Development and Characterization of Metal-Doped Modified CO Oxidation Catalyst for Coalbed Methane with Strong Adsorption and Water Resistance
Abstract
1. Introduction
2. Results and Discussion
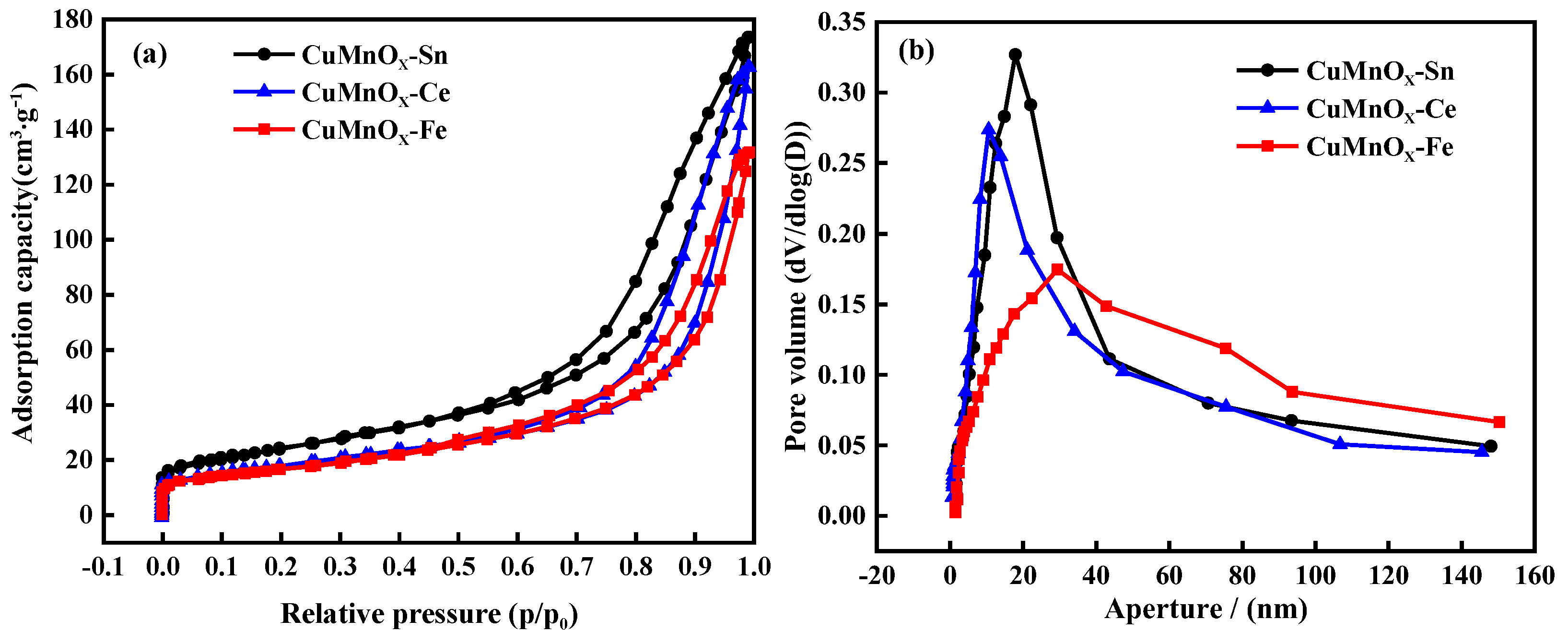
2.1. Optimal Selection of Doped Metals
2.2. Feature Testing and Optimization of Preparation Conditions
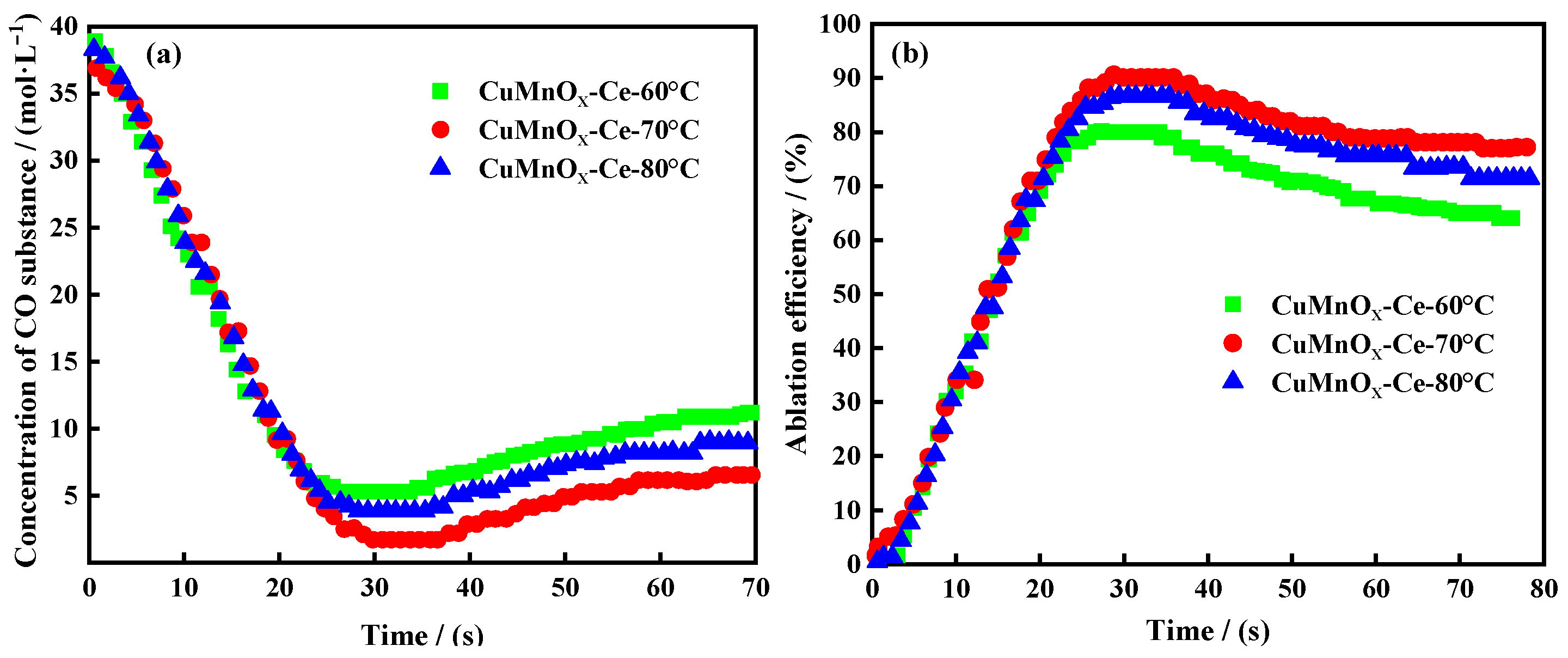
2.2.1. Testing of Ablation Characteristics of Catalysts at Different Precipitation Temperatures
2.2.2. Analysis of Surface Crystal Structure of Catalysts at Different Precipitation Temperatures
2.2.3. Analysis of Pore Characteristics of Catalysts at Different Precipitation Temperatures
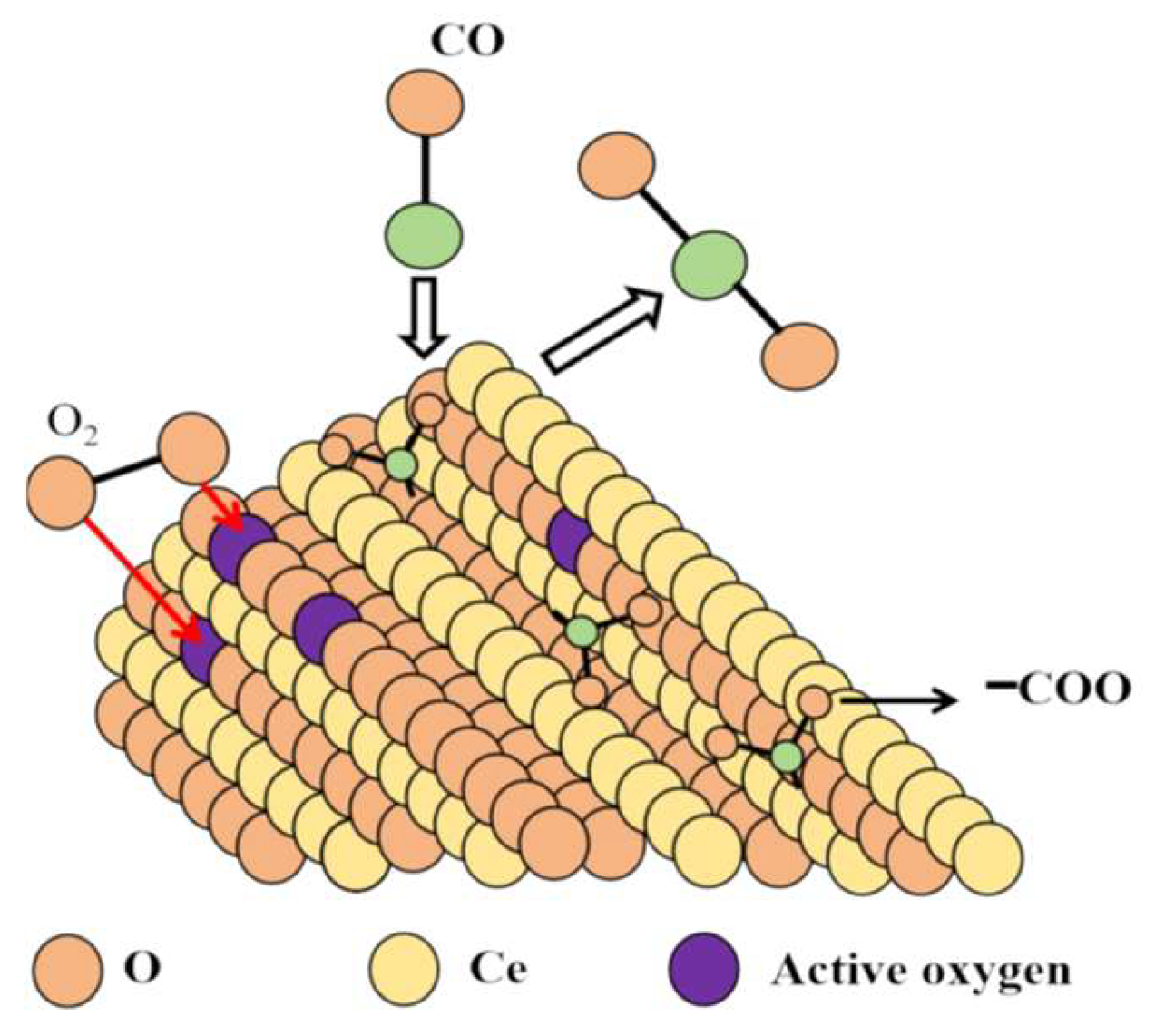
2.2.4. Analysis of the Ablation Process of Cu Mn-Type Co Oxidation Catalyst
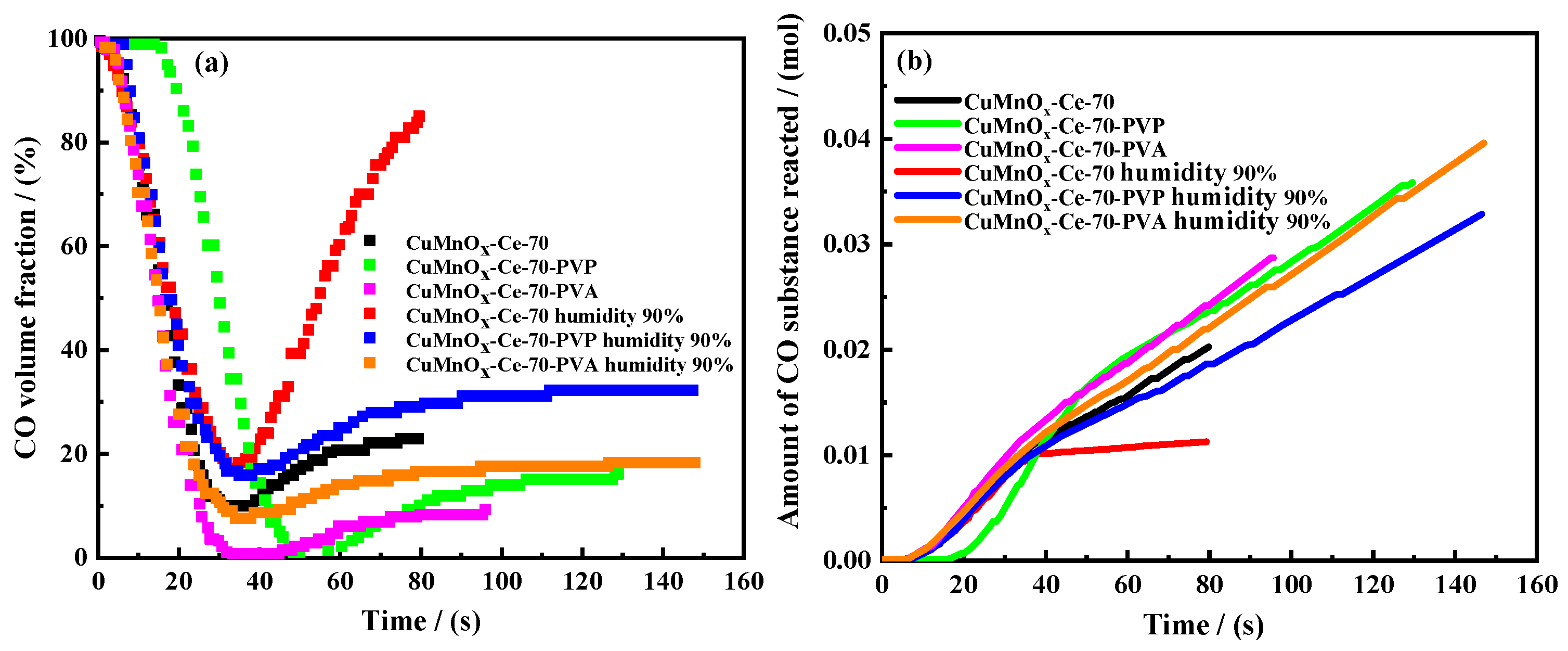
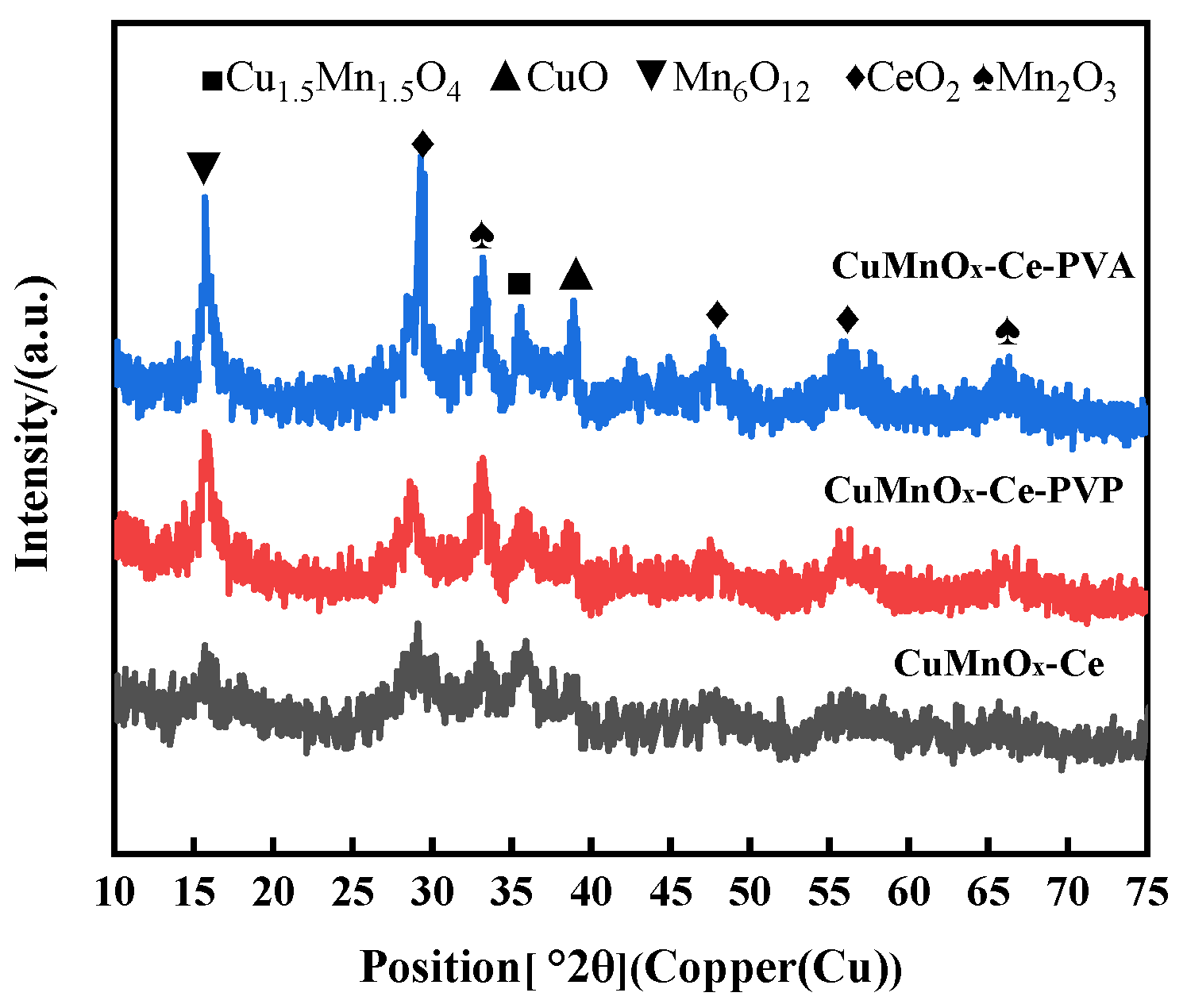
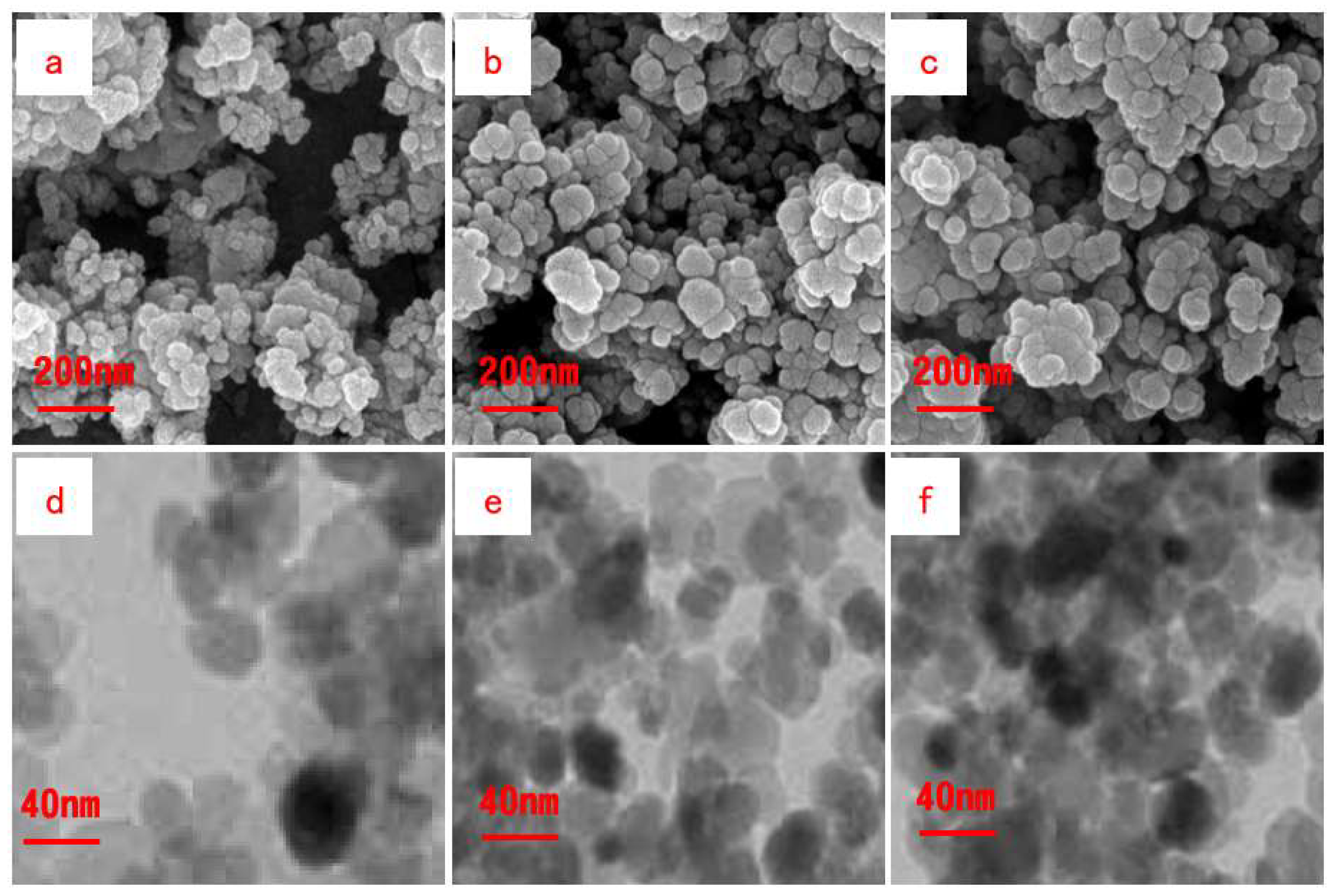
2.3. Water Resistance Optimization
2.3.1. Ablation Characteristics Test
2.3.2. Analysis of Surface Crystal Structure
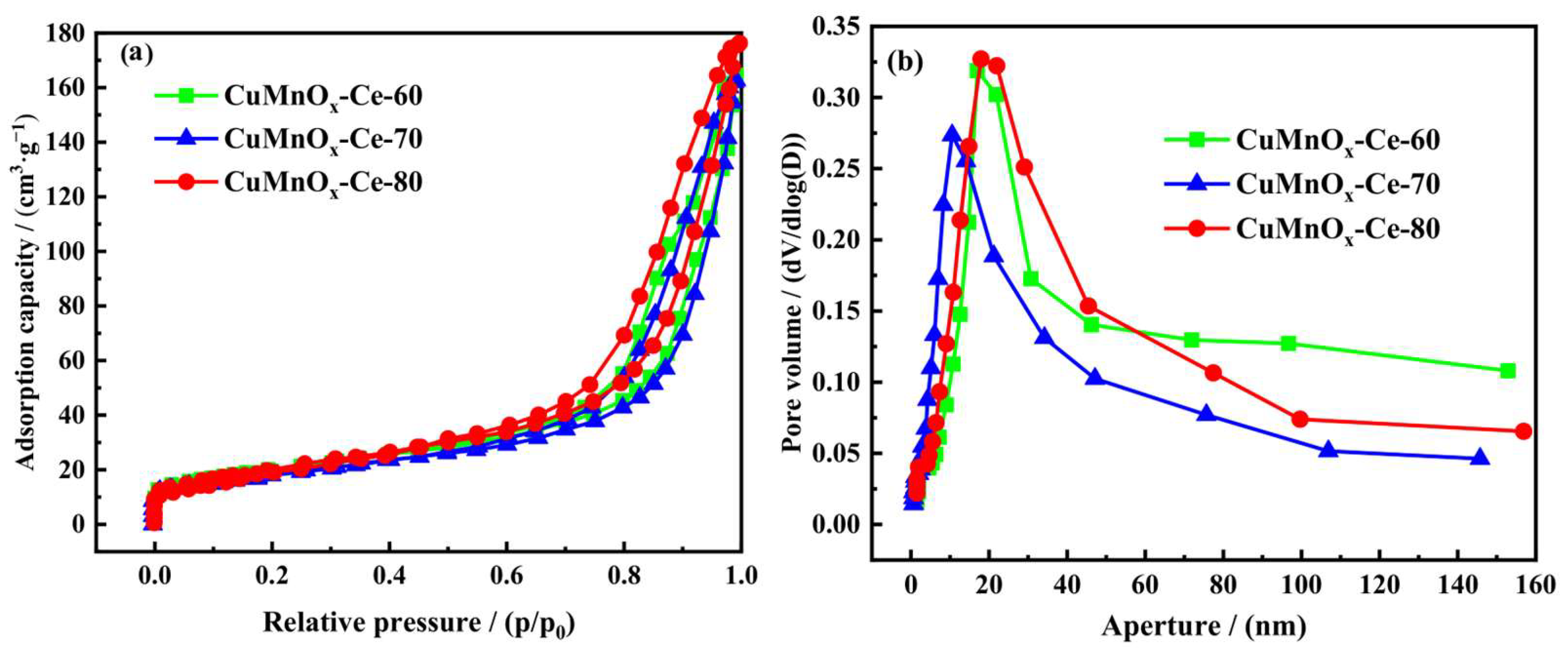
2.3.3. Analysis of Pore Characteristics
2.3.4. Hydrophobicity Analysis of Catalysts
2.4. Evaluation of Water-Resistant Steam Ablation Process of Cu Mn-Type Co Oxidation Catalyst
3. Experimental
3.1. Experimental Materials
3.2. Sample Preparation
3.3. Characterization of Samples
3.4. Performance Evaluation Methods
- (1)
- Experimental setup and gas flow path
- (2)
- Experimental Procedure
- (3)
- Reaction mechanism and reaction conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Liu, X.; Huang, Z.; Cao, M.; Zhang, M.; Zhang, Y.; Jia, K. Analysis of the explosion risk of low-concentration oxygen-bearing coal bed methane in the low-temperature environment. Case Stud. Therm. Eng. 2024, 59, 104468. [Google Scholar] [CrossRef]
- Cheng, X.; Song, J.; Wen, H.; Fan, S.; Liu, M.; Mi, W.; Yu, Z.; Liu, Y.; Li, R. Mitigation of coal spontaneous combustion and en-hanced coalbed methane recovery using liquid CO2: Mechanisms, field applications, and implications for mines. J. CO2 Util. 2024, 90, 102987. [Google Scholar] [CrossRef]
- Li, X.; Si, X.; Li, X.; Zhang, S.; Yang, T.; Hu, L.; Nie, B.; Li, Z. Methane release and energy evolution in coal and its impli-cation for gas outburst. Phys. Fluids 2025, 37, 022001. [Google Scholar] [CrossRef]
- Sidorenko, S.; Trushnikov, V.; Sidorenko, A. Methane Emission Estimation Tools as a Basis for Sustainable Underground Mining of Gas-Bearing Coal Seams. Sustainability 2024, 16, 3457. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Qin, Y. Coal wettability in coalbed methane production: A critical review. Fuel 2021, 303, 121277. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: A review. Environ. Chem. Lett. 2020, 18, 79–96. [Google Scholar] [CrossRef]
- Hu, G.; Xu, J.; Zhang, F.; Zhao, C.; Qin, W.; Zhu, Y. Coal and coalbed methane co-extraction technology based on the ground movement in the Yangquan coalfield, China. Energies 2015, 8, 6881–6897. [Google Scholar] [CrossRef]
- Duan, J.; Mao, S.; Xie, P.; Lang, J.; Li, A.; Tong, J.; Qin, M.; Xu, J.; Shen, Z. Key emergency response technologies for abrupt air pollution accidents in China. J. Environ. Sci. 2023, 123, 235–254. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, M.; Niu, Y.; Yi, L.; Yi, H.; Zhou, Y.; Zhao, S.; Tang, X.; Gao, F. Advances in CO catalytic oxidation on typical noble metal catalysts: Mechanism, performance and optimization. Chem. Eng. J. 2024, 495, 153523. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Zanella, R. Catalytic oxidation of propane and carbon monoxide by Pd nanoparticles on Mn/TiO2 catalysts. Catal. Lett. 2024, 154, 155–169. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, J.; Pan, C. The Surface/Interface Modulation of Platinum Group Metal (PGM)-Free Catalysts for VOCs and CO Catalytic Oxidation. ACS Appl. Mater. Interfaces 2024, 16, 37379–37389. [Google Scholar] [CrossRef] [PubMed]
- Nyathi, T.M.; Fadlalla, M.I.; Claeys, M. In situ and Operando Characterisation in the Preferential Oxidation of Carbon Monoxide over Base Metal Oxide Catalysts: A Review. ChemCatChem 2024, 16, e202400285. [Google Scholar] [CrossRef]
- Mancilla, A.; Mendoza-Cruz, R.; Portales, B.; Zanella, R. Catalytic oxidation of carbon monoxide at low temperature using AuRh/TiO2 bimetallic catalysts: Effect of the synthesis method. Mater. Today Chem. 2024, 38, 102092. [Google Scholar] [CrossRef]
- Beg, M.B.; Ali, L.; Shittu, T.; Khaleel, A.; Vermeire, F.H.; Altarawneh, M. Non-Noble Catalysts Formulations using CuO-CeO2/Nb2O5 for Low-temperature catalytic Oxidation of Carbon Monoxide. J. Environ. Chem. Eng. 2024, 12, 113177. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Tang, H.; Zou, Y.; Cen, W.; Zhu, T. Simultaneously accomplishing selective catalytic reduction of NO and catalytic oxidation of CO in a catalyst coupling system. Fuel 2024, 360, 130616. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, X.; Xia, Y.; Xu, Z.; Song, M.; Wang, Z.; Guo, Q.; Jiang, Z. Realizing the high loading amount of active Cu on Al2O3 to boost its CO catalytic oxidation. J. Colloid Interface Sci. 2024, 673, 669–678. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Catalytic Oxidation of Hydrocarbons. Catalysts 2024, 14, 111. [Google Scholar] [CrossRef]
- He, Z.; Hou, Y.; Wei, J.; Ren, S.; Wu, W. Efficient catalytic oxidation of biomass to formic acid coupled with low-energy formaldehyde production from methanol. Green Chem. 2024, 26, 2170–2182. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Xu, Y.; Zheng, Y.; Huang, F. Effect of silica on generation and stability of Cu+ cations in CuO/CeO2 catalysts for CO oxidation in H2-rich atmosphere. Int. J. Hydrogen Energy 2024, 53, 1065–1075. [Google Scholar] [CrossRef]
- Zeng, Y.; Rong, W.; Zhang, S.; Zhong, Q.; Zhong, Z. Ti species regulated CuO/CeO2 catalyst for efficient simultaneously NH3-SCR denitration and CO oxidation under oxygen-rich conditions. Fuel 2024, 358, 130277. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Jie, W.; Liu, Y.; Li, C.; Song, G.; Gong, X.; Liu, Q.; Qiu, M.; Kawi, S.; et al. MOF-derived high oxygen vacancies CuO/CeO2 catalysts for low-temperature CO preferential oxidation. J. Colloid Interface Sci. 2024, 674, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, F.; Yang, H.; Yin, S.; Wu, C.E.; Zhou, T.; Xu, J.; Xu, L.; Chen, M. Constructing Efficient CuO-Based CO Oxi-dation Catalysts with Large Specific Surface Area Mesoporous CeO2 Nanosphere Support. Nanomaterials 2024, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, J.; Gong, W.; Xu, A.; Li, X.; Qiu, Y. Modulating electronic Metal–Support interactions of Pt-CuO/CeO2 catalyst via quenching to optimize catalytic activity. Sep. Purif. Technol. 2023, 332, 125864. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Yang, Y.; Wang, L. Rheology of film-forming solutions and physical properties of tara gum film reinforced with polyvinyl alcohol (PVA). Food Hydrocoll. 2014, 63, 677–684. [Google Scholar] [CrossRef]
- Soud, S.A.; Hasoon, B.A.; Abdulwahab, A.I.; Hussein, N.N.; Maeh, R.K. Synthesis and characterization of plant extracts loaded PVA/PVP blend films and evaluate their biological activities. EurAsian J. BioSci. 2020, 14, 2921–2931. [Google Scholar]
- Jin, W.; Liu, Y.; Xue, H.; Yu, J.; Mao, D. CO Oxidation over Cu/Ce Binary Oxide Prepared via the Solvothermal Method: Effects of Cerium Precursors on Properties and Catalytic Behavior. Catalysts 2024, 14, 856. [Google Scholar] [CrossRef]
- de Freitas Silva, T.; Assaf, J.M.; Lucrédio, A.F.; Reis, C.G.M.; Almeida, K.A. Influence of La2O3 Addition on CuO/CeO2 Catalysts for the Water-Gas Shift Reaction. Langmuir 2025, 41, 3108–3120. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Zhang, M.; Song, B.; Han, Y.; Wang, S.; Ren, Z.; Wang, L.; Yin, P.; Wei, M. Highly Active MnCoO x Catalyst toward CO Preferential Oxidation. ACS Catal. 2024, 14, 1281–1291. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, J.; Sun, W.; Shen, Z. Fabrication of homogeneously Cu2+/La3+-doped CeO2 nanosheets and their application in CO oxidation. J. Mater. Chem. A 2017, 5, 9717–9722. [Google Scholar] [CrossRef]
- Sodpiban, O.; Kessaratikoon, T.; Smith, J.; Ren, G.; Del Gobbo, S.; Das, S.; Chi, M.; D’elia, V.; Gates, B.C. Catalysts Prepared from Atomically Dispersed Ce(III) on MgO Rival Bulk Ceria for CO Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 55885–55894. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Liu, B. Recent Advances of Monolithic Metal Mesh-Based Catalysts for CO Oxidation. ChemCatChem 2024, 16, e202401122. [Google Scholar] [CrossRef]
- Zhigalina, O.M.; Morozova, O.S.; Khmelenin, D.N.; Firsova, A.A.; Silchenkova, O.V.; Vorobieva, G.A.; Bukhtiyarov, A.V.; Cherkovskiy, E.N.; Basu, V.G. Effect of Copper Particle Size on the Surface Structure and Catalytic Activity of Cu–CeO2 Nanocomposites Prepared by Mechanochemical Synthesis in the Preferential CO Oxidation in a H2-Rich Stream (CO-PROX). Catalysts 2024, 14, 222. [Google Scholar] [CrossRef]
- Bai, Z.; Jiang, X.Z.; Luo, K.H. Effects of Electric Field on Chemical Looping Combustion: A DFT Study of CO Oxidation on CuO (111) Surface. ACS Omega 2024, 9, 21082–21088. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Liu, K.; Shen, X.; Chen, X.; Fang, Y.; Zhang, Y.; Yang, X. Steam activation significantly promotes the CO oxidation performance of Pt/CeZrO2 catalyst: Enhancement effect and mechanism. Mol. Catal. 2024, 555, 113916. [Google Scholar] [CrossRef]
- Gong, S.; Zhu, G.; Xie, X.; Rao, F.; Zhu, L.; An, Y.; Shi, X.; Huang, Y.; Liu, P.; Hojamberdiev, M. Engineering the CuO/MnO2 interface with multivalent conversion for low-temperature and efficient CO oxidation. Appl. Surf. Sci. 2024, 652, 159326. [Google Scholar] [CrossRef]
- Mane, R.; Kim, H.; Han, K.; Kim, H.; Lee, S.S.; Roh, H.-S.; Lee, C.; Jeon, Y. Important factors of the A-site deficient Mn perovskites design affecting the CO oxidation activity. Catal. Today 2024, 425, 114347. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Wang, R. CeO2 nanorods supported CuOx-RuOx bimetallic catalysts for low temperature CO oxidation. J. Colloid Interface Sci. 2024, 654, 1378–1392. [Google Scholar] [CrossRef]
- Fan, J.; Hu, S.; Li, C.; Wang, Y.; Chen, G. Effect of loading method on catalytic performance of Pt/CeO2 system for CO oxidation. Mol. Catal. 2024, 558, 114013. [Google Scholar] [CrossRef]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Jia, B.; Liu, J.; Kang, J.; Zhang, G.; Lv, D.; Wang, Y. Investigating NH3-SCR coupled with CO oxidation reaction mechanisms on titanium nanotube-loaded CuMnFe composite metal catalysts. Appl. Surf. Sci. 2024, 652, 159299. [Google Scholar] [CrossRef]
- Ren, Y.; Song, C.; Wang, H.; Qu, Z. Accelerated Dual Activation of Lattice Oxygen and Molecule Oxygen over CoMn2O4 Catalysts for VOC Oxidation: Promoting the Role of Oxygen Vacancies. ACS Catal. 2024, 14, 4340–4351. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Liu, T.; Xu, M.; Dong, Y.; Wang, C.-A. Constructing morphologically stable supported noble metal catalysts in heterogeneous catalysis: Mechanisms and strategies. Nano Energy 2024, 129, 109975. [Google Scholar] [CrossRef]














| CO Elimination Methods | Advantage | Drawbacks |
|---|---|---|
| CoSorb method | Suitable for low concentrations of CO; high selectivity. | Complex equipment, high cost; adsorbent is easily saturated and needs to be regenerated. |
| pressure swing adsorption method | Recyclable, adjustable operating pressure. | High energy consumption; limited adsorbent lifetime. |
| catalytic oxidation method | Highly efficient CO removal at low temperatures; fast reaction rates. | Catalysts are susceptible to humidity and sulfur toxicity; preparation process needs to be optimized. |
| porous media adsorption method | Simple equipment, wide range of applications. | Decreased adsorption effect at low temperatures, easily affected by humidity. |
| Catalyst | CuMnOx-Ce-70 | CuMnOx-Ce-70-PVP | CuMnOx-Ce-70-PVA |
|---|---|---|---|
| Water contact angle | 46.04° | 52.03° | 53.69° |
| Type | Chemical Formula | Purity | Manufacturer |
|---|---|---|---|
| Polyvinylpyrrolidone | [C6H9NO]n | 99.80% | Aladdin Reagent Company (Shanghai, China) |
| 50% manganese nitrate solution | Mn(NO3)2 | 50 wt.% | |
| stannic chloride | SnCl4·5H2O | 99.70% | |
| Cerium nitrate | Ce(NO3)3·6H2O | 99.50% | |
| Iron nitrate | Fe(NO3)3·9H2O | 99.70% | |
| Polyvinyl alcohol | [C2H4O]n | 99.80% | |
| Copper nitrate | Cu(NO3)2·3H2O | 99% | |
| Anhydrous sodium carbonate | Na2CO3 | 99.70% |
| Instrument and Equipment | Manufacturer |
|---|---|
| FZG-1 vacuum drying oven | Guangdong Huanrui Testing Equipment Co., Ltd. (Dongguan, China) |
| XPR504SDR/AC electronic balance | Mettler Toledo Technology (China) Co., Ltd. (Shanghai, China) |
| SX2-5-12NP Box type Resistance Furnace | Yiheng Scientific Equipment Co., Ltd. (Yongkang, China) |
| SHZ-D (III) circulating water vacuum pump | Gongyi Yuhua Equipment Co., Ltd. (Gongyi, China) |
| PHS-3C digital acidity meter | Zhengzhou Baojing Electronic Technology Co., Ltd. (Zhengzhou, China) |
| MS-H280-Pro digital heating magnetic stirrer | Shengke Instrument Co., Ltd. (Xi’an, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Guo, P.; Liu, J. Development and Characterization of Metal-Doped Modified CO Oxidation Catalyst for Coalbed Methane with Strong Adsorption and Water Resistance. Catalysts 2025, 15, 299. https://doi.org/10.3390/catal15040299
Fan Y, Guo P, Liu J. Development and Characterization of Metal-Doped Modified CO Oxidation Catalyst for Coalbed Methane with Strong Adsorption and Water Resistance. Catalysts. 2025; 15(4):299. https://doi.org/10.3390/catal15040299
Chicago/Turabian StyleFan, Yanyang, Ping Guo, and Jun Liu. 2025. "Development and Characterization of Metal-Doped Modified CO Oxidation Catalyst for Coalbed Methane with Strong Adsorption and Water Resistance" Catalysts 15, no. 4: 299. https://doi.org/10.3390/catal15040299
APA StyleFan, Y., Guo, P., & Liu, J. (2025). Development and Characterization of Metal-Doped Modified CO Oxidation Catalyst for Coalbed Methane with Strong Adsorption and Water Resistance. Catalysts, 15(4), 299. https://doi.org/10.3390/catal15040299









