Photothermal Effect of Carbon-Doped Carbon Nitride Synergized with Localized Surface Plasmon Resonance of Ag Nanoparticles for Efficient CO2 Photoreduction
Abstract
1. Introduction
2. Results and Discussions
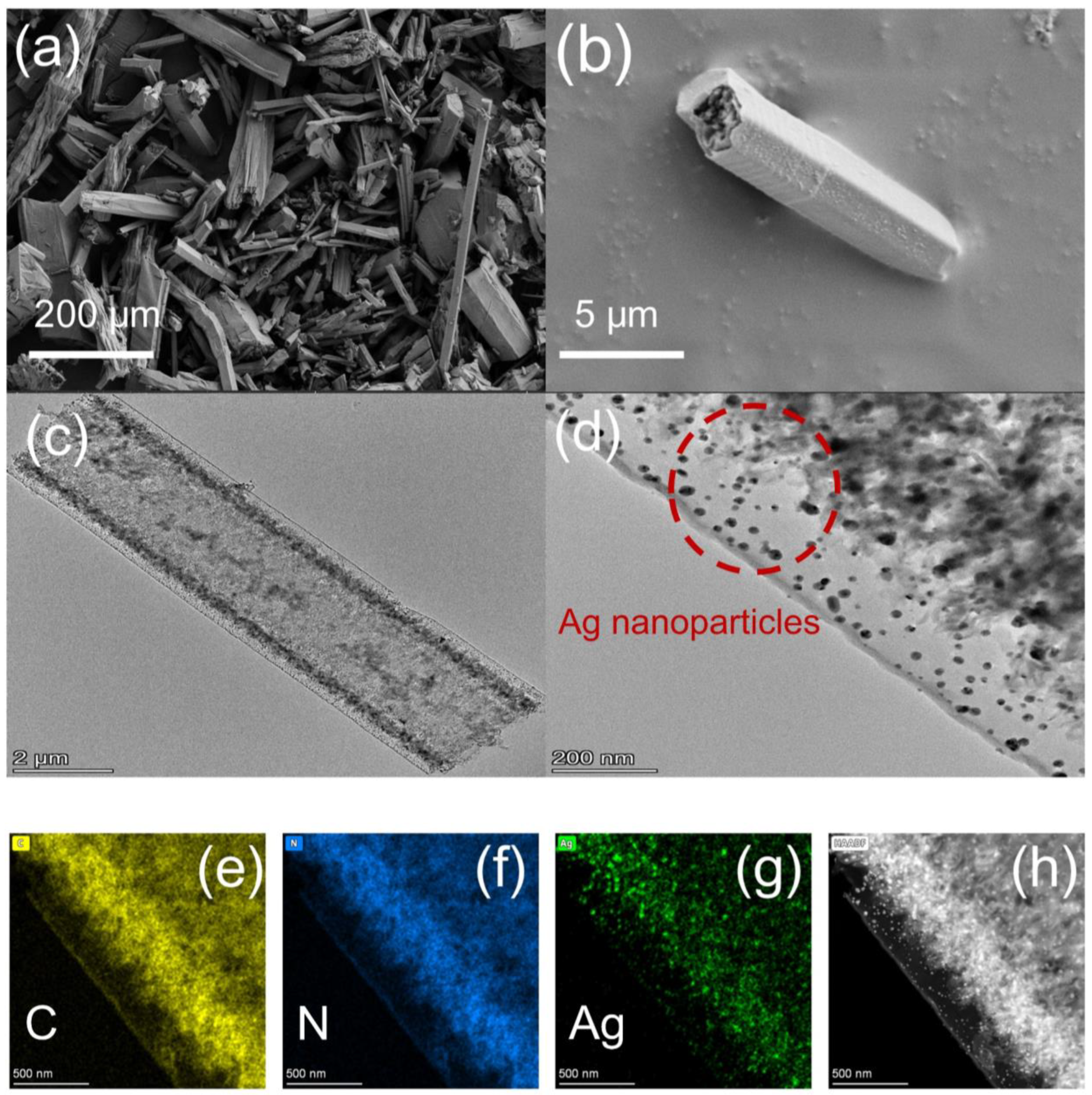
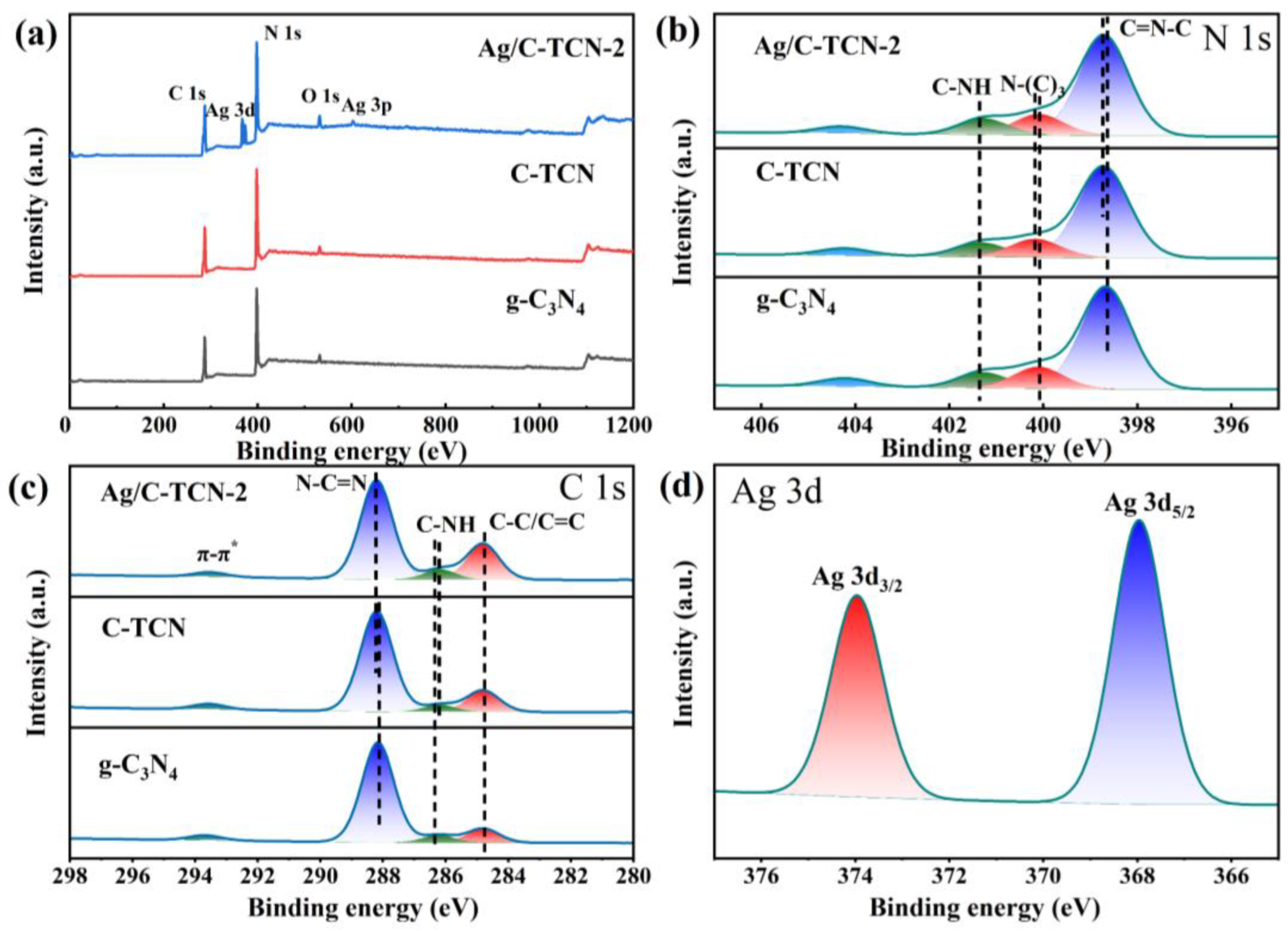
2.1. Structure Characterization
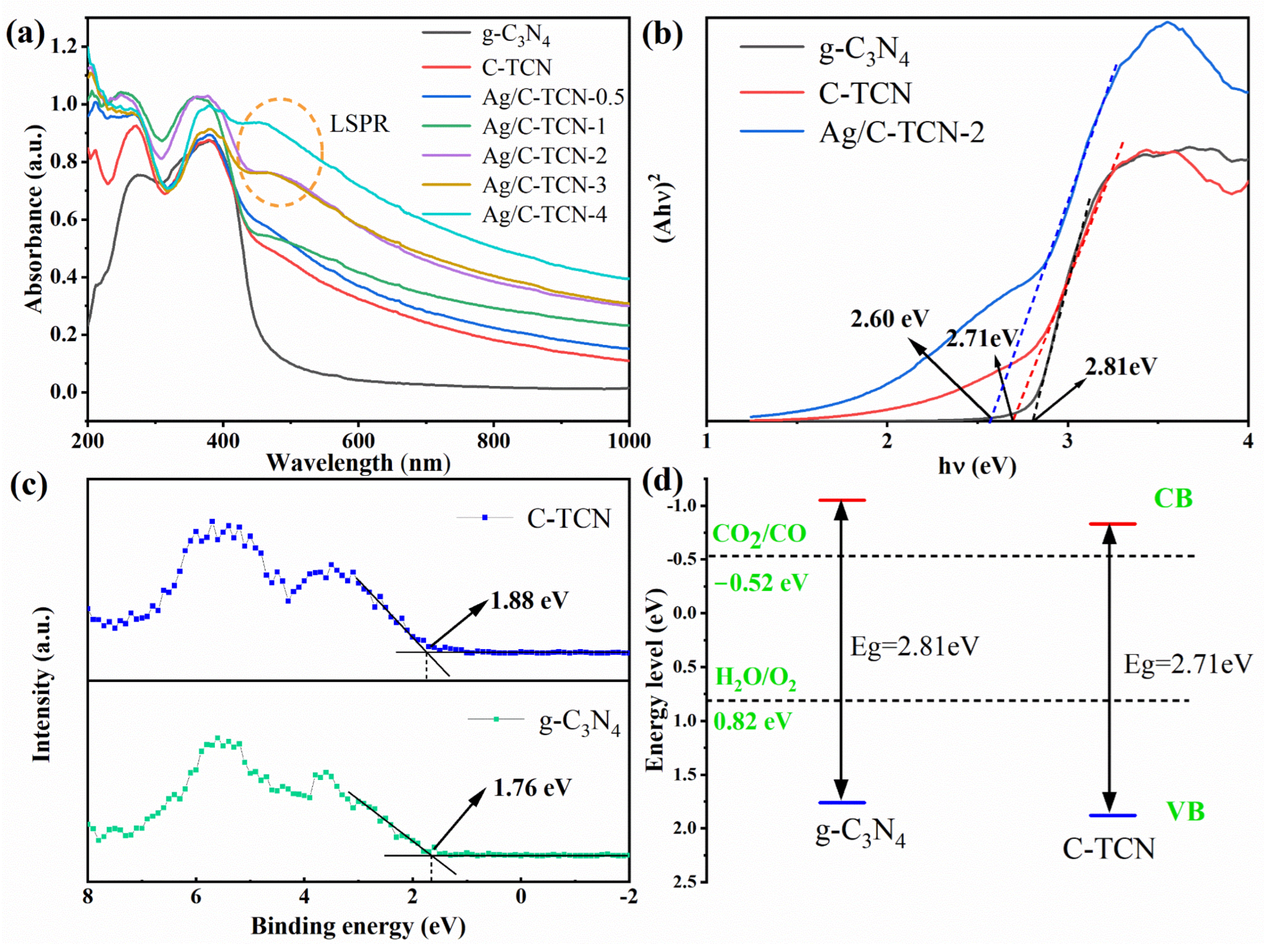
2.2. Optical Absorption and Band Structure
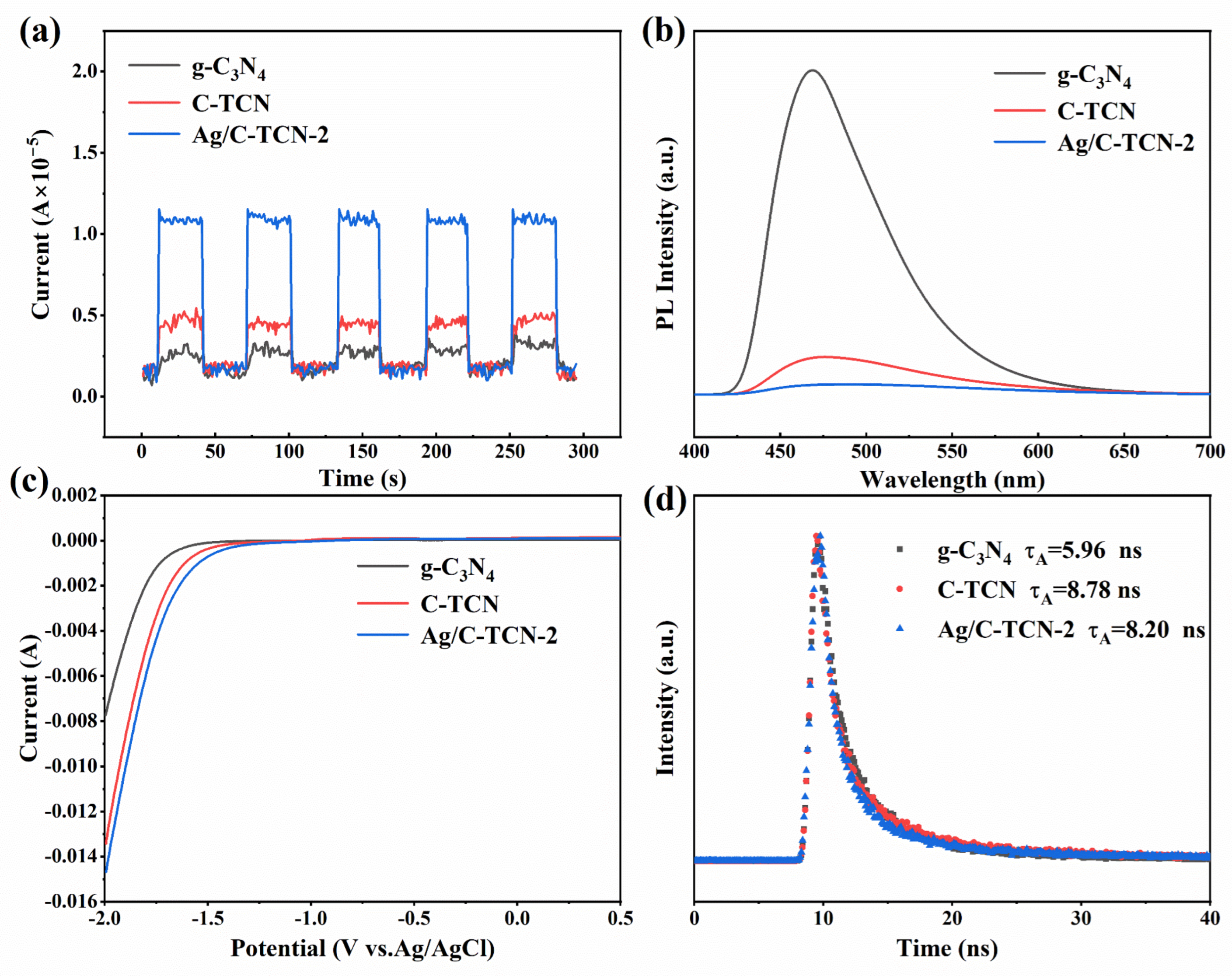
2.3. Photoelectrochemical Properties
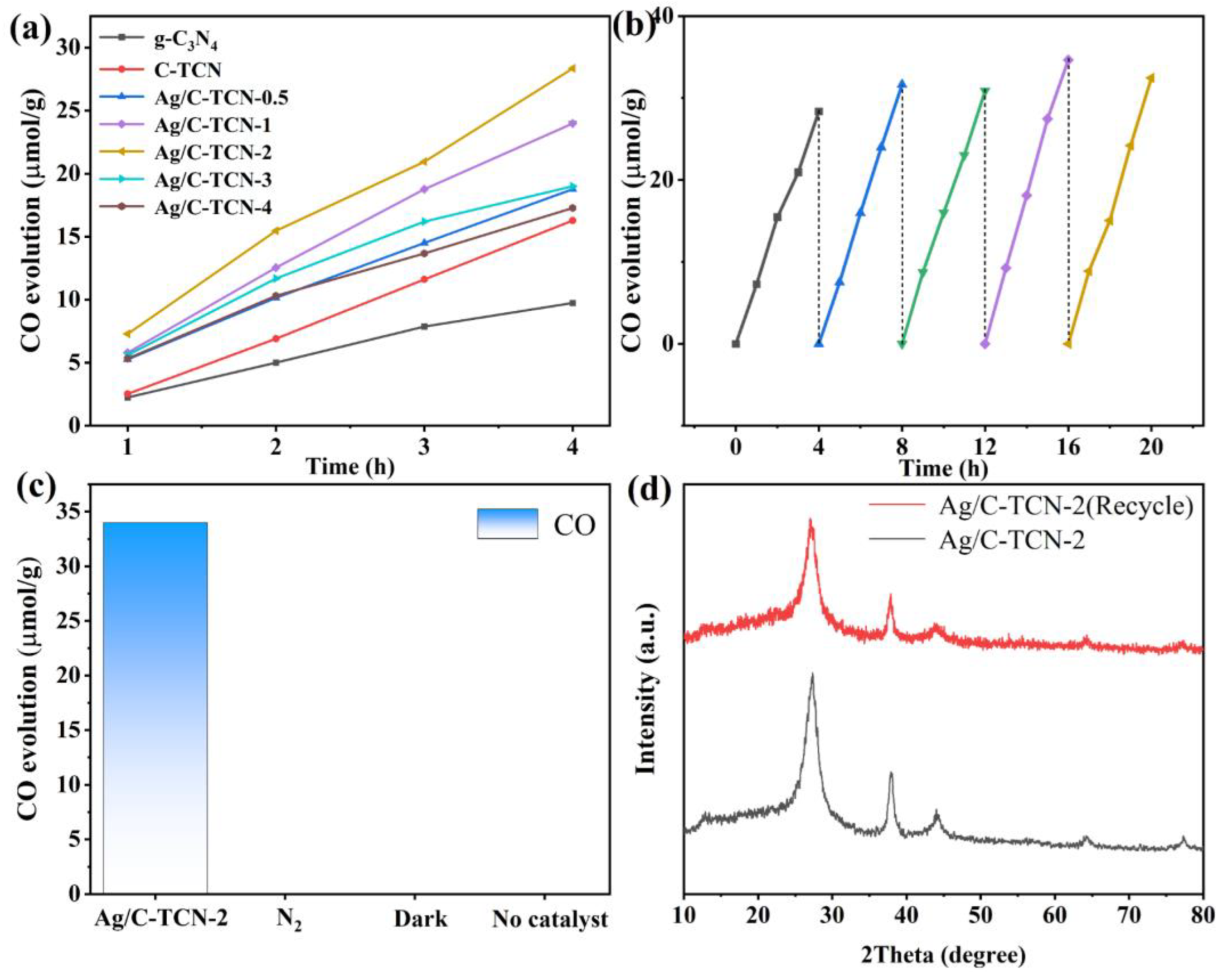
2.4. Photocatalytic CO2 Reduction Performance
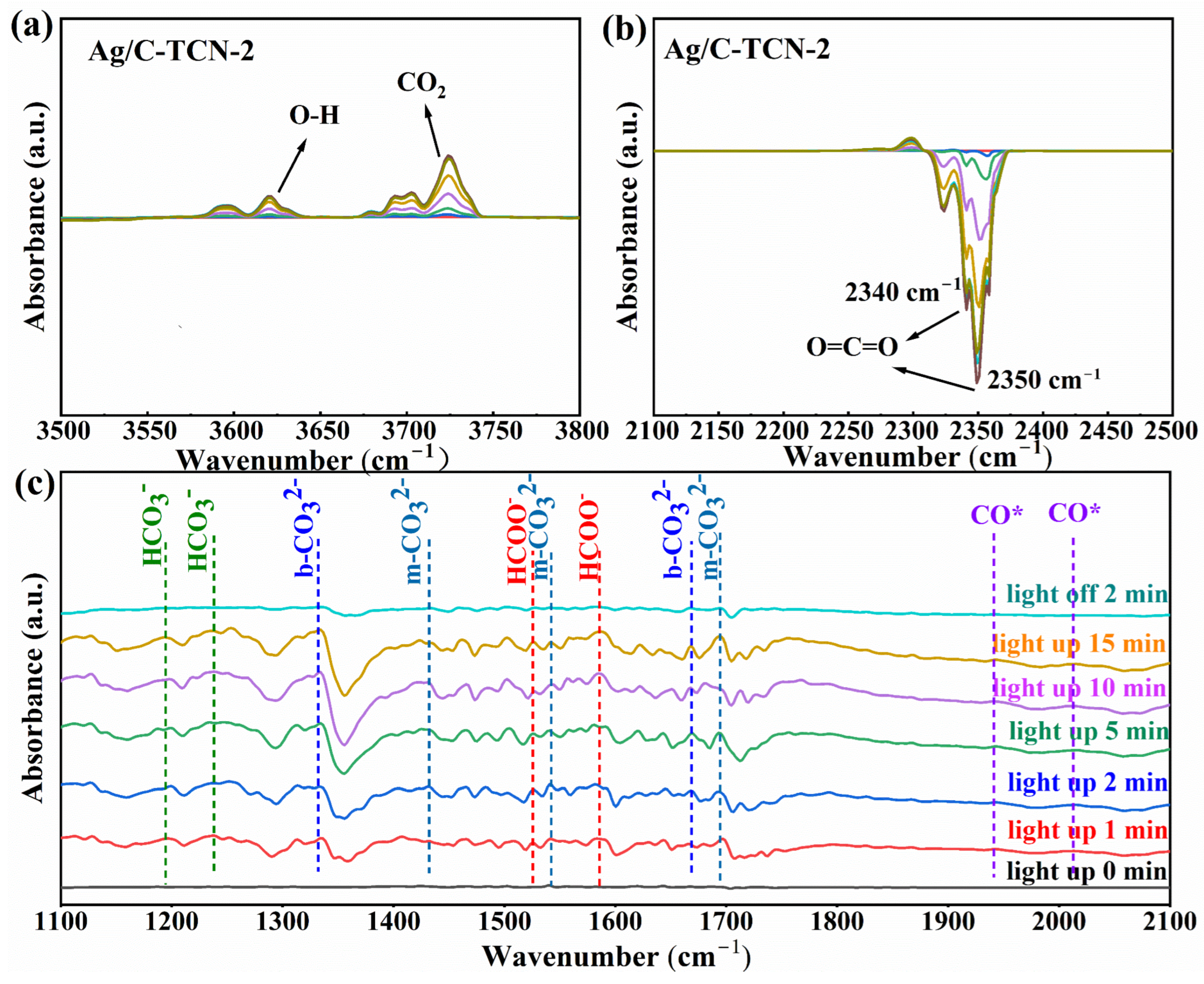
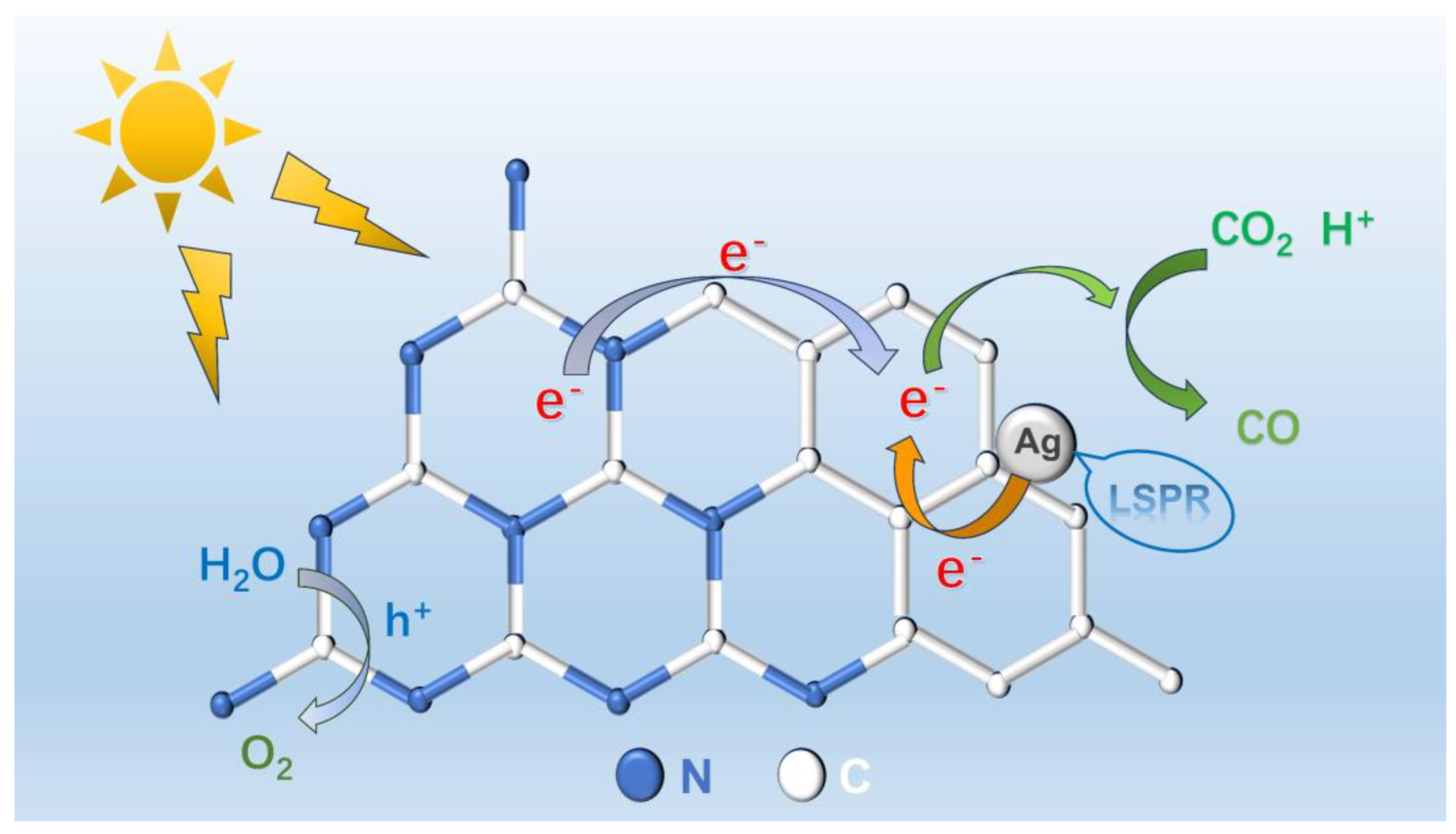
2.5. Reaction Process and Mechanism
3. Materials and Methods
3.1. Materials
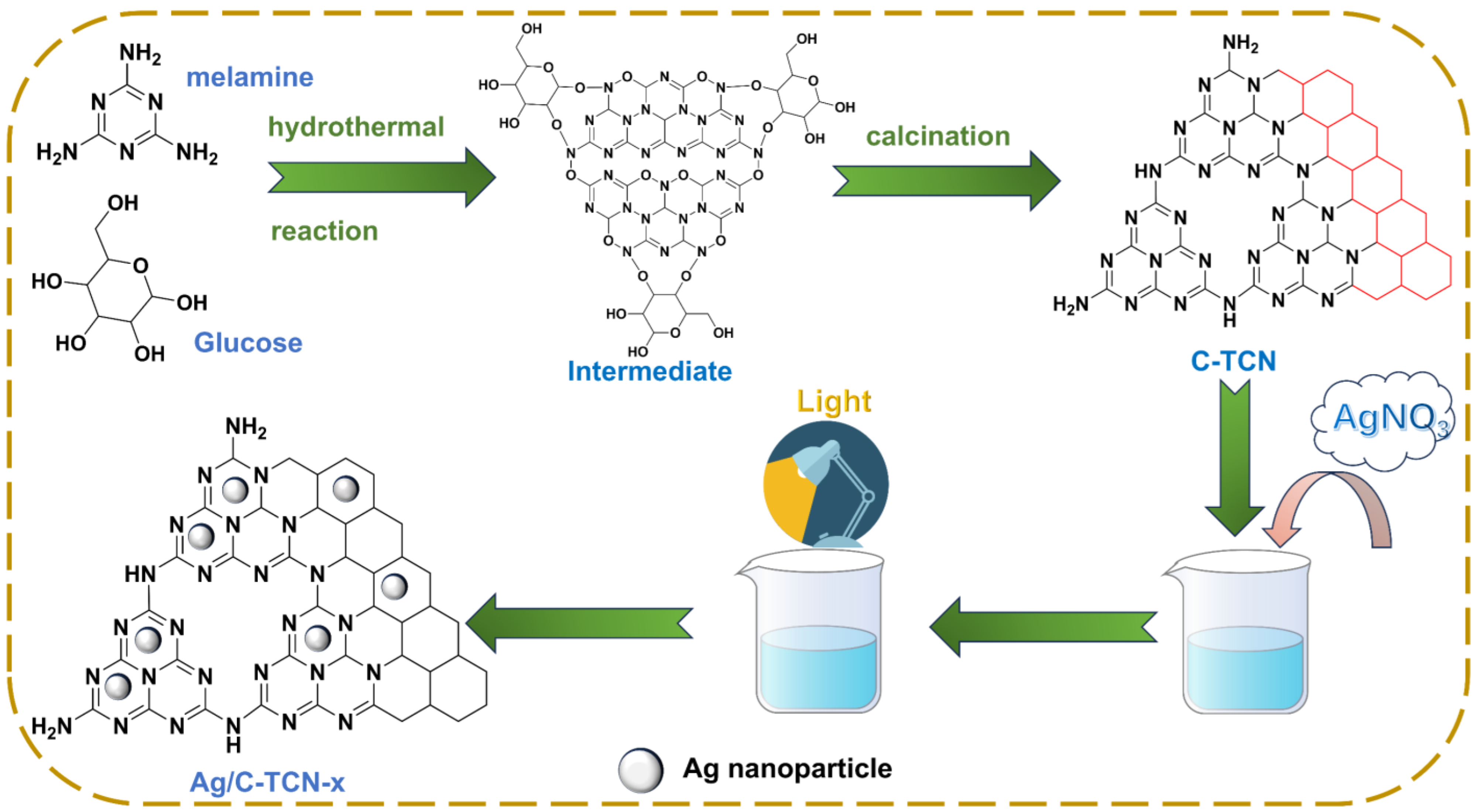
3.2. Preparation of Samples
3.2.1. Synthesis of C-TCN
3.2.2. Synthesis of Ag/C-TCN-x
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhang, P.; Gu, F.; Tong, L.; Jiang, J.; Zuo, Y.; Dong, H. Atomically dispersed Au confined by oxygen vacancies in Au-θ-Al2O3/Au/PCN hybrid for boosting photocatalytic CO2 reduction driven by multiple built-in electric fields. Chem. Eng. J. 2023, 476, 146514. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Wang, M.; Zhou, W.; Liu, X.; Zhu, Z.; Song, X.; Huo, P. Graphitic Carbon Nitride Modified with 2-Aminothiophene-3-Carbonitrile to Boost Molecular Dipole and n → π* Electronic Transition for Photoreduction of Carbon Dioxide. ACS Appl. Nano Mater. 2023, 6, 14513–14526. [Google Scholar] [CrossRef]
- Song, X.; Li, G.; Zhou, W.; Wu, Y.; Liu, X.; Zhu, Z.; Huo, P.; Wang, M. Construction of Au-modified CN-based donor-acceptor system coupled with dual photothermal effects for efficient photoreduction of carbon dioxide. J. Colloid Interface Sci. 2024, 664, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhou, Y.; Zhang, S.; Zheng, X.; Xu, Q. CO2-Induced 2D Ni-BDC Metal–Organic Frameworks with Enhanced Photocatalytic CO2 Reduction Activity. Adv. Mater. Interfaces 2021, 8, 2100205. [Google Scholar] [CrossRef]
- Wu, H.; Liang, R.; Song, G.; Hu, Z.; Zhang, X.; Zhou, M. Enhanced interfacial charge transfer on Bi metal@defective Bi2Sn2O7 quantum dots towards improved full-spectrum photocatalysis: A combined experimental and theoretical investigation. Chin. Chem. Lett. 2024, 35, 109131. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Che, H.; Tang, C.; Liu, B.; Ao, Y. In-situ growing Ni2P on CdS@g-C3N4 composites for highly efficient synergistically photocatalytic H2 evolution and antibiotic degradation. Surf. Interfaces 2024, 47, 104205. [Google Scholar] [CrossRef]
- Mo, Z.; Di, J.; Yan, P.; Lv, C.; Zhu, X.; Liu, D.; Song, Y.; Liu, C.; Yu, Q.; Li, H.; et al. An All-Organic D-A System for Visible-Light-Driven Overall Water Splitting. Small 2020, 16, 2003914. [Google Scholar] [CrossRef]
- Gao, S.; Liu, S.; Wang, D.; Zhu, C.; Shi, W.; Tao, H.; Wang, X.; Yang, F. Boosting carrier separation over ultrathin g-C3N4 by f-ionic intercalation for improved photocatalytic activity. Appl. Surf. Sci. 2024, 644, 158808. [Google Scholar] [CrossRef]
- Tang, X.; Shen, W.; Li, D.; Li, B.; Wang, Y.; Song, X.; Zhu, Z.; Huo, P. Research on cobalt-doping sites in g-C3N4 framework and photocatalytic reduction CO2 mechanism insights. J. Alloys Compd. 2023, 954, 170044. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhang, X.-H.; Wu, J.; Li, N.; Zheng, Y.-Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Zeng, W.; Dong, Y.; Ye, X.; Zhang, Z.; Zhang, T.; Guan, X.; Guo, L. Crystalline carbon nitride with in-plane built-in electric field accelerates carrier separation for excellent photocatalytic hydrogen evolution. Chin. Chem Lett. 2024, 35, 109252. [Google Scholar] [CrossRef]
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Huang, Y.; Liu, Q.; Liu, J.; Hu, F.; et al. Fast Photoelectron Transfer in (Cring)–C3N4 Plane Heterostructural Nanosheets for Overall Water Splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Z.; Shen, Y.; Qin, H.; Yuan, H.; Lu, J.; Guo, F.; Li, C.; Shi, W. Efficient photothermal-assisted photocatalytic H2 production using carbon dots-infused g-C3N4 nanoreactors synthesized via one-step template-free thermal polymerization. Chem. Eng. J. 2024, 488, 151041. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, W.; Zhu, Q.; Wang, X.; Wang, S.; Fan, F.; Li, C. The Polarization Effect in Surface-Plasmon-Induced Photocatalysis on Au/TiO2 Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 18218–18223. [Google Scholar] [CrossRef]
- Xi, G.; Ouyang, S.; Li, P.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. Ultrathin W18O49 Nanowires with Diameters below 1 nm: Synthesis, Near-Infrared Absorption, Photoluminescence, and Photochemical Reduction of Carbon Dioxide. Angew. Chem. Int. Ed. 2012, 51, 2395–2399. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Ma, C.; Zhu, Z.; Song, X.; Wang, H.; Huo, P.; Li, X. Local surface plasma resonance effect enhanced Z-scheme ZnO/Au/g-C3N4 film photocatalyst for reduction of CO2 to CO. Appl. Catal. B Environ. 2021, 283, 119638. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Plasmonic Photocatalysts for Sunlight-Driven Reduction of CO2: Details, Developments, and Perspectives. ChemSusChem 2020, 13, 3967–3991. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Li, X.; Xiao, Q.; Luo, W.; Xu, J. In-situ formation of electron-deficient Pd sites on AuPd alloy nanoparticles under irradiation enabled efficient photocatalytic Heck reaction. Chin. J. Catal. 2023, 46, 72–83. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Gao, P.F.; Jing, C.J.; Zhou, J.; Liang, W.T.; Lei, G.; Feng, W.; Li, Y.F.; Huang, C.Z. Microscopic electron counting during plasmon-driven photocatalytic proton coupled electron transfer on a single silver nanoparticle. Appl. Catal. B Environ. 2021, 291, 120090. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Xi, X.; Nie, Z.; Lim, F.y.; Ong, S.L.; Hu, J. Design of dual-LSPR Bi/W18O49 composite and its red-light driven photocatalytic property in the photocatalytic degradation of PPCPs. Chem. Eng. J. 2024, 481, 148119. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Lu, X.; Chen, M.; Shi, W. Near infrared-driven photocatalytic overall water splitting: Progress and perspective. Chin. J. Catal. 2024, 58, 105–122. [Google Scholar] [CrossRef]
- Anyika, T.; Hong, I.; Ndukaife, J.C. Mirror-Enhanced Plasmonic Nanoaperture for Ultrahigh Optical Force Generation with Minimal Heat Generation. Nano Lett. 2023, 23, 11416–11423. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2021, 18, 2101638. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, W.; Chen, H.; Ji, H. A review on g-C3N4 decorated with silver for photocatalytic energy conversion. Chin. Chem. Lett. 2024, 35, 108567. [Google Scholar] [CrossRef]
- Wang, F.; Guo, J.; Han, L.; Shen, H.; Zhu, L.; Chen, S. Oxygen vacancy-engineered BiOCl nanoflake with silver decoration for enhanced photocatalytic CO2 reduction at solid-gas interface. Chem. Eng. J. 2023, 478, 147365. [Google Scholar] [CrossRef]
- Ding, J.; Bu, Y.; Ou, M.; Yu, Y.; Zhong, Q.; Fan, M. Facile decoration of carbon fibers with Ag nanoparticles for adsorption and photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 202, 314–325. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Imade, E.E.; Oyewo, O.A.; Onwudiwe, D.C. Silver functionalized gC3N4: Photocatalytic potency for chromium(VI) reduction, and evaluation of the antioxidant and antimicrobial properties. J. Photochem. Photobiol. A Chem. 2022, 432, 114107. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Shen, W.; Wu, H.; Li, D.; Li, B.; Liang, J.; Tang, X.; Zhao, J.; et al. Fabrication of graphene modified CeO2/g-C3N4 heterostructures for photocatalytic degradation of organic pollutants. Chin. J. Struct. Chem. 2023, 42, 100194. [Google Scholar] [CrossRef]
- Ke, Y.; Yu, Z.; Lin, X.; Yuan, Y. Synergistic effect of n-π* electronic transitions in porous ultrathin graphitic carbon nitride nanosheets for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2023, 627, 157305. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, J.; Kirk, C.H.; Wang, H.; Sun, J.; Liu, Y.; Liu, B.; Zhang, T.; Jiang, S.; et al. Efficient photocatalytic hydrogen evolution reaction promoted via a synergistic strategy of S-scheme heterojunction structure combined with surface plasmon resonance effect. Chem. Eng. J. 2023, 466, 143184. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Wu, J.; Yan, P.; Xu, L.; Lei, Y.; Yuan, S.; et al. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B Environ. 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Ren, X.; Guo, M.; Xue, L.; Xu, L.; Li, L.; Yang, L.; Wang, M.; Xin, Y.; Ding, F.; Wang, Y. Photoelectrochemical performance and S-scheme mechanism of ternary GO/g-C3N4/TiO2 heterojunction photocatalyst for photocatalytic antibiosis and dye degradation under visible light. Appl. Surf. Sci. 2023, 630, 157446. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z. Ultrasonic-assisted synthesis of porous S-doped carbon nitride ribbons for photocatalytic reduction of CO2. Ultrason. Sonochem. 2023, 92, 106273. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Z.; Du, F.; Kong, W.; Li, Z.; Zhou, W. CdS nanocages@defective-CoNi-LDH with bilayer porous hollow frameworks toward optimized sono-photocatalytic performance. J. Mater. Chem. A 2022, 10, 16439–16447. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Wang, T.; Ali, A.; Zeng, H. Alkali-assisted fabrication of holey carbon nitride nanosheet with tunable conjugated system for efficient visible-light-driven water splitting. Appl. Catal. B Environ. 2018, 224, 877–885. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Z.; Shao, B.; He, Q.; Pan, Y.; Zhang, X.; Sun, J.; He, M.; Ge, L.; Cheng, C.; et al. Enhanced piezo-photocatalytic degradation of organic pollutants by cambered wall lamellar structure of porous tubular g-C3N4. Nano Energy 2024, 120, 109137. [Google Scholar] [CrossRef]
- Fu, X.; Huang, H.; Tang, G.; Zhang, J.; Sheng, J.; Tang, H. Recent advances in g-C3N4-based direct Z-scheme photocatalysts for environmental and energy applications. Chin. J. Struct. Chem. 2024, 43, 100214. [Google Scholar] [CrossRef]
- Yan, P.; Ji, F.; Zhang, W.; Mo, Z.; Qian, J.; Zhu, L.; Xu, L. Engineering surface bromination in carbon nitride for efficient CO2 photoconversion to CH4. J. Colloid Interface Sci. 2023, 634, 1005–1013. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Zhang, H.; Zhao, J.; Shi, H.; Zhang, M.; Yang, H.; Zheng, Z.; Yang, P. Nitrogen vacancies in polymeric carbon nitrides promote CO2 photoreduction. J. Catal. 2022, 409, 12–23. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ren, X.; Li, W.; Sun, J.; Wang, X.; Huang, Y.; Guo, Y.; Zeng, H. Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; You, X.; An, B.; Liu, F.; Duan, X.; Wang, Y.; Liu, C.; Zhao, C. Visible-light-responsive Cl/S co-doped carbon nitride nanotubes for photocatalytic denitrification: A new reaction pathway dominated by photo-electrons. Appl. Catal. B Environ. 2022, 305, 121018. [Google Scholar] [CrossRef]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-Light-Responsive Graphitic Carbon Nitride: Rational Design and Photocatalytic Applications for Water Treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-S.; Li, Q.; Chu, C.; Liu, Q.; Li, Z.; Mao, S. Synergetic regulation of electronic structure of graphitic carbon nitride through phosphorus and carbon co-doping for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2024, 482, 149155. [Google Scholar] [CrossRef]
- Wang, D.; Xue, G.; Zhen, Y.; Fu, F.; Li, D. Monodispersed Ag nanoparticles loaded on the surface of spherical Bi2WO6 nanoarchitectures with enhanced photocatalytic activities. J. Mater. Chem. 2012, 22, 4751–4758. [Google Scholar] [CrossRef]
- Li, J.; Lou, Z.; Li, B. Nanostructured materials with localized surface plasmon resonance for photocatalysis. Chin. Chem. Lett. 2022, 33, 1154–1168. [Google Scholar] [CrossRef]
- Yu, Z.; Guan, C.; Yue, X.; Xiang, Q. Infiltration of C-ring into crystalline carbon nitride S-scheme homojunction for photocatalytic hydrogen evolution. Chin. J. Catal. 2023, 50, 361–371. [Google Scholar] [CrossRef]
- Gu, Y.; Feng, H.; Zhao, J.; Cui, M.; Li, Y.; Li, Z. Rational construction of edge-grafted g-C3N4 via cross-linking aromatic compounds with C F bonds for efficient photocatalytic H2 evolution. Chem. Eng. J. 2023, 476, 146555. [Google Scholar] [CrossRef]
- Che, H.; Gao, X.; Chen, J.; Hou, J.; Ao, Y.; Wang, P. Iodide-Induced Fragmentation of Polymerized Hydrophilic Carbon Nitride for High-Performance Quasi-Homogeneous Photocatalytic H2O2 Production. Angew. Chem. Int. Ed. 2021, 60, 25546–25550. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef]
- Dash, S.; Tripathy, S.P.; Subudhi, S.; Acharya, L.; Ray, A.; Behera, P.; Parida, K. Ag/Pd bimetallic nanoparticle-loaded Zr-MOF: An efficacious visible-light-responsive photocatalyst for H2O2 and H2 production. Energy Adv. 2024, 3, 1073–1086. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Lei, J.; Zhou, N.; Sang, S.; Meng, S.; Low, J.; Li, Y. Unraveling the roles of atomically-dispersed Au in boosting photocatalytic CO2 reduction and aryl alcohol oxidation. Chin. J. Catal. 2024, 65, 163–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, S.; Yang, D.; Liang, H.; Wu, Y.; Hao, J.; Wang, Y.; Liu, J.; Wang, Y. Enhanced visible-light photocatalytic hydrogen evolution using two-dimensional carbon nitride sheets with the removal of amine groups. Chin. Chem. Lett. 2024, 35, 108551. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Zhu, X.; Ding, P.; Zhong, K.; Liu, J.; Hua, Y.; Hu, Q.; Yi, J.; Xu, H.; et al. Unraveling the unique role of brown graphitic carbon nitride in robust CO2 photoreduction. Appl. Surf. Sci. 2023, 615, 156173. [Google Scholar] [CrossRef]
- Niu, Q.; Dong, S.; Tian, J.; Huang, G.; Bi, J.; Wu, L. Rational Design of Novel COF/MOF S-Scheme Heterojunction Photocatalyst for Boosting CO2 Reduction at Gas–Solid Interface. ACS Appl. Mater. Interfaces 2022, 14, 24299–24308. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhao, X.; Wang, L.; Chen, H.; Wang, S.; Xu, X.; Shi, L.; Zhang, L.-C.; Zhu, Y.; et al. Aligning potential differences within carbon nitride based photocatalysis for efficient solar energy harvesting. Nano Energy 2021, 89, 106357. [Google Scholar] [CrossRef]
- Yin, S.; Zhou, Y.; Liu, Z.; Wang, H.; Zhao, X.; Zhu, Z.; Yan, Y.; Huo, P. Elucidating protonation pathways in CO2 photoreduction using the kinetic isotope effect. Nat. Commun. 2024, 15, 437. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Chen, G.; Chu, X.; Liu, X.; Naisa, C.; Pohl, D.; Löffler, M.; Feng, X. Accessing parity-forbidden d-d transitions for photocatalytic CO2 reduction driven by infrared light. Nat. Commun. 2023, 14, 4034. [Google Scholar] [CrossRef]
- Zu, X.; Zhao, Y.; Li, X.; Chen, R.; Shao, W.; Wang, Z.; Hu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Ultrastable and Efficient Visible-light-driven CO2 Reduction Triggered by Regenerative Oxygen-Vacancies in Bi2O2CO3 Nanosheets. Angew. Chem. Int. Ed. 2021, 60, 13840–13846. [Google Scholar] [CrossRef]
- Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. Recent advances in crystalline carbon nitride for photocatalysis. J. Mater. Sci. Technol. 2021, 91, 224–240. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Hu, J.; Li, Z.; Zhang, W.; Zhang, J.; Wang, Z.; Guo, X.; Yan, C.; Yuan, H.; et al. Polymeric carbon nitride loaded with atomic Cu sites for improved CO2 photocatalytic conversion performance. J. Power Sources 2023, 577, 233188. [Google Scholar] [CrossRef]
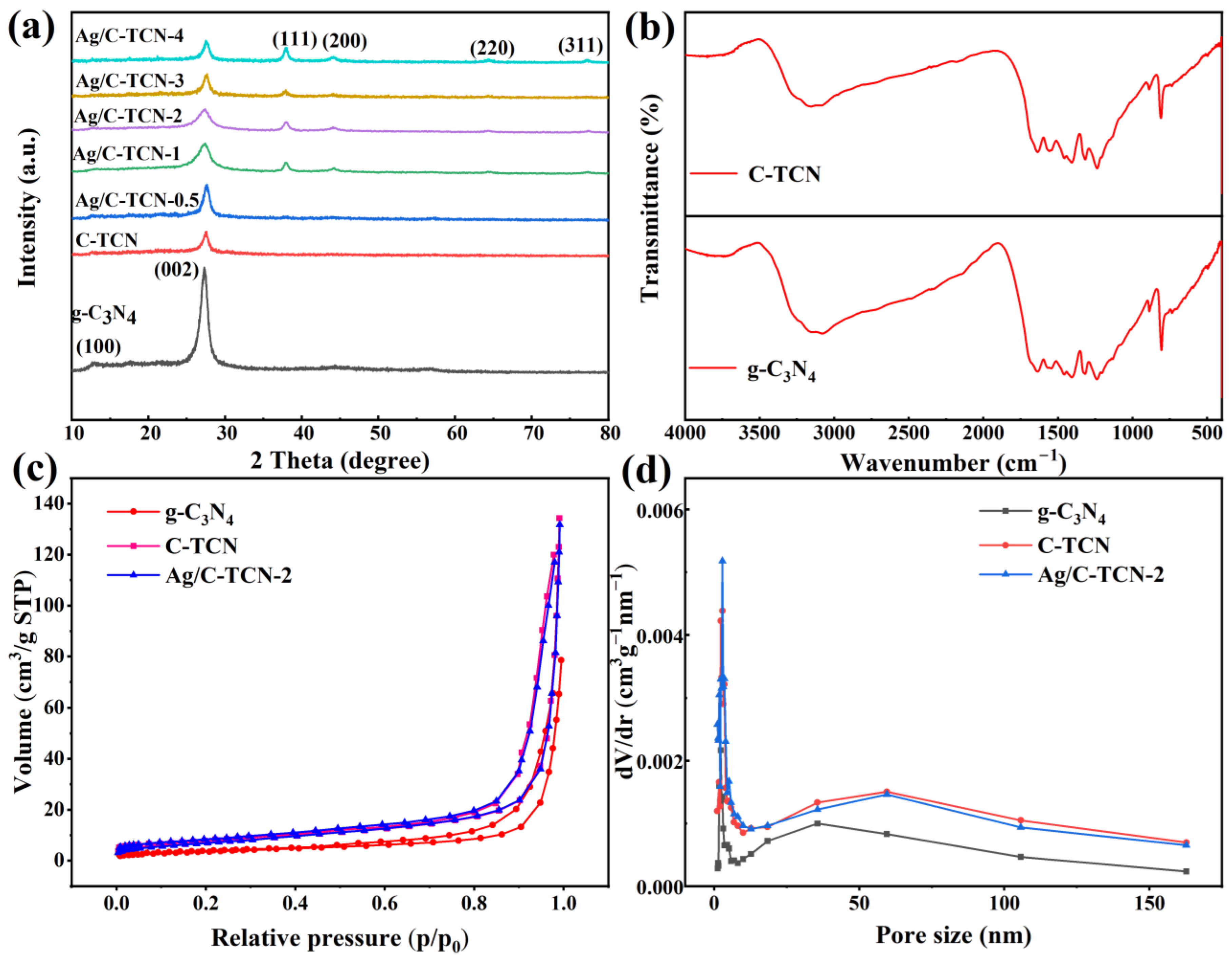

| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| g-C3N4 | 13.580 | 0.104 | 30.610 |
| C-TCN | 26.415 | 0.205 | 31.035 |
| Ag/C-TCN-2 | 25.995 | 0.191 | 29.418 |
| Catalyst | C (Atom%) | N (Atom%) | Ag (Atom%) |
|---|---|---|---|
| g-C3N4 | 44.32 | 55.68 | 0 |
| C-TCN | 47.79 | 52.21 | 0 |
| Ag/C-TCN-2 | 48.64 | 49.74 | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Xu, S.; Yang, F.; Liu, X.; Wang, M.; Liu, X.; Zhou, W.; Zhang, J.; Yang, Y.; Huo, P. Photothermal Effect of Carbon-Doped Carbon Nitride Synergized with Localized Surface Plasmon Resonance of Ag Nanoparticles for Efficient CO2 Photoreduction. Catalysts 2025, 15, 369. https://doi.org/10.3390/catal15040369
Song X, Xu S, Yang F, Liu X, Wang M, Liu X, Zhou W, Zhang J, Yang Y, Huo P. Photothermal Effect of Carbon-Doped Carbon Nitride Synergized with Localized Surface Plasmon Resonance of Ag Nanoparticles for Efficient CO2 Photoreduction. Catalysts. 2025; 15(4):369. https://doi.org/10.3390/catal15040369
Chicago/Turabian StyleSong, Xianghai, Sheng Xu, Fulin Yang, Xiang Liu, Mei Wang, Xin Liu, Weiqiang Zhou, Jisheng Zhang, Yangyang Yang, and Pengwei Huo. 2025. "Photothermal Effect of Carbon-Doped Carbon Nitride Synergized with Localized Surface Plasmon Resonance of Ag Nanoparticles for Efficient CO2 Photoreduction" Catalysts 15, no. 4: 369. https://doi.org/10.3390/catal15040369
APA StyleSong, X., Xu, S., Yang, F., Liu, X., Wang, M., Liu, X., Zhou, W., Zhang, J., Yang, Y., & Huo, P. (2025). Photothermal Effect of Carbon-Doped Carbon Nitride Synergized with Localized Surface Plasmon Resonance of Ag Nanoparticles for Efficient CO2 Photoreduction. Catalysts, 15(4), 369. https://doi.org/10.3390/catal15040369









