Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction C1 Product Selectivity
Abstract
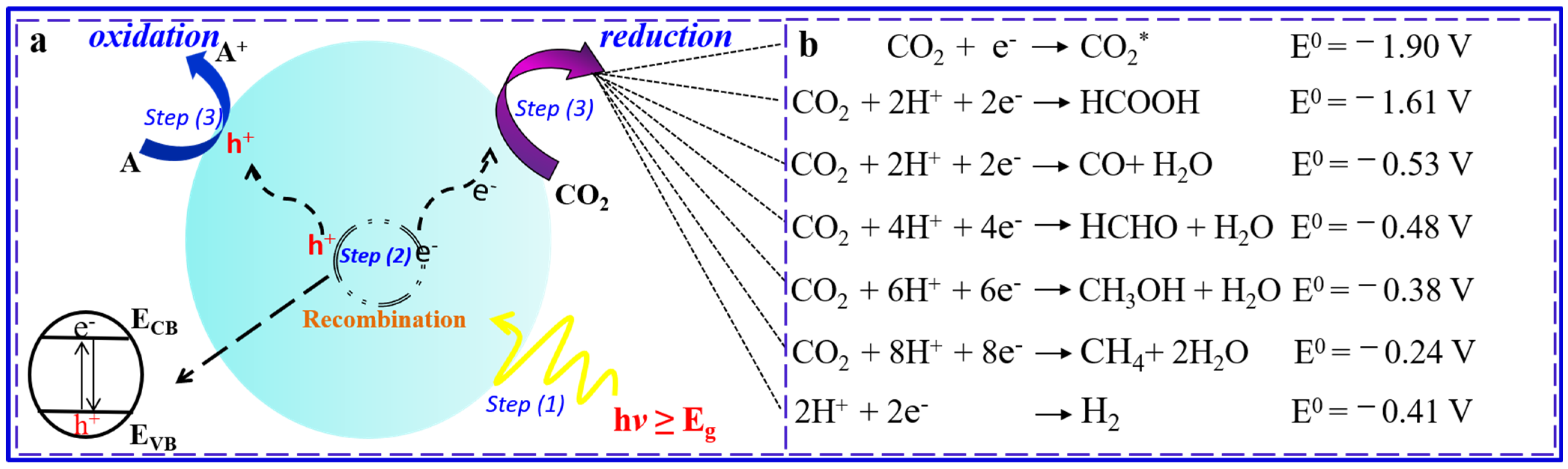
:1. Introduction
2. The g-C3N4 and Its Composite for Photocatalytic CO2RR Selectivity
2.1. In the Context of Charge Carrier Transmission and Reaction Dynamics
2.1.1. Heteroatom Doping (Electron Property)
2.1.2. Metal Co-Catalysts
2.1.3. Single-Atom
2.1.4. Defects and Crystalline Regulation
2.1.5. Morphological Adjustment
2.1.6. Construction of Heterostructures
2.2. CO2 Adsorption and Intermediate Desorption
2.2.1. Heteroatom Doping
2.2.2. Metal Co-Catalysts
2.2.3. Single Atom
2.2.4. Morphological Adjustment
2.2.5. Fabricating Heterostructures
2.2.6. Metal–Organic Frameworks/g-C3N4
2.3. Proton Regulation to Control the Competitiveness of HER
2.3.1. Hydrophobic Surface Modification
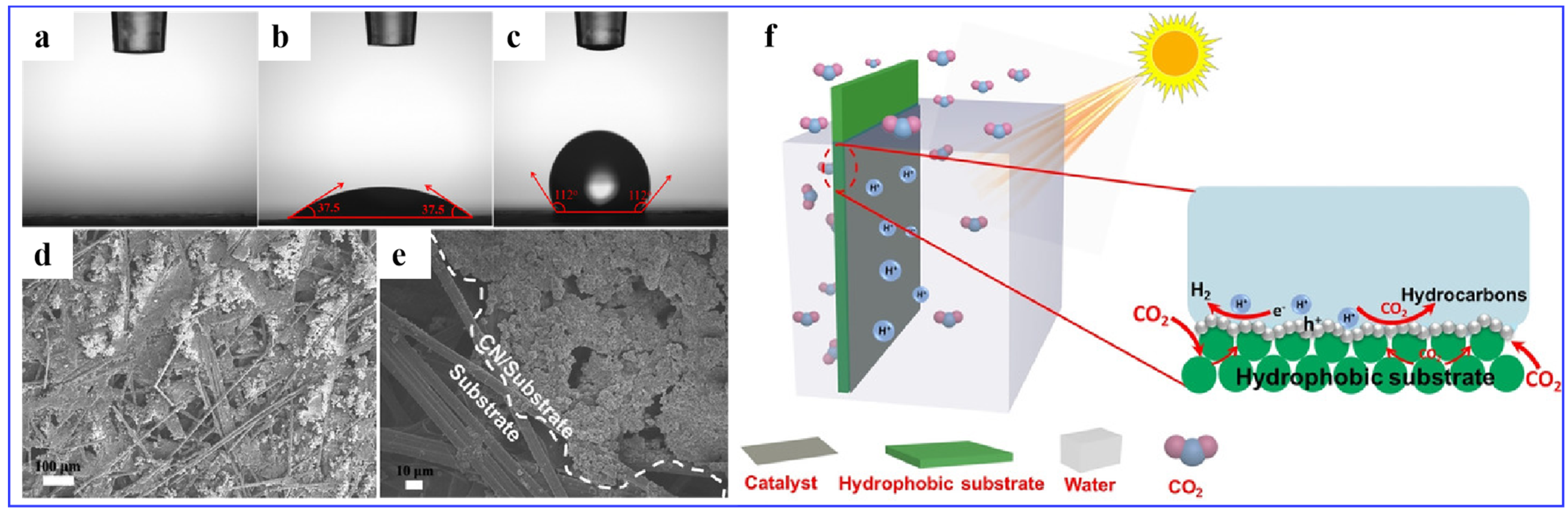
2.3.2. Hydrophobic Substrate Modification
2.3.3. Metal and Organic Co-Catalysts
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Si, S.; Shou, H.; Mao, Y.; Bao, X.; Zhai, G.; Song, K.; Wang, Z.; Wang, P.; Liu, Y.; Zheng, Z. Low-coordination single Au atoms on ultrathin ZnIn2S4 nanosheets for selective photocatalytic CO2 reduction towards CH4. Angew. Chem. Int. Ed. 2022, 61, e202209446. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, D.; Yang, X.; Chen, J.; Guo, Y.; Zhang, T. Vibration Isolation Performance Analysis of a Nonlinear Fluid Inerter-Based Hydro-Pneumatic Suspension. Int. J. Struct. Stab. Dyn. 2024, 22, 2650079. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Luo, H.; Mao, Y. Construction of biochar assisted S-scheme of CeO/g-CN with enhanced photoreduction CO2 to CO activity and selectivity. Mater. Res. Bull. 2025, 181, 113085. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, X.; Meng, H.; Dong, F.; Yang, S. Design, modeling, and simulation of a novel transducer for vibration energy recovery system of speed bump. Trans. Can. Soc. Mech. Eng. 2023, 47, 225–238. [Google Scholar] [CrossRef]
- Song, S.; Gao, Z.; Guo, X.; Chen, G. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Tech. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, C.; Wang, G.; He, B.; Hao, B.; Han, Y.; Wang, B.; Bao, R.; Syed, T.N.; et al. A Review of Precision Irrigation Water-Saving Technology under Changing Climate for Enhancing Water Use Efficiency, Crop Yield, and Environmental Footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): An overview. Grasas Aceites 2019, 70, 3417–3495. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.Q.; Duan, W.Y.; Liu, C.J. Assessment of the environmental comfort of lactating sows via improved analytic hierarchy process and fuzzy comprehensive evaluation. Int. J. Agric. Biol. Eng. 2022, 15, 58–67. [Google Scholar] [CrossRef]
- Nazir, M.; Li, G.; Nazir, M.; Zulfiqar, F.; Siddique, K.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Iqbal, B.; Alabbosh, K.F.; Jalal, A.; Suboktagin, S.; Elboughdiri, N. Sustainable food systems transformation in the face of climate change: Strategies, challenges, and policy implications. Food Sci. Biotechnol. 2025, 34, 871–883. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Sun, H.; Taghizadeh-Hesary, F. How Does Climate Change Affect Rice Yield in China? Agriculture 2020, 10, 441. [Google Scholar] [CrossRef]
- Akhlaq, M.; Zhang, C.; Yan, H.; Ou, M.; Zhang, W.; Liang, S.; Ikram, A. Response of tomato growth to continuous elevated CO2 concentration under controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 51–59. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Xue, R.; Li, M.; Adnan, R.M.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO in a solid food: A novel method to enhance the low-frequency ultrasound effect on immersion freezing process. Innov. Food Sci. Emerg. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Xu, C.; Chen, Y.; Sun, W.; Liu, Q.; Dong, Q. Modeling inhibition effects of subsp CICC 6257 on growth of in ground pork stored at CO-rich atmospheres. Lwt-Food Sci. Technol. 2018, 97, 811–817. [Google Scholar] [CrossRef]
- Khan, K.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil NO and CO emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, J.; Hu, H.; Pan, Q.; Cui, L.; Hu, Y. Determination of Critical Crop Water Stress Index of Tea under Drought Stress Based on the Intercellular CO Concentration. Agronomy 2024, 14, 2073–4395. [Google Scholar] [CrossRef]
- Junaid, N.; Li, H.; Pan, X.; Zaman, M.; Anjum, S.; Yang, F.; Akbar, N.; Azamat, U. Modeling effects of climate change on crop phenology and yield of wheat-maize cropping system and exploring sustainable solutions. J. Sci. Food Agric. 2025, 105, 3679–3700. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Ali, S.; Li, H.; Ouyang, Q.; Chen, Q. Self-Cleaning-Mediated SERS Chip Coupled Chemometric Algorithms for Detection and Photocatalytic Degradation of Pesticides in Food. J. Agric. Food Chem. 2021, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zhang, L.; Tollerud, J.O.; Dong, M.; Zhu, Y.; Halbich, R.; Vogl, T.; Liang, K.; Nguyen, H.T.; Wang, F.; et al. Supertransport of excitons in atomically thin organic semiconductors at the 2D quantum limit. Light-Sci. Appl. 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light-Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, W.; Liu, S.; Haruna, S.A.; Zareef, M.; Ahmad, W.; Hassan, M.M.; Li, H.; Chen, Q. A tailorable and recyclable TiO NFSF/Ti@Ag NPs SERS substrate fabricated by a facile method and its applications in prohibited fish drugs detection. J. Food Meas. Charact. 2022, 16, 2890–2898. [Google Scholar] [CrossRef]
- Cheng, K.; Du, J.; Xu, F.; Wang, Z.; Zhang, L.; Bai, M.; Wang, X.; Liu, J. Preparation and characterization of zein-based active packaging films integrated with TiO nanotube arrays. Food Packag. Shelf 2024, 45, 101348. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.Y.; Holmes, M.; Povey, M.; et al. Agar/TiO/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Meng, S.; Liu, D.; Li, Y.; Dong, N.; Chen, T.; You, T. Engineering the Signal Transduction between CdTe and CdSe Quantum Dots for Ratiometric Photoelectrochemical Immunoassay of Cry1Ab Protein. J. Agric. Food Chem. 2022, 70, 13583–13591. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.H.; Ma, S.; Li, L.; You, T.Y. Quantification of zearalenone in mildewing cereal crops using an innovative photoelectrochemical aptamer sensing strategy based on ZnO-NGQDs composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef]
- Zhu, A.; Jiao, T.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q. Dispersive micro solid phase extraction based ionic liquid functionalized ZnO nanoflowers couple with chromatographic methods for rapid determination of aflatoxins in wheat and peanut samples. Food Chem. 2022, 391, 133277. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Liu, Y.; Rashid, A.; Qayum, A.; Zhou, C.; Han, X.; Ren, X.; Chi, Z.; Chi, R.; et al. Preparation technology and preservation mechanism of novel Ag NPs-loaded ZIF-67 packaging film. Food Packag. Shelf 2024, 45, 101338. [Google Scholar] [CrossRef]
- Shi, Y.; Rong, X.; Chen, C.; Wu, M.; Takai, Y.; Qiu, X.; Wang, C.; Shimasaki, Y.; Oshima, Y. Effects of ZIF-8 Nanoparticles on the Survival, Development, and Locomotor Activity of Early-life-stages of Zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 211–216. [Google Scholar]
- Zhang, J.; Zhang, J.; Zhang, X.; Huang, X.; Shi, J.; Sobhy, R.; Khalifa, I.; Zou, X. Ammonia-Responsive Colorimetric Film of Phytochemical Formulation (Alizarin) Grafted onto ZIF-8 Carrier with Poly (vinyl alcohol) and Sodium Alginate for Beef Freshness Monitoring. J. Agric. Food Chem. 2024, 72, 11706–11715. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, Z.; Sun, Y.; Sun, M.; Duan, J.; Tian, Y.; Du, D.; Li, M. Cascade Amplifying Electrochemical Bioanalysis for Zearalenone Detection in Agricultural Products: Utilizing a Glucose-Fenton-HQ System on Bimetallic-ZIF@CNP Nanocomposites. Foods 2024, 13, 3192. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef]
- Ma, S.; Pan, L.; You, T.; Wang, K. g-C3N4/Fe3O4 Nanocomposites as Adsorbents Analyzed by UPLC-MS/MS for Highly Sensitive Simultaneous Determination of 27 Mycotoxins in Maize: Aiming at Increasing Purification Efficiency and Reducing Time. J. Agric. Food Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, W.; Ma, W.; Guo, S.; Meng, H.; Xu, N. Mechanism of a dual-phase Ti-Nb alloy exhibiting near-linear elastic deformation. Rare Met. 2024, 43, 2282–2289. [Google Scholar] [CrossRef]
- Meijerink, A. A new route towards polarized luminescence: 0D/2D nanocomposites. Light-Sci. Appl. 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Sun, L.; Zhou, Y.; Li, X.; Li, J.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Enhanced electron–hole separation in SnS2/Au/g-C3N4 embedded structure for efficient CO2 photoreduction. Chem. Eng. J. 2021, 406, 126776. [Google Scholar] [CrossRef]
- Ham, R.; Nielsen, C.; Pullen, S.; Reek, J. Supramolecular Coordination Cages for Artificial Photosynthesis and Synthetic Photocatalysis. Chem. Rev. 2023, 123, 5225–5261. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal electric field in carbon nitride-based heterojunctions for photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Guo, Y.; Yang, L.; Lv, M.; Tang, X.; Li, S. Enhanced photoreduction CO2 activity on g-C3N4: By synergistic effect of nitrogen defective-enriched and porous structure, and mechanism insights. Chem. Eng. J. 2020, 388, 124288. [Google Scholar] [CrossRef]
- Cui, E.; Lu, Y.; Li, Z.; Chen, Z.; Ge, C.; Jiang, J. Interfacial B-O bonding modulated S-scheme B-doped N-deficient C3N4/O-doped-C3N5 for efficient photocatalytic overall water splitting. Chin. Chem. Lett. 2025, 36, 110288. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, X.; Zong, H.; Zeng, G.; Miao, H.; Mo, Z.; Hossain, M.S.; Yan, J.; Wang, L.; Xu, H. Strongly coupled NH2NH-modified high crystallinity Graphene quantum dots/Carbon Nitride for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 36818–36824. [Google Scholar] [CrossRef]
- Cai, J.; Huang, H.; Zhu, Z.; Han, D.; Hu, B.; Li, H.; Tang, X. Preparation of Biochar-Loaded Er3+-Doped BiOCl Ultrathin Nanosheets Composite Photocatalysts and Their Photodegradation Performance of TC-HCl. Catalysts 2024, 14, 874. [Google Scholar] [CrossRef]
- Lei, D.; Su, D.; Maier, S. New insights into plasmonic hot-electron dynamics. Light-Sci. Appl. 2024, 13, 243. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, Y.; Xu, Y.; Zhang, Q.; Shi, X.; Li, D.; Tian, D.; Jiang, D. Improved charge transfer in polymeric carbon nitride synergistically induced by the aromatic rings modification and Schottky junctions for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2023, 463, 142395. [Google Scholar] [CrossRef]
- Yang, J.; Yang, K.; Zhu, X.; Wang, Z.; Yang, Z.; Ding, X.; Zhong, K.; He, M.; Li, H.; Xu, H. Band engineering of non-metal modified polymeric carbon nitride with broad spectral response for enhancing photocatalytic CO2 reduction. Chem. Eng. J. 2023, 461, 141841. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Esakkimuthu, S.; Chen, H.; Yang, J.; Mu, M.; Gong, X. Life cycle assessment of carbon-based adsorbent preparation from algal biomass. J. Clean. Prod. 2023, 427, 139269. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Jiang, H.; Chen, S.; Huo, P. MOFs-derived C-In2O3/g-C3N4 heterojunction for enhanced photoreduction CO2. J. Environ. Chem. Eng. 2021, 9, 106469. [Google Scholar] [CrossRef]
- Zhou, F.; Fang, X.; Zhang, Y.; Yang, W.; Zhou, W.; Zhou, H.; Liu, Q.; Wu, J.; Qi, F.; Shen, Y. Synergetic effects of Cu cluster-doped g-C3N4 with multiple active sites for CO2 reduction to C2 products: A DFT study. Fuel 2023, 353, 129202. [Google Scholar] [CrossRef]
- Shen, W.; Qi, Q.; Hu, B.; Zhu, Z.; Huo, P.; Jiang, J.; Tang, X. Studying bimetals copper-indium for enhancing PCN photocatalytic CO2 reduction activity and selectivity mechanism. J. Ind. Eng. Chem. 2025, 145, 384–394. [Google Scholar] [CrossRef]
- Wu, H.; Han, D.; Hu, B.; Liang, J.; Tang, X.; Zhu, Z.; Huo, P. Insights into enhanced photocatalytic CO2 reduction on carbon nitride: A strategy of simultaneous O, S co-doping. J. Environ. Chem. Eng. 2025, 13, 116121. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Tang, S.; Liang, Y.; Zhang, D.; Jin, X.; Wang, Q.; Sun, W.; Zheng, L.; Li, W. Integrating configuration, doping and heterojunction into the g-C3N4-based photocatalyst for water splitting. Carbon 2024, 218, 118723. [Google Scholar] [CrossRef]
- Pan, Y.; Si, W.; Li, Y.; Tan, H.; Liang, J.; Hou, F. Promoting exciton dissociation by metal ion modification in polymeric carbon nitride for photocatalysis. Chin. Chem. Lett. 2024, 35, 109877. [Google Scholar] [CrossRef]
- Fu, J.; Liu, K.; Jiang, K.; Li, H.; An, P.; Li, W.; Zhang, N.; Li, H.; Xu, X.; Zhou, H. Graphitic Carbon Nitride with Dopant Induced Charge Localization for Enhanced Photoreduction of CO2 to CH4. Adv. Sci. 2019, 6, 1900796. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Tang, X.; Reeti, K.; Huo, P.; Wong, J.W.-C.; Zhao, J. Sulfur-doped g-C3N4 for efficient photocatalytic CO2 reduction: Insights by experiment and first-principles calculations. Catal. Sci. Technol. 2021, 11, 1725–1736. [Google Scholar] [CrossRef]
- Song, X.; Li, X.; Zhang, X.; Wu, Y.; Ma, C.; Huo, P.; Yan, Y. Fabricating C and O co-doped carbon nitride with intramolecular donor-acceptor systems for efficient photoreduction of CO2 to CO. Appl. Catal. B Environ. 2020, 268, 118736. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, J.; Chen, K.; Ali, A.; Zeng, L.; Zhao, H.; Hu, W.; Zhu, L.; Xu, X. Potassium-Doped g-C3N4 Achieving Efficient Visible-Light-Driven CO2 Reduction. ACS Sustain. Chem. Eng. 2020, 8, 8214–8222. [Google Scholar] [CrossRef]
- Ojha, N.; Bajpai, A.; Kumar, S. Enhanced and selective photocatalytic reduction of CO2 by H2O over strategically doped Fe and Cr into porous boron carbon nitride. Catal. Sci. Technol. 2020, 10, 2663–2680. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Wang, H.; Wu, Z. Insight into the enhanced CO2 photocatalytic reduction performance over hollow-structured Bi-decorated g-C3N4 nanohybrid under visible-light irradiation. J. CO2 Util. 2018, 28, 126–136. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Zhao, Y.; Zhang, J.; Zhang, C.; Wang, Z.; Chen, G. Selective photocatalytic reduction of CO2 into CH4 over Pt- Cu2O TiO2 nanocrystals: The interaction between Pt and Cu2O cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Lee, D.-E.; Moru, S.; Bhosale, R.; Jo, W.-K.; Tonda, S. Cu–Ni core–shell bimetallic cocatalyst decorated polymeric carbon nitride for highly efficient and selective methane production from photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 599, 153973. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Xiao, W.; Cheng, B. Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4–Pt nanocomposite photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 11492–11501. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Shi, X.; An, P.; Zhang, Q.; Song, Q.; Jiang, D.; Tian, D.; Li, D. Synergy of nitrogen vacancies and Fe2P cocatalyst on graphitic carbon nitride for boosting photocatalytic CO2 conversion. Chem. Eng. J. 2022, 446, 137096. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.-T.; Liu, X.-Y.; Shi, X.; Wang, Z.-Y.; Tang, J.-Y.; Pan, W.-G. 0D NiS2 quantum dots modified 2D g-C3N4 for efficient photocatalytic CO2 reduction. Colloids Surf. A 2020, 600, 124912. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Zuo, C.; Li, R.; Zhou, Y.; Zhang, Y.; Wu, B. Few-layer porous carbon nitride anchoring Co and Ni with charge transfer mechanism for photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 625, 722–733. [Google Scholar] [CrossRef]
- Huang, P.; Huang, J.; Pantovich, S.A.; Carl, A.D.; Fenton, T.G.; Caputo, C.A.; Grimm, R.L.; Frenkel, A.I.; Li, G. Selective CO2 Reduction Catalyzed by Single Cobalt Sites on Carbon Nitride under Visible-Light Irradiation. J. Am. Chem. Soc. 2018, 140, 16042–16047. [Google Scholar] [CrossRef]
- Cheng, L.; Yin, H.; Cai, C.; Fan, J.; Xiang, Q. Single Ni Atoms Anchored on Porous Few-Layer g-C3N4 for Photocatalytic CO2 Reduction: The Role of Edge Confinement. Small 2020, 16, 2002411. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. [Google Scholar] [CrossRef]
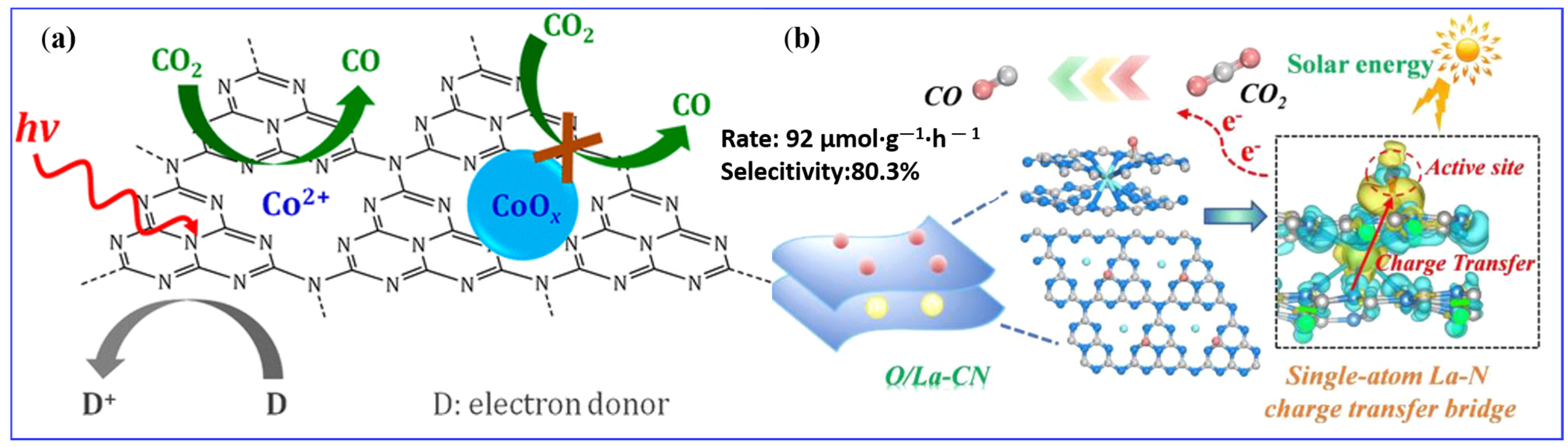
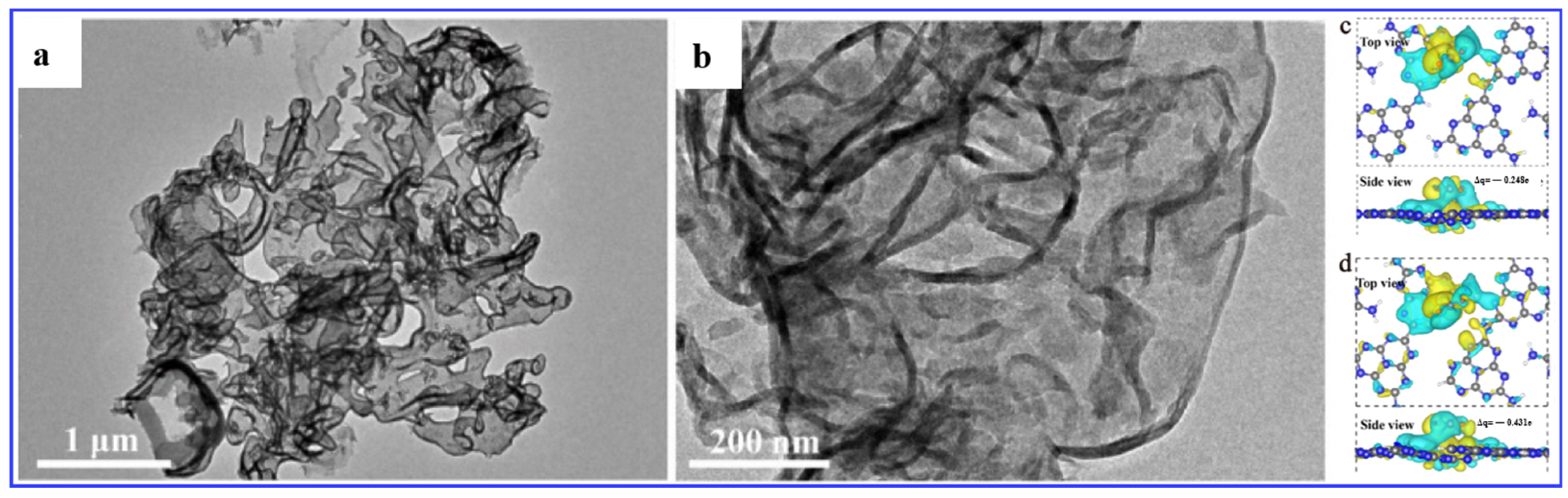
- Chen, P.; Lei, B.; Dong, X.; Wang, H.; Sheng, J.; Cui, W.; Li, J.; Sun, Y.; Wang, Z.; Dong, F. Rare-Earth Single-Atom La–N Charge-Transfer Bridge on Carbon Nitride for Highly Efficient and Selective Photocatalytic CO2 Reduction. ACS Nano 2020, 14, 15841–15852. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Yu, J.G.; Cao, S.W. Designing Defective Crystalline Carbon Nitride to Enable Selective CO2 Photoreduction in the Gas Phase. Adv. Funct. Mater. 2019, 29, 1900093. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-Vacancy Modified Graphitic Carbon Nitride: Enhanced CO2 Photocatalytic Reduction Performance and Mechanism Probing. J. Mater. Chem. A 2019, 7, 1556–1563. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, S.; Li, M.; Zhang, J. Selective Photocatalytic CO2 Reduction to CH4 on Tri-s-triazine-Based Carbon Nitride via Defects and Crystal Regulation: Synergistic Effect of Thermodynamics and Kinetics. ACS Appl. Mater. Interfaces 2022, 14, 25417–25426. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Huang, L.; Tian, L.; Shalom, M.; Xiong, C.; Zhang, H.; Jia, Q.; Zhang, S.; Liang, F. Ultrathin Mesoporous Graphitic Carbon Nitride Nanosheets with Functional Cyano Group Decoration and Nitrogen-Vacancy Defects for Efficient Selective CO2 Photoreduction. Nanoscale 2021, 13, 12634–12641. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253–1260. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Zhou, R.; Yang, H.; Yan, Z.; Wu, D.; Nkoom, M. Switching g-C3N4 morphology from double-walled to single-walled microtubes induced high photocatalytic H2-production performance. J. Alloys Compd. 2020, 820, 153166. [Google Scholar] [CrossRef]
- Sun, L.; Wang, W.; Zhang, C.; Cheng, M.; Zhou, Y.; Yang, Y.; Ouyang, Z. Multiple optimization strategies for improving photocatalytic performance of the h-BN/flower-ring g-C3N4 heterostructures, Morphology engineering and internal electric field effect. Chem. Eng. J. 2022, 446, 137027. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Jiang, W.; Zhu, Z.; Wang, Y.; Liu, Z.; Teng, F. Structural reconstruction of carbon nitride with tailored electronic structure, A bifunctional photocatalyst for cooperative artificial photosynthesis and selective phenylcarbinol oxidation. Appl. Catal. B Environ. 2021, 298, 120517. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Chen, H.S.; Yang, P.; Jiang, S.P. Fusiform-Shaped g-C3N4 Capsules with Superior Photocatalytic Activity. Small 2020, 16, 2003910. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, D.; Xiang, Q. Nanosheet-assembled hierarchical flower-like g-C3N4 for enhanced photocatalytic CO2 reduction activity. Chem. Commun. 2020, 56, 2443–2446. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Gan, L.; Ji, X.; Chen, F.; Peng, X.; Zhang, R. 3D macropore carbon-vacancy g-C3N4 constructed using polymethylmethacrylate spheres for enhanced photocatalytic H2 evolution and CO2 reduction. J. Energy Chem. 2021, 53, 139–146. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Li, H.; Han, D.; Song, X.; Huo, P. Regulation CN reduction of CO2 products selectivity by adjusting the number of V sites and mechanism exploration. Fuel 2025, 388, 134509. [Google Scholar] [CrossRef]
- Lei, K.; Wang, D.; Ye, L.; Kou, M.; Deng, Y.; Ma, Z.; Kong, Y. A metal-free donor–acceptor covalent organic framework photocatalyst for visible-light-driven reduction of CO2 with H2O. ChemSusChem 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Thiruppathiraja, T.; Rhimi, B.; Zhang, N.; Zhou, M.; Shi, W.; Jiang, Z. Theoretical investigations of hydroxyl-functionalized iron-doped phthalocyanine photocatalyst for efficient CO2 reduction to methanol and methane. Chem. Eng. J. 2025, 305, 121121. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Fan, H.; Liu, X.; Cao, J.; Liu, H.; Vomiero, A. Oxygen vacancy-rich Ni2P2O7 modified g-C3N4 heterojunction for highly-efficient CO2 photoreduction. Chem. Eng. J. 2024, 500, 157174. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Shangguan, Y.; Wu, X.; Chen, H. Selective CO2 photoreduction to CH4 mediated by dimension-matched 2D/2D Bi3NbO7/g-C3N4 S-scheme heterojunction. Chin. J. Catal. 2022, 43, 246–254. [Google Scholar] [CrossRef]
- Wu, J.; Li, K.; Li, J.; Du, J.; Li, X.; Song, C.; Guo, X. An S-scheme heterojunction constructed from α-Fe2O3 and In-doped carbon nitride for high-efficiency CO2 photoreduction. Catal. Sci. Technol. 2022, 12, 1520–1529. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, D.; Yan, J.; Lee, L.Y.S. Photocatalytic CO2 reduction enabled by interfacial S-scheme heterojunction between ultrasmall copper phosphosulfide and g-C3N4. ACS Appl. Mater. Interfaces 2021, 13, 9762–9770. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Zhang, J.; Liang, C.; Dai, K. Fabrication of novel CoO/porous graphitic carbon nitride S-scheme heterojunction for efficient CO2 photoreduction. Mater. Lett. 2021, 282, 128722. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Wang, Z.; Dai, K.; Pan, C.; Liang, C. Efficient interfacial charge transfer of 2D/2D porous carbon nitride/bismuth oxychloride step-scheme heterojunction for boosted solar-driven CO2 reduction. J. Colloid Interface Sci. 2021, 585, 684–693. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. Constructing S-scheme 2D/0D g-C3N4/TiO2 NPs/MPs heterojunction with 2D-Ti3AlC2 MAX cocatalyst for photocatalytic CO2 reduction to CO/CH4 in fixed-bed and monolith photoreactors. J. Mater. Sci. Technol. 2022, 106, 195–210. [Google Scholar] [CrossRef]
- Meng, F.; Qu, C.; Wang, L.; Yang, D.; Zhao, Z.; Ye, Q. ZIF-67-derived NiCo2O4 hollow nanocages coupled with g-C3N4 nanosheets as Z-scheme photocatalysts for enhancing CO2 reduction. J. Colloid Interface Sci. 2025, 684, 492–502. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Dai, K. Graphitic carbon nitride/antimonene van der Waals heterostructure with enhanced photocatalytic CO2 reduction activity. J. Mater. Sci. Technol. 2022, 116, 192–198. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Zhang, X.; Li, X.; Zhu, Z.; Ma, C.; Yang, G. Boosting charge carriers separation and migration efficiency via fabricating all organic van der Waals heterojunction for efficient photoreduction of CO2. Chem. Eng. J. 2021, 408, 127292. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Mohamed, R.M.; Ismail, A.; Alhaddad, M. Z-scheme g-C3N4 nanosheet photocatalyst decorated with mesoporous CdS for the photoreduction of carbon dioxide. Ceram. Int. 2021, 47, 17210–17219. [Google Scholar] [CrossRef]
- Madhusudan, P.; Shi, R.; Xiang, S.; Jin, M.; Chandrashekar, B.N.; Wang, J.; Wang, W.; Peng, O.; Amini, A.; Cheng, C. Construction of highly efficient Z-scheme ZnxCd1-xS/Au@g-C3N4 ternary heterojunction composite for visible-light-driven photocatalytic reduction of CO2 to solar fuel. Appl. Catal. B Environ. 2021, 282, 19600. [Google Scholar] [CrossRef]
- Bhosale, R.; Jain, S.; Vinod, C.P.; Kumar, S.; Ogale, S. Direct Z-Scheme g-C3N4/FeWO4 Nanocomposite for Enhanced and Selective Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 6174–6183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, J.; Sun, Y.; Jia, S.; Liu, Z.; Tang, X.; Zhu, Z. Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight. Catalysts 2024, 14, 546. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, H.; Zhang, W.; Zhu, G.; Yang, W.; Son, N.; Kang, M.; Liu, C. Z-scheme SnFe2O4-graphitic carbon nitride: Reusable, magnetic catalysts for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2020, 383, 123172. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T. Heterostructured AgX/g-C3N4 (X = Cl and Br) nanocomposites via a sonication-assisted deposition-precipitation approach: Emerging role of halide ions in the synergistic photocatalytic reduction of carbon dioxide. Appl. Catal. B Environ. 2016, 180, 530–543. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Huang, H.; Guo, Y.; Zhang, Y. Bi4NbO8Cl {001} nanosheets coupled with g-C3N4 as 2D/2D heterojunction for photocatalytic degradation and CO2 reduction. J. Hazard. Mater. 2020, 381, 121159. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Wang, M.; Cheng, R.; Zhang, L.; Yao, H.; Shi, J. Core-shell LaPO4/g-C3N4 nanowires for highly active and selective CO2 reduction. Appl. Catal. B Environ. 2017, 201, 629–635. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhang, J.; Tao, F.; Xu, J. Construction of NiO/g-C3N4 p-n heterojunctions for enhanced photocatalytic CO2 reduction. J. Solid State Chem. 2022, 308, 122878. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B Environ. 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Hu, J.; Yang, T.; Yang, X.; Qu, J.; Cai, Y.; Li, C.M. Highly Selective and Efficient Solar-Light-Driven CO2 Conversion with an Ambient-Stable 2D/2D Co2P@BP/g-C3N4 Heterojunction. Small 2021, 18, 2105376. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Liu, Z.; Zhu, Z.; Tang, X.; Wang, Y. Study on optical properties of alkali metal doped g-C3N4 and their photocatalytic activity for reduction of CO2. Chem. Phys. Lett. 2020, 751, 137467. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Tan, Y.-C.; Tan, L.-L.; Li, N.; Ng, Y.H.; Wen, X.; Chai, S.P. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Huo, P.; Yan, Y.; Zhu, Z.; Dai, J.; Liu, Z.; Li, Z.; Xi, H. Insight into the Effect of the Cl 3p Orbital on g-C3N4 Mimicking Photosynthesis under CO2 Reduction. J. Phys. Chem. C 2021, 125, 9646–9656. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, and Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Wei, S.; Li, X.; Zhou, Y. Mechanistic insights into CO2 reduction on Cu/Mo-loaded two-dimensional g-C3N4(001). Phys. Chem. Chem. Phys. 2017, 19, 4405–4410. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Guan, H.; Yu, J.; Cao, S. Potassium/oxygen co-doped polymeric carbon nitride for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 563, 150310. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, R.K.; Park, N.-J.; Baeg, J.-O. Facile One-Pot Two-Step Synthesis of Novel in Situ Selenium-Doped Carbon Nitride Nanosheet Photocatalysts for Highly Enhanced Solar Fuel Production from CO2. ACS Appl. Nano Mater. 2017, 1, 47–54. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Gao, C.; Wu, X. The enhanced photo-catalytic CO2 reduction performance of g-C3N4 with high selectivity by coupling CoNiSx. Mater. Res. Bull. 2021, 144, 111488. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Zheng, F.; Zhao, J.; Lee, L.Y.S. Designing charge transfer route at the interface between WP nanoparticle and g-C3N4 for highly enhanced photocatalytic CO2 reduction reaction. Appl. Catal. B Environ. 2021, 286, 119879. [Google Scholar] [CrossRef]
- Li, J.; Yan, P.; Li, K.; You, J.; Wang, H.; Cui, W.; Cen, W.; Chu, Y.; Dong, F. Cu supported on polymeric carbon nitride for selective CO2 reduction into CH4: A combined kinetics and thermodynamics investigation. J. Mater. Chem. A. 2019, 7, 17014–17021. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, D.; Cheng, L.; Xiang, Q. Crystalline carbon nitride supported copper single atoms for photocatalytic CO2 reduction with nearly 100% CO selectivity. ACS Nano 2020, 14, 10552–105561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Chen, J.W.; Zhong, L.; Zhen, W.; Tay, Y.Y.; Li, S.; Wang, Y.G.; Huang, L.; Xue, C. Synergistic effect of Ru-N4 sites and Cu-N3 sites in carbon nitride for highly selective photocatalytic reduction of CO2 to methane. Appl. Catal. B Environ. 2022, 307, 121154. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Z.; Zhou, B.; Shen, J.; He, H.; Ali, A.; Zhong, Q.; Xiong, Y.; Gao, C.; Alsaedi, A. In situ no-slot joint integration of half-metallic C (CN)3 cocatalyst into g-C3N4 scaffold: An absolute metal-free in-plane heterosystem for efficient and selective photoconversion of CO2 into CO. Appl. Catal. B Environ. 2020, 264, 118470. [Google Scholar] [CrossRef]
- Lang, Q.; Yang, Y.; Zhu, Y.; Hu, W.; Jiang, W.; Zhong, S.; Gong, P.; Teng, B.; Zhao, L.; Bai, S. High-index facet engineering of PtCu cocatalysts for superior photocatalytic reduction of CO2 to CH4. J. Mater. Chem. A 2017, 5, 6686–6694. [Google Scholar] [CrossRef]
- Cao, S.; Li, Y.; Zhu, B.; Jaroniec, M.; Yu, J. Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4. J. Catal. 2017, 349, 208–217. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, L.; Jiang, K.; Liu, K.; Wang, Z.; Qiu, X.; Li, H.; Hu, J.; Pan, H.; Lu, Y.R.; et al. Activation of CO2 on graphitic carbon nitride supported single-atom cobalt sites. Chem. Eng. J. 2021, 415, 128982. [Google Scholar] [CrossRef]
- Shi, X.; Huang, Y.; Bo, Y.; Duan, D.; Wang, Z.; Cao, J.; Zhu, G.; Ho, W.; Wang, L.; Huang, T.; et al. Highly selective photocatalytic CO2 methanation with water vapor on single-atom platinum-decorated defective carbon nitride. Angew. Chem. Int. Ed. 2022, 134, e202203063. [Google Scholar] [CrossRef]
- Wang, J.; Heil, T.; Zhu, B.; Tung, C.W.; Yu, J.; Chen, H.; Antonietti, M.; Cao, S. A single Cu-center containing enzyme-mimic enabling full photosynthesis under CO2 reduction. ACS Nano 2020, 14, 8584–8593. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Ren, Z.; Liu, X.; Wang, M.; Wu, Y.; Zhou, W.; Zhu, Z.; Huo, P. Insights into the greatly improved catalytic performance of N-doped BiOBr for CO2 photoreduction. Acta Phys.-Chim. Sin. 2025, 41, 100055. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Yuan, K.; Sewell, C.D.; Zhang, Z.; Fang, X.; Lin, Z. Robust route to highly porous graphitic carbon nitride microtubes with preferred adsorption ability via rational design of one-dimension supramolecular precursors for efficient photocatalytic CO2 conversion. Nano Energy 2020, 77, 105104. [Google Scholar] [CrossRef]
- Song, X.; Wang, M.; Liu, W.; Li, X.; Zhu, Z.; Huo, P.; Yan, Y. Thickness regulation of graphitic carbon nitride and its influence on the photocatalytic performance towards CO2 reduction. Appl. Surf. Sci. 2022, 577, 151810. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 117854. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Li, H.; Li, H.; Qi, W.; Zhang, Q.; Zhu, J.; Zhao, P.; Chen, L. Rational design of 3D carbon nitrides assemblies with tunable nano-building blocks for efficient visible-light photocatalytic CO2 conversion. Appl. Catal. B Environ. 2022, 316, 121612. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, G.; Zhang, L.; Li, G.; An, T. Highly efficient and selective photoreduction of CO2 to CO with nanosheet g-C3N4 as compared with its bulk counterpart. Environ. Res. 2021, 195, 110880. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, B.; Guo, Q.; Guo, S.; Peng, Z.; Liu, J.; Tian, Q.; Yang, Y.; Xu, Q.; Liu, Z.; et al. Van der Waals heterojunction for selective visible-light-driven photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 284, 119733. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A hierarchical Z-scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, H.; Liu, B.; Guo, H. Z-scheme Au@Void@g-C3N4/SnS yolk–shell heterostructures for superior photocatalytic CO2 reduction under visible light. ACS Appl. Mater. Interfaces 2018, 10, 34123–34131. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Xie, L.; Geng, K.; Wu, J.; Meng, X.; Hou, H. Rational construction of metal organic framework hybrid assemblies for visible light-driven CO2 conversion. Inorg. Chem. 2023, 62, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Ikreedeegh, R.R.; Tahir, M. Indirect Z-scheme heterojunction of NH2-MIL-125 (Ti) MOF/g-C3N4 nanocomposite with RGO solid electron mediator for efficient photocatalytic CO2 reduction to CO and CH4. J. Environ. Chem. Eng. 2021, 9, 105600. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tasleem, S.; Hossen, M.A. Facile fabrication of binary g-C3N4/NH2-MIL-125(Ti) MOF nanocomposite with Z-scheme heterojunction for efficient photocatalytic H2 production and CO2 reduction under visible light. Fuel 2024, 360, 130561. [Google Scholar] [CrossRef]
- Sonowal, K.; Nandal, N.; Basyach, P.; Kalita, L.; Jain, S.L.; Saikia, L. Photocatalytic reduction of CO2 to methanol using Zr (IV)-based MOF composite with g-C3N4 quantum dots under visible light irradiation. J. CO2 Util. 2022, 57, 101905. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Li, S.; Peng, X.; Xiong, Y. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters. Appl. Catal. B Environ. 2017, 211, 1–10. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the g-C3N4 nanosheet with B–H bonding decorated metal–organic framework for CO2 activation and photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Zeng, Y.; Guo, H.; Wan, S.; Ou, M.; Zhang, S.; Zhong, Q. Amino-assisted NH2-UiO-66 anchored on porous g-C3N4 for enhanced visible-light-driven CO2 reduction. ACS Appl. Mater. Interfaces 2019, 11, 30673–30681. [Google Scholar] [CrossRef]
- Dao, X.Y.; Xie, X.F.; Guo, J.H.; Zhang, X.Y.; Kang, Y.S.; Sun, W.Y. Boosting photocatalytic CO2 reduction efficiency by heterostructures of NH2-MIL-101(Fe)/g-C3N4. ACS Appl. Energy Mater. 2020, 3, 3946–3954. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Zhu, Y.; Liang, Z.; Pei, A.; Wu, C.L.; Wang, H.; Lee, H.R.; Liu, K.; Chu, S.; et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018, 1, 592–600. [Google Scholar] [CrossRef]
- Song, X.; Li, G.; Liu, X.; Xu, S.; Zhou, W.; Zhu, Z.; Wu, Y. Achieving efficient photoreduction of CO2 by simultaneously facilitating the splitting of H2O and the conversion of *CO intermediates. Chem. Eng. J. 2025, 505, 159308. [Google Scholar] [CrossRef]
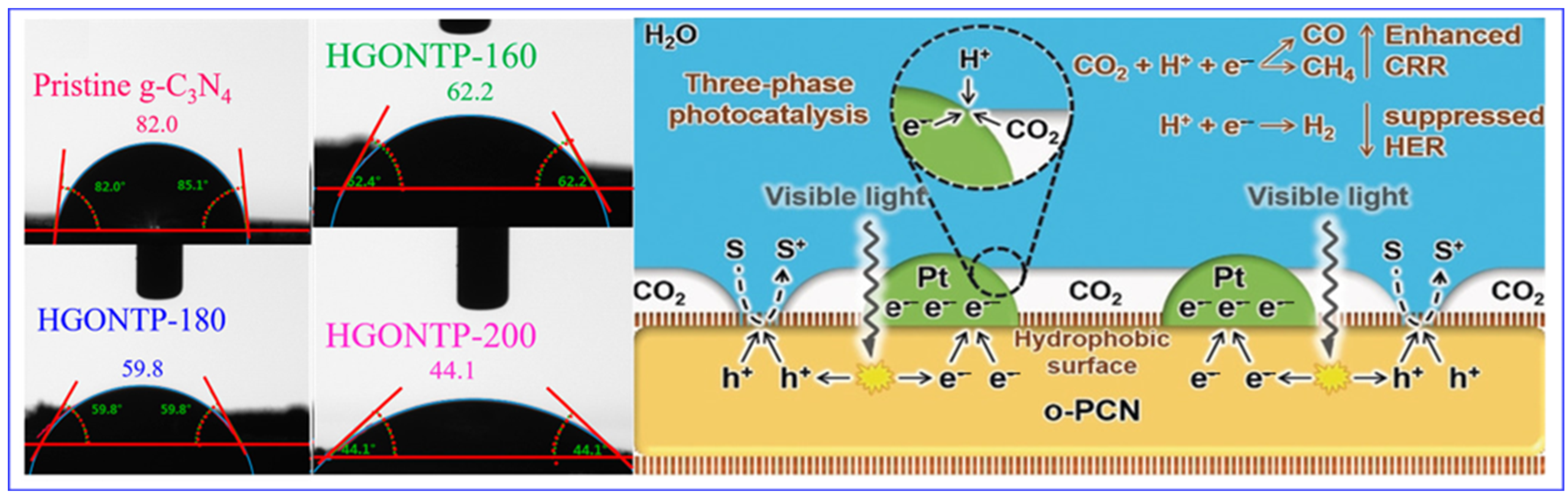
- Li, A.; Cao, Q.; Zhou, G.; Schmidt, B.V.; Zhu, W.; Yuan, X.; Huo, H.; Gong, J.; Antonietti, M. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface. Angew. Chem. Int. Ed. 2019, 58, 14549–14555. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energ. Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.; Cao, S.; Cui, C.; Cheng, B. Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl. Surf. Sci. 2015, 358, 350–355. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, X.; Tang, Y.; Liu, C.; Luo, S. Efficient photocatalytic hydrogen evolution and CO2 reduction: Enhanced light absorption, charge separation, and hydrophilicity by tailoring terminal and linker units in g-C3N4. ACS Appl. Mater. Interfaces 2020, 12, 19607–19615. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, K.; Cheng, B.; Yu, J.; Jiang, L.; Antonietti, M.; Cao, S. Improving artificial photosynthesis over carbon nitride by gas–liquid–solid interface management for full light-induced CO2 reduction to C1 and C2 fuels and O2. ChemSusChem 2020, 13, 1730–1734. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, F.; Wang, D.; Cai, X.; Meng, C.; Xie, H.; Zhang, J.; Bai, S. Lattice Engineering on Metal Cocatalysts for Enhanced Photocatalytic Reduction of CO2 into CH4. ChemSusChem 2018, 11, 3524–3533. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, T.; Chao, D. Organic terpyridine molecule as an efficient cocatalyst for metal–free CO2 photoreduction mediated by mesoporous graphitic carbon nitride. Chem. Eng. J. 2022, 429, 132348. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Wang, W.; Li, H.; Zhao, J.; Tang, X. Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction C1 Product Selectivity. Catalysts 2025, 15, 408. https://doi.org/10.3390/catal15050408
Zhu Z, Wang W, Li H, Zhao J, Tang X. Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction C1 Product Selectivity. Catalysts. 2025; 15(5):408. https://doi.org/10.3390/catal15050408
Chicago/Turabian StyleZhu, Zhi, Wei Wang, Hongping Li, Jun Zhao, and Xu Tang. 2025. "Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction C1 Product Selectivity" Catalysts 15, no. 5: 408. https://doi.org/10.3390/catal15050408
APA StyleZhu, Z., Wang, W., Li, H., Zhao, J., & Tang, X. (2025). Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction C1 Product Selectivity. Catalysts, 15(5), 408. https://doi.org/10.3390/catal15050408









