Fe3O4@SiO2@WO3 Multifunctional Composite Photocatalyst with Magnetic Core and Dual Shells
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Characterization
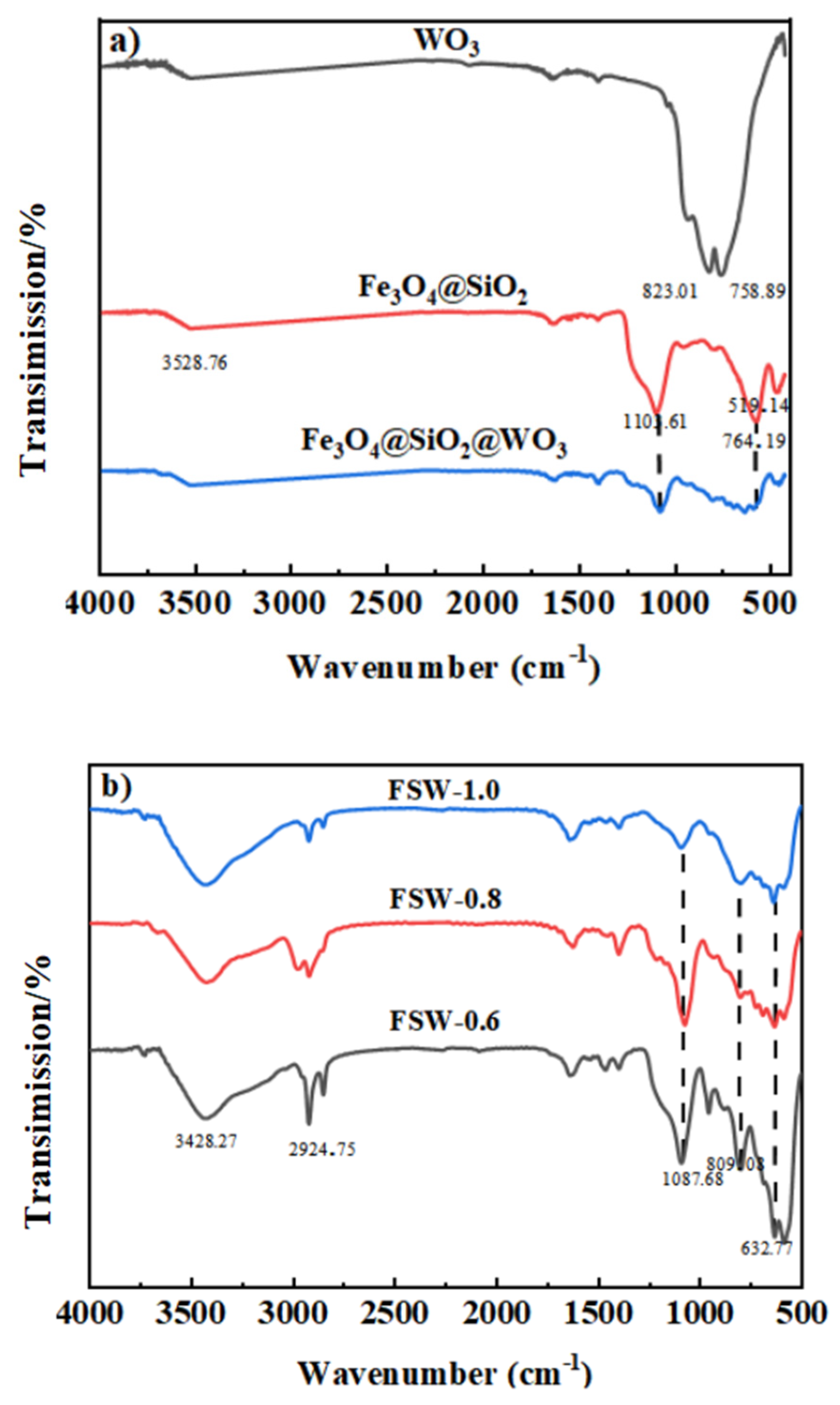
2.2. FT-IR Spectroscopy
2.3. Raman Spectroscopy
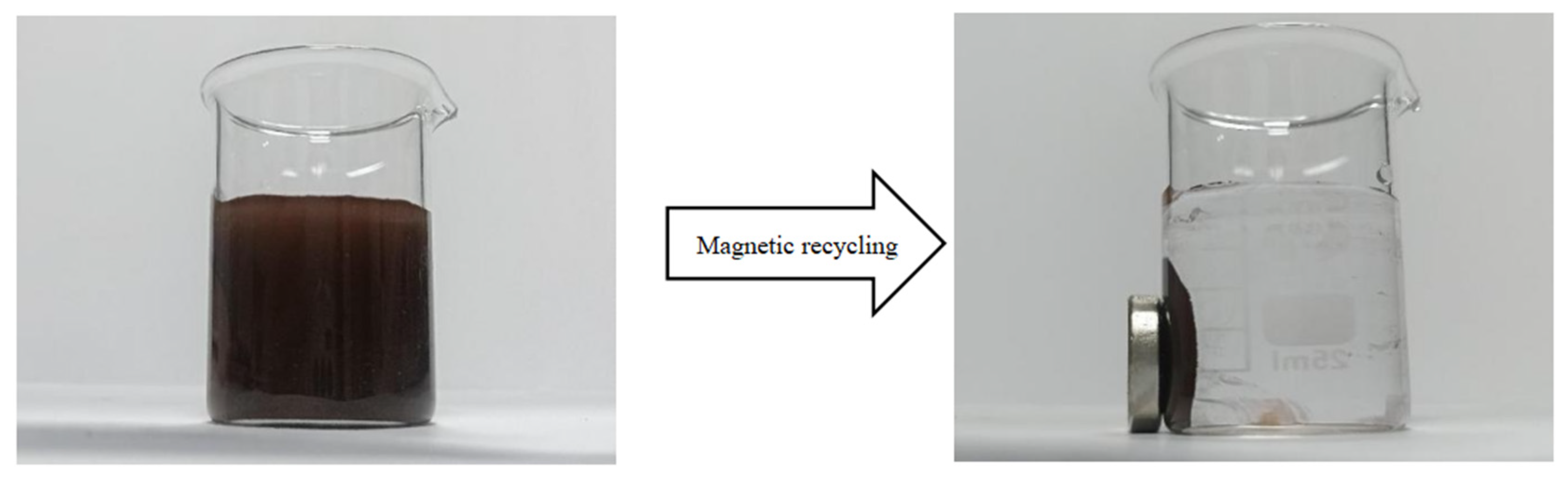
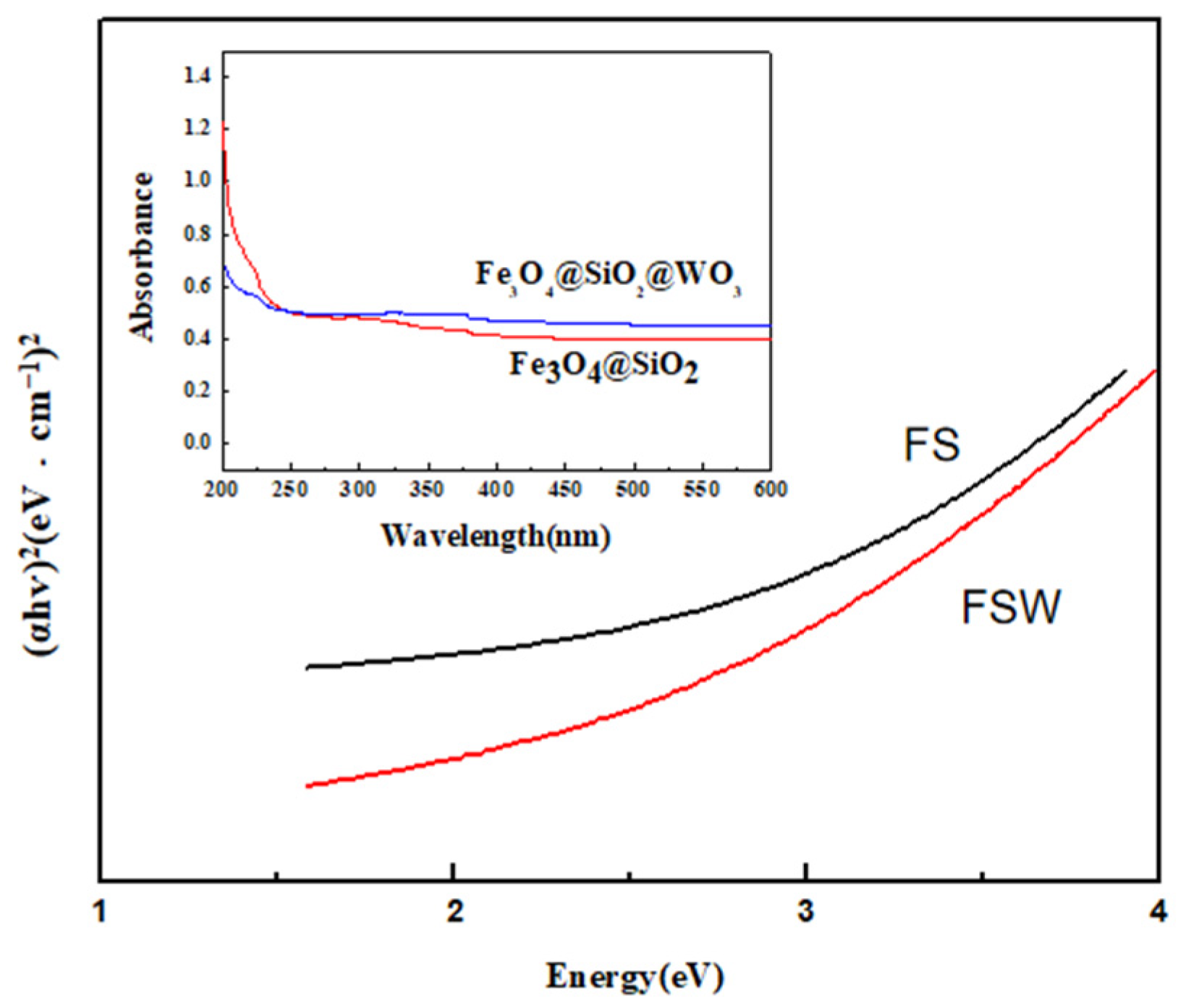
2.4. Magnetic Response Test
2.5. Catalytic Activity Analysis
2.5.1. Catalyst Degradation
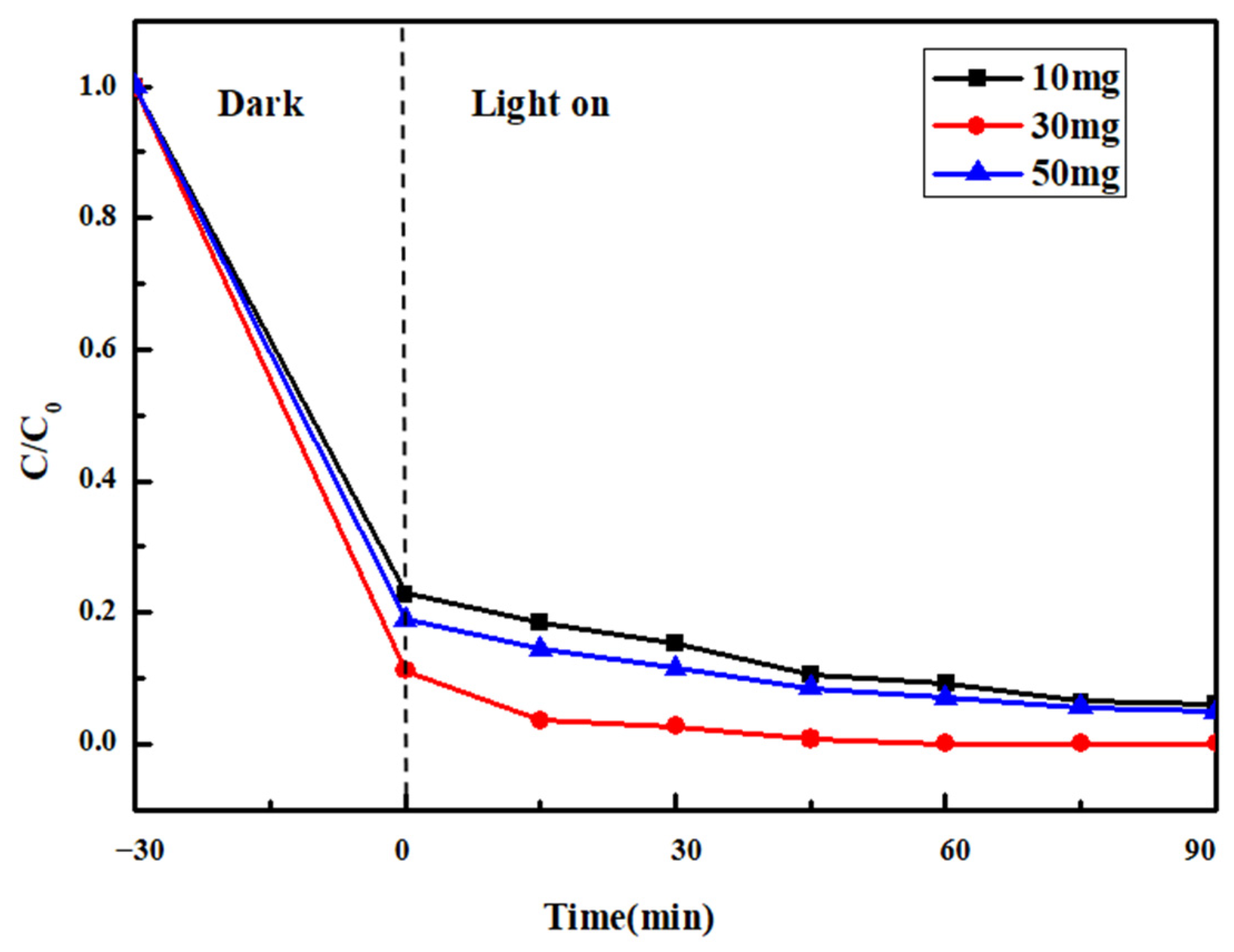
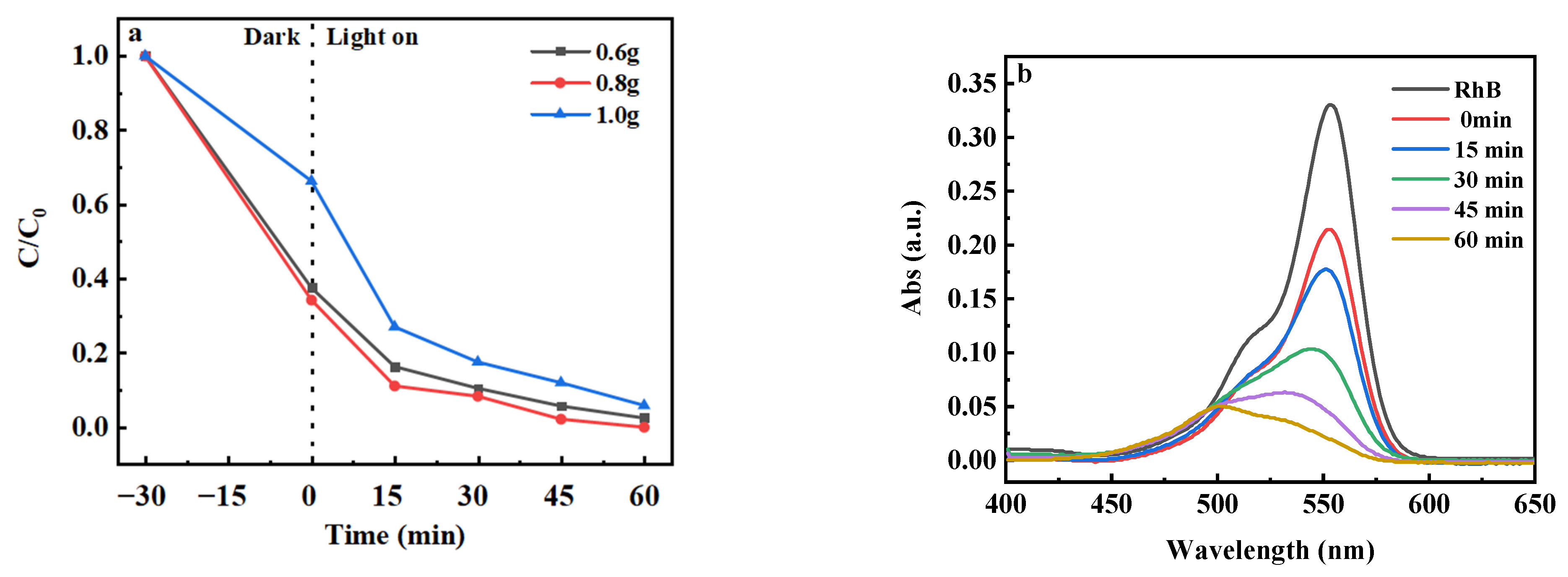
2.5.2. Comparison of Catalyst Amount
2.5.3. Influence of Catalyst Doping Ratio
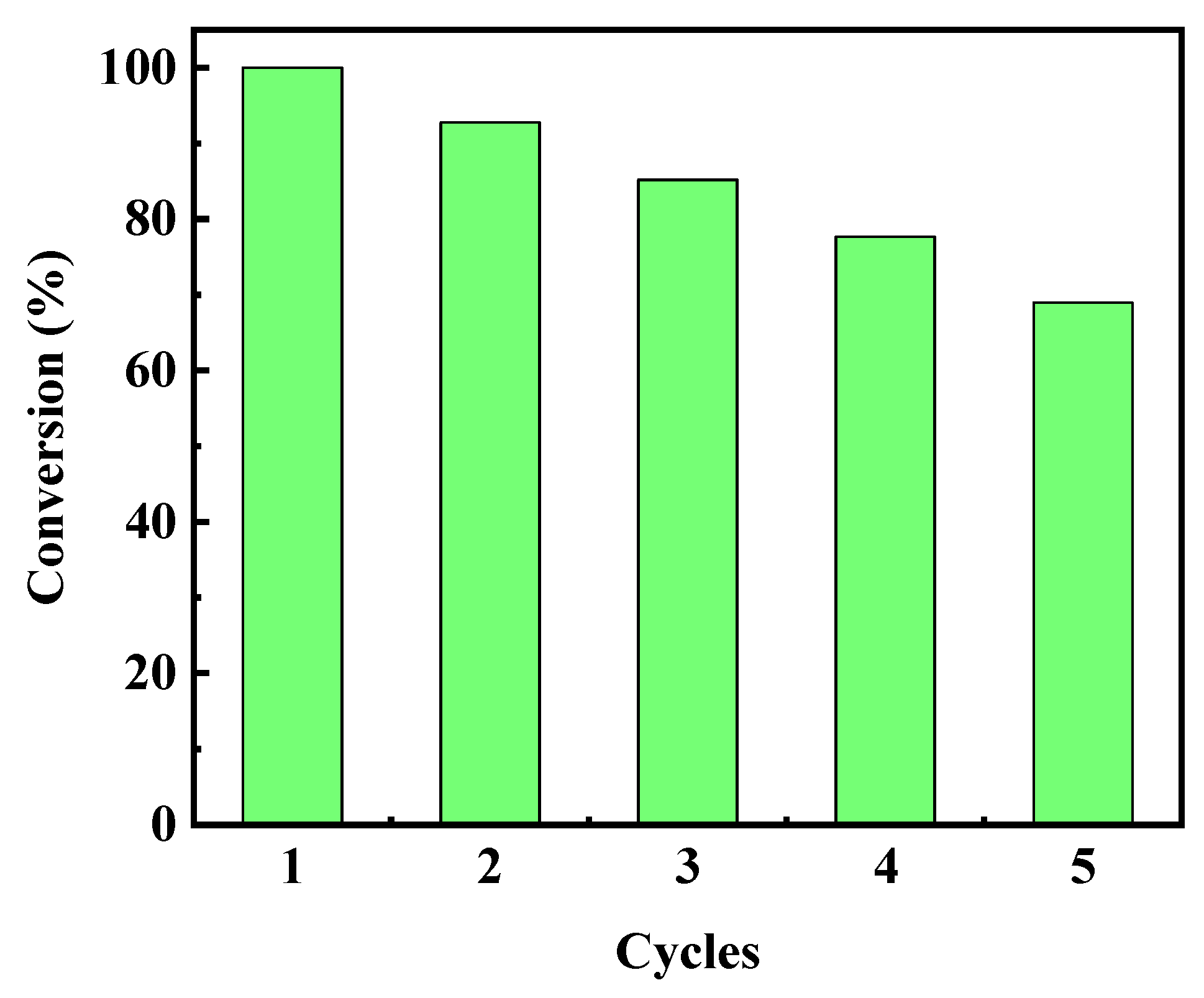
2.5.4. Catalyst Stability Test
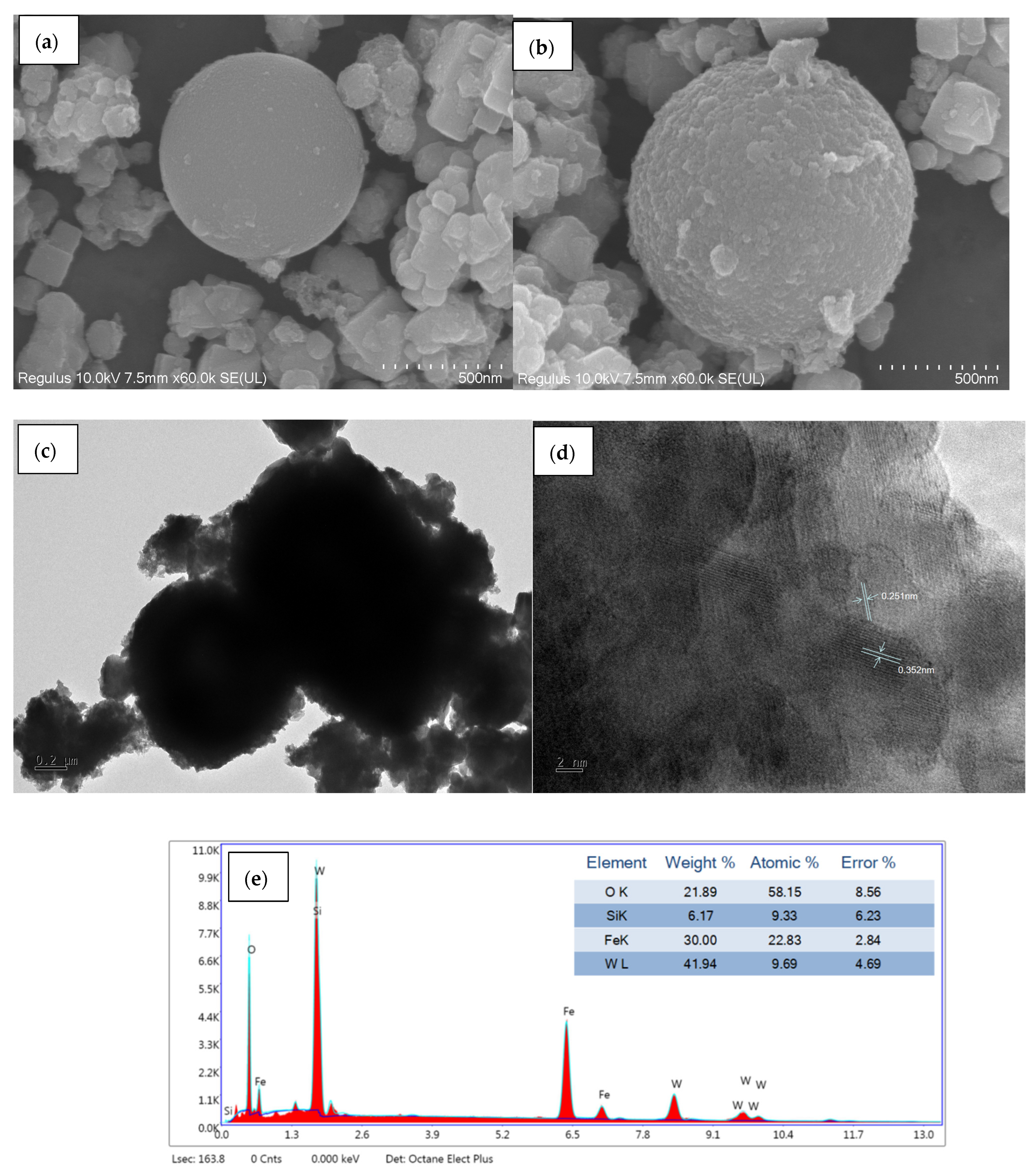
2.6. Catalyst Morphology
2.7. Catalyst Mechanism Model
3. Materials and Methods
3.1. Preparation of Fe3O4
3.2. Synthesis of Fe3O4@SiO2
3.3. Synthesis of Fe3O4@SiO2@WO3
3.4. Characterization of Catalysts
3.5. Evaluation of Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, H.; Wang, C.; Zhang, Y.; Cheng, X.; Wang, J.; Yang, B.; Du, E. ROS reevaluation for degradation of 4-chloro-3,5-dimethylphenol (PCMX) by UV and UV/persulfate processes in the water: Kinetics, mechanism, DFT studies and toxicity evolution. Chem. Eng. J. 2020, 390, 124610. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zhou, Y. Efficiently activate peroxymonosulfate by Fe3O4@MoS2 for rapid degradation of sulfonamides. Chem. Eng. J. 2021, 422, 130126. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765, 142794. [Google Scholar] [CrossRef]
- Sadeghi, M.; Liu, W.; Zhang, T.; Stavropoulos, P.; Levy, B. Role of photoinduced charge carrier separation distance in heterogeneous photocatalysis: Oxidative degradation of CH3OH vapor in contact with Pt/TiO2 and cofumed TiO2−Fe2O3. J. Phys. Chem. 1996, 100, 19466–19474. [Google Scholar] [CrossRef]
- Faust, B.C.; Hoffmann, M.R.; Bahnemann, D.W. Photocatalytic oxidation of sulfur dioxide in aqueous suspensions of. alpha.-iron oxide (Fe2O3). J. Phys. Chem. 1989, 93, 6371–6381. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Maruyama, R.; Kumagai, H.; Maeda, K.; Notes, T.T.A. Hybrid photocatalyst constructed using polymerized metal complexes and semiconductor powders for photocatalytic carbon dioxide reduction. Chem. Lett. 2024, 5, upae085. [Google Scholar] [CrossRef]
- Li, P.; Li, Q.; Wang, G.; Lu, Y.; Duan, L.; Bai, J.; Sarina, S.; Liu, J. Metal-free polymeric and molecular disorder/order semiconductor heterojunctions for the visible-light photocatalytic Minisci reaction. J. Mater. Chem. A Mater. Energy Sustain. 2024, 12, 13760–13769. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Chen, W.H.; You, F. Sustainable energy management and control for Decarbonization of complex multi-zone buildings with renewable solar and geothermal energies using machine learning, robust optimization, and predictive control. Appl. Energy 2024, 372, 123802. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, L.; Liu, Y.; Zhang, Z.; Yao, S.; Huang, X.; Yin, Y.; Sun, K.; Yang, T.; Zou, B.; et al. An innovative eco-friendly organic semiconductor-biochar photocatalytic adsorbent for efficient and stable removal mechanism of oxytetracycline hydrochloride under light illumination. Sep. Purif. Technol. 2025, 357, 130054. [Google Scholar] [CrossRef]
- Khoshnam, M.; Jazi, H.S. Synthesis and characterization of magnetic-photocatalytic Fe3O4/SiO2/α-Fe2O3 nano core-shell. J. Surfin. 2021, 7, 101322. [Google Scholar] [CrossRef]
- Shagolsem, B.S.; Singh, N.M. An environmentally friendly photocatalyst for the breakdown of RhB, MB, 2,4-DCP, and TCAA: Reusable Fe3O4/Ag doped g-C3N4. J. Mol. Liq. 2024, 413, 125913. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Zhong, J.; Xiong, C.; Zhu, H.; Ding, Y.; Jin, Z.; Guo, W.; Chen, J.; Li, J.; et al. Highly Efficient Photodegradation of RHB-MO-MB Mixture Dye Wastewater by WO3/Fe3O4/Diatomite under Different Condition. ECS J. Solid State Sci. Technol. 2023, 12, 093009. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide–properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Lee, J.; Son, N.; Shin, H.; Kim, J.; Park, N.-K.; Kim, U.S.; Kang, M. An eco-efficient dual-function technology: Magnetically recoverable rGO-WO3/Fe3O4 ternary heterojunction catalytic system simultaneously performing malachite green photolysis and Cr(VI) reduction. J. Clean. Prod. 2024, 459, 142533. [Google Scholar] [CrossRef]
- Chinchillas-Chinchillas, M.J.; Garrafa-Gálvez, H.E.; Castro-Beltran, A. Turnera diffusa and its novel application in the synthesis of ZnO semiconductor nanoparticles with high photocatalytic power. Appl. Phys. A Mater. Sci. Process. 2024, 130, 710. [Google Scholar] [CrossRef]
- Yadav, P.; Vignesh, D.; Patnaik, M.; Priyadarshani, M.; Rout, E. Investigation of structural and optoelectronic integrity of Sm3+ doped CaWO4 for LED applications. Ceram. Int. 2024, 50, 35203–35213. [Google Scholar] [CrossRef]
- Gandon, A.; Nguyen, C.; Kaliaguine, S. Synthesis of magnetic core@dual shell Fe3O4@SiO2@WO3 nanocatalysts for olefin double bond oxidative cleavage. Can. J. Chem. Eng. 2021, 9, 2003–2013. [Google Scholar] [CrossRef]
- Maham, R.; Ijaz, A.B.; Nimra, N. Synthesis and photocatalytic degradation of rhodamine B using ternary zeolite/WO3/Fe3O4 composite. Nanotechnology 2021, 32, 345705. [Google Scholar]
- Morel, A.L.; Sergei, I. Sonochemical approach to the synthesis of Fe3O4@SiO2 core-shell nanoparticles with tunable properties. ACS Nano. 2008, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]


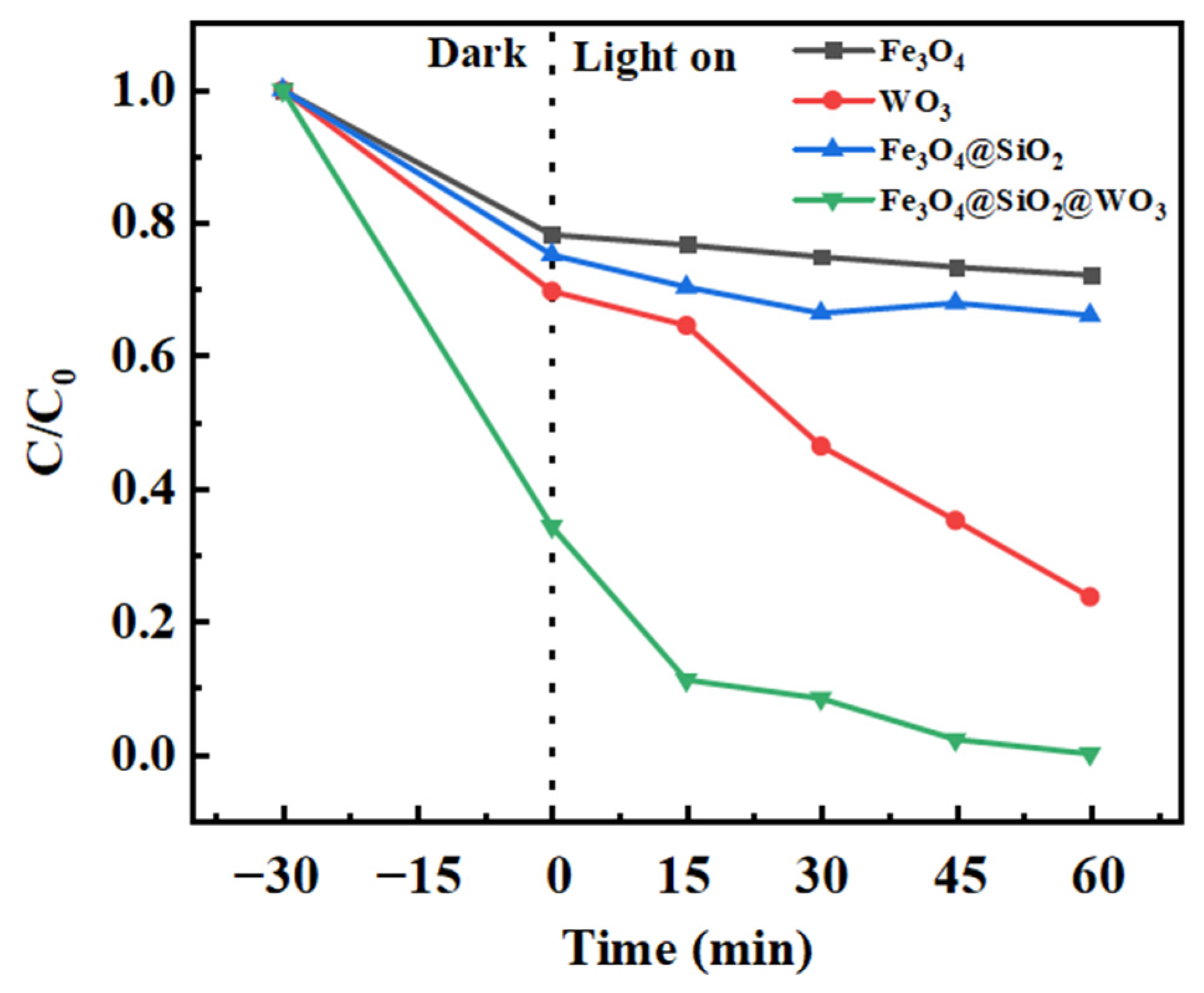

| Catalyst | Degradation Percentage (%) |
|---|---|
| Fe3O4 | 22.12 |
| Fe3O4@SiO2 | 33.65 |
| WO3 | 66.06 |
| Fe3O4@SiO2@WO3 | 100.00 |
| Catalyst Amount (mg) | Degradation Percentage (%) |
|---|---|
| 10 | 81.60 |
| 30 | 87.17 |
| 50 | 84.46 |
| Doping Amount (g) | Degradation Percentage (%) |
|---|---|
| 0.6 | 97.54 |
| 0.8 | 99.80 |
| 1.0 | 94.15 |
| Catalyst | Degradation Percentage (%) | References |
|---|---|---|
| WO3/Fe3O4/Diatomite | 78% | [15] |
| Zeolite/WO3/Fe3O4 | 97% | [21] |
| Fe3O4@SiO2@WO3 | 99.80% | This study |
| Number of Cycles | Degradation Percentage (%) |
|---|---|
| 1 | 100.00 |
| 2 | 92.74 |
| 3 | 85.20 |
| 4 | 77.65 |
| 5 | 68.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chu, J. Fe3O4@SiO2@WO3 Multifunctional Composite Photocatalyst with Magnetic Core and Dual Shells. Catalysts 2025, 15, 314. https://doi.org/10.3390/catal15040314
Wang X, Chu J. Fe3O4@SiO2@WO3 Multifunctional Composite Photocatalyst with Magnetic Core and Dual Shells. Catalysts. 2025; 15(4):314. https://doi.org/10.3390/catal15040314
Chicago/Turabian StyleWang, Xin, and Jia Chu. 2025. "Fe3O4@SiO2@WO3 Multifunctional Composite Photocatalyst with Magnetic Core and Dual Shells" Catalysts 15, no. 4: 314. https://doi.org/10.3390/catal15040314
APA StyleWang, X., & Chu, J. (2025). Fe3O4@SiO2@WO3 Multifunctional Composite Photocatalyst with Magnetic Core and Dual Shells. Catalysts, 15(4), 314. https://doi.org/10.3390/catal15040314






