High-Efficiency Biodiesel Production Using ZnO-Modified Starfish-Based Catalysts
Abstract
1. Introduction
2. Results
2.1. Structural and Surface Characterization
2.1.1. XRD Analysis
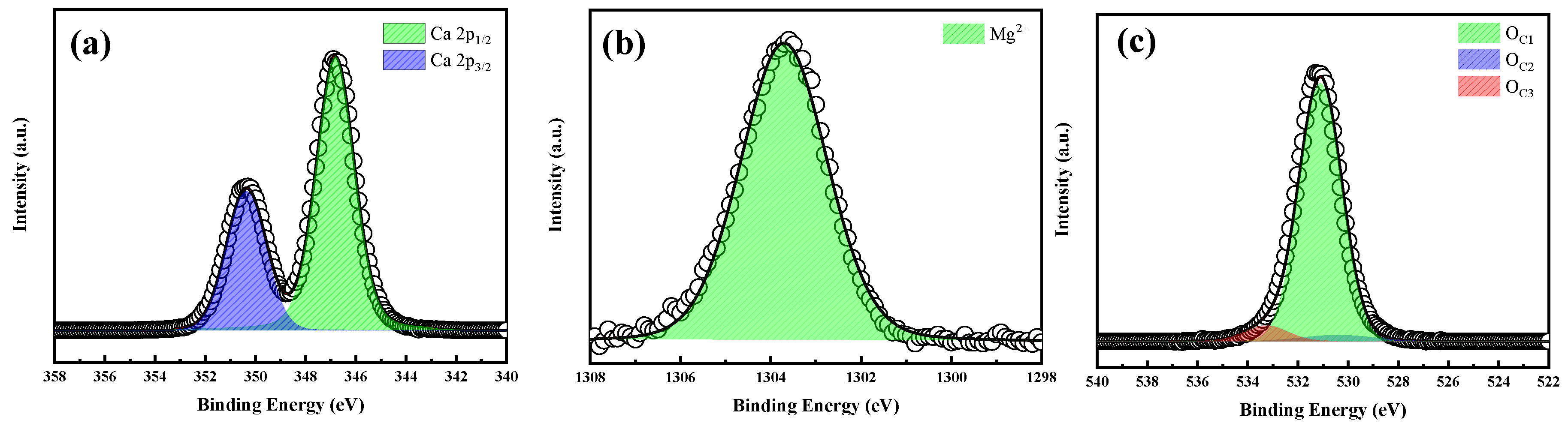
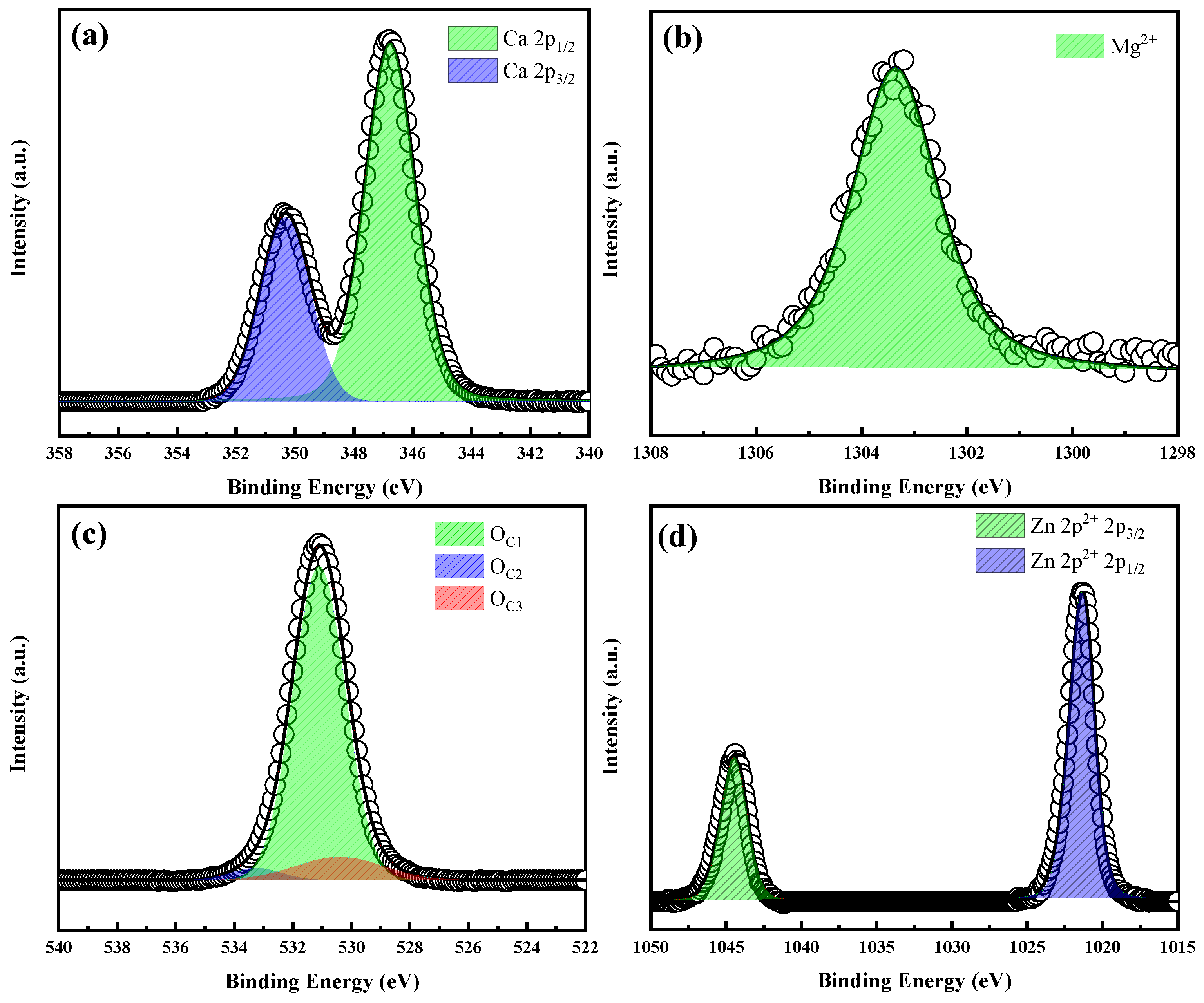
2.1.2. XPS Analysis
2.1.3. Morphological Analysis by SEM
2.1.4. BET Surface Area and Porosity Analysis
2.2. Catalytic Performance in Biodiesel Production
2.2.1. Basicity Measurement by HCl Titration
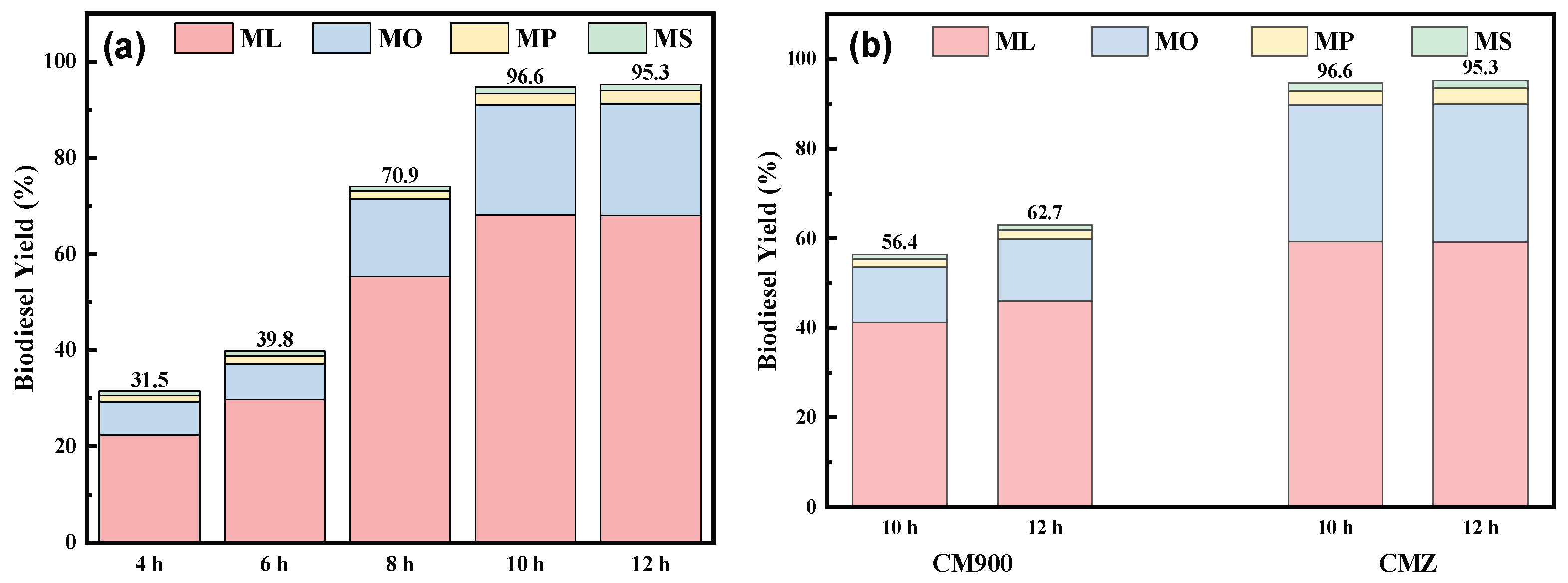
2.2.2. Biodiesel Yield and Reaction Efficiency
3. Discussion
3.1. Effect of Zn Doping on Catalyst Structure
3.2. Surface Chemistry and Catalytic Activity
3.3. Role of Surface Area and Porosity in Catalysis
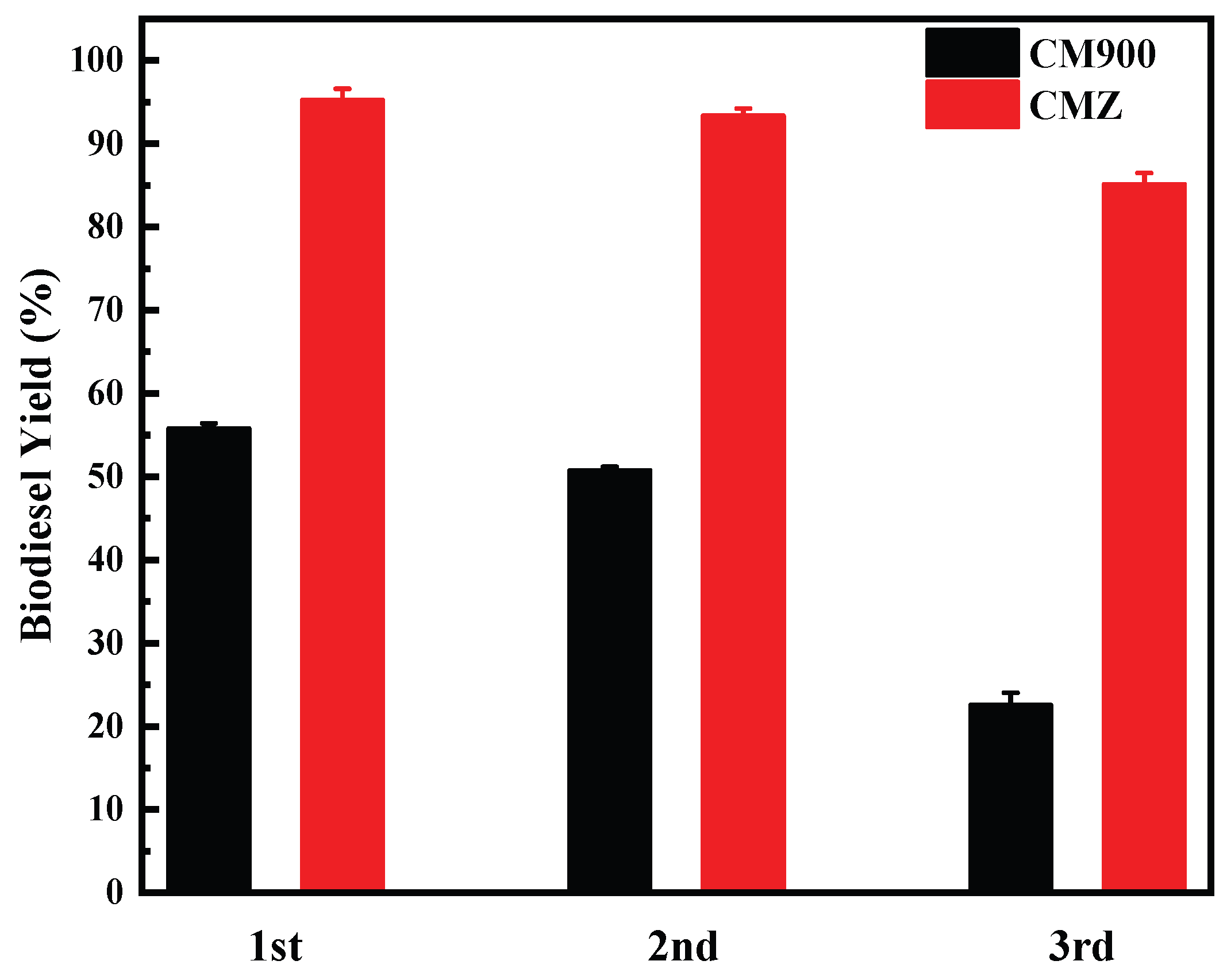
3.4. Catalytic Performance and Reusability
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.2.1. Calcination of Starfish-Derived Catalyst (CM900)
4.2.2. Zinc Doping via Hydrothermal Treatment (CMZ)
4.3. Catalyst Characterization
- X-ray diffraction (XRD): Structural analysis was conducted using an X-ray diffractometer (Rigaku, Ultima IV, Tokyo, Japan) with Cu Kα radiation (40 kV, 40 mA).
- Field Emission Scanning Electron Microscopy (FE-SEM): The morphology of the catalysts was examined using a MIRA3 SEM (TESCAN, Brno, Czech Republic).
- X-ray photoelectron spectroscopy (XPS): The surface chemical composition was analyzed with an X-ray photoelectron spectrometer (JEOL, JPS-9010 MC, Tokyo, Japan) at the Korea Basic Science Institute (Busan, Republic of Korea), using Al Kα monochromatic radiation (hν = 1486.6 eV).
- Brunauer–Emmett–Teller (BET) Surface Area Analysis: The surface area and pore volume were measured to assess catalyst textural properties.
4.4. Transesterification of Grapeseed Oil
- Methanol/oil molar ratio: 10:1.
- Catalyst loading: 1 wt.% (based on the total weight of oil and methanol).
- Reaction temperature: 68 °C.
- Stirring speed: 1500 rpm.
- Reaction time: 4, 6, 8, 10, and 12 h.
4.5. Catalytic Performance Evaluation
4.5.1. Basicity Measurement via HCl Titration
4.5.2. Biodiesel Yield Analysis via HPLC
- Oven temperature: 40 °C.
- Flow rate: 0.5 mL/min.
- Injection volume: 1 µL.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction. |
| XPS | X-ray photoelectron spectroscopy. |
| SEM | Scanning Electron Microscopy. |
| BET | Brunauer–Emmett–Teller. |
| CM900 | Calcined starfish catalyst at 900 °C. |
| CMZ | Zn-doped calcined starfish catalyst (900 °C). |
| FAME | Fatty acid methyl ester. |
| HPLC | High-Performance Liquid Chromatography. |
References
- Newell, R.; Raimi, D.; Villanueva, S.; Prest, B. Global energy outlook 2021: Pathways from Paris. Resour. Future 2021, 8, 39. [Google Scholar]
- Bunce, M.; Snyder, D.; Adi, G.; Hall, C.; Koehler, J.; Davila, B.; Kumar, S.; Garimella, P.; Stanton, D.; Shaver, G. Optimization of soy-biodiesel combustion in a modern diesel engine. Fuel 2011, 90, 2560–2570. [Google Scholar] [CrossRef]
- Ma, F.R.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sust. Energ. Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Beveridge, T.H.J.; Girard, B.; Kopp, T.; Drover, J.C.G. Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: Varietal effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef]
- Fernández, C.M.; Ramos, M.J.; Pérez, A.; Rodríguez, J.F. Production of biodiesel from winery waste: Extraction, refining and transesterification of grape seed oil. Bioresour. Technol. 2010, 101, 7019–7024. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenerg. Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Faria, E.A.; Marques, J.S.; Dias, I.M.; Andrade, R.D.A.; Suarez, P.A.Z.; Prado, A.G.S. Nanosized and Reusable SiO/ZrO Catalyst for Highly Efficient Biodiesel Production by Soybean Transesterification. J. Braz. Chem. Soc. 2009, 20, 1732–1737. [Google Scholar] [CrossRef]
- Tahvildari, K.; Anaraki, Y.N.; Fazaeli, R.; Mirpanji, S.; Delrish, E. The study of CaO and MgO heterogenic nano-catalyst coupling on transesterification reaction efficacy in the production of biodiesel from recycled cooking oil. J. Environ. Health Sci. 2015, 13, 73. [Google Scholar] [CrossRef]
- Arenas-Quevedo, M.G.; Manríquez, M.E.; Wang, J.A.; Elizalde-Solís, O.; Gonzalez-Garcia, J.; Zúñiga-Moreno, A.; Chen, L.F. ZnO-Doped CaO Binary Core-Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification. Inorganics 2024, 12, 51. [Google Scholar] [CrossRef]
- Yan, S.L.; Lu, H.F.; Liang, B. Supported CaO catalysts used in the transesterification of rapeseed oil for the purpose of biodiesel production. Energ. Fuel 2008, 22, 646–651. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Lee, H.V.; Hussein, M.Z.; Yunus, R. Calcium-based mixed oxide catalysts for methanolysis of oil to biodiesel. Biomass Bioenerg. 2011, 35, 827–834. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shan, R.; Li, S.Y.; Shi, J.F. A biomimetic silicification approach to synthesize CaO-SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 2015, 153, 48–55. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shan, R.; Shi, J.F.; Yan, B.B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A.; Ali, D.M.H. Utilization of waste cockle shell (Andara granosa) in biodiesel production from palm olein: Optimization using response surface methodology. Fuel 2011, 90, 2353–2358. [Google Scholar] [CrossRef]
- Wang, A.A.N.; Quan, W.X.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for efficient biodiesel production. Rsc. Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Radón, A.; Hawelek, L.; Lukowiec, D.; Kubacki, J.; Wlodarczyk, P. Dielectric and electromagnetic interference shielding properties of high entropy (Zn,Fe,Ni,Mg,Cd)Fe2O4 ferrite. Sci. Rep. 2019, 9, 20078. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.M.; Samundsett, C.; Bullock, J.; Hettick, M.; Allen, T.; Yan, D.; Peng, J.; Wu, Y.L.; Cui, J.; Javey, A.; et al. Conductive and Stable Magnesium Oxide Electron-Selective Contacts for Efficient Silicon Solar Cells. Adv. Energy Mater. 2017, 7, 1601863. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Salim, S.M.; Izriq, R.; Almaky, M.M.; Al-Abbassi, A.A. Synthesis and characterization of ZnO nanoparticles for the production of biodiesel by transesterification: Kinetic and thermodynamic studies. Fuel 2022, 321, 124135. [Google Scholar] [CrossRef]
- Shu, H.B.; Wang, X.Y.; Wu, Q.; Liu, L.; Liang, Q.Q.; Yang, S.Y.; Ju, B.W.; Yang, X.K.; Zhang, X.Y.; Wang, Y.P.; et al. The effect of ammonia concentration on the morphology and electrochemical properties of LiFePO4 synthesized by ammonia assisted hydrothermal route. Electrochim. Acta 2012, 76, 120–129. [Google Scholar] [CrossRef]
- FoodsafetyKorea, Grapeseed Oil Nutrition Detail. Available online: https://various.foodsafetykorea.go.kr/nutrient/general/food/detail.do?dbGrpCm=A&searchTextPre=&searchTextListStr=&sortOrder=DESC&sortFieldCnt=1&searchProcCode=LOG_IDX&searchLogType=main&searchSubOrderby=&searchDetailMode=all&searchGroup=&searchCrtMth=1&searchMaker=&searchSource=&searchClass=&searchQc=&searchHcln=&searchReduct=&searchText=%ED%8F%AC%EB%8F%84%EC%94%A8%EC%9C%A0&searchOper=AND&searchGroupText=&searchMakerText=&searchOrderby=CAL&searchPageCnt=10&pagenum=1&totalListCnt=1&pageblock=10&pagesize=10&searchFoodCd=R114-026000000-0000&searchRegionCd=ZZ&searchMonthCd=AVG (accessed on 29 June 2024).
- TANABE, K.; YAMAGUCHI, T. Measurement of acidity and basicity of silica-alumina and acidity of zinc sulfide by calorimetric titration. J. Res. Inst. Catal. Hokkaido Univ. 1966, 14, 93–100. [Google Scholar]


| Catalyst | SBET (m2·g−1) | Vp (cm3·g−1) | Pore Size (Å) |
|---|---|---|---|
| CM900 | 12 ± 5% | 0.031 ± 5% | 106 ± 5% |
| CMZ | 19 ± 5% | 0.047 ± 5% | 101 ± 5% |
| Basic Strength (mmol·g−1) | Site Density (Sites·g−1) | |
|---|---|---|
| CM900 | 2.48 | 1.49 × 1021 |
| CMZ | 2.68 | 1.61 × 1021 |
| Catalysts | Ca (%) | Mg (%) | Zn (%) |
|---|---|---|---|
| CMZ | 2% | 1% | 0.5% |
| CMZ 1st reuse | 2% | 1% | 0.5% |
| CMZ 2nd reuse | 2% | 1% | 0.5% |
| CMZ 3rd reuse | 2% | 1% | 0.5% |
| Catalysts | Reaction Temperature (°C) | Catalyst Amount (wt %) | Methanol/Oil Molar Ratio | Reaction Time (h) | Biodiesel Yield (%) |
|---|---|---|---|---|---|
| CMZ | 68 | 1 | 10:1 | 10 | 94.7 |
| CaO/MgO [14] | 64.5 | 10 | 18:1 | 3.5 | 92 |
| CaO [15] | 65 | 4 | 15:1 | 6 | 85 |
| CaMgO [15] | 65 | 4 | 15:1 | 6 | 83 |
| CaZnO [15] | 65 | 4 | 15:1 | 6 | 81 |
| CaO (0Si5Ca) [16] | 65 | 9 | 15:1 | 3 | 90.2 |
| CaO-SiO2 (2Si5Ca) [16] | 65 | 9 | 15:1 | 8 | 80.1 |
| CaO (Ostrich eggshell) [17] | 60 | 8 | 9:1 | 1 | 92.7 |
| Linoleic Acid (mg) | Oleic Acid (mg) | Palmitic Acid (mg) | Stearic Acid (mg) |
|---|---|---|---|
| 694.55 | 139.38 | 61.71 | 35.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.; Lee, S.; Li, O.L. High-Efficiency Biodiesel Production Using ZnO-Modified Starfish-Based Catalysts. Catalysts 2025, 15, 372. https://doi.org/10.3390/catal15040372
Ha J, Lee S, Li OL. High-Efficiency Biodiesel Production Using ZnO-Modified Starfish-Based Catalysts. Catalysts. 2025; 15(4):372. https://doi.org/10.3390/catal15040372
Chicago/Turabian StyleHa, Jeyoung, Sungho Lee, and Oi Lun Li. 2025. "High-Efficiency Biodiesel Production Using ZnO-Modified Starfish-Based Catalysts" Catalysts 15, no. 4: 372. https://doi.org/10.3390/catal15040372
APA StyleHa, J., Lee, S., & Li, O. L. (2025). High-Efficiency Biodiesel Production Using ZnO-Modified Starfish-Based Catalysts. Catalysts, 15(4), 372. https://doi.org/10.3390/catal15040372






