Advances in the Preparation of Carrier-Based Composite Photocatalysts and Their Applications
Abstract
:1. Introduction
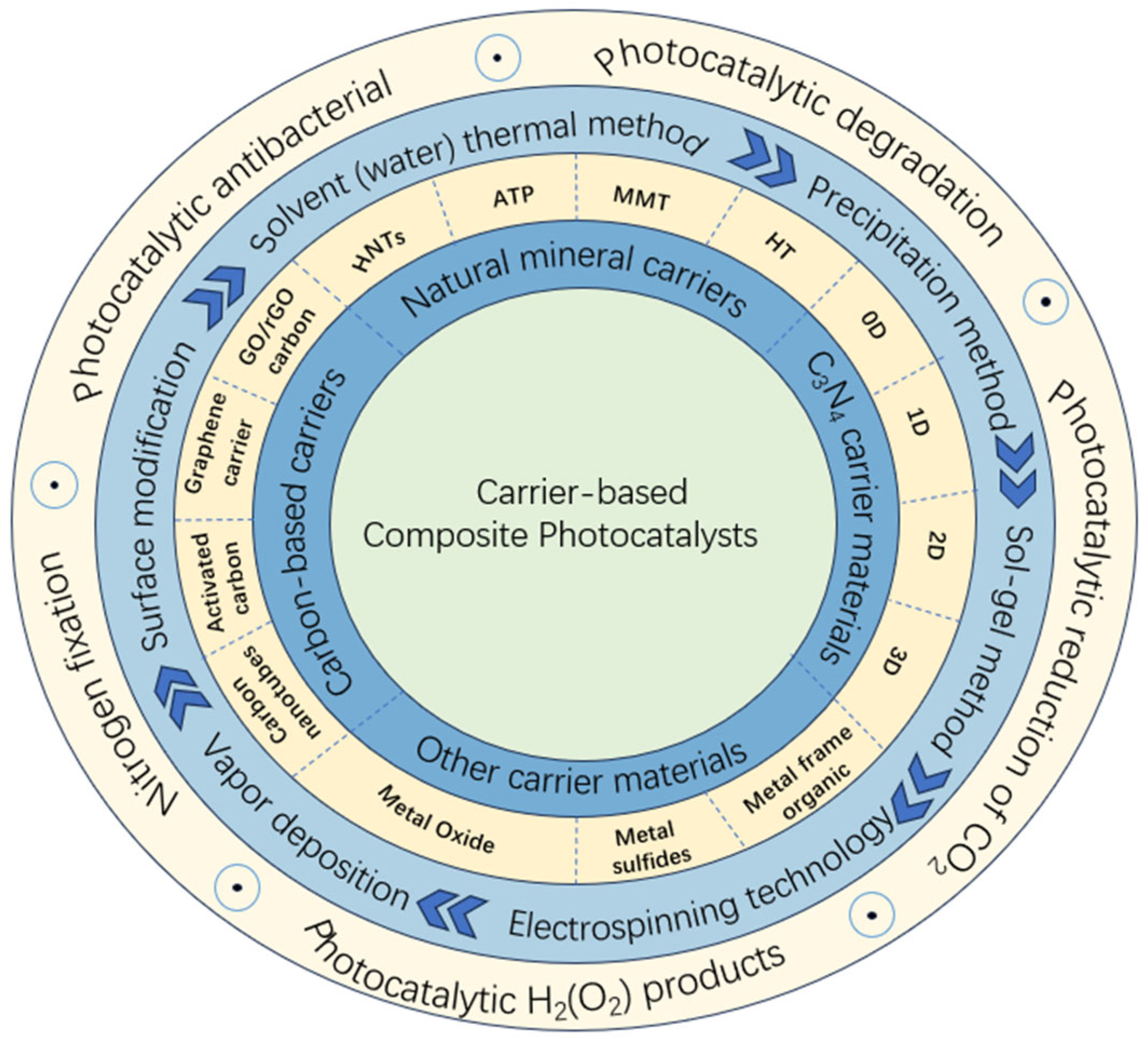
2. Classification of Carrier Materials for Photocatalysts
2.1. Natural Mineral Carriers
2.1.1. Halloysite Nanotubes
2.1.2. Attapulgite
2.1.3. Montmorillonite
2.1.4. Hydrotalcite
2.2. Carbon-Based Carriers
2.2.1. Graphene Carrier Materials
2.2.2. Carbon Nanotubes
2.2.3. Activated Carbon
2.3. C3N4 Carrier Material
2.3.1. One-Dimensional Carbon Nitride
2.3.2. Two-Dimensional Sheet Carbon Nitride
2.3.3. Three-Dimensional Carbon Nitride
2.4. Other Carrier Materials
3. Preparation of Carrier-Based Composite Photocatalytic Materials
3.1. Solvent (Water) Thermal Method
3.2. Precipitation Method
3.3. Sol–Gel Method
3.4. Electrospinning Technology
3.5. Vapor Deposition
3.6. Surface Modification
3.7. Ball Milling
4. Application of Carrier-Based Composite Photocatalytic Materials in Environmental Photocatalysis
4.1. Photocatalytic Degradation
4.2. Photocatalytic Reduction of CO2
4.3. Photocatalytic H2(O2) Products
4.4. Nitrogen Fixation
4.5. Photocatalytic Antibacterial Agents
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Hassan, M.; Ali, S.; Li, H.; Ou, O.; Chen, Q. Self-cleaning-mediated SERS chip coupled chemometric algorithms for detection and photocatalytic degradation of pesticides in food. J. Agric. Food. Chem. 2021, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, S.; Barak, N.; Bauer, D.; Davidson, F.; Gibney, B.; Hubbard, S.; Westerhof, P. Sustaining water resources: Environmental and economic impact. ACS Sustain. Chem. Eng. 2019, 7, 2879–2888. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, W.; Liu, S.; Haruna, S.; Zareef, M.; Ahmad, W.; Hassan, M.; Chen, Q. A tailorable and recyclable TiO2 NFSF/Ti@ Ag NPs SERS substrate fabricated by a facile method and its applications in prohibited fish drugs detection. J. Food Meas. Charact. 2022, 16, 2890–2898. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO2 in a solid food: A novel method to enhance the low-frequency ultrasound effect on immersion freezing process. Innov. Food Sci. Emerg. Technol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Xu, C.; Chen, Y.; Sun, W.; Liu, Q.; Dong, Q. Modeling inhibition effects of Lactobacillus plantarum subsp. plantarum CICC 6257 on growth of Listeria monocytogenes in ground pork stored at CO2-rich atmospheres. LWT 2018, 97, 811–817. [Google Scholar] [CrossRef]
- Akhlaq, M.; Zhang, C.; Yan, H.; Ou, M.; Zhang, W.; Liang, S.; Ikram, R. Response of tomato growth to continuous elevated CO2 concentration under controlled environment. Int. J. Agri. Biol. Eng. 2022, 15, 51–59. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO2 enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Khan, K.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, J.; Hu, H.; Pan, Q.; Cui, L.; Hu, Y. Determination of critical crop water stress index of tea under drought stress based on the intercellular CO2 concentration. Agronomy 2024, 14, 2154. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Taha, T.A.; Alenad, A.M.; Uddin, I.; Hayat, A.; Ali, T.; Shah, R.; Irfan, A.; Khan, W. A superficial intramolecular alignment of carbon nitride through conjugated monomer for optimized photocatalytic CO2 reduction. Catalysts 2021, 11, 935. [Google Scholar] [CrossRef]
- Ye, L.; Wang, H.; Jin, X.; Su, Y.; Wang, D.; Xie, H.; Liu, X.; Liu, X. Synthesis of olive-green few-layered BiOI for efficient photoreduction of CO2 into solar fuels under visible/near-infrared light. Sol. Energy Mater. Sol. Cells 2016, 144, 732–739. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Liu, G.; Zhang, C.; Liu, G.; Xu, S.; Cui, P.; Li, D. Rapid synthesis of BiOCl graded microspheres with highly exposed (110) facets and oxygen vacancies at room temperature to enhance visible light photocatalytic activity. Catal. Commun. 2019, 130, 105769. [Google Scholar] [CrossRef]
- Pellegrino, F.; Sordello, F.; Mino, L.; Hodoroaba, V.; Martra, G.; Maurino, V. Formic acid photoreforming for hydrogen production on shape-controlled anatase TiO2 nanoparticles: Assessment of the role of fluorides, {101}/{001} surfaces ratio, and platinization. ACS Catal. 2019, 9, 6692–6697. [Google Scholar] [CrossRef]
- Wang, K.; Endo-Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Boucle, J.; Ohtani, B.; Kowalska, E.; Herlin-Boime, N. Carbon/graphene-modified titania with enhanced photocatalytic activity under UV and vis irradiation. Materials 2019, 12, 4158. [Google Scholar] [CrossRef]
- Wu, H.; Han, D.; Hu, B.; Liang, J.; Tang, X.; Zhu, Z.; Huo, P. Insights into enhanced photocatalytic CO2 reduction on carbon nitride: A strategy of simultaneous O, S co-doping. J. Environ. Chem. Eng. 2025, 13, 116121. [Google Scholar] [CrossRef]
- Ruiz, C.; Rincón, L.; Contreras, R.; Sidney, C.; Almarza, J. Sustainable and negative carbon footprint Solid-based NaOH technology for CO2 capture. ACS Sustain. Chem. Eng. 2020, 8, 19003–19012. [Google Scholar] [CrossRef]
- Schuur, E.; Vogel, J.; Crummer, K.; Lee, H.; Sickman, J.; Osterkamp, T. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 2009, 459, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Bernardo, J. An approach to energy and climate issues aiming at carbon neutrality. Renew. Energy Focus 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Chi, C.; Khosrowabadi, K.; Sheehan, S. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 2018, 4, 2571–2586. [Google Scholar]
- Raza, A.; Hu, Y.; Lu, Y. Improving carbon flux estimation in tea plantation ecosystems: A machine learning ensemble approach. Eur. J. Agron. 2024, 160, 127297. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.; Zhang, T.; Chen, Q.; Qiu, F.; Peng, Y.; Tang, S. Fabrication of functional biomass carbon aerogels derived from sisal fibers for application in selenium extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using magnetic multiwalled carbon nanotubes as modified QuEChERS adsorbent for simultaneous determination of multiple mycotoxins in grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.; Han, C.; Tang, Z.; Xu, Y. Photocorrosion inhibition of semiconductor-based photocatalysts: Basic principle, current development and future perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2021, 301, 120802. [Google Scholar] [CrossRef]
- Gerber, I.; Serp, P. A Theory/experience description of support effects in Carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Chang, C.; Tseng, T.; Chen, B.; Wu, Y.; Huang, C.; Chen, J. Silver nanoparticle-mediated synthesis of fluorescent thiolated gold nanoclusters. Nanomaterials 2021, 11, 2835. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Katwal, R.; Sharma, G.; Naushad, M.; Khan, M.; Ala’a, H. Novel guar gum/Al2O3 nanocomposite as an effective photocatalyst for the degradation of malachite green dye. Int. J. Biol. Macromol. 2016, 87, 366–374. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Ma, R.; Xue, Y.; Zheng, S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater 2017, 243, 281–290. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of horseradish peroxidase on multi-armed magnetic graphene oxide composite: Improvement of loading amount and catalytic activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Yu, Z.; Zhan, B.; Ge, B.; Zhu, Y.; Dai, Y.; Zhou, G.; Yu, F.; Wang, P.; Huang, B.; Zhan, J. Synthesis of high efficient and stable plasmonic photocatalyst Ag/AgNbO3 with specific exposed crystal-facets and intimate heterogeneous interface via combustion route. Appl. Surf. Sci. 2019, 488, 485–493. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Janczarek, M.; Wei, Z.; Wang, K.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Bactericidal properties of plasmonic photocatalysts composed of noble-metal nanoparticles on faceted ana-tase titania. J. Nanosci. Nanotechnol. 2019, 19, 442–452. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Jing, J.; Hou, J. The photocatalytic phenol degradation mechanism of Ag-modified ZnO nanorods. J. Mater. Chem. C 2020, 8, 3000–3009. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Huang, M.; Huang, W.; Li, Y.; Feng, J. Interfacial engineering strategy to improve the stabilizing effect of curcumin-loaded nanostructured lipid carriers. Food Hydrocoll. 2022, 127, 107552. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y. New perspective on natural plant protein-based nanocarriers for bioactive ingredients delivery. Foods 2022, 11, 1701. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Xie, W.; Li, D.; Yan, G.; Guo, S.; Pan, C.; Wu, J. In situ fabrication of I-doped Bi2O2CO3/g-C3N4 heterojunctions for enhanced photodegradation activity under visible light. J. Hazard. Mater. 2020, 385, 121622. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, Q.; Wang, L.; Han, J.; Wu, Y.; Sun, Z.; Li, W.; Li, X.; Xu, L.; Feng, C. Fabrication of Ag-modified porous ZnMgO nanorods with enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 16962–16970. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X. Experimental and theoretical studies of natural mineral attapulgite supported iron-based oxygen carriers in chemical looping hydrogen production. Fuel 2023, 347, 128439. [Google Scholar] [CrossRef]
- Shang, A.; Liu, H.; Luo, M.; Xia, Y.; Yang, X.; Li, H.; Gan, R. Sweet tea (Lithocarpus polystachyus rehd.) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 917–934. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, Z.; Ma, M.; Li, P.; Lu, J.; Hou, Y.; Duo, S. Enhanced visible-light photocatalytic performance of sapo-5-based g-C3N4 composite for rhodamine B (RhB) degradation. Materials 2019, 12, 3948. [Google Scholar] [CrossRef]
- Azam, K.; Akhtar, S.; Gong, Y.; Routledge, M.; Ismail, A.; Oliveira, C.; Ali, H. Evaluation of the impact of activated carbon-based filtration system on the concentration of aflatoxins and selected heavy metals in roasted coffee. Food Control 2021, 121, 107583. [Google Scholar] [CrossRef]
- Shao, Z.; Xing, C.; Xue, M.; Fang, Y.; Li, P. Selective removal of Pb (II) from yellow rice wine using magnetic carbon-based adsorbent. J. Sci. Food Agri. 2023, 103, 6929–6939. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Li, R.; Deng, C.; Gao, L.; Deng, H.; Jing, Y.; Jiang, D.; Yao, Q.; Zhong, C.; et al. Dielectric screening modulating charge carrier recombination in high-performance of CsPbI2Br carbon-based perovskite solar cells. Chem. Eng. J. 2024, 500, 157370. [Google Scholar] [CrossRef]
- Ma, S.; Pan, L.G.; You, T.; Wang, K. g-C3N4/Fe3O4 nanocomposites as adsorbents analyzed by UPLC-MS/MS for highly sensitive simultaneous determination of 27 mycotoxins in maize: Aiming at increasing purification efficiency and reducing time. J. Agri. Food Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, C.; He, W.; Wei, Y.; Zhao, Z.; Liu, J. The Z-scheme g-C3N4/3DOM-WO3 photocatalysts with enhanced activity for CO2 photoreduction into CO. Chin. Chem. Lett. 2022, 33, 939–942. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Qiu, F.; Zhang, T.; Xu, J. Controllable preparation of FeOOH/CuO@ WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef] [PubMed]
- Barimah, A.; Guo, Z.; Agyekum, A.; Guo, C.; Chen, P.; El-Seedi, H.; Hesham, R.; Zou, X.; Chen, Q. Sensitive label-free Cu2O/Ag fused chemometrics SERS sensor for rapid detection of total arsenic in tea. Food Control 2021, 130, 108341. [Google Scholar] [CrossRef]
- Liu, L.; Dai, K.; Zhang, J.; Li, L. Plasmonic Bi-enhanced ammoniated alpha-MnS/Bi2MoO6 S-scheme heterostructure for visible-light-driven CO2 reduction. J. Colloid Interface Sci. 2021, 604, 844–855. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Che, H.; Tang, C.; Liu, B.; Ao, Y. In-situ growing Ni2P on CdS@g-C3N4 composites for highly efficient synergistically photocatalytic H2 evolution and antibiotic degradation. Surf. Interfaces 2024, 47, 104205. [Google Scholar] [CrossRef]
- Liu, W.; Wang, P.; Ao, Y.; Chen, J.; Gao, X.; Jia, B.; Ma, T. Directing charge transfer in a chemical-bonded BaTiO3@ReS2 Schottky heterojunction for piezoelectric enhanced photocatalysis. Adv. Mater. 2022, 34, 2202508. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal–organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, J.; Zhi, Y.; Ma, S.; Liu, X. Porous organic polymers for light-driven organic transformations. Chem. Soc. Rev. 2022, 51, 2444–2490. [Google Scholar] [CrossRef]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Yang, D.; Tao, Y.; Ding, X.; Han, B. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Xu, Q.; Mei, Z.; Duan, L.; Guo, H. Understanding dual-vacancy heterojunction for boosting photocatalytic CO2 reduction with highly selective conversion to CH4. Appl. Catal. B Environ. 2022, 316, 121679. [Google Scholar] [CrossRef]
- Yan, C.; Xu, M.; Cao, W.; Chen, Q.; Song, X.; Huo, P.; Zhou, W.; Wang, H. Fabricated S-scheme BiOBr/Cu2O heterojunction photocatalyst for adjusting conversion of CO2 to CH4. J. Environ. Chem. Eng. 2023, 11, 111479. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, X.; Jiang, E.; Yan, Y.; Zhou, P.; Huo, P. Boosting water decomposition by sulfur vacancies for efficient CO2 photo-reduction. Energy Environ. Sci. 2022, 15, 1556–1562. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, J.; Wang, J.; Zhang, Y. Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl. Soil Ecol. 2022, 170, 104266. [Google Scholar] [CrossRef]
- Dzah, C.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Li, C.; Lin, L. Enhancing the antibacterial activity of thyme oil against Salmonella on eggshell by plasma-assisted process. Food Control 2016, 70, 183–190. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Lin, L. Antibacterial activity of liposome containing curry plant essential oil against Bacillus cereusin rice. J. Food Saf. 2017, 37, e12302. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Chen, R.; Liu, X. Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light. RSC Adv. 2014, 4, 406–408. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Yamashita, H.; Mori, K.; Kuwahara, Y.; Kamegawa, T.; Wen, M.; Verma, P.; Che, M. Single-site and nano-confined photocatalysts designed in porous materials for environmental uses and solar fuels. Chem. Soc. Rev. 2018, 47, 8072–8096. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Ruan, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Prishchenko, D.; Zenkov, E.; Mazurenko, V.; Fakhrullin, R.; Lvov, Y.; Mazurenko, V. Molecular dynamics of the halloysite nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Yan, C. Facile Preparation of Attapulgite-Supported Ag-AgCl Composite Photocatalysts for Enhanced Degradation of Tetracycline. Catalysts 2024, 14, 464. [Google Scholar] [CrossRef]
- González, B.; Trujillano, R.; Vicente, M.; Rives, V.; Korili, S.; Gil, A. Photocatalytic degradation of trimethoprim on doped Ti-pillared montmorillonite. Appl. Clay Sci. 2019, 167, 43–49. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Wang, C.; Zhuang, C.; Yao, Y.; Li, Z.; Wu, C.; Feng, J.; Zou, Z. 2D high-entropy hydrotalcites. Small 2021, 17, 2103412. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Lazzara, G. Colloidal stability of halloysite clay nanotubes. Ceram. Int. 2019, 45, 2858–2865. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93. [Google Scholar] [CrossRef]
- Lim, S.; Park, S.; Sohn, D. Modification of halloysite nanotubes for enhancement of gas-adsorption capacity. Clays. Clay. Miner. 2020, 68, 189–196. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Jia, L.; Bi, N.; Zhao, T. Metal-enhanced fluorescence detection and degradation of tetracycline by silver nanoparticle-encapsulated halloysite nano-lumen. J. Hazard. Mater. 2020, 386, 121630. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A. 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.; Vega-Verduga, C.; Díaz, D.; Zumeta-Dubé, I. Transformation of Bismuth and beta-Bi2O3 nanoparticles into (BiO)2CO3 and (BiO)4(OH)2CO3 by capturing CO2: The role of halloysite nanotubes and “Sunlight” on the crystal shape and size. Cryst. Growth Des. 2018, 18, 4334–4346. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Si, S.; Shou, H.; Mao, Y.; Bao, X.; Zhai, G.; Song, K.; Wang, Z.; Wang, P.; Liu, Y.; Zheng, Z.; et al. Low-Coordination single Au atoms on ultrathin ZnIn2S4 nanosheets for selective photocatalytic CO2 reduction towards CH4. Angew. Chem. Int. Ed. 2022, 134, 202209446. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; You, Y.; Zeng, C.; Xiao, X.; Ma, T.; Huang, H. Bifunctional hydrogen production and storage on 0D-1D heterojunction of Cd0.5Zn0.5S@halloysites. Adv. Funct. Mater. 2019, 29, 1903825. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, Y.; Zhang, D.; Wang, H.; Sun, X.; Li, Z. Iridium complex-linked porous organic polymers for recyclable, broad-scope photocatalysis of organic transformations. Green Chem. 2020, 22, 136–143. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, C.; Wang, J.; Xiao, G.; Luo, G. Green synthesis of Ag-TiO2 supported on porous glass with enhanced photocatalytic performance for oxidative desulfurization and removal of dyes under visible light. ACS Sustainable. Chem. Eng. 2018, 6, 13276–13286. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Wu, S. Recent progress in photocatalytic antibacterial. ACS Appl. Bio Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef]
- Yu, K.; Jing, S.; Tong, Z. Photo-activated graphene oxide to enhance photocatalytic reduction of CO2. ACS Appl. Mater. Interfaces 2020, 12, 3580–3591. [Google Scholar]
- Zhu, C.; Zhu, M.; Sun, Y.; Zhou, Y.; Gao, J.; Huang, H.; Kang, Z. Carbon-supported oxygen vacancy-rich Co3O4 for robust photocatalytic H2O2 production via coupled water oxidation and oxygen reduction reaction. ACS Appl. Energy Mater. 2019, 2, 8737–8746. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723–129735. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Alietti, A.; Brindley, G. Summary of recommendations of AIPEA nomenclature committee. Clays Clay Miner. 1980, 28, 73–78. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Kong, Y.; Cui, B.; Li, D. Nanocomposite of attapulgite-Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. Energy 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Guo, R.; Wang, G.; Liu, W. Clever use of natural clay materials in the synthesis of non-symmetric carbonates by utilizing CO2 as a feedstock: Ag/attapulgite nano-catalyst. Dalton Trans. 2020, 49, 10232–10239. [Google Scholar] [CrossRef]
- Wang, W.; Fan, M.; Zhang, Z.; Wu, L.; Liu, Y.; Cao, Y.; Peng, W. In-situ nanofication of Cu-doped BiVO4 on montmorillonite nanosheets with enhanced photocatalytic efficiency for phenolic degradation. Sep. Purif. Technol. 2025, 361, 131341. [Google Scholar] [CrossRef]
- Fatimah, I.; Syu’aib, Y.; Ramanda, G.; Kooli, F.; Sagadevan, S.; Oh, W. Facile synthesis of highly active and reusable NiO/montmorillonite photocatalyst for tetracycline removal by photocatalytic oxidation. Inorg. Chem. Commun. 2025, 172, 113731. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Liu, G.; Cao, Y. Synthesis, characterization and photocatalytic activity of inexpensive and non-toxic Fe2O3− Fe3O4 nano-composites supported by montmorillonite and modified by graphene. Compos. Part B Eng. 2017, 114, 211–222. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, H.; Peng, T.; Lv, X. Comparison of the phase transition and degradation of methylene blue of TiO2, TiO2/montmorillonite mixture and TiO2/montmorillonite composite. Front. Chem. 2019, 7, 538. [Google Scholar] [CrossRef]
- Liu, K.; Wang, L.; Fu, T.; Zhang, H.; Lu, C.; Tong, Z.; Yang, Y.; Peng, Y. Oxygen-functionalized Ti3C2 MXene/exfoliated montmorillonite supported S-scheme BiOBr/Bi2MoO6 heterostructures for efficient photocatalytic quinolone antibiotics degradation. Chem. Eng. J. 2023, 457, 141271. [Google Scholar] [CrossRef]
- Mohamed, I.; Wu, X.; Zhu, J.; Abd El-Lateef, H.; Mousa, H.; Xing, F. Microstructure and interface analyses of novel external anode mortar incorporated calcined hydrotalcite nanoparticles towards an enhanced impressed current cathodic protection. J. Taiwan Inst. Chem. Eng. 2023, 145, 104803. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, G.; Jin, Z. ZnCdS/NiAl hydrotalcite S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 292–304. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Zou, Y.; Zhang, N.; Zhang, Y.; Wu, Y.; Wang, S. Charge transfer modulated activity of carbon-based electrocatalysts. Adv. Energy Mater. 2020, 10, 1901227. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Carbon-based nanocages: A new platform for advanced energy storage and conversion. Adv. Mater. 2020, 32, 1904177. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.; Batool, A.; Gong, J. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Zhu, Y.; Sokolowski, J.; Song, X.; He, Y.; Mei, Y.; Wu, G. Engineering local coordination environments of atomically dispersed and heteroatom-coordinated single metal site electrocatalysts for clean energy-conversion. Adv. Energy Mater. 2020, 10, 1902844. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef]
- Ma, B.; Blanco, M.; Calvillo, L.; Chen, L.; Chen, G.; Lau, T.; Granozzi, G. Hybridization of molecular and graphene materials for CO2 photocatalytic reduction with selectivity control. J. Am. Chem. Soc. 2021, 143, 8414–8425. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, L.; Nie, H.; Huang, H.; Li, Y.; Yao, S.; Lu, T. Facile electron delivery from graphene template to ultrathin metal-organic layers for boosting CO2 photoreduction. Nat. Commun. 2021, 12, 21084–21089. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Javey, A.; Guo, J.; Wang, Q.; Lundstrom, M.; Dai, H. Ballistic carbon nanotube field-effect transistors. Nature 2003, 424, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wei, W.; Jiang, L.; Zhu, F.; Du, D. Use of carbon nanotubes as a solid support to establish quantitative (centrifugation) and qualitative (filtration) immunoassays to detect gentamicin contamination in commercial milk. J. Agric. Food Chem. 2016, 64, 7874–7881. [Google Scholar] [CrossRef]
- Bai, Y.; Yue, H.; Wang, J.; Shen, B.; Sun, S.; Wang, S.; Wei, F. Super-durable ultralong carbon nanotubes. Science 2020, 369, 1104–1106. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, R.; Ye, X.; Zhu, Z.; Xie, H.; Shen, B.; Wei, F. Carbon nanotube bundles with tensile strength over 80 GPa. Nature Nanotechnol. 2018, 13, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Magnin, Y.; Amara, H.; Ducastelle, F.; Loiseau, A.; Bichara, C. Entropy driven stability of chiral single-walled carbon nanotubes. Science 2018, 362, 212–215. [Google Scholar] [CrossRef]
- Jeyagopal, R.; Chen, Y.; Ramadoss, M.; Marimuthu, K.; Wang, B.; Li, W.; Zhang, X. A three-dimensional porous CoSnS@CNT nanoarchitecture as a highly efficient bifunctional catalyst for boosted OER performance and photocatalytic degradation. Nanoscale 2020, 12, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Chen, X.; Fischer, A.; Thomas, A.; Antonietti, M.; Wang, X. Condensed graphitic carbon nitride nanorods by nanoconfinement: Promotion of crystallinity on photocatalytic conversion. Chem. Mater. 2011, 23, 4344–4348. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Antonietti, M. Mesoporous g-C3N4 nanorods as multifunctional supports of ultrafine metal nanoparticles: Hydrogen generation from water and reduction of nitrophenol with tandem catalysis in one step. Chem. Sci. 2012, 3, 2170. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Ye, X.; Guo, F.; Wang, X. Helical graphitic carbon nitrides with photocatalytic and optical activities. Angew. Chem. Int. Ed. Engl. 2014, 53, 11926–11930. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Z.; Rao, D.; Wang, Y.; Shi, Q.; Liu, Y.; Wu, H.; Deng, K.; Liu, H.; Lu, R. Efficient band structure tuning, charge separation, and visible-light response in ZrS2-based van der waals heterostructures. Energy Environ. Sci. 2016, 9, 841–849. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.; Guo, Y.; Zhang, Z.; Fang, X. Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 248–258. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Interactions between ZnO nanoparticles and amorphous g-C3N4 nanosheets in thermal formation of g-C3N4/ZnO composite materials: The annealing temperature effect. Appl. Surf. Sci. 2018, 458, 369–381. [Google Scholar] [CrossRef]
- Yang, P.; Guo, S.; Yu, X.; Zhang, F.; Yu, B.; Zhang, H.; Liu, Z. Photocatalytic reduction of carbon dioxide over quinacridone nanoparticles supported on reduced graphene oxide. Ind. Eng. Chem. Res. 2019, 58, 9636–9643. [Google Scholar] [CrossRef]
- Lin, P.; Shen, J.; Yu, X.; Liu, Q.; Li, D.; Tang, H. Construction of Ti3C2 MXene/O-doped g-C3N4 2D-2D schottky-junction for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2019, 45, 24656–24663. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, X.; Fan, W.; Liu, Z.; Huo, P.; Wang, T.; Li, C. Studying of Co-doped g-C3N4 and modified with Fe3O4 quantum dots on removing tetracycline. J. Alloys Compd. 2019, 775, 248–258. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Chen, X. Phosphorus-doped graphitic carbon nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Tran, D.; Pham, C.; Ngoc, T.; Phi, H.; Ta, Q.; Truong, D.; Vo, V. One-step synthesis of oxygen doped g-C3N4 for enhanced visible-light photodegradation of Rhodamine B. J. Phys. Chem. Solids 2021, 151, 109900. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhai, H.; Zhang, Q.; Huang, B.; Wang, Z.; Zhang, X. Synthesis of synergetic phosphorus and cyano groups (CN) modified g-C3N4 for enhanced photocatalytic H2 production and CO2 reduction under visible light irradiation. Appl. Catal. B Environ. 2018, 232, 521–530. [Google Scholar] [CrossRef]
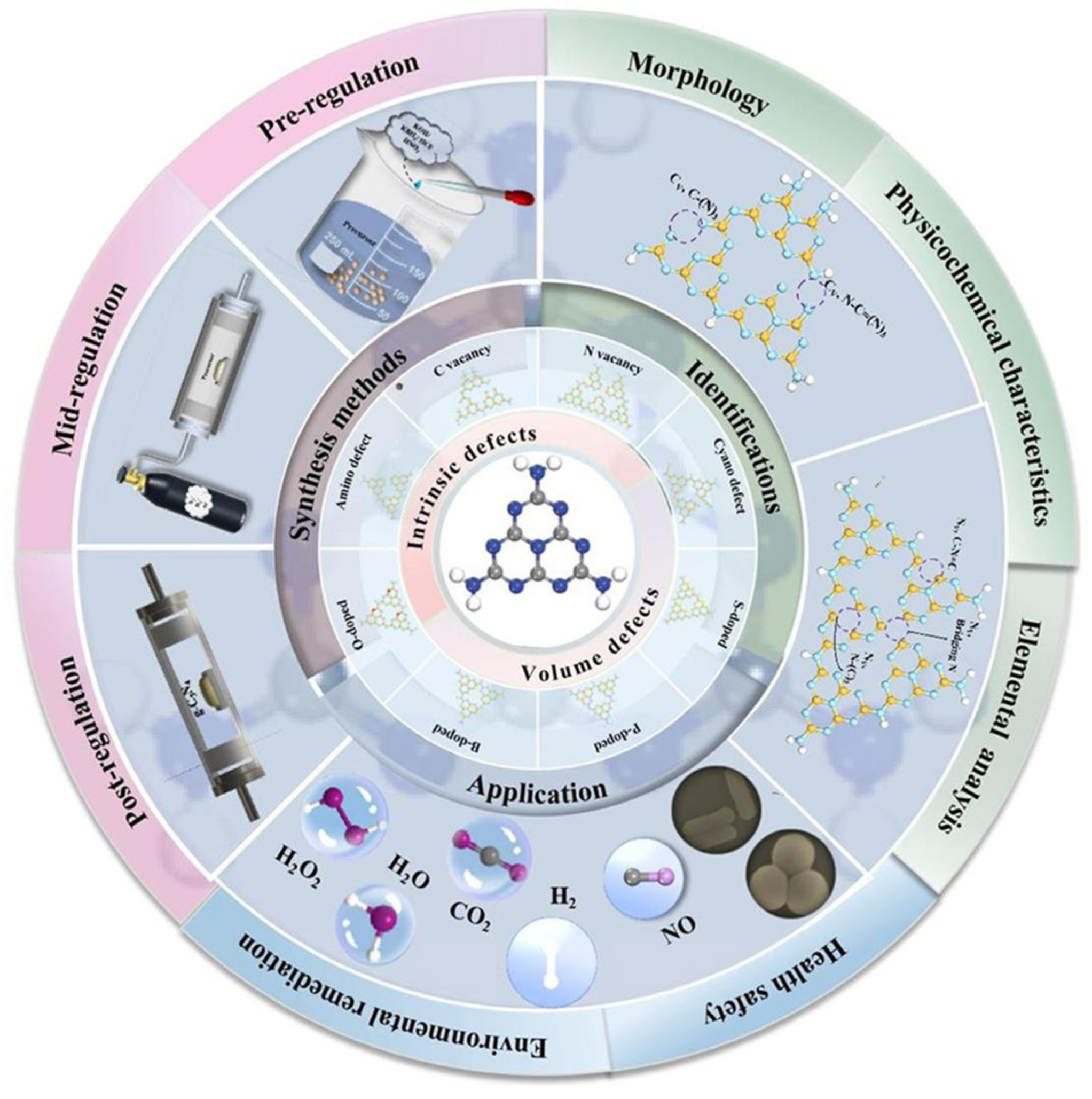
- Chen, M.; Sun, M.; Cao, X.; Wang, H.; Xia, L.; Jiang, W.; Huang, M.; He, L.; Zhao, X.; Zhou, Y. Progress in preparation, identification and photocatalytic application of defective g-C3N4. Coord. Chem. Rev. 2024, 510, 215849. [Google Scholar] [CrossRef]
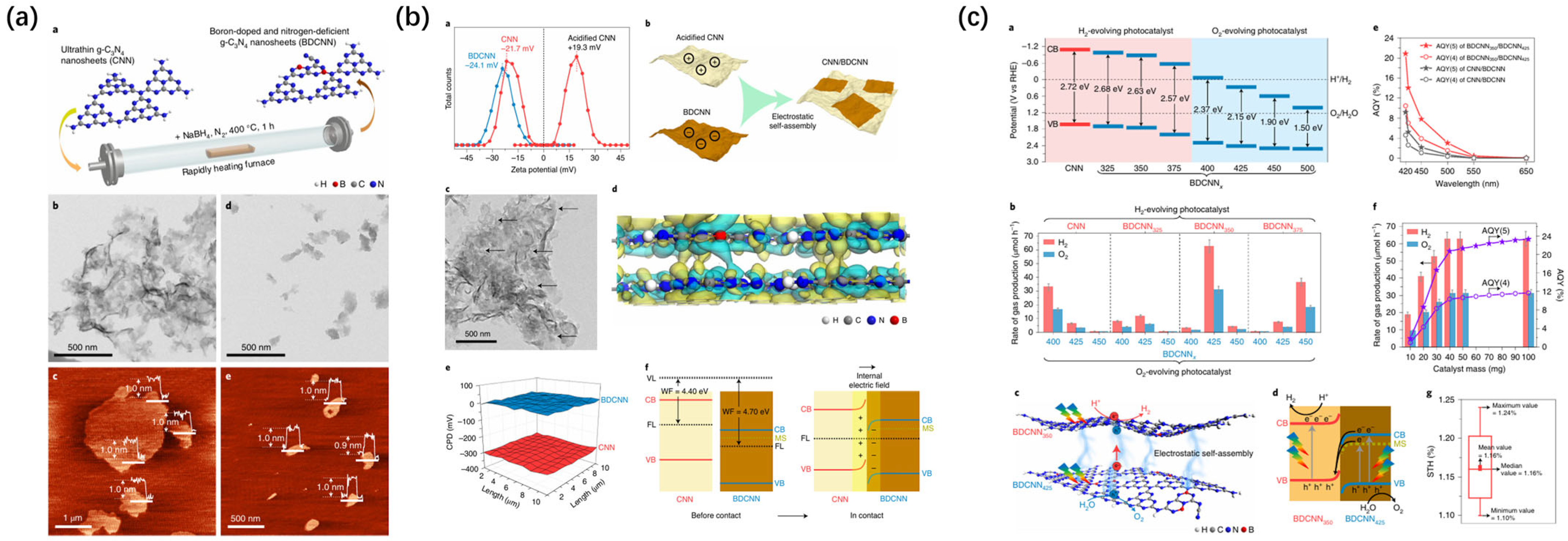
- Zhao, D.; Wang, Y.; Dong, C.; Huang, Y.; Chen, J.; Xue, F.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.; Kang, F.; Yang, Q. Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogene evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Shen, Y.; Zhou, Z.; Yu, J.; Li, Y.; Wei, W.; Liu, S.; Zhang, Y. Environment-friendly preparation of porous graphite-phase polymeric carbon nitride using calcium carbonate as templates, and enhanced photoelectrochemical activity. J. Mater. Chem. A 2015, 3, 5126–5131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, X.; Antonietti, M.; Li, H. Boron- and fluorine-ontaining mesoporous carbon nitride polymers: Metal-free catalysts for cyclohexane oxidation. Angew. Chem.-Int. Edit. 2010, 49, 3356–3359. [Google Scholar] [CrossRef]
- Li, N.; Gao, X.; Su, J.; Gao, Y.; Ge, L. Metallic WO2-decorated g-C3N4 nanosheets as noble-metal-free photocatalysts for efficient photocatalysis. Chin. J. Catal. 2023, 47, 161–170. [Google Scholar] [CrossRef]
- Zeng, J.; Han, C.; Wang, B.; Cao, G.; Yao, C.; Li, X. Construction of plasmonic CuS/attapulgite nanocomposites toward photothermal reforming of biomass for hydrogen production. J. Alloys Compd. 2024, 985, 174038. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Liu, E.; Xu, J.; Sun, J.; Shi, H. Biochar decorated Bi4O5Br2/g-C3N4 S-scheme heterojunction with enhanced photocatalytic activity for Norfloxacin degradation. J. Mater. Sci. Technol. 2024, 198, 1–11. [Google Scholar] [CrossRef]
- Cao, G.; Xing, H.; Gui, H.; Yao, C.; Chen, Y.; Chen, Y.; Li, X. Plasmonic quantum dots modulated nano-mineral toward photothermal reduction of CO2 coupled with biomass conversion. Nano Res. 2024, 17, 5061–5072. [Google Scholar] [CrossRef]
- Mohamed, I.; Hossam, G.; Shaker, A.; Nassr, L. Surface functionalization of TiO2 nanoparticles with hydrophobic tridentate schiff base for a promising photocatalytic reduction of toxic Cr (VI) in aqueous solutions. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Huang, Y.; Ma, M.; Liu, K.; Dou, X.; Wang, Z. Engineering the photocatalytic behaviors of g-C3N4 based metal-free materials for degradation of a representative antibiotic. Adv. Funct. Mater. 2020, 30, 2002353. [Google Scholar] [CrossRef]
- Song, X.; Li, G.; Zhou, W.; Wu, Y.; Liu, X.; Zhu, Z.; Huo, P.; Wang, M. Construction of Au-modified CN-based donor-acceptor system coupled with dual photothermal effects for efficient photoreduction of carbon dioxide. J. Colloid Interface Sci. 2024, 664, 868–881. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient photocatalytic overall water splitting induced by a giant internal electric field of g-C3N4/rGO/PDIP Z-scheme heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, M.; Yuan, S.; Meng, L.; Ding, W.; Su, S.; Cao, Y.; Wang, Y.; Li, X. Boron-doped graphene quantum dot/bismuth molybdate composite photocatalysts for efficient photocatalytic nitrogen fixation reactions. J. Colloid Interface Sci. 2023, 650, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Sillanpaa, M.; Almutairi, T.; Mohammed, A.; Ali, S. Excellent photocatalytic and antibacterial activities of bio-activated carbon decorated magnesium oxide nanoparticles. Chemosphere 2023, 312, 137327. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Chen, W.; Zhang, L.; Qi, Z.; Bai, G.; Fan, Y.; Liu, C.; Xiao, C.; Li, W.; et al. Contribution of surface plasmonic resonance to enhanced photocatalytic antibacterial performance of graphene-based two-dimensional heterojunction. Chem. Eng. J. 2023, 460, 141720. [Google Scholar] [CrossRef]




| Material | Efficiency (Relative) | Light Absorption Range (nm) | Charge Separation Capability | Practical Application |
|---|---|---|---|---|
| Hydrotalcite | Moderate | UV | Ordinary | Environmental purification |
| Attapulgite | Slightly below | UV | Ordinary | Absorbent |
| Montmorillonite | Slightly below | UV | Ordinary | Absorbent |
| Carbon nanotubes | High | visible area | Large | Composite photocatalysts |
| Graphene carrier materials | High | Visible region and near infrared | Large | Composite photocatalysts |
| g-C3N4 | Medium–high | visible area | Slightly higher | Environmental purification |
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Solvent (water) thermal method | High crystallinity and good particle size control | Higher energy consumption |
| Precipitation method | Higher energy consumption | Low product purity |
| Sol–gel method | Uniformity of composition and variety of forms | High cost and complex drying process |
| Electrospinning technology | Controlled fiber morphology | Limited production |
| Vapor deposition | Continuous production with high purity | Process is complex |
| Surface modification | Flexible and long life | Flexible and long life |
| Ball milling | Environmentally friendly, good nano-sizing effect | Many crystal defects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yan, C.; Xu, M.; Song, X. Advances in the Preparation of Carrier-Based Composite Photocatalysts and Their Applications. Catalysts 2025, 15, 286. https://doi.org/10.3390/catal15030286
Wang H, Yan C, Xu M, Song X. Advances in the Preparation of Carrier-Based Composite Photocatalysts and Their Applications. Catalysts. 2025; 15(3):286. https://doi.org/10.3390/catal15030286
Chicago/Turabian StyleWang, Huiqin, Chenlong Yan, Mengyang Xu, and Xianghai Song. 2025. "Advances in the Preparation of Carrier-Based Composite Photocatalysts and Their Applications" Catalysts 15, no. 3: 286. https://doi.org/10.3390/catal15030286
APA StyleWang, H., Yan, C., Xu, M., & Song, X. (2025). Advances in the Preparation of Carrier-Based Composite Photocatalysts and Their Applications. Catalysts, 15(3), 286. https://doi.org/10.3390/catal15030286






