Cellulosome Systems in the Digestive Tract: Underexplored Enzymatic Machine for Lignocellulose Bioconversion
Abstract
1. Introduction
2. Cellulosome: Conservation and Diversity
2.1. Fundamental Framework of Cellulosomes
2.2. Species-Specific Diversity of Cellulosomes
3. Applications of Cellulosome System in Lignocellulose Bioconversion and Biotechnology
3.1. Biomass Conversion
3.2. Other Biotechnological Applications
4. Cellulosomes in Rumen Microbiota of Herbivores
4.1. Cellulosomes of Rumen Ruminococcus
4.1.1. Cellulosomes of R. flavefaciens
4.1.2. Cellulosomes of R. albus
4.2. Cellulosomes of Rumen Fungi
5. Cellulosomes in the Intestinal Tract of Primates
5.1. Cellulosomes in the Human Gut
5.2. Cellulosomes in the Gut of NHPs
6. Cellulosomes in Termite Guts
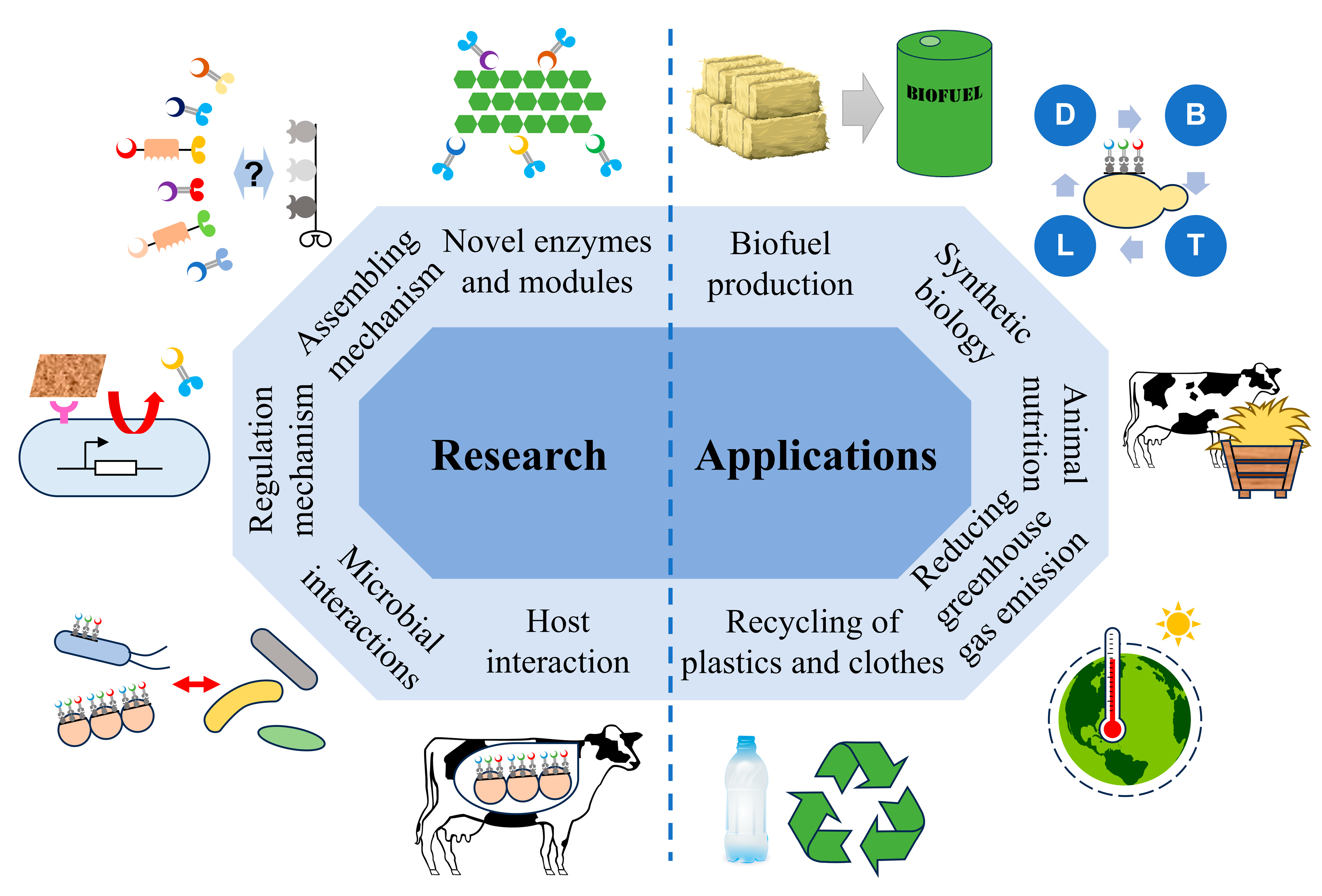
7. Research and Application Prospect of Digestive Tract Cellulosomes
- Enzymatic characterization of cellulosomal components, as most enzymes in digestive tract cellulosomes remain uncharacterized, and their roles in lignocellulose degradation have not been experimentally validated. Discovering novel enzymes and functional modules will enhance our understanding of these systems and provide new tools for lignocellulose biorefineries.
- Elucidating the structural organization and interaction mechanisms of cellulosomal components to understand how their arrangement contributes to functionality across different digestive tract environments.
- Exploring the regulation of cellulosome production in digestive tract microorganisms, as novel regulatory mechanisms are likely to be uncovered.
- Investigating the interactions between cellulosome-producing microorganisms and other gut microbes, which may reveal their roles in host health and functionality.
- Clarifying the contributions of cellulosome-producing microorganisms to greenhouse gas emissions (e.g., methane) and feed efficiency in livestock, which could inform strategies to reduce emissions and enhance productivity in the livestock industry.
- Lignocellulose Biorefineries: The high efficiency of cellulosomes has already been leveraged for biofuel production and lignocellulose bio-saccharification [9,57]. Novel cellulosomal components from digestive tracts may enhance the activity and versatility of cellulosomes in biorefinery applications.
- Environmental Sustainability: Digestive tract cellulosomes may mitigate greenhouse gas emissions from livestock [21] and enhance the composting of lignocellulosic agricultural waste [144]. Additionally, cellulosomal modules could be engineered to assemble plastic-degrading enzymes, improving the biotreatment of plastic waste and reducing pollution [145,146].
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCB | lignocellulosic biomass |
| CBM | carbohydrate-binding module |
| GH | glycoside hydrolase |
| SLH | S-layer homology |
| CBP | consolidated bioprocessing |
| CBS | consolidated bio-saccharification |
| CAZymes | carbohydrate-active enzymes |
| PUL | polysaccharide utilization loci |
| NCDD | non-catalytic dockerin domain |
| DDP | dockerin domain protein |
| CMC | carboxymethyl cellulose |
| SCFA | short-chain fatty acids |
| NHP | non-human primate |
References
- Mohanty, B.; Abdullahi, I.I. Bioethanol production from lignocellulosic waste—A review. Biosci. Biotechnol. Res. Asia 2016, 13, 1153–1161. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, D.; Mao, G.; Wang, F.; Song, A. Availability of lignocellulose from forestry waste for use as a biofuel in China. 3 Biotech 2018, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Waste paper: An underutilized but promising source for nanocellulose mining. Waste Manag. 2020, 102, 281–303. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nguyen, A.Q.; Johir, M.A.; Guo, W.S.; Ngo, H.H.; Chaves, A.V.; Nghiem, L.D. Application of rumen and anaerobic sludge microbes for bio harvesting from lignocellulosic biomass. Chemosphere 2019, 228, 702–708. [Google Scholar] [CrossRef]
- Ilic, N.; Milic, M.; Beluhan, S.; Dimitrijevic-Brankovic, S. Cellulases: From lignocellulosic biomass to improved production. Energies 2023, 16, 3598. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of lignocellulose hydrolysate in different biorefinery strategies: Nutrients and inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Kenig, R.; Lamed, R. Adherence of Clostridium thermocellum to cellulose. J. Bacteriol. 1983, 156, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Lamed, R.; Setter, E.; Bayer, E.A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 1983, 156, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Setter, E.; Lamed, R. Organization and distribution of the cellulosome in Clostridium thermocellum. J. Bacteriol. 1985, 163, 552–559. [Google Scholar] [CrossRef]
- Lamed, R.; Setter, E.; Kenig, R.; Bayer, E.A. The cellulosome: A discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities. Biotechnol. Bioeng. Symp. 1983, 13, 163–181. [Google Scholar]
- Artzi, L.; Bayer, E.A.; Moraïs, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef]
- Bayer, E.A.; Lamed, R.; White, B.A.; Flint, H.J. From cellulosomes to cellulosomics. Chem. Rec. 2008, 8, 364–377. [Google Scholar] [CrossRef]
- Alves, V.D.; Fontes, C.; Bule, P. Cellulosomes: Highly efficient cellulolytic complexes. Subcell. Biochem. 2021, 96, 323–354. [Google Scholar] [CrossRef]
- Desvaux, M. Clostridium cellulolyticum: Model organism of mesophilic cellulolytic clostridia. FEMS Microbiol. Rev. 2005, 29, 741–764. [Google Scholar] [CrossRef]
- Ben David, Y.; Dassa, B.; Borovok, I.; Lamed, R.; Koropatkin, N.M.; Martens, E.C.; White, B.A.; Bernalier-Donadille, A.; Duncan, S.H.; Flint, H.J.; et al. Ruminococcal cellulosome systems from rumen to human. Environ. Microbiol. 2015, 17, 3407–3426. [Google Scholar] [CrossRef]
- Feng, Y.; Yeoman, C.J. When cellulosomes meet humans. Green Carbon 2024, 2, 337–338. [Google Scholar] [CrossRef]
- Han, Y.J.; Chang, X.; Xiang, H.; Fang, Y.; Hao, L.Z.; Gu, Y.; Han, X.Y.; Zhao, B.; Zhao, Z.J.; Zhao, C.Z.; et al. Exploring biomimetic potential of ruminant digestion strategies for lignocellulosic biomass utilization: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 188, 113887. [Google Scholar] [CrossRef]
- Chassard, C.; Delmas, E.; Robert, C.; Bernalier-Donadille, A. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol. Ecol. 2010, 74, 205–213. [Google Scholar] [CrossRef]
- Chassard, C.; Delmas, E.; Robert, C.; Lawson, P.A.; Bernalier-Donadille, A. Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int. J. Syst. Evol. Microbiol. 2012, 62, 138–143. [Google Scholar] [CrossRef]
- Moraïs, S.; Winkler, S.; Zorea, A.; Levin, L.; Nagies, F.S.P.; Kapust, N.; Lamed, E.; Artan-Furman, A.; Bolam, D.N.; Yadav, M.P.; et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science 2024, 383, eadj9223. [Google Scholar] [CrossRef]
- Munir, R.I.; Schellenberg, J.; Henrissat, B.; Verbeke, T.J.; Sparling, R.; Levin, D.B. Comparative analysis of carbohydrate active enzymes in Clostridium termitidis CT1112 reveals complex carbohydrate degradation ability. PLoS ONE 2014, 9, e104260. [Google Scholar] [CrossRef]
- Miller, M.E.B.; Antonopoulos, D.A.; Rincon, M.T.; Band, M.; Bari, A.; Akraiko, T.; Hernandez, A.; Thimmapuram, J.; Henrissat, B.; Coutinho, P.M.; et al. Diversity and strain specificity of plant cell wall degrading enzymes revealed by the draft genome of Ruminococcus flavefaciens FD-1. PLoS ONE 2009, 4, e6650. [Google Scholar] [CrossRef]
- Dassa, B.; Borovok, I.; Ruimy-Israeli, V.; Lamed, R.; Flint, H.J.; Duncan, S.H.; Henrissat, B.; Coutinho, P.; Morrison, M.; Mosoni, P.; et al. Rumen cellulosomics: Divergent fiber-degrading strategies revealed by comparative genome-wide analysis of six ruminococcal strains. PLoS ONE 2014, 9, e99221. [Google Scholar] [CrossRef]
- Miller, M.E.B.; Yeoman, C.J.; Chia, N.; Tringe, S.G.; Angly, F.E.; Edwards, R.A.; Flint, H.J.; Lamed, R.; Bayer, E.A.; White, B.A. Phage–bacteria relationships and CRISPR elements revealed by a metagenomic survey of the rumen microbiome. Environ. Microbiol. 2012, 14, 207–227. [Google Scholar] [CrossRef]
- Vodovnik, M.; Duncan, S.H.; Reid, M.D.; Cantlay, L.; Turner, K.; Parkhill, J.; Lamed, R.; Yeoman, C.J.; Miller, M.E.B.; White, B.A.; et al. Expression of cellulosome components and type IV pili within the extracellular proteome of Ruminococcus flavefaciens 007. PLoS ONE 2013, 8, e65333. [Google Scholar] [CrossRef]
- Suen, G.; Stevenson, D.M.; Bruce, D.C.; Chertkov, O.; Copeland, A.; Cheng, J.-F.; Detter, C.; Detter, J.C.; Goodwin, L.A.; Han, C.S.; et al. Complete genome of the cellulolytic ruminal bacterium Ruminococcus albus 7. J. Bacteriol. 2011, 193, 5574–5575. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Ramachandran, U.; Zhang, X.; Munir, R.; Sparling, R.; Levin, D.B. Draft genome sequence of the cellulolytic, mesophilic, anaerobic bacterium Clostridium termitidis strain CT1112 (DSM 5398). Genome Announc. 2013, 1, e00281-13. [Google Scholar] [CrossRef] [PubMed]
- Haitjema, C.H.; Gilmore, S.P.; Henske, J.K.; Solomon, K.V.; de Groot, R.; Kuo, A.; Mondo, S.J.; Salamov, A.A.; LaButti, K.; Zhao, Z.Y.; et al. A parts list for fungal cellulosomes revealed by comparative genomics. Nat. Microbiol. 2017, 2, 17087. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.S.; Nabi, M.; Zhang, P.Y.; Zhang, G.M.; Cai, Y.J.; Wang, Q.Y.; Zhou, Z.Y.; Ding, Y.R. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110335. [Google Scholar] [CrossRef]
- Smith, S.P.; Bayer, E.A. Insights into cellulosome assembly and dynamics: From dissection to reconstruction of the supramolecular enzyme complex. Curr. Opin. Struct. Biol. 2013, 23, 686–694. [Google Scholar] [CrossRef]
- Bule, P.; Pires, V.M.R.; Alves, V.D.; Carvalho, A.L.; Prates, J.; Ferreira, L.M.A.; Smith, S.P.; Gilbert, H.J.; Noach, I.; Bayer, E.A.; et al. Higher order scaffoldin assembly in Ruminococcus flavefaciens cellulosome is coordinated by a discrete cohesin-dockerin interaction. Sci. Rep. 2018, 8, 6987. [Google Scholar] [CrossRef]
- Doi, R.H.; Kosugi, A. Cellulosomes: Plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2004, 2, 541–551. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, J.; Feng, Y.; Mohr, G.; Lambowitz, A.M.; Cui, G.Z.; Liu, Y.J.; Cui, Q. The contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum analyzed by using thermotargetrons. Biotechnol. Biofuels 2014, 7, 80. [Google Scholar] [CrossRef]
- Xu, Q.; Resch, M.G.; Podkaminer, K.; Yang, S.H.; Baker, J.O.; Donohoe, B.S.; Wilson, C.; Klingeman, D.M.; Olson, D.G.; Decker, S.R.; et al. Dramatic performance of Clostridium thermocellum explained by its wide range of cellulase modalities. Sci. Adv. 2016, 2, e1501254. [Google Scholar] [CrossRef]
- Chen, C.; Cui, Z.; Song, X.; Liu, Y.-J.; Cui, Q.; Feng, Y. Integration of bacterial expansin-like proteins into cellulosome promotes the cellulose degradation. Appl. Microbiol. Biotechnol. 2016, 100, 2203–2212. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.W.; Dong, S.; You, C.; Moraïs, S.; Bayer, E.A.; Liu, Y.J.; Xuan, J.S.; Cui, Q.; Mizrahi, I.; et al. A cellulosomal double-dockerin module from Clostridium thermocellum shows distinct structural and cohesin-binding features. Protein Sci. 2024, 33, e4937. [Google Scholar] [CrossRef] [PubMed]
- Meguro, H.; Morisaka, H.; Kuroda, K.; Miyake, H.; Tamaru, Y.; Ueda, M. Putative role of cellulosomal protease inhibitors in Clostridium cellulovorans based on gene expression and measurement of activities. J. Bacteriol. 2011, 193, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
- Levy-Assaraf, M.; Voronov-Goldman, M.; Rozman Grinberg, I.; Weiserman, G.; Shimon, L.J.W.; Jindou, S.; Borovok, I.; White, B.A.; Bayer, E.A.; Lamed, R.; et al. Crystal structure of an uncommon cellulosome-related protein module from Ruminococcus flavefaciens that resembles papain-Like cysteine peptidases. PLoS ONE 2013, 8, e56138. [Google Scholar] [CrossRef]
- Murray, W.D.; Sowden, L.C.; Colvin, J.R. Bacteroides cellulosolvens sp. nov., a cellulolytic species from sewage sludge. Int. J. Syst. Bacteriol. 1984, 34, 185–187. [Google Scholar] [CrossRef]
- Zhivin, O.; Dassa, B.; Moraïs, S.; Utturkar, S.M.; Brown, S.D.; Henrissat, B.; Lamed, R.; Bayer, E.A. Unique organization and unprecedented diversity of the Bacteroides (Pseudobacteroides) cellulosolvens cellulosome system. Biotechnol. Biofuels 2017, 10, 211. [Google Scholar] [CrossRef]
- Pages, S.; Belaich, A.; Belaich, J.P.; Morag, E.; Lamed, R.; Shoham, Y.; Bayer, E.A. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: Prediction of specificity determinants of the dockerin domain. Proteins 1997, 29, 517–527. [Google Scholar] [CrossRef]
- Haimovitz, R.; Barak, Y.; Morag, E.; Voronov-Goldman, M.; Shoham, Y.; Lamed, R.; Bayer, E.A. Cohesin-dockerin microarray: Diverse specificities between two complementary families of interacting protein modules. Proteomics 2008, 8, 968–979. [Google Scholar] [CrossRef]
- Izquierdo, J.A.; Goodwin, L.; Davenport, K.W.; Teshima, H.; Bruce, D.; Detter, C.; Tapia, R.; Han, S.S.; Land, M.; Hauser, L.; et al. Complete genome sequence of Clostridium clariflavum DSM 19732. Stand. Genom. Sci. 2012, 6, 104–115. [Google Scholar] [CrossRef]
- Artzi, L.; Morag, E.; Barak, Y.; Lamed, R.; Bayer, E.A. Clostridium clariflavum: Key cellulosome players are revealed by proteomic analysis. mBio 2015, 6, e00411-15. [Google Scholar] [CrossRef]
- Gold, N.D.; Martin, V.J.J. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J. Bacteriol. 2007, 189, 6787–6795. [Google Scholar] [CrossRef]
- Raman, B.; Pan, C.; Hurst, G.B.; Rodriguez, M.; McKeown, C.K.; Lankford, P.K.; Samatova, N.F.; Mielenz, J.R. Impact of pretreated switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: A quantitative proteomic analysis. PLoS ONE 2009, 4, e5271. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.A.; Pattathil, S.; Guseva, A.; Hahn, M.G.; Lynd, L.R. Comparative analysis of the ability of Clostridium clariflavum strains and Clostridium thermocellum to utilize hemicellulose and unpretreated plant material. Biotechnol. Biofuels 2014, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; Hörl, M.; Fuhrer, T.; Cui, J.X.; Zhou, J.L.; Maloney, M.I.; Amador-Noguez, D.; Tian, L.; Sauer, U.; Lynd, L.R. Glycolysis without pyruvate kinase in Clostridium thermocellum. Metab. Eng. 2017, 39, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Liu, W.-H.; Fan, Z.-Y.; Liu, Y.-Y.; Liang, C.-Y.; Xu, J.-L. Research progress on the conversion of lignocellulose to ethanol by Clostridium thermocellum. Adv. New Renew. Energy 2020, 8, 28–34. [Google Scholar] [CrossRef]
- Mazzoli, R.; Olson, D.G. Chapter Three—Clostridium thermocellum: A microbial platform for high-value chemical production from lignocellulose. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 113, pp. 111–161. [Google Scholar]
- Lynd, L.R.; Elamder, R.T.; Wyman, C.E. Likely features and costs of mature biomass ethanol technology. Appl. Biochem. Biotechnol. 1996, 57, 741–761. [Google Scholar] [CrossRef]
- Lynd, L.R.; Liang, X.Y.; Biddy, M.J.; Allee, A.; Cai, H.; Foust, T.; Himmel, M.E.; Laser, M.S.; Wang, M.; Wyman, C.E. Cellulosic ethanol: Status and innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef]
- Qi, K.; Chen, C.; Yan, F.; Feng, Y.; Bayer, E.A.; Kosugi, A.; Cui, Q.; Liu, Y.-J. Coordinated β-glucosidase activity with the cellulosome is effective for enhanced lignocellulose saccharification. Bioresour. Technol. 2021, 337, 125441. [Google Scholar] [CrossRef]
- Tian, Q.-Q.; Liang, L.; Zhu, M.-J. Enhanced biohydrogen production from sugarcane bagasse by Clostridium thermocellum supplemented with CaCO3. Bioresour. Technol. 2015, 197, 422–428. [Google Scholar] [CrossRef]
- Ban, Y.J.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef]
- Bayer, E.A.; Morag, E.; Lamed, R. The cellulosome—A treasure-trove for biotechnology. Trends Biotechnol. 1994, 12, 379–386. [Google Scholar] [CrossRef]
- Lilly, M.; Fierobe, H.P.; van Zyl, W.H.; Volschenk, H. Heterologous expression of a Clostridium minicellulosome in Saccharomyces cerevisiae. FEMS Yeast Res. 2009, 9, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Oh, J.; Singh, S.; Chen, R.Z.; Chen, W. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 2009, 75, 6087–6093. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Zhang, Z.J.; Yu, X.Y.; Xue, Y.X.; Tan, T.W. Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production. Proc. Natl. Acad. Sci. USA 2012, 109, 13260–13265. [Google Scholar] [CrossRef]
- Tsai, S.L.; DaSilva, N.A.; Chen, W. Functional display of complex cellulosomes on the yeast surface via adaptive assembly. ACS Synth. Biol. 2013, 2, 14–21. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.H.; Lee, K.; Hahn, J.S. Cellulosic ethanol production using a yeast consortium displaying a minicellulosome and β-glucosidase. Microb. Cell Factories 2013, 12, 14. [Google Scholar] [CrossRef]
- Gunnoo, M.; Cazade, P.-A.; Galera-Prat, A.; Nash, M.A.; Czjzek, M.; Cieplak, M.; Alvarez, B.; Aguilar, M.; Karpol, A.; Gaub, H.; et al. Nanoscale engineering of designer cellulosomes. Adv. Mater. 2016, 28, 5619–5647. [Google Scholar] [CrossRef]
- Sun, Q.; Tsai, S.-L.; Chen, W. Chapter Fourteen—Artificial scaffolds for enhanced biocatalysis. In Methods in Enzymology; Schmidt-Dannert, C., Quin, M.B., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 617, pp. 363–383. [Google Scholar]
- Liu, M.; Wang, Y.; Jiang, H.; Han, Y.; Xia, J. Synthetic multienzyme assemblies for natural product biosynthesis. ChemBioChem 2023, 24, e202200518. [Google Scholar] [CrossRef]
- Fierer, J.O.; Tovar-Herrera, O.E.; Weinstein, J.Y.; Kahn, A.; Moraïs, S.; Mizrahi, I.; Bayer, E.A. Affinity-induced covalent protein-protein ligation via the SpyCatcher-SpyTag interaction. Green Carbon 2023, 1, 33–42. [Google Scholar] [CrossRef]
- Hyeon, J.E.; Kang, D.H.; Han, S.O. Signal amplification by a self-assembled biosensor system designed on the principle of dockerin-cohesin interactions in a cellulosome complex. Analyst 2014, 139, 4790–4793. [Google Scholar] [CrossRef]
- Shpigel, E.; Goldlust, A.; Efroni, G.; Avraham, A.; Eshel, A.; Dekel, M.; Shoseyov, O. Immobilization of recombinant heparinase I fused to cellulose-binding domain. Biotechnol. Bioeng. 1999, 65, 17–23. [Google Scholar] [CrossRef]
- Hyeon, J.E.; Kang, D.H.; Kim, Y.I.; Jeon, S.D.; You, S.K.; Kim, K.Y.; Kim, S.W.; Han, S.O. Production of functional agarolytic nano-complex for the synergistic hydrolysis of marine biomass and its potential application in carbohydrate-binding module-utilizing one-step purification. Process Biochem. 2012, 47, 877–881. [Google Scholar] [CrossRef]
- Puniya, A.K.; Singh, R.; Kamra, D.N. (Eds.) Rumen Microbiology: From Evolution to Revolution; Springer: New Delhi, India, 2015; pp. 1–379. [Google Scholar]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M. Biotechnological utilization of animal gut microbiota for valorization of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2020, 104, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Lapébie, P.; Lombard, V.; Drula, E.; Terrapon, N.; Henrissat, B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019, 10, 2043. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.Z.; Han, J.L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Aurilia, V.; Martin, J.C.; McCrae, S.I.; Scott, K.P.; Rincon, M.T.; Flint, H.J. Three multidomain esterases from the cellulolytic rumen anaerobe Ruminococcus flavefaciens 17 that carry divergent dockerin sequences. Microbiology 2000, 146, 1391–1397. [Google Scholar] [CrossRef]
- Rincón, M.T.; McCrae, S.I.; Kirby, J.; Scott, K.P.; Flint, H.J. EndB, a multidomain family 44 cellulase from Ruminococcus flavefaciens 17, binds to cellulose via a novel cellulose-binding module and to another R. flavefaciens protein via a dockerin domain. Appl. Environ. Microbiol. 2001, 67, 4426–4431. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Rincon, M.T.; Lamed, R.; Martin, J.C.; McCrae, S.I.; Aurilia, V.; Shoham, Y.; Bayer, E.A.; Flint, H.J. Cellulosomal scaffoldin-like proteins from Ruminococcus flavefaciens. J. Bacteriol. 2001, 183, 1945–1953. [Google Scholar] [CrossRef]
- Rincon, M.T.; Ding, S.Y.; McCrae, S.I.; Martin, J.C.; Aurilia, V.; Lamed, R.; Shoham, Y.; Bayer, E.A.; Flint, H.J. Novel organization and divergent dockerin specificities in the cellulosome system of Ruminococcus flavefaciens. J. Bacteriol. 2003, 185, 703–713. [Google Scholar] [CrossRef]
- Rincón, M.T.; Martin, J.C.; Aurilia, V.; McCrae, S.I.; Rucklidge, G.J.; Reid, M.D.; Bayer, E.A.; Lamed, R.; Flint, H.J. ScaC, an adaptor protein carrying a novel cohesin that expands the dockerin-binding repertoire of the Ruminococcus flavefaciens 17 cellulosome. J. Bacteriol. 2004, 186, 2576–2585. [Google Scholar] [CrossRef]
- Rincon, M.T.; Čepeljnik, T.; Martin, J.C.; Lamed, R.; Barak, Y.; Bayer, E.A.; Flint, H.J. Unconventional mode of attachment of the Ruminococcus flavefaciens cellulosome to the cell surface. J. Bacteriol. 2005, 187, 7569–7578. [Google Scholar] [CrossRef] [PubMed]
- Jindou, S.; Borovok, I.; Rincon, M.T.; Flint, H.J.; Antonopoulos, D.A.; Berg, M.E.; White, B.A.; Bayer, E.A.; Lamed, R. Conservation and divergence in cellulosome architecture between two strains of Ruminococcus flavefaciens. J. Bacteriol. 2006, 188, 7971–7976. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.T.; Cepeljnik, T.; Martin, J.C.; Barak, Y.; Lamed, R.; Bayer, E.A.; Flint, H.J. A novel cell surface-anchored cellulose-binding protein encoded by the sca gene cluster of Ruminococcus flavefaciens. J. Bacteriol. 2007, 189, 4774–4783. [Google Scholar] [CrossRef]
- Jindou, S.; Brulc, J.M.; Levy-Assaraf, M.; Rincon, M.T.; Flint, H.J.; Berg, M.E.; Wilson, M.K.; White, B.A.; Bayer, E.A.; Lamed, R.; et al. Cellulosome gene cluster analysis for gauging the diversity of the ruminal cellulolytic bacterium Ruminococcus flavefaciens. FEMS Microbiol. Lett. 2008, 285, 188–194. [Google Scholar] [CrossRef]
- Rincon, M.T.; Dassa, B.; Flint, H.J.; Travis, A.J.; Jindou, S.; Borovok, I.; Lamed, R.; Bayer, E.A.; Henrissat, B.; Coutinho, P.M.; et al. Abundance and diversity of dockerin-containing proteins in the fiber-degrading rumen bacterium, Ruminococcus flavefaciens FD-1. PLoS ONE 2010, 5, e12476. [Google Scholar] [CrossRef]
- Brulc, J.M.; Yeoman, C.J.; Wilson, M.K.; Berg Miller, M.E.; Jeraldo, P.; Jindou, S.; Goldenfeld, N.; Flint, H.J.; Lamed, R.; Borovok, I.; et al. Cellulosomics, a gene-centric approach to investigating the intraspecific diversity and adaptation of Ruminococcus flavefaciens within the rumen. PLoS ONE 2011, 6, e25329. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Israeli-Ruimy, V.; Bule, P.; Jindou, S.; Dassa, B.; Moraïs, S.; Borovok, I.; Barak, Y.; Slutzki, M.; Hamberg, Y.; Cardoso, V.; et al. Complexity of the Ruminococcus flavefaciens FD-1 cellulosome reflects an expansion of family-related protein-protein interactions. Sci. Rep. 2017, 7, 42355. [Google Scholar] [CrossRef]
- Grinberg, I.R.; Yin, G.; Borovok, I.; Miller, M.E.B.; Yeoman, C.J.; Dassa, B.; Yu, Z.; Mizrahi, I.; Flint, H.J.; Bayer, E.A.; et al. Functional phylotyping approach for assessing intraspecific diversity of Ruminococcus albus within the rumen microbiome. FEMS Microbiol. Lett. 2015, 362, 1–10. [Google Scholar] [CrossRef]
- Duarte, M.; Alves, V.D.; Correia, M.; Caseiro, C.; Ferreira, L.M.A.; Romão, M.J.; Carvalho, A.L.; Najmudin, S.; Bayer, E.A.; Fontes, C.M.G.A.; et al. Structure-function studies can improve binding affinity of cohesin-dockerin interactions for multi-protein assemblies. Int. J. Biol. Macromol. 2023, 224, 55–67. [Google Scholar] [CrossRef]
- Salama-Alber, O.; Jobby, M.K.; Chitayat, S.; Smith, S.P.; White, B.A.; Shimon, L.J.W.; Lamed, R.; Frolow, F.; Bayer, E.A. Atypical cohesin-dockerin complex responsible for cell surface attachment of cellulosomal components: Binging fidelity, promiscuity, and structural buttresses. J. Biol. Chem. 2013, 288, 16827–16838. [Google Scholar] [CrossRef] [PubMed]
- Voronov-Goldman, M.; Yaniv, O.; Gul, O.; Yoffe, H.; Salama-Alber, O.; Slutzki, M.; Levy-Assaraf, M.; Jindou, S.; Shimon, L.J.W.; Borovok, I.; et al. Standalone cohesin as a molecular shuttle in cellulosome assembly. FEBS Lett. 2015, 589, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Bule, P.; Alves, V.D.; Leitão, A.; Ferreira, L.M.A.; Bayer, E.A.; Smith, S.P.; Gilbert, H.J.; Najmudin, S.; Fontes, C.M.G.A. Single binding mode integration of hemicellulose-degrading enzymes via adaptor scaffoldins in Ruminococcus flavefaciens cellulosome. J. Biol. Chem. 2016, 291, 26658–26669. [Google Scholar] [CrossRef] [PubMed]
- Bule, P.; Alves, V.D.; Israeli-Ruimy, V.; Carvalho, A.L.; Ferreira, L.M.A.; Smith, S.P.; Gilbert, H.J.; Najmudin, S.; Bayer, E.A.; Fontes, C.M.G.A. Assembly of Ruminococcus flavefaciens cellulosome revealed by structures of two cohesin-dockerin complexes. Sci. Rep. 2017, 7, 759. [Google Scholar] [CrossRef]
- Schoeler, C.; Malinowska, K.H.; Bernardi, R.C.; Milles, L.F.; Jobst, M.A.; Durner, E.; Ott, W.; Fried, D.B.; Bayer, E.A.; Schulten, K.; et al. Ultrastable cellulosome-adhesion complex tightens under load. Nat. Commun. 2014, 5, 5635. [Google Scholar] [CrossRef]
- Goyal, D.; Kumar, K.; Centeno, M.S.J.; Thakur, A.; Pires, V.M.R.; Bule, P.; Fontes, C.M.G.A.; Goyal, A. Molecular cloning, expression and biochemical characterization of a family 5 glycoside hydrolase first endo-mannanase (RfGH5_7) from Ruminococcus flavefaciens FD-1 v3. Mol. Biotechnol. 2019, 61, 826–835. [Google Scholar] [CrossRef]
- Mondal, S.; Thakur, A.; Fontes, C.M.G.A.; Goyal, A. A trimodular family 16 glycoside hydrolase from the cellulosome of Ruminococcus flavefaciens displays highly specific licheninase (EC 3.2.1.73) activity. Microbiology 2021, 167, 001055. [Google Scholar] [CrossRef]
- Gavande, P.V.; Nath, P.; Kumar, K.; Ahmed, N.; Fontes, C.M.G.A.; Goyal, A. Highly efficient, processive and multifunctional recombinant endoglucanase RfGH5_4 from Ruminococcus flavefaciens FD-1 v3 for recycling lignocellulosic plant biomasses. Int. J. Biol. Macromol. 2022, 209, 801–813. [Google Scholar] [CrossRef]
- Venditto, I.; Luis, A.S.; Rydahl, M.; Schückel, J.; Fernandes, V.O.; Vidal-Melgosa, S.; Bule, P.; Goyal, A.; Pires, V.M.R.; Dourado, C.G.; et al. Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc. Natl. Acad. Sci. USA 2016, 113, 7136–7141. [Google Scholar] [CrossRef]
- Lamed, R.; Naimark, J.; Morgenstern, E.; Bayer, E.A. Specialized cell surface structures in cellulolytic bacteria. J. Bacteriol. 1987, 169, 3792–3800. [Google Scholar] [CrossRef]
- Ohara, H.; Karita, S.; Kimura, T.; Sakka, K.; Ohmiya, K. Characterization of the cellulolytic complex (cellulosome) from Ruminococcus albus. Biosci. Biotechnol. Biochem. 2000, 64, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ezer, A.; Matalon, E.; Jindou, S.; Borovok, I.; Atamna, N.; Yu, Z.T.; Morrison, M.; Bayer, E.A.; Lamed, R. Cell surface enzyme attachment is mediated by family 37 carbohydrate-binding modules, unique to Ruminococcus albus. J. Bacteriol. 2008, 190, 8220–8222. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarivonina, H.; Terrie, C.; Chambon, C.; Forano, E.; Mosoni, P. Proteomic identification of CBM37-containing cellulases produced by the rumen cellulolytic bacterium Ruminococcus albus 20 and their putative involvement in bacterial adhesion to cellulose. Arch. Microbiol. 2009, 191, 379–388. [Google Scholar] [CrossRef]
- Devillard, E.; Goodheart, D.B.; Karnati, S.K.R.; Bayer, E.A.; Lamed, R.; Miron, J.; Nelson, K.E.; Morrison, M. Ruminococcus albus 8 mutants defective in cellulose degradation are deficient in two processive endocellulases, Cel48A and Cel9B, both of which possess a novel modular architecture. J. Bacteriol. 2004, 186, 136–145. [Google Scholar] [CrossRef]
- Christopherson, M.R.; Dawson, J.A.; Stevenson, D.M.; Cunningham, A.C.; Bramhacharya, S.; Weimer, P.J.; Kendziorski, C.; Suen, G. Unique aspects of fiber degradation by the ruminal ethanologen Ruminococcus albus 7 revealed by physiological and transcriptomic analysis. BMC Genom. 2014, 15, 1066. [Google Scholar] [CrossRef]
- Ohara, H.; Noguchi, J.; Karita, S.; Kimura, T.; Sakka, K.; Ohmiya, K. Sequence of egV and Properties of EgV, a Ruminococcus albus Endoglucanase Containing a Dockerin Domain. Biosci. Biotechnol. Biochem. 2000, 64, 80–88. [Google Scholar] [CrossRef]
- Wilson, C.A.; Wood, T.M. Studies on the cellulase of the rumen anaerobic fungus Neocallimastix frontalis, with special reference to the capacity of the enzyme to degrade crystalline cellulose. Enzyme Microb. Technol. 1992, 14, 258–264. [Google Scholar] [CrossRef]
- Henske, J.K.; Gilmore, S.P.; Knop, D.; Cunningham, F.J.; Sexton, J.A.; Smallwood, C.R.; Shutthanandan, V.; Evans, J.E.; Theodorou, M.K.; O’Malley, M.A. Transcriptomic characterization of Caecomyces churrovis: A novel, non-rhizoid-forming lignocellulolytic anaerobic fungus. Biotechnol. Biofuels 2017, 10, 305. [Google Scholar] [CrossRef]
- Fanutti, C.; Ponyi, T.; Black, G.W.; Hazlewood, G.P.; Gilbert, H.J. The conserved noncatalytic 40-residue sequence in cellulases and hemicellulases from anaerobic fungi functions as a protein docking domain. J. Biol. Chem. 1995, 270, 29314–29322. [Google Scholar] [CrossRef]
- Bayer, E.A.; Belaich, J.P.; Shoham, Y.; Lamed, R. The cellulosomes: Multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 2004, 58, 521–554. [Google Scholar] [CrossRef]
- Steenbakkers, P.J.M.; Harhangi, H.R.; Bosscher, M.W.; van der Hooft, M.M.C.; Keltjens, J.T.; van der Drift, C.; Vogels, G.D.; Den Camp, H. β-glucosidase in cellulosome of the anaerobic fungus Piromyces sp. strain E2 is a family 3 glycoside hydrolase. Biochem. J. 2003, 370, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Paul, S.S.; Puniya, A.K.; van der Giezen, M.; Shaw, C.; Edwards, J.E.; Fliegerova, K. Anaerobic fungi: Past, present, and future. Front. Microbiol. 2020, 11, 584893. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Youssef, N.H.; Hanafy, R.A.; Couger, M.B.; Stajich, J.E.; Wang, Y.; Baker, K.; Dagar, S.S.; Griffith, G.W.; Farag, I.F.; et al. Horizontal gene transfer as an indispensable driver for evolution of Neocallimastigomycota into a distinct gut-dwelling fungal lineage. Appl. Environ. Microbiol. 2019, 85, e00988-19. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Ben David, Y.; Bensoussan, L.; Duncan, S.H.; Koropatkin, N.M.; Martens, E.C.; Flint, H.J.; Bayer, E.A. Enzymatic profiling of cellulosomal enzymes from the human gut bacterium, Ruminococcus champanellensis, reveals a fine-tuned system for cohesin-dockerin recognition. Environ. Microbiol. 2016, 18, 542–556. [Google Scholar] [CrossRef]
- Moraïs, S.; Barak, Y.; Lamed, R.; Wilson, D.B.; Xu, Q.; Himmel, M.E.; Bayer, E.A. Paradigmatic status of an endo- and exoglucanase and its effect on crystalline cellulose degradation. Biotechnol. Biofuels 2012, 5, 78. [Google Scholar] [CrossRef]
- Vazana, Y.; Moraïs, S.; Barak, Y.; Lamed, R.; Bayer, E.A. Interplay between Clostridium thermocellum family 48 and family 9 cellulases in cellulosomal versus noncellulosomal states. Appl. Environ. Microbiol. 2010, 76, 3236–3243. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Sathitsuksanoh, N.; Zhang, Y.H.P. Glycoside hydrolase family 9 processive endoglucanase from Clostridium phytofermentans: Heterologous expression, characterization, and synergy with family 48 cellobiohydrolase. Bioresour. Technol. 2010, 101, 5534–5538. [Google Scholar] [CrossRef]
- Ze, X.; David, Y.B.; Laverde-Gomez, J.A.; Dassa, B.; Sheridan, P.O.; Duncan, S.H.; Louis, P.; Henrissat, B.; Juge, N.; Koropatkin, N.M.; et al. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic Firmicutes bacterium Ruminococcus bromii. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef]
- Clayton, J.B.; Gomez, A.; Amato, K.; Knights, D.; Travis, D.A.; Blekhman, R.; Knight, R.; Leigh, S.; Stumpf, R.; Wolf, T.; et al. The gut microbiome of nonhuman primates: Lessons in ecology and evolution. Am. J. Primatol. 2018, 80, e22867. [Google Scholar] [CrossRef]
- Milton, K.; McBee, R.H. Rates of fermentative digestion in the howler monkey, Alouatta palliata (primates: Ceboidea). Comp. Biochem. Physiol. A Physiol. 1983, 74, 29–31. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Li, J.; Dai, L.; Xiong, C.; Tang, X.; Yang, Y.; Mu, Y.; Zhou, J.; Ding, J.; et al. Metagenomic analysis of the Rhinopithecus bieti fecal microbiome reveals a broad diversity of bacterial and glycoside hydrolase profiles related to lignocellulose degradation. BMC Genom. 2015, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Shively, C.A.; Register, T.C.; Appt, S.E.; Yadav, H.; Colwell, R.R.; Fanelli, B.; Dadlani, M.; Graubics, K.; Nguyen, U.T.; et al. Diet, obesity, and the gut microbiome as determinants modulating metabolic outcomes in a non-human primate model. Microbiome 2021, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Houtkamp, I.M.; van Zijll Langhout, M.; Bessem, M.; Pirovano, W.; Kort, R. Multiomics characterisation of the zoo-housed gorilla gut microbiome reveals bacterial community compositions shifts, fungal cellulose-degrading, and archaeal methanogenic activity. Gut Microbiome 2023, 4, e12. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fei, H.-L.; Luo, Z.-H.; Gao, S.-M.; Wang, P.-D.; Lan, L.-Y.; Zhao, X.-F.; Huang, L.-N.; Fan, P.-F. Gut microbiome responds compositionally and functionally to the seasonal diet variations in wild gibbons. npj Biofilms Microbiomes 2023, 9, 21. [Google Scholar] [CrossRef]
- Brune, A.; Friedrich, M. Microecology of the termite gut: Structure and function on a microscale. Curr. Opin. Microbiol. 2000, 3, 263–269. [Google Scholar] [CrossRef]
- Maurice, N.; Erdei, L. Termite Gut Microbiome. In Termites and Sustainable Management: Volume 1—Biology, Social Behaviour and Economic Importance; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 69–99. [Google Scholar]
- Brune, A.; Dietrich, C. The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef]
- Wenzel, M.; Schönig, I.; Berchtold, M.; Kämpfer, P.; König, H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 2002, 92, 32–40. [Google Scholar] [CrossRef]
- Ni, J.F.; Tokuda, G. Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol. Adv. 2013, 31, 838–850. [Google Scholar] [CrossRef]
- Hethener, P.; Brauman, A.; Garcia, J.L. Clostridium termitidis sp. nov., a cellulolytic bacterium from the gut of the wood-feeding termite, Nasutitermes lujae. Syst. Appl. Microbiol. 1992, 15, 52–58. [Google Scholar] [CrossRef]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandezgarayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A.E. The phylogeny of the genus Clostridium: Proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, B.; Dai, L.R.; Lawson, P.A.; Zheng, Z.Z.; Liu, L.Y.; Deng, Y.; Zhang, H.; Cheng, L. Petroclostridium xylanilyticum gen. nov., sp. nov., a xylan-degrading bacterium isolated from an oilfield, and reclassification of clostridial cluster III members into four novel genera in a new Hungateiclostridiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 3197–3211. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.I.; Spicer, V.; Shamshurin, D.; Krokhin, O.V.; Wilkins, J.; Ramachandran, U.; Sparling, R.; Levin, D.B. Quantitative proteomic analysis of the cellulolytic system of Clostridium termitidis CT1112 reveals distinct protein expression profiles upon growth on α-cellulose and cellobiose. J. Proteom. 2015, 125, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.I.; Spicer, V.; Krokhin, O.V.; Shamshurin, D.; Zhang, X.L.; Taillefer, M.; Blunt, W.; Cicek, N.; Sparling, R.; Levin, D.B. Transcriptomic and proteomic analyses of core metabolism in Clostridium termitidis CT1112 during growth on α-cellulose, xylan, cellobiose and xylose. BMC Microbiol. 2016, 16, 91. [Google Scholar] [CrossRef]
- Xu, C.; Huang, R.; Teng, L.; Jing, X.; Hu, J.; Cui, G.; Wang, Y.; Cui, Q.; Xu, J. Cellulosome stoichiometry in Clostridium cellulolyticum is regulated by selective RNA processing and stabilization. Nat. Commun. 2015, 6, 6900. [Google Scholar] [CrossRef]
- Riederer, A.; Takasuka, T.E.; Makino, S.; Stevenson, D.M.; Bukhman, Y.V.; Elsen, N.L.; Fox, B.G. Global gene expression patterns in Clostridium thermocellum as determined by microarray analysis of chemostat cultures on cellulose or cellobiose. Appl. Environ. Microbiol. 2011, 77, 1243–1253. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Miyata, R.; Noda, N.; Tamaki, H.; Kinjyo, K.; Aoyagi, H.; Uchiyama, H.; Tanaka, H. Influence of feed components on symbiotic bacterial community structure in the gut of the wood-feeding higher termite Nasutitermes takasagoensis. Biosci. Biotechnol. Biochem. 2007, 71, 1244–1251. [Google Scholar] [CrossRef]
- Marynowska, M.; Goux, X.; Sillam-Dussès, D.; Rouland-Lefèvre, C.; Halder, R.; Wilmes, P.; Gawron, P.; Roisin, Y.; Delfosse, P.; Calusinska, M. Compositional and functional characterisation of biomass-degrading microbial communities in guts of plant fibre- and soil-feeding higher termites. Microbiome 2020, 8, 96. [Google Scholar] [CrossRef]
- Watanabe, H.; Tokuda, G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef]
- Senadheera, U.E.; Jayasanka, D.J.; Udayanga, D.; Hewawasam, C. Natural and designer cellulosomes: A potential tool for enhancing microbial additive-mediated lignocellulosic agricultural waste composting. Bioresour. Technol. Rep. 2024, 25, 101695. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Yan, F.; Dong, W.; Sun, Y.; Wei, R.; Feng, Y. Optimized whole-cell depolymerization of polyethylene terephthalate to monomers using engineered Clostridium thermocellum. J. Hazard. Mater. 2025, 488, 137441. [Google Scholar] [CrossRef] [PubMed]
- Aer, L.; Jiang, Q.; Zhong, L.; Si, Q.; Liu, X.; Pan, Y.; Feng, J.; Zeng, H.; Tang, L. Optimization of polyethylene terephthalate biodegradation using a self-assembled multi-enzyme cascade strategy. J. Hazard. Mater. 2024, 476, 134887. [Google Scholar] [CrossRef]


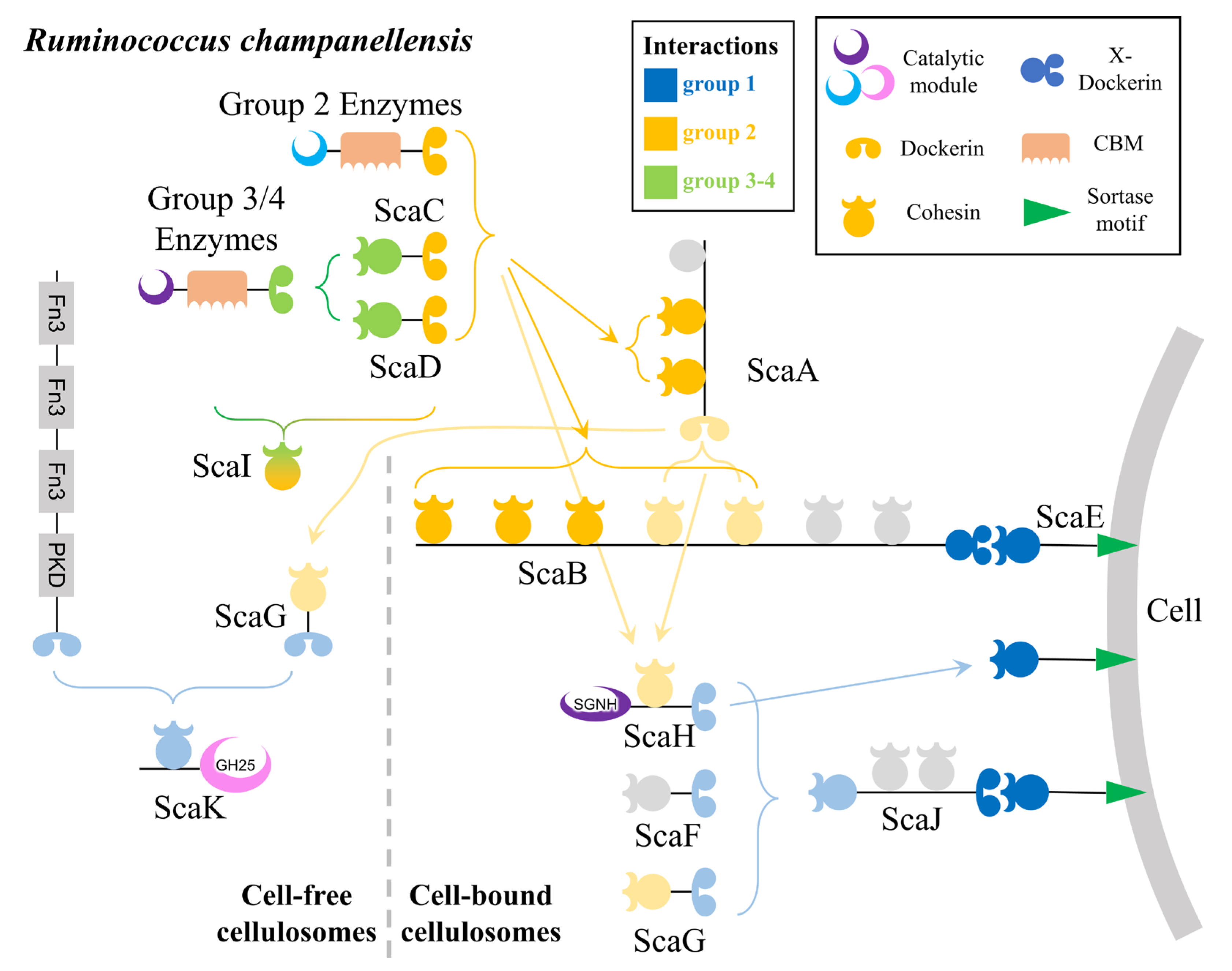
| Host | Strain | Scaffoldins/Cohesins | Dockerin-Containing Proteins/CAZymes | Genome Accession Number | Reference |
|---|---|---|---|---|---|
| Cow | Ruminococcus flavefaciens FD-1 | 17/27 | 223/154 | ACOK00000000 | [27,28] |
| Cow | Ruminococcus flavefaciens 17 | 11/21 | 180/123 | AFNE00000000 | [28,29] |
| Cow | Ruminococcus flavefaciens 007c | 10/16 | 183/122 | ATAX01000000 | [28,30] |
| Cow | Ruminococcus albus 7 | 1/1 | 90/122 | GCA_000179635.2 | [28,31] |
| Cow | Ruminococcus albus 8 | 0/0 | 62/114 | GCF_000178155.2 | [28] |
| Sheep | Ruminococcus albus SY3 | 1/1 | 58/124 | GCF_000586615.1 | [28] |
| Human | Ruminococcus champanellensis 18P13 | 11/20 | 64/107 | FP929052.1 | [20,24] |
| Human and nonhuman primate | Ruminococcus primaciens, Ruminococcus hominiciens and Ruminococcus ruminiciens | - | - | - | [25] |
| Termite | Clostridium termitidis CT1112 | 5/5 | 22/355 | AORV00000000 | [26,32] |
| Sheep | Anaeromyces robustus | 26/- | 276/- | MCFG00000000 | [33] |
| Goat | Neocallimastix californiae | 55/- | 422/- | MCOG00000000 | [33] |
| Horse | Piromyces finnis | 14/- | 227/- | MCFH00000000 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Zhang, M.; Chen, C.; Feng, Y.; Xuan, J. Cellulosome Systems in the Digestive Tract: Underexplored Enzymatic Machine for Lignocellulose Bioconversion. Catalysts 2025, 15, 387. https://doi.org/10.3390/catal15040387
Qi J, Zhang M, Chen C, Feng Y, Xuan J. Cellulosome Systems in the Digestive Tract: Underexplored Enzymatic Machine for Lignocellulose Bioconversion. Catalysts. 2025; 15(4):387. https://doi.org/10.3390/catal15040387
Chicago/Turabian StyleQi, Jiajing, Mengke Zhang, Chao Chen, Yingang Feng, and Jinsong Xuan. 2025. "Cellulosome Systems in the Digestive Tract: Underexplored Enzymatic Machine for Lignocellulose Bioconversion" Catalysts 15, no. 4: 387. https://doi.org/10.3390/catal15040387
APA StyleQi, J., Zhang, M., Chen, C., Feng, Y., & Xuan, J. (2025). Cellulosome Systems in the Digestive Tract: Underexplored Enzymatic Machine for Lignocellulose Bioconversion. Catalysts, 15(4), 387. https://doi.org/10.3390/catal15040387









