High-Pressure CO2 Photoreduction, Flame Spray Pyrolysis and Type-II Heterojunctions: A Promising Synergy
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. XRD Analysis
2.3. N2 Physisorption Analysis
2.4. DRS UV–Vis Analysis
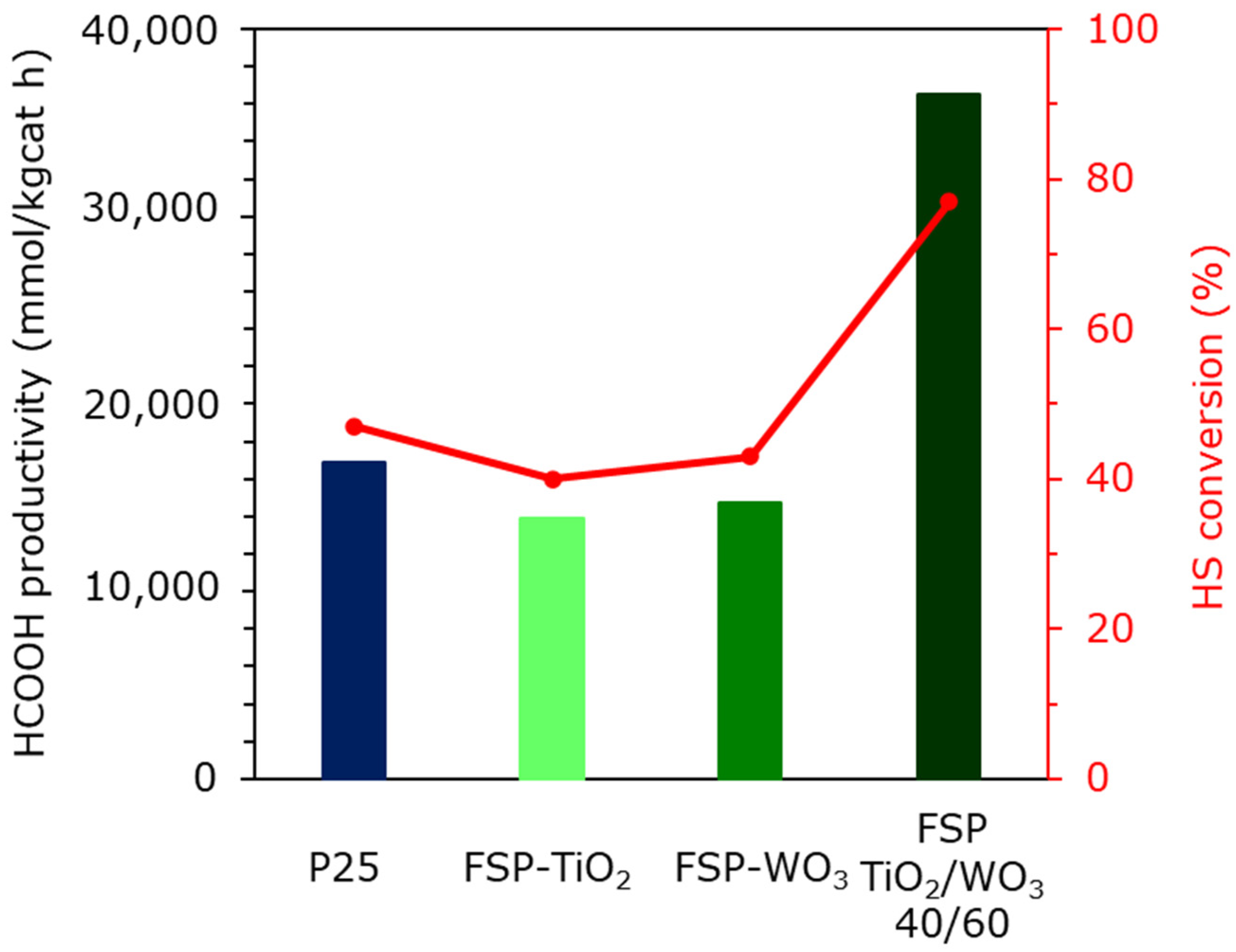
2.5. Photoreduction of CO2
| Catalyst | Reaction Medium | Light Source | Productivity (mol/kgcat h) | Reference |
|---|---|---|---|---|
| Cu2O | Aqueous suspension in 0.3 M NaHCO3, 2 M glycerol as HS | Arc Lamp 1000 W Xenon | 2.76 | [36] |
| Ag/TiO2 | Aqueous suspension in 0.2 M NaHCO3 | Arc Lamp 1000 W Xenon | 3.89 | [37] |
| Co3O4 | Aqueous suspension in water | LED lamp 21 W, λ = 510 | HCOOH 4.53 (mmol/kgcat·h) HCHO 0.62 (mmol/kgcat·h) | [38] |
| ZnO-Cu2O NPs | Aqueous suspension in water and ZnO-Cu2O 300 W Xe lamp 0.2 M Na2CO3 | 300 W Xe lamp | CH4 1080 (mmol/kgcat·h) CO 1.4 (mmol/kgcat·h) | [39] |
| TiO2 FSP | Aqueous suspension in water, HS 1.66 g/L of Na2SO3 | Medium Hg-pressure UV lamp 250 W | 13.9 | This work |
| WO3/TiO2 (40/60) FSP | Aqueous suspension in water, HS 1.66 g/L of Na2SO3 | Medium Hg-pressure UV lamp 250 W | 36.5 | This work |
3. Materials and Methods
3.1. Materials Used
3.2. Flame Spray Pyrolysis
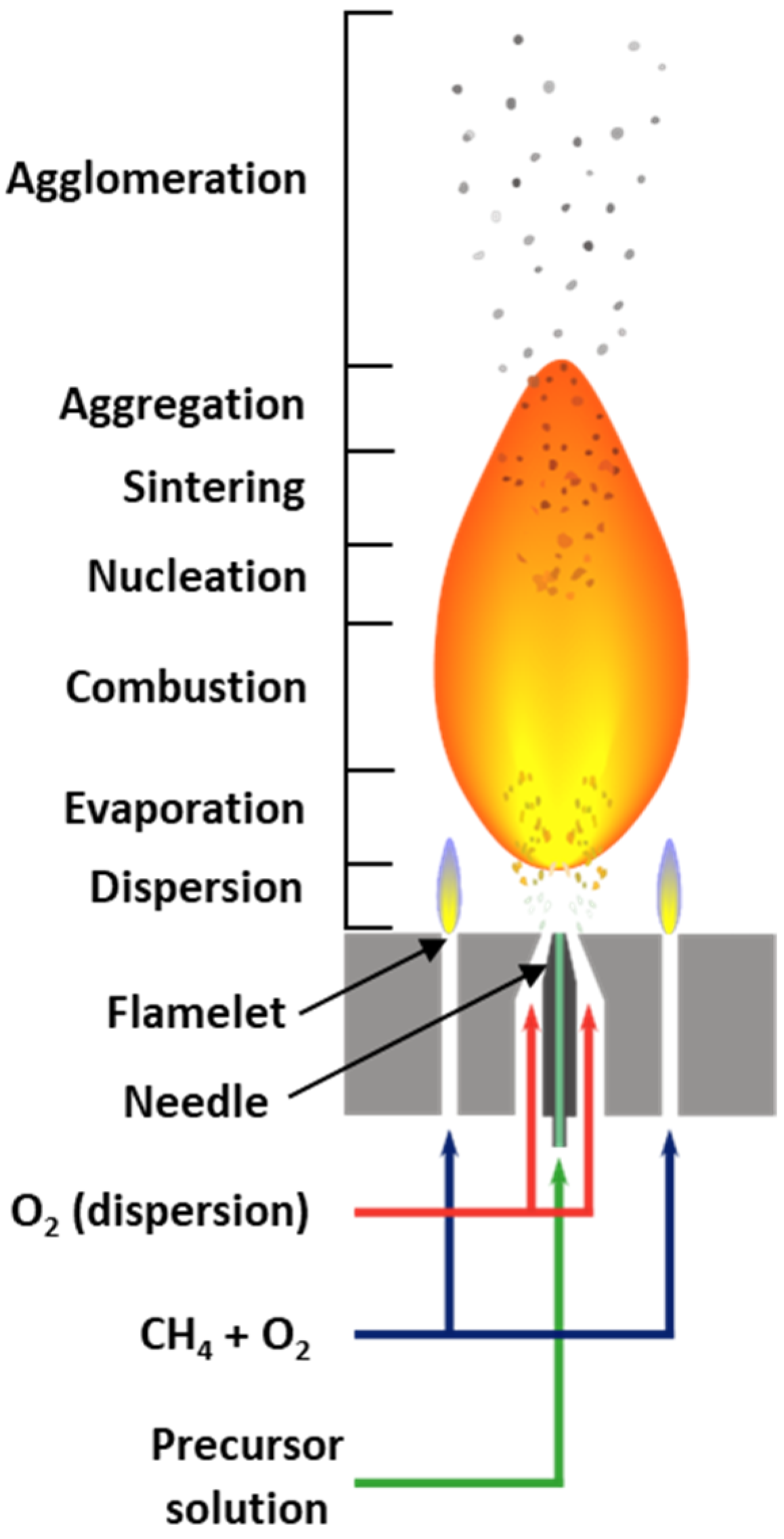
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.5. Photoreduction Setup
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soon, W.; Baliunas, S.L.; Robinson, A.B.; Robinson, Z.W. Environmental Effects of Increased Atmospheric Carbon Dioxide. Clim. Res. 1999, 13, 149–164. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent Advancements in Engineering Approach towards Design of Photo-Reactors for Selective Photocatalytic CO2 Reduction to Renewable Fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Albo, J.; Luis, P.; Irabien, A. Carbon Dioxide Capture from Flue Gases Using a Cross-Flow Membrane Contactor and the Ionic Liquid 1-Ethyl-3-Methylimidazolium Ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- Das, S.; Wan Daud, W.M.A. A Review on Advances in Photocatalysts towards CO2 Conversion. RSC Adv. 2014, 4, 20856–20893. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and Photoelectrocatalytic Reduction of CO2 Using Heterogeneous Catalysts with Controlled Nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef]
- Sorcar, S.; Hwang, Y.; Grimes, C.A.; In, S.I. Highly Enhanced and Stable Activity of Defect-Induced Titania Nanoparticles for Solar Light-Driven CO2 Reduction into CH4. Mater. Today 2017, 20, 507–515. [Google Scholar] [CrossRef]
- Liao, F.; Zeng, Z.; Eley, C.; Lu, Q.; Hong, X.; Tsang, S.C.E. Electronic Modulation of a Copper/Zinc Oxide Catalyst by a Heterojunction for Selective Hydrogenation of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2012, 51, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Beigi, A.; Fatemi, S.; Salehi, Z. Synthesis of Nanocomposite CdS/TiO2 and Investigation of Its Photocatalytic Activity for CO2 Reduction to CO and CH4 under Visible Light Irradiation. J. CO2 Util. 2014, 7, 23–29. [Google Scholar] [CrossRef]
- Lan, H.; Tang, Y.; Zhang, X.; You, S.; Tang, Q.; An, X.; Liu, H.; Qu, J. Decomplexation of Cu(II)-EDTA over Oxygen-Doped g-C3N4: An Available Resource towards Environmental Sustainability. Chem. Eng. J. 2018, 345, 138–146. [Google Scholar] [CrossRef]
- Kohno, Y.; Tanaka, T.; Funabiki, T.; Yoshida, S. Reaction Mechanism in the Photoreduction of CO2 with CH4 over ZrO2. Phys. Chem. Chem. Phys. 2000, 2, 5302–5307. [Google Scholar] [CrossRef]
- Lu, X.; Xie, J.; Chen, X.; Li, X. Engineering MPx (M = Fe, Co or Ni) Interface Electron Transfer Channels for Boosting Photocatalytic H2 Evolution over g-C3N4/MoS2 Layered Heterojunctions. Appl. Catal. B Environ. 2019, 252, 250–259. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Sun, L.; Liu, R.; Cui, S. Studies of Z-Scheme WO3-TiO2/Cu2ZnSnS4 Ternary Nanocomposite with Enhanced CO2 Photoreduction under Visible Light Irradiation. J. CO2 Util. 2020, 37, 260–271. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- El-Yazeed, W.S.A.; Ahmed, A.I. Photocatalytic Activity of Mesoporous WO3/TiO2 Nanocomposites for the Photodegradation of Methylene Blue. Inorg. Chem. Commun. 2019, 105, 102–111. [Google Scholar] [CrossRef]
- Gao, L.; Du, J.; Ma, T. Cysteine-Assisted Synthesis of CuS-TiO2 Composites with Enhanced Photocatalytic Activity. Ceram. Int. 2017, 43, 9559–9563. [Google Scholar] [CrossRef]
- Gan, T.; Li, Y.; Wang, X.Z.; Wang, X.T.; Wang, C.W. Cu2 ZnSnS4@TiO2 P-n Heterostructured Nanosheet Arrays: Controllable Hydrothermal Synthesis and Enhanced Visible Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2017, 408, 60–67. [Google Scholar] [CrossRef]
- Wei, R.B.; Huang, Z.L.; Gu, G.H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.Q. Dual-Cocatalysts Decorated Rimous CdS Spheres Advancing Highly-Efficient Visible-Light Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Ji, Y.; Xu, Y.; Xie, M. A Novel Phase Retrieval Method from Three-Wavelength in-Line Phase-Shifting Interferograms Based on Positive Negative 2$π$ Phase Shifts. J. Mod. Opt. 2018, 65, 8–15. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Patience, G.S.; Boffito, D.C. Spray Dried TiO2/WO3 Heterostructure for Photocatalytic Applications with Residual Activity in the Dark. Appl. Catal. B Environ. 2018, 226, 311–323. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-Scheme Heterojunction for High-Efficiency Visible-Light-Driven Photocatalytic CO2 Reduction. Nanoscale 2021, 13, 4359–4389. [Google Scholar] [CrossRef]
- Li, J.; Tu, P.; Yang, Q.; Cui, Y.; Gao, C.; Zhou, H.; Lu, J.; Bian, H. A Novel Type-II–II Heterojunction for Photocatalytic Degradation of LEV Based on the Built-in Electric Field: Carrier Transfer Mechanism and DFT Calculation. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhang, W.; Jin, B.; Feng, Q.; Huang, J.; Jiao, Z. Advances in Z-Scheme Semiconductor Photocatalysts for the Photoelectrochemical Applications: A Review. Carbon Energy 2022, 4, 294–331. [Google Scholar] [CrossRef]
- Lei, X.; Yin, X.; Meng, S.; Li; Wang, H.; Xi, H.; Yang, J.; Xu, X.; Yang, Z.; Lei, Z. Fabrication of Type II Heterojunction in ZnIn2S4@ZnO Photocatalyst for Efficient Oxidative Coupling of Benzylamine under Visible Light. Mol. Catal. 2022, 519. [Google Scholar] [CrossRef]
- Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-Scheme Heterojunction TiO2/CdS Nanocomposite Nanofiber as H2-Production Photocatalyst. ChemCatChem 2019, 11, 6301–6309. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Matsunaga, H.; Ishiku, T.; Kamimura, S.; Ohno, T.; Yamakata, A. Trapping-Induced Enhancement of Photocatalytic Activity on Brookite TiO2 Powders: Comparison with Anatase and Rutile TiO2 Powders. ACS Catal. 2017, 7, 2644–2651. [Google Scholar] [CrossRef]
- De Castro, I.A.; Avansi, W.; Ribeiro, C. WO3/TiO2 Heterostructures Tailored by the Oriented Attachment Mechanism: Insights from Their Photocatalytic Properties. CrystEngComm 2014, 16, 1514–1524. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide – Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Gao, X.; Yu, B.; Gao, Y.; Han, Z. TiO2-Rutile/Anatase Homojunction with Enhanced Charge Separation for Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2021, 46, 26358–26366. [Google Scholar] [CrossRef]
- Kutorglo, E.M.; Schwarze, M.; Nguyen, A.D.; Tameu, S.D.; Huseyinova, S.; Tasbihi, M.; Görke, O.; Primbs, M.; Šoóš, M.; Schomäcker, R. Efficient Full Solar Spectrum-Driven Photocatalytic Hydrogen Production on Low Bandgap TiO2/Conjugated Polymer Nanostructures. RSC Adv. 2023, 13, 24038–24052. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Li, C.; Chen, Y.; Zhang, Z. Optimal Monolayer WO3 Nanosheets/TiO2 Heterostructure and Its Photocatalytic Performance under Solar Light. Chem. Phys. Lett. 2022, 804, 139861. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.; Schmidt, W.G. The Electronic Structure and Optical Response of Rutile, Anatase and Brookite TiO2. J. Phys. Condens. Matter 2012, 24. [Google Scholar] [CrossRef] [PubMed]
- NIST. Formic Acid. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=64-18-6 (accessed on 2 December 2024).
- Tommasi, M.; Conte, F.; Alam, M.I.; Ramis, G.; Rossetti, I. Highly Efficient and Effective Process Design for High-Pressure CO2 Photoreduction over Supported Catalysts. Energies 2023, 16, 4990. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of Modification Strategies towards Enhanced Charge Carrier Separation and Photocatalytic Degradation Activity of Metal Oxide Semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Pan, H.; Heagy, M.D. Photons to Formate—A Review on Photocatalytic Reduction of CO2 to Formic Acid. Nanomaterials 2020, 10, 2422. [Google Scholar] [CrossRef]
- Pan, H.; Chowdhury, S.; Premachandra, D.; Olguin, S.; Heagy, M.D. Semiconductor Photocatalysis of Bicarbonate to Solar Fuels: Formate Production from Copper(I) Oxide. ACS Sustain. Chem. Eng. 2018, 6, 1872–1880. [Google Scholar] [CrossRef]
- Pan, H.; Heagy, M.D. Plasmon-Enhanced Photocatalysis: Ag/TiO2 Nanocomposite for the Photochemical Reduction of Bicarbonate to Formic Acid. MRS Adv. 2019, 4, 425–433. [Google Scholar] [CrossRef]
- Mendoza, J.A.; Kim, H.K.; Park, H.K.; Park, K.Y. Photocatalytic Reduction of Carbon Dioxide Using Co3O4 Nanoparticles under Visible Light Irradiation. Korean J. Chem. Eng. 2012, 29, 1483–1486. [Google Scholar] [CrossRef]
- Bae, K.-L.; Kim, J.; Lim, C.K.; Nam, K.M.; Song, H. Colloidal Zinc Oxide-Copper(I) Oxide Nanocatalysts for Selective Aqueous Photocatalytic Carbon Dioxide Conversion into Methane. Nat. Commun. 2017, 8, 1156. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Rossetti, I. High Pressure Photoreduction of CO2: Effect of Catalyst Formulation, Hole Scavenger Addition and Operating Conditions. Catalysts 2018, 8, 430. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-Spray Pyrolysis Preparation of Perovskites for Methane Catalytic Combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia Michałand Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Masuda, Y.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C.; Ramakrishna, S. Synthesis of Hierarchical WO3 Nanostructured Thin Films with Enhanced Electrochromic Performance for Switchable Smart Windows. RSC Adv. 2015, 5, 96416–96427. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G. A Novel High-Pressure Photoreactor for CO2 Photoconversion to Fuels. RSC Adv. 2014, 4, 28883–28885. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Abdi, F.F.; Ng, Y.H. Heterogeneous Photocatalysts: An Overview of Classic and Modern Approaches for Optical, Electronic, and Charge Dynamics Evaluation. Chem. Soc. Rev. 2019, 48, 1255–1271. [Google Scholar] [CrossRef]
- Yamada, Y.; Kanemitsu, Y. Determination of Electron and Hole Lifetimes of Rutile and Anatase TiO2 Single Crystals. Appl. Phys. Lett. 2012, 101, 133907. [Google Scholar] [CrossRef]


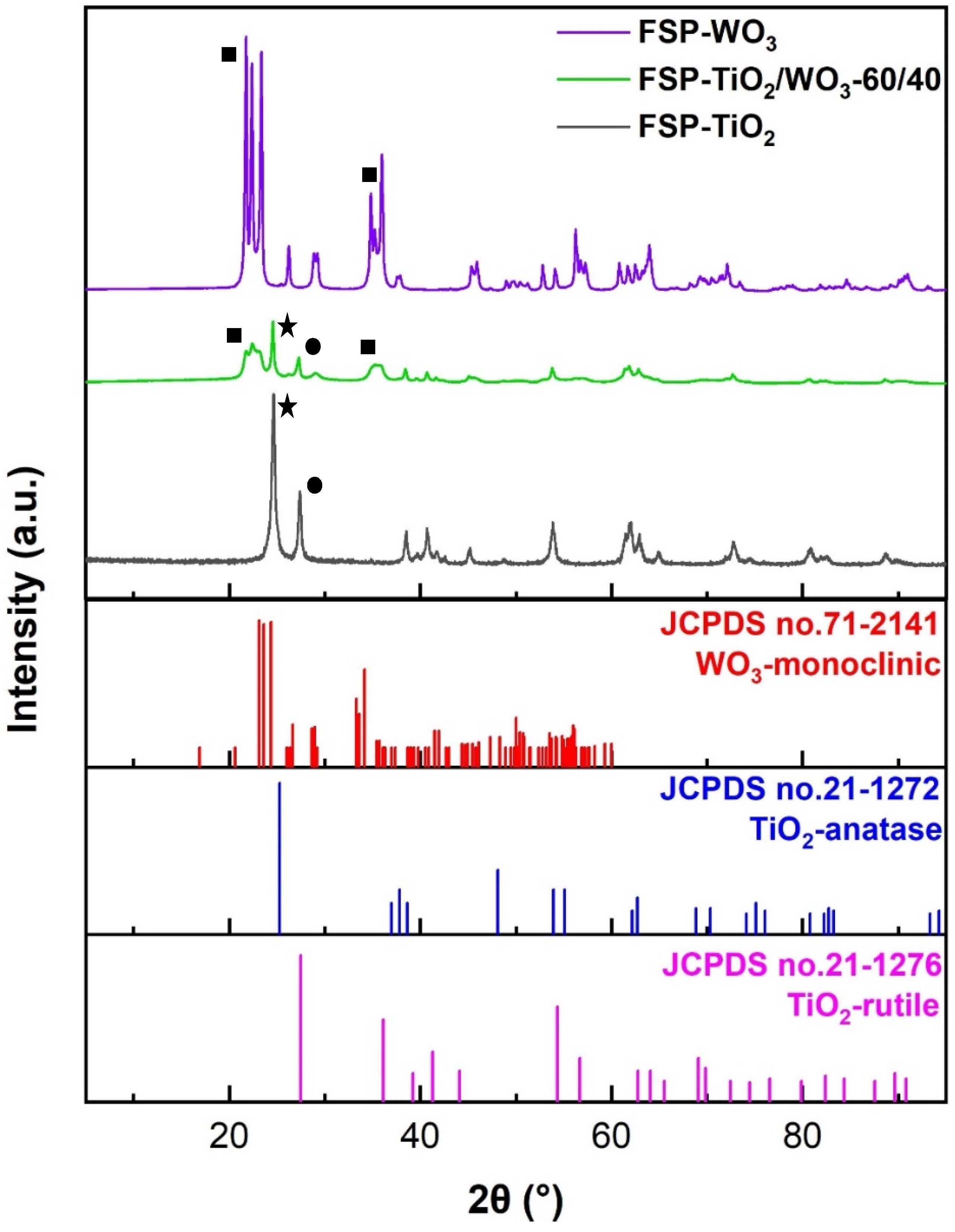

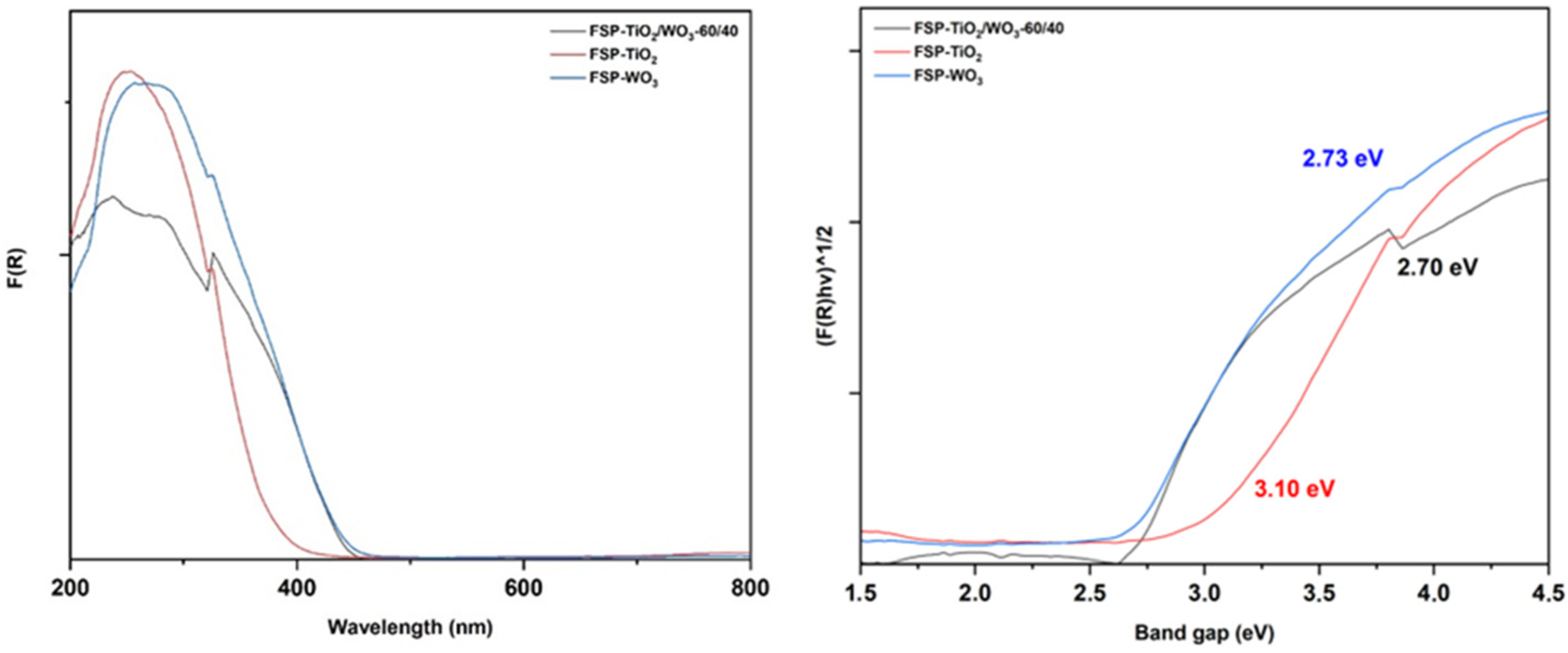
| Sample | BET SSA (m2/g) | Total Pore Volume (cm3/g) | BJH ads. Pore Width (nm) | Crystallite Size (nm) | Phase % | BG (eV) |
|---|---|---|---|---|---|---|
| P25 | 52.7 | 0.225 | 18.7 | 15(A); 26(R) | 78(A); 22(R) | 3.22 |
| FSP-TiO2 | 29.7 | 0.109 | 14.5 | 20(A); 24(R) | 48(A); 52(R) | 3.10 |
| FSP-WO3 | 3.4 | 0.015 | 35.0 | 42 | / | 2.73 |
| TiO2/WO3 60/40 wt | 29.2 | 0.077 | 9.8 | 22(A); 16(R) | 45(A); 65(R) | 2.7 |
| Catalyst | HS Conv. (%) | HCOOH (mol/kgcat h) | HCOOH (g/kgcat h) | Total Stored Energy (J) in 6 h |
|---|---|---|---|---|
| P25 | 47 | 16.9 | 778 | 195 |
| FSP TiO2 | 40 | 13.9 | 640 | 160 |
| FSP WO3 | 43 | 14.7 | 677 | 169 |
| FSP TiO2/WO3 60/40 | 77 | 36.5 | 1680 | 406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasi, M.; Gramegna, A.; Degerli, S.N.; Galli, F.; Rossetti, I. High-Pressure CO2 Photoreduction, Flame Spray Pyrolysis and Type-II Heterojunctions: A Promising Synergy. Catalysts 2025, 15, 383. https://doi.org/10.3390/catal15040383
Tommasi M, Gramegna A, Degerli SN, Galli F, Rossetti I. High-Pressure CO2 Photoreduction, Flame Spray Pyrolysis and Type-II Heterojunctions: A Promising Synergy. Catalysts. 2025; 15(4):383. https://doi.org/10.3390/catal15040383
Chicago/Turabian StyleTommasi, Matteo, Alice Gramegna, Simge Naz Degerli, Federico Galli, and Ilenia Rossetti. 2025. "High-Pressure CO2 Photoreduction, Flame Spray Pyrolysis and Type-II Heterojunctions: A Promising Synergy" Catalysts 15, no. 4: 383. https://doi.org/10.3390/catal15040383
APA StyleTommasi, M., Gramegna, A., Degerli, S. N., Galli, F., & Rossetti, I. (2025). High-Pressure CO2 Photoreduction, Flame Spray Pyrolysis and Type-II Heterojunctions: A Promising Synergy. Catalysts, 15(4), 383. https://doi.org/10.3390/catal15040383








