Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea
Abstract
:1. Introduction
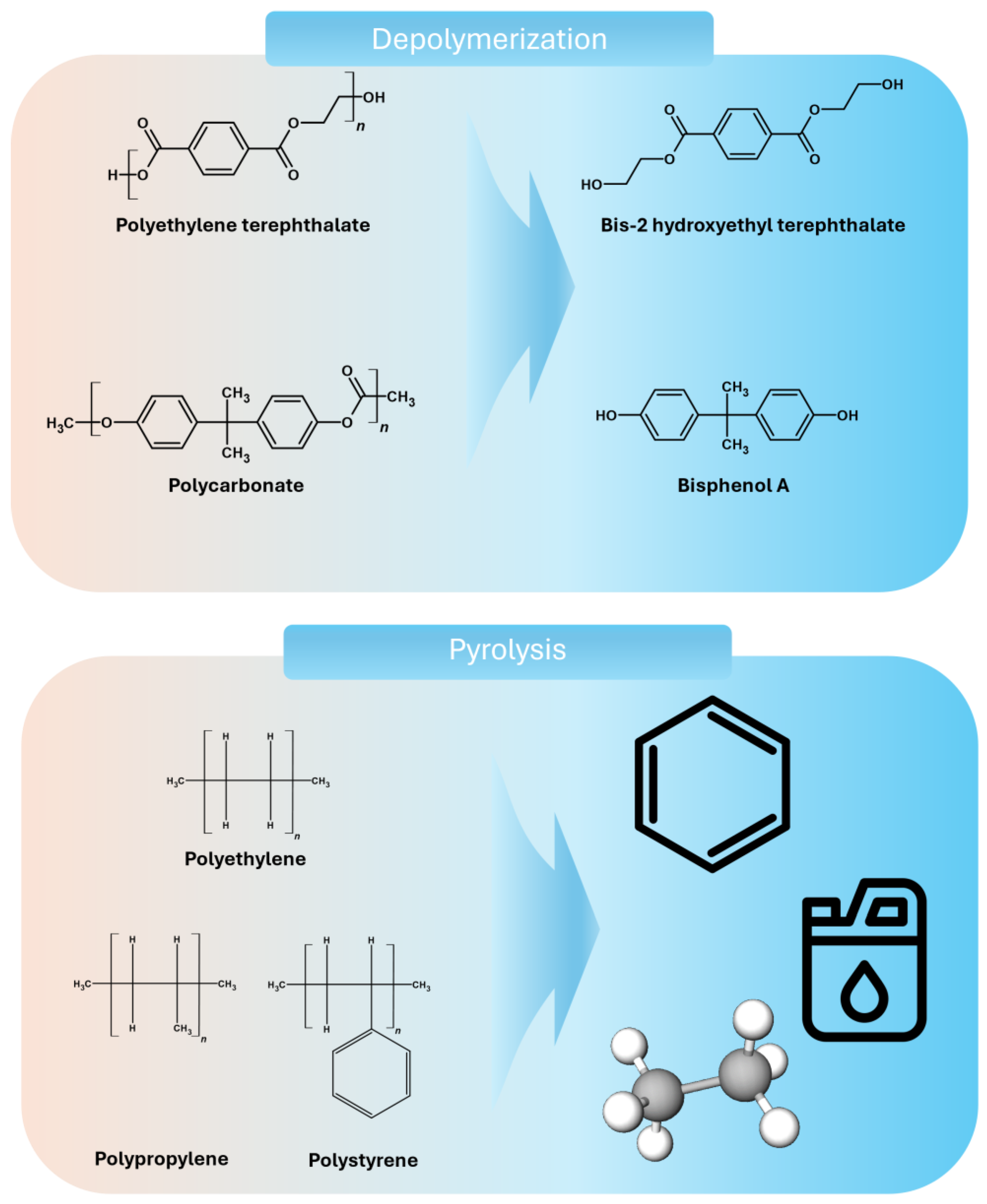
2. Mechanisms of Chemical Recycling of Plastics
2.1. Categorization of Chemical Recycling Methodologies
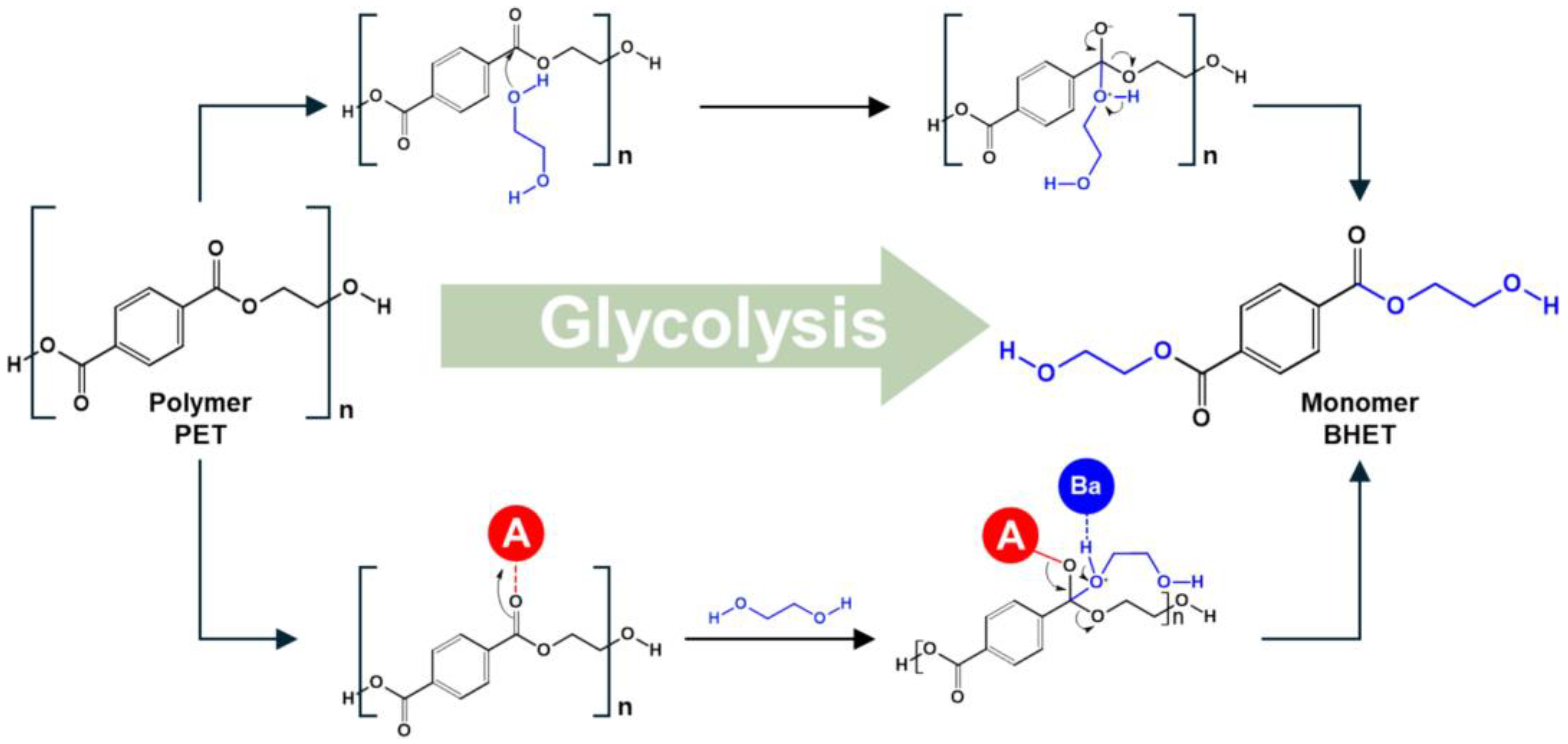
2.2. Mechanisms of Depolymerization
2.3. Mechanisms of Pyrolysis
3. Depolymerization
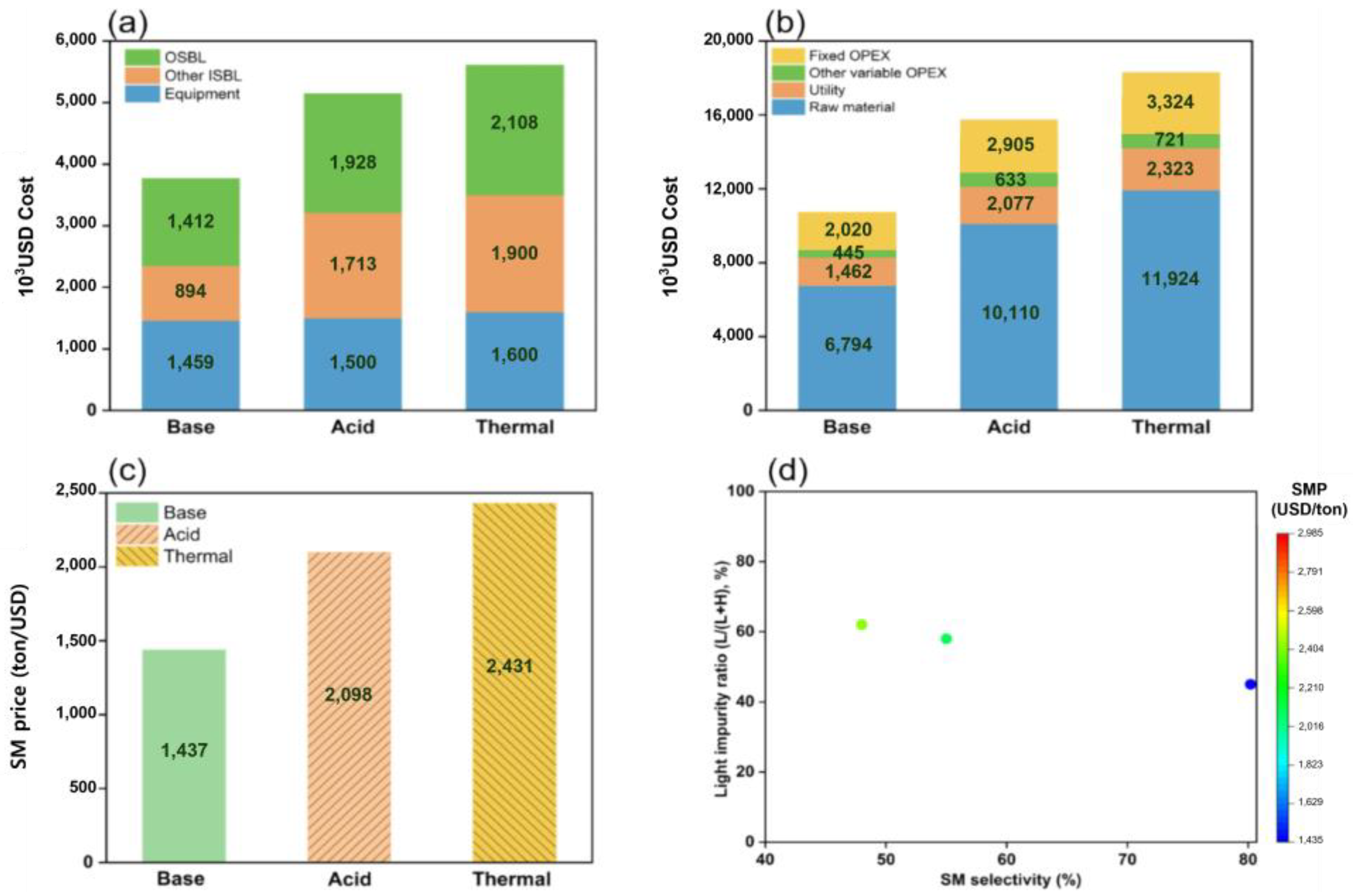
3.1. Depolymerization of PET

3.2. Depolymerization of Polycarbonate
4. Pyrolysis
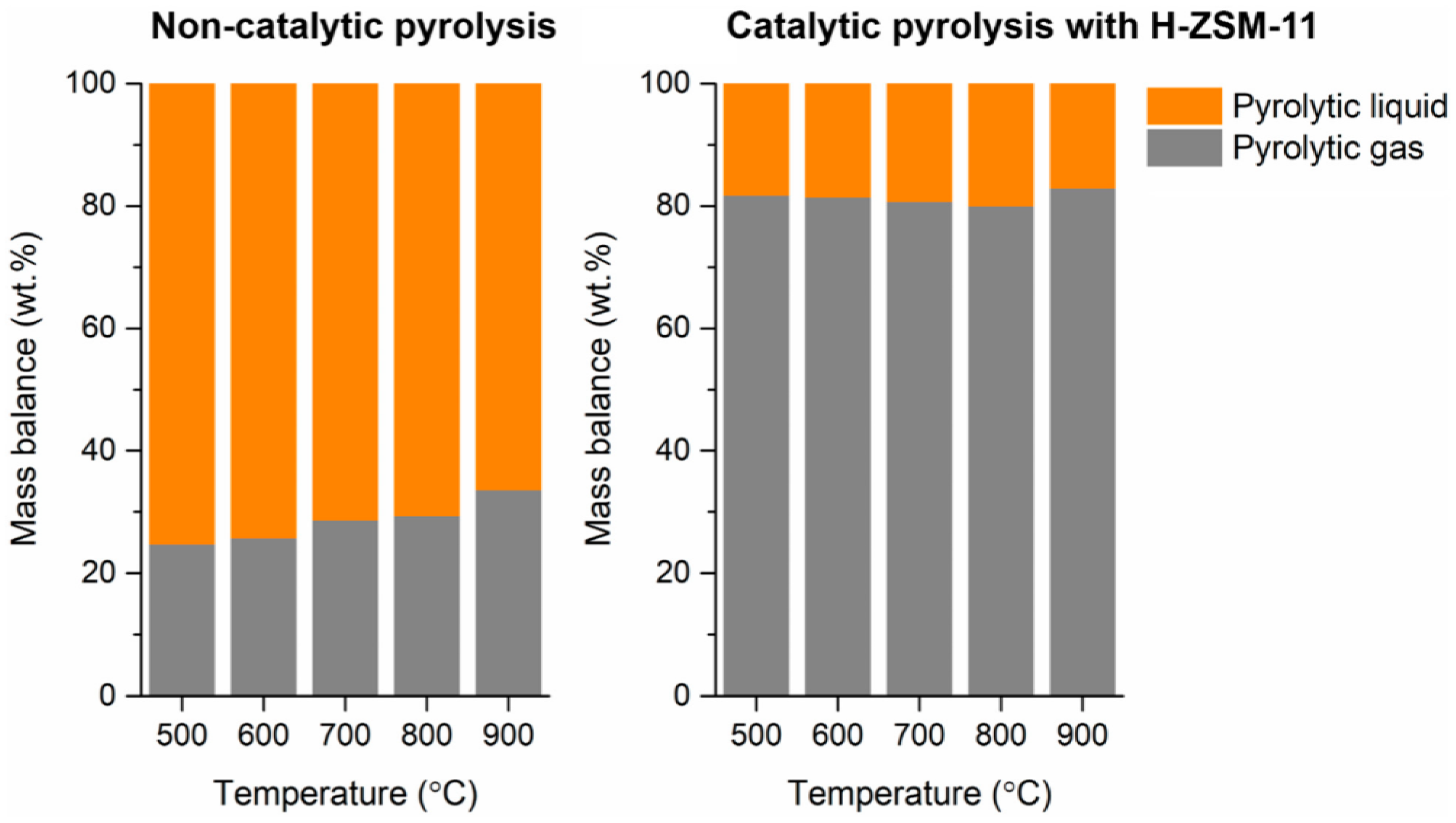
4.1. Pyrolysis of PE
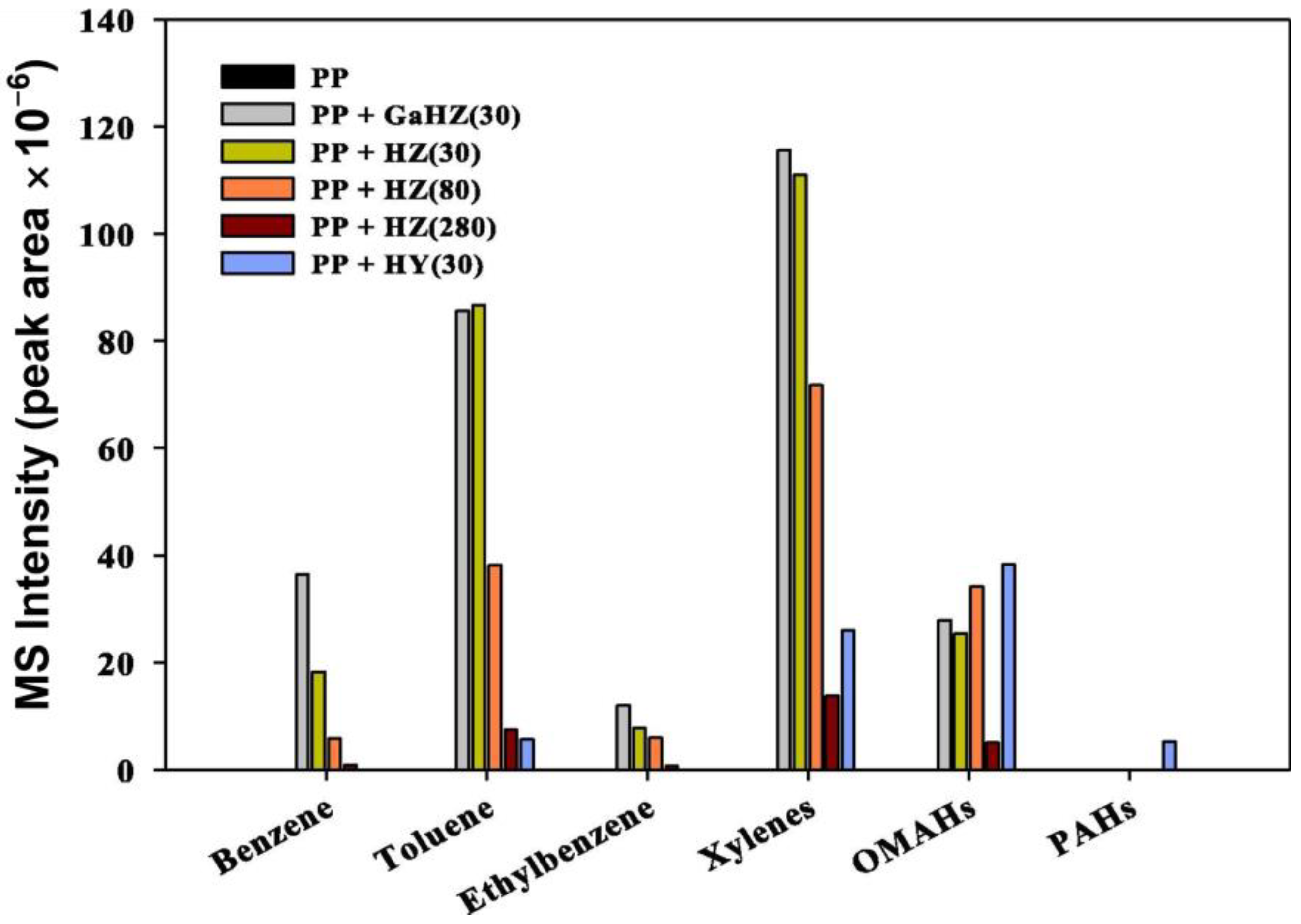
4.2. Pyrolysis of Polypropylene
4.3. Pyrolysis of Polystyrene
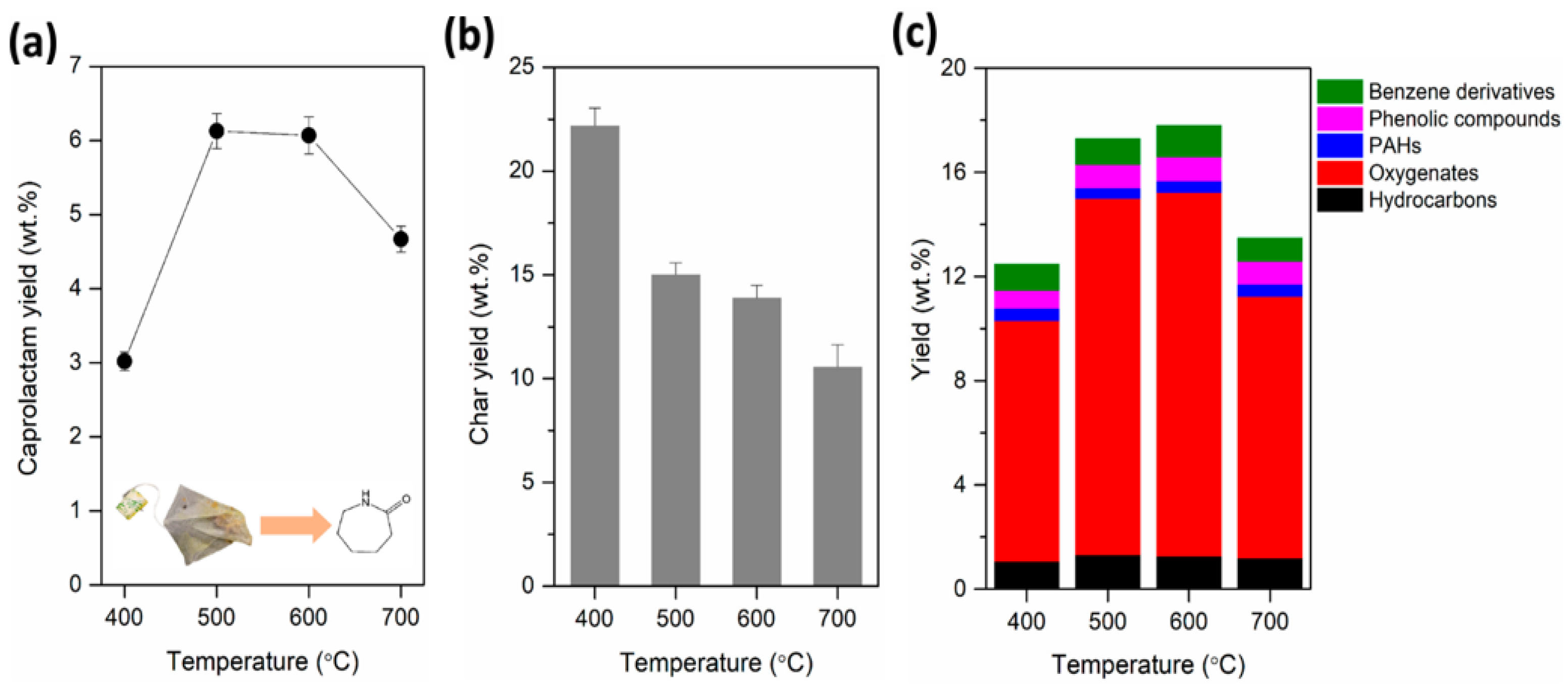
4.4. Pyrolysis of Nylon
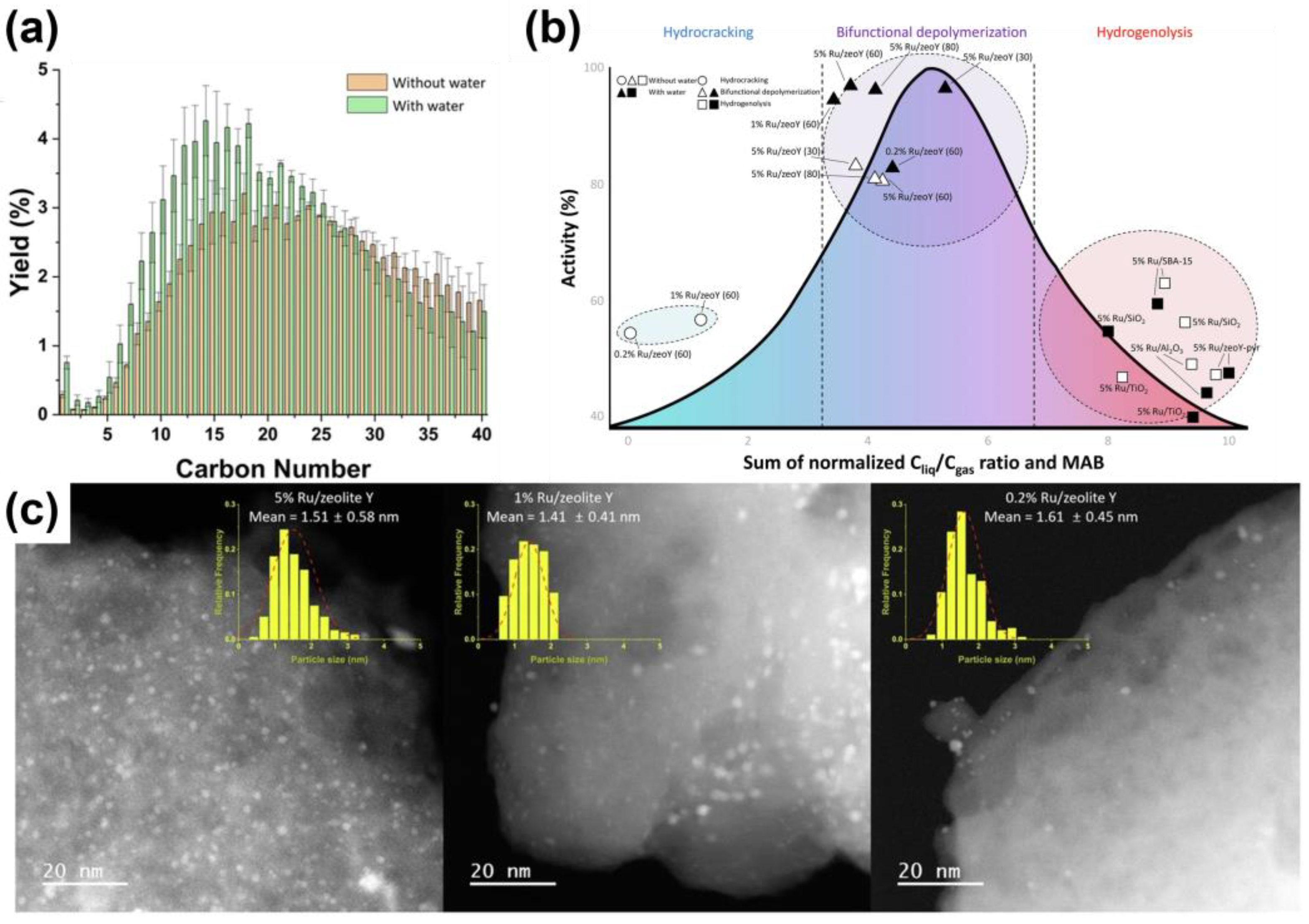
4.5. Pyrolysis of Other Plastics Including Mixed Plastics
5. Chemical Recycling Industry in South Korea
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to Fuel: A Review. Renewable Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Kwon, T.; Jeong, H.; Kim, M.; Jung, S.; Ro, I. Catalytic Approaches to Tackle Mixed Plastic Waste Challenges: A Review. Langmuir 2024, 40, 17212–17238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Zheng, L.; Hu, T.; Yan, M.; Wu, C. Recycling and Depolymerisation of Poly(Ethylene Terephthalate): A Review. Polym. Chem. 2024, 15, 585–608. [Google Scholar] [CrossRef]
- Kim, D.; Han, M.; Kim, N.; Kim, J.; Jung, S.P. Waste Plastic Pyrolysis Industry: Current Status and Prospects. J. Korean Soc. Environ. Eng. 2024, 46, 395–407. [Google Scholar] [CrossRef]
- Park, J.; Jang, Y.; Son, M. Dynamic Plastics Flow Analysis for Korea between 1982-2020. J. Korean Soc. Environ. Eng. 2023, 45, 127–137. [Google Scholar] [CrossRef]
- Kwon, T.; Ro, I. Advancing Towards a Sustainable Future: Recent Trends in Catalytic Upcycling of Waste Plastics. Korean Chem, Engin. Res. 2023, 61, 505–516. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of Microplastics in Water and Aquatic Systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef]
- Lee, M.Y.; Cho, N.H.; Lee, S.J.; Um, N.; Jeon, T.W.; Kang, Y.Y. Application of Material Flow Analysis for Plastic Waste Management in the Republic of Korea. J. Environ. Manag. 2021, 299, 113625. [Google Scholar] [CrossRef]
- Colombani, D. Chain-Growth Control in Free Radical Polymerization. Prog. Polym. Sci. 1997, 22, 1649–1720. [Google Scholar] [CrossRef]
- Stille, J.K. Step-Growth Polymerization. J. Chem. Educ. 1981, 58, 862–866. [Google Scholar] [CrossRef]
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; De Los Rios, J.P.; Fieser, M.E. Catalytic Methods for Chemical Recycling or Upcycling of Commercial Polymers. Mater. Horiz. 2021, 8, 1084–1129. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.; Rubio Arias, J.J.; Thielemans, W. Chemolytic Depolymerisation of PET: A Review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, T.; Hwang, H.; Sung, Y.; Kim, B.H. Development of Glycolysis Catalysts for PET Wastes Including Polyester Textiles. Fibers Polym. 2024, 26, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Wang, W.; Ji, G.; Zhao, M.; Li, A. Kinetics, Product Evolution, and Mechanism for the Pyrolysis of Typical Plastic Waste. ACS Sustain. Chem. Eng. 2022, 10, 91–103. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Meng, H.; Tong, H.; Cai, X.; Liu, J. Studies on Pyrolysis Mechanisms of Syndiotactic Polystyrene Using DFT Method. Chem. Phys. Lett. 2020, 747, 137334. [Google Scholar] [CrossRef]
- Kim, S.-H.H.-T.W.-S. Production Properties of Pyrolytic Matter of PP and PS Plastics in n Low Temperature Pyrolysis Condition. J. Korea Acad.-Ind. Coop. Soc. 2007, 8, 867–873. [Google Scholar]
- Gao, F. Pyrolysis of Waste Plastics into Fuels. Ph.D. Thesis, University of Canterbury. Chemical and Process Engineering, Christchurch, New Zealand, 2010. [Google Scholar] [CrossRef]
- Cho, H.; Jin, A.; Kim, S.J.; Kwon, Y.; Lee, E.; Shin, J.J.; Kim, B.H. Conversion of Polyethylene to Low-Molecular-Weight Oil Products at Moderate Temperatures Using Nickel/Zeolite Nanocatalysts. Materials 2024, 17, 1863. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of Ethylene Glycol and Terephthalic Acid from Biomass for Producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Lee, C.; Jang, Y.C.; Choi, K.; Kim, B.; Song, H.; Kwon, Y. Recycling, Material Flow, and Recycled Content Demands of Polyethylene Terephthalate (PET) Bottles towards a Circular Economy in Korea. Environments 2024, 11, 25. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical Recycling of Polyester Textile Wastes: Shifting towards Sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Schade, A.; Melzer, M.; Zimmermann, S.; Schwarz, T.; Stoewe, K.; Kuhn, H. Plastic Waste Recycling—A Chemical Recycling Perspective. ACS Sustain. Chem. Eng. 2024, 12, 12270–12288. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the Catalytic Glycolysis of Polyethylene Terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef] [PubMed]
- Abedsoltan, H. A Focused Review on Recycling and Hydrolysis Techniques of Polyethylene Terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Han, M. Depolymerization of PET Bottle via Methanolysis and Hydrolysis. In Recycling of Polyethylene Terephthalate Bottles; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 85–108. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eissa, A.M.F.; Moustafa, M.E.; Eshaq, G.; Rabie, A.M.; Elmetwally, A.E. Cu- and Zn-Acetate-Containing Ionic Liquids as Catalysts for the Glycolysis of Poly(Ethylene Terephthalate). Polym. Degrad. Stab. 2014, 110, 364–377. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, B.; Woo, D.; Han, M. Depolymerization of PET by Ethylene Glycol. Korean Chem. Eng. Res. 2009, 47, 683–687. [Google Scholar]
- Sangalang, A.; Bartolome, L.; Kim, D.H. Generalized Kinetic Analysis of Heterogeneous PET Glycolysis: Nucleation-Controlled Depolymerization. Polym. Degrad. Stab. 2015, 115, 45–53. [Google Scholar] [CrossRef]
- Park, R.; Sridhar, V.; Park, H. Taguchi Method for Optimization of Reaction Conditions in Microwave Glycolysis of Waste PET. J. Mater. Cycles Waste Manag. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- Duldner, M.M.; Bartha, E.; Capitanu, S.; Nica, S.; Coman, A.E.; Tincu, R.; Sarbu, A.; Filip, P.I.; Apostol, S.; Garea, S. Attempts to Upcycle PET Wastes into Bio-Based Long-Lasting Insulating Materials. Rev. Chim. 2019, 70, 2301–2307. [Google Scholar] [CrossRef]
- Yue, Q.F.; Wang, C.X.; Zhang, L.N.; Ni, Y.; Jin, Y.X. Glycolysis of Poly(Ethylene Terephthalate) (PET) Using Basic Ionic Liquids as Catalysts. Polym. Degrad. Stab. 2011, 96, 399–403. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep Eutectic Solvents as Highly Active Catalysts for the Fast and Mild Glycolysis of Poly(Ethylene Terephthalate)(PET). Green. Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Le, N.H.; Van Ngoc, T.T.; Shong, B.; Cho, J. Low-Temperature Glycolysis of Polyethylene Terephthalate. ACS Sustain. Chem. Eng. 2022, 10, 17261–17273. [Google Scholar] [CrossRef]
- Park, G.; Bartolome, L.; Lee, K.G.; Lee, S.J.; Kim, D.H.; Park, T.J. One-Step Sonochemical Synthesis of a Graphene Oxide–Manganese Oxide Nanocomposite for Catalytic Glycolysis of Poly(Ethylene Terephthalate). Nanoscale 2012, 4, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, X.; Ju, Z.; Sun, P.; Xin, J.; Yao, X.; Zhou, Q.; Zhang, S. Ultrafast Homogeneous Glycolysis of Waste Polyethylene Terephthalate via a Dissolution-Degradation Strategy. Ind. Eng. Chem. Res. 2018, 57, 16239–16245. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Jin, S.B.; Park, H.J.; Park, S.H.; Jeon, H.; Suh, H.; Park, Y.-J.; Seo, D.; Hwang, S.Y.; Kim, D.H.; et al. Large-Scale Fast Fluid Dynamic Processes for the Syntheses of 2D Nanohybrids of Metal Nanoparticle-Deposited Boron Nitride Nanosheet and Their Glycolysis of Poly(Ethylene Terephthalate). Adv. Mater. Interfaces 2020, 7, 2000599. [Google Scholar] [CrossRef]
- Son, S.G.; Jin, S.B.; Kim, S.J.; Park, H.J.; Shin, J.; Ryu, T.; Jeong, J.M.; Choi, B.G. Exfoliated Manganese Oxide Nanosheets as Highly Active Catalysts for Glycolysis of Polyethylene Terephthalate. FlatChem 2022, 36, 100430. [Google Scholar] [CrossRef]
- Han, N.; Lee, K.; Lee, J.; Jo, J.H.; An, E.J.; Lee, G.; Chi, W.S.; Lee, C. Dual-Porous ZIF-8 Heterogeneous Catalysts with Increased Reaction Sites for Efficient PET Glycolysis. Chemosphere 2024, 364, 143187. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, J.I.; Choi, J.W.; Jeong, S.M.; Lee, P.S.; Hong, D.Y. A Highly Porous MgAl2O4 Spinel-Supported Mn3O4 as a Reusable Catalyst for Glycolysis of Postconsumer PET Waste. J. Ind. Eng. Chem. 2022, 115, 251–262. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Hwang, J.; Im, E.; Moon, G.D. Optimizing PET Glycolysis with an Oyster Shell-Derived Catalyst Using Response Surface Methodology. Polymers 2022, 14, 656. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, I.; Kim, S.H.; Kim, J.F.; Choi, Y.H.; Lee, H. Chemical Recycling of Polyethylene Terephthalate Using a Micro-Sized MgO-Incorporated SiO2 Catalyst to Produce Highly Pure Bis(2-Hydroxyethyl) Terephthalate in High Yield. Chem. Eng. J. 2024, 499, 155865. [Google Scholar] [CrossRef]
- Cha, Y.; Park, Y.J.; Kim, D.H. Hydrodynamic Synthesis of Fe2O3@MoS2 0D/2D-N anocomposite Material and Its Application as a Catalyst in the Glycolysis of Polyethylene Terephthalate. RSC Adv. 2021, 11, 16841–16848. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, E.J.; Kim, J.; An, K. Efficient Fe3O4 Nanoparticle Catalysts for Depolymerization of Polyethylene Terephthalate. Green Chem. 2023, 25, 8160–8171. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into Terephthalic Acid in Neutral Media Catalyzed by the ZSM-5 Acidic Catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-Energy Catalytic Methanolysis of Poly(Ethyleneterephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar] [CrossRef]
- Haba, O.; Itakura, I.; Ueda, M.; Kuze, S. Synthesis of Polycarbonate from Dimethyl Carbonate and Bisphenol-A Through a Non-Phosgene Process. J. Polym. Sci. Part A Poly. Chem. 1999, 37, 2087–2093. [Google Scholar] [CrossRef]
- Kausar, A. A Review of Filled and Pristine Polycarbonate Blends and Their Applications. J. Plast. Film Sheeting 2018, 34, 60–97. [Google Scholar] [CrossRef]
- Antonakou, E.V.; Achilias, D.S. Recent Advances in Polycarbonate Recycling: A Review of Degradation Methods and Their Mechanisms. Waste Biomass Valorization 2013, 4, 9–21. [Google Scholar] [CrossRef]
- Do, T.; Baral, E.R.; Kim, J.G. Chemical Recycling of Poly(Bisphenol A Carbonate): 1,5,7-Triazabicyclo[4.4.0]-Dec-5-Ene Catalyzed Alcoholysis for Highly Efficient Bisphenol A and Organic Carbonate Recovery. Polymer 2018, 143, 106–114. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, S.; Lee, H.S.; Shin, H.G.; Yoo, Y.; Baral, E.R.; Lee, J.H.; Kwak, J.; Kim, J.G. Chemical Upcycling of Waste Poly(Bisphenol A Carbonate) to 1,4,2-Dioxazol-5-Ones and One-Pot C−H Amidation. ChemSusChem 2021, 14, 4301–4306. [Google Scholar] [CrossRef]
- Seo, S.; Lee, S.H.; Lee, J.; Lee, E.; Lee, J.; Hong, M.; Seo, J.H. Polycarbonate Ethanolysis with Imidazolium-Based Ionic Liquids: Recovery of High-Purity BPA and Value-Added by-Product. Mater. Today Sustain. 2024, 25, 100665. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J. Feedstock Recycling of Polycarbonate Waste via Thermochemical Conversion Supported by Municipal Solid Waste Incinerator Bottom Ash. Chemosphere 2024, 368, 143748. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Yoo, K.; Borchardt, L.; Kim, J.G. Chemical Recycling of Polycarbonate and Polyester without Solvent and Catalyst: Mechanochemical Methanolysis. Green Chem. 2024, 26, 2087–2093. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Characteristics of a New Type Continuous Two-Stage Pyrolysis of Waste Polyethylene. Energy 2019, 166, 343–351. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial Degradation of Low Density Polyethylene (LDPE): A Review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Falcão, A.N.; Gil, M.H. Modification of LDPE Molecular Structure by Gamma Irradiation for Bioapplications. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 513–520. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Shaidullin, N.M.; Chalykh, A.E.; Matsko, M.A.; Shapagin, A.V.; Batyrshin, A.Z.; Shandryuk, G.A.; Nifant’ev, I.E. Low-Temperature Mechanical Properties of High-Density and Low-Density Polyethylene and Their Blends. Polymers 2021, 13, 1821. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, H.J.; Kim, D.S.; Lee, B.-H. Distribution Characteristics of Pyrolysis Products of Polyethylene. Polymer 2008, 32, 157–162. [Google Scholar]
- Manos, G.; Garforth, A.; Dwyer, J. Catalytic Degradation of High-Density Polyethylene over Different Zeolitic Structures. Ind. Eng. Chem. Res. 2000, 39, 1198–1202. [Google Scholar] [CrossRef]
- Park, D.W.; Hwang, E.Y.; Kim, J.R.; Choi, J.K.; Kim, Y.A.; Woo, H.C. Catalytic Degradation of Polyethylene over Solid Acid Catalysts. Polym. Degrad. Stab. 1999, 65, 193–198. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, K.H.; Shin, D.H. Investigation of Catalytic Degradation of High-Density Polyethylene by Hydrocarbon Group Type Analysis. J. Anal. Appl. Pyrolysis. 2003, 70, 383–398. [Google Scholar] [CrossRef]
- Lee, N.; Joo, J.; Lin, K.Y.A.; Lee, J. Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11. Polymers 2021, 13, 1198. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Ahn, B.; Kang, K.H.; Won, W.; Ro, I. Unraveling the Role of Water in Mechanism Changes for Economically Viable Catalytic Plastic Upcycling. Nat. Commun. 2024, 15, 10239. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, Y.K.; Jeong, K.E.; Kim, T.W.; Chae, H.J.; Park, S.H.; Jeon, J.K.; Kim, S.S. Catalytic Degradation of Polyethylene over SBA-16. Korean J. Chem. Eng. 2010, 27, 1446–1451. [Google Scholar] [CrossRef]
- Chung, T.C.; Rhubright, D. Synthesis of Functionalized Polypropylene. Macromolecules 1991, 24, 970–972. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Kang, H.K.; Yu, M.J.; Park, S.H.; Jeon, J.-K.; Kim, S.-C.; Park, Y.K. Catalytic Pyrolysis of Miscanthus and Random Polypropylene over SAPO-11. Polymer 2013, 37, 379–386. [Google Scholar] [CrossRef]
- Pyo, S.; Kim, Y.M.; Park, Y.; Lee, S.B.; Yoo, K.S.; Ali Khan, M.; Jeon, B.H.; Jun Choi, Y.; Hoon Rhee, G.; Park, Y.K. Catalytic Pyrolysis of Polypropylene over Ga Loaded HZSM-5. J. Ind. Eng. Chem. 2021, 103, 136–141. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of Polystyrene–Clay Nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]
- Marquez, C.; Martin, C.; Linares, N.; De Vos, D. Catalytic Routes towards Polystyrene Recycling. Mater. Horiz. 2023, 10, 1625–1640. [Google Scholar] [CrossRef]
- Selpiana; Santoso, B.; Pramayuda, D.; Ismoro, D.S. Physical Properties Analysis of the Liquid Produced by Polystyrene Pyrolysis. J. Phys. Conf. Ser. 2019, 1282, 012072. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Two-Stage Pyrolysis of Polystyrene: Pyrolysis Oil as a Source of Fuels or Benzene, Toluene, Ethylbenzene, and Xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, G.C.; Bae, S.Y.; Yi, S.C.; Moon, S.K.; Kumazawa, H. Pyrolysis of Polystyrene in a Batch-Type Stirred Vessel. Korean J. Chem. Eng. 1999, 16, 161–165. [Google Scholar] [CrossRef]
- Song, H.S.; Hyun, J.C. An Optimization Study on the Pyrolysis of Polystyrene in a Batch Reactor. Korean J. Chem. Eng. 1999, 16, 316–324. [Google Scholar] [CrossRef]
- Park, J.J.; Park, K.; Kim, J.S.; Maken, S.; Song, H.; Shin, H.; Park, J.W.; Choi, M.J. Characterization of Styrene Recovery from the Pyrolysis of Waste Expandable Polystyrene. Energy Fuels 2003, 17, 1576–1582. [Google Scholar] [CrossRef]
- Lee, C.G.; Cho, Y.J.; Song, P.S.; Kang, Y.; Kim, J.S.; Choi, M.J. Effects of Temperature Distribution on the Catalytic Pyrolysis of Polystyrene Waste in a Swirling Fluidized-Bed Reactor. Catal. Today 2003, 79–80, 453–464. [Google Scholar] [CrossRef]
- Kim, J.; Yun, G.N.; Song, I.H.; Song, K.H.; Baek, S.J.; Kim, J.; Hwang, D.W.; An, J.; Min, J. Process Modeling and Assessment of Waste Polystyrene Pyrolysis: Comparing Catalytic and Thermal Methods. Chem. Eng. J. 2025, 505, 159261. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Feng, H.; Li, D.; Xia, Z.; Yang, R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers 2023, 15, 2161. [Google Scholar] [CrossRef]
- Charles, J.; Ramkumaar, G.R.; Azhagiri, S.; Gunasekaran, S. FTIR and Thermal Studies on Nylon-66 and 30% Glass Fibre Reinforced Nylon-66. J. Chem. 2009, 6, 23–33. [Google Scholar] [CrossRef]
- Tonsi, G.; Maesani, C.; Alini, S.; Ortenzi, M.A.; Pirola, C. Nylon Recycling Processes: A Brief Overview. Chem. Eng. Trans. 2023, 100, 727–732. [Google Scholar] [CrossRef]
- Minor, A.J.; Goldhahn, R.; Rihko-Struckmann, L.; Sundmacher, K. Chemical Recycling Processes of Nylon 6 to Caprolactam: Review and Techno-Economic Assessment. Chem. Eng. J. 2023, 474, 145333. [Google Scholar] [CrossRef]
- Kim, S.; Lee, N.; Lee, J. Pyrolysis for Nylon 6 Monomer Recovery from Teabag Waste. Polymers 2020, 12, 2695. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.T.; Oh, L.S.; Kim, H.J.; Lee, J. Marine Waste Upcycling—Recovery of Nylon Monomers from Fishing Net Waste Using Seashell Waste-Derived Catalysts in a CO2-Mediated Thermocatalytic Process. J. Mater. Chem. A 2022, 10, 20024–20034. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.K. Catalytic Pyrolysis of Polyethylene and Polypropylene over Desilicated Beta and Al-MSU-F. Catalysts 2018, 8, 501. [Google Scholar] [CrossRef]
- Koo, J.K.; Kim, S.W.; Seo, Y.H. Characterization of Aromatic Hydrocarbon Formation from Pyrolysis of Polyethylene-Polystyrene Mixtures. Resour. Conserv. Recycl. 1991, 5, 365–382. [Google Scholar] [CrossRef]
- Park, C.; Kim, S.; Kwon, Y.; Jeong, C.; Cho, Y.; Lee, C.G.; Jung, S.; Choi, K.Y.; Lee, J. Pyrolysis of Polyethylene Terephthalate over Carbon-Supported Pd Catalyst. Catalysts 2020, 10, 496. [Google Scholar] [CrossRef]
- Oh, D.; Lee, H.W.; Kim, Y.M.; Park, Y.K. Catalytic Pyrolysis of Polystyrene and Polyethylene Terephthalate over Al-MSU-F. Energy Procedia 2018, 144, 111–117. [Google Scholar] [CrossRef]
- Temporal, R. SK Chemicals Look to Establish Plastic Recycling Solution Centre in Korea. Interplas Insights 2025, 25 February. Available online: https://interplasinsights.com/plastics-environment-news/plastics-recycling-innovations-news/sk-chemicals-plastic-recycling-centre-korea/ (accessed on 2 April 2025).
- Loop Industries and SK Geocentric Sigh Joint Venture Agreement to Commercialize Loop’s Technology in the Asian Market. Available online: https://www.loopindustries.com/cms/loop-industries-and-sk-geo-centric-sign-joint-venture-agreement-to-commercialize-loops-technology-in-the-asian-market (accessed on 19 April 2025).
- Terracle. Official Website. Available online: https://terracle.im/index.html (accessed on 15 April 2025).
- Step Closer to Resource Circulating Ecosystem–LOTTE Chemical Begins Test Production of Chemically Recycled PET (C-rPET). Available online: https://www.lottechem.com/en/media/news/613/view.do (accessed on 15 April 2025).
- LOTTE Chemical Applies Recycled Plastic in 25 kg PE and PP Packaging Bags. LOTTE Chemical 2024, 28 March. Available online: https://www.lottechem.com/en/media/news/901/view.do (accessed on 2 April 2025).
- LG Chem. Supercritical Pyrolysis, a Technology That Converts Plastic Waste into Initial Raw Material. LG Chem Blog 2023, 13 May. Available online: https://blog.lgchem.com/en/2023/05/19_supercritical_pyrolysis/ (accessed on 2 April 2025).
- HD Hyundai Oilbank Integrated Report 2022. Available online: https://www.hd.com/common/en/docs/FY2022%20HD%20Hyundai%20Oilbank.pdf (accessed on 15 April 2025).
- GS Caltex. Green Transformation. Available online: https://www.gscaltex.com/en/future/Greentransformation (accessed on 15 April 2025).
- Ko, M.J.; Yoo, N.H.; Yeo, H.Y.; Koo, B.C.; Yoon, S.J.; Yoo, J.W.; Jeong, J. Composition and Method for Depolymerization of Cured Epoxy Resin Using Transition Metal Salts; Korea Institute of Science and Technology (KIST): Seoul, Republic of Korea, 2017. [Google Scholar] [CrossRef]
- Park, S.; Heo, J. Alkali-Activated Carbon and Electrode for Supercapacitor Using the Samer; Inha University Industry-Academic Cooperation Foundation: Incheon, Republic of Korea, 2015. [Google Scholar] [CrossRef]
- Lee, J.; Hong, M.; Seo, J.; Seo, S.; Lee, S.; Lee, E.; Lee, J. Catalyst for Depolymerizing Polycarbonate-Based Resin, Preparation Method Thereof, and Decomposition Method of Polycarbonate-Based Resin, Recoverization Method of Catalyst for Depolymerizing Polycarbonate-Based Resin Using the Same; Korea Intellectual Property Office: Daejeon, Republic of Korea, 2023. [CrossRef]
- Park, S.J.; Kang, S.H.; Seo, M.K.; Min, H.K. Catalyst for Depolymerization of Polystyrene and Depolymerization Process of Polystyrene Using the Same; Lotte Chemical: Seoul, Republic of Korea, 2021. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Seo, K. Method for Decomposing of Polycarbonate Using Ethanol; Korea Intellectual Property Office: Daejeon, Republic of Korea, 2022; Volume 21. [CrossRef]
- Song, U.; Park, H. Plastic Recycling in South Korea: Problems, Challenges, and Policy Recommendations in the Endemic Era. J. Ecol. Environ. 2024, 48, 74–84. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, P.; Amadei, A.M.; Klenert, D.; Nessi, S.; Tonini, D.; Tosches, D.; Ardente, F.; Saveyn, H. Environmental and Economic Assessment of Plastic Waste Recycling. Resour. Conserv. Recycl. 2025, 215, 108099. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Strategies to Achieve a Carbon Neutral Society: A Review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Jerdy, A.C.; Pham, T.; González-Borja, M.Á.; Atallah, P.; Soules, D.; Abbott, R.; Lobban, L.; Crossley, S. Impact of the Presence of Common Polymer Additives in Thermal and Catalytic Polyethylene Decomposition. Appl. Catal. B Environ. 2023, 325, 122348. [Google Scholar] [CrossRef]
- Aguiar, M.I.S.; Sousa, A.F.; Teixeira, G.; Tavares, A.P.M.; Ferreira, A.M.; Coutinho, J.A.P. Enhancing Plastic Waste Recycling: Evaluating the Impact of Additives on the Enzymatic Polymer Degradation. Catal. Today 2024, 429, 114492. [Google Scholar] [CrossRef]
- Hinton, Z.R.; Kots, P.A.; Soukaseum, M.; Vance, B.C.; Vlachos, D.G.; Epps, T.H.; Korley, L.T.J. Antioxidant-Induced Transformations of a Metal-Acid Hydrocracking Catalyst in the Deconstruction of Polyethylene Waste. Green Chem. 2022, 24, 7332–7339. [Google Scholar] [CrossRef]
- Ngu, J.; Najmi, S.; Selvam, E.; Vance, B.; Yang, P.; Vlachos, D.G. Catalytic Deconstruction of Organic Additive-Containing Plastics. Nat. Chem. Eng. 2025, 2, 220–228. [Google Scholar] [CrossRef]




| Catalysts | Temperature | Time | Product | Yield | Ref. | |
|---|---|---|---|---|---|---|
| PET | Zinc acetate | 230 °C | 6 h | BHET | 71% | 28 |
| Zinc acetate | 250 °C | 0.5 h | BHET | 65% | 30 | |
| Potassium acetate | 153 °C | 2 h | BHET | 86.5% | 34 | |
| Mn3O4 | 300 °C | 80 min | BHET | 96.4% | 35 | |
| Pd/hBN | 100 °C | 0.5 h | BHET | 92.1% | 37 | |
| MnO2 nanosheet | 200 °C | 0.5 h | BHET | 100% | 38 | |
| ZIF-8 | 180 °C | 4 h | BHET | 76.1% | 39 | |
| Mn3O4/p-spMgAl800 | 190 °C | 3 h | BHET | 97.6% | 40 | |
| Oyster shell | 195 °C | 1 h | BHET | 68.6% | 41 | |
| MgO-doped SiO2 | 196 °C | 2 h | BHET | 95.1% | 42 | |
| Fe2O3@MoS2 | 225 °C | 3 h | BHET | 90% | 43 | |
| Fe3O4 | 195 °C | 2 h | BHET | 93.5% | 44 | |
| ZSM-5-25 | 230 °C | 0.5 h | TPA | 100% | 45 | |
| K2CO3 | 25 °C | 24 h | DMT | 93.1% | 46 | |
| PC | TBD | 75 °C | 12 h | BPA | >98% | 50 |
| [EMIM][Ac] | 90 °C | 10 h | BPA | 99.9% | 52 | |
| MSW-IBA | 600 °C | BPA | 25.9 wt.% | 53 | ||
| PE | HZSM-5 | 450 °C | 1 h | Oil | 10.9 wt.% | 61 |
| Gas | 88.4 wt.% | |||||
| NZ | 450 °C | 1 h | Oil | 65.1 wt.% | ||
| Gas | 34.9 wt.% | |||||
| ZSM-5 | 450 °C | 0.5 h | Oil | 35 wt.% | 62 | |
| Gas | 63.5 wt.% | |||||
| Zeolite Y | 450 °C | 0.5 h | Oil | 71.5 wt.% | ||
| Gas | 27 wt.% | |||||
| H-ZSM-11 | 700 °C | Oil | 19.2 wt.% | 63 | ||
| Gas | 80.8 wt.% | |||||
| Ni/Zeolite | 350 °C | 2 h | Oil | 25 wt.% | 19 | |
| Gas | 58 wt.% | |||||
| Ru/Zeolite | 250 °C | 3 h | Oil | 88.2 wt.% | 64 | |
| Gas | 1.4 wt.% | |||||
| Al-SBA-16 | 430 °C | 2 h | Oil | 75.8 wt.% | 65 | |
| Gas | 20.6 wt.% | |||||
| PS | 400 °C | 0.5 h | Styrene | 71.6 wt.% | 77 | |
| BaO | 350 °C | 1 h | Styrene | 61.7 wt.% | 78 | |
| Fe2O3 | 450 °C | 20 min | Styrene | 71.5 wt.% | 79 | |
| K2O/SiO2 | 375 °C | 1 h | Styrene | 80.2 wt.% | 80 | |
| Nylon | 500 °C | Caprolactam | 6.2 wt.% | 85 | ||
| Shell waste | 500 °C | Caprolactam | 80 wt.% | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, T.; Shin, I.; Choi, J.; Lee, S.; Hwang, H.; Kim, M.; Kim, B.H. Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea. Catalysts 2025, 15, 414. https://doi.org/10.3390/catal15050414
Jang T, Shin I, Choi J, Lee S, Hwang H, Kim M, Kim BH. Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea. Catalysts. 2025; 15(5):414. https://doi.org/10.3390/catal15050414
Chicago/Turabian StyleJang, Taemin, Ik Shin, Jungwook Choi, Sohyeon Lee, Hyein Hwang, Minchang Kim, and Byung Hyo Kim. 2025. "Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea" Catalysts 15, no. 5: 414. https://doi.org/10.3390/catal15050414
APA StyleJang, T., Shin, I., Choi, J., Lee, S., Hwang, H., Kim, M., & Kim, B. H. (2025). Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea. Catalysts, 15(5), 414. https://doi.org/10.3390/catal15050414






