Butanol Production by Ethanol Condensation: Improvements and Limitations in the Rational Design of Cu-Ni-MgO/Graphite Catalysts
Abstract
:1. Introduction
2. Results
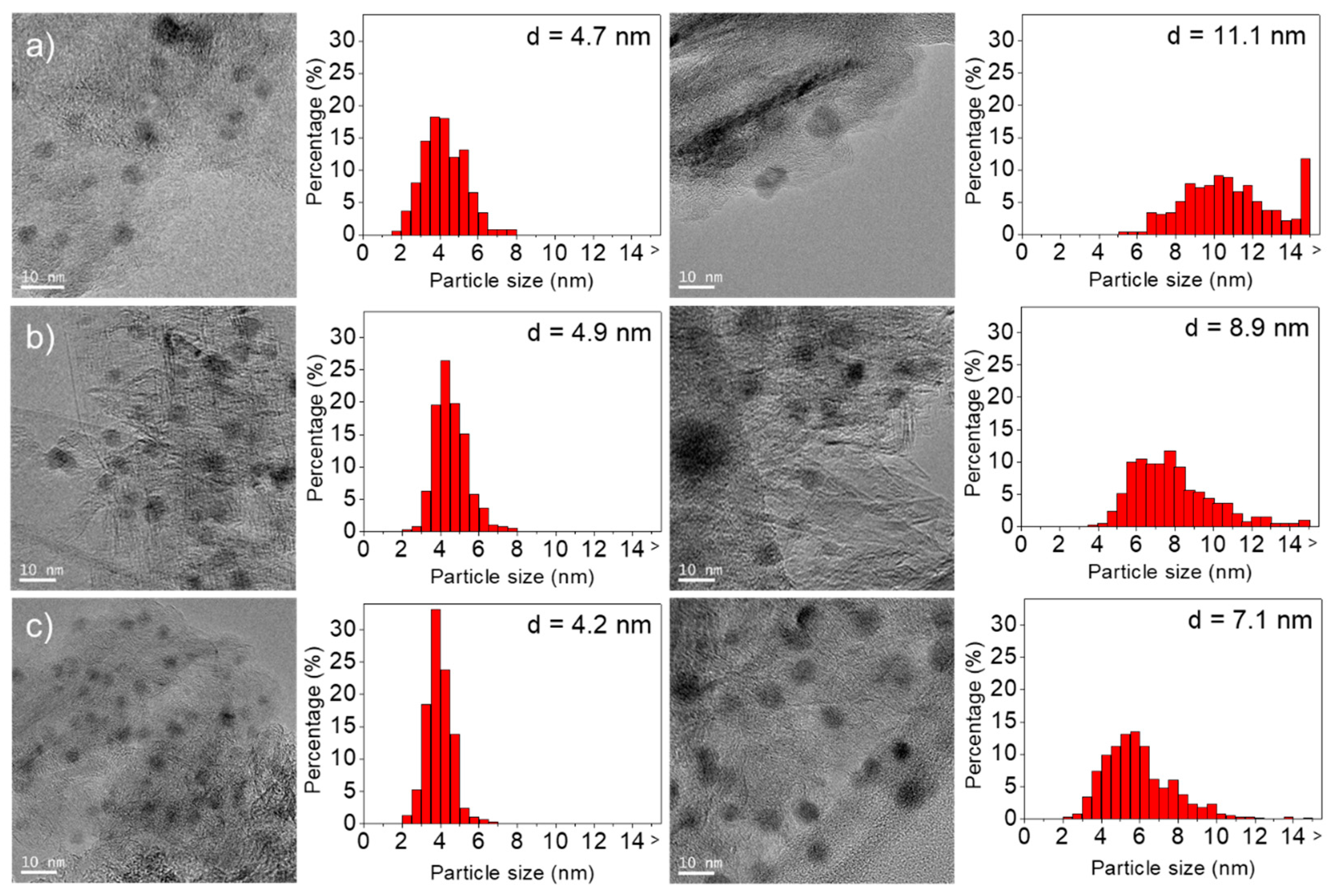
2.1. Characterization of the Multicomponent Catalysts
2.2. Characterization of the Catalytic Surfaces
2.3. Comparative Study of Catalytic Materials in the Ethanol to Butanol Reaction
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, B.; Ma, S.; Liang, S.; Wang, Z.; Liu, Y.; Mao, S.; Ban, H.; Wang, L.; Wang, Y. Efficient Conversion of Ethanol to 1-Butanol over Adjacent Acid–Base Dual Sites via Enhanced C–H Activation. ACS Catal. 2023, 13, 4866–4872. [Google Scholar] [CrossRef]
- Onyestyák, G. Carbon supported alkaline catalysts for Guerbet coupling of bioethanol. Period. Polytech. Chem. Eng. 2018, 62, 91–96. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent Advances in Catalytic Conversion of Ethanol to Chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Earley, J.H.; Bourne, R.A.; Watson, M.J.; Poliakoff, M. Continuous catalytic upgrading of ethanol to n-butanol and C4 products over Cu/CeO2 catalysts in supercritical CO2. Green Chem. 2015, 17, 3018–3025. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic Upgrading of Bioethanol to Fuel Grade Biobutanol: A Review. Ind Eng. Chem. Res. 2015, 54, 7181–7194. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L. Bio-aviation fuel conversion technologies. Renew. Sust. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Ordóñez, S. Ethanol catalytic condensation over Mg-Al mixed oxides derived from hydrotalcites. Catal. Today 2011, 164, 436–442. [Google Scholar] [CrossRef]
- Marcu, I.C.; Tichit, D.; Fajula, F.; Tanchoux, N. Catalytic valorization of bioethanol over Cu-Mg-Al mixed oxide catalysts. Catal. Today 2009, 147, 231–238. [Google Scholar] [CrossRef]
- Sun, Z.; Vasconcelos, A.C.; Bottari, G.; Stuart, M.C.A.; Bonura, G.; Cannilla, C.; Frusteri, F.; Barta, K. Efficient Catalytic Conversion of Ethanol to 1-Butanol via the Guerbet Reaction over Copper- and Nickel-Doped Porous. ACS Sustain. Chem. Eng. 2017, 5, 1738–1746. [Google Scholar] [CrossRef]
- Ho, C.R.; Shylesh, S.; Bell, A.T. Mechanism and Kinetics of Ethanol Coupling to Butanol over Hydroxyapatite. ACS Catal. 2016, 6, 939–948. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquo, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Makshina, E.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.; Sels, B. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef]
- Jordison, T.; Lira, C.; Miller, D. Condensed-Phase Ethanol Conversion to Higher Alcohols. Ind. Eng. Chem. Res. 2015, 54, 10991–11000. [Google Scholar] [CrossRef]
- Shimizu, K. Heterogeneous catalysis for the direct synthesis of chemicals by borrowing hydrogen methodology. Catal. Sci. Technol. 2015, 5, 1412–1427. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; He, L.; Li, L.; Pan, X.; Wang, A.; Wang, X.; Zhang, T. Upgrading ethanol to n-butanol over highly dispersed Ni–MgAlO catalysts. J. Catal. 2016, 344, 184–193. [Google Scholar] [CrossRef]
- Ogo, S.; Onda, A.; Iwasa, Y.; Hara, K.; Fukuoka, A.; Yanagisawa, K. 1-Butanol synthesis from ethanol over strontium phosphate hydroxyapatite catalysts with various Sr/P ratios. J. Catal. 2012, 296, 24–30. [Google Scholar] [CrossRef]
- Cimino, S.; Apuzzo, J.; Lisi, L. MgO Dispersed on Activated Carbon as Water Tolerant Catalyst for the Conversion of Ethanol into Butanol. Appl. Sci. 2019, 9, 1371. [Google Scholar] [CrossRef]
- Perrone, O.M.; Lobefaro, F.; Aresta, M.; Nocito, F.; Boscolo, M.; Dibenedetto, A. Butanol synthesis from ethanol over CuMgAl mixed oxides modified with palladium (II) and indium (III). Fuel Process. Tech. 2018, 177, 353–357. [Google Scholar] [CrossRef]
- Ungureanu, A.; Dragoi, B.; Chirieac, A.; Ciotonea, C.; Royer, S.; Duprez, D.; Dumitriu, E. Composition-dependent morphostructural properties of Ni-Cu oxide nanoparticles confined within the channels of ordered mesoporous SBA-15 silica. ACS Appl. Mater. Interfaces 2013, 5, 3010–3025. [Google Scholar] [CrossRef]
- Riittonen, T.; Toukoniitty, E.; Madnani, D.K.; Leino, A.-R.; Kordas, K.; Szabo, M.; Sapi, A.; Arve, K.; Wärnå, J.; Mikkola, J.-P. One-Pot Liquid-Phase Catalytic Conversion of Ethanol to 1-Butanol over Aluminium Oxide—The Effect of the Active Metal on the Selectivity. Catalysts 2012, 2, 68–84. [Google Scholar] [CrossRef]
- Benito, P.; Vaccari, A.; Antonetti, C.; Licursi, D.; Schiarioli, N.; Rodriguez-Castellon, E.; Raspolli Galletti, A.M. Tunable copper-hydrotalcite derived mixed oxides for sustainable ethanol condensation to n-butanol in liquid phase. J. Clean. Prod. 2019, 209, 1614–1623. [Google Scholar] [CrossRef]
- Nezam, I.; Peereboom, L.; Miller, D.J. Continuous condensed-phase ethanol conversion to higher alcohols: Experimental results and techno-economic analysis. J. Clean. Prod. 2019, 209, 1365–1375. [Google Scholar] [CrossRef]
- Eslava, J.L.; Sun, X.; Gascon, J.; Kapteijn, F.; Rodríguez-Ramos, I. Ruthenium particle size and cesium promotion effects in Fischer-Tropsch synthesis over high-surface-area graphite supported catalysts. Catal. Sci. Technol. 2017, 7, 1235–1244. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Badenes, P.; Rodríguez-Ramos, I. Study of some factors affecting the Ru and Pt dispersions over high surface area graphite-supported catalysts. Appl. Catal. A 1998, 173, 313–321. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Morales, M.V.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Tunable selectivity of Ni catalysts in the hydrogenation reaction of 5-hydroxymethylfurfural in aqueous media: Role of the carbon supports. Carbon 2021, 182, 265–275. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Feng, S.; Liu, P.; Hao, F.; Xiong, W.; Luo, H. Multi-walled carbon nanotubes supported nickel nanoparticles doped with magnesia and copper for adiponitrile hydrogenation with high activity and chemoselectivity under mild conditions. Chem. Eng. J. 2018, 346, 203–216. [Google Scholar] [CrossRef]
- Patil, P.T.; Liu, D.; Liu, Y.; Chang, J.; Borgna, A. Improving 1,3-butadiene yield by Cs promotion in ethanol conversion. Appl. Catal. A 2017, 543, 67–74. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, I.; Kang, J.; Jeong, H.; Park, J.K.; Park, J.H.; Jung, J. Alcohol-assisted low temperature methanol synthesis from syngas over Cu/ZnO catalysts: Effect of pH value in the co-precipitation step. J. Mol. Catal. A Chem. 2015, 400, 132–138. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Wang, H.-H.; Zhao, T.-J.; Zhang, B.; Su, H.; Xue, Z.-H.; Li, X.-H.; Chen, J.-S. Schottky Barrier Induced Coupled Interface of Electron-Rich N-Doped Carbon and Electron-Deficient Cu: In-Built Lewis Acid–Base Pairs for Highly Efficient CO2 Fixation. J. Am. Chem. Soc. 2019, 141, 38–41. [Google Scholar] [CrossRef]
- López-Olmos, C.; Morales, M.V.; Guerrero-Ruiz, A.; Ramirez-Barria, C.; Asedegbega-Nieto, E.; Rodríguez-Ramos, I. Continuous Gas-Phase Condensation of Bioethanol to 1-Butanol over Bifunctional Pd/Mg and Pd/Mg–Carbon Catalysts. ChemSusChem 2018, 11, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Faba, L.; Díaz, E.; Ordóñez, S. Tuning the selectivities of Mg-Al mixed oxides for ethanol upgrading reactions through the presence of transition metals. Appl. Catal. A 2018, 559, 167–174. [Google Scholar] [CrossRef]
- Almohalla, M.; Gallegos-Suarez, E.; Arcoya, A.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Comparative study of bioethanol transformation catalyzed by Ru or Pt nanoparticles supported on KL zeolite. Catal. Sci. Technol. 2016, 6, 521–529. [Google Scholar] [CrossRef]
- Patel, A.D.; Telalović, S.; Bitter, J.H.; Worrell, E.; Patel, M.K. Analysis of sustainability metrics and application to the catalytic production of higher alcohols from ethanol. Catal. Today 2015, 239, 56–79. [Google Scholar] [CrossRef]
- Bisarya, A.; Kathuria, L.; Kanu Das, K.; Yasmin, E.; Jasra, R.V.; Dholed, S.; Kumar, A. State-of-the-art advances in homogeneous molecular catalysis for the Guerbet upgrading of bio-ethanol to fuel-grade bio-butanol. Chem. Commun. 2025, 61, 2906–2925. [Google Scholar] [CrossRef]
- López Olmos, C. Producción Catalítica de Compuestos Químicos a Partir de Materiales Renovables. Ph.D. Thesis, Universidad Nacional de Educación a Distancia, Madrid, Spain, 2019. Available online: https://hdl.handle.net/20.500.14468/17508 (accessed on 31 January 2025).




| Material | SBET (m2/g) |
|---|---|
| HSAG | 399 |
| Mg/HSAG | 317 |
| 5Cu-Mg/HSAG | 270 |
| 5Ni-Mg/HSAG | 303 |
| 4Cu1Ni-Mg/HSAG | 243 |
| 4Cu1Ni-Mg/HSAG * | 214 |
| Catalyst | Weak Acids (%) | Medium Acids (%) | Strong Acids (%) | Areas of NH3 TPD (a.u.) |
|---|---|---|---|---|
| 5Cu/HSAG | 35 | 31 | 34 | 1.3 |
| 5Ni/HSAG | 31 | 26 | 43 | 1.3 |
| Mg/HSAG | 36 | 33 | 29 | 1.6 |
| 5Cu-Mg/HSAG | 34 | 31 | 35 | 2.6 |
| 5Ni-Mg/HSAG | 29 | 32 | 39 | 2.0 |
| 4Cu1Ni-Mg/HSAG | 40 | 31 | 29 | 1.9 |
| 4Cu1Ni-Mg/HSAG * | 29 | 30 | 41 | 1.7 |
| Catalyst | Weak Bases (%) | Medium Bases (%) | Strong Bases (%) | Total Uptakes (µmol CO2/g) |
|---|---|---|---|---|
| Mg/HSAG | 26 | 55 | 19 | 65 |
| Mg/HSAG * | 23 | 56 | 21 | 85 |
| 5Cu-Mg/HSAG | 26 | 62 | 12 | 54 |
| 5Ni-Mg/HSAG | 13 | 49 | 38 | 86 |
| 4Cu1Ni-Mg/HSAG | 15 | 77 | 8 | 70 |
| 4Cu1Ni-Mg/HSAG * | 12 | 70 | 18 | 69 |
| Selectivity (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Conv. (%) | ButOH | Ac | CO | CH4 | 1,1-DEE | Others | d (nm) a | d (nm) b |
| Cu/HSAG | 17 | 4 | 52 | 0 | 0 | 31 | 13 | 4.7 | 11.1 |
| Ni/HSAG | 23 | 7 | 24 | 15 | 25 | 1 | 28 | 4.9 | 8.9 |
| 4Cu-1Ni/HSAG | 20 | 5 | 39 | 8 | 12 | 13 | 23 | 4.2 | 7.1 |
| 5Cu-Mg/HSAG | 26 | 26 | 23 | 0 | 0 | 15 | 36 | 7.5 | 8.9 |
| 5Ni-Mg/HSAG | 23 | 24 | 12 | 15 | 25 | 2 | 22 | 4.5 | 5.5 |
| 2.5Cu2.5Ni-Mg/HSAG | 28 | 32 | 13 | 11 | 15 | 2 | 27 | 5.8 | 9.3 |
| 4Cu1Ni-Mg/HSAG | 24 | 39 | 22 | 4 | 6 | 4 | 25 | 6.6 | 7.1 |
| 4.75Cu0.25Ni-Mg/HSAG | 28 | 31 | 23 | 2 | 2 | 10 | 32 | 6.8 | 8.5 |
| 4Cu1Ni-Mg/HSAG * | 20 | 44 | 21 | 3 | 2 | 3 | 27 | 8.8 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ramos, I.; Lopez-Olmos, C.; Guerrero-Ruiz, A. Butanol Production by Ethanol Condensation: Improvements and Limitations in the Rational Design of Cu-Ni-MgO/Graphite Catalysts. Catalysts 2025, 15, 272. https://doi.org/10.3390/catal15030272
Rodríguez-Ramos I, Lopez-Olmos C, Guerrero-Ruiz A. Butanol Production by Ethanol Condensation: Improvements and Limitations in the Rational Design of Cu-Ni-MgO/Graphite Catalysts. Catalysts. 2025; 15(3):272. https://doi.org/10.3390/catal15030272
Chicago/Turabian StyleRodríguez-Ramos, Inmaculada, Cristina Lopez-Olmos, and Antonio Guerrero-Ruiz. 2025. "Butanol Production by Ethanol Condensation: Improvements and Limitations in the Rational Design of Cu-Ni-MgO/Graphite Catalysts" Catalysts 15, no. 3: 272. https://doi.org/10.3390/catal15030272
APA StyleRodríguez-Ramos, I., Lopez-Olmos, C., & Guerrero-Ruiz, A. (2025). Butanol Production by Ethanol Condensation: Improvements and Limitations in the Rational Design of Cu-Ni-MgO/Graphite Catalysts. Catalysts, 15(3), 272. https://doi.org/10.3390/catal15030272











