjPPK | Acinetobacter johnsonii PPK |
| AMP | adenosine-5′-monophosphate |
| AMPDA | AMP deaminase |
| AprT | adenine PRT |
| AtADK | Arabidopsis thaliana adenosine kinase |
| ATP | adenosine-5′-triphosphate |
| ß-RFAS | 4-(D-ribofuranosyl)aminobenzene synthases |
| 5-BrU | 5-bromo uracil |
| 5-Br-dUTP | 5-bromo dUTP |
| CD | cytosine deaminase |
| CDA | cytidine deaminase |
| CDP | cytidine-5′-diphosphate |
| CK | creatine kinase |
| 6-Cl-P | 6-chloro purine |
| 2,6-Cl-P | 2,6-dichloropurine |
| 6-Cl-dPTP | 6-chloro purine 5′-triphosphate |
| 2,6-Cl-dPTP | 2,6-dichloropurine 5′-triphosphate |
| CMP | cytidine-5′-monophosphate |
| CMPDA | CMP deaminase |
| CTP | cytidine-5′-triphosphate |
| CTP-S | CTP synthase |
| 5-FU | 5-fluoro uracil |
| 5-F-dUTP | 5-fluoro dUTP |
| 2,6-D | 2,6-diamino purine |
| 5′-deoxyNTP/dNTP | deoxynucleoside-5′-triphosphate |
| DmdNK | deoxynucleoside kinase of Drosophila melanogaster |
| dNK | deoxynucleoside kinase |
| 2,6-dPTP | 2,6-diamino purine 5′-triphosphate |
| EcAPT | Escherichia coli adenine PRT |
| EcAPT | Escherichia coli hypoxanthine PRT |
| GD | guanine deaminase |
| GDA | guanosine deaminase |
| GDP | guanosine-5′-diphosphate |
| glyD | guanine PRT |
| GMP | guanosine-5′-monophosphate |
| GMPK | guanosine kinase |
| GMP-S | GMP synthase |
| GTP | guanosine-5′-triphosphate |
| IMP | inosine-5′-monophosphate |
| IMPDH | IMP dehydrogenase |
| LhPPK | Lampropedia hyalina PPK |
| MjNK | Methanocaldococcus jannaschii NK |
| MrPPK | Meiothermus ruber wildtype PPK |
| 5′-NDP | nucleoside-5′-diphosphate |
| NDPK | NDP kinases |
| NdT | nucleoside 2′-deoxyribosyltransferase |
| NH | nucleoside hydrolase |
| NK | nucleoside kinase |
| 5′-NMP | nucleoside-5′-monophosphate |
| NMPK | NMP kinase |
| NP | nucleoside phosphorylase |
| NPT | nucleoside phosphotransferase |
| NSAP | nonspecific acid phosphatase |
| NT | 5′-nucleotidase |
| 5′-NTP | nucleoside-5′-triphosphate |
| PCR | polymerase chain reaction |
| PDN2′H | pyrimidine nucleoside 2′-hydroxylase |
| PK | pyruvate kinase |
| ppGpp | guanosine tetraphosphate |
| PpnN | nucleosidase PpnN |
| PPK | polyphosphate kinase |
| pppGpp | guanosine pentaphosphate |
| PR | ribose-5′-phosphate |
| PRPP | 5-phospho-D-ribosyl-α-1-pyrophosphate |
| PrsA | PRPP synthase |
| PRT | phosphoribosyl transferase |
| PUS | pseudouridylate synthase |
| RbsK | ribokinase |
| RNR | ribonucleotide reductase |
| ScADK | Saccharomyces cerevisiae adenosine kinase |
| SlPPK | Sulfurovum lithotrophicum PPK |
| SmPPK | Sinorhizobium meliloti PPK |
| TMP | thymidine-5′-monophosphate |
| TMP-S | TMP synthase |
| TVNrdJm | RNR of Thermus virus TV74-23 |
| UDP | uridine-5′-diphosphate |
| UK | uridine kinase |
| UMP | uridine-5′-monophosphate |
| UraP | uracil PRT |
| UTP | uridine-5′-triphosphate |
| YeiN | pseudouridylate synthase |
References
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry; W. H. Freeman Publishing: New York, NY, USA, 2002; p. 1050. [Google Scholar]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Physiological Concentrations of Purines and Pyrimidines. Mol. Cell Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Burnstock, G. THE DOUBLE LIFE OF ATP. Sci. Am. 2009, 301, 84. [Google Scholar] [CrossRef]
- Andexer, J.N.; Richter, M. Emerging Enzymes for ATP Regeneration in Biocatalytic Processes. ChemBioChem 2015, 16, 380–386. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.-Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Boraston, A.B. (P)PpGpp and the Stringent Response: An Emerging Threat to Antibiotic Therapy. ACS Infect. Dis. 2019, 5, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; Maertens, J.; Beauprez, J.; Soetaert, W.; De Mey, M. Biotechnological Advances in UDP-Sugar Based Glycosylation of Small Molecules. Biotechnol. Adv. 2015, 33, 288–302. [Google Scholar] [CrossRef]
- Wagner, G.K.; Pesnot, T.; Field, R.A. A Survey of Chemical Methods for Sugar-Nucleotide Synthesis. Nat. Prod. Rep. 2009, 26, 1172–1194. [Google Scholar] [CrossRef]
- Hollenstein, M. Nucleoside Triphosphates—Building Blocks for the Modification of Nucleic Acids. Molecules 2012, 17, 13569–13591. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef]
- Espinasse, A.; Lembke, H.K.; Cao, A.A.; Carlson, E.E. Modified Nucleoside Triphosphates in Bacterial Research for in Vitro and Live-Cell Applications. RSC Chem. Biol. 2020, 1, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.L.Y.; Schiess, G.H.A.; Pâ, C.; Miranda, M.; Weber, G.; Astakhova, K. Pseudouridine and N1-Methylpseudouridine as Potent Nucleotide Analogues for RNA Therapy and Vaccine Development. Chem. Biol. 2024, 5, 418. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Phosphorylation of Nucleosides and Nucleoside Analogs by Mammalian Nucleoside Monophosphate Kinases. Pharmacol. Ther. 2000, 87, 189–198. [Google Scholar] [CrossRef]
- Tsesmetzis, N.; Paulin, C.B.J.; Rudd, S.G.; Herold, N. Nucleobase and Nucleoside Analogues: Resistance and Re-Sensitisation at the Level of Pharmacokinetics, Pharmacodynamics and Metabolism. Cancers 2018, 10, 240. [Google Scholar] [CrossRef]
- Gollnest, T.; de Oliveira, T.D.; Rath, A.; Hauber, I.; Schols, D.; Balzarini, J.; Meier, C. Membrane-Permeable Triphosphate Prodrugs of Nucleoside Analogues. Angew. Chemie 2016, 128, 5341–5344. [Google Scholar] [CrossRef]
- Rachwalak, M.; Romanowska, J.; Sobkowski, M.; Stawinski, J. Nucleoside Di-and Triphosphates as a New Generation of Anti-HIV Pronucleotides. Chemical and Biological Aspects. Appl. Sci. 2021, 11, 2248. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle-MRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Johansson, N.G.; Eriksson, S. Structure-Activity Relationships for Phosphorylation of Nucleoside Analogs to Monophosphates by Nucleoside Kinases. Acta Biochim. Polonica. 1996, 43, 143–160. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Bærentsen, R.L.; Fuhrer, T.; Sauer, U.; Gerdes, K.; Brodersen, D.E. (P)PpGpp Regulates a Bacterial Nucleosidase by an Allosteric Two-Domain Switch. Mol. Cell 2019, 74, 1239–1249.e4. [Google Scholar] [CrossRef]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.; Agrofoglio, L.A.; Janin, J. Human and Viral Nucleoside/Nucleotide Kinases Involved in Antiviral Drug Activation: Structural and Catalytic Properties. Antiviral Res. 2010, 86, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Munch-Petersen, B.; Johansson, K.; Ecklund, H. Structure and Function of Cellular Deoxyribonucleoside Kinases. Cell Mol. Life Sci. 2002, 59, 1327–1346. [Google Scholar] [CrossRef] [PubMed]
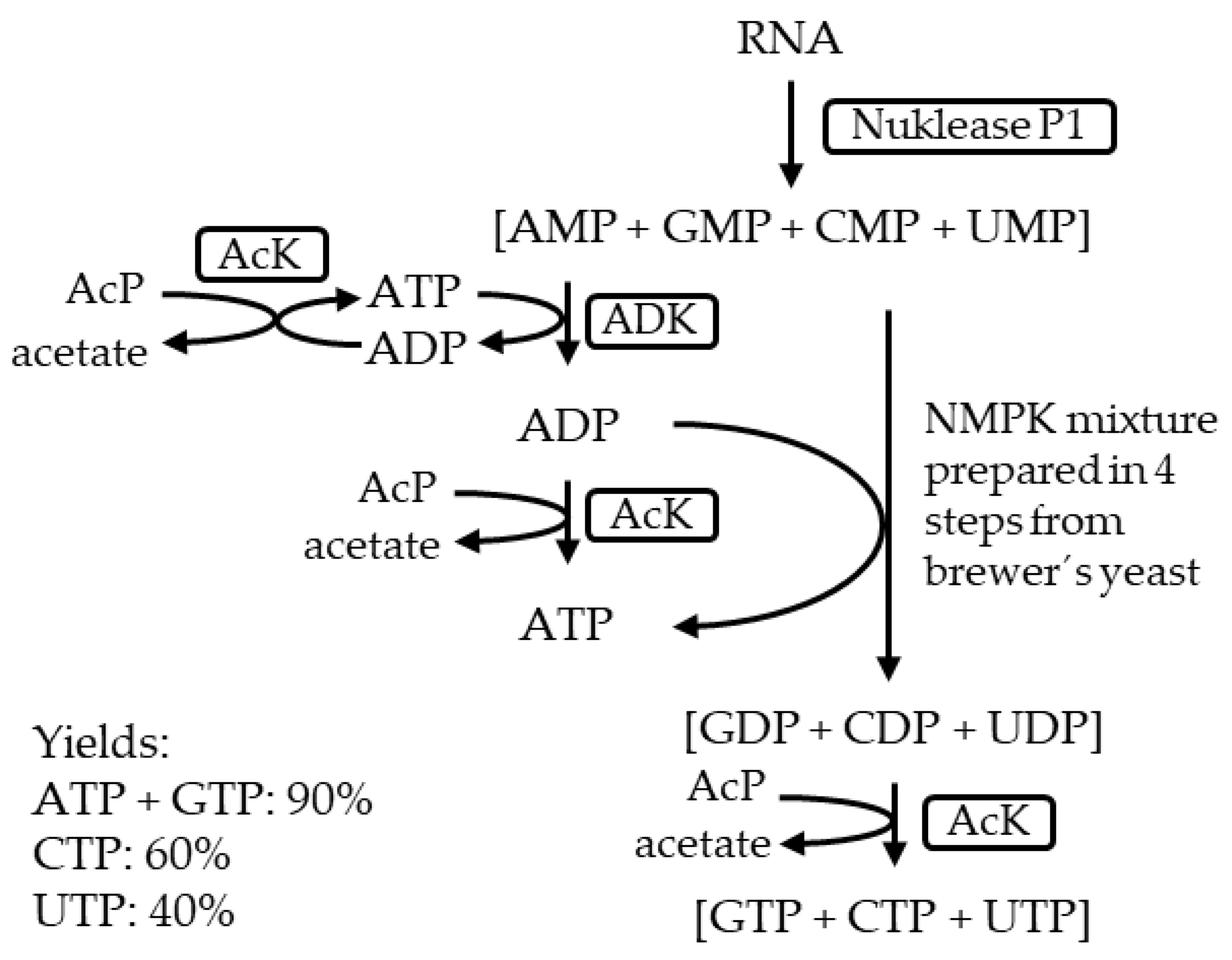
- Haynie, S.L.; Whitesides, G.M. Preparation of a Mixture of Nucleoside Triphosphates Suitable for Use in Synthesis of Nucleotide Phosphate Sugars from Ribonucleic Acid Using Nuclease P1, a Mixture of Nucleoside Monophosphokinases and Acetate Kinase. Appl. Biochem. Biotechnol. 1990, 23, 205–220. [Google Scholar] [CrossRef]
- Del Arco, J.; Fernandez-Lucas, J. Purine and Pyrimidine Phosphoribosyltransferases: A Versatile Tool for Enzymatic Synthesis of Nucleoside-5’-Monophosphates. Curr. Pharm. Des. 2017, 23, 6898–6912. [Google Scholar] [CrossRef]
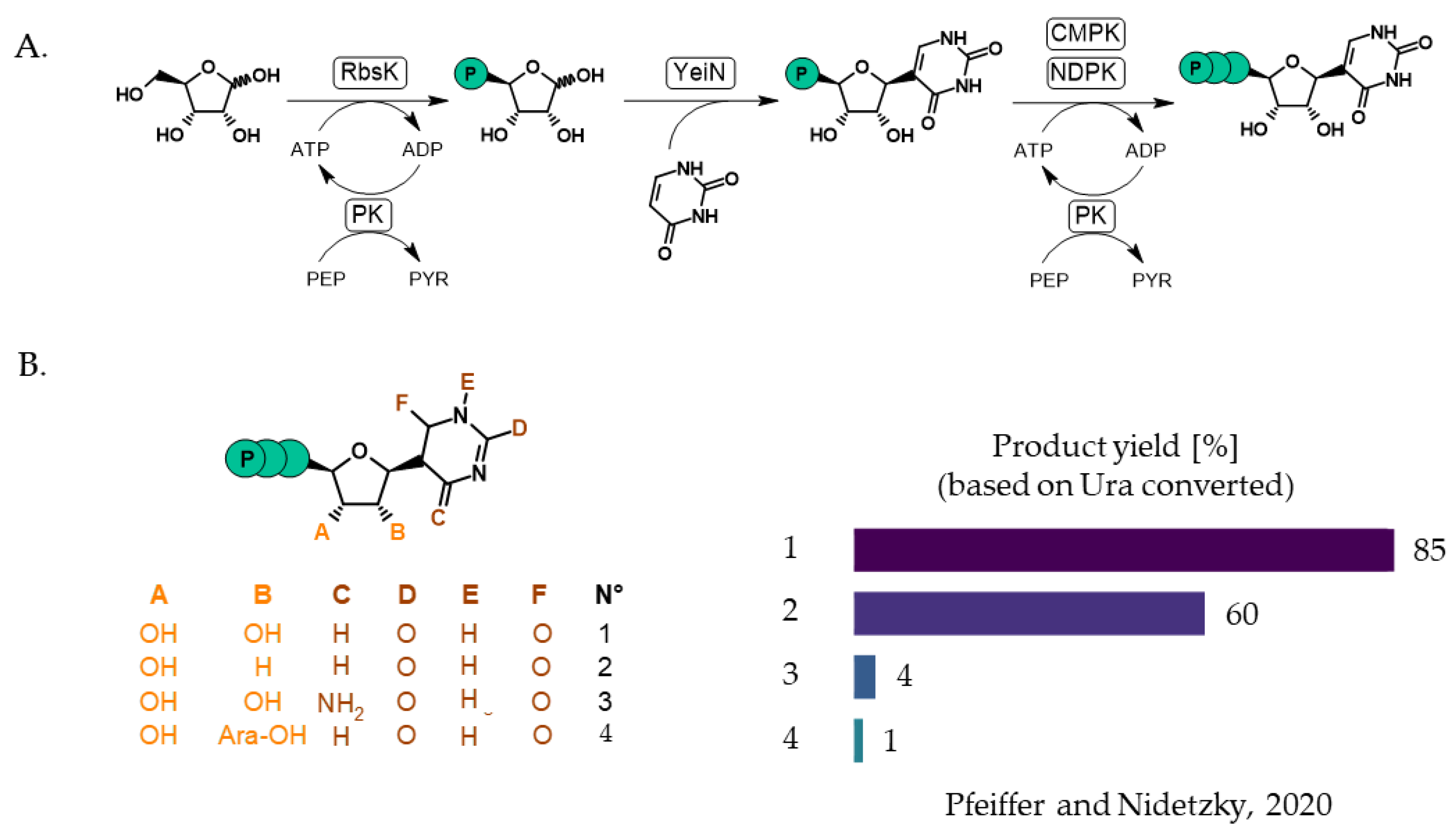
- Ribar, A.; Pfeiffer, M.; Correspondence, B.N. Phosphorylation-Condensation Cascade for Biocatalytic Synthesis of C-Nucleosides. Chem. Catal. 2024, 4, 101127. [Google Scholar] [CrossRef]
- Bennett, L.L.; Allan, W.; Arnett, G.; Shealy, Y.F.; Shewach, D.S.; Mason, W.S.; Fourel, I.; Parker, W.B. Metabolism in Human Cells of the D and L Enantiomers of the Carbocyclic Analog of 2’-Deoxyguanosine: Substrate Activity with Deoxycytidine Kinase, Mitochondrial Deoxyguanosine Kinase, and 5’-Nucleotidase. Antimicrob. Agents Chemother. 1998, 42, 1045–1051. [Google Scholar] [CrossRef]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Substrate Specificity and Phosphorylation of Antiviral and Anticancer Nucleoside Analogues by Human Deoxyribonucleoside Kinases and Ribonucleoside Kinases. Pharmacol. Ther. 2003, 100, 119–139. [Google Scholar] [CrossRef]
- Long, M.C.; Shaddix, S.C.; Moukha-Chafiq, O.; Maddry, J.A.; Nagy, L.; Parker, W.B. Structure-Activity Relationship for Adenosine Kinase from Mycobacterium Tuberculosis. II. Modifications to the Ribofuranosyl moiety. Biochem. Pharmacol. 2008, 75, 1588–1600. [Google Scholar] [CrossRef]
- Arnfors, L.; Hansen, T.; Schönheit, P.; Ladenstein, R.; Meining, W. Structure of Methanocaldococcus jannaschii Nucleoside Kinase: An Archaeal Member of the Ribokinase Family. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 1085–1097. [Google Scholar] [CrossRef]
- Elkin, S.R.; Kumar, A.; Price, C.W.; Columbus, L. A Broad Specificity Nucleoside Kinase from Thermoplasma acidophilum. Proteins Struct. Funct. Bioinform. 2013, 81, 568–582. [Google Scholar] [CrossRef]
- Piškur, J.; Sandrini, M.P.B.; Knecht, W.; Munch-Petersen, B. Animal Deoxyribonucleoside Kinases: ‘Forward’ and ‘Retrograde’ Evolution of Their Substrate Specificity. FEBS Lett. 2004, 560, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Ramaswamy, S.; Ljungcrantz, C.; Knecht, W.; Piskur, J.; Munch-Petersen, B.; Eriksson, S.; Eklund, H. Structural Basis for Substrate Specificities of Cellular Deoxyribonucleoside Kinases. Nat. Struct. Biol. 2001, 8, 616–620. [Google Scholar] [CrossRef]
- Munch-Petersen, B.; Piskur, J.; Søndergaard, L. Four Deoxynucleoside Kinase Activities from Drosophila Melanogaster Are Contained within a Single Monomeric Enzyme, a New Multifunctional Deoxynucleoside Kinase. J. Biol. Chem. 1998, 273, 3926–3931. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Munch-Petersen, B.; Eklund, H. Structural Studies of Nucleoside Analog and Feedback Inhibitor Binding to Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. FEBS J. 2008, 275, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila melanogaster Deoxyribonucleoside Kinase (DmdNK) as a High Performing Biocatalyst for the Synthesis of Purine Arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Eriksson, S. Mammalian Deoxyribonucleoside Kinases. Pharmacol. Ther. 1995, 67, 155–186. [Google Scholar] [CrossRef]
- Welin, M. Nordlund, Understanding Specificity in Metabolic Pathways-Structural Biology of Human Nucleotide Metabolism. Biochem. Biophys. Res. Commun. 2010, 396, 157–163. [Google Scholar] [CrossRef]
- Médici, R.; Garaycoechea, J.I.; Valino, A.L.; Pereira, C.A.; Lewkowicz, E.S.; Iribarren, A.M. A Comparative Study on Phosphotransferase Activity of Acid Phosphatases from Raoultella planticola and Enterobacter aerogenes on Nucleosides, Sugars, and Related Compounds. Appl. Microbiol. Biotechnol. 2014, 98, 3013–3022. [Google Scholar] [CrossRef]
- Valino, A.L.; Iribarren, A.M.; Lewkowicz, E. New Biocatalysts for One Pot Multistep Enzymatic Synthesis of Pyrimidine Nucleoside Diphosphates from Readily Available Reagents. J. Mol. Catal. B Enzym. 2015, 114, 58–64. [Google Scholar] [CrossRef]
- Zinchenko, I.; Barai, V.N.; Zalashko, L.M.; Poopeiko, N.E.; Sivets, G.G.; a Mikhailopulo, I.; Pricota, I. Enzymatic Synthesis of Nucleoside 5’-Mono and -Triphosphates. FEBS Lett. 1990, 260, 254–256. [Google Scholar] [CrossRef]
- Tasnádi, G.; Jud, W.; Hall, M.; Baldenius, K.; Ditrich, K.; Faber, K. Evaluation of Natural and Synthetic Phosphate Donors for the Improved Enzymatic Synthesis of Phosphate Monoesters. Adv. Synth. Catal. 2018, 360, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Mihara, Y.; Yamada, H. A Novel Selective Nucleoside Phosphorylating Enzyme from Morganella morganii. J. Biosci. Bioeng. 1999, 87, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhang, L.; Sun, L.H.; Li, X.J.; Wan, N.W.; Zheng, Y.G. Enzymatic Production of 5′-Inosinic Acid by a Newly Synthesised Acid Phosphatase/Phosphotransferase. Food Chem. 2012, 134, 948–956. [Google Scholar] [CrossRef]
- Mihara, Y.; Utagawa, T.; Yamada, H.; Asano, Y. Phosphorylation of Nucleosides by the Mutated Acid Phosphatase from Morganella morganii. Appl. Environ. Microbiol. 2000, 66, 2811–2816. [Google Scholar] [CrossRef]
- Yuan, H.; fan Jia, Z.; hua He, J.; guang Fan, X.; Chen, N. Production of 5′-Inosinic Acid by Whole-Cell Biocatalyst Expressing a Mutated Acid Phosphatase/Phosphotransferase. In Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 444, pp. 605–614. [Google Scholar]
- Okuyama, K.; Shibuya, S.; Hamamoto, T.; Noguchi, T. Enzymatic Synthesis of 2′-Deoxyguanosine with Nucleoside Deoxyribosyltransferase-II. Biosci. Biotechnol. Biochem. 2003, 67, 989–995. [Google Scholar] [CrossRef]
- Genz, F.; Friedrich, F.; Lönarz, C.; Einsle, O.; Jung, M.; Mu, M.; Fessner, N.D. Identification and Characterization of Pyrimidine Nucleoside 2′-Hydroxylase. ACS Catal. 2024, 15, 3611–3618. [Google Scholar] [CrossRef]
- Panayiotou, C.; Solaroli, N.; Johansson, M.; Karlsson, A. Evidence of an Intact N-Terminal Translocation Sequence of Human Mitochondrial Adenylate Kinase 4. Int. J. Biochem. Cell Biol. 2010, 42, 62–69. [Google Scholar] [CrossRef]
- Nucleoside Triphospates and Their Analogs. Chemistry, Biotechnology, and Biological Applications, 1st ed.; Vaghefi, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Teplyakov, A.; Sebastiao, P.; Obmolova, G.; Perrakis, A.; Brush, G.S.; Bessman, M.J.; Wilson, K.S. Crystal Structure of Bacteriophage T4 Deoxynucleotide Kinase with Its Substrates DGMP and ATP. EMBO J. 1996, 15, 3487–3497. [Google Scholar] [CrossRef]
- Caillat, C.; Topalis, D.; Agrofoglio, L.A.; Pochet, S.; Balzarini, J.; Deville-Bonne, D.; Meyer, P. Crystal Structure of Poxvirus Thymidylate Kinase: An Unexpected Dimerization Has Implications for Antiviral Therapy. Proc. Natl. Acad. Sci. USA 2008, 105, 16900–16905. [Google Scholar] [CrossRef]
- Torrents, E.; Aloy, P.; Gibert, I.; Rodríguez-Trelles, F. Ribonucleotide Reductases: Divergent Evolution of an Ancient Enzyme. J. Mol. Evol. 2002, 55, 138–152. [Google Scholar] [CrossRef]
- Gonin, P.; Xu, Y.; Milon, L.; Dabernat, S.; Morr, M.; Kumar, R.; Lacombe, M.L.; Janin, J.; Lascu, I. Catalytic Mechanism of Nucleoside Diphosphate Kinase Investigated Using Nucleotide Analogues, Viscosity Effects, and x-Ray Crystallography. Biochemistry 1999, 38, 7265–7272. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Moréra, S.; Janin, J.; Lascu, I.; Véron, M. X-Ray Structure of Nucleoside Diphosphate Kinase Complexed with Thymidine Diphosphate and Mg2+ at 2-Å Resolution. Biochemistry 1994, 33, 9062–9069. [Google Scholar] [CrossRef]
- Eckstein, F.; Goody, R.S. Synthesis and Properties of Diastereoisomers of Adenosine 5′-(O-1-Thiotriphosphate) and Adenosine 5′-(O-2-Thiotriphosphate). Biochemistry 1976, 15, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Peliska, J.A.; O’Leary, M.H. Sulfuryl Transfer Catalyzed by Phosphokinases. Biochemistry 1991, 30, 1049–1057. [Google Scholar] [CrossRef]
- Kamiya, H.; Kasai, H. Preparation of 8-Hydroxy-DGTP and 2-Hydroxy-DATP by a Phosphate Transfer Reaction by Nucleoside-Diphosphate Kinase. Nucleosides Nucleotides 1999, 18, 307–310. [Google Scholar] [CrossRef]
- Wu, W.; Bergstrom, D.E.; Davisson, V.J. A Combination Chemical and Enzymatic Approach for the Preparation of Azole Carboxamide Nucleoside Triphosphate. J. Org. Chem. 2003, 68, 3860–3865. [Google Scholar] [CrossRef]
- Imazawa, M.; Eckstein, F. Synthesis of Sugar-Modified Nucleoside 5’-Triphosphates with Partially Purified Nucleotide Kinases from Calf Thymus. Biochim. Biophys. Acta 1979, 570, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bao, J.; Gu, X.; Xin, X.; Chen, C.; Ryu, D.D.Y. Substrate Promiscuity of Pyruvate Kinase on Various Deoxynucleoside Diphosphates for Synthesis of Deoxynucleoside Triphosphates. Enzyme Microb. Technol. 2008, 43, 455–459. [Google Scholar] [CrossRef]
- Kim, M.J.; Whitesides, G.M. Enzyme-Catalyzed Synthesis of Nucleoside Triphosphates from Nucleoside Monophosphates–ATP from AMP and Ribavirin 5’-Triphosphate from Ribavirin 5’-Monophosphate. Appl. Biochem. Biotechnol. 1987, 16, 95–108. [Google Scholar] [CrossRef]
- Cardeilhac, T.; Cohen, S.S. Some Metabolic Properties of Nucleotides of 1-Beta-D-Arabinofuranosylcytosine. Cancer Res. 1964, 24, 1595–1603. [Google Scholar]
- Da Costa, C.P.; Fedor, M.J.; Scott, L.G. 8-Azaguanine Reporter of Purine Ionization States in Structured RNAs. J. Am. Chem. Soc. 2007, 129, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, I.; Boudou, V.; Pierra, C.; Gosselin, G.; Johnson, R.A. Enzymatic Synthesis of Unlabeled and Beta-(32)P-Labeled Beta-L-2’, 3’-Dideoxyadenosine-5’-Triphosphate as a Potent Inhibitor of Adenylyl Cyclases and Its Use as Reversible Binding Ligand. J. Biol. Chem. 1999, 274, 34735–34741. [Google Scholar] [CrossRef]
- Scott, L.G.; Geierstanger, B.H.; Williamson, J.R.; Hennig, M. Enzymatic Synthesis and 19 F NMR Studies of 2-Fluoroadenine-Substituted RNA. J. Am. Chem. Soc. 2004, 126, 11776–11777. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Inouye, M. Adenylate Kinase Complements Nucleoside Diphosphate Kinase Deficiency in Nucleotide Metabolism. Proc. Natl. Acad. Sci. USA 1996, 93, 5720–5725. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Conserva, F.; Panayiotou, C.; Karlsson, A.; Solaroli, N. The Human Adenylate Kinase 9 Is a Nucleoside Mono- and Diphosphate Kinase. Int. J. Biochem. Cell Biol. 2013, 45, 925–931. [Google Scholar] [CrossRef]
- Pospísilová, H.; Sebela, M.; Novák, O.; Frébort, I. Hydrolytic Cleavage of N6-Substituted Adenine Derivatives by Eukaryotic Adenine and Adenosine Deaminases. Biosci. Rep. 2008, 28, 335–347. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.H.P.J. Enzymatic Regeneration and Conservation of ATP: Challenges and Opportunities. Crit. Rev. Biotechnol. 2020, 41, 16–33. [Google Scholar] [CrossRef]
- Tavanti, M.; Hosford, J.; Lloyd, R.C.; Brown, M.J.B. ATP Regeneration by a Single Polyphosphate Kinase Powers Multigram-Scale Aldehyde Synthesis in Vitro. Green. Chem. 2021, 23, 828–837. [Google Scholar] [CrossRef]
- Motomura, K.; Hirota, R.; Okada, M.; Ikeda, T.; Ishida, T.; Kuroda, A. A New Subfamily of Polyphosphate Kinase 2 (Class III PPK2) Catalyzes Both Nucleoside Monophosphate Phosphorylation and Nucleoside Diphosphate Phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2602–2608. [Google Scholar] [CrossRef]
- Mordhorst, S.; Andexer, J.N. Round, Round We Go–Strategies for Enzymatic Cofactor Regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef]
- Kornberg, S.R. Adenosine Triphosphate Synthesis from Polyphosphate by an Enzyme from Escherichia Coli. BBA Biochim. Biophys. Acta 1957, 26, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Shiba, T. Use of Escherichia Coli Polyphosphate Kinase for Oligosaccharide Synthesis. Biosci. Biotechnol. Biochem. 1998, 62, 1594–1596. [Google Scholar] [CrossRef]
- Kuroda, A.; Kornberg, A. Polyphosphate Kinase as a Nucleoside Diphosphate Kinase in Escherichia Coli and Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. USA 1997, 94, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, J.C.; Teleki, A.; Jendrossek, D. A Universal Polyphosphate Kinase: PPK2c of Ralstonia Eutropha Accepts Purine and Pyrimidine Nucleotides Including Uridine Diphosphate. Appl. Microbiol. Biotechnol. 2020, 104, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Kulmer, S.T.; Gutmann, A.; Lemmerer, M.; Nidetzky, B. Biocatalytic Cascade of Polyphosphate Kinase and Sucrose Synthase for Synthesis of Nucleotide-Activated Derivatives of Glucose. Adv. Synth. Catal. 2017, 359, 292–301. [Google Scholar] [CrossRef]
- Benčić, P.; Keppler, M.; Kuge, M.; Qiu, D.; Schütte, L.M.; Häner, M.; Strack, K.; Jessen, H.J.; Andexer, J.N.; Loenarz, C. Non-Canonical Nucleosides: Biomimetic Triphosphorylation, Incorporation into MRNA and Effects on Translation and Structure. FEBS J. 2023, 290, 4899–4920. [Google Scholar] [CrossRef]
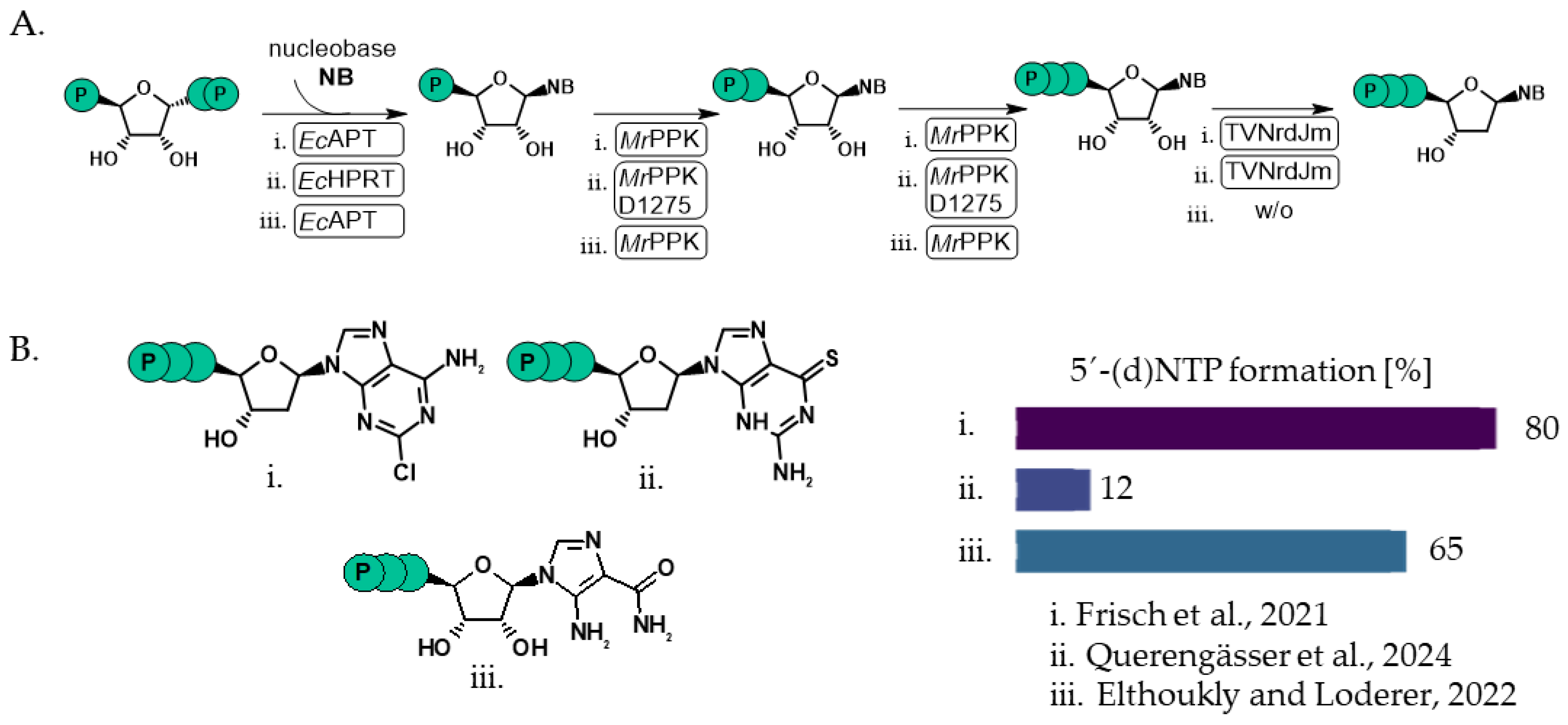
- Querengässer, T.; Wenzlaff, J.; Loderer, C. Broadening the Substrate Scope of a Polyphosphate Kinase for Canonical and Non-Canonical Nucleotides. ChemCatChem 2024, 16, e202400181. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kato, T.; Takenishi, T. A Novel Method for Phosphorylation of Nucleosides to 5′-Nucleotides. Tetrahedron Lett. 1967, 50, 5065–5068. [Google Scholar] [CrossRef]
- Ludwig, J.; Eckstein, F. Rapid and Efficient Synthesis of Nucleoside 5′-O-(1-Thiotriphosphates), 5′-Triphosphates and 2′,3′-Cyclophosphorothioates Using 2-Chloro-4H-1,3,2-Benzodioxaphosphorin-4-One. J. Org. Chem. 1989, 54, 631–635. [Google Scholar] [CrossRef]
- Kovács, T.; Ötvös, L. Simple Synthesis of 5-Vinyl- and 5-Ethynyl-2′-Deoxyuridine-5′-Triphosphates. Tetrahedron Lett. 1988, 29, 4525–4528. [Google Scholar] [CrossRef]
- Gillerman, I.; Fischer, B. An Improved One-Pot Synthesis of Nucleoside 5′-Triphosphate Analogues. Nucleosides Nucleotides Nucleic Acids 2010, 29, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Caton-Williams, J.; Smith, M.; Carrasco, N.; Huang, Z. Protection-Free One-Pot Synthesis of 2 0-Deoxynucleoside 5 0-Triphosphates and DNA Polymerization. Org. Lett. 2011, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.; Srinivasan, B. Recent Advances in the Syntheses of Nucleoside Triphosphates. Curr. Org. Synth. 2014, 10, 903–934. [Google Scholar] [CrossRef]
- Burgess, K.; Cook, D. Syntheses of Nucleoside Triphosphates. Chem. Rev. 2000, 100, 2047–2059. [Google Scholar] [CrossRef]
- Johnson, D.C.; Widlanski, T.S. Overview of the Synthesis of Nucleoside Phosphates and Polyphosphates. Curr. Protoc. Nucleic Acid. Chem. 2004, 15, 13.1.1–13.1.31. [Google Scholar] [CrossRef]
- Rozzell, J.D. Commercial Scale Biocatalysis: Myths and Realities. Bioorg Med. Chem. 1999, 7, 2253–2261. [Google Scholar] [CrossRef]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar]
- Baughn, R.L.; Adalsteinsson, O.; Whitesides, G.M. Large-Scale Enzyme-Catalyzed Synthesis of ATP from Adenosine and Acetyl Phosphate. Regeneration of ATP from AMP. J. Am. Chem. Soc. 1978, 100, 304–306. [Google Scholar] [CrossRef]
- Hennig, M.; Scott, L.G.; Sperling, E.; Bermel, W.; Williamson, J.R. Synthesis of 5-Fluoropyrimidine Nucleotides as Sensitive NMR Probes of RNA Structure. J. Am. Chem. Soc. 2007, 129, 14911–14921. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Nidetzky, B. Biocatalytic Cascade Transformations for the Synthesis of C-Nucleosides and N-Nucleoside Analogs. Curr. Opin. Biotechnol. 2023, 79, 102873. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Nidetzky, B. Reverse C-Glycosidase Reaction Provides C-Nucleotide Building Blocks of Xenobiotic Nucleic Acids. Nat. Commun. 2020, 11, 6270. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Maršić, T.; Loderer, C. A Novel One-Pot Enzyme Cascade for the Biosynthesis of Cladribine Triphosphate. Biomolecules 2021, 11, 346. [Google Scholar] [CrossRef]
- Eltoukhy, L.; Loderer, C. A Multi-Enzyme Cascade for the Biosynthesis of AICA Ribonucleoside Di- and Triphosphate. ChemBioChem 2022, 23, e202100596. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, Z.; Ning, X.; Xu, W.; Li, Z. In Vitro Biosynthesis of ATP from Adenosine and Polyphosphate. Bioresour. Bioprocess. 2021, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Nikel, P.; Andexer, J.N.; Lütz, S.; Rosenthal, K. A Multi-Enzyme Cascade Reaction for the Production of 2′3′-CGAMP. Biomolecules 2021, 11, 590. [Google Scholar] [CrossRef]
- Becker, M.; Nowak, I.; Hildebrand, K.; Lütz, S.; Rosenthal, K. Development of a Multi-Enzyme Cascade for 2′3′-CGAMP Synthesis from Nucleosides. Catal. Sci. Technol. 2024, 14, 3335–3345. [Google Scholar] [CrossRef]
- Mordhorst, S.; Siegrist, J.; Müller, M.; Richter, M.; Andexer, J.N. Catalytic Alkylation Using a Cyclic S-Adenosylmethionine Regeneration System. Angew. Chemie Int. Ed. 2017, 56, 4037–4041. [Google Scholar] [CrossRef]
- Popadić, D.; Mhaindarkar, D.; Dang Thai, M.H.N.; Hailes, H.C.; Mordhorst, S.; Andexer, J.N. A Bicyclic S-Adenosylmethionine Regeneration System Applicable with Different Nucleosides or Nucleotides as Cofactor Building Blocks. RSC Chem. Biol. 2021, 2, 883–891. [Google Scholar] [CrossRef]
- Schultheisz, H.; Szymczyna, B.; Scott, L.; Williamson, J. Pathway Engineered Enzymatic de Novo Purine Nucleotide Synthesis. ACS Chem. Biol. 2008, 5, 313–320. [Google Scholar] [CrossRef]
- Schultheisz, H.L.; Szymczyna, B.R.; Scott, L.G.; Williamson, J.R. Enzymatic de Novo Pyrimidine Nucleotide Synthesis. J. Am. Chem. Soc. 2010, 133, 297–304. [Google Scholar] [CrossRef]
- Kaminski, A.; Labesse, G. Phosphodeoxyribosyltransferases, Designed Enzymes for Deoxyribonucleotides Synthesis. J. Biol. Chem. 2013, 288, 6534–6541. [Google Scholar] [CrossRef]
- Siedentop, R.; Prenzel, T.; Waldvogel, S.R.; Rosenthal, K.; Lütz, S. Reaction Engineering and Comparison of Electroenzymatic and Enzymatic ATP Regeneration Systems. ChemElectroChem 2023, 10, e202300332. [Google Scholar] [CrossRef]
- Chenault, H.K.; Simon, E.S.; Whitesides, G. Cofactor Regeneration for Enzyme-Catalysed Synthesis. Biotechnol. Genet. Eng. Rev. 1988, 6, 221–270. [Google Scholar] [CrossRef]
- Ruccolo, S.; Brito, G.; Christensen, M.; Itoh, T.; Mattern, K.; Stone, K.; Strotman, N.A.; Sun, A.C. Electrochemical Recycling of Adenosine Triphosphate in Biocatalytic Reaction Cascades. J. Am. Chem. Soc. 2022, 144, 22582–22588. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Noguchi, T. Inorganic Polyphosphate Kinase and Adenylate Kinase Participate in the Polyphosphate:AMP Phosphotransferase Activity of Escherichia Coli. Proc. Natl. Acad. Sci. USA 2002, 97, 14168–14171. [Google Scholar] [CrossRef]
- Scism, R.A.; Bachmann, B.O. Five-Component Cascade Synthesis of Nucleotide Analogues in an Engineered Self-Immobilized Enzyme Aggregate. ChemBioChem 2010, 11, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.L.; Hahn, T.M.; Reynolds, K.K.; Shewach, D.S. Kinetic Analysis of Human Deoxycytidine Kinase with the True Phosphate Donor Uridine Triphosphate. Biochemistry 1997, 36, 7540–7547. [Google Scholar] [CrossRef]
- Li, Z.; Ning, X.; Zhao, Y.; Zhang, X.; Xiao, C.; Li, Z. Efficient One-Pot Synthesis of Cytidine 5′-Monophosphate Using an Extremophilic Enzyme Cascade System. J. Agric. Food Chem. 2020, 68, 9188–9194. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Zhu, J. Enzymatic Production of L-Theanine by γ-Glutamylmethylamide Synthetase Coupling with an ATP Regeneration System Based on Polyphosphate Kinase. Process Biochem. 2016, 51, 1458–1463. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehlau, M.; Westarp, S.; Neubauer, P.; Kurreck, A. Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts 2025, 15, 270. https://doi.org/10.3390/catal15030270
Fehlau M, Westarp S, Neubauer P, Kurreck A. Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts. 2025; 15(3):270. https://doi.org/10.3390/catal15030270
Chicago/Turabian StyleFehlau, Maryke, Sarah Westarp, Peter Neubauer, and Anke Kurreck. 2025. "Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs" Catalysts 15, no. 3: 270. https://doi.org/10.3390/catal15030270
APA StyleFehlau, M., Westarp, S., Neubauer, P., & Kurreck, A. (2025). Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts, 15(3), 270. https://doi.org/10.3390/catal15030270










