Ca-, Mg-, Sc-, and Y-Stabilized Zirconia: High-Performance Support Material for Dry Reforming of Methane and Solid-Electrolyte Material for Fuel Cell
Abstract
1. Introduction
2. Synthetic Strategy for Preparation of Stabilized Zirconia Material
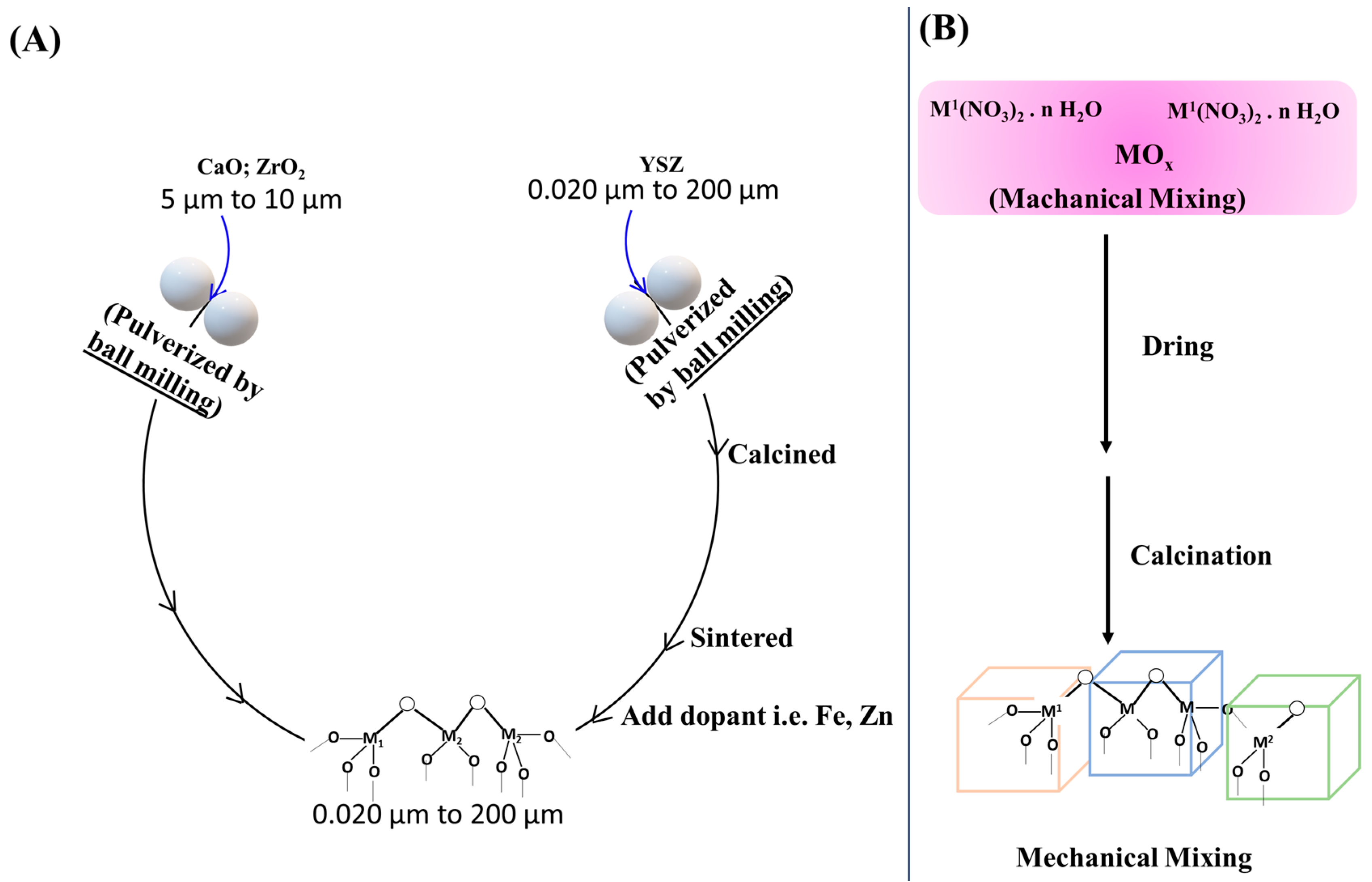
2.1. Top-to-Bottom Approach
2.2. Bottom-to-Top Approach
2.2.1. Impregnation Method
2.2.2. Vapour Deposition
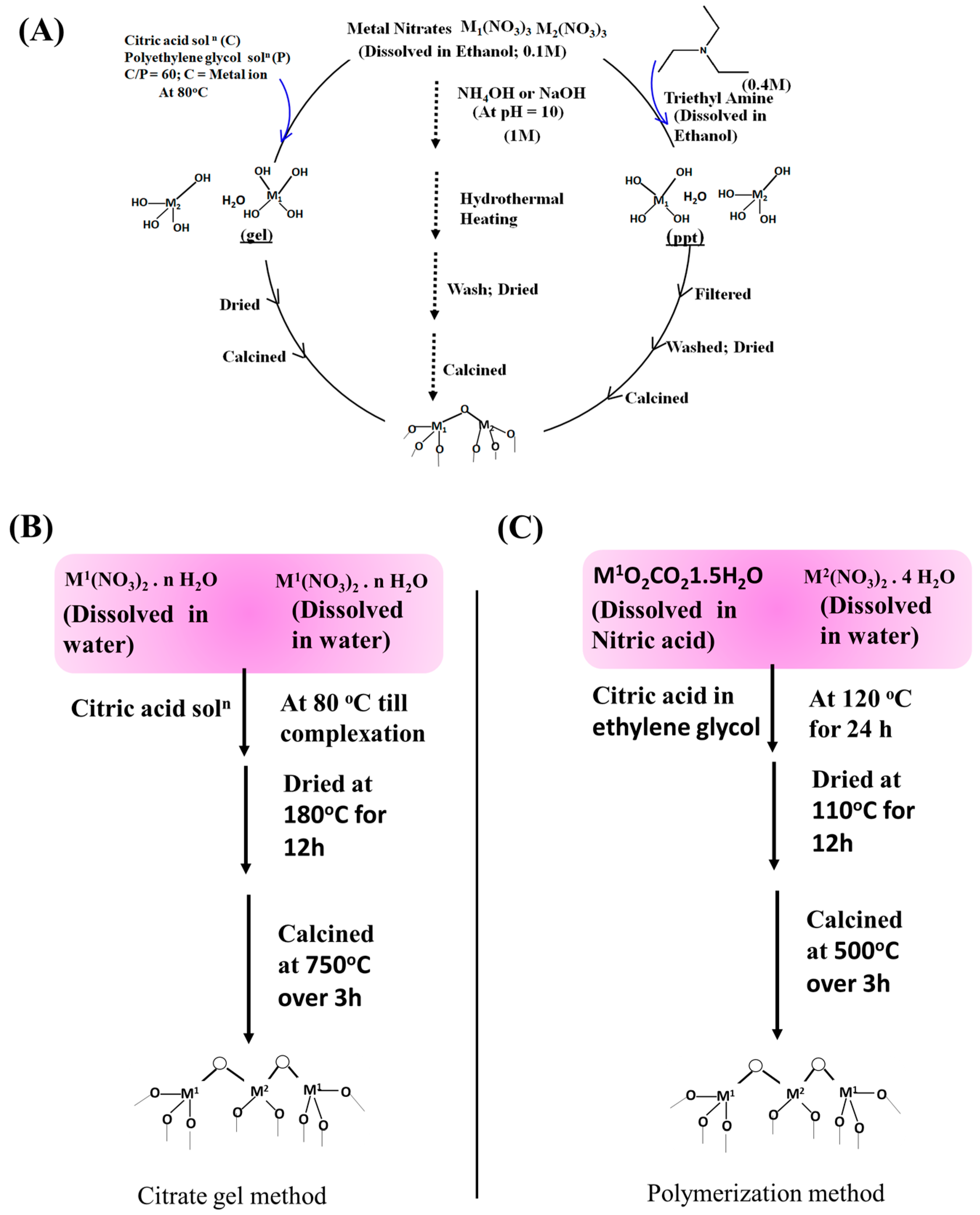
2.2.3. Co-Precipitation
2.2.4. Sol-Gel Method
2.2.5. Hydrothermal Method
3. The Current Status of Stabilized Zirconia as Support for DRM and as a Solid Electrolyte for Fuel Cells
3.1. Calcia Stabilized Zirconia
3.1.1. The Reducibility Profile of Calcia Stabilized Zirconia
3.1.2. Calcia Stabilized Zirconia-Based Support for Carrying Active Sites in DRM
3.1.3. Calcia Stabilized Zirconia-Based Electrolyte for Fuel Cell
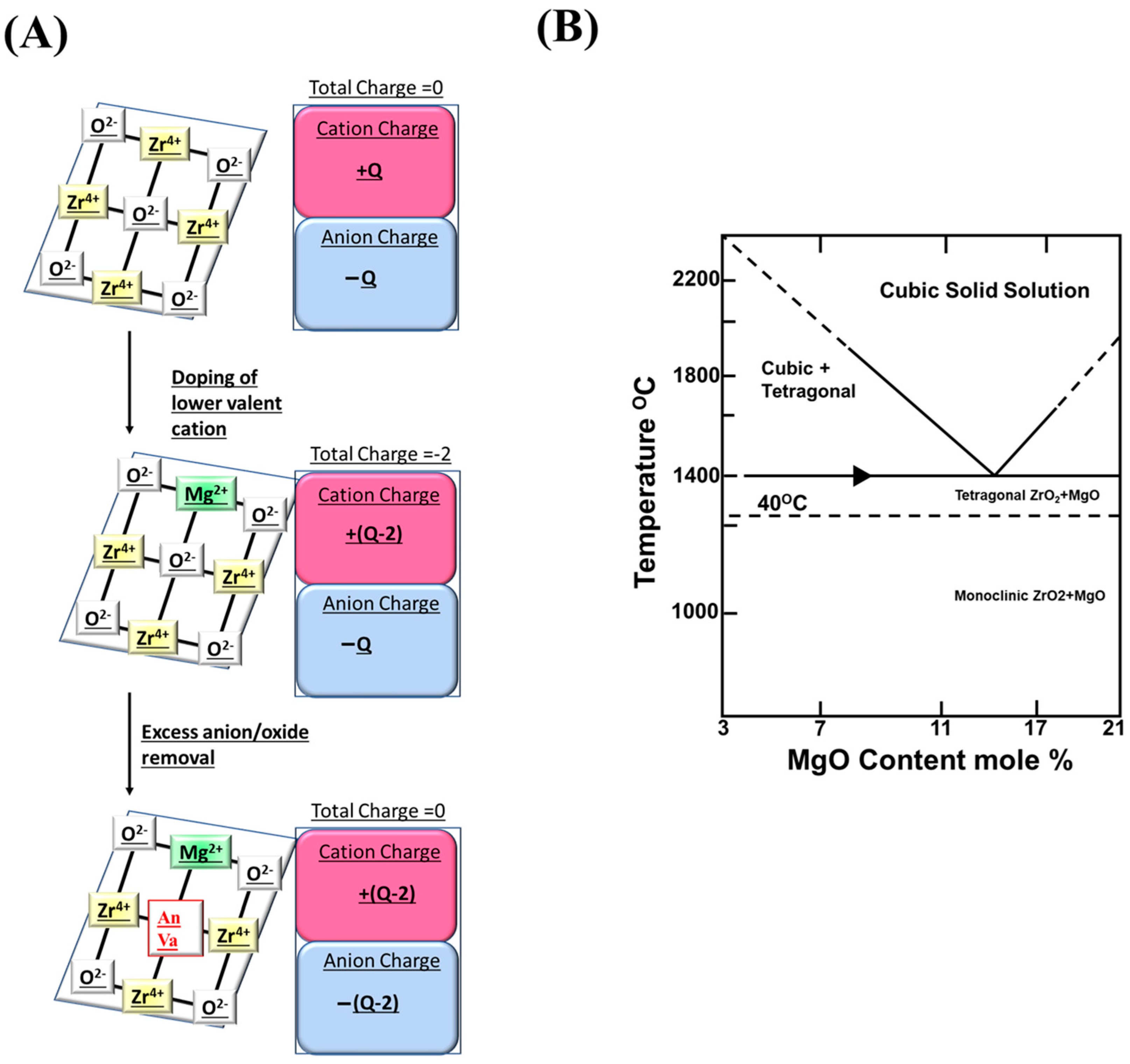
3.2. Magnesium-Stabilized Zirconia
3.2.1. Reducibility Profile of Magnesium-Stabilized Zirconia
3.2.2. Magnesium-Stabilized Zirconia-Based Support for Carrying Active Sites in DRM
3.2.3. Magnesia Stabilized Zirconia-Based Electrolyte
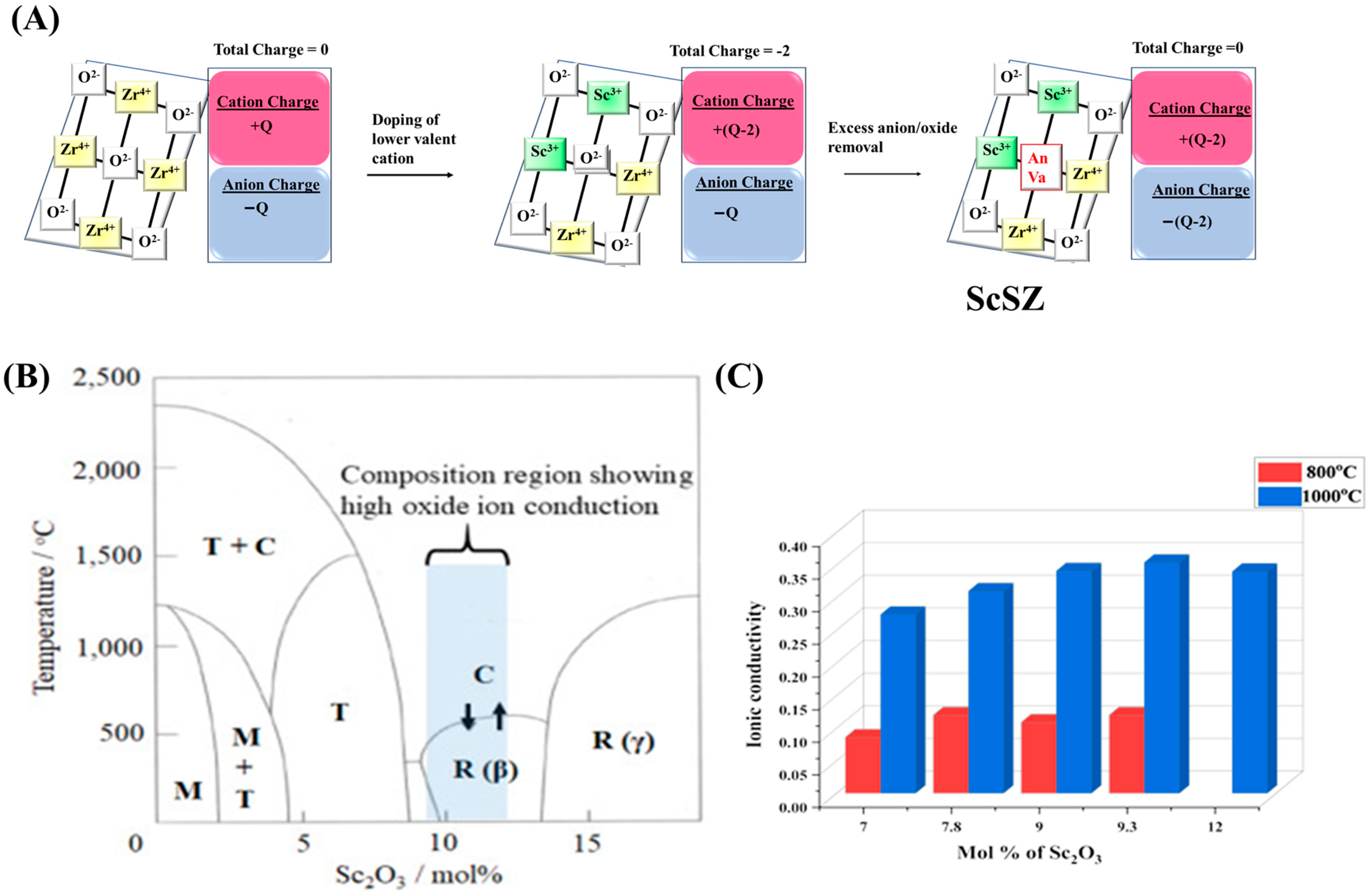
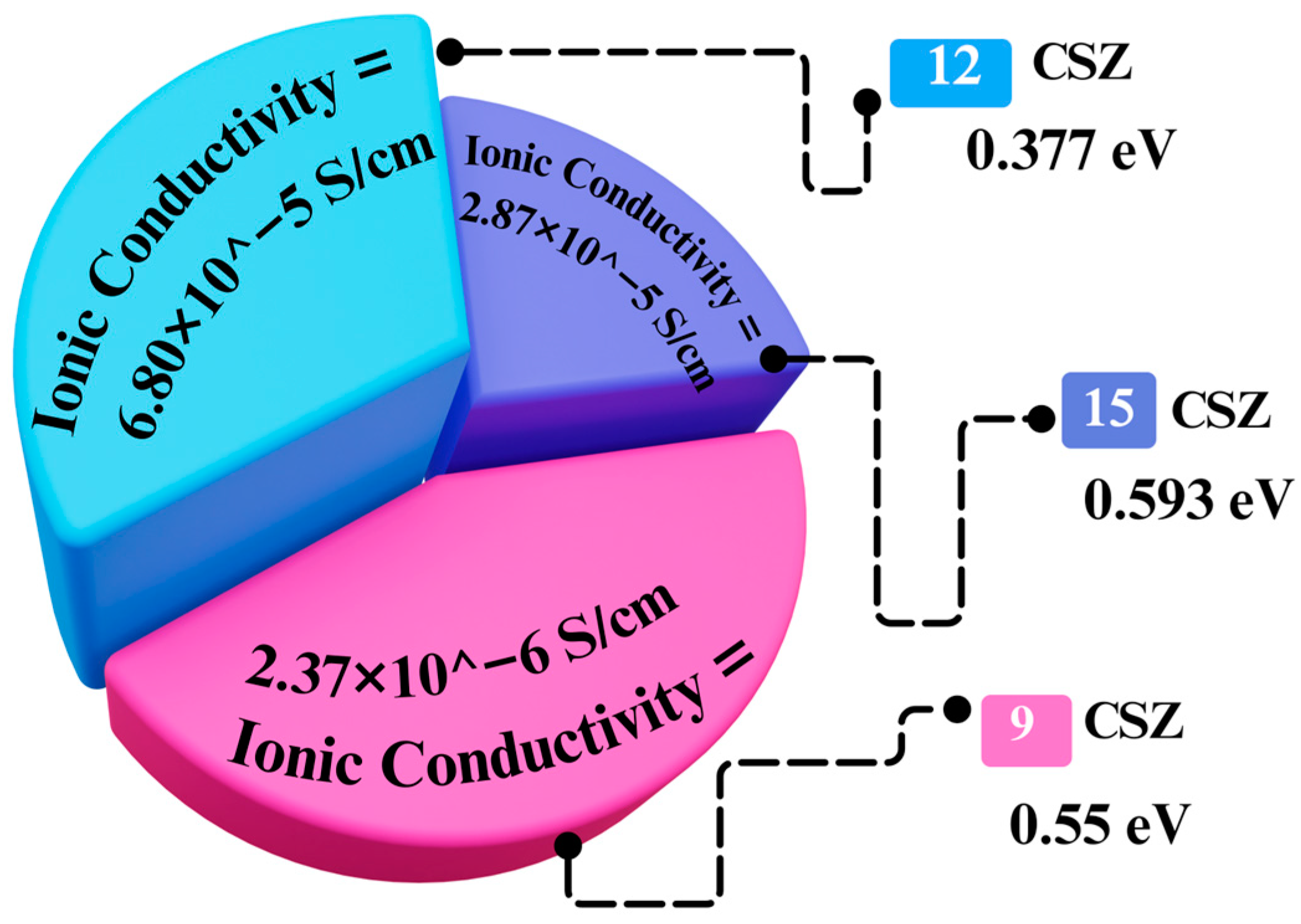
3.3. Scandia Stabilized Zirconia
3.3.1. Scandia Stabilized Zirconia-Based Support for Carrying Active Sites in DRM
3.3.2. Scandia Stabilized Zirconia-Based Electrolyte
3.4. Yttria Stabilized Zirconia
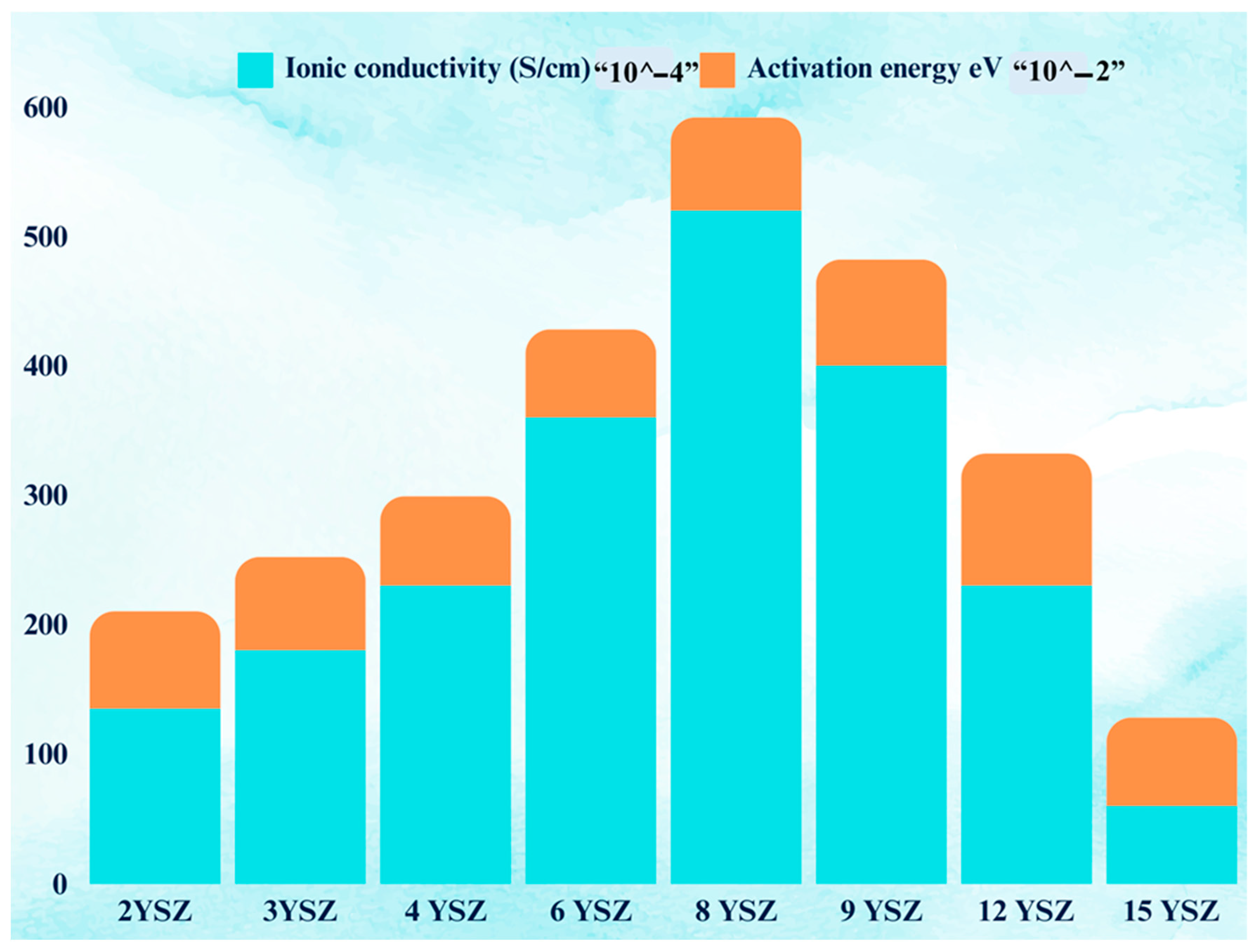
3.4.1. Reducibility Profile of Yttria Stabilized Zirconia
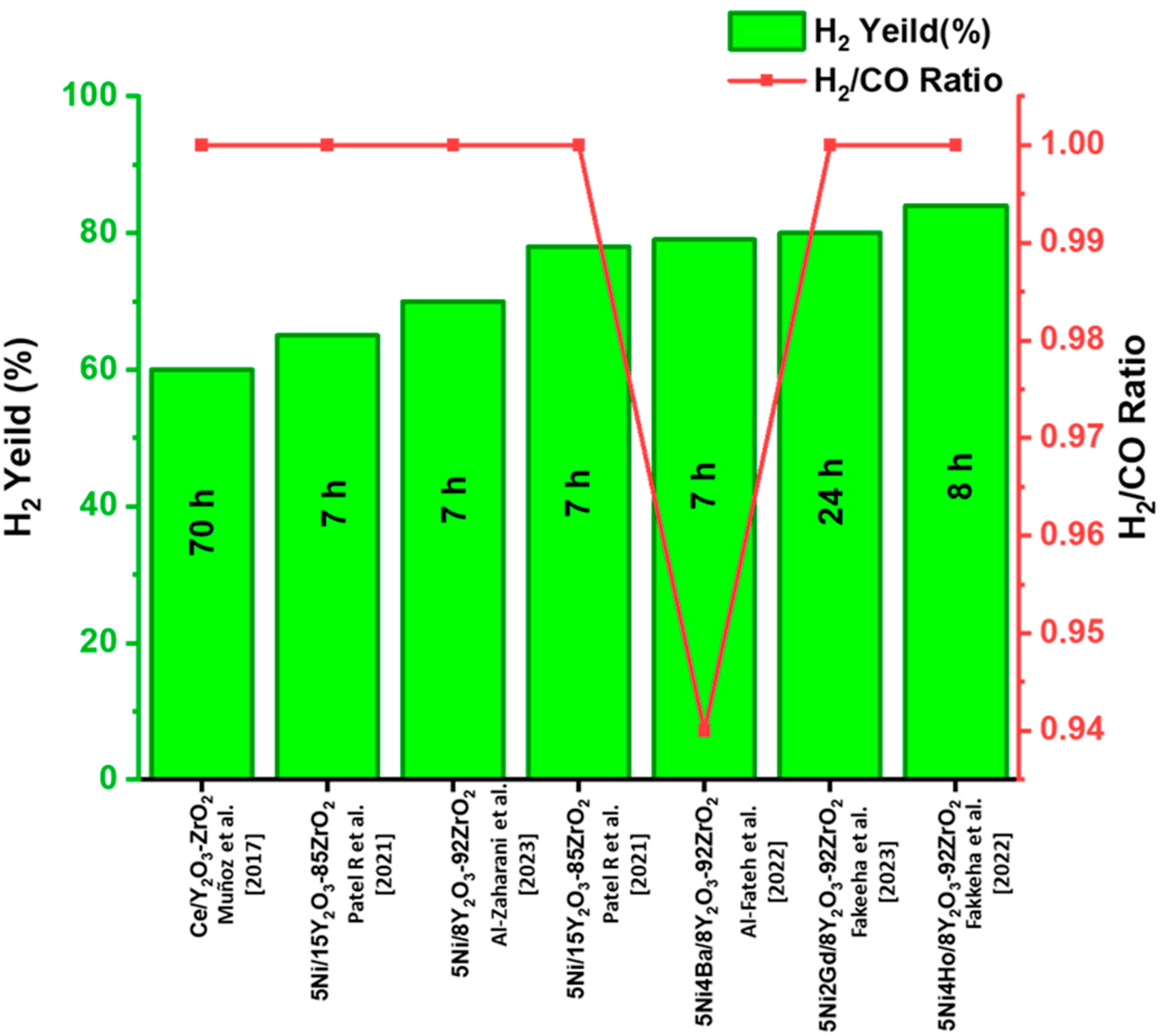
3.4.2. Yttria-Stabilized Zirconia-Based Support for Carrying Active Sites in DRM
3.4.3. Yttria Stabilized Zirconia-Based Electrolyte

4. Discussion
5. Conclusions and Future Prescriptive
5.1. Doped ZrO2 as Thermally Stable Carrier
5.2. Doped ZrO2 as an Ion-Conducting Medium
5.2.1. Generation of Ion Conducting Phases
5.2.2. Grain Boundary Conductivity
5.2.3. Thickness/Dispersion
5.2.4. Clustering Effect
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alipour, Z.; Babu Borugadda, V.; Wang, H.; Dalai, A.K. Syngas Production through Dry Reforming: A Review on Catalysts and Their Materials, Preparation Methods and Reactor Type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of Methane: Effects of Active Metals, Supports, and Promoters. Catal. Rev. 2023, 66, 2209–2307. [Google Scholar] [CrossRef]
- Swaan, H.M.; Rouanet, R.; Widyananda, P.; Mirodatos, C. Partial Oxidation of Methane over Nickel- and Cobalt-Based Catalysts. In Natural Gas Conversion IV; de Pontes, M., Espinoza, R.L., Nicolaides, C.P., Scholtz, J.H., Scurrell, M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 107, pp. 447–453. ISBN 0167-2991. [Google Scholar]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragón, F. Dry Reforming of Methane over LaNi1-YByO3±δ (B = Mg, Co) Perovskites Used as Catalyst Precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous Silica Supported Ni-Based Catalysts for Methane Dry Reforming: A Review of Recent Studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Wischert, R.; Laurent, P.; Copéret, C.; Delbecq, F.; Sautet, P. γ-Alumina: The Essential and Unexpected Role of Water for the Structure, Stability, and Reactivity of “Defect” Sites. J. Am. Chem. Soc. 2012, 134, 14430–14449. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Patel, R.; El Hassan, N.; Al-Zahrani, S.A.; Al-Awadi, A.S.; Frusteri, L.; Bayahia, H.; Alharth, A.I.; Al-Fatesh, A.S.; Kumar, R. Holmium Promoted Yttria-Zirconia Supported Ni Catalyst for H2 Production via Dry Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 38242–38257. [Google Scholar] [CrossRef]
- Rajput, Y.B.; Al-Fatesh, A.S.; Osman, A.I.; Bayazed, M.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Almubaddel, F.S.; Alothman, O.; Kumar, R. Enhancing Hydrogen Production via Dry Reforming of Methane: Optimization of Co and Ni on Scandia-Ceria-Zirconia Supports for Catalytic Efficiency and Economic Feasibility. Fuel 2024, 378, 132843. [Google Scholar] [CrossRef]
- Alwadai, N.; Abahussain, A.A.M.; Shrivastava, V.K.; Al-zahrani, S.A.; Fakeeha, A.H.; Alarifi, N.; Bayazed, M.O.; Banabdwin, K.M.; Kumar, R.; Al-fatesh, A. The Synergistic Effect of Pore Architect and Reducibility in Ceria-Promoted Ni Molecular Sieve for Methane Dry Reforming. Catalysts 2024, 14, 852. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of Solid Oxide Fuel Cell Materials: Cathode, Anode, and Electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar]
- Zhang, J.; Lenser, C.; Menzler, N.H.; Guillon, O. Comparison of Solid Oxide Fuel Cell (SOFC) Electrolyte Materials for Operation at 500 °C. Solid State Ion. 2020, 344, 115138. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent Advances in High Temperature Electrolysis Using Solid Oxide Fuel Cells: A Review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Hajimolana, S.A.; Hussain, M.A.; Daud, W.M.A.W.; Soroush, M.; Shamiri, A. Mathematical Modeling of Solid Oxide Fuel Cells: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1893–1917. [Google Scholar]
- Buonomano, A.; Calise, F.; d’Accadia, M.D.; Palombo, A.; Vicidomini, M. Hybrid Solid Oxide Fuel Cells-Gas Turbine Systems for Combined Heat and Power: A Review. Appl. Energy 2015, 156, 32–85. [Google Scholar]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar]
- Wachsman, E.D.; Marlowe, C.A.; Lee, K.T. Role of Solid Oxide Fuel Cells in a Balanced Energy Strategy. Energy Environ. Sci. 2012, 5, 5498–5509. [Google Scholar] [CrossRef]
- Gómez, S.Y.; Hotza, D. Current Developments in Reversible Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2016, 61, 155–174. [Google Scholar]
- Kannan, M.; Singh, S.; Prasad, R.R. Oxide Ion Conductivity of Mechanically Produced Calcia Stabilised Zirconia for Oxygen Sensing Applications. Mater. Res. 2022, 25, e20220090. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Yu, H.; Guan, F.; Hou, Z.; Cui, D.; Zhang, Y. Bimetallic Ni-Co Catalysts for Co-Production of Methane and Liquid Fuels from Syngas. Catal. Today 2021, 369, 167–174. [Google Scholar] [CrossRef]
- Selvaraj, T.; Johar, B.; Khor, S.F. Iron/Zinc Doped 8 mol% Yttria Stabilized Zirconia Electrolytes for the Green Fuel Cell Technology: A Comparative Study of Thermal Analysis, Crystalline Structure, Microstructure, Mechanical and Electrochemical Properties. Mater. Chem. Phys. 2019, 222, 309–320. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Wang, J.; Zhang, H. Recent Advances during CH4 Dry Reforming for Syngas Production: A Mini Review. Int. J. Hydrogen Energy 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- Parsa, M.; Qi, Y.; Di Nuzzo, J.J.; Moussakhani, Y.; Tirto, A.; Chaffee, A.L. Regenerable Carbon Honeycomb Monoliths Directly Prepared from Brown Coal: A Novel Carbon Product. Chem. Eng. J. 2023, 471, 144699. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Catalysts for CO2 Reforming of CH4: A Review. J. Mater. Chem. A 2021, 9, 12495–12520. [Google Scholar] [CrossRef]
- Liang, F.; Yang, J.; Zhao, Y.; Zhou, Y.; Yan, Z.; He, J.; Yuan, Q.; Wu, J.; Liu, P.; Zhong, Z.; et al. A Review of Thin Film Electrolytes Fabricated by Physical Vapor Deposition for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2022, 47, 36926–36952. [Google Scholar] [CrossRef]
- Ettalibi, O.; Samid, A.; Achak, O.; Mansori, M.; Chafik, T. Towards a Low-Cost Solid Oxide Electrolyte Based on Local Mineral. Mater. Chem. Phys. 2024, 315, 128949. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abu Hassan, S.H.; Shaari, N.; Yahaya, A.Z.; Boon Kar, Y. A Review on Recent Status and Challenges of Yttria Stabilized Zirconia Modification to Lowering the Temperature of Solid Oxide Fuel Cells Operation. Int. J. Energy Res. 2020, 44, 631–650. [Google Scholar] [CrossRef]
- Capotondo, F.; Bishop, M.T.; Palmerini, F.; Smitshuysen, A.L.; Pirou, S.; Sudireddy, B.R.; Hagen, A. Fabrication Framework for Metal Supported Solid Oxide Cells via Tape Casting. J. Power Sources 2024, 613, 234812. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Advanced Modification of Scandia-Stabilized Zirconia Electrolytes for Solid Oxide Fuel Cells Application—A Review. Int. J. Energy Res. 2021, 45, 4871–4887. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Rafique, M.; Usman, M.; Ain, Q.u.; Ghaffar, A. Evaluation of Densification Effects on the Properties of 8 Mol % Yttria Stabilized Zirconia Electrolyte Synthesized by Cost Effective Coprecipitation Route. Ceram. Int. 2021, 47, 2857–2863. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-Based Bimetallic Heterogeneous Catalysts for Energy and Environmental Applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Courtin, E.; Boy, P.; Rouhet, C.; Bianchi, L.; Bruneton, E.; Poirot, N.; Laberty-Robert, C.; Sanchez, C. Optimized Sol-Gel Routes to Synthesize Yttria-Stabilized Zirconia Thin Films as Solid Electrolytes for Solid Oxide Fuel Cells. Chem. Mater. 2012, 24, 4540–4548. [Google Scholar] [CrossRef]
- Bellido, J.D.A.; De Souza, J.E.; M’Peko, J.-C.; Assaf, E.M. Effect of Adding CaO to ZrO2 Support on Nickel Catalyst Activity in Dry Reforming of Methane. Appl. Catal. A Gen. 2009, 358, 215–223. [Google Scholar] [CrossRef]
- Lai, Q.; Chen, J.; Chang, F.; Pei, J.; Liang, Y.; Chen, X.; Feng, Q.; Cen, Z.; Luo, N. Cold Sintering Process Assisted Sintering for 8YSZ Ceramic: A Way of Achieving High Density and Electrical Conductivity at a Reduced Sintering Temperature. Ceram. Int. 2023, 49, 14744–14749. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic Steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Wongkamhaeng, K.; Dawson, D.V.; Holloway, J.A.; Denry, I. Effect of Surface Modification on In-Depth Transformations and Flexural Strength of Zirconia Ceramics. J. Prosthodont. 2019, 28, E364–E375. [Google Scholar] [CrossRef] [PubMed]
- Ferrage, L.; Bertrand, G.; Lenormand, P. Dense Yttria-Stabilized Zirconia Obtained by Direct Selective Laser Sintering. Addit. Manuf. 2018, 21, 472–478. [Google Scholar] [CrossRef]
- Zhang, N.; Asle Zaeem, M. Competing Mechanisms between Dislocation and Phase Transformation in Plastic Deformation of Single Crystalline Yttria-Stabilized Tetragonal Zirconia Nanopillars. Acta Mater. 2016, 120, 337–347. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Li, W.; Leu, M.C.; Watts, J.L.; Hilmas, G.E. Additive Manufacturing and Mechanical Characterization of High Density Fully Stabilized Zirconia. Ceram. Int. 2017, 43, 6082–6088. [Google Scholar] [CrossRef]
- Singh, R.; Chavan, S.B. Processing and Properties of Scandia-Doped Zirconia Electrolyte for Intermediate Temperature SOFC. ECS Trans. 2007, 7, 2207–2212. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel Cell Materials and Components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid Oxide Fuel Cells (SOFCs): A Review of an Environmentally Clean and Efficient Source of Energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar]
- Etsell, T.H.; Flengas, S.N. The Electrical Properties of Solid Oxide Electrolytes. Chem. Rev. 1970, 70, 339–376. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Pan, B.; Kong, W.; Chen, Y.; Zhang, W.; Sun, Y. Temperature-Dependent Anti-Coking Behaviors of Highly Stable Ni-CaO-ZrO2 Nanocomposite Catalysts for CO2 Reforming of Methane. Chem. Eng. J. 2017, 320, 63–73. [Google Scholar] [CrossRef]
- Sun, N.; Wen, X.; Wang, F.; Peng, W.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Kang, J. Catalytic Performance and Characterization of Ni–CaO–ZrO2 Catalysts for Dry Reforming of Methane. Appl. Surf. Sci. 2011, 257, 9169–9176. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and Durable Alkaline Earth Metal Substituted Perovskite Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Dudek, M.; Bućko, M.M. Electrical Properties of Stoichiometric and Non-Stoichiometric Calcium Zirconate. In Proceedings of the Solid State Ionics, Kyoto, Japan, 9–13 November 2003; Volume 157. [Google Scholar]
- Mahato, N.; Gupta, A.; Balani, K. Doped Zirconia and Ceria-Based Electrolytes for Solid Oxide Fuel Cells: A Review. Nanomater. Energy 2012, 1, 27–45. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.H.; Gao, Y.H.; Lu, X.G.; Ding, W.Z. Thermodynamic Reassessment of ZrO2-CaO System. J. Am. Ceram. Soc. 2009, 92, 1098–1104. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. One-Step Preparation of CaO-Doped Partially Stabilized Zirconia from Fused Zirconia. Ceram. Int. 2020, 46, 6484–6490. [Google Scholar] [CrossRef]
- Tong, X.; Bowman, W.J.; Mejia-Giraldo, A.; Crozier, P.A.; Mebane, D.S. New Data-Driven Interacting-Defect Model Describing Nanoscopic Grain Boundary Compositions in Ceramics. J. Phys. Chem. C 2020, 124, 23619–23625. [Google Scholar] [CrossRef]
- Ramírez-González, J.; West, A.R. Electrical Properties of Calcia-Stabilised Zirconia Ceramics. J. Eur. Ceram. Soc. 2020, 40, 5602–5611. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A Review of Zirconia-Based Solid Electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar]
- Prasad, R.R.; Yadav, K.L.; Puri, D.; Daniel, B.S.S. Conductivity Measurement of Calcia Stabilized Zirconia Prepared by Mechanical Route. Adv. Mater. Res. 2012, 585, 245–249. [Google Scholar]
- Kim, B.-J.; Park, H.-R.; Lee, Y.-L.; Ahn, S.-Y.; Kim, K.-J.; Hong, G.-R.; Roh, H.-S. Customized Ni-MgO-ZrO2 Catalysts for the Dry Reforming of Methane Using Coke Oven Gas: Optimizing the MgO Content. J. CO2 Util. 2023, 68, 102379. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry Reforming of Methane with Carbon Dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Fakeeha, A.H.; Kasim, S.O.; Khatri, J.; Ibrahim, A.A.; Arasheed, R.; Alabdulsalam, M.; Lanre, M.S.; Osman, A.I.; et al. Promotional Effect of Magnesium Oxide for a Stable Nickel-Based Catalyst in Dry Reforming of Methane. Sci. Rep. 2020, 10, 13861. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Fernández, J.J.; Ruíz, W.; Mondragón, F.; Moreno, A. Effect of MgO Addition on the Basicity of Ni/ZrO2 and on Its Catalytic Activity in Carbon Dioxide Reforming of Methane. Catal. Commun. 2009, 11, 240–246. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Kurdi, A.; Al-Baqmaa, Y.A.; Ibrahim, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Performance Study of Methane Dry Reforming on Ni/ZrO2 Catalyst. Energies 2022, 15, 3841. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Abdullah, B.; Hasnain, S.M.W.; Farooqi, S.A. Syngas Production via Dry Reforming of Methane over Ni/MgO-ZrO2 Catalyst. IOP Conf. Ser. Earth Environ. Sci. 2023, 1261, 012028. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Bulushev, D.A.; Beloshapkin, S.; Chansai, S.; Ross, J.R.H. Potassium-Doped Ni–MgO–ZrO2 Catalysts for Dry Reforming of Methane to Synthesis Gas. Top. Catal. 2013, 56, 1686–1694. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials Properties, Advantages and Shortcomings for Applications Volume 1: Conventional Approaches; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar]
- Banerjee, S.; Mukhopadhyay, P. Phase Transformations—Examples from Titanium and Zirconium Alloys; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12. [Google Scholar]
- Hannink, R.; Swain, M.V. The Development of Zirconia Transformation Toughened Ceramics in Australia. J. Aust. Ceram. Soc. 2014, 50, 1–14. [Google Scholar]
- Deng, W.; Li, Y. High-Temperature Electrical Properties of Polycrystalline MgO-Doped ZrO2. Mater. Res. Bull. 2019, 113, 182–189. [Google Scholar] [CrossRef]
- Wen, T.; Jin, E.; Yuan, L.; Yu, J.; Zhou, Y.; Tian, C. Structure and Ionic Conductivity of ZrO2 (MgO)/CaO-Al2O3 Bilayer System Used as Solid Electrolyte for Sulfur Sensor. Mater. Res. Bull. 2019, 117, 113–119. [Google Scholar]
- Devanathan, R.; Thevuthasan, S.; Gale, J.D. Defect Interactions and Ionic Transport in Scandia Stabilized Zirconia. Phys. Chem. Chem. Phys. 2009, 11, 5506–5511. [Google Scholar] [CrossRef]
- Hansen, K.K. Solid State Electrochemical DeNOx-An Overview. Appl. Catal. B Environ. 2010, 100, 427–432. [Google Scholar] [CrossRef]
- Valadez Huerta, G.; Reus, L.; Kabelac, S. A Diffusivity Study of (Sc2O3)0.1(CeO2)0.01(ZrO2)0.89 between 1100 and 1500 K at Zero Pressure with Molecular Dynamics. J. Chem. Eng. Data 2018, 63, 1955–1960. [Google Scholar] [CrossRef]
- Mahmud, L.S.; Muchtar, A.; Somalu, M.R. Challenges in Fabricating Planar Solid Oxide Fuel Cells: A Review. Renew. Sustain. Energy Rev. 2017, 72, 105–116. [Google Scholar]
- Dwivedi, S. Solid Oxide Fuel Cell: Materials for Anode, Cathode and Electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Spirin, A.; Ivanov, V.; Nikonov, A.; Lipilin, A.; Paranin, S.; Khrustov, V.; Spirina, A. Scandia-Stabilized Zirconia Doped with Yttria: Synthesis, Properties, and Ageing Behavior. Solid State Ionics 2012, 225, 448–452. [Google Scholar]
- Yamamoto, O.; Arati, Y.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Electrical Conductivity of Stabilized Zirconia with Ytterbia and Scandia. Solid State Ionics 1995, 79, 137–142. [Google Scholar] [CrossRef]
- Chowdari, B.V.R.; Dissanayake, M.A.K.L.; Careem, M.A. Solid State Ionics: New Developments. In Proceedings of the Fifth Asian Conference on Defence Technology, Hsinchu, Taiwan, 20–22 November 1996. [Google Scholar]
- Nakayama, S.; Tokunaga, R.; Takata, M.; Kondo, S.; Nakajima, Y. Crystal Phase, Electrical Properties, and Solid Oxide Fuel Cell Electrolyte Application of Scandia-Stabilized Zirconia Doped with Rare Earth Elements. Open Ceram. 2021, 6, 100136. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T. Oxygen-Ion Conducting Electrolyte Materials for Solid Oxide Fuel Cells. Ionics 2000, 6, 1–21. [Google Scholar]
- Palaci, Y.; Timurkutluk, B. Design and Fabrication of Stair-Step-Type Electrolyte Structure for Solid Oxide Fuel Cells. Int. J. Energy Res. 2013, 37, 631–637. [Google Scholar] [CrossRef]
- Yamamoto, O.; Arachi, Y.; Sakai, H.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Zirconia Based Oxide Ion Conductors for Solid Oxide Fuel Cells. Ionics 1998, 4, 403–408. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T.; Milosevic, D. Scandia–Zirconia Electrolytes for Intermediate Temperature Solid Oxide Fuel Cell Operation. Solid State Ion. 2000, 136–137, 91–99. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, A.; Sanbui, M.; Omar, S. Oxygen-Ion Conduction in Scandia-Stabilized Zirconia-Ceria Solid Electrolyte (XSc2O3–1CeO2–(99−x)ZrO2, 5 ≤ x ≤ 11). J. Am. Ceram. Soc. 2017, 100, 659–668. [Google Scholar] [CrossRef]
- Kharton, V.V.; Naumovich, E.N.; Vecher, A.A. Research on the Electrochemistry of Oxygen Ion Conductors in the Former Soviet Union. I. ZrO2-Based Ceramic Materials. J. Solid State Electrochem. 1999, 3, 61–81. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, S.H.; Wackerl, J.; Jung, D.H.; Suhr, D.S.; Choi, S.Y.; Peck, D.H. Fabrication of Scandia-Stabilized Zirconia Electrolyte with a Porous and Dense Composite Layer for Solid Oxide Fuel Cells. Ceram. Int. 2012, 38, S485–S488. [Google Scholar]
- Tillborg, H.; Nilsson, A.; Hernnäs, B.; Mårtensson, N.; Palmer, R.E. X-Ray and UV Photoemission Studies of Mono-, Bi- and Multilayers of Physisorbed Molecules: O2 and N2 on Graphite. Surf. Sci. 1993, 295, 1–12. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Kasim, S.O.; Fakeeha, A.H.; Lanre, M.S.; Abasaeed, A.E.; Abu-Dahrieh, J.K.; Al-Fatesh, A.S. Dry Reforming of Methane with Ni Supported on Mechanically Mixed Yttria-Zirconia Support. Catal. Lett. 2022, 152, 3632–3641. [Google Scholar] [CrossRef]
- Patel, R.; Fakeeha, A.H.; Kasim, S.O.; Sofiu, M.L.; Ibrahim, A.A.; Abasaeed, A.E.; Kumar, R.; Al-Fatesh, A.S. Optimizing Yttria-Zirconia Proportions in Ni Supported Catalyst System for H2 Production through Dry Reforming of Methane. Mol. Catal. 2021, 510, 111676. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, R.; Srivastava, V.K.; Ibrahim, A.A.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alquraini, A.A.; Kumar, R. Barium-Promoted Yttria–Zirconia-Supported Ni Catalyst for Hydrogen Production via the Dry Reforming of Methane: Role of Barium in the Phase Stabilization of Cubic ZrO2. ACS Omega 2022, 7, 16468–16483. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Srivastava, V.K.; Ibrahim, A.A.; Abahussain, A.A.M.; Abu-Dahrieh, J.K.; Alotibi, M.F.; Kumar, R. Hydrogen Production from Gadolinium-Promoted Yttrium-Zirconium-Supported Ni Catalysts through Dry Methane Reforming. ACS Omega 2023, 8, 22108–22120. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.A.; Al-Fatesh, A.S.; Kaydouh, M.-N.; Al Otaibi, A.; Francesco, F.; Fakeeha, A.H.; El Hassan, N. High Carbon-Resistant Nickel Supported on Yttria–Zirconia Catalysts for Syngas Production by Dry Reforming of Methane: The Promoting Effect of Cesium. Alex. Eng. J. 2023, 74, 371–386. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Al-Fatesh, A.S.; Kumar, R.; Lanre, M.S.; Frusteri, F.; AlReshaidan, S.B.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Promotional Effect of Addition of Ceria over Yttria-Zirconia Supported Ni Based Catalyst System for Hydrogen Production through Dry Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 20838–20850. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Calvino, J.J.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Arias, D.C.; Pérez-Omil, J.A.; Hernández-Garrido, J.C.; González-Leal, J.M.; Cauqui, M.A.; Yeste, M.P. Highly Stable Ceria-Zirconia-Yttria Supported Ni Catalysts for Syngas Production by CO2 Reforming of Methane. Appl. Surf. Sci. 2017, 426, 864–873. [Google Scholar] [CrossRef]
- Xia, X.; Oldman, R.; Catlow, R. Computational Modeling Study of Bulk and Surface of Yttria-Stabilized Cubic Zirconia. Chem. Mater. 2009, 21, 3576–3585. [Google Scholar] [CrossRef]
- Jiao, Z.; Shikazono, N. 3D Reconstruction Size Effect on the Quantification of Solid Oxide Fuel Cell Nickel–Yttria-Stabilized-Zirconia Anode Microstructural Information Using Scanning Electron Microscopy-Focused Ion Beam Technique. Sci. Bull. 2016, 61, 1317–1323. [Google Scholar] [CrossRef]
- Phongpreecha, T.; Nicholas, J.D.; Bieler, T.R.; Qi, Y. Computational Design of Metal Oxides to Enhance the Wetting and Adhesion of Silver-Based Brazes on Yttria-Stabilized-Zirconia. Acta Mater. 2018, 152, 229–238. [Google Scholar] [CrossRef]
- Asadikiya, M.; Sabarou, H.; Chen, M.; Zhong, Y. Phase Diagram for a Nano-Yttria-Stabilized Zirconia System. RSC Adv. 2016, 6, 17438–17445. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Nowotny, M.K.; Sorrell, C.C. Charge Transfer at Oxygen/Zirconia Interface at Elevated Temperatures Part 1: Basic Properties and Terms. Adv. Appl. Ceram. 2005, 104, 147–153. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Feng, J.; Pan, W. Phase Transformation Behavior in Air Plasma Sprayed Yttria Stabilized Zirconia Coating. J. Alloys Compd. 2018, 750, 189–196. [Google Scholar] [CrossRef]
- Celik, E.; Negi, R.S.; Bastianello, M.; Boll, D.; Mazilkin, A.; Brezesinski, T.; Elm, M.T. Tailoring the Protonic Conductivity of Porous Yttria-Stabilized Zirconia Thin Films by Surface Modification. Phys. Chem. Chem. Phys. 2020, 22, 11519–11528. [Google Scholar] [CrossRef] [PubMed]
- Mat, Z.B.A.; Madya; Kar, Y.B.; Hassan, S.H.B.A.; Talik, N.A.B. Proton Exchange Membrane (PEM) and Solid Oxide (SOFC) Fuel Cell Based Vehicles-a Review. In Proceedings of the 2017 2nd IEEE International Conference on Intelligent Transportation Engineering, ICITE 2017, Singapore, 1–3 September 2017. [Google Scholar]
- Santos, T.H.; Grilo, J.P.F.; Loureiro, F.J.A.; Fagg, D.P.; Fonseca, F.C.; Macedo, D.A. Structure, Densification and Electrical Properties of Gd3+ and Cu2+ Co-Doped Ceria Solid Electrolytes for SOFC Applications: Effects of Gd2O3 Content. Ceram. Int. 2018, 44, 2745–2751. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Senthil Kumar, S.; Aruna, S.T. Properties and Development of Ni/YSZ as an Anode Material in Solid Oxide Fuel Cell: A Review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar]
- Saebea, D.; Authayanun, S.; Patcharavorachot, Y.; Chatrattanawet, N.; Arpornwichanop, A. Electrochemical Performance Assessment of Low-Temperature Solid Oxide Fuel Cell with YSZ-Based and SDC-Based Electrolytes. Int. J. Hydrogen Energy 2018, 43, 921–931. [Google Scholar] [CrossRef]
- Vonk, V.; Khorshidi, N.; Stierle, A.; Dosch, H. Atomic Structure and Composition of the Yttria-Stabilized Zirconia (111) Surface. Surf. Sci. 2013, 612, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.H.; Zeng, Y.P.; Jiang, D. Properties of Microstructure-Controllable Porous Yttria-Stabilized Ziroconia Ceramics Fabricated by Freeze Casting. Int. J. Appl. Ceram. Technol. 2008, 5, 198–203. [Google Scholar] [CrossRef]
- Della Negra, M.; Foghmoes, S.P.V.; Klemensø, T. Complementary Analysis Techniques Applied on Optimizing Suspensions of Yttria Stabilized Zirconia. Ceram. Int. 2016, 42, 14443–14451. [Google Scholar] [CrossRef]
- Bae, K.; Son, K.S.; Kim, J.W.; Park, S.W.; An, J.; Prinz, F.B.; Shim, J.H. Proton Incorporation in Yttria-Stabilized Zirconia during Atomic Layer Deposition. Int. J. Hydrogen Energy 2014, 39, 2621–2627. [Google Scholar] [CrossRef]
- Marinopoulos, A.G. Protons in Cubic Yttria-Stabilized Zirconia: Binding Sites and Migration Pathways. Solid State Ionics 2018, 315, 116–125. [Google Scholar] [CrossRef]
- Singhal, S.C. Solid Oxide Fuel Cells for Stationary, Mobile, and Military Applications. Solid State Ionics 2002, 152–153, 405–410. [Google Scholar] [CrossRef]
- Rahmawati, F.; Permadani, I.; Syarif, D.G.; Soepriyanto, S. Electrical Properties of Various Composition of Yttrium Doped-Zirconia Prepared from Local Zircon Sand. Int. J. Technol. 2017, 8, 939. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, S.; Bian, Y.; Zhang, M.; Ding, W. Effect of Sintering Temperature on Mechanical Properties of Magnesia Partially Stabilized Zirconia Refractory. Ceram. Int. 2016, 42, 10593–10598. [Google Scholar] [CrossRef]
- Muccillo, E.N.S.; Kleitz, M. Impedance Spectroscopy of Mg-Partially Stabilized Zirconia and Cubic Phase Decomposition. J. Eur. Ceram. Soc. 1996, 16, 453–465. [Google Scholar] [CrossRef]
- Makinose, Y.; Yamada, T.; Kubota, Y. Ionic Conductivity of Electrolytes Composed of Oleate-Capped Yttria-Stabilized Zirconia Nanoparticles. ACS Omega 2023, 8, 48728–48734. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Choi, I.W.; Kim, Y.J.; Lee, S.; Jeong, W.; Yu, W.; Cho, G.Y.; Cha, S.W. Nanocrystal Engineering of Thin-Film Yttria-Stabilized Zirconia Electrolytes for Low-Temperature Solid-Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2023, 15, 42659–42666. [Google Scholar] [CrossRef] [PubMed]
- Ciacchi, F.T.; Badwal, S.P.S. The System Y2O3-Sc2O3-ZrO2: Phase Stability and Ionic Conductivity Studies. J. Eur. Ceram. Soc. 1991, 7, 197–206. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T.; Rajendran, S.; Drennan, J. An Investigation of Conductivity, Microstructure and Stability of Electrolyte Compositions in the System 9 Mol% (Sc2O3-Y2O3)-ZrO2(Al2O3). Solid State Ionics 1998, 109, 167–186. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Bredikhin, S.I.; Burmistrov, I.N.; Eliseeva, G.M.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; et al. Phase Compositions, Structures and Properties of Scandia-Stabilized Zirconia Solid Solution Crystals Co-Doped with Yttria or Ytterbia and Grown by Directional Melt Crystallization. Solid State Ionics 2020, 346, 115218. [Google Scholar] [CrossRef]
- Subramanian, Y.; Veena, R.; Ali, S.A.M.; Kumar, A.; Gubediran, R.K.; Dhanasekaran, A.; Gurusamy, D.; Muniandi, K. Artificial Intelligence Technique Based Performance Estimation of Solid Oxide Fuel Cells. Mater. Today Proc. 2023, 80, 2573–2576. [Google Scholar]
- Minkiewicz, J.; Jones, G.M.; Ghanizadeh, S.; Bostanchi, S.; Wasely, T.J.; Yamini, S.A.; Nekouie, V. Large-Scale Manufacturing of Solid-State Electrolytes: Challenges, Progress, and Prospects. Open Ceram. 2023, 16, 100497. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly Dispersed and Stable Ni Nanoparticles Confined by MgO on ZrO2 for CO2 Methanation. Appl. Surf. Sci. 2019, 481, 1538–1548. [Google Scholar] [CrossRef]
- Wu, H.; Duan, Y.; Liu, K.; Lv, D.; Qin, L.; Shi, L.; Tang, G. First-Principles Study of Phase Transition and Band Structure of ZrO2 under Pressure. J. Alloys Compd. 2015, 645, 352–357. [Google Scholar] [CrossRef]
- Arifin, N.A.; Afifi, A.A.; Samreen, A.; Hafriz, R.S.R.M.; Muchtar, A. Characteristic and Challenges of Scandia Stabilized Zirconia as Solid Oxide Fuel Cell Material–In Depth Review. Solid State Ionics 2023, 399, 116302. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for Solid Oxide Fuel Cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Atkinson, A. Transport Properties of Solid Oxide Electrolyte Ceramics: A Brief Review. Solid State Ionics 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified Approach for Molecular Dynamics and Density-Functional Theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.A. Going against the Grain: Atomistic Modeling of Grain Boundaries in Solid Electrolytes for Solid-State Batteries. ACS Mater. Au 2024, 4, 1–13. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. Climbing Image Nudged Elastic Band Method for Finding Saddle Points and Minimum Energy Paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Xu, H.; Cao, G.; Shen, Y.; Yu, Y.; Hu, J.; Wang, Z.; Shao, G. Enabling Argyrodite Sulfides as Superb Solid-State Electrolyte with Remarkable Interfacial Stability Against Electrodes. Energy Environ. Mater. 2022, 5, 852–864. [Google Scholar] [CrossRef]
- Mishra, A.K.; Rajput, S.; Karamta, M.; Mukhopadhyay, I. Exploring the Possibility of Machine Learning for Predicting Ionic Conductivity of Solid-State Electrolytes. ACS Omega 2023, 8, 16419–16427. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, S.A.; Rajput, Y.; Chaudhary, K.J.; Al-Fatesh, A.S.; Ali, F.A.A.; El-Toni, A.M.; Abahussain, A.A.M.; Alshareef, R.; Kumar, R.; Osman, A.I. Ca-, Mg-, Sc-, and Y-Stabilized Zirconia: High-Performance Support Material for Dry Reforming of Methane and Solid-Electrolyte Material for Fuel Cell. Catalysts 2025, 15, 300. https://doi.org/10.3390/catal15040300
Al-Zahrani SA, Rajput Y, Chaudhary KJ, Al-Fatesh AS, Ali FAA, El-Toni AM, Abahussain AAM, Alshareef R, Kumar R, Osman AI. Ca-, Mg-, Sc-, and Y-Stabilized Zirconia: High-Performance Support Material for Dry Reforming of Methane and Solid-Electrolyte Material for Fuel Cell. Catalysts. 2025; 15(4):300. https://doi.org/10.3390/catal15040300
Chicago/Turabian StyleAl-Zahrani, Salma A., Yuvrajsinh Rajput, Kirankumar J. Chaudhary, Ahmed S. Al-Fatesh, Fekri Abdulraqeb Ahmed Ali, Ahmed Mohamed El-Toni, Abdulaziz A. M. Abahussain, Rayed Alshareef, Rawesh Kumar, and Ahmed I. Osman. 2025. "Ca-, Mg-, Sc-, and Y-Stabilized Zirconia: High-Performance Support Material for Dry Reforming of Methane and Solid-Electrolyte Material for Fuel Cell" Catalysts 15, no. 4: 300. https://doi.org/10.3390/catal15040300
APA StyleAl-Zahrani, S. A., Rajput, Y., Chaudhary, K. J., Al-Fatesh, A. S., Ali, F. A. A., El-Toni, A. M., Abahussain, A. A. M., Alshareef, R., Kumar, R., & Osman, A. I. (2025). Ca-, Mg-, Sc-, and Y-Stabilized Zirconia: High-Performance Support Material for Dry Reforming of Methane and Solid-Electrolyte Material for Fuel Cell. Catalysts, 15(4), 300. https://doi.org/10.3390/catal15040300









