In Situ Reinforced g-C3N4/CoO/CoP Ternary Composite for Enhanced Photocatalytic H2 Production
Abstract
:1. Introduction
2. Results and Discussion
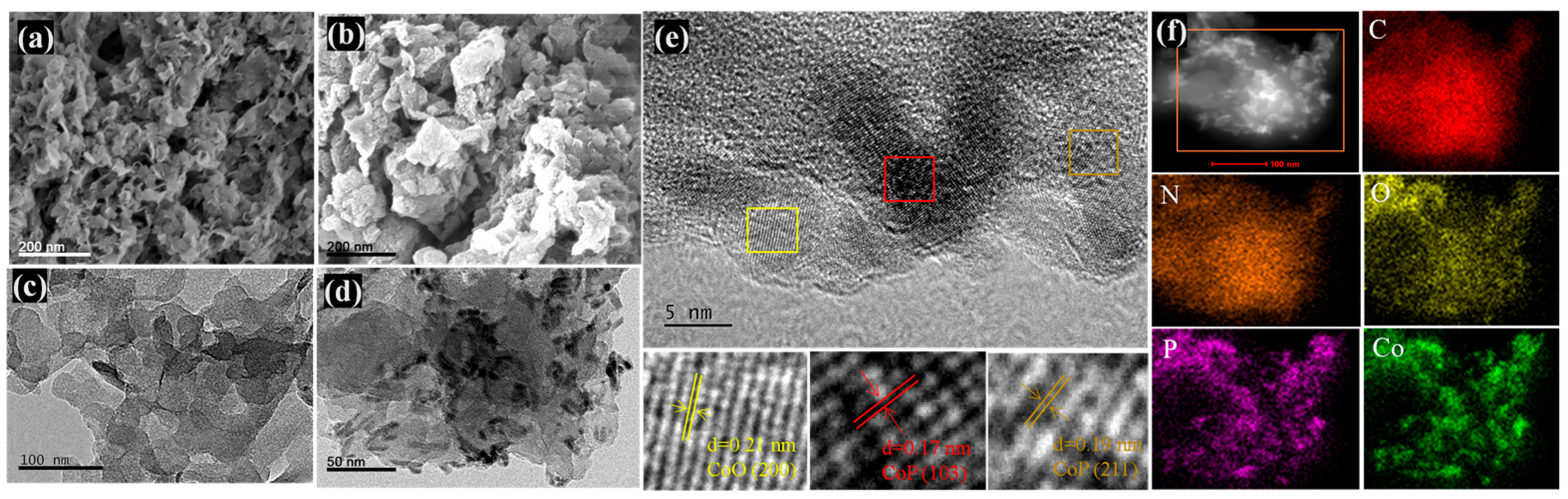
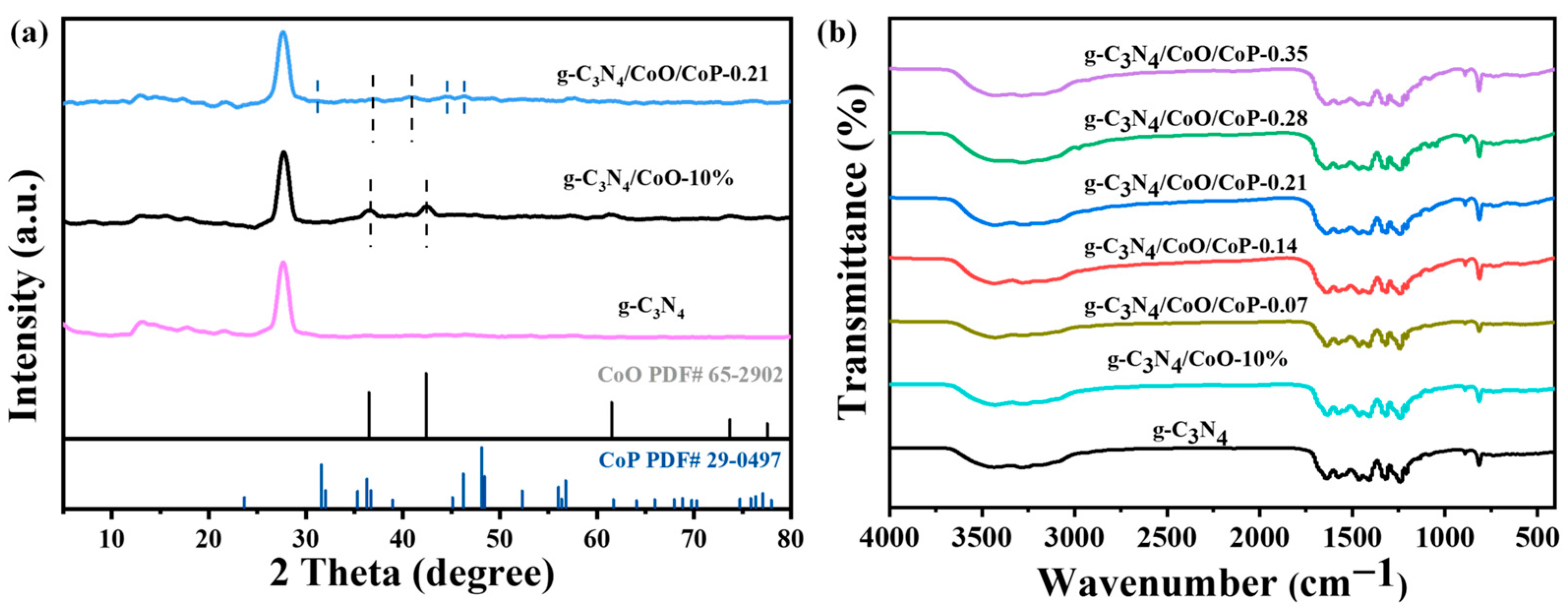
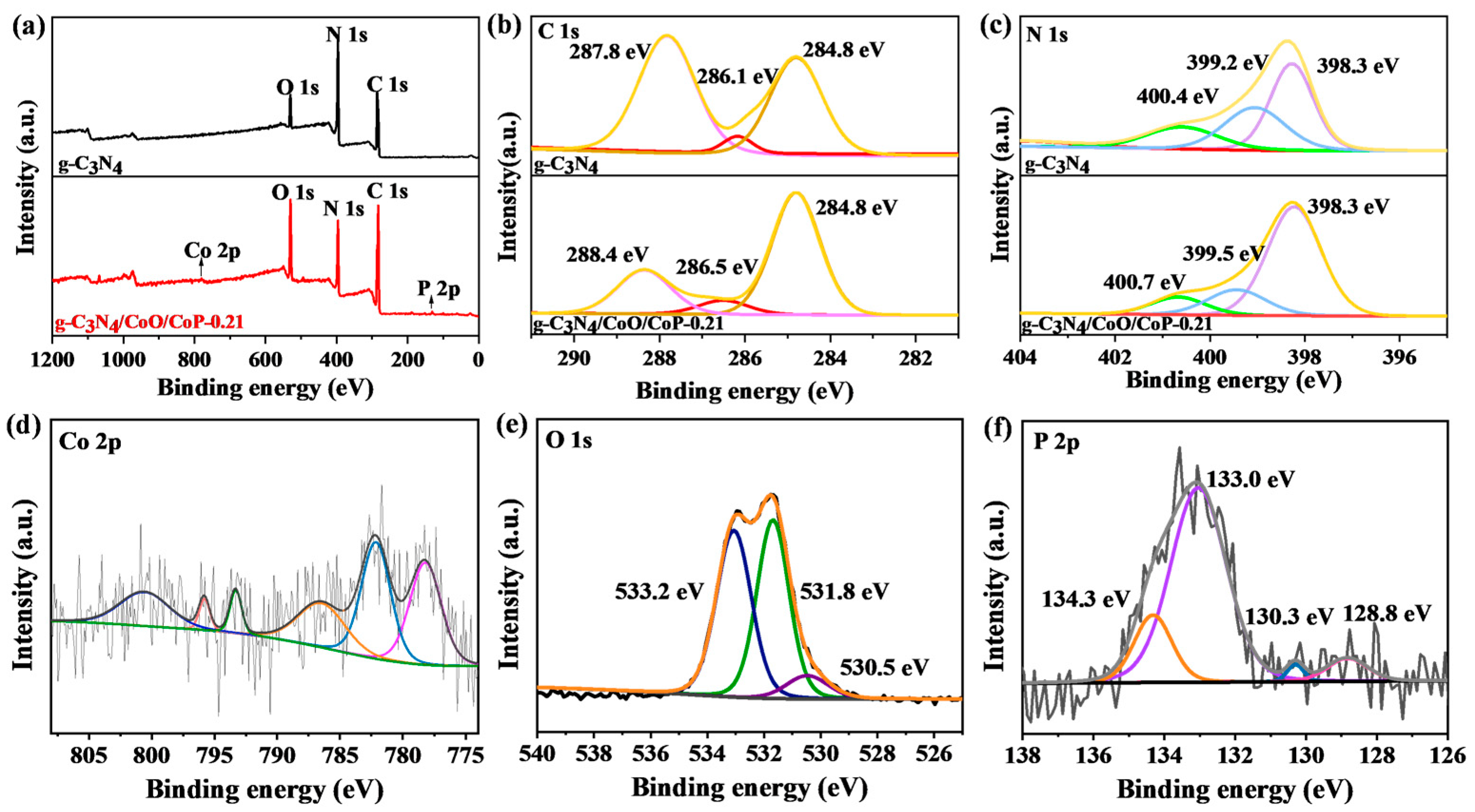
2.1. Morphology and Structure of g-C3N4/CoO/CoP
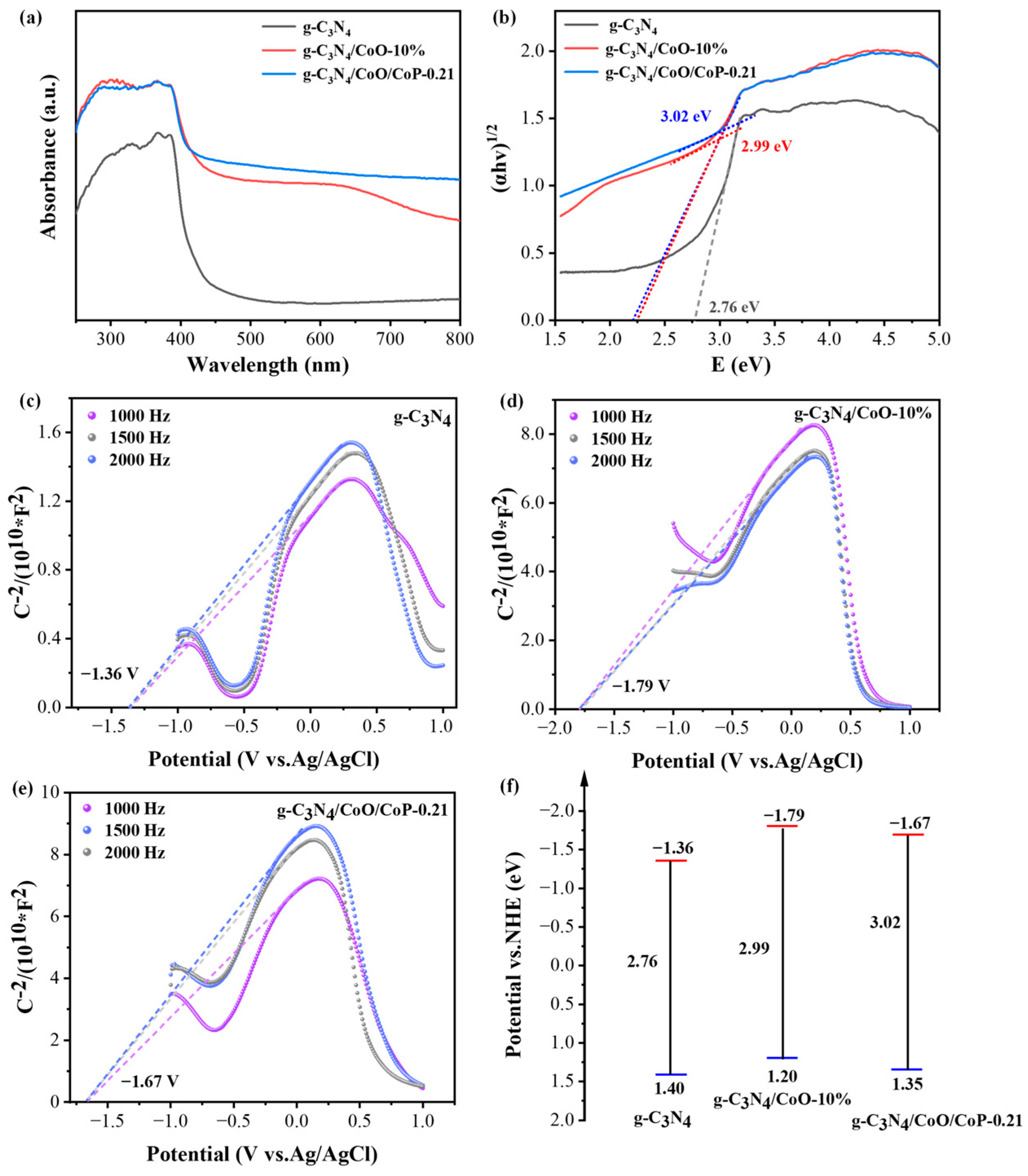
2.2. Narrowed Band Gap and Extended Light Absorption of g-C3N4/CoO/CoP
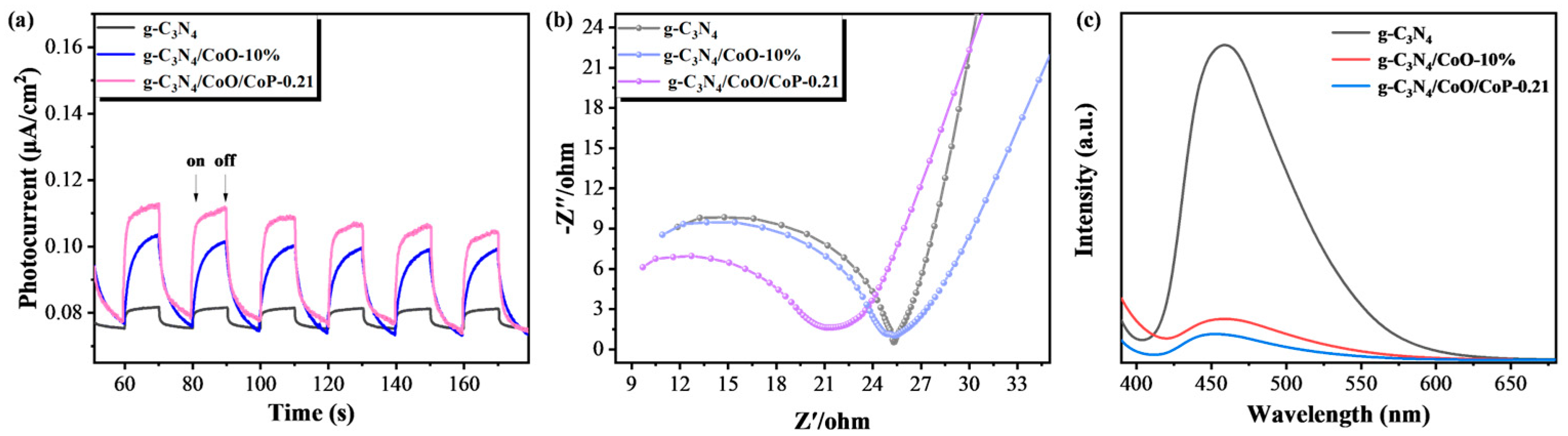
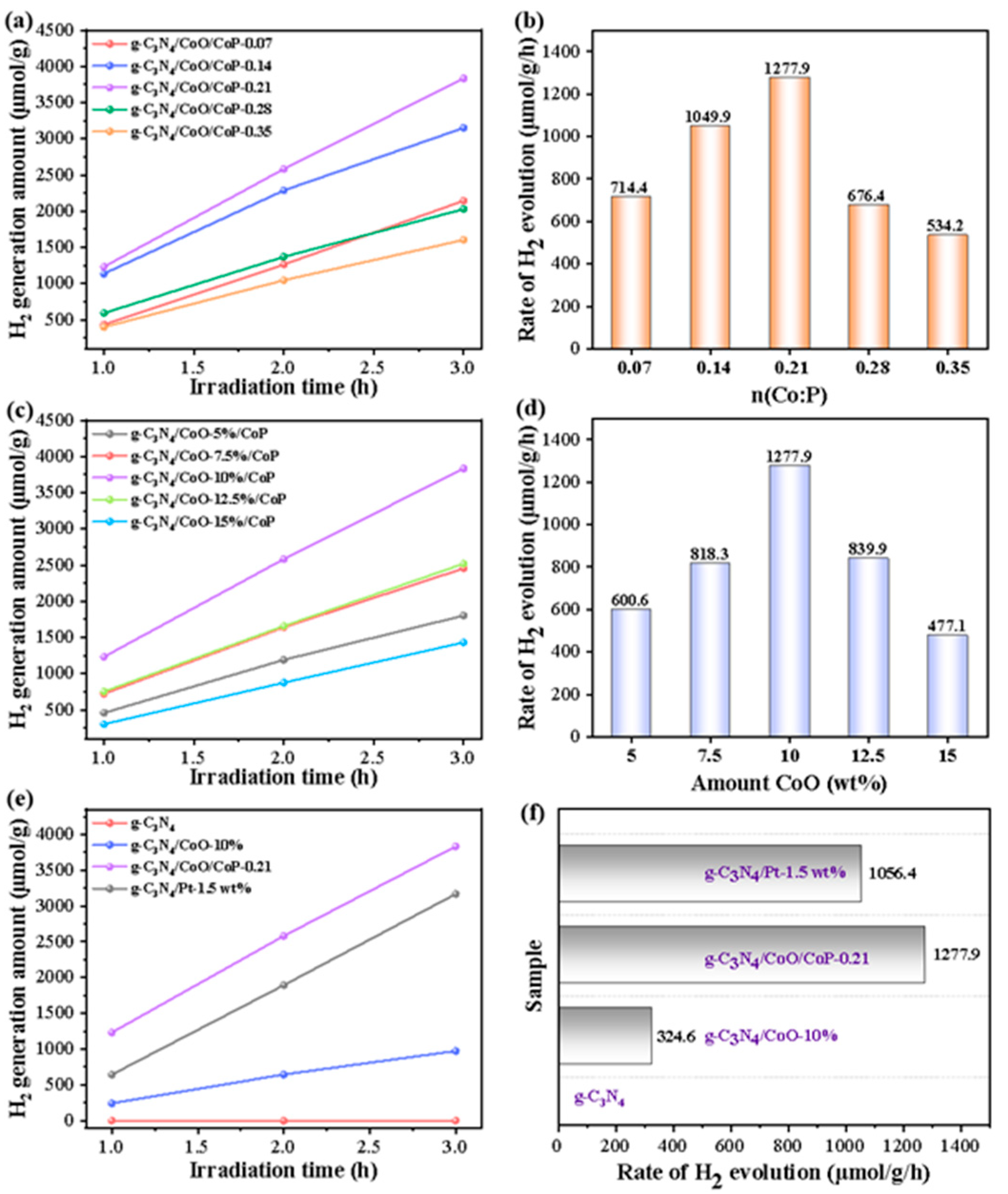
2.3. Enhancement and Mechanism of Photocatalytic Hydrogen Activity
3. Experimental
3.1. Material Synthesis
3.1.1. Synthesis of g-C3N4
3.1.2. Synthesis of g-C3N4/CoO
3.1.3. Synthesis of g-C3N4/CoO/CoP
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ma, Y.; Hu, X.; Liu, E.; Fan, J. Enhanced photocatalytic H2 production over dual-cocatalyst-modified g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 434–445. [Google Scholar]
- Jing, L.; Xu, Y.; Xie, M.; Li, Z.; Wu, C.; Zhao, H.; Zhong, N.; Wang, J.; Wang, H.; Yan, Y.; et al. Cyano-Rich g-C3N4 in Photochemistry: Design, Applications, and Prospects. Small 2024, 20, 1613–6810. [Google Scholar]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [PubMed]
- Song, Y.; Li, Z.; Li, S.; Yang, C.; Huang, L.; Zhang, X.; Wang, Q.; Zhang, H. Enhancing the photocatalytic efficiency of sulfamethoxazole by regulating the band gap structure of g-C3N4 through phosphorus element doping. J. Water Process Eng. 2024, 58, 2214–7144. [Google Scholar]
- Jiang, R.; Lu, G.; Zhou, R.; Yang, H.; Yan, Z.; Wu, D.; Liu, J.; Nkoom, M. Switching g-C3N4 morphology from double-walled to single-walled microtubes induced high photocatalytic H2-production performance. J. Alloys Compd. 2020, 820, 0925–8388. [Google Scholar]
- Wang, Y.; Zhang, X.; Liu, Y.; Zhao, Y.; Song, Y.; Yang, P. Crystallinity and phase controlling of g-C3N4 /CdS hetrostructures towards high efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2019, 44, 30151–30159. [Google Scholar]
- Ong, W.; Tian, L.; Ng, Y.; Yong, S.; Chai, S. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar]
- Chen, W.; Fang, J.; Zhang, Y.; Chen, G.; Zhao, S.; Zhang, C.; Xu, R.; Bao, J.; Zhou, Y.; Xiang, X. CdS nanosphere-decorated hollow polyhedral ZCO derived from a metal-organic framework (MOF) for effective photocatalytic water evolution. Nanoscale 2018, 10, 4463–4474. [Google Scholar]
- Bala, S.; Mondal, I.; Goswami, A.; Pal, U.; Mondal, R. Co-MOF as a sacrificial template: Manifesting a new Co3O4/TiO2 system with a p-n heterojunction for photocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 20288–20296. [Google Scholar]
- Liao, L.; Zhang, Q.; Su, Z.; Zhao, Z.; Wang, Y.; Li, Y.; Lu, X.; Wei, D.; Feng, G.; Yu, Q.; et al. Efficient solar water-splitting using a nanocrystalline CoO photocatalyst. Nat. Nanotechnol. 2014, 9, 69–73. [Google Scholar]
- Zhang, J.; Yu, Z.; Gao, Z.; Ge, H.; Zhao, S.; Chen, C.; Chen, S.; Tong, X.; Wang, M.; Zheng, Z.; et al. Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2017, 56, 816–820. [Google Scholar]
- Peng, S.; Cao, Y.; Zhou, F.; Xu, Z.; Li, Y. CoP decorated with Co3O4 as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Appl. Surf. Sci. 2019, 487, 315–321. [Google Scholar]
- Zhang, F.; Zhang, J.; Li, J.; Jin, X.; Li, Y.; Wu, M.; Kang, X.; Hu, T.; Wang, X.; Ren, W.; et al. Modulating charge transfer dynamics for g-C3N4 through a dimension and interface engineered transition metal phosphide co-catalyst for efficient visible-light photocatalytic hydrogen generation. J. Mater. Chem. A 2019, 7, 6939–6945. [Google Scholar]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Anchoring CoP nanoparticles on the octahedral CoO by self-phosphating for enhanced photocatalytic overall water splitting activity under visible light. Chin. J. Chem. Eng. 2021, 40, 114–123. [Google Scholar]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Lee, S. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171. [Google Scholar]
- Peng, C.; Chen, B.; Qin, Y.; Yang, S.; Li, C.; Zuo, Y.; Liu, S.; Yang, J. Facile ultrasonic synthesis of CoO quantum dot/graphene nanosheet composites with high lithium storage capacity. ACS Nano 2012, 6, 1074–1081. [Google Scholar]
- Cao, S.; Chen, Y.; Wang, C.; Lv, X.; Fu, W. Spectacular photocatalytic hydrogen evolution using metal-phosphide/CdS hybrid catalysts under sunlight irradiation. Chem. Commun. 2015, 51, 8708–8711. [Google Scholar]
- Sun, B.; Yu, H.; Yang, Y.; Li, H.; Zhai, C.; Qian, D.; Chen, M. New complete assignment of X-ray powder diffraction patterns in graphitic carbon nitride using discrete Fourier transform and direct experimental evidence. Phys. Chem. Chem. Phys. 2017, 19, 26072–26084. [Google Scholar]
- Zha, Q.; Yuan, F.; Qin, G.; Ni, Y. Cobalt-based MOF-on-MOF two-dimensional heterojunction nanostructures for enhanced oxygen evolution reaction electrocatalytic activity. Inorg. Chem. 2020, 59, 1295–1305. [Google Scholar]
- Shi, R.; Chen, G.; Ma, W.; Zhang, D.; Qiu, G.; Liu, X. Shape-controlled synthesis and characterization of cobalt oxides hollow spheres and octahedra. Dalton Trans. 2012, 41, 5981–5987. [Google Scholar]
- Chen, Z.; Chong, B.; Wells, N.; Yang, G.; Wang, L. Constructing a coplanar heterojunction through enhanced π-π conjugation in g-C3N4 for efficient solar-driven water splitting. Chin. Chem. Lett. 2022, 33, 2579–2584. [Google Scholar]
- Liu, Q.; Shen, J.; Yu, X.; Yang, X.; Liu, W.; Yang, J.; Tang, H.; Xu, H.; Li, H.; Li, Y.; et al. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl. Catal. B Environ. 2019, 248, 84–94. [Google Scholar]
- Lai, J.-Y.; Zhang, W.-D.; Yu, Y.-X. Building sp carbon-bridged g-C3N4-based electron donor-π-acceptor unit for efficient photocatalytic water splitting. Mol. Catal. 2021, 505, 111518. [Google Scholar]
- Yang, F.; Chen, Y.; Cheng, G.; Chen, S.; Luo, W. Ultrathin nitrogen-doped carbon coated with CoP for efficient hydrogen evolution. ACS Catal. 2017, 7, 3824–3831. [Google Scholar]
- Giribabu, G.; Murali, G.; Reddy, D.; Liu, C.; Vijayalakshmi, R. Structural, optical and magnetic properties of Co doped CdS nanoparticles. J. Alloy. Compd. 2023, 581, 363–368. [Google Scholar]
- Sun, Y.; Zhou, Y.; Zhu, C.; Hu, L.; Han, M.; Wang, A.; Hunag, H.; Liu, Y.; Kang, Z. A Pt-Co3O4-CD electrocatalyst with enhanced electrocatalytic performance and resistance to CO poisoning achieved by carbon dots and Co3O4 for direct methanol fuel cells. Nanoscale 2017, 9, 5467–5474. [Google Scholar]
- Luo, B.; Song, R.; Geng, J.; Liu, X.; Jing, D.; Wang, M.; Cheng, C. Towards the prominent cocatalytic effect of ultra-small CoP particles anchored on g-C3N4 nanosheets for visible light driven photocatalytic H2 production. Appl. Catal. B Environ. 2019, 256, 117819. [Google Scholar]
- Grosvenor, A.P.; Wik, S.D.; Cavell, R.G.; Mar, A. Examination of the bonding in binary transition-metal monophosphides MP (M=Cr, Mn, Fe, Co) by X-Ray photoelectron spectroscopy. Inorg. Chem. 2005, 44, 8988–8998. [Google Scholar]
- Tian, X.; Sun, Y.; He, J.; Wang, X.; Zhao, J.; Qiao, S.; Li, F. Surface P atom grafting of g-C3N4 for improved local spatial charge separation and enhanced photocatalytic H2 production. J. Mater. Chem. A 2019, 7, 7628–7635. [Google Scholar]
- Ren, P.; Zhang, T.; Jain, N.; Ching, H.; Jaworski, A.; Barcaro, G.; Monti, S.; Silvestre, J.; Celorrio, V.; Chouhan, L.; et al. An atomically dispersed Mn-photocatalyst for generating hydrogen peroxide from seawater via the water oxidation reaction (WOR). J. Am. Chem. Soc. 2023, 145, 16584–16596. [Google Scholar]
- Ai, G.; Li, H.; Liu, S.; Mo, R.; Zhong, J. Solar water splitting by TiO2/CdS/Co-Pi nanowire array photoanode enhanced with Co-Pi as hole transfer relay and CdS as light absorber. Adv. Funct. Mater. 2015, 25, 5706–5713. [Google Scholar]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar]
- Wang, H.; Tang, Q.; Wu, Z. Construction of few-layer Ti3C2 MXene and boron-doped g-C3N4 for enhanced photocatalytic CO2 reduction. ACS Sustain. Chem. Eng. 2021, 9, 8425–8434. [Google Scholar]
- Zhang, W.; Chen, R.; Yin, Z.; Wang, X.; Wang, Z.; Fan, F.; Ma, Y. Surface assistant charge separation in PEC Cu2S−Ni/Cu2O cathode. ACS Appl. Mater. Interfaces 2019, 11, 34000–34009. [Google Scholar]
- Li, Y.; Li, Y.; Yang, C.; Yu, C.; Gan, L. CoO/g-C3N4 p-n heterojunction catalyst in-situ loading CoP for enhanced photocatalytic H2 evolution. Appl. Surf. Sci. 2023, 639, 158180. [Google Scholar]




| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|
| g-C3N4 | 89.8 | 0.16 |
| g-C3N4/CoO-10% | 91.9 | 0.18 |
| g-C3N4/CoO/CoP-0.21 | 46.9 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wang, Z.; Yang, X.; Li, Z.; Li, Y. In Situ Reinforced g-C3N4/CoO/CoP Ternary Composite for Enhanced Photocatalytic H2 Production. Catalysts 2025, 15, 315. https://doi.org/10.3390/catal15040315
Han Y, Wang Z, Yang X, Li Z, Li Y. In Situ Reinforced g-C3N4/CoO/CoP Ternary Composite for Enhanced Photocatalytic H2 Production. Catalysts. 2025; 15(4):315. https://doi.org/10.3390/catal15040315
Chicago/Turabian StyleHan, Yanan, Zhaohui Wang, Xiuyuan Yang, Zhongjun Li, and Yike Li. 2025. "In Situ Reinforced g-C3N4/CoO/CoP Ternary Composite for Enhanced Photocatalytic H2 Production" Catalysts 15, no. 4: 315. https://doi.org/10.3390/catal15040315
APA StyleHan, Y., Wang, Z., Yang, X., Li, Z., & Li, Y. (2025). In Situ Reinforced g-C3N4/CoO/CoP Ternary Composite for Enhanced Photocatalytic H2 Production. Catalysts, 15(4), 315. https://doi.org/10.3390/catal15040315








